The phylogenetic placement of the enigmatic pseudoscorpion family Menthidae (Pseudoscorpiones): a revised superfamily assignment based on new molecular data
Mark S. Harvey A B * , Ligia R. Benavides
A B * , Ligia R. Benavides  C , Terrence L. Miller D , Julia G. Cosgrove
C , Terrence L. Miller D , Julia G. Cosgrove  C , Gonzalo Giribet
C , Gonzalo Giribet  C and Michael G. Rix
C and Michael G. Rix  D
D
A
B
C
D
Abstract
Pseudoscorpions are an ancient arachnid group with a fossil record that extends to the Devonian, with all modern families having likely evolved during the Mesozoic. One of the rarest pseudoscorpion families, Menthidae, is sporadically distributed around the world, and ever since its description has been included in the superfamily Garypoidea. Based on new Sanger sequencing and phylotranscriptomic data, Menthidae are inferred to be a member of the superfamily Neobisioidea, and the sister-group to a clade that includes Gymnobisiidae, Neobisiidae and some Syarinidae.
Keywords: Arachnida, Iocheirata, morphology, Neobisioidea, phylogeny, phylotranscriptomic analysis, Sanger sequencing.
Introduction
The arachnid order Pseudoscorpiones is an ancient, moderately diverse group of arachnids with ~4060 species and 479 genera (World Pseudoscorpiones Catalog 2025). The smallest adults are less than 1 mm in body length and the largest are ~1 cm. Although their fossil record is incomplete, the earliest fossils are cuticular fragments from the Middle Devonian (Schawaller et al. 1991; Judson 2012), with all subsequent fossils from the Mesozoic and Cenozoic being readily assigned to modern families (Harms and Dunlop 2017), suggesting deep origins for all clades (see also Benavides et al. 2019). Judson (2012) regarded Dracochela Schawaller, Shear & Bonamo, 1991 as a stem-group pseudoscorpion, but Benavides et al. (2019) included it in its own suborder of crown-group pseudoscorpions. Pseudoscorpions have a worldwide distribution and can be found in most terrestrial ecosystems.
The past three decades has seen various phylogenetic hypotheses for the entire order with a morphology-based cladistic treatment (Harvey 1992), and molecular phylogenies based on three genes (Murienne et al. 2008; Arabi et al. 2012; Harvey et al. 2016b) or transcriptomes (Benavides et al. 2019). Despite iterative modifications to the classification, the results of these analyses are now converging on a phylogenetic classification that comprises three modern suborders: Heterosphyronida with Chthoniidae and Pseudotyrannochthoniidae (superfamily Chthonioidea); Atoposphyronida with Feaellidae and Pseudogarypidae (Feaelloidea); and Iocheirata with six superfamilies including Neobisioidea (Bochicidae, Gymnobisiidae, Hyidae, Ideoroncidae, Neobisiidae, Parahyidae and Syarinidae), Garypoidea (Garypidae, Geogarypidae, Hesperolpiidae, Menthidae and Olpiidae), Garypinoidea (Garypinidae and Larcidae), Sternophoroidea (Sternophoridae), Cheiridioidea (Cheiridiidae and Pseudochiridiidae) and Cheliferoidea (Atemnidae, Cheliferidae, Chernetidae and Withiidae). Confidence in the strength of the various phylogenies has grown over time such that changes to the pseudoscorpion classification have been implemented to better reflect the phylogenetic treatments. For example, Murienne et al. (2008) recovered a paraphyletic Garypoidea, which was only formalised based on the more comprehensive phylogenomic treatment by Benavides et al. (2019) with the recognition of a separate superfamily Garypinoidea in addition to a reduced Garypoidea.
Previous molecular studies have included species from most pseudoscorpion families, thus providing relatively good coverage across the phylogenetic breadth of the order. However, two enigmatic families – Menthidae and Pseudochiridiidae – have so far lacked any molecular data and have never been included in molecular analyses. Both are rarely encountered and are sparsely distributed around the world.
Ever since the genus Menthus Chamberlin, 1930 and the family Menthidae were first erected (Chamberlin 1930), they have been mostly considered to be garypoids with the greatest morphological similarity (Fig. 1) to Olpiidae (Chamberlin 1931; Beier 1932; Muchmore 1982; Harvey and Muchmore 1990). The only exception was the inclusion of Olpiidae and Menthidae in their own superfamily Olpioidea based on the results of a morphological cladistic analysis (Harvey 1992). Olpioidea was abandoned by Benavides et al. (2019), and both families were returned to the Garypoidea. Menthids differ from the other garypoid families in a suite of unusual features such as the presence of 11 trichobothria on the fixed chelal finger and hand, the absence of the venom gland in the movable chelal finger and the presence of a specialised articulation joint between coxae II and III (Chamberlin 1930, 1931; Harvey and Muchmore 1990; Harvey 1992). Nevertheless, their affinities have never been questioned.
The recent collection of fresh specimens of the menthid Thenmus aigialites Harvey, 1990 from eastern Australia has allowed us to test the phylogenetic position of this enigmatic family, and to compare it with independent transcriptome data obtained from Menthus californicus Chamberlin, 1930 from south-western USA. To our surprise, menthids were recovered within the superfamily Neobisioidea, which prompted a renewed examination of their morphological traits.
Methods
We analysed two independent data sets, incorporating a wide variety of different pseudoscorpion taxa, with the aim of robustly assessing the phylogenetic position of Menthidae. These included a Sanger sequencing-based phylogeny with new sequence data and a novel menthid transcriptome that was added to the phylogenomic analysis of Benavides et al. (2019). The results from these two molecular independent data sets (different taxa, different markers) were used to investigate the systematic position of Menthidae. Chitrella or any of its putative relatives (i.e. members of the syarinid subfamily Chitrellinae) could not be included in the phylogenomic analysis.
The first dataset was an extension of previous Sanger amplicon studies using the mitochondrial cytochrome c oxidase subunit 1 (COI), and two nuclear genes, the small ribosomal subunit (18S rRNA, 18S) and the large ribosomal subunit (28S rRNA, 28S) (Murienne et al. 2008; Arabi et al. 2012; Harvey et al. 2015, 2016a, 2016b, 2020), with the inclusion of two menthids. Thenmus aigialites was sequenced for all three markers, based on a newly collected specimen from eastern Queensland. Menthus californicus (MCZ:IZ:149277) was also included based on a 1047-bp fragment of 18S (GenBank Accession: MW463272.1) extracted from the transcriptome reads. As the monophyly of Iocheirata has been convincingly demonstrated in all previous Sanger-based (Murienne et al. 2008; Arabi et al. 2012; Harvey et al. 2016b) and transcriptomic (Benavides et al. 2019; Ontano et al. 2021; Ballesteros et al. 2022) studies, only two outgroups from the other suborders were added, Austrochthonius sp. representing Heterosphyronida and Neopseudogarypus scutellatus Morris, 1948 for Atoposphyronida. Menthidae is unequivocally a member of the Iocheirata due to the presence of a venom apparatus in the fixed chelal finger (Chamberlin 1930; Harvey 1992). The number of exemplars in Cheliferoidea was also reduced (six exemplars; four families), as there was no evidence that menthids are closely related to them based on morphological criteria, including the presence of two pairs of eyes in most menthids, separate metatarsi and tarsi and the lack of spermathecae in the female genital system (Chamberlin 1931; Harvey 1992). The number of exemplars in other superfamilies were as follows: Neobisioidea (28 exemplars; 6 families); Garypoidea (27 exemplars; 4 families); Garypinoidea (10 exemplars; 2 families); Sternophoroidea (1 exemplar; 1 family); and Cheiridioidea (1 exemplar; 1 family) (Table 1).
| Family | Species | Repository and number | COI | 18S | 28S | |
|---|---|---|---|---|---|---|
| Superfamily Chthonioidea | ||||||
| Chthoniidae | Austrochthonius sp. | WAM T135835 | OR067318 | OR039602 | OR059142 | |
| Superfamily Feaelloidea | ||||||
| Pseudogarypidae | Neopseudogarypus scutellatus Morris, 1948 | WAM T104213 | OR067276 | OR039591 | OR059173 | |
| Superfamily Neobisioidea | ||||||
| Gymnobisiidae | Gymnobisium inukshuk Harvey & Giribet, 2016 | MCZ:IZ:21577 | KU057099 | KU057097 | KU057098 | |
| Gymnobisium sp. | WAM T132111 | KU156712 | KU156705 | – | ||
| Mirobisium sp. 1 | DNA102450; MCZ:IZ:130497 | EU559547 | EU559369 | EU559473 | ||
| Mirobisium sp. 2 | WAM T135413 | – | KU156706 | KU156710 | ||
| Hyidae | Hya minuta (Tullgren, 1905) | WAM T146845 | OR067360 | OR039622 | OR059105 | |
| Indohya humphreysi (Harvey, 1993) | WAM T136663 | OR067321 | OR039605 | OR059139 | ||
| Indohya typhlops Harvey, 1993 | WAM T158275 | OR067370 | OR039627 | OR059095 | ||
| Ideoroncidae | Dhanus sumatranus (Redikorzev, 1922) | MCZ:IZ:49986 | – | MW463270 | MW513995 | |
| WAM T135069 | OR067316 | OR039600 | – | |||
| Pseudalbiorix veracruzensis (Hoff, 1945) | MCZ:IZ:130499 | EU559567 | EU559427 | EU559474 | ||
| 'Genus indet.’ sp. JA-2011 | MNHN-JAD70 | JN018183 | JN018300 | JN018397 | ||
| Menthidae | Menthus californicus Chamberlin, 1930 | MCZ:IZ:149277 | – | – | MW463272 | |
| Thenmus aigialites Harvey, 1990 | WAM T165567 | PQ834818 | PQ834806 | PQ834808 | ||
| Neobisiidae | Bisetocreagris sp. 1 | MNHN-JAD69 | JN018181 | JN018298 | JN018395 | |
| Halobisium occidentale Beier, 1931 | WAM T126234 | PQ834817 | PQ834805 | PQ834807 | ||
| Lissocreagris sp. JM-2008 | MCZ:IZ:130501 | EU559555 | EU559392 | EU559450 | ||
| Microbisium parvulum (Banks, 1895) | MCZ:IZ:130502 | EU559558 | EU559371 | EU559476 | ||
| Neobisium carcinoides (Hermann, 1804) | WAM T143210 | OR067357 | OR039620 | OR059108 | ||
| Novobisium tenue (Chamberlin, 1930) | MCZ:IZ:-130504 | EU559559 | EU559407 | EU559452 | ||
| Roncus transsilvanicus Beier, 1928 | MCZ:IZ:130505 | MF124523 | MF124385 | |||
| Stenohya hamata (Leclerc & Mahnert, 1988) | MCZ:IZ:130507 | EU559498 | EU559370 | EU559475 | ||
| Tuberocreagris lata (Hoff, 1945) | MCZ DNA102419 | EU559552 | EU559406 | EU559451 | ||
| Parahyidae | Parahya submersa (Bristowe, 1931) | MCZ:IZ:130517; WAM T57298 | EU559548 | EU559426 | EU559478 | |
| Syarinidae | Alocobisium sp. | WAM T146854 | OR067364 | OR039626 | OR059101 | |
| Anysrius chamberlini Harvey, 1998 | WAM T127771 | OR067305 | OR039596 | OR059150 | ||
| Chitrella cala (Chamberlin, 1930) | MCZ:IZ:130522 | EU559551 | EU559373 | EU559479 | ||
| Ideobisium sp. JM-2008 | MCZ:IZ:130523 | EU559549 | EU559429 | EU559458 | ||
| Ideoblothrus pisolitus Harvey & Edward, 2007 | WAM T138535 | OR067333 | OR039615 | OR059131 | ||
| Nannobisium sp. JM-2008 | MCZ:IZ:130525 | EU559561 | EU559375 | EU559481 | ||
| Syarinus sp. JM-2008 | MCZ DNA102402; MCZ:IZ:130522; MCZ:IZ:130526 | EU559550 | EU559386 | EU559437 | ||
| Superfamily Garypoidea | ||||||
| Garypidae | Ammogarypus lawrencei Beier, 1962 | WAM T132031 | MN058676 | MN065589 | MN065614 | |
| Anagarypus australianus Muchmore, 1978 | WAM T144963 | MN058692 | MN065607 | MN065632 | ||
| Anchigarypus californicus (Banks, 1909) | WAM T92262 | MN058668 | MN065584 | MN065610 | ||
| Anchigarypus japonicus (Beier, 1952) | WAM T140775 | MN058687 | MN065603 | MN065628 | ||
| Garypus beauvoisii (Audouin, 1826) | WAM T143813 | MN058691 | MN065606 | MN065631 | ||
| Garypus latens Harvey, 2020 | WAM T143502 | MN058690 | MN065605 | MN065630 | ||
| Synsphyronus gracilis Harvey, 1987 | WAM T122371 | MN058669 | MN065585 | MN065611 | ||
| Synsphyronus xynus Cullen & Harvey, 2021 | WAM T133129 | MN058679 | MN065595 | MN065620 | ||
| Thaumastogarypus robustus Beier, 1947 | WAM T132035 | MN058678 | MN065592 | MN065617 | ||
| Thaumastogarypus sp. | WAM T132030 | MN058675 | MN065588 | MN065613 | ||
| Geogarypidae | Afrogarypus purcelli (Ellingsen, 1912) | Afrogarypus_purcelli_1 | KP331817 | – | KP297850 | |
| Geogarypus connatus Harvey, 1986 | WAM T135408 | OR359475 | – | OR372786 | ||
| WAM T135409 | – | OR359929 | OR372785 | |||
| Geogarypus deceptor Neethling & Haddad, 2016 | MCZ:IZ:130496 | EU559560 | EU559385 | EU559436 | ||
| Geogarypus longidigitatus (Rainbow, 1897) | WAM T146858 | MN058693 | MN065608 | – | ||
| Geogarypus minor (L. Koch, 1873) | MNHN-JAD10 | JN018180 | JN018297 | JN018394 | ||
| Hesperolpiidae | Apolpium parvum Hoff, 1945 | MCZ:IZ:130509 | EU559541 | EU559380 | EU559489 | |
| Nanolpium sp. JM-2008 | MCZ:IZ:130513 | EU559543 | EU559390 | EU559445 | ||
| Pachyolpium sp. JM-2008 | MCZ:IZ:130514 | EU559542 | EU559421 | EU559488 | ||
| Progarypus sp. JM-2008 | MCZ:IZ:130515 | EU559538 | EU559420 | EU559490 | ||
| Olpiidae | Antillolpium sp. 772A | sp. 772A | KX263395 | – | KX263356 | |
| Austrohorus sp. | WAM T135388 | MN058681 | MN065597 | MN065622 | ||
| Beierolpium bornemisszai (Beier, 1966) | MCZ:IZ:130510 | EU559545 | EU559378 | EU559486 | ||
| Beierolpium sp. | WAM T136468 | MN058684 | MN065600 | MN065625 | ||
| Calocheiridius cf. termitophilus | MCZ:IZ:130511 | EU559544 | EU559359 | EU559460 | ||
| Euryolpium sp. JM-2008 | MCZ:IZ:130512 | EU559546 | EU559379 | EU559487 | ||
| Euryolpium sp. | WAM T136709 | MN058685 | MN065601 | MN065626 | ||
| Indolpium sp. | WAM T136397 | MN058683 | MN065599 | MN065624 | ||
| Superfamily Garypinoidea | ||||||
| Garypinidae | Amblyolpium sp. PSE215 | WAM T146503 | OR359473 | OR359932 | OR372787 | |
| Aldabrinus rixi Harvey, 2023 | WAM T143175 | OR359471 | OR359931 | OR372788 | ||
| Garypinus sp. JA-2011 | MNHN-JAD71 | JN018179 | JN018296 | JN018393 | ||
| Nobilipinus karenae Harvey, 2023 | BRUN001; BMKB | OR359476 | OR359936 | OR372784 | ||
| Protogarypinus giganteus Beier, 1954 | WAM T147599 | OR359480 | OR359938 | OR372783 | ||
| Pseudogarypinus cooperi Muchmore, 1980 | WAM T63231; MCZ:IZ:130495 | EU559566 | EU559423 | EU559485 | ||
| Serianus sp. | WAM T152657 | OR359484 | OR359942 | OR372779 | ||
| Solinus sp. PSE214 | WAM T152660 | OR359485 | OR359943 | OR372777 | ||
| Larcidae | Larca granulata (Banks, 1891) | WAM T143199 | – | OR359930 | OR372789 | |
| Larca lata (Hansen, 1885) | MCZ:IZ:130500 | EU559563 | EU559425 | – | ||
| Superfamily Cheiridioidea | ||||||
| Cheiridiidae | Cheiridiinae sp. JM-2008 | WAM T65474 | EU559570 | EU559424 | EU559483 | |
| Superfamily Sternophoroidea | ||||||
| Sternophoridae | Afrosternophorus sp. JM-2008 | MCZ:IZ:130520 | EU559568 | EU559360 | EU559461 | |
| Superfamily Cheliferoidea | ||||||
| Atemnidae | Oratemnus curtus (Beier, 1954) | WAM T65458 | EU559531 | EU559361 | – | |
| Stenatemnus sp. JM-2008 | MCZ:IZ:130455 | EU559529 | EU559394 | EU559448 | ||
| Cheliferidae | Chelifer cancroides (Linnaeus, 1758) | WAM T130755 | KT354337 | KT354350 | KT354343 | |
| Chernetidae | Calymmachernes angulatus Beier, 1954 | MCZ:IZ:130462 | EU559525 | EU559383 | EU559496 | |
| Megachernes sp. | WAM T143612 | OQ330006 | OQ540495 | OQ517183 | ||
| Withiidae | Withius sp. 1 JM-2008 | MCZ:IZ:130528 | EU559571 | EU559417 | EU559435 | |
For the Sanger-sequencing dataset, fragments of COI, 18S and 28S were sequenced using standard amplicon methodologies, as outlined in previous studies on pseudoscorpions (Murienne et al. 2008; Harvey et al. 2015, 2016a, 2016b, 2020) – with the exception mentioned above for Menthus californicus. The molecular methods used for the polymerase chain reaction (PCR) amplification of each of these genes for more recently sequenced specimens followed Harvey et al. (2015, 2016a, 2020), with PCR purification and Sanger bidirectional sequencing conducted by the Australian Genome Research Facility (AGRF; Perth). Chromatograms were edited using the Geneious software package (ver. 2021.2, Biomatters Ltd, see https://www.geneious.com/, accessed June 2021), and resulting nucleotide sequences for all taxa are deposited in GenBank (Table 1). The sequences were aligned using the MAFFT (ver. 7.490, see https://mafft.cbrc.jp/alignment/software/; Katoh et al. 2002; Katoh and Standley 2013) plug-in within Geneious with the default settings. A total of 3657 aligned base pairs (bp) were analysed, including 658 bp for COI, 1795 bp for 18S and 1204 bp for 28S. The concatenated alignment was analysed using maximum likelihood (ML) and Bayesian inference (BI). ML analysis was run in the web version of IQ-TREE (ver. 2.2.0, see https://github.com/iqtree/iqtree2; Trifinopoulos et al. 2016; Minh et al. 2020), and the data were partitioned per gene. The substitution model option was set to Auto, and branch support was assessed with 5000 replicates of the ultrafast bootstrap approximation (Hoang et al. 2018) (bootstrap support or BS hereafter). BI was implemented in MrBayes (ver. 3.2.7, see https://github.com/NBISweden/MrBayes/; Ronquist et al. 2012); each partition had a unique GTR substitution model with gamma-distributed rate variation across sites and a proportion of invariant sites (GTR + Γ + Ι). The analysis consisted of two runs of 5 million generations each, with four chains, sampling every 100 generations, evaluating convergence of the independent runs through the standard deviation of split frequencies in Tracer (ver. 1.7.2, see https://github.com/beast-dev/tracer/releases/tag/v1.7.2; Rambaut et al. 2018). A consensus tree was estimated after discarding a burn-in of 10% of the sampled trees.
Both Sanger-sequencing analyses were rooted on Austrochthonius sp., a member of the superfamily Chthonioidea (World Pseudoscorpiones Catalog 2025), which represents the sister taxon to the suborders Atoposphyronida + Iocheirata (Benavides et al. 2019).
The phylotranscriptomic dataset was an expanded version of the transcriptomic study undertaken by Benavides et al. (2019), with the addition of Menthus californicus (see https://mczbase.mcz.harvard.edu/guid/MCZ:IZ:149277), and the exclusion of Cybella gelanggi Harvey, 2018 due to the low number of BUSCO genes present in this assembly (Supplementary Table S1). The majority of the 26 pseudoscorpion families were included in the study, with 23 included in both the phylotranscriptomic and Sanger datasets. The exceptions were Bochicidae for which we lacked Sanger data, and Amblyolpiidae and Olpiidae for which we lacked transcriptomic data.
The raw reads of M. californicus were processed following the same pipeline as for the other taxa in Benavides et al. (2019). BUSCO (Manni et al. 2021a) was used to quantify the completeness of our assemblies (Table S1) by comparing them with the Arthropoda database (Manni et al. 2021b).
The Orthologous Matrix (OMA) database standalone (ver. 2.6.0, see https://github.com/DessimozLab/OmaStandalone; Altenhoff et al. 2019) was used to infer orthologous genes across our samples; each OMA-generated orthogroup was aligned individually using MAFFT (ver. 7.309; Katoh and Standley 2013) eliminating positions with more than 80% missing data with a custom script (trimEnds.sh). One matrix was assembled targeting a minimum gene occupancy of 75% meaning that an OMA orthogroup was selected if present in at least 75% of the taxa. Our resulting matrix consisted of 235 loci (58,041 aa).
The transcriptomic matrix was analysed under maximum likelihood (ML) and Bayesian inference (BI). ML analysis was run in IQ-TREE multicore version (ver. 2.2.2.7, see https://github.com/iqtree/iqtree2) on the FAS Research Computer cluster, performing a gene-partitioned analysis (PART) with the option MFP + MERGE, which selects the best-fit partitioning scheme by merging partitions to reduce over-parameterisation and increase model fit, and including the LG4 mixture models. Nodal support was calculated using 1000 ultrafast bootstrap replicates (-bb 1000; Hoang et al. 2018). The unpartitioned matrix was used to run a BI analysis in ExaBayes (ver. 1.5.1, see https://github.com/aberer/exabayes; Aberer et al. 2014) under the default GTR model (Lartillot and Philippe 2004). The Markov Chain Monte Carlo (MCMC) was configured with two runs and two coupled chains per independent run, each with one cold and three hot chains sampling every 500 until the average standard deviation of split frequencies (ASDSF) was <0.02. Tracer (ver. 1.7.2; Rambaut et al. 2018) was used to check parameter convergence and effective sample size (ESS) of the MCMC runs. The runs converged after ~1.7 × 106 generations and the first 25% of trees were discarded as burn-in.
Divergence time inference
To estimate divergence dates we employed an approximate likelihood Bayesian inference in MCMCtree (see https://github.com/dosreislab/mcmctree; Yang and Rannala 2006), contained in PAML (ver. 4.10.7, see https://github.com/abacus-gene/paml; Yang 2007), with a node-calibrated Bayesian relaxed clock. CODEML was used to estimate an approximate substitution rate as well as a gradient (g) and Hessian (H) of the branch lengths without the clock (usedata = 3). The resulting gradient and Hessian were then used to estimate divergence times with the approximate likelihood method (usedata = 2; dos Reis and Yang 2011; see https://github.com/abacus-gene/paml-tutorial) and the transcriptomics-based IQ-TREE topology as a guide tree. We implemented a uniform prior distribution for all node calibrations, an independent rate model, a birth–death prior on the divergence time, and the LG protein model with gamma rates among sites (Le and Gascuel 2008). Twelve nodes were calibrated with soft minima from fossil evidence with crown groups defined in Table 2.
| Node in tree | Taxon | Oldest fossil | Deposit | Reference | Notes | Maxima (Ma) | Minima (Ma) | Label in MCMCtree | |
|---|---|---|---|---|---|---|---|---|---|
| ROOT | Euchelicerata | 528 | 476 | ||||||
| Suborder Palaeosphyronida | |||||||||
| 1 | Dracochelidae | Dracochela deprehendor Schawaller, Shear & Bonamo, 1991 | Paleozoic: Givetian–Eifelian: Gilboa shales | Judson (2012) | Split Pseudoscorpions–sister group | 500.5 | 382.7 | B (3.82, 5.00) | |
| Suborder Heterosphyronida | |||||||||
| 2 | Chthonioidea: Chthoniidae | Weygoldtiella plausus Harvey, Cosgrove, Harms, Selden, Shih & Wang, 2018 | Mesozoic: Cretaceous: lowermost Cenomanian: Burmese amber | Harvey et al. (2018) | Split Chthoniidae–Pseudotyranochthoniidae | 382.7 | 99 | B (0.99, 3.82) | |
| Suborder Atoposphyronida | |||||||||
| 3 | Feaelloidea: Feaellidae | Archaeofeaella hendrickxi Kolesnikov, Turbanov, Eskov, Propistsova, Bashkuev & Taylor, 2022 | Mesozoic: Upper Triassic: late Carnian or early Norian | Kolesnikov et al. (2022) | Split Pseudogarypidae–Feaellidae | 382.7 | 227 | B (2.27, 3.82) | |
| Suborder Iocheirata | |||||||||
| 4 | Neobisioidea: Ideoroncidae | Proalbiorix compactus Geißler, Kotthoff, Hammel, Harvey & Harms, 2022 | Mesozoic: Cretaceous: lowermost Cenomanian: Burmese amber | Geißler et al. (2022) | Split Ideoroncidae–Bochichidae + Hyidae | 382.7 | 99 | B (0.99, 3.82) | |
| 5 | Neobisioidea: Hyidae | Hya fynni Röschmann, Harvey, Hou, Harms, Kotthoff, Hammel, Ren & Loria, 2024 | Mesozoic: Cretaceous: lowermost Cenomanian: Burmese amber | Röschmann et al. (2024) | Split Hyidae–Bochichidae | 382.7 | 99 | B (0.99, 3.82) | |
| 6 | Neobisioidea: Neobisiidae | Neobisium henderickxi Judson, 2003 | Cenozoic: Eocene: Lutetian: Baltic amber | Judson (2003) | Split Neobisiidae–Gymnobisiidae | 382.7 | 49 | B (0.49, 3.82) | |
| 7 | Garypoidea: Geogarypidae | Geogarypus gorskii Henderickx, 2005 | Cenozoic: Eocene: Lutetian: Baltic amber | Henderickx (2005) | Split Geogarypidae–Other Garypoidea | 382.7 | 49 | B (0.49, 3.82) | |
| 8 | Garypinoidea: Garypinidae | Amblyolpium burmiticum (Cockerell, 1920) | Mesozoic: Cretaceous: lowermost Cenomanian: Burmese amber | Judson (1997) | Split Protogarypinus– Pseudogarypinus + Larca | 382.7 | 99 | B (0.99, 3.82) | |
| 9 | Cheiridioidea: Cheiridiidae | Electrobisium acutum (Cockerell, 1917) | Mesozoic: Cretaceous: lowermost Cenomanian: Burmese amber | Judson (2000) | Split Cheiridiidae–Sternophoridae | 382.7 | 99 | B (0.99, 3.82) | |
| 10 | Cheliferoidea: Chernetidae | Chernetidae sp. indet. | Mesozoic: Cretaceous: lowermost Cenomanian: Burmese amber | Benavides et al. (2019) | Split Chernetidae–rest of Cheliferoidea | 382.7 | 99 | B (0.99, 3.82) | |
| 11 | Cheliferoidea: Cheliferidae | Heurtaultia rossiorum Judson, 2009 | Mesozoic: Cretaceous: late Albian: Archingeay amber | Judson (2009) | Split Cheliferidae–Withiidae + Attemnidae | 382.7 | 99 A | B (1.00, 3.82) | |
| 12 | Cheliferoidea: Atemnidae | Progonatemnus succineus Beier, 1955 | Cenozoic: Eocene: Lutetian: Baltic amber | Beier (1955) | Split Withiidae–Attemnidae | 99 | 49 | B (0.49, 1.00) | |
The phylogeny was dated using the oldest identified fossils for individual families (Table 2), as explained in Benavides et al. (2019). The rationale for the splits between individual clades remains the same. However, three new calibration points were added to our analyses: to constrain the split between Neobisiidae and Gymnobisiidae the fossil neobisiid Neobisium henderickxi Judson, 2003 from Baltic amber was used. Geogarypus gorskii Henderickx, 2005, also from Baltic amber, was used to constrain the split between Geogarypidae and Hesperolpiidae + Garypidae. Lastly, Progonatemnus succineus Beier, 1955, constrained the split between Atemnidae and Withiidae. The transfer of Amblyolpium to Amblyolpiidae by Gao et al. (2025) post-dated the present analysis, so we retained the former familial placement (Table 2). This change does not affect the outcomes of the dating analysis.
Four independent MCMC chains were run with a burn-in of 5000, sampling frequency of 10, and a sample size of 50,000. Convergence of the runs and ESS were assessed in Tracer (ver. 1.7.2; Rambaut et al. 2018). A phylogeny annotated with all calibration distribution parameters can be found in Supplementary Fig. S2.
Results
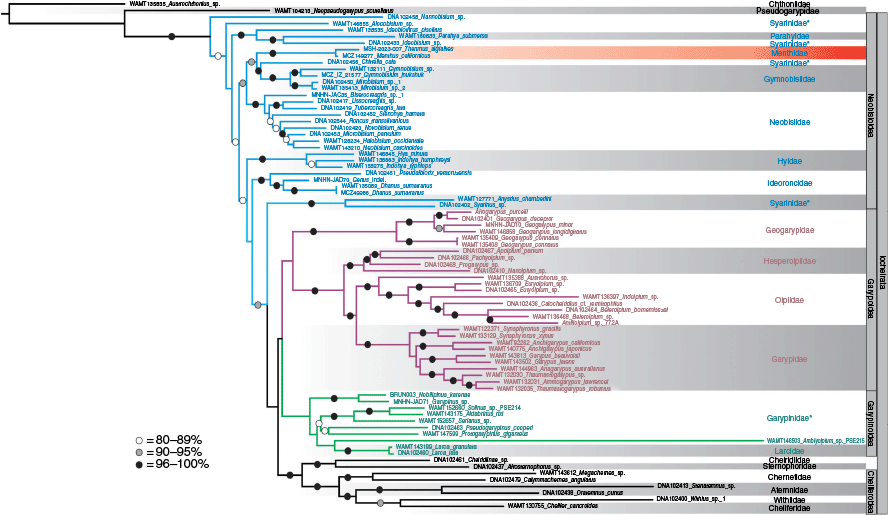
For the discussion, we draw on the Sanger and transcriptome IQ-TREE analyses as our reference trees, but the resulting topologies for all our analyses (ML – Sanger, Fig. 2; BI – Sanger, Fig. S2; ML – Transcriptomes, Fig. 3; BI – Transcriptomes, Fig. S3 of the Supplementary material), are virtually identical and unambiguously place Menthidae within the superfamily Neobisioidea, and not within Garypoidea or Olpioidea as previously postulated (Chamberlin 1930, 1931; Harvey 1992; Benavides et al. 2019). In the Sanger-based analysis, Thenmus and Menthus were recovered as sister taxa (Fig. 2) in a clade with Gymnobisiidae and the syarinid Chitrella Beier, 1932 (BS = 93%), which was in turn sister group to all nine genera of Neobisiidae. The phylotranscriptomic analysis (Fig. 3) placed Menthus as a sister group to a clade that includes Gymnobisium (Gymnobisiidae) and Neobisiidae, albeit with 55% BS. The strong concordance between both analyses strongly supports menthids being a neobisioid and a member of the clade that also contains Gymnobisiidae and Neobisiidae.
Maximum likelihood phylogeny of selected pseudoscorpions, based on alignment of concatenated cytochrome c oxidase subunit I (COI), 18S rRNA (18S) and 28S rRNA (28S). Bootstrap support values are presented for nodes greater than 80%.
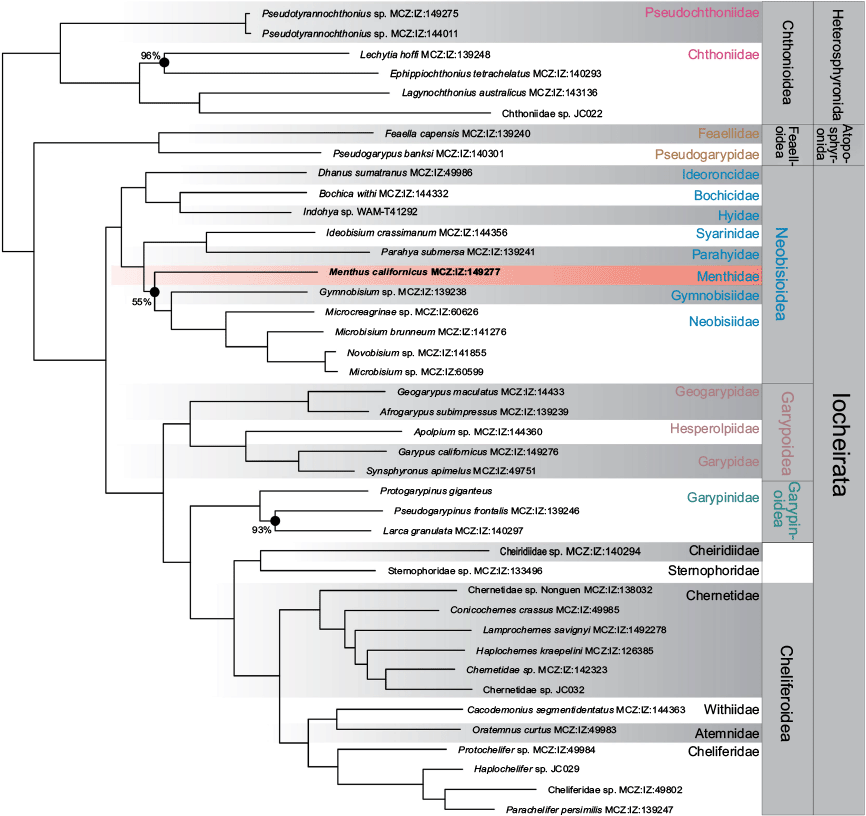
Pseudoscorpion phylogeny inferred from the maximum likelihood analysis of 235 lociorthogroups (58,041 aa; 75% occupancy) in IQ-TREE partitioned by gene and model search including LG4 mixture models and accounting for heterotachy (lnL = −1,232,140.595). Nodes without values have maximal support.
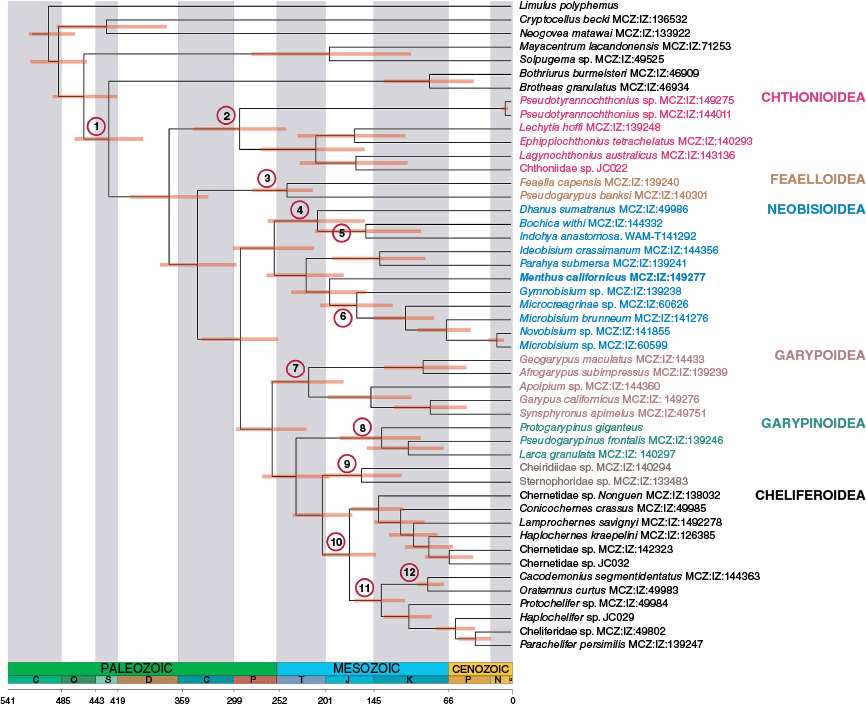
The dating analysis infers that menthids diverged from Gymnobisiidae + Neobisiidae in the early Mesozoic with a median expected divergence in the early Jurassic (Fig. 4). Gymnobisiidae and Neobisiidae then diverged in the mid-Mesozoic, and all such dates are consistent with the dating analysis presented by Benavides et al. (2019). Indeed, all pseudoscorpion families have arisen during the Mesozoic, as evidenced by our analysis that is predicated on numerous Cretaceous fossils that are readily assigned to modern families (Table 2).
MCMCtree chronogram showing divergence time estimates for Pseudoscorpiones evolution obtained from the analysis of 235 loci (58,041 aa; 75% occupancy) with 95% highest posterior density (HPD) bars under a uniform prior. Numbers on nodes indicate the placement of the fossils used to calibrate the dating analysis.
The superfamily Neobisioidea sensu Benavides et al. (2019) was not found to be monophyletic with various taxa either as sister group to the entire Iocheirata, i.e. Parahyidae and some Syarinidae, or as sister group to Panctenata, i.e. Hyidae, Ideoroncidae and some Syarinidae (Fig. 2).
Garypinidae was rendered paraphyletic by the inclusion of Larcidae and Amblyolpiidae. It should be noted that this clade lacked support in the analysis (Fig. 2), and that a different result was obtained by Gao et al. (2025) using a larger dataset. Similar problems were found by Harvey et al. (2020) whereby the garypid subfamilies Garypinae and Synsphyroninae were non-monophyletic despite very convincing morphological synapomorphies to the contrary.
Discussion
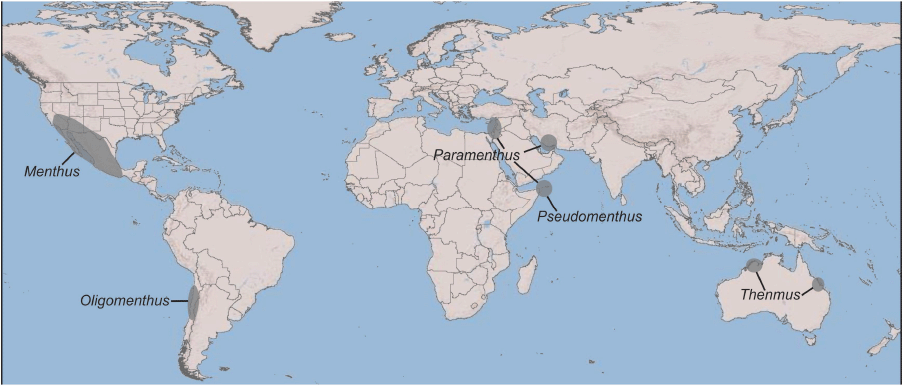
Menthids are one of the rarest pseudoscorpion families with only 5 genera and 12 valid species described to date. Their worldwide distribution is sporadic, with each genus restricted to isolated regions in both the northern and southern hemispheres: Menthus Chamberlin, 1930 (four species) in the south-western USA and Mexico; Oligomenthus Beier, 1962 in Argentina and Chile (two species); Paramenthus Beier, 1963 (two species) and Pseudomenthus Mahnert, 2007 (two species) in the Middle East, Iran and Yemen; and Thenmus Muchmore, 1990 (two species) in northern Australia (Fig. 5). They display a potentially vicariant pattern that is difficult to reconcile with dispersal hypotheses, as menthids are small, seemingly delicate arthropods that rely on humid microclimates that have never been found in a phoretic relationship with other arthropods, unlike some other pseudoscorpion taxa (e.g. Beier 1948; Muchmore 1971). Indeed, menthids are small, fragile pseudoscorpions that do not survive outside of their mesic environment, e.g. after being transferred to glass vials (M. S. Harvey, T. L. Miller and M. G. Rix, unpubl. data). A more plausible explanation is that they are Mesozoic relicts with Pangean origins but have only survived in isolated regions of the world due to subsequent extinction events, a result that remains to be tested by including multiple menthids in a dated phylogeny. However, the split from its sister taxon largely overlaps with a time of a unified Pangea, when the family probably initiated its diversification. This Pangean-wide pattern is also evident in other pseudoscorpion clades such as Pseudotyrannochthoniidae (Harms et al. 2024), Pseudogarypidae (Harvey and Šťáhlavský 2010), Syarinidae (Harvey 1998) and Garypinidae (Harvey and Šťáhlavský 2010). A similar pattern also occurs in some early lineages of the Opiliones superfamily Triaenonychoidea, the family Buemarinoidae (Derkarabetian et al. 2021). The superfamily Neobisioidea is thought to have arisen in the late Paleozoic with all of the families established by the Triassic (Benavides et al. 2019).
Menthids (Fig. 6) possess some very unusual morphological features (Chamberlin 1931; Muchmore 1982; Harvey 1992). They are the only adult pseudoscorpions that have 11 trichobothria on the fixed chelal finger and hand (Harvey and Muchmore 1990; Harvey 2006; Mahnert 2007) (Fig. 11). Most other pseudoscorpions have eight trichobothria, although all species of Ideoroncidae have supernumerary trichobothria on both chelal fingers (e.g. Mahnert 1984; Harvey and Muchmore 2013; Harvey and Du Preez 2014; Harvey 2016), even in Cretaceous taxa (Geißler et al. 2022). Several families have reduced numbers of trichobothria, most likely due to neoteny (e.g. Harvey 1992), including Neobisiidae, Syarinidae, Garypidae, Geogarypidae, Olpiidae, Garypinidae, Larcidae, Cheiridiidae, Sternophoridae, Chernetidae and Cheliferidae. The condylar articulation between coxae II and III of the prosoma in Menthidae (Chamberlin 1931, fig. 7B, H) (Fig. 9) is unparalleled in other pseudoscorpions, even though its function is unknown.
Images of Menthus californicus Chamberlin, 1930 (WAM T109850) showing important taxonomic features of menthids: 6, body, dorsal; 7, body, ventral; 8, left movable cheliceral finger, dorsal; 9, cephalothorax, ventral; 10, left pedipalp, dorsal; 11, left chela showing supernumerary trichobothria a, c and d. Scale lines: 0.5 mm.
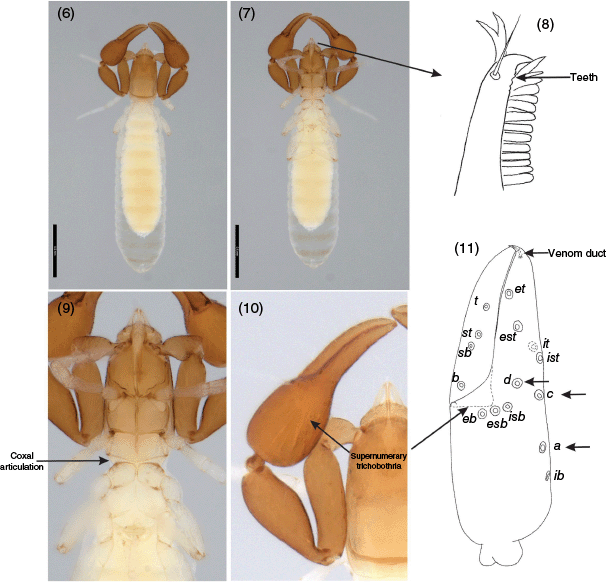
Despite having been placed in Garypoidea (Chamberlin 1930, 1931), our results solidly support its placement in Neobisioidea. This placement actually makes sense under the light of several morphological characters that menthids share with the rest of the neobisoideans that were not previously given sufficient attention. These include the following six characteritics.
Cheliceral movable finger with a sub-apical lobe that is sometimes divided into two or three sublobes
Harvey and Muchmore (1990) noted that menthids had up to three subapical teeth on the movable cheliceral finger, which is wholly consistent with a placement in Neobisioidea. Although these teeth are small and difficult to observe, they have been illustrated for most genera (Harvey and Muchmore 1990; Mahnert 2007; Nassirkhani and Shoushtari 2015) (Fig. 8).
Cheliceral lamina exterior present
This feature is a relatively good character to distinguish neobisioids, which usually lack a lamina exterior, from garypoids and garypinoids, which usually possess one. Ideoroncids are the only recorded neobisioids with a lamina exterior, and garypinids frequently lack one (e.g. Harvey 2023).
Posterior maxillary lyrifissure a closed loop, except in Menthidae, where it is composed of two interlocking U-shaped fissures
The posterior maxillary lyrifissure of menthids was reported by Chamberlin (1931) to be composed of two interlocking U-shaped fissures, whereas in all other garypoids it is a closed loop. The interlocking morphology is characteristic of all neobisioids and is thus entirely consistent with Menthidae being a member of Neobisioidea.
Serrula exterior fused to movable finger for practically its full length
The serrula exterior of neobisioids is usually hemictenal and semi-fused, with the distal lamellae raised free (Judson 2012). This state corresponds to the partially fused state coded by Harvey (1992) in a cladistic analysis of pseudoscorpion relationships. The panctenal state is fully fused to the movable finger such that the serrula is attached to the fingers for its whole length (Judson 2012), which was extremely well illustrated for a species of Synsphyronus (Garypidae) by Engel (2012). There have been no detailed illustrations of menthid serrulae but previous studies have implied that they are panctenal, whereas, in fact, they are hemictenal with the distal lamellae free, although this is difficult to see in specimens (Fig. 8).
Serrula interior with basal blades fused to form a velum, and distal blades not lingulate: Chamberlin (1931) noted that garypoids, including menthids, had a velum, but examination of specimens of Menthus californicus, M. mexicanus, Thenmus aigilaites and T. augustus lodged in WAM failed to reveal the presence of a velum.
Junction between femora and patellae of the posterior legs strongly oblique
The morphology of the posterior leg pairs III and IV is fairly consistent in Neobisioidea, with the femur and patella usually being approximately equal in size and the suture line perpendicular (e.g. Chamberlin 1931; Harvey 1992). By contrast, the femora of all Garypoidea, Garypinoidea, Cheiridioidea and Cheliferoidea (i.e. Panctenata) with the exception of Sternophoroidea, is much smaller than the patella and the suture is strongly oblique (e.g. Chamberlin 1931; Harvey 1992). Menthids also have small femora and an oblique suture (e.g. Harvey and Muchmore 1990). This morphology is, however, not unprecedented among Neobisioidea. For example, both genera of Syarininae (Syarinus Chamberlin, 1930 and Anysrius Harvey, 1998), have small femora (Chamberlin 1931; Harvey 1998), as do all species of the bochicid subfamily Bochicinae (e.g. Muchmore 1973, 1984, 1998; Dumitresco and Orghidan 1977).
Rallum (then called the flagellum) composed of one to four blades
The number of blades in the cheliceral rallum of garypoids and garypinoids varies with one, three or four blades, often with moderately strong fidelity: Geogarypidae with one blade; Garypidae and Olpiidae with three or rarely two blades; Hesperolpiidae with two or three blades; and Garypinoidea with four or very rarely three blades. Neobisioids have a widely variable number of blades ranging from 1 to 10 blades. Menthidae have four or rarely three blades (e.g. Harvey and Muchmore 1990; Mahnert 2007), which fits well within the range found in neobisioids.
There are several other features that are consistent with a menthid placement in Neobisioidea.
The venom apparatus of menthids is only present in the fixed finger and is completely absent in the movable finger (Fig. 11). In all garypoids and garypinoids, the venom apparatus is present in both fingers, but is often absent in neobisioids (Chamberlin 1931; Harvey 1992). It is lost in the fixed finger of Gymnobisiidae, some Hyidae (the genus Indohya Beier, 1974), some Bochicidae (Vachonium Chamberlin, 1947 and the subfamily Leucohyinae), and from the movable finger in all Neobisiidae, Parahyidae and Syarinidae. The loss of the venom apparatus in the movable finger of menthids is therefore in accord with its inferred relationship as sister to the clade Gymnobisiidae + Neobisiidae + Parahyidae + Syarinidae, with an interesting change of fingers in gymnobisiids. Like Neobisiidae, Parahyidae and Syarinidae, the venom duct of menthids is very short and terminates in the nodus ramosus almost immediately, whereas it is longer in the other families.
The presence of three auxiliary trichobothria on the fixed chelal finger and hand resulting in a total of 11 trichobothria (Fig. 10, 11) is a definitive autapomorphy for adult Menthidae, which is unparalleled in any other pseudoscorpions (Harvey 1992). Most pseudoscorpions have 8 trichobothria although some have reduced numbers due to neoteny, and all Ideoroncidae have supernumerary trichobothria resulting in 17–31 on the fixed chelal finger and hand, and 9–14 on the movable finger (e.g. Mahnert 1984; Harvey and Muchmore 2013; Harvey and Du Preez 2014; Harvey 2016).
The position of trichobothrium ib on the medio-dorsal surface of the hand of menthids resembles that found in some other neobisioids including Ideoroncidae, Bochicidae and most Syarinidae (e.g. Arcanobisiinae, Chitrellinae, and most Ideobisiinae, but not Syarininae) (e.g. Beier 1952; Mahnert 1984; Muchmore 1996, 1998; Zaragoza 2010; Harvey and Muchmore 2013; Gardini 2015; Harvey 2016). In some other neobisioids such as Hyidae, ib is located on the dorsal surface but in a more distal position close to the base of the fingers (Harvey 1993; Harvey et al. 2023). There are no garypoids or garypinoids that have ib situated dorsally on the hand.
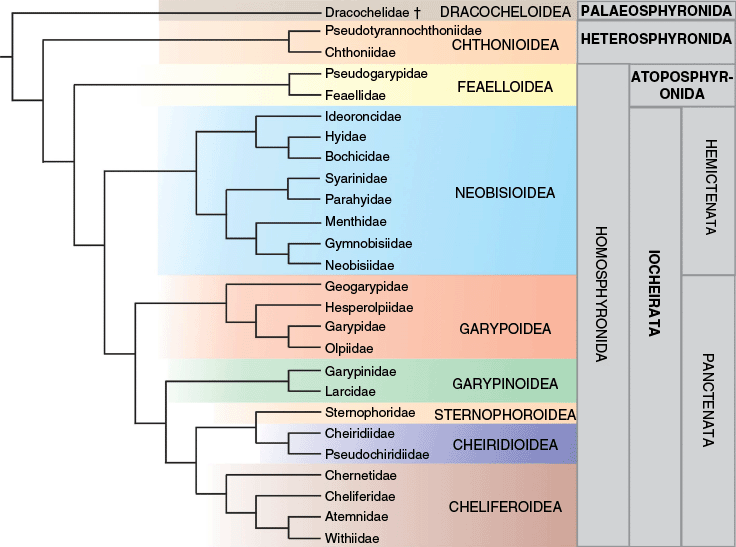
Taking into account all of the molecular evidence, it is possible to add Menthidae to a summary phylogeny as sister group to a clade of Gymnobisiidae + Neobisiidae (Fig. 12). The discovery of a group of neobisioid pseudoscorpions with distinctive morphological traits (Chamberlin 1930, 1931; Harvey and Muchmore 1990) and an isolated phylogenetic placement (Fig. 2, 3) suggests that there is much more to discover among the many disparate clades of Neobisioidea. In particular, the non-monophyly of the family Syarinidae remains a vexatious problem that should be best resolved with a more robust phylogenomic dataset and better taxon representation.
Summary tree depicting the relationships of pseudoscorpion families based on Benavides et al. (2019) and this study. Suborders Palaeosphyronida, Heterosphyronida, Atoposphyronida and Iocheirata in bold; group Homosphyronida and infraorders Hemictenata and Panctenata in regular font.
Data availability
Sanger sequences generated in this study are available in GenBank as outlined in Table 1. New transcriptomic data have been deposited in the NCBI Sequence Read Archive (BioProject PRJNA534138). Supplementary tree files, data matrices and tables are publicly available at the Harvard Dataverse (Benavides Silva 2024).
Conflicts of interest
M. S. Harvey, L. R. Benavides and G. Giribet are editors for Invertebrate Systematics. Despite this relationship, they took no part in the review and acceptance of this manuscript, in line with the publishing policy. The authors declare that they have no further conflicts of interest.
Declaration of funding
The specimens of Thenmus aigialites were collected using research funds from the Queensland Museum’s Biodiversity and Geosciences Program.
Acknowledgements
Some of the sequence data were supplied by the Western Australian Museum’s Molecular Systematics Unit and we thank Nerida Wilson and Melissa Danks for their assistance. The phylogenomic computations in this paper were run on the FASRC Cannon cluster supported by the FAS Division of Science Research Computing Group at Harvard University. Finally, we acknowledge the two anonymous reviewers who provided useful comments that improved this work.
References
Aberer AJ, Kobert K, Stamatakis A (2014) ExaBayes: massively parallel Bayesian tree inference for the whole-genome era. Molecular Biology and Evolution 31, 2553-2556.
| Crossref | Google Scholar | PubMed |
Altenhoff AM, Levy J, Zarowiecki M, Tomiczek B, Warwick Vesztrocy A, Dalquen DA, Müller S, Telford MJ, Glover NM, Dylus D, Dessimoz C (2019) OMA standalone: orthology inference among public and custom genomes and transcriptomes. Genome Research 29, 1152-1163.
| Crossref | Google Scholar | PubMed |
Arabi J, Judson ML, Deharveng L, Lourenço WR, Cruaud C, Hassanin A (2012) Nucleotide composition of CO1 sequences in Chelicerata (Arthropoda): detecting new mitogenomic rearrangements. Journal of Molecular Evolution 74, 81-95.
| Crossref | Google Scholar | PubMed |
Ballesteros JA, Santibáñez-López CE, Baker CM, Benavides LR, Cunha TJ, Gainett G, Ontano AZ, Setton EVW, Arango CP, Gavish-Regev E, Harvey MS, Wheeler WC, Hormiga G, Giribet G, Sharma PP (2022) Comprehensive species sampling and sophisticated algorithmic approaches refute the monophyly of Arachnida. Molecular Biology and Evolution 39, msac021.
| Crossref | Google Scholar | PubMed |
Beier M (1932) Pseudoscorpionidea I. Subord. Chthoniinea et Neobisiinea. Tierreich 57, 1-258 [In German].
| Google Scholar |
Beier M (1948) Phoresie und Phagophilie bei Pseudoscorpionen. Österreichische Zoologische Zeitschrift 1, 441-497 Available at https://www.zobodat.at/pdf/OEZ_01_0441-0497.pdf [In German].
| Google Scholar |
Beier M (1952) On some Pseudoscorpionidea from Malaya and Borneo. Bulletin of the Raffles Museum 24, 96-108 Available at https://lkcnhm.nus.edu.sg/wp-content/uploads/sites/11/app/uploads/2017/06/24brm096-108.pdf.
| Google Scholar |
Beier M (1955) Pseudoscorpione im baltischen Bernstein aus dem Geologischen Staatsinstitut in Hamburg. Mitteilungen aus dem Mineralogisch-Geologischen Staatsinstitut in Hamburg 24, 48-54.
| Google Scholar |
Benavides LR (2025) Datasets and Supplementary material of ‘The phylogenetic placement of the enigmatic pseudoscorpion family Menthidae (Pseudoscorpiones): a revised superfamily assignment based on new molecular data’. Harvard Dataverse 2025, V2 [Dataset, published 17 October 2024].
| Crossref | Google Scholar |
Benavides LR, Cosgrove JG, Harvey MS, Giribet G (2019) Phylogenomic interrogation resolves the backbone of the Pseudoscorpiones Tree of Life. Molecular Phylogenetics and Evolution 139, 106509.
| Crossref | Google Scholar | PubMed |
Chamberlin JC (1930) A synoptic classification of the false scorpions or chela-spinners, with a report on a cosmopolitan collection of the same. Part II. The Diplosphyronida (Arachnida-Chelonethida). Annals and Magazine of Natural History 5(10), 1-48.
| Crossref | Google Scholar |
Chamberlin JC (1931) The arachnid order Chelonethida. Stanford University Publications, Biological Sciences 7(1), 1-284.
| Google Scholar |
Derkarabetian S, Baker CM, Hedin M, Prieto CE, Giribet G (2021) Phylogenomic re-evaluation of Triaenonychoidea (Opiliones: Laniatores), and systematics of Triaenonychidae, including new families, genera and species. Invertebrate Systematics 35, 133-157.
| Crossref | Google Scholar |
dos Reis M, Yang Z (2011) Approximate likelihood calculation on a phylogeny for Bayesian estimation of divergence times. Molecular Biology and Evolution 28(7), 2161-2172.
| Crossref | Google Scholar | PubMed |
Engel R (2012) Novel discovery of lamellar papillae on the grooming organ in Synsphyronus (Garypidae: Pseudoscorpiones). Arthropod Structure & Development 41, 265-269.
| Crossref | Google Scholar | PubMed |
Gao Z, Zhang F, Harvey MS (2025) New Asian pseudoscorpions improve the phylogenetic resolution of Garypinoidea (Pseudoscorpiones). Invertebrate Systematics 39, IS24098.
| Crossref | Google Scholar | PubMed |
Gardini G (2015) The species of the pseudoscorpion genus Pseudoblothrus (Pseudoscorpiones: Syarinidae) in Italy (on Italian pseudoscorpions XLVIII). Arachnologische Mitteilungen 49, 21-33.
| Crossref | Google Scholar |
Geißler C, Kotthoff U, Hammel J, Harvey MS, Harms D (2022) The first fossil of the pseudoscorpion family Ideoroncidae (Arachnida: Pseudoscorpiones): a new taxon from the mid-Cretaceous of northern Myanmar. Cretaceous Research 130, 105030.
| Crossref | Google Scholar |
Harms D, Dunlop JA (2017) The fossil history of pseudoscorpions (Arachnida: Pseudoscorpiones). Fossil Record 20, 215-238.
| Crossref | Google Scholar |
Harms D, Harvey MS, Roberts JD, Loria SF (2024) Tectonically driven climate change and the spread of temperate biomes: Insights from dragon pseudoscorpions (Pseudotyrannochthoniidae), a globally distributed arachnid lineage. Journal of Biogeography 51, 1032-1048.
| Crossref | Google Scholar |
Harvey MS (1992) The phylogeny and classification of the Pseudoscorpionida (Chelicerata: Arachnida). Invertebrate Taxonomy 6, 1373-1435.
| Crossref | Google Scholar |
Harvey MS (1993) The systematics of the Hyidae (Pseudoscorpionida: Neobisioidea). Invertebrate Taxonomy 7, 1-32.
| Crossref | Google Scholar |
Harvey MS (1998) Pseudoscorpion groups with bipolar distributions: a new genus from Tasmania related to the Holarctic Syarinus (Arachnida, Pseudoscorpiones, Syarinidae). Journal of Arachnology 26, 429-441.
| Google Scholar |
Harvey MS (2006) New species and records of the pseudoscorpion family Menthidae (Pseudoscorpiones). Records of the Western Australian Museum 23, 167-174.
| Crossref | Google Scholar |
Harvey MS (2016) The systematics of the pseudoscorpion family Ideoroncidae (Pseudoscorpiones, Neobisioidea) in the Asian region. Journal of Arachnology 44, 272-329.
| Crossref | Google Scholar |
Harvey MS (2023) A preliminary phylogeny for the pseudoscorpion family Garypinidae (Pseudoscorpiones: Garypinoidea), with new taxa and remarks on the Australasian fauna. Invertebrate Systematics 37, 623-676.
| Crossref | Google Scholar |
Harvey MS, Du Preez G (2014) A new troglobitic ideoroncid pseudoscorpion (Pseudoscorpiones: Ideoroncidae) from southern Africa. Journal of Arachnology 42, 106-110.
| Crossref | Google Scholar |
Harvey MS, Muchmore WB (1990) The systematics of the family Menthidae (Pseudoscorpionida). Invertebrate Taxonomy 3, 941-964.
| Crossref | Google Scholar |
Harvey MS, Muchmore WB (2013) The systematics of the pseudoscorpion family Ideoroncidae (Pseudoscorpiones: Neobisioidea) in the New World. Journal of Arachnology 41, 229-290.
| Crossref | Google Scholar |
Harvey MS, Šťáhlavský F (2010) A review of the pseudoscorpion genus Oreolpium (Pseudoscorpiones: Garypinidae), with remarks on the composition of the Garypinidae and on pseudoscorpions with bipolar distributions. Journal of Arachnology 38, 294-308.
| Crossref | Google Scholar |
Harvey MS, Lopes PC, Goldsmith GR, Halajian A, Hillyer MJ, Huey JA (2015) A novel symbiotic relationship between sociable weaver birds (Philetairus socius) and a new cheliferid pseudoscorpion (Pseudoscorpiones: Cheliferidae) in southern Africa. Invertebrate Systematics 29, 444-456.
| Crossref | Google Scholar |
Harvey MS, Abrams KM, Beavis AS, Hillyer MJ, Huey JA (2016a) Pseudoscorpions of the family Feaellidae (Pseudoscorpiones: Feaelloidea) from the Pilbara region of Western Australia show extreme short-range endemism. Invertebrate Systematics 30, 491-508.
| Crossref | Google Scholar |
Harvey MS, Huey JA, Hillyer MJ, McIntyre E, Giribet G (2016b) The first troglobitic species of Gymnobisiidae (Pseudoscorpiones, Neobisioidea), from Table Mountain (Western Cape Province, South Africa) and its phylogenetic position. Invertebrate Systematics 30, 75-85.
| Crossref | Google Scholar |
Harvey MS, Cosgrove JG, Harms D, Selden PA, Shih C, Wang C-C (2018) The oldest chthonioid pseudoscorpion (Arachnida: Pseudoscorpiones: Chthonioidea: Chthoniidae): a new genus and species from mid-Cretaceous Burmese amber. Zoologischer Anzeiger 273, 102-111.
| Crossref | Google Scholar |
Harvey MS, Hillyer MJ, Carvajal JI, Huey JA (2020) Supralittoral pseudoscorpions of the genus Garypus (Pseudoscorpiones: Garypidae) from the Indo-West Pacific region, with a review of the subfamily classification of Garypidae. Invertebrate Systematics 34, 34-87.
| Crossref | Google Scholar |
Harvey MS, Burger MAA, Abrams KM, Finston TL, Huey JA, Perina G (2023) The systematics of the pseudoscorpion genus Indohya (Pseudoscorpiones: Hyidae) in Australia. Zootaxa 5342, 1-119.
| Crossref | Google Scholar | PubMed |
Henderickx H (2005) A new Geogarypus from Baltic Amber (Pseudoscorpiones: Geogarypidae). Phegea 33, 87-92.
| Google Scholar |
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35(2), 518-522.
| Crossref | Google Scholar | PubMed |
Judson MLI (1997) Catalogue of the pseudoscorpion types (Arachnida: Chelonethi) in the Natural History Museum, London. Occasional Papers on Systematic Entomology 11, 1-54.
| Google Scholar |
Judson MLI (2000) Electrobisium acutum Cockerell, a cheiridiid pseudoscorpion from Burmese amber, with remarks on the validity of the Cheiridioidea (Arachnida, Chelonethi). Bulletin of the Natural History Museum, Geology 56, 79-83.
| Google Scholar |
Judson MLI (2003) Baltic amber pseudoscorpions (Arachnida, Chelonethi): a new species of Neobisium (Neobisiidae) and the status of Obisium rathkii Koch & Berendt. Geodiversitas 25, 445-450.
| Google Scholar |
Judson MLI (2009) Cheliferoid pseudoscorpions (Arachnida, Chelonethi) from the Lower Cretaceous of France. Geodiversitas 31, 61-71.
| Crossref | Google Scholar |
Judson MLI (2012) Reinterpretation of Dracochela deprehendor (Arachnida: Pseudoscorpiones) as a stem-group pseudoscorpion. Palaeontology 55, 261-283.
| Crossref | Google Scholar |
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772-780.
| Crossref | Google Scholar | PubMed |
Katoh K, Misawa K, Kuma K-i, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30, 3059-3066.
| Crossref | Google Scholar | PubMed |
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12), 1647-1649.
| Crossref | Google Scholar |
Kolesnikov VB, Turbanov IS, Eskov KY, Propistsova EA, Bashkuev AS (2022) First non-amber Mesozoic pseudoscorpion from Upper Triassic deposits of eastern Europe, with a description of two new fossil subfamilies (Arachnida, Pseudoscorpiones, Feaellidae). Papers in Palaeontology 8(5), e1466.
| Crossref | Google Scholar |
Lartillot N, Philippe H (2004) A Bayesian mixture model for across-site heterogeneities in the amino-acid replacement process. Molecular Biology and Evolution 21(6), 1095-1109.
| Crossref | Google Scholar | PubMed |
Le SQ, Gascuel O (2008) An improved general amino acid replacement matrix. Molecular Biology and Evolution 25, 1307-1320.
| Crossref | Google Scholar | PubMed |
Mahnert V (1984) Beitrag zu einer besseren Kenntnis der Ideoroncidae (Arachnida: Pseudoscorpiones), mit Beschreibung von sechs neuen Arten. Revue Suisse de Zoologie 91, 651-686 [In German].
| Crossref | Google Scholar |
Mahnert V (2007) Pseudoscorpions (Arachnida: Pseudoscorpiones) of the Socotra Archipelago, Yemen. Fauna of Arabia 23, 271-307.
| Google Scholar |
Manni M, Berkeley MR, Seppey M, Zdobnov EM (2021a) BUSCO: assessing genomic data quality and beyond. Current Protocols 1, e323.
| Crossref | Google Scholar |
Manni M, Berkeley MR, Seppey M, Simão FA, Zdobnov EM (2021b) BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Molecular Biology and Evolution 38(10), 4647-4654.
| Crossref | Google Scholar |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530-1534.
| Crossref | Google Scholar | PubMed |
Muchmore WB (1971) Phoresy by North and Central American pseudoscorpions. Proceedings of the Rochester Academy of Science 12, 79-97.
| Google Scholar |
Muchmore WB (1973) New and little known pseudoscorpions, mainly from caves in Mexico (Arachnida, Pseudoscorpionida). Bulletin of the Association for Mexican Cave Studies 5, 47-62.
| Google Scholar |
Muchmore WB (1984) Troglobochica, a new genus from caves in Jamaica, and redescription of the genus Bochica Chamberlin (Pseudoscorpionida, Bochicidae). Journal of Arachnology 12, 61-68.
| Google Scholar |
Muchmore WB (1996) A remarkable new genus and species of Pseudoscorpionida (Syarinidae) from a cave in Arizona. Southwestern Naturalist 41, 145-148.
| Google Scholar |
Muchmore WB (1998) Review of the family Bochicidae, with new species and records (Arachnida: Pseudoscorpionida). Insecta Mundi 12, 117-132.
| Google Scholar |
Murienne J, Harvey MS, Giribet G (2008) First molecular phylogeny of the major clades of Pseudoscorpiones (Arthropoda: Chelicerata). Molecular Phylogenetics and Evolution 49, 170-184.
| Crossref | Google Scholar | PubMed |
Nassirkhani M, Shoushtari RV (2015) The first record of the family Menthidae Chamberlin (Arachnida: Pseudoscorpiones) from Iran. The Journal of Zoology Studies 1, 27-31.
| Google Scholar |
Ontano AZ, Gainett G, Aharon S, Ballesteros JA, Benavides LR, Corbett KF, Gavish-Regev E, Harvey MS, Monsma S, Santibáñez-López CE, Setton EVW, Zehms JT, Zeh JA, Zeh DW, Sharma PP (2021) Taxonomic sampling and rare genomic changes overcome long-branch attraction in the phylogenetic placement of pseudoscorpions. Molecular Biology and Evolution 38, 2446-2467.
| Crossref | Google Scholar | PubMed |
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67, 901-904.
| Crossref | Google Scholar | PubMed |
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3), 539-542.
| Crossref | Google Scholar | PubMed |
Röschmann LM, Harvey MS, Hou Y, Harms D, Kotthoff U, Hammel JU, Ren D, Loria SF (2024) First fossil species of family Hyidae (Arachnida: Pseudoscorpiones) confirms 99 million years of ecological stasis in a Gondwanan lineage. PeerJ 12, 17515.
| Crossref | Google Scholar | PubMed |
Schawaller W, Shear WA, Bonamo PM (1991) The first Paleozoic pseudoscorpions (Arachnida, Pseudoscorpionida). American Museum Novitates 3009, 1-24.
| Google Scholar |
Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44(W1), W232-W235.
| Crossref | Google Scholar | PubMed |
World Pseudoscorpiones Catalog (2025) World Pseudoscorpiones Catalog. (Natural History Museum) Available at https://wac.nmbe.ch/order/pseudoscorpiones/3 [Verified 22 January 2025]
Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution 24, 1586-1591.
| Crossref | Google Scholar | PubMed |
Yang Z, Rannala B (2006) Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds. Molecular Biology and Evolution 23, 212-226.
| Crossref | Google Scholar | PubMed |
Zaragoza JA (2010) Arcanobisium, a remarkable new genus, representing a new subfamily with a relictual distribution from eastern Spain (Arachnida: Pseudoscorpiones: Syarinidae). Zootaxa 2491, 41-60.
| Crossref | Google Scholar |