Taxonomy and systematics of the Australasian gum nut orb-weaving spider genus Carepalxis (Araneae, Araneidae)
Pedro de S. Castanheira A B * , Dimitar Dimitrov
A B * , Dimitar Dimitrov  C , Renner L. C. Baptista B , Nikolaj Scharff D and Volker W. Framenau A E F
C , Renner L. C. Baptista B , Nikolaj Scharff D and Volker W. Framenau A E F
A
B
C
D
E
F
Abstract
We revised the orb-weaving spider genus Carepalxis L. Koch, 1872 and tested its monophyly using Maximum Likelihood and Bayesian Inference phylogenetic analyses, comparing our results to a previously published family-level dataset on world-wide Araneidae. We studied the placement of the genus and the classification of the informally termed clade ‘backobourkiines’ using phylogenetic analyses based on two mitochondrial genes, cytochrome c oxidase subunit 1 (COI) and 16S rRNA (16S), and two nuclear genes, 28S rRNA (28S) and 18S rRNA (18S). Approximately 12,000 araneid records (vials) from major Australian and overseas collections were examined during our taxonomic revision. All phylogenetic analyses supported a monophyletic ‘backobourkiines’ clade, but found a polyphyletic Carepalxis, with its Australasian representatives being part of the ‘backobourkiines’ and the Neotropical species being related to the Neotropical Ocrepeira Marx, 1883. Consequently, the genus was revised to include seven endemic Australian species, Carepalxis montifera L. Koch, 1872 (type species), C. bilobata Keyserling, 1886, C. ferreirasousai sp. nov., C. kolla sp. nov., C. megalostylus sp. nov., C. tholos sp. nov. and C. tuberculata Keyserling, 1886 (=C. furcifera (Keyserling, 1886) syn. nov.), in addition to C. beelzebub (van Hasselt, 1873) (=C. suberosa Thorell, 1881 syn. nov. = C. tuberculifera (Thorell, 1881) comb. nov., syn. nov. = C. tricuspidata Chrysanthus, 1961 syn. nov.), which is present in Australia, Indonesia (West Papua) and Papua New Guinea. The following new combinations for Neotropical species previously placed in Carepalxis were proposed: Ocrepeira camelus (Simon, 1895) comb. nov., Ocrepeira gibbosa (O. Pickard-Cambridge, 1889) comb. nov., Ocrepeira perpera (Petrunkevitch, 1911) comb. nov., Ocrepeira quasimodo (Ferreira-Sousa & Motta, 2022) comb. nov., and Ocrepeira topazio (Ferreira-Sousa & Motta, 2022) comb. nov. Within the backobourkiines, Carepalxis can be recognised by the presence of two cephalic humps in females and two enlarged megaspines apically on tibia II of males (both here considered synapomorphies of the genus), an anteriorly elevated abdomen usually with numerous tubercles, humps or sigilla in addition to the humeral humps, an elongated male pedipalp median apophysis bearing a small projection, and a female epigyne with broad lateral lobes, and, whenever present, conspicuous transverse slits instead of baso-lateral flaps.
ZooBank: urn:lsid:zoobank.org:pub:4F888132-4EE9-417F-9A82-7F9C470C9FB3
Keywords: Australia, backobourkiines, cephalic morphology, mimicry, molecular systematics, new combination, New Guinea, new synonymy, phylogenetic analysis, taxonomic revision.
Introduction
The orb-weaving spider genus Carepalxis L. Koch, 1872, with C. montifera L. Koch, 1872 collected at Port Mackay in Queensland designated as type species, was originally diagnosed by the shape of the female carapace with two conspicuous dorsal anterior humps (Koch 1872). The genus currently includes a further 11 species; 4 from Australia: Carepalxis beelzebub (van Hasselt, 1873), C. bilobata Keyserling, 1886, C. furcifera (Keyserling, 1886) and C. tuberculata Keyserling, 1886); 2 from the island of New Guinea: Carepalxis suberosa Thorell, 1881 from Papua New Guinea and C. tricuspidata Chrysanthus, 1961 from West Papua, Indonesia; and 5 from the Neotropical Region: Carepalxis camelus Simon, 1895, C. gibbosa O. Pickard-Cambridge, 1889, C. perpera (Petrunkevitch, 1911), C. quasimodo Ferreira-Sousa & Motta, 2022 and C. topazio Ferreira-Sousa & Motta, 2022 (World Spider Catalog, ver. 25.5, see http://wsc.nmbe.ch, accessed 14 January 2025).
The Australian and New Guinean species of Carepalxis, colloquially called gum nut orb-weavers due to their mimicry of eucalypt tree fruit, i.e. ‘gum nuts’ (Main 1999), have never been formally revised. The only modern illustrations are those of female C. tuberculata in an illustrated guide to the genera of Australian orb-weaving spiders (Davies 1988). In the Americas, the genus was represented by three species based on females only, whereas the only Carepalxis male known at that time was that of C. tuberculata from Australia, though not illustrated by Levi (1992). Further studies on Carepalxis included those on the biogeography and natural history of the genus (Main 1999), and the transfer of Verrucosa furcifera (Keyserling, 1886) to Carepalxis, adding a second known male to the genus (Framenau 2019). Most recently, two species of Carepalxis from Brazil were described, including the first male of the genus from the Americas, noting that the pedipalp morphology of this male is more similar to that of Ocrepeira Marx, 1883, thereby questioning the monophyly of Carepalxis (Ferreira-Sousa and Motta 2022).
A recent molecular phylogenetic study of Araneidae Clerck, 1757 included many Australian species and one Australian representative of Carepalxis nested within a clade informally termed ‘backobourkiines’ and sister to Singa nitidula C. L. Koch, 1844 (Scharff et al. 2020). No illustrations of male pedipalps of Australasian Carepalxis have ever been published and therefore it is currently not possible to evaluate the putative synapomorphies of this clade, for example the base of the median apophysis forming an arch over the radix (e.g. Framenau et al. 2010, 2021a, 2021b, 2022; Framenau 2011; Joseph and Framenau 2012; Framenau and Castanheira 2022).
The aim of this study was two fold. Firstly, to test the monophyly of the genus Carepalxis as currently defined, with representatives from the Neotropics and Australia, and to test its placement within the backobourkiines sensu Scharff et al. (2020) with an expanded taxon sample. Secondly, we revise the Australasian species of Carepalxis as a further contribution to the understanding of the Australasian orb-weaving spider diversity.
Methods
Molecular analyses
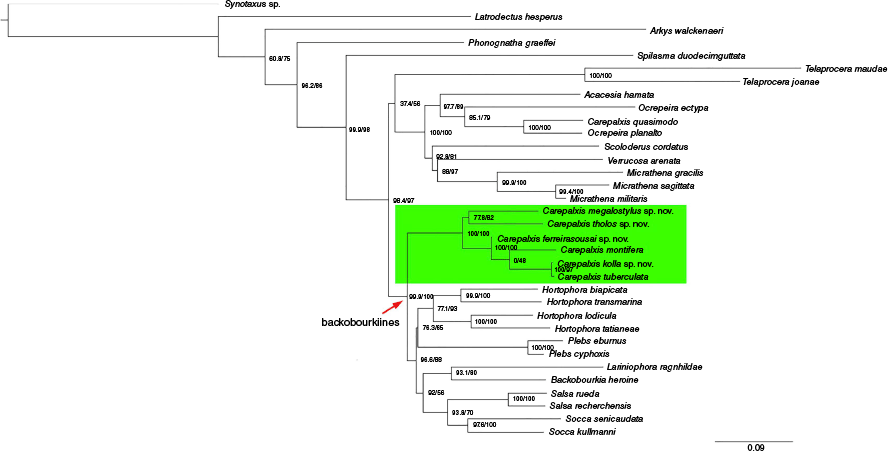
We used two different datasets to test phylogenetic hypotheses in relation to Carepalxis. To test relationships within Carepalxis (including its Neotropical representatives) and the genus’ affiliations to other putative backobourkiines, we analysed a dataset of 34 terminals (called ‘limited dataset’), of which 19 were sequenced specifically for this study, and 15 came directly from the Scharff et al. (2020) dataset (Fig. 1, 2, Table 1). Within the terminals sequenced by us, we included seven species of Carepalxis (incl. one from the Neotropics), one species of Ocrepeira Marx, 1883 (Neotropics), and Australian backobourkiines in the genera Backobourkia Framenau, Dupérré, Blackledge & Vink, 2010, Hortophora Framenau & Castanheira, 2021, Lariniophora Framenau, 2011, Plebs Joseph & Framenau, 2012, Salsa Framenau & Castanheira, 2022, and Socca Framenau, Castanheira & Vink, 2022. Outgroups were chosen based on recent phylogenetic studies on araneids (e.g. Kallal and Hormiga 2018; Scharff et al. 2020), and we sequenced an Australian species of Arkys Walckenaer, 1837 (Arkyidae) to complement the outgroup selection. The genus Synotaxus Simon, 1895 (family Synotaxidae Simon, 1895) was used to root the tree (Scharff et al. 2020). We analysed this limited dataset using both Maximum Likelihood and Bayesian Inference (see below respectively at Fig. 1, 2).
Maximum Likelihood preliminary multi-loci phylogenetic analysis of the backobourkiines focusing on Carepalxis with a limited dataset of 34 terminals and based on COI, 16S, 18S and 28S.
Bayesian Inference preliminary multi-loci phylogenetic analysis of the backobourkiines focusing on Carepalxis with a limited dataset of 34 terminals and based on COI, 16S, 18S and 28S.
| Species | Collection details | Sequenced for this study | Specimen used in ‘complete dataset’ | GenBank: COI | GenBank: 16S | GenBank: 18S | GenBank: 28S | |
|---|---|---|---|---|---|---|---|---|
| Acacesia hamata (Hentz, 1847) | USA, Florida, Gainesville (29°41′N, 82°24′W), 18–19 Aug 2003 | – | X | MK420048 | MK420173 | MK426050 | MK425946 | |
| Arkys walckenaeri Simon, 1879 | Australia, Western Australia, Murdoch University (32°04′S, 115°50′E), 24 Mar 2023, F Bokhari (WAM T171336) | X | – | PV065955 | PV065542 | PV065561 | PV065589 | |
| Backobourkia heroine (L. Koch, 1871) | Australia, Western Australia, Gypsum Lake, Beekeepers Nature Reserve (30°11′S, 115°00′E), 30 Nov 2008 (WAM T171337) | X | – | PV065951 | PV065540 | PV065544 | PV065584 | |
| Carepalxis ferreirasousai sp. nov. | Australia, South Australia Munyeroo Conservation Park, 17 km SSE Moonabbie (33°23′S, 137°21′E) (SAM NN30840) | X | X | PV065943 | PV065533 | PV065554 | PV065582 | |
| Carepalxis kolla sp. nov. | Australia, South Australia, Gluepot Reserve, (33°44′S, 140°01′E), 26 Nov 2000–06 Dec 2000 (SAM NN18561) | X | X | PV065941 | PV065531 | PV065552 | PV065581 | |
| Carepalxis megalostylus sp. nov. | Australia, South Australia, Nelshaby (33°07′S, 138°04′E), 02 Nov 2004, B Pavy (SAM NN30829) | X | X | PV065945 | PV065530 | PV065555 | PV065579 | |
| Carepalxis montifera L. Koch, 1872 | Australia, South Australia, Lake Goolangarie (Lake Goyder) (27°01′S, 140°10′E), 13 Dec 2000, GM Tomlinson (SAM NN30837) | X | X | PV065944 | PV065532 | PV065553 | PV065583 | |
| Carepalxis quasimodo Ferreira-Sousa & Motta, 2022 | Brazil, Bahia, Jaborandi, Fazenda Trijunção (14°38′S, 45°48′W), 18 Nov 2018, PC Motta et al. (UNB 9274) | X | X | PV065953 | PV065536 | PV065559 | PV065587 | |
| Carepalxis tholos sp. nov. | Australia, Western Australia, Mt Ragged Campsite, Cape Arid National Park (33°27′S, 123°27′E), 01 Jan 2008, VW Framenau & ML Thomas (WAM T81454) | X | X | PV065940 | PV065529 | PV065550 | PV065578 | |
| Carepalxis tuberculata Keyserling, 1886 | Australia, South Australia, Karte Conservation Park, 23 km NW Pinnaroo (35°07′S, 140°43′E), 26 Mar 2000, D Hirst (SAM NN30846) | X | X | PV065942 | – | PV065556 | PV065580 | |
| Hortophora biapicata (L. Koch, 1871) | Australia, Western Australia, Fitzgerald River National Park, Hamersley Inlet Campground (33°57′S, 119°54′E), 29 Jan 2023, RLC Baptista (WAM T171338) | X | X | PV065939 | PV065541 | PV065546 | PV065575 | |
| Hortophora lodicula (Keyserling, 1887) | Australia, Victoria, Grampians, Mackenzie Falls (37°06′S, 142°24′E), 20 Jan 2023, RLC Baptista (WAM T171339) | X | X | PV065938 | PV065528 | PV065557 | PV065574 | |
| Hortophora tatianeae Framenau & Castanheira, 2021 | Australia, Tasmania, Tasman National Park, Fortescue Bay (43°08′S, 147°57′E), 13 Jan 2023, RLC Baptista (WAM T171340) | X | X | PV065937 | PV065527 | PV065551 | PV065573 | |
| Hortophora transmarina (Keyserling, 1865) | Australia, Queensland, Tamborine National Park, Witches Falls (27°56′27″S, 153°10′48″E), 15 Apr 2002, N Scharff & S Larsen | – | X | MK420105 | MK420224 | MK426103 | MK425993 | |
| Lariniophora ragnhildae (Strand, 1917) | Australia, Western Australia, Lake Lefroy (31°29′S, 121°42′E), 27 Nov 2013, E Volschenk (WAM T171341) | X | – | PV065952 | PV065539 | PV065549 | – | |
| Latrodectus hesperus Chamberlin & Ivie, 1935 | USA, California, Riverside, 29 Jan 2003 (CAR) | – | X | MK420122 | MK420238 | MK426121 | MK426046 | |
| Micrathena gracilis (Walckenaer, 1805) | USA, Ohio | – | X | MK420136 | MK420251 | MK426133 | MK426013 | |
| Micrathena militaris (Fabricius, 1775) | Dominican Republic: W of Barahona Haitian Village (18°04′N, 71°06′W), Jul 2004, J Huff | – | X | – | MK420252 | MK426134 | MK426014 | |
| Micrathena sagittata (Walckenaer, 1841) | USA, Florida, Gainesville, 7 Jul 1997 | – | X | MK420137 | MK420253 | MK426135 | MK426015 | |
| Ocrepeira ectypa (Walckenaer, 1841) | USA, Oklahoma, Norman (35°13′N, 97°26′W), H Guariousco | – | X | MK420146 | MK420262 | MK426144 | MK426020 | |
| Ocrepeira planalto Ferreira-Sousa & Motta, 2022 | Brazil, Bahia, Jaborandi, Fazenda Trijunção (14°38′S, 45°48′W), 18 Nov 2018, PC Motta et al. (UNB 9280) | X | X | PV065954 | PV065537 | PV065560 | PV065588 | |
| Phonognatha graeffei (Keyserling, 1865) | Australia, Sydney, Apr 2003 | – | X | FJ607582 | FJ607469 | FJ607508 | FJ607543 | |
| Plebs cyphoxis (Simon, 1908) | Australia, Western Australia, Hedges Road, Hovea (31°53′S, 116°06′E), 17 Oct 2021, VW Framenau (WAM T171342) | X | X | PV065947 | – | PV065545 | PV065585 | |
| Plebs eburnus (Keyserling, 1886) | Australia, Tasmania, Launceston, Trevallyn Nature Recreation Area, Stolen Spice Trail (41°26′S, 147°05′E), 10 Jan 2023, RLC Baptista (WAM T171343) | X | X | PV065946 | PV065534 | PV065547 | PV065586 | |
| Salsa recherchensis (Main, 1954) | Australia, Western Australia, Stirling Range National Park, Ellen Peak Trail (34°23′S, 118°17′E), 7 Nov 2007, VW Framenau (WAM T81440) | – | X | MK420071 | MK420192 | MK426068 | MK425963 | |
| Salsa rueda Framenau & Castanheira, 2022 | Australia, Tasmania, Tasman National Park, Fortescue Bay (43°08′S 147°57′E), 13 Jan 2023, RLC Baptista (WAM T171344) | X | X | PV065950 | PV065538 | PV065543 | PV065572 | |
| Scoloderus cordatus (Taczanowski, 1879) | USA, Florida, Gainesville, 18–19 Aug 2003 | – | X | MK420161 | MK420275 | MK426160 | MK426035 | |
| Socca kullmanni Framenau, Castanheira & Vink, 2022 | Australia, Tasmania, Lake Pedder, McPartlan Pass Boat Ramp (42°51′S, 146°10′E), 16 Jan 2023, RLC Baptista (WAM T171345) | X | X | PV065948 | – | PV065548 | PV065577 | |
| Socca senicaudata (Simon, 1908) | Australia, Western Australia, Fitzgerald River National Park, Hamersley Inlet Campground (33°57′S, 119°54′E), 29 Jan 2023, RLC Baptista (WAM T171346) | X | X | PV065949 | – | PV065558 | PV065576 | |
| Spilasma duodecimguttata (Keyserling, 1879) | French Guinea, Commune Regina, Les Nouragues Field Station (4°04′08.6″N, 52°40′08.2″W), 13–26 Nov 2005, I Agnarsson et al. | – | X | MK420163 | MK420276 | MK426162 | MK426036 | |
| Synotaxus sp. | Costa Rica, Heredia province, La Selva, 01–05 Jul 2008 | – | X | MK420172 | MK420284 | MK426171 | MK426043 | |
| Telaprocera joanae Harmer & Framenau, 2008 | Australia, Queensland, Lamington National Park (28°13′S, 153°08′E), Feb 2007, A. Harmer (WAM T85256) | – | X | MK420164 | MK420277 | MK426163 | MK426037 | |
| Telaprocera maudae Harmer & Framenau, 2008 | Australia, Queensland, Lamington National Park (28°13′S, 153°08′E), Feb 2007, A. Harmer (WAM T85257) | – | X | MK420165 | MK420278 | MK426164 | – | |
| Verrucosa arenata (Walckenaer, 1841) | – | X | MK420168 | MK420281 | MK426167 | MK426039 |
The collection information Verrucosa arenata is not available.
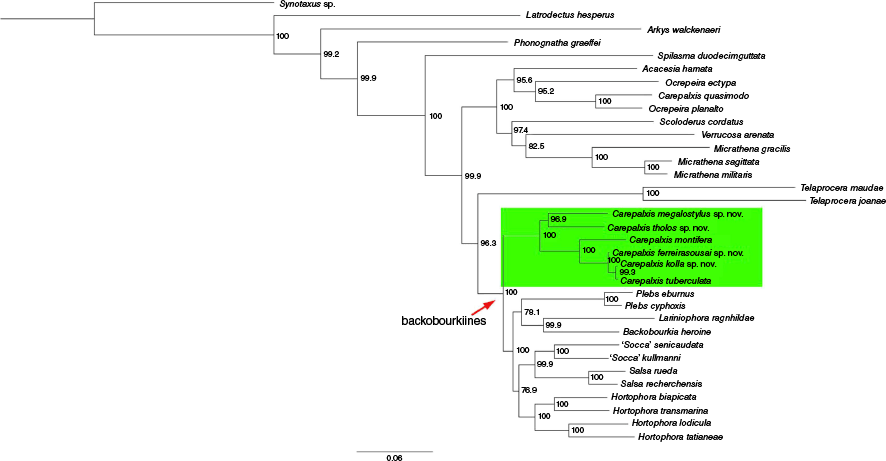
To explore the effects of an extended backobourkiines dataset on the phylogenetic hypothesis of Scharff et al. (2020), we added 14 species sequenced as part of this study, including all Carepalxis and Ocrepeira planato Ferreira-Sousa & Motta, 2022 (Table 1, Fig. S1 of the Supplementary material) to the ‘complete dataset’ of Scharff et al. (2020). This new phylogenetic analysis was carried out using Maximum Likelihood, and used all four genes mentioned above, differing from the original Scharff et al. (2020) dataset by the absence of the gene Histone 3 (H3).
DNA extractions and Next Generation Sequencing were carried out at the Institute for Immunology and Infectious Diseases (IIID) at Murdoch University. Genomic DNA was extracted from ethanol-preserved spiders. For most species, two to four legs were removed from one side of the body incubated overnight in 400 μL of lysis buffer and 40 μL of Protease solvent (QIAamp DNA Blood Mini Kit extraction kit from Qiagen) at room temperature (22–23°C). They were homogenised on the Tissuelyser for 2 min (25 mHz) and DNA was extracted as per manufacturer instructions and eluted in a final volume of 50 μL of Elution buffer. Concentration and quality of extracted DNA were determined by spectrophotometric evaluation of 1 μL of sample using Nanodrop. An aliquot of 3 μL of each sample was analysed by 1% agarose gel electrophoresis to verify DNA integrity.
Genomic DNA libraries were prepared for whole genome sequencing using the Twist Library Preparation kit EF V 2.0 with Enzymatic Fragmentation (Twist Biosciences) as per the manufacturer’s protocol. Indexed libraries were quantified using Quantus Fluorometer E6150 (Promega) and pooled to equimolar amounts. Samples were sequenced on an Illumina Novaseq. 6000 using a 2 × 150-bp paired-end chemistry kit (Illumina Inc., CA, USA), as per the manufacturer’s instructions. An average of 50 million paired-end reads were targeted per sample.
Raw reads were trimmed and filtered using default options with fastp (ver. 0.23.4, see https://github.com/OpenGene/fastp; Chen 2023), keeping only the reads longer than 50 bp after trimming. To extract COI and 16S we used NOVOplasty (ver. 4.3.3, see https://github.com/ndierckx/NOVOPlasty; Dierckxsens et al. 2017) to assemble de novo mitochondrial genomes and MITOS2 (Bernt et al. 2013) to annotate them. We used a COI sequence from the araneid species Cyclosa japonica Bösenberg & Strand, 1906 that was obtained from GenBank (Accession number MK512575) as a seed in the NOVOplasty runs. The two nuclear genes, 18S and 28S were extracted from the clean reads also using NOVOplasty but seeded with 18S and 28S seeds from Cyclosa conica (Pallas, 1772) (Accession number EU003343). After assembling of mitochondrial genomes and mining the data for the two nuclear genes we obtained fragments of two mitochondrial cytochrome c oxidase I (COI) and 16S rRNA (16S), and two nuclear 28S rDNA (28S) and 18S rDNA (18S) genes, providing ~4163 bp of data per taxon. Accession numbers of newly generated sequences, and data that were downloaded from GenBank to be used at our ‘limited dataset’ phylogenies (Fig. 1, 2) are shown in Table 1.
After contig assembly and editing, sequences for each gene fragment were subjected to multiple sequence alignment using the online version of MAFFT (ver. 7, see https://mafft.cbrc.jp/alignment/server/index.html; Katoh and Standley 2013). Alignments were built using the L-INS-I strategy and individual alignments were concatenated using FASconCAT-G (ver. 1.05.01, see https://github.com/PatrickKueck/FASconCAT-G; Kück and Meusemann 2010). Alignments of protein coding genes were translated into amino acids and checked for stop codons as an additional quality control step.
Best fit models of molecular evolution and Maximum Likelihood (ML) analyses were performed in the same run with IQ-TREE (ver. 1.6.12, see http://iqtree.cibiv.univie.ac.at/; Trifinopoulos et al. 2016). A priori data were partitioned by gene and best fit models of molecular evolution and best partition scheme were estimated by IQ-TREE prior to the ML analyses. Nodal support was assessed using ultrafast bootstrap (Hoang et al. 2018) using 10,000 replicates and SH-aLRT branch test using 1000 iterations. The best fit models for each partition for the limited dataset were as follows: GTR + F + I + G for 16S, TNe + I + G4 for 18S, GTR + F + I + G4 for 28S and K3Pu + F + I + G4 for COI. Best fit models for the complete dataset were: GTR + F + I + G4 for 16S, TNe + G4 for 18S, TIM3 + F + G4 for 28S and GTR + F + I + G4 for COI. The original partition by gene was retained.
Bayesian Inference (BI) analysis of the concatenated limited dataset was carried out in the software MrBayes (ver. 3.2.7, see https://github.com/NBISweden/MrBayes/; Ronquist et al. 2012). Two independent searches were done, each with four Markov chains, for 10,000,000 generations, saving a tree every 1000 generations. Of these, 2,500,000 generations were considered burn in (25%), corresponding to 2501 trees. Considering that the models that are unavailable on MrBayes were excluded a priori, GTR + I + G was the model that we used for each partition, which was selected by Akaike criterion of information (AIC) in jModelTest2 (ver. 2.1.1, see https://github.com/ddarriba/jmodeltest2; Guindon and Gascuel 2003; Darriba et al. 2012). BI analysis demonstrated sufficient convergence in independent analyses and parameter mixing, which were calculated by average standard deviation of split frequencies <0.005, parameter Potential Scale Reduction Factor = 1.00, and effective sampling size (ESS) values >200 as checked in Tracer (ver. 1.7, see https://github.com/beast-dev/tracer/releases/tag/v1.7; Rambaut et al. 2018). Clade support of BI analysis is read as Bayesian posterior probabilities (PP).
Taxonomic revision
All orb-weaving spider specimens in the major Australian museum collections and in overseas collections where historical type material is lodged were examined, totalling almost 12,000 records (vials). Descriptions and terminology follow recent publications on the backobourkiines (e.g. Framenau and Castanheira 2022; Castanheira and Framenau 2023). The generic and species descriptions are based on recently collected specimens in lieu of often poorly preserved historic type specimens. Colour patterns were described based on specimens preserved in ~75% ethanol. To expand the male pedipalp and to clarify the female genitalia we submerged these structures alternatively for ~10 min in 10% KOH and 10 min in distilled water for a complete pedipalp expansion and for the female genitalia to be sufficiently cleared to allow the visualisation of internal structures.
Morphology
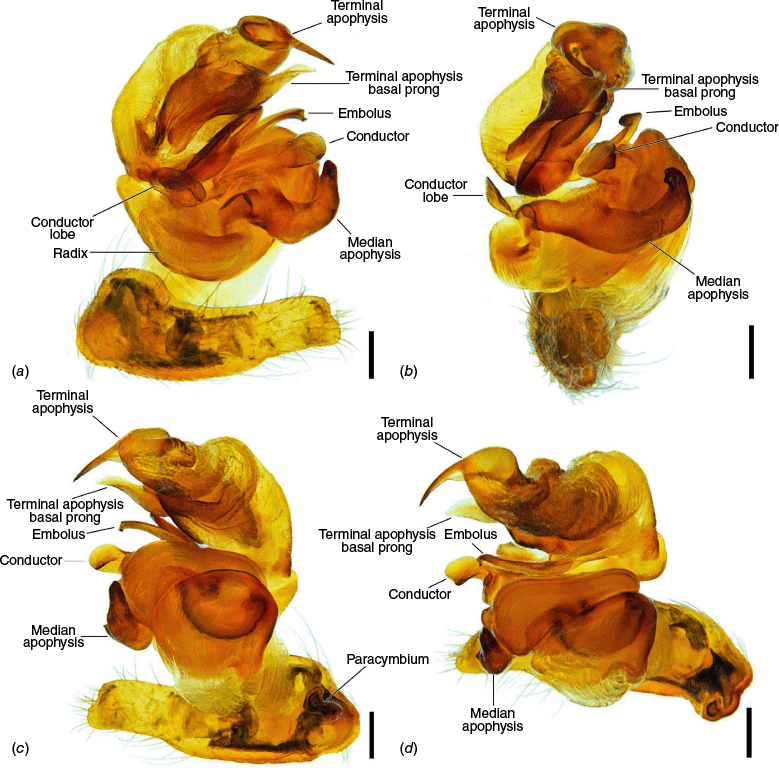
The terminology of the views of the male pedipalp considers their position as a limb i.e. the full view of the bulb with the cymbium in the background is a retrolateral (not ventral), as in Araneidae the pedipalp is twisted so that the cymbium is situated mesally (see also Framenau and Castanheira 2022; Castanheira and Framenau 2023). The term ‘conductor lobe’ is preferred over ‘paramedian apophysis’ for a structure that arises at the base of the conductor in the male pedipalp (Scharff and Coddington 1997; Framenau et al. 2010, 2021b). The term ‘terminal apophysis basal prong’ is used to name a projection on the base of the terminal apophysis of Carepalxis (e.g. Fig. 10c–e, 11a–d). However, this term has been previously used to name similar structures in different araneid genera such as ‘terminal apophysis prong’ in the Australasian Salsa Framenau & Castanheira, 2022 (e.g. Framenau and Castanheira 2022, fig. 3A–C) or ‘basal prong’ in the Neotropical Alpaida O. Pickard-Cambridge, 1889 (e.g. Baptista et al. 2018, fig. 1C, 3C). Our interpretation of these structures does not necessarily suggest homologies to these structures, primarily serving to better communicate the pedipalp morphology of males.
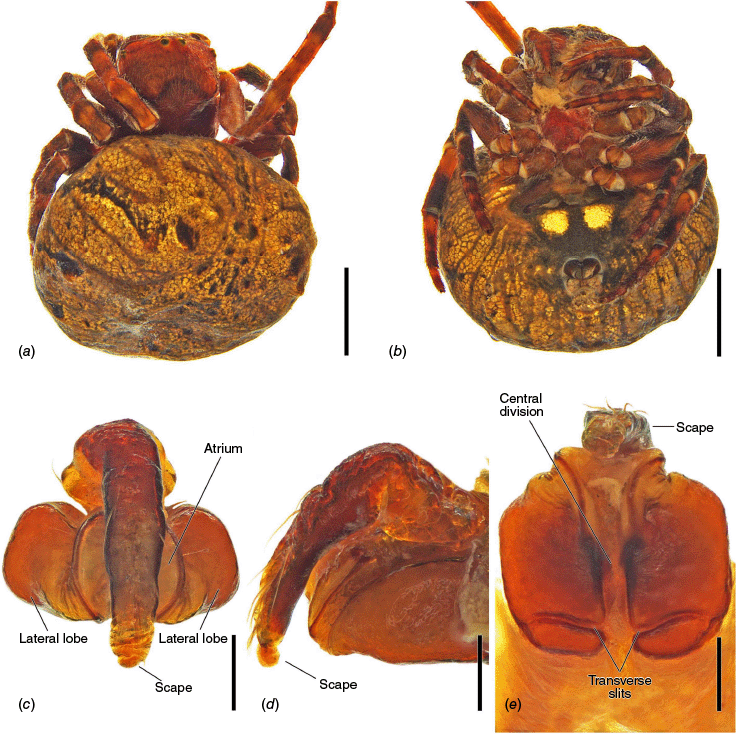
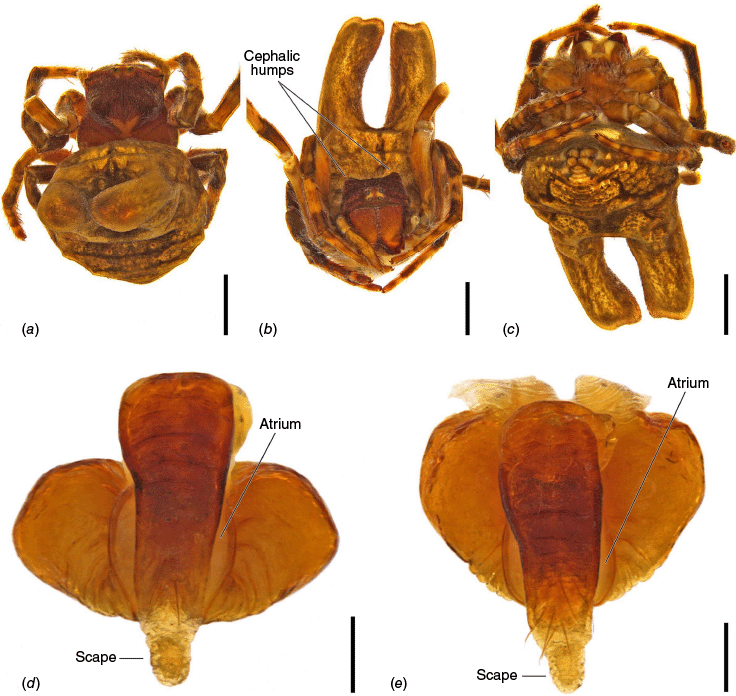
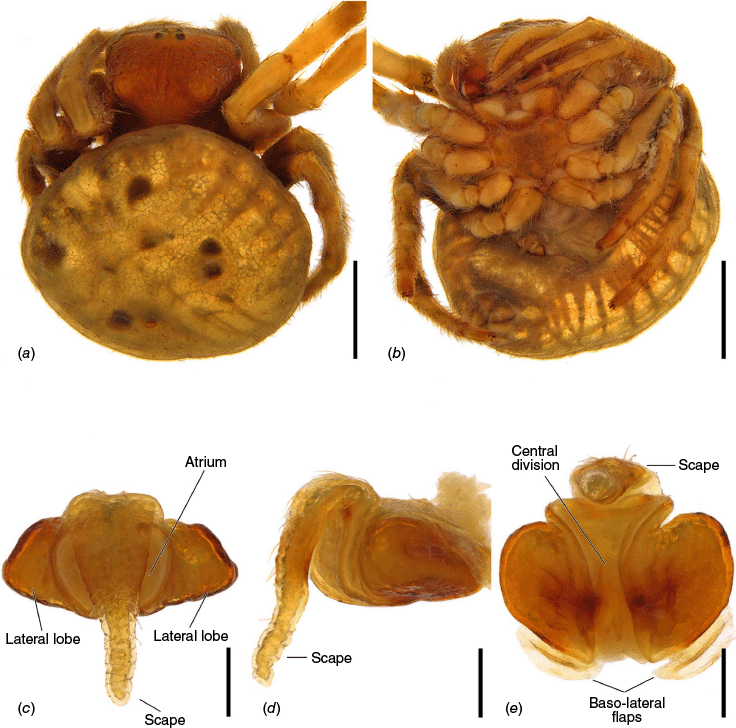
The epigyne has two main parts, the base (encapsulating the internal genitalia) and the scape. We refer to the central part of the base in ventral view as the atrium, which becomes the central division in posterior view (see Framenau et al. 2021a, 2021b, 2022; Framenau and Castanheira 2022; Castanheira and Framenau 2023). For the first time we use the term ‘epigyne transverse slits’ to refer to the transverse invagination of the terminal portion of the posterior plate of the epigyne at the end of the central division, partially separating the posterior plate in two parts (e.g. Fig. 5f, 6e, 18e). The most terminal portion of the posterior plate of the epigyne after the slits differ from the baso-lateral flaps, which are two independent fleshy structures that are outwardly projected (e.g. Fig. 9e, 14e, 21e), and are also present in, for example, Hortophora (Framenau et al. 2021a, fig. 7E, 16E, 22E).
Images, measurements and map preparations
Microscopic photographs were taken at the Harry Butler Institute, Murdoch University (Australia) in different focal planes with a Leica DMC4500 digital camera mounted on a Leica M205C stereomicroscope and combined using Leica Application Suite X (ver. 3.6.0.20104, see https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/). All photos were edited and combined into plates with Photoshop CC (ver. 2023, Adobe).
All measurements are given in millimetres using the same Leica Application Suite mentioned above. They were taken with an accuracy of 0.1 mm, except for eye and labium measurements taken with an accuracy of 0.01 mm. Pedipalp total length of segments measurements is femur + patella + tibia + tarsus, whereas legs total length of segments measurements is femur + patella + tibia + metatarsus + tarsus.
Maps were compiled in the software package QGIS (ver. 3.2.6, see https://qgis.org/en/site/, accessed 14 July 2023). For specimens collected during our recent field trip, geographic coordinates were obtained by pin-pointing the locations using Google Earth Pro (ver. 7.3.6.9345) to the nearest tenths of a second latitude and longitude. For museum specimens, these data were extracted directly from original labels or the registration information, or estimated to the closest minute of latitude and longitude in Google Earth Pro.
Abbreviations
ALE, anterior lateral eyes; AME, anterior median eyes; PLE, posterior lateral eyes; PME, posterior median eyes; TL, total length.
AM, Australian Museum, Sydney (Australia); CVIC, La Trobe University, Bendigo (Australia); MNHN, Muséum national d’Histoire naturelle, Paris (France); MSNG, Museo Civico di Storia Naturale Giacomo Doria, Genoa (Italy); NHMD, Natural History Museum of Denmark, Copenhagen (Denmark); NMV, Museums Victoria, Melbourne (Australia); QM, Queensland Museum, Brisbane (Australia); RMNH, Rijksmuseum van Natuurlijke Historie (currently Naturalis Biodiversity Centre), Leiden (Netherlands); SAM, South Australian Museum, Adelaide (Australia); UNB, Universidade de Brasília, Brasília (Brazil); WAM, Western Australian Museum, Perth (Australia); ZMH, Zoologisches Institut und Zoologisches Museum, Universität Hamburg (Germany).
Results
Molecular analyses
Both the analyses of the limited (Fig. 1, 2) and complete (Fig. S1) datasets suggest that Carepalxis, as currently circumscribed, is not monophyletic. In all analyses, the Neotropical C. quasimodo nests within two species of Ocrepeira (incl. its type species O. ectypa (Walckenaer, 1841)) within a well-supported Neotropical clade composed of Scoloderus Simon, 1887, Verrucosa McCook, 1888, Micrathena Sundevall, 1833, and Acacesia Simon, 1895 (Fig. 1, 2, S1). The ML and BI analyses of the limited dataset differ in the position of the Australian genus Telaprocera, but its position as sister to the Neotropical clade in the ML analysis is not well supported (Fig. 1).
Both analyses of the limited dataset show the Australasian representatives of Carepalxis forming a well-supported sister clade to all other sampled backobourkiines combined (Fig. 1, 2). By contrast, the ML analysis of the complete dataset resulted in Carepalxis as sister to a clade composed of Backobourkia heroine (L. Koch, 1871) and Parawixia dehaani (Doleschall, 1859) at the base of the backobourkiines clade (Fig. S1).
The relationships within Australasian Carepalxis in the ML and BI analyses of the limited dataset are largely congruent with the only difference being that C. ferreirasousai sp. nov. and C. montifera swapped their position (Fig. 1, 2). The analysis of the complete dataset agrees with the topology of the BI of the limited dataset in relation to our ingroup (Fig. 2) but includes a currently unidentified ‘Carepalxis sp. 250’ (Fig. S1). There appear to be two stable clades in Australasian Carepalxis, one represented by C. megalostylus sp. nov. and C. tholos sp. nov. and one representing all other species, with C. kolla sp. nov. and C. tuberculata most derived (Fig. 1, 2, S1).
Taxonomic revision
Australasian Carepalxis spiders are comparatively rare in collections; a total of 34 males, 176 females and 22 juveniles in 130 records were examined for this study excluding the type material of previously described species (Table 2). Following our revision, Carepalxis contains eight species, seven of which are exclusively found in Australia, and one that occurs in Australia and New Guinea (Table 2).
| Species | Comments | Distribution | Type specimen | Other material examined | |
|---|---|---|---|---|---|
| Carepalxis montifera L. Koch, 1872 | Type species of Carepalxis; males unknown. 2 female syntypes of C. tuberculata are C. montifera | NSW, Qld, SA, WA | Holotype female, Rockhampton (Qld) (ZMH-A0001729) | 26 females, 7 juveniles (in 12 records) | |
| C. beelzebub (Hasselt, 1873) | Senior synonym of C. suberosa (Thorell, 1881), C. tuberculifera (Thorell, 1881), and C. tricuspidata Chrysanthus, 1961; male described for the first time | Qld, SA, Tas., Vic., WA, also in New Guinea | Holotype female, Wangaratta (Vic.) (RMNH.ARA.5784) | 6 males, 25 females, 4 juveniles (in 20 records) | |
| C. bilobata Keyserling, 1886 | Male unknown | NSW, Qld, Vic. | 2 females and one juvenile syntypes, Peak Downs (Qld) (ZMH-A0001734) | 5 females (in 4 records) | |
| C. ferreirasousai sp. nov. | Male unknown | ACT, NSW, SA, WA | Holotype female, Munyeroo Conservation Park, 17 km SSE Moonabbie (SA) (SAM NN30840) | 14 females, 3 juveniles (in 10 records) | |
| C. megalostylus sp. nov. | Male unknown | NSW, Qld, SA | Holotype female, Nelshaby (SA) (SAM NN30829) | 8 females, 1 juvenile (in 8 records) | |
| C. kolla sp. nov. | Only known by the holotype female | SA | Holotype female, Gluepot Reserve, (SA) (SAM NN18561) | 1 female | |
| C. tholos sp. nov. | ACT, NSW, Qld, SA, Tas., Vic. | Holotype male, W of Bookabie (SA) (SAM NN30842) | 7 males, 11 females, 1 juvenile (in 18 records) | ||
| C. tuberculata Keyserling, 1886 | Senior synonym of Carepalxis furcifera (Keyserling, 1886) | ACT, NSW, NT, Qld, SA, Vic, WA | Lectotype female, Sydney (NSW), Peak Downs and Rockhampton (Qld) (ZMH-A0001776) | 21 males, 86 females, 6 juveniles (in 57 records) |
Abbreviations: NSW, New South Wales; SA, South Australia; Tas., Tasmania; Vic., Victoria; WA, Western Australia.
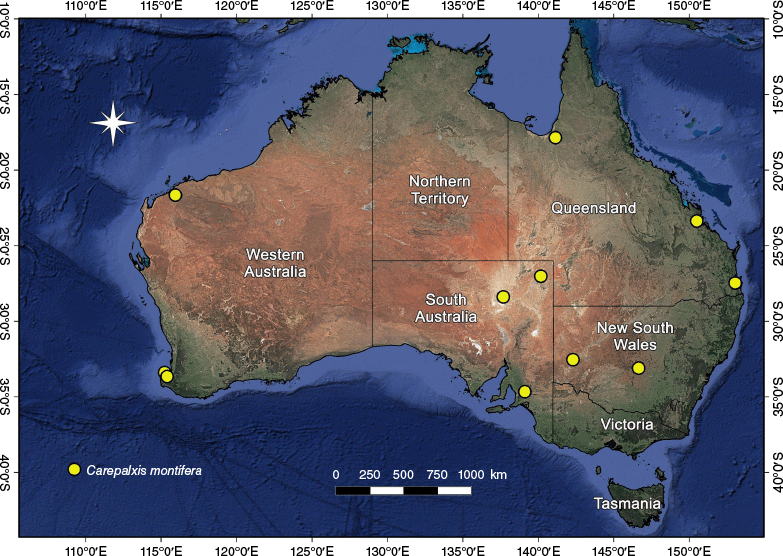
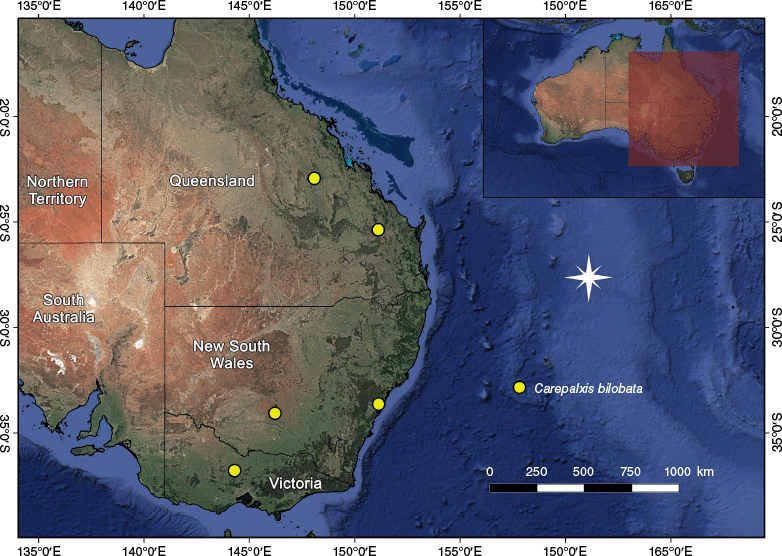
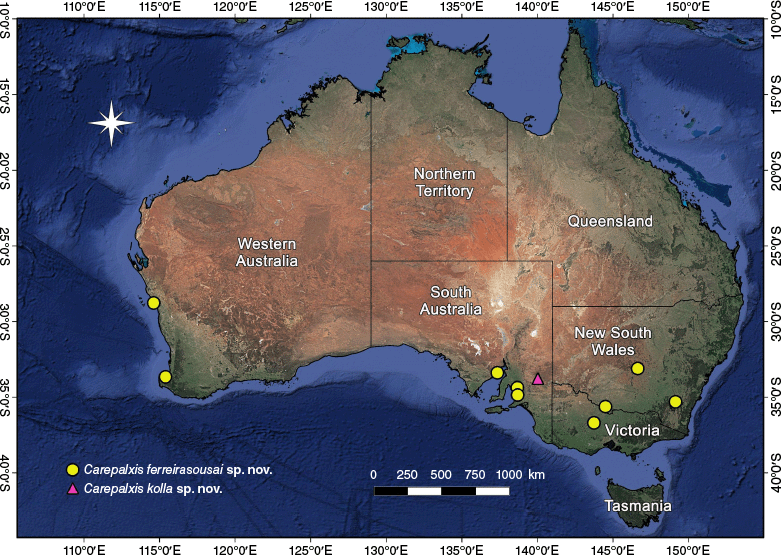
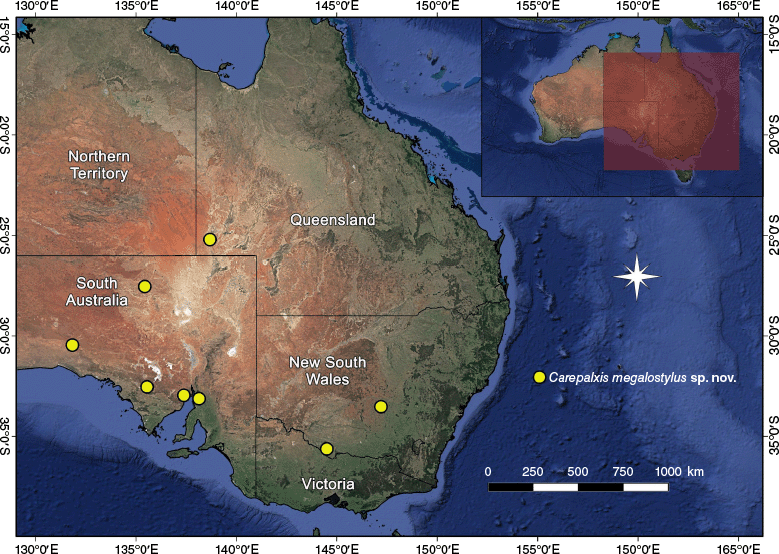
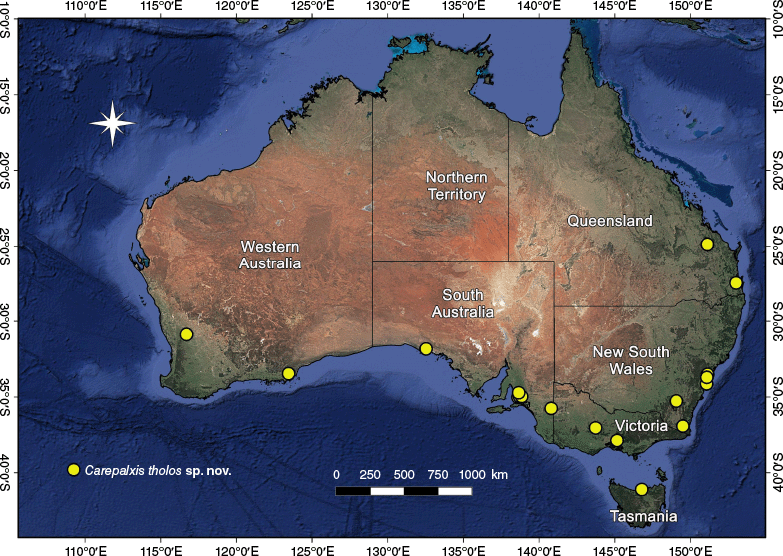
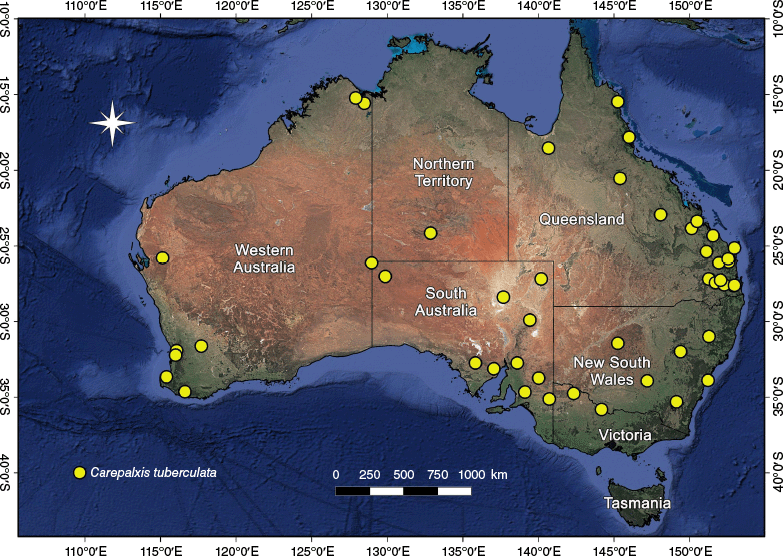
In Australia, the highest diversity of Carepalxis is in the south-eastern states, where all species occur; five species were recorded from Western Australia and the Northern Territory (Table 2). Carepalxis tuberculata is the most common and widespread species, with 21 males, 86 females and 6 juveniles (excluding type material) found in all mainland states and territories, but not Tasmania (Fig. 33).
Taxonomy
Genus Carepalxis L. Koch, 1872
Type species: Carepalxis montifera L. Koch, 1872
Species of Carepalxis differ from other backobourkiines by a pair of cephalic humps, a synapomorphy of Carepalxis, whereas similar humps are present as a convergence in a few Ocrepeira species and also Paraplectanoides crassipes Keyserling, 1886, the latter representing a distantly related genus placed as sister to Nephilinae Simon, 1894, a basal clade in the family Araneidae (e.g. Fig. 7b; Hormiga et al. 2023, fig. 1a–f, 2b, e). Males are characterised by the presence of a series of macrosetae on tibia II, including two highly modified and enlarged macrosetae (megaspines), another synapomorphy of Carepalxis (similar to Neotropical Verrucosa), compared to a maximum of one macroseta in some Hortophora Framenau & Castanheira, 2021 and Novakiella Court & Forster, 1993. Species of Carepalxis can also be identified by the anteriorly elevated abdomen (e.g. Fig. 22a, 27a), which may carry numerous tubercles, humps or sigilla in addition to the humeral humps (e.g. Fig. 14a, 26a). Other backobourkiines may have one (e.g. in Salsa) or a maximum of five posterior tubercles (in Socca and Acroaspis Karsch, 1878). Males also have the following diagnostic characters: median apophysis elongated and strongly curved (similar to some Salsa but comparatively larger) and terminal apophysis with an elongated basal prong (somewhat similar in position to Salsa and Alpaida), apically elongated, inflated, and ending in a long and pointed tip (e.g. Fig. 10c–e, 11a–d; Baptista et al. 2018, fig. 1C, 3C; Framenau and Castanheira 2022, fig. 3A–C), whereas females are also identified by the wide lateral lobes on the epigyne that accommodate large spermathecae; an elevated scape with enlarged basis, not completely covering the atrium, and being longer than the epigyne area (e.g. Fig. 4a–n, 5d–f, 9c–e, 14c–e). Some species present ‘transverse slits’ in posterior view (e.g. Fig. 5f, 6e, 18e), whereas others bear baso-lateral flaps (e.g. Fig. 9e, 14e, 21e), the latter structure present in some other backobourkiines like Hortophora (e.g. Framenau et al. 2021a; Fig. 3a–n, 4d–f, 8c–e, 13c–e).
Male leg II. (a) Carepalxis beelzebub (van Hasselt, 1873) (QM S121046), retrolateral view; (b) ventral view; (c) prolateral view. (d) Carepalxis tholos sp. nov. (AM KS.91104), retrolateral view; (e) ventral view; (f) prolateral view. (g) Carepalxis tuberculata Keyserling, 1886 (SAM NN30841), retrolateral view; (h) ventral view; (i) prolateral view. Scale bars: 0.2 mm.
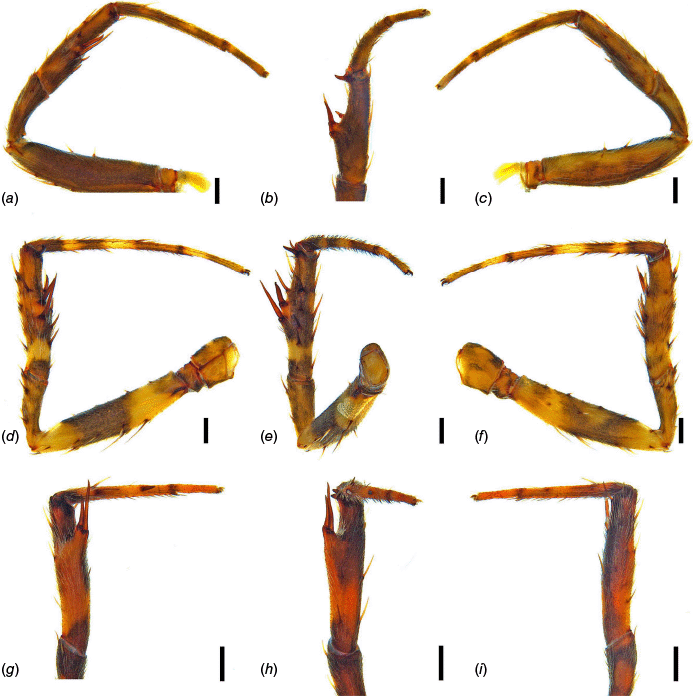
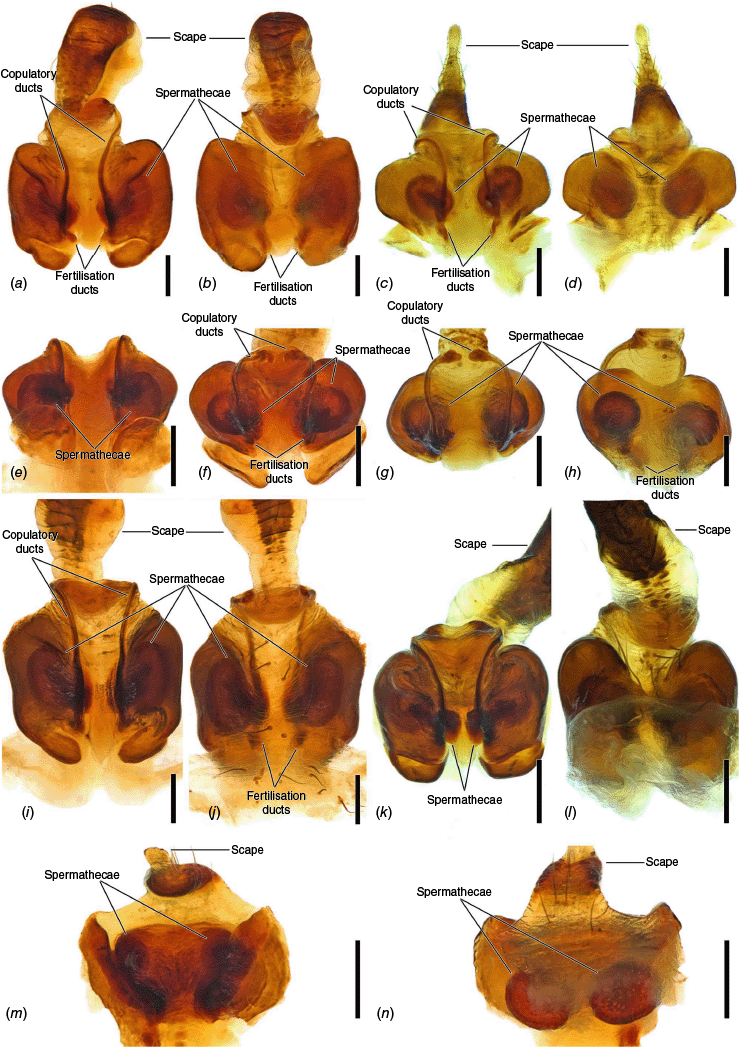
Female epigyne, cleared. (a) Carepalxis montifera L. Koch, 1872 (SAM NN30815), posterior view; (b) anterior view. (c) Carepalxis beelzebub (van Hasselt, 1873) (QM S121046), posterior view; (d) anterior view. (e) Carepalxis bilobata Keyserling, 1886 (AM KS.76355), posterior view; (f) anterior view. (g) Carepalxis megalostylus sp. nov. (SAM NN30839), posterior view; (h) anterior view. (i) Carepalxis ferreirasousai sp. nov. (AM KS.91254), posterior view; (j) anterior view. (k) Carepalxis tuberculata Keyserling, 1886 (SAM NN30835), posterior view; (l) anterior view. (m) Carepalxis tholos sp. nov. (QM W874), posterior view; (n) anterior view. Scale bars: 0.2 mm.
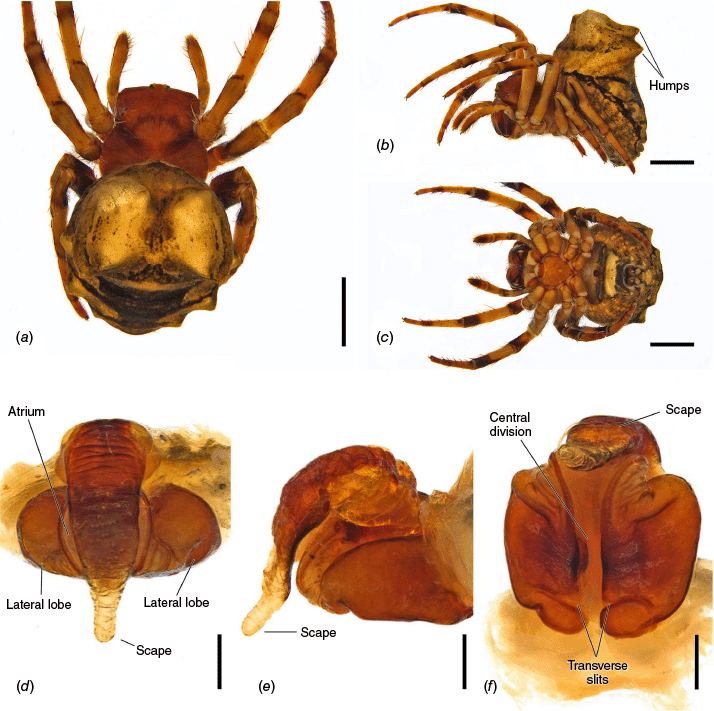
Carepalxis montifera L. Koch, 1872, female (SAM NN30815). (a) Dorsal habitus; (b) lateral habitus; (c) ventral habitus; (d) epigyne, ventral view; (e) epigyne, lateral view; (f) epigyne, posterior view. Scale bars: (a–c) 2 mm; (d–f) 0.2 mm.
Carepalxis montifera L. Koch, 1872, females. (a) Carepalxis montifera L. Koch, 1872 holotype (ZMH-A0001729), dorsal habitus; (b) epigyne posterior view. (c) Carepalxis montifera L. Koch, 1872 (syntype of Carepalxis tuberculata Keyserling, 1886) from Rockhampton (ZMH-A00016656), epigyne apico-ventral view; (d) epigyne lateral view; (e) epigyne posterior view. Scale bars: (a) 1 mm; (b–e) 0.1 mm.
Carepalxis montifera L. Koch, 1872, female variation (QM W1654). (a) Dorsal habitus; (b) frontal habitus; (c) ventral habitus; (d) epigyne, ventral view; (e) epigyne, apico-ventral view. Scale bars: (a–c) 2 mm; (d, e) 0.2 mm.
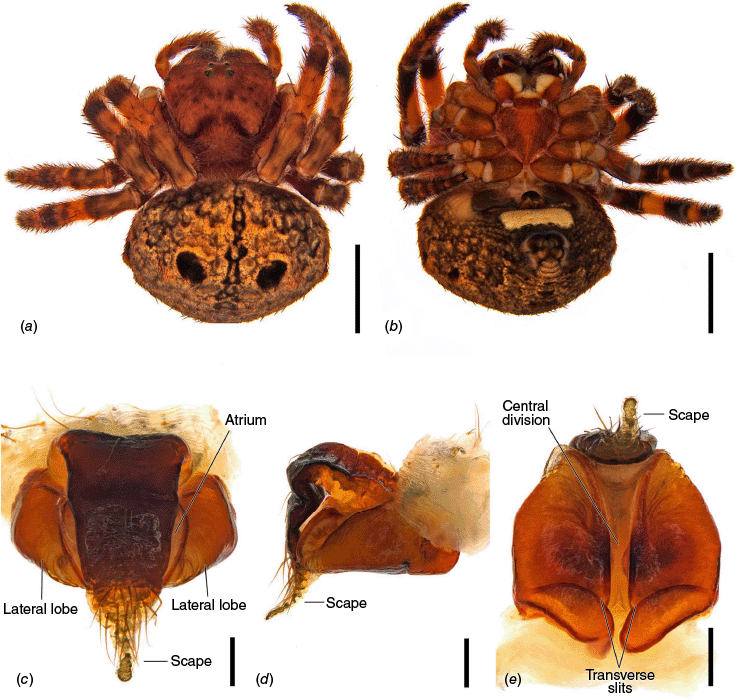
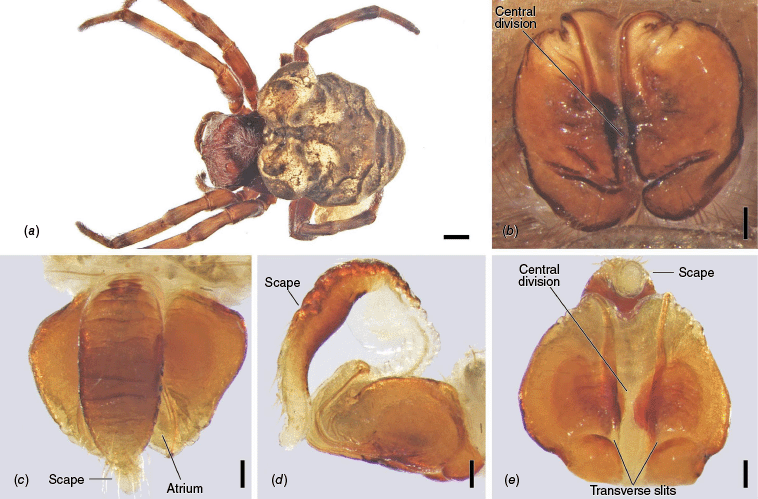
Carepalxis beelzebub (van Hasselt, 1873), female (SAM NN30834). (a) Dorsal habitus; (b) ventral habitus; (c) epigyne, ventral view; (d) epigyne, lateral view; (e) epigyne, posterior view. Scale bars: (a, b) 2 mm; (c–e) 0.2 mm.
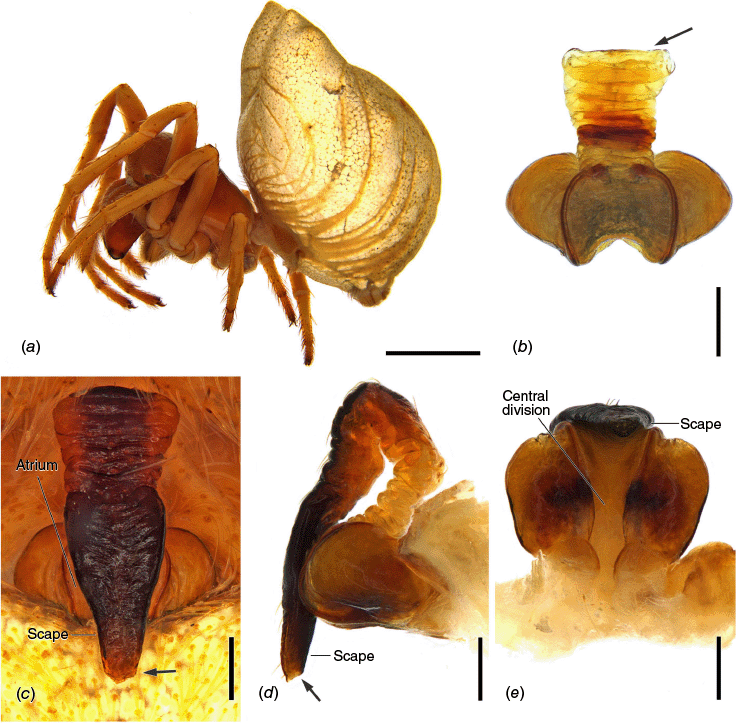
Carepalxis beelzebub (van Hasselt, 1873), male (AM KS.32866). (a) Dorsal habitus; (b) ventral habitus; (c) left pedipalp, ventral view; (d) left pedipalp, retrolateral view; (e) left pedipalp, dorsal view. Scale bars: (a, b) 2 mm; (c–e) 0.2 mm. Black arrow points to the median apophysis’ stump.
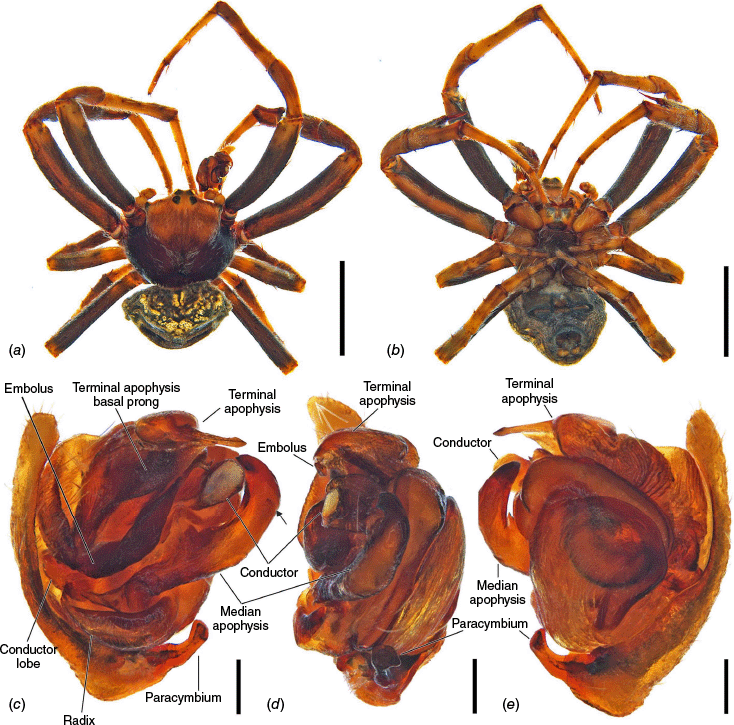
Medium-sized orb-weaving spiders, males (~TL 4.0–4.8) smaller than females (~TL 6.5–8.4). Carapace slightly longer than wide, elevated anteriorly, with cephalic region relatively wider, rectangular and with two posterior humps in females (e.g. Fig. 5a, 7b), whereas the cephalic region is narrow and flat in males (Fig. 10a, 24a, 30a); colouration variable from yellowish-brown to reddish-brown, generally with a dark pattern behind the cephalic region and covered with yellowish-white setae (e.g. Fig. 5a, 9a, 10a, 14a, 18a). Fovea absent. Posterior median eyes generally largest, row of posterior eyes slightly recurved, lateral eyes almost touching, posterior lateral eyes separated from posterior median eyes by more than their diameter; anterior median eyes raised from the carapace. Chelicerae fangs with three or four promarginal teeth (two in C. beelzebub females, and one in C. tholos sp. nov. males), and three retromarginal teeth (four in both sexes of C. beelzebub, and one in C. tholos sp. nov. males). Legs: Leg formula I > II > IV > III. Tibiae II of males stronger than tibiae I, thinner distally provided with two strong retrolateral macrosetae on large bases in association with other thick macrosetae (Fig. 3a–i). Labium wider than long, with anterior pale edge (e.g. Fig. 5c, 7c, 9c, 10b). Maxillae with glabrous antero-mesal section (e.g. Fig. 5c, 7c, 9c, 10b). Sternum longer than wide, comparatively narrower in males than females, its colour varying from yellowish-brown to dark brown and sometimes with dark contour, covered by sparse to dense setae (e.g. Fig. 5c, 7c, 9c, 10b, 14b). Abdomen generally oval or oblong, but sometimes dome shaped (e.g. C. tholos sp. nov., see Fig. 27a), with extreme intra- and interspecific variation, specifically in relation to humeral humps being inconspicuous or absent (e.g. C. beelzebub, see Fig. 9a), but sometimes composed of two strong projections (e.g. C. montifera, see Fig. 5a, 6a, 7a–c; or C. megalostylus sp. nov., see Fig. 22a), or forming two flat elongated humps (e.g. C. montifera (variation), see Fig. 7b); abdomen otherwise generally without specialised setae, sigilla (except for the female of C. beelzebub, see Fig. 9a), condyles or other specific structures, colour dorsally highly variable with tones from golden- or pale-yellow to olive grey and reddish-brown (e.g. Fig. 5a, 9a, 10a, 14a, 16a, c) and generally with black patches or spots varying from small (e.g. Fig. 9a, 19a, 24a) to large in size (e.g. Fig. 5a, 18a, 21a). Venter of variable colour, centrally generally dark brown or grey and sometimes with two large white spots (Fig. 14b, 19b, 29b) or a transversal white band (e.g. Fig. 5c, 18b). Male pedipalp patella with a single long macroseta (e.g. Fig. 25a–d, 30c–e); paracymbium thin and slanted with a wide tip (e.g. Fig. 10c, e, 30e, 31c); median apophysis elongated, C shaped, with outer margin bearing an inconspicuous projection (C. beelzebub, Fig. 10c), a strong excavation (C. tuberculata, Fig. 30c), or a short projection (C. tholos sp. nov., Fig. 24c), and inner margin with a strong sclerotised tip pointing ventrally (e.g. Fig. 10c, 24c, 30c), base forming and arch over the radix (proposed synapomorphy of backobourkiines, see Scharff et al. 2020); radix not pronounced, canoe shaped and partially or entirely sclerotised (e.g. Fig. 10c, 24c, 30c); basal conductor lobe very conspicuous, with scale-like impressions distally (e.g. Fig. 10c, 24c, 30c); terminal apophysis basally with a long and strong prong that tapers to a thick tip (e.g. Fig. 10a, 25a, 31a), and apically elongated, inflated, curving basally ending in a straight pointed tip (e.g. Fig. 9c, 24c, 30c); conductor massive, rounded and fleshy with sclerotised borders (e.g. Fig. 11a, 25a, 31a); embolus strong, slanted, almost straight, uncapped, with a large curved and carved tip (e.g. Fig. 10c, 11a, 24c, 25a, 30c, 31a). Epigyne wider than long, partially to strongly sclerotised (e.g. Fig. 5d, 7d, 9c), with very thick and rounded lateral lobes (e.g. C. montifera, Fig. 5d), or with converging and more pointed lateral lobes (e.g. C. beelzebub, Fig. 9c); atrium almost as wide as long, concave, and accommodating the sclerotised scape (e.g. Fig. 5d, e, 9c, e, 14c, e); scape with strong base sometimes elevated and wider anteriorly, and tapering to its tip, which overlaps the epigyne area (e.g. Fig. 5d, e, 9c, e, 14c, d, 18c, d); central division with straight sides, medially narrow (e.g. Fig. 5f, 18e, 19e, 29e), wide (e.g. Fig. 9e, 14e, 21e), or very wide (Fig. 26e); baso-lateral flaps oblong (e.g. C. beelzebub, Fig. 9e) or oval (e.g. C. bilobata, Fig. 14e), and, whenever absent, species will have two transverse slits dividing the terminal end of the posterior plate (e.g. Fig. 5f, 18e, 19e, 29e); spermathecae enlarged and normally occupying most of the lateral borders of the epigyne (Fig. 4a–n).
Carepalxis montifera L. Koch, 1872; C. beelzebub (Hasselt, 1873); C. bilobata Keyserling, 1886; C. ferreirasousai sp. nov.; C. kolla sp. nov.; C. megalostylus sp. nov.; C. tholos sp. nov.; C. tuberculata Keyserling, 1886 (Table 2).
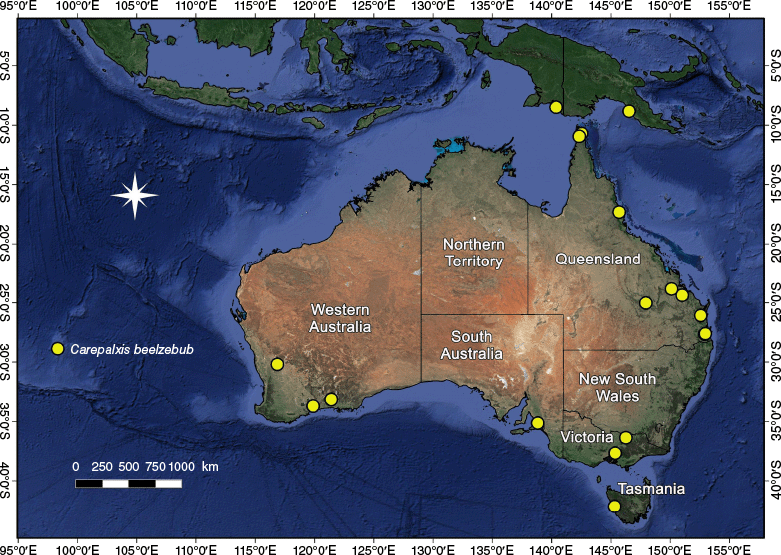
Carepalxis is widespread in Australia with records for all states and territories (Fig. 8, 13, 17, 20, 23, 28, 33). Additionally, C. beelzebub has been found in New Guinea, both in West Papua (Indonesia) and in Papua New Guinea (Fig. 13).
Male of C. bilobata, C. ferreirasousai sp. nov., C. kolla sp. nov., C. megalostylus sp. nov., C. montifera unknown.
| 1 | Tibia of leg II with pair of long macrosetae surpassing joint between tibia and metatarsus (Fig. 3g, h); median apophysis of pedipalp with spatulate projection apically fused to the sclerite’s tip (e.g. Fig. 30c, 31c, d)...C. tuberculata Tibia of leg II with the pair of long macrosetae not surpassing the joint between tibia and metatarsus (Fig. 3a, b, d, e); median apophysis of pedipalp without spatulate projection (Fig. 10c, d, 11a, b, 24d, 25b, d)...2 |
| 2 | Median apophysis of pedipalp with small triangular projection apically; embolus long with slender tip (Fig. 10c, 11a, b)...C. beelzebub Median apophysis of pedipalp with a small projection medially, and embolus thick and short (Fig. 24c, 25a, b)...C. tholos sp. nov. |
| 1 | Epigyne with baso-lateral flaps (Fig. 9e, 14e, 21e, 22e, 26e)...2 Epigyne without baso-lateral flaps, but with transverse slits on the posterior plate (Fig. 5f, 18e, 19e, 29e)...5 |
| 2 | Epigyne with scape surpassing the epigyne area by more than half of the scape’s length (Fig. 14c, d, 15b, 16b, d, 21c, d)...3 Epigyne with scape surpassing the epigyne area by less than the half of the scape’s length (Fig. 9c, d, 12a, b, 26c, d, 27b)...4 |
| 3 | Epigyne with scape medially almost completely covering the atrium; baso-lateral flaps small, not laterally projected (Fig. 21c, e, 22c, e)...C. megalostylus sp. nov. Epigyne scape medially ~1/3–1/2 of the atrium width; baso-lateral flaps conspicuous and laterally projected (Fig. 14c, e, 15b, 16b, d)...C. bilobata |
| 4 | Epigyne with atrium almost as wide as long and lateral lobes broad and converging to a vertex (Fig. 9c, 12a, b)...C. beelzebub Epigyne with atrium wider than long and lateral lobes semicircular and not thick (Fig. 26c, d, 27b)...C. tholos sp. nov. |
| 5 | Width of epigyne scape medially ~1/2 of atrium width (Fig. 29c)...C. tuberculata Width of epigyne scape medially ~2/3 of atrium width (Fig. 5d, 18c, 19c)...6 |
| 6 | Abdomen ventrally with two large white patches (Fig. 19b). Epigyne atrium slightly longer than wide (Fig. 19c)...C. kolla sp. nov. Abdomen ventrally with a single wide white patch (Fig. 5c, 18b). Epigyne atrium approximately as long as wide or wider than long (e.g. Fig. 5d, 18c)...7 |
| 7 | Epigyne scape winkled, elevated near its base, and tapering to a broad rounded tip (Fig. 5d, e)...C. montifera Epigyne scape not winkled or elevated near its base, and tapering to thin flattened tip (Fig. 18c, d)...C. ferreirasousai sp. nov. |
Carepalxis montifera L. Koch, 1872
(Fig. 1, 2, 4a, b, 5a–f, 6a–e, 7a, e, 8, 32c, S1.)
Carepalxis montifera L. Koch, 1872,pp. 123–125, pl. 10, fig. 1. – Rainbow (1911), p. 197.
Holotype. Female of Carepalxis montifera, Rockhampton (23°22′S, 150°30′E, Queensland, AUSTRALIA), ZMH-A0001729, examined (photos).
AUSTRALIA: New South Wales: 2 females, Kinchega National Park, 32°32′S, 142°17′E, 15 May 1969, AM KS109267; 1 female, Lane Paddock, ‘Brotherony’, via Condolobin, 33°06′S, 146°39′E, 14 March 2002, AM KS91268. Queensland: 1 female, Hemmant, 27°28′S, 153°03′E, QM W1654; 2 females (originally syntypes of C. tuberculata), Rockhampton, 23°22′S,150°30′E, ZMH-A00016656; 1 female, Norman River, 20 km SSE Normanton, 17°51′S, 141°08′E, 08 January 1993, NMV K-13315. South Australia: 1 female, Coopers Creek, 28°23′S, 137°41′E, SAM NN30815; 2 females, 1 juvenile, Eden Valley, 34°39′S, 139°06′E, SAM NN30817–30819; 2 females, Lake Goolangarie (Lake Goyder), 27°01′S, 140°10′E, 13 December 2000, SAM NN30837–NN30838. Western Australia: 1 female, Robe River near Northwestern Highway, 21°39′S, 115°57′E, 13 July 2000, WAM T41817; 2 females, 1 juvenile, Sabina River, 33°23′S, 115°14′E, 12 January 1984, WAM T37203–5; 1 female, Sabina River, 33°23′S, 115°14′E, WAM T37207; 1 female, same data as previous, WAM T37234; 11 females, 3 juveniles, same data as previous, WAM T37209–22.
Females of Carepalxis montifera are most similar to those of C. tuberculata as both species share an epigyne with rounded lateral lobes and a scape reaching over the epigyne area by ~1/4 of the scape’s length (Fig. 5d, e, 6c, d, 7d, 29c, d, 32a–c), differing for example from C. kolla sp. nov. whose scape surpasses the epigyne area by 1/5 of the scape’s length (Fig. 19c). However, C. montifera can be identified by an abdomen having elevated humps dorsally and ventrally bearing a wide white patch (Fig. 5a–c) v. an abdomen dorsally without conspicuous humps and centrally with two white spots in C. tuberculata (Fig. 29a, b); and an epigyne scape that is less elevated and anteriorly wider exposing less of the atrium and tapering to its tip (v. approximately the same width from base to tip in C. tuberculata) (Fig. 5d, e, 6c, d, 7d, 22c, d, 25a–c). Male unknown.
Total length 7.5. Carapace 2.4 long, 3.3 wide; with orange–brown background and dusky streaks posteriorly (Fig. 5a). Eye diameter AME 0.13, ALE 0.08, PME 0.13, PLE 0.07; row of eyes: AME 0.48, PME 0.61, PLE 2.50. Chelicerae orange–brown at their base and reddish-brown distally (Fig. 5b), three promarginal teeth of similar size and three retromarginal teeth of similar size. Legs orange–brown, mottled dark, base of femora darker (Fig. 5a–c). Pedipalp length of segments: 1.0 + 0.6 + 0.7 + 1.2 = 3.5. Leg formula I > II > IV > IV; length of segments: I – 3.2 + 1.9 + 2.3 + 2.0 + 1.0 = 10.4, II – 3.0 + 1.7 + 2.0 + 1.9 + 1.0 = 9.6, III – 1.9 + 1.1 + 1.1 + 1.0 + 0.8 = 5.9, IV – 2.6 + 1.6 + 1.6 + 1.7 + 0.8 = 8.3. Labium 0.47 long, 0.80 wide, and maxillae base orange and apically beige (Fig. 5c). Sternum 1.3 long, 1.5 wide, orange–brown mottled lighter and with yellow setae laterally (Fig. 5c). Abdomen 5.4 long, 5.5 wide; oblong, two extremely elevated humeral humps and six tubercles on edges (Fig. 5a–c); dorsum golden-brown with sparse black spots and a large black patch posteriorly (Fig. 5a); sides golden-brown with two black contiguous patches and dusky streaks (Fig. 5b, c); venter with black background and covered by a large beige area (Fig. 5c). Epigyne oblong with wide semicircular sides (Fig. 5d); atrium almost as wide as long and slightly convex forming a shallow rim to accommodate the scape (Fig. 5d–f); scape elongated, base wrinkled and slightly elevated, wide anteriorly and strongly tapering to a rounded blunt tip that is slightly projected centrally, overreaching the epigyne area by ~1/4 of its length (Fig. 5d, e); central division with parallel sides slightly widening towards atrium (Fig. 5f); posterior plate with two somewhat almost straight transverse slits, dividing the posterior plate on its terminal portion forming two longer than wide lobes (Fig. 5f); baso-lateral absent (Fig. 5f); spermathecae oval, enlarged, and occupying most of genital area (Fig. 4a, b).
Total length females 5.4–8.4 (n = 5). Humeral humps can vary in size and shape, from two large turrets (Fig. 5a, b, 6a) to two elongated and slender flaps (Fig. 7a–c). Epigyne scape width is also variable medially, with a variable degree of exposure of the atrium (e.g. Fig. 8c, 7d, e, 32c).
The holotype of C. montifera is a female from Rockhampton (Queensland). It has the more commonly found elevated turret-like dorsal humps and the scape of the epigyne is broken off, even though still matching well with the specimen we used in our redescription of the species (SAM NN30815) (Fig. 5a–f, 6a, b). However, as described in the variation section above, the width of the epigyne scape can vary, and this can be amplified by the position of the scape during examination. Viewed from a more apical angle, with the scape slightly more detached from the atrium, the viewer can get the illusion of a different shape, with the scape appearing not to be elevated, and occupying most of the atrium width, if not completely covering it. This situation was initially observed in one of the syntypes of C. tuberculata, also from Rockhampton, from which we received photos (ZMH-A00016656) (Fig. 6c–e). On Fig. 6d (lateral view) one can clearly see that the scape is elevated (or protruding) at its base, whereas on Fig. 6c (apico-ventral view), as the photo was taken more apically, the scape elevation is barely visible, causing the illusion that the scape is wider than it really is. After observing several specimens of C. montifera, such as the one with long elongated humeral humps (ventral view, Fig. 7c v. apico-ventral view, Fig. 7d), we judged that by the variation in scape angle mentioned above, but also by the shape of the terminal portion of the posterior plate after the transverse slits, which matches with the holotype of the genus type species (Fig. 6b, e), the specific syntype ZMH-A00016656 of C. tuberculata was initially misidentified and is, in fact, C. montifera. By contrast, the second syntype specimen of C. tuberculata from the same vial from Rockhampton (ZMH-A00016656) (Fig. 32c) has the typical tapering of the scape present in C. montifera in comparison to C. tuberculata and was also originally misidentified and included in the type series of the latter species.
According to the original labels where the collection date was available, specimens of C. montifera were found between December and March, with additional four specimens collected in May, and one in July. Therefore, the species is considered as mainly summer to early autumn mature, whereas for the northern-most regions of Australia, the only specimen with collection date was found during the wet season in January. No habitat data were given on the original labels. Some specimens were collected in nests of mud wasps (genus Pison Spinola, 1808).
The species is widely distributed in Australia, with records from New South Wales, Queensland, South Australia and Western Australia (Fig. 8). Occurrence in the Northern Territory is likely.
Carepalxis beelzebub (van Hasselt, 1873)
(Fig. 3a–c, 4c, 9a–e, 10a–e, 11a–d, 12a, 13.)
Epeira beelzebub van Hasselt, 1873, pp. 240 –243, pl. 12, fig. A–C.
Carepalxis beelzibub (van Hasselt). – Rainbow (1911), p. 197 (misspelled).
Carepalxis suberosa Thorell, 1881, pp. 48–51. New synonymy.
Acroaspis tuberculifera Thorell, 1881, pp. 52–55. – Rainbow (1911), p. 198. New synonymy.
Carepalxis tricuspidata Chrysanthus, 1961, pp. 205–207, fig. 50–53. New synonymy.
Holotype. Female of Carepalxis beelzebub, Wangaratta (36°21′S, 146°16′E, Victoria, AUSTRALIA), RMNH.ARA.5784, examined (photos). Syntype male and female of Acroaspis tuberculifera Thorell, 1881, Somerset (Cape York) (10°41′28.2″S, 142°31′56.5″E, Queensland, AUSTRALIA), year 1875, LM D’Albertis coll., MSNG, examined (photos). Holotype female of Carepalxis suberosa Thorell, 1881, Yule Island (8°49′S, 146°32′E, PAPUA NEW GUINEA), year 1875, LM D’Albertis coll., MSNG, examined (photos). Holotype female of Carepalxis tricuspidata Chrysanthus, 1961, Merauke (8°29′S, 140°23′E, West Papua, INDONESIA), c. 1956–1957, Br. Monulf coll., RMNH.ARA.971, not examined.
AUSTRALIA: 1 female, no data, QM. Queensland: 1 female, Atherton Plateau, Rose Gums Wilderness Retreat, 12.4 km at 059°ENE Malanda, 17°18′S, 145°42′E, 15–16 March 2006, NHMD; 1 male, 1 juvenile, Burster Creek, 10°56′S, 142°20′E, QM S83476; 1 male, Camira, 27°38′S 152°55′E, QM S19591; 1 male, same locality as previous, 01 October 1990, QM S17806; 2 females, same locality as previous, 04 December 1987, QM S2465; 2 females, Burra, via Gympie, 26°04′S, 152°35′E, 04 September 1993, QM S124029; 4 females, 1 juvenile, Inala, Brisbane, 27°36′S, 152°58′E, 16 February 1992, QM S124030; 1 male, Kroombit Tops, Northern Escarpment, 24°22′S, 151°01′E, QM S121042; 1 female, Mt Moffat, 25°01′S, 147°57′E, QM S121043; 1 female, Pheasant Creek, Rockhampton area, 23°50′S, 150°07′E, 04 January 1999, QM S124032. South Australia: 1 female, Blakiston, 35°07′S, 138°50′E, SAM NN30834. Tasmania: 1 female, north Tasmania, no exact locality, 19 March 1929, AM KS.29130; 1 female, no exact locality, AM KS.29129; 1 male, Strahan, 42°09′S, 145°19′E, 08 December 1973, AM KS.32866. Victoria: 4 females, 2 juveniles, no exact locality, NMV K-13319; 2 males, 1 female, Yarra Glen, 37°39′S, 145°22′E, QM S121046. Western Australia: 2 females, 20 km S of Ravensthorpe, 33°41′S, 119°53′E, 02 January 1994, WAM T31075–6; 1 male, East Nugadong Nature Reserve, 30°11′S, 116°52′E, WAM T75382; 1 female, Grass Patch, 33°08′S, 121°25′E, 24 December 1992, WAM T37191.
Females of C. beelzebub are most similar to those of C. bilobata and C. megalostylus sp. nov. as all three species share epigyne in which the lateral lobes have acute projections and scape reaching posteriorly past the genital area by at least 1/2 of the scape’s length (Fig. 9c, d, 14c, d, 15b, 16b, d, 21c, d). Carepalxis beelzebub differs from these other two species by a scape base that is not elevated with the scape’s tip reaching past the genital area by only approximately the atrium length (Fig. 9c, d). Males of C. beelzebub are most similar to those of C. tholos sp. nov., both differing from C. tuberculata by leg tibia II having shorter pair of macrosetae that do not reach the metatarsus. In addition, the pedipalp median apophysis is less elongated and without an apical excavation, and the terminal apophysis is less inflated (Fig. 3a–h, 10c, d, 24c, d, 30c, d). Carepalxis beelzebub males differ from those of C. tholos sp. nov. by an inconspicuous stump (small projection) on the outer margin of the median apophysis; the terminal apophysis is not strongly curved basally with a wider triangular tip; and the embolus is medially thinner with an enlarged tip basally projected (Fig. 10c, d, 27c, d).
Total length 6.5. Carapace 2.1 long, 2.7 wide; orange–brown and covered by pale yellow setae (Fig. 9a). Eye diameter AME 0.13, ALE 0.09, PME 0.14, PLE 0.08; row of eyes: AME 0.49, PME 0.63, PLE 2.74. Chelicerae orange–brown, with two promarginal teeth of similar size and four retromarginal teeth (basal largest). Legs yellowish-brown, base of femora darker (Fig. 9a, b). Pedipalp length of segments: 0.9 + 0.4 + 0.7 + 1.2 = 3.2. Leg formula I > II > IV > III; length of segments: I – 3.5 + 1.1 + 1.9 + 1.7 + 0.9 = 9.1, II – 3.1 + 1.2 + 1.7 + 1.6 + 0.8 = 8.4, III – 1.8 + 0.9 + 1.0 + 1.0 + 0.7 = 5.4, IV – 2.8 + 1.5 + 1.6 + 1.4 + 0.8 = 8.1. Labium 0.49 long, 0.75 wide, and maxillae base orange and apically beige (Fig. 9b). Sternum 1.2 long, 1.5 wide, orange with centre mottled dark (Fig. 9b). Abdomen 4.6 long, 5.8 wide; rounded, dorsum without noticeable humps or tubercles, golden-brown with six dark sigilla, two anteriorly, two centralised and four posteriorly (Fig. 9a); laterally with golden-brown streaks (Fig. 9b); venter golden-brown with sparse guanine spots (Fig. 9b). Epigyne approximately twice as wide as long with projected pointed sides, atrium large, almost as wide as long and slightly convex (Fig. 9c); scape elongated, not elevated at base, much wider anteriorly and tapering to a rounded blunt tip extended beyond the epigyne area by approximately half of its length (Fig. 9c, d); central division with parallel sides (Fig. 8e); baso-lateral flaps elongated and narrow (Fig. 9e); spermathecae oval, slanted and almost their diameter apart (Fig. 4c, d).
Total length 4.0. Carapace 1.8 long, 2.3 wide, dark brown with cephalic area light brown and whitish setae around cephalic area and borders of carapace (Fig. 10a). Eye diameter AME 0.13, ALE 0.11, PME 0.14, PLE 0.07; row of eyes: AME 0.45, PME 0.53, PLE 1.67. Chelicerae light brown; with four promarginal teeth (basal largest) and four retromarginal teeth (basal largest). Legs light brown, mottled dark on joints and dark brown ventrally on femora (Fig. 3a–c, 10a, b). Leg tibia II strong and bearing two adjoined, thick and long macrosetae at approximately half of its length, followed by one short and thick macroseta with a very wide base near the outer margin of the pair of macrosetae; tibia somewhat reducing in thickness after the macrosetae bases and ending in one long and transversal macroseta on the distalmost portion of it at the articulation with metatarsus (Fig. 3a–c). Leg formula I > II > IV > III; length of segments: I – 2.9 + 1.3 + 2.3 + 1.9 + 1.0 = 9.4, II – 2.7 + 1.2 + 2.0 + 2.1 + 1.0 = 9.0, III – 1.7 + 0.7 + 0.8 + 0.7 + 0.5 = 4.4, IV – 2.3 + 0.6 + 1.0 + 1.2 + 0.6 = 5.7. Labium 0.28 long, 0.33 wide, dark brown and maxillae light brown, both beige apically (Fig. 10b). Sternum 0.8 long, 0.8 wide, dark brown (Fig. 10b). Abdomen 2.5 long, 2.3 wide, oblong; dorsum dark brown covered by guanine patches (Fig. 10a); sides dark grey with black streaks (Fig. 9b); venter dark brown (Fig. 10b). Pedipalp (Fig. 10c–e, 11a–d) length of segments: 0.4 + 0.2 + 0.3 + 1.2 = 2.1; paracymbium short, strong and curved apically; median apophysis elongated, reaching over the conductor, bearing and inconspicuous stump on the outer margin that does not form a proper projection, and a sclerotised curved tip; conductor lobe enlarged, located behind the stipes; terminal apophysis basally with a curved, flattened and pointed prong, and apically inflated, ending in a long and thin projection with an enlarged tip pointing laterally; conductor enlarged, oval and fleshy; embolus elongated, medially slender and tapering to an enlarged hook-like tip basally projected.
Total length females 6.5–8.9 (n = 5). Total length males 3.9–4.3 (n = 4). The size of the abdominal tubercles varies slightly among studied specimens. Also, the thickness of the base of the scape of females varied somewhat among the specimens (e.g. Fig. 9c v. 12a v. 12b). One female had the scape broken off (QM S124032).
The genitalia of males and females of C. beelzebub were compared to the genitalia of three similar looking species. The variation among all type material examined or assessed by published descriptions and illustrations (see above at the taxonomic list) falls within the variation of specimens here considered to belong to C. beelzebub, including the drawings of the female epigyne of Carepalxis tricuspidata Chrysanthus, 1961 (Chrysanthus 1961, fig. 50–53, not examined) and the photos we received of the female holotype epigyne of Carepalxis suberosa Thorell, 1881 (Fig. 12b), and the female epigyne and male pedipalp of Acroaspis tuberculifera (Thorell, 1881) (Fig. 12a, b). Therefore, taking into account these morphological similarities, we propose the following synonymies: Carepalxis beelzebub = C. suberosa syn. nov. = C. tuberculifera comb. nov. & syn. nov. = C. tricuspidata Chrysanthus, 1961 syn. nov.
According to the original labels, specimens of C. beelzebub were collected in September, October, December, January, February and March. Therefore, the species is considered as mainly spring and summer mature for most of Australia, whereas in Queensland and New Guinea, the species was collected during the wet season. No habitat data were given on any labels. One sample was collected in nests of mud wasps (genus Pison).
Carepalxis beelzebub has been found in five Australian states: Queensland, South Australia, Tasmania, Victoria and Western Australia, but based on these distribution records it is likely to occur more widely. It has also been found in West Papua (Indonesia) and Papua New Guinea (Fig. 13).
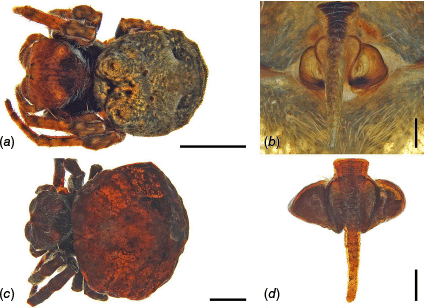
Carepalxis beelzebub (van Hasselt, 1873), junior synonyms. (a) Carepalxis tuberculifera (Thorell, 1881) (MSNG), female syntype, epigyne ventral view; (b) Carepalxis suberosa Thorell, 1881 (MSNG), female holotype, epigyne ventral view.
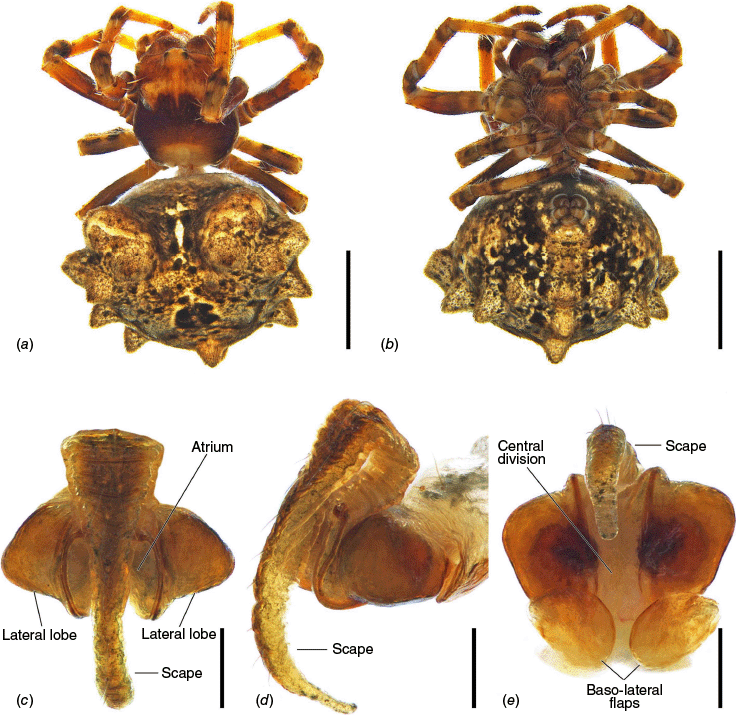
Carepalxis bilobata Keyserling, 1886, female (AM KS.76355). (a) Dorsal habitus; (b) ventral habitus; (c) epigyne, ventral view; (d) epigyne, lateral view; (e) epigyne, posterior view. Scale bars: (a, b) 2 mm; (c–e) 0.2 mm.
Carepalxis bilobata Keyserling, 1886
(Fig. 4e, f, 14a–e, 15a, b, 16a–d, 17.)
Carepalxis bilobata Keyserling, 1886, pp. 118, 119, pl. 9, fig. 4. – Rainbow (1911), p. 197.
2 females and 1 juvenile syntypes of Carepalxis bilobata from Peak Downs (22°56′S 148°05′, Queensland, AUSTRALIA), ZMH-A0001734, examined (photos).
AUSTRALIA: New South Wales: 1 female, Cocoparra National Park, The Woolshed Flat campsite, 34°04′S, 146°13′E, 15 March 2002, AM KS.76355; 1 female, Ku-ring-gai Chase National Park, 33°38′S 151°08′E, AM KS.59248. Queensland: 2 females, Eidsvold, 25°22′S, 151°07′E, SAM NN30825, NN30826. Victoria: 1 female, La Trobe University Flora Hill, 36°47′S, 144°18′E, 16 September 1996, CVIC 1227.
Females of C. bilobata share with C. beelzebub and C. megalostylus sp. nov. the projected lateral lobes of the epigyne and the scape tip reaching posteriorly past the epigyne area by at least half of scape’s length (Fig. 9c, d, 14c, d, 15b, 16b, d, 21c, d). Carepalxis bilobata and C. megalostylus sp. nov. can be differentiated from C. beelzebub by a more elongated scape that has an elevated base, reaching posteriorly past the epigyne area by more than its width (Fig. 14c, d, 15b, 16b, d, 21c, d). Carepalxis bilobata differs from C. megalostylus sp. nov. by the epigyne atrium not being almost completely covered by the scape and the much wider baso-lateral flaps (Fig. 14c, e, 15b, 16b, d). Males unknown.
Total length 6.5. Carapace 2.6 long, 2.1 wide; light brown with darker streaks on cephalic area and a large dark brown band centrally and on the borders (Fig. 14a). Eye diameter AME 0.11, ALE 0.08, PME 0.12, PLE 0.07; row of eyes: AME 0.46, PME 0.54, PLE 1.81. Chelicerae brown, four promarginal teeth (apical and third largest) and three retromarginal teeth of similar size. Legs yellowish-brown, mottled dark on joints and ventral portion of femora darker (Fig. 14a, b). Pedipalp length of segments: 0.7 + 0.3 + 0.5 + 0.7 = 2.2. Leg formula I > II > IV > III; length of segments: I – 2.1 + 1.0 + 1.7 + 1.4 + 0.8 = 7.0, II – 1.9 + 0.9 + 1.4 + 1.2 + 0.7 = 6.1, III – 1.2 + 0.6 + 0.6 + 0.7 + 0.6 = 3.7, IV – 1.8 + 0.7 + 0.9 + 1.1 + 0.6 = 5.1. Labium 0.37 long, 0.66 wide and maxillae base brown and apically beige (Fig. 14b). Sternum 0.9 long, 0.8 wide, light brown mottled dark (Fig. 14b). Abdomen 3.9 long, 5.1 wide; oblong, dorsum covered by tubercles, golden-brown with a dark pattern on folium and scattered black spots (Fig. 14a); sides golden-brown mottled in black (Fig. 14b); venter black (Fig. 14b). Epigyne approximately twice as wide as long with almost as wide as long projected sides, atrium large, subquadrate and concave, not covered by the scape (Fig. 14c); scape elongated, elevated at its base, much wider anteriorly, curved medially and tapering to a rounded pointed tip that reaches posteriorly past the epigyne area by approximately half of the scape’s length (Fig. 14c, d); central division very wide with parallel sides (Fig. 14e); baso-lateral flaps enlarged, oval and membranous (Fig. 14e); spermathecae spherical, apart by their diameter, and occupying most of the epigyne lateral lobes (Fig. 4e, f).
Total length females 6.5–8.9 (n = 3). Carapace and legs uniformly dark brown (Fig. 16c). Abdominal humeral humps vary in size, forming two lobes anteriorly (Fig. 15a) or being inconspicuous (Fig. 16c), whereas the abdominal tubercles can be very conspicuous and pointed as in the redescription of the species (Fig. 14a), or not noticeable (Fig. 15a, 16a, c). Epigyne scape is generally not curved medially, but almost straight (Fig. 15b, 16b, d).
Only two specimens had collection dates on their original labels, indicating they were collected in September and March. No habitat information was available on the original labels.
Carepalxis bilobata has been found in three Australian states: New South Wales, Queensland and Victoria (Fig. 17).
Carepalxis ferreirasousai sp. nov.
(Fig. 1, 2, 4i, j, 18a–e, 20, S1.)
ZooBank: urn:lsid:zoobank.org:act:4B18A14D-9B40-45E0-92AC-81D58E7FDE3B
Holotype. Female, Munyeroo Conservation Park, 17 km SSE Moonabbie (33°23′S, 137°21′E, South Australia, AUSTRALIA), 22–30 September 2002, SAM NN30840.
AUSTRALIA: Australian Capital Territory: 2 females, Canberra, 35°18′S, 149°08′E, QM S121045. New South Wales: 1 female, Caldwell, 35°37′S, 144°30′E, NMV K-13317; 1 female, Woolshed 3, ‘Brotherony’, via Condolobin, 33°06′S, 146°38′E, 02 March 2002, AM KS.91254. South Australia: 1 female, Hamley Bridge, 34°21′S, 138°40′E, February 1898, SAM NN30833; 1 female, Hope Valley, Adelaide, 34°51′S, 138°41′E, 20 January 1925, SAM NN30832. Victoria: 1 female, Gowar East, 36°33′S, 143°25′E, 15 February 1909, NMV K-13312. Western Australia: 2 females, Geraldton, 28°46′S, 114°37′E, AM KS.34298; 2 males, Sabina River, 33°39′S, 115°24′E, collected in nests of mud dauber wasps, 27 January 1984, WAM T37231–32; 2 females, 3 juveniles, same locality as previous, 27 January 1994, WAM T37226–30.
The specific epithet is a patronym in honour of the Brazilian arachnologist Leonardo Ferreira Sousa, who described Care-palxis quasimodo and sent us tissue of this and Ocrepeira planalto for our molecular analysis.
Females of C. ferreirasousai sp. nov. can be distinguished from all Carepalxis by the shape of the epigyne scape being heavily sclerotised, broadly triangular and tapering to a narrow, flat and slightly elevated tip; and the central division, which is narrow and ends in two strong and curved slits, dividing the terminal portion of the posterior plate (Fig. 18c–e). Males are unknown.
Total length 6.8. Carapace 2.7 long, 2.4 wide; orange–brown with sparse dark spots and a dark contour around the cephalic region (Fig. 18a). Eye diameter AME 0.13, ALE 0.08, PME 0.10, PLE 0.07; row of eyes: AME 0.56, PME 0.71, PLE 2.67. Chelicerae dark-brown, four promarginal teeth (apical and third largest) and three retromarginal teeth of similar size. Legs orange–brown, dark annulated, and with large dark brown patches dorsally and ventrally on femur (Fig. 18a, b). Pedipalp length of segments: 0.9 + 0.7 + 0.7 + 1.1 = 3.4. Leg formula I > II > IV > III; length of segments: I – 3.6 + 1.9 + 2.3 + 2.0 + 1.0 = 10.8, II – 3.0 + 1.8 + 2.0 + 1.9 + 0.9 = 9.6, III – 1.9 + 1.2 + 1.1 + 1.0 + 0.8 = 6.0, IV – 2.7 + 1.7 + 1.7 + 1.5 + 0.9 = 8.5. Labium 0.55 long, 0.75 wide, and maxillae base orange–brown and apically beige (Fig. 18b). Sternum 1.4 long, 1.6 wide, orange–brown with a wide dark contour (Fig. 18b). Abdomen 5.8 long, 4.9 wide; rounded, dorsum without noticeable humps or tubercles, golden-brown with two central dark spots and a dark stripe on folium (Fig. 18a); sides golden-brown with dark streaks (Fig. 18b); venter black with a large rectangular white patch (Fig. 18b). Epigyne wider than long with semi-circular sides, atrium slightly wider than long and little concave (Fig. 18c, e); scape medially wide and triangular, flat, with wide straight base, gradually tapering to a narrow blunt and slightly elevated tip that reaches posteriorly past the epigyne area by less than half of the scape’s length (Fig. 18c, d); central division narrow with parallel sides (Fig. 18e); central division narrow ending in two strong and curved transverse slits dividing the terminal end of the posterior plate into two prominent portions (Fig. 18e); baso-lateral flaps absent (Fig. 18e); spermathecae kidney shaped, very elongated, not touching by ~1/2 of its diameter, and occupying most of the epigyne lateral lobes (Fig. 4i, j).
Total length females 6.0–6.8 (n = 4). Very little morphological variation was detected in the specimens we examined.
According to the original labels, specimens of C. ferreirasousai were collected in January, February, March and September, possibly indicating spring to summer maturity for the species. No habitat information was provided on labels. Specimens in the samples WAM T37226–32 were collected in nests of mud wasps (genus Pison Spinola, 1808), an apparent important parasitoid of this species.
Carepalxis ferreirasousai sp. nov. specimens were collected in southern Australia, from the Australian Capital Territory, New South Wales, South Australia, Victoria to Western Australia (Fig. 20).
Carepalxis kolla sp. nov.
ZooBank: urn:lsid:zoobank.org:act:70C8BCE1-86B8-4F70-8D35-526B6CD99380
Holotype. Female, Gluepot Reserve, 8.5 km W–WNW Gluepot Homestead (33°44′S, 140°01′E, South Australia, AUSTRALIA), 26 November–6 December 2000, SAM NN18561.
The specific name, ‘kolla’, is a Greek noun (κόλλα) used in apposition that means ‘glue’ and refers to the species type locality, Gluepot Reserve.
Females of C. kolla sp. nov. are most similar to the those of C. montifera and C. tuberculata as they share an epigyne with conspicuous rounded lateral lobes, and a scape with base elevated and its tip surpassing the epigyne area by less than 1/3 of the scape’s length (Fig. 5d, e, 19c, d, 29c, d). However, C. kolla sp. nov. and C. montifera differ from C. tuberculata by the epigyne scape that almost completely covers the atrium (Fig. 5d, e, 19c, d), whereas C. kolla sp. nov. can be differentiated from C. montifera by the longer than wide epigyne atrium, and a scape with a more acute tip reaching over the epigyne area by 1/5 of the scape’s length (v. atrium as long as wide, and scape reaching over the epigyne area by 1/4 of scape’s length in C. montifera) (Fig. 19c, d). Males are unknown.
Total length 6.4. Carapace 2.4 long, 2.5 wide; orange–brown with sparse white setae (Fig. 19a). Eye diameter AME 0.14, ALE 0.08, PME 0.13, PLE 0.07; row of eyes: AME 0.56, PME 0.67, PLE 2.40. Chelicerae dark-brown, four promarginal teeth (third largest) and three retromarginal teeth (second largest). Legs orange–brown, with dark ventral patches (Fig. 19a, b). Pedipalp length of segments: 1.0 + 0.5 + 0.7 + 1.2 = 3.4. Leg formula I > II > IV > III; length of segments: I – 3.2 + 1.8 + 2.2 + 1.9 + 0.9 = 10.0, II – 3.0 + 1.6 + 2.0 + 1.6 + 0.8 = 9.0, III – 2.0 + 1.2 + 1.1 + 1.0 + 0.7 = 6.0, IV – 2.7 + 1.4 + 1.7 + 1.7 + 0.8 = 8.3. Labium 0.46 long, 0.76 wide and maxillae base orange–brown and apically beige (Fig. 19b). Sternum 1.2 long, 1.4 wide, orange–brown with wide light contour (Fig. 19b). Abdomen 4.1 long, 5.4 wide; rounded, without noticeable humps or tubercles (Fig. 19a); dorsum golden-brown with two central dark spots and sparse dark spots (Fig. 19a); sides olive-brown (Fig. 19b); venter black with two large subquadrate white patches (Fig. 19b). Epigyne with semi-circular sides, atrium longer than wide and slightly concave (Fig. 19c, e); scape elongated, elevated on its base and slightly wider above atrium, almost completely covering it and tapering to a triangular tip that overreaches the epigyne area by ~1/5 of its length (Fig. 19c, d); central division relatively narrow with parallel sides ending in two transverse slits (Fig. 19e); baso-lateral flaps absent (Fig. 19e); spermathecae were not cleared to better preserve the holotype.
The holotype of C. kolla sp. nov. was collected at the end of November–beginning of December. No habitat data were cited on the original label.
Carepalxis kolla sp. nov. is only known from its type locality in south-eastern South Australia (Fig. 20).
Carepalxis megalostylus sp. nov.
(Fig. 1, 2, 4g, h, 21a–e, 22a–e, 23, S1.)
ZooBank: urn:lsid:zoobank.org:act:650249BE-BA56-490A-BF02-656C04FADF69
Holotype. Female, Euglo State Forest (33°31′S, 147°12′E, New South Wales, AUSTRALIA), 11 December 1998, H Smith leg., beating vegetation, AM KS.54361.
AUSTRALIA: New South Wales: 1 female, Caldwell, 35°37′S, 144°30′E, NMV K-13320. Queensland: 1 female, Muncoonie Lakes, Simpson Desert, 25°12′S, 138°41′E, QM S121044. South Australia: 1 female, Chillunie Well, 09 December 1989, SAM NN30839; 1 female, Middleback Station, 32°57′S, 137°23′E, SAM NN30857; 1 female, Oodnadatta, 27°32′S, 135°26′E, SAM NN445; 1 female, 1 juvenile, Ooldea, 30°27′S, 131°50′E, SAM NN30848–30849; 1 female, Nelshaby, 33°07′S, 138°08′E, 02 November 2004, SAM NN30829.
The specific epithet, ‘megalostylus’, is a compound noun in apposition derived from the Greek megalo (μεγάλο) – large, and stylus (στύλος) – pen or scape, and refers to the large epigyne scape of females.
The epigynes of female C. megalostylus sp. nov., C. bilobata and C. beelzebub are similar with projected lateral lobes, and a scape exceeding the epigyne area by more than 50% of the scape’s length (Fig. 9c, d, 14c, d, 15b, 16b, d, 21c, d). Carepalxis megalostylus sp. nov. and C. bilobata females differ from those of C. beelzebub by having a much longer scape with elevated base, reaching posteriorly beyond the epigyne area by more than the atrium length (v. approximately atrium length in C. beelzebub) (Fig. 9c, 12a, b, 14c, d, 15b, 16b, d, 21c, d). Carepalxis megalostylus sp. nov. females differ from those of C. bilobata by the scape almost completely covering the epigyne atrium and by the smaller inwardly projected baso-lateral flaps (Fig. 21c, e, 22c, e). Males are unknown.
Total length 4.8. Carapace 2.3 long, 1.9 wide; orange–brown, centrally with a large dark brown patch, and covered by pale yellow setae (Fig. 21a). Eye diameter AME 0.11, ALE 0.07, PME 0.12, PLE 0.06; row of eyes: AME 0.42, PME 0.50, PLE 1.87. Chelicerae dark brown, three promarginal teeth (median largest) and three retromarginal teeth (basal largest). Legs orange–brown with dark patches on joints and distally on femur and tibia (Fig. 21a, b). Pedipalp length of segments: 0.6 + 0.3 + 0.5 + 1.0 = 2.4. Leg formula I > II > IV > III; length of segments: I – 2.4 + 1.0 + 1.6 + 1.4 + 0.7 = 7.1, II – 2.2 + 0.7 + 1.2 + 1.4 + 0.7 = 6.2, III – 1.2 + 0.7 + 0.8 + 0.7 + 0.5 = 3.9, IV – 1.7 + 0.8 + 1.1 + 1.0 + 0.6 = 5.2. Labium 0.28 long, 0.55 wide and maxillae base orange and apically beige (Fig. 21b). Sternum 1.0 long, 1.0 wide, orange–brown with three central lighter faint spots (Fig. 21b). Abdomen 2.4 long, 2.2 wide; rounded, with short humps and tubercles, dorsum coated in golden-brown with scattered black spots, and folium with two dark central patches, one oblong followed by a large rounded one (Fig. 21a); sides and venter golden-brown with scattered black spots (Fig. 21b). Epigyne wider than long, with conspicuous rounded and projected sides, atrium subquadrate and deeply concave (Fig. 21c, e); scape flattened, elongated, extremely elevated and wide on its base with wrinkled sides, and tapering to a large tip that reaches posteriorly beyond the epigyne area by ~1/2 of the scape’s length (Fig. 21c, d); central division very wide with parallel sides (Fig. 21e); baso-lateral flaps very small and fleshy (Fig. 21e); internal genitalia with spermathecae spherical, separated by more than half of their diameter and occupying most of the epigyne lateral lobes, with copulatory openings located apically near the base of the scape (Fig. 4g, h).
Total length females 4.8–6.9 (n = 5). The abdomen shape is variable, with some specimens having two conspicuously elongated and pointed humps (e.g. Fig. 22a). The epigyne scape is broken off in most of the specimens checked with the break point either closer to its base (Fig. 22b) or often at a point closer to the tip of the scape (Fig. 22c, d). The central division can also slightly vary in width (Fig. 22e).
The only specimens with dates available in the original labels showed them to be collected in November–December. No habitat data were available for any specimen.
Carepalxis megalostylus sp. nov. has been found in New South Wales, Queensland and South Australia (Fig. 23).
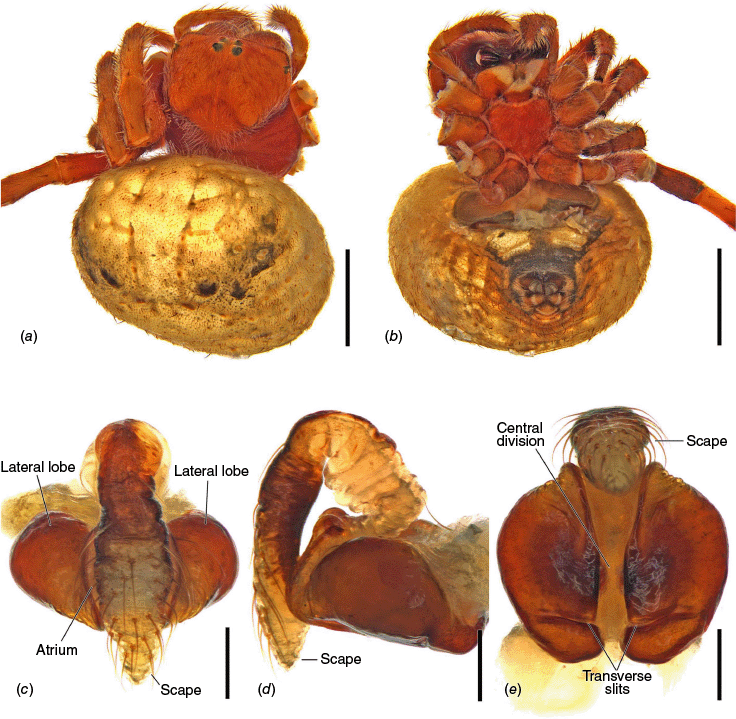
Carepalxis megalostylus sp. nov., female holotype (AM KS.54361). (a) Dorsal habitus; (b) ventral habitus; (c) epigyne, ventral view; (d) epigyne, lateral view; (e) epigyne, posterior view. Scale bars: (a, b) 2 mm; (c–e) 0.2 mm.
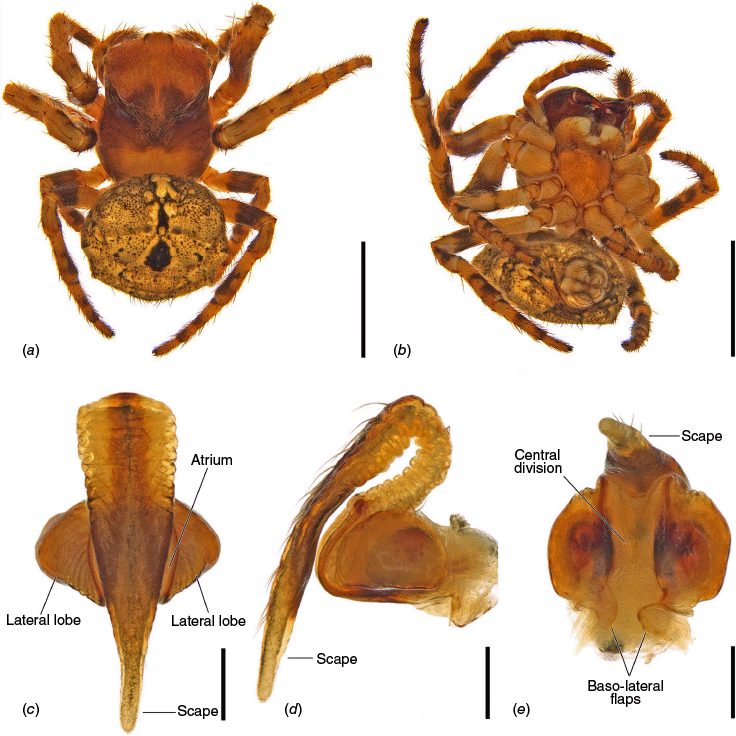
Carepalxis megalostylus sp. nov., female variations. (a) Lateral habitus (SAM NN30848); (b) broken off epigyne scape at base, ventral view (SAM NN30857); (c) broken off epigyne scape after atrium, ventral view (SAM NN30829); (d) broken off epigyne scape after atrium, lateral view (SAM NN30829); (e) broken off epigyne scape after the atrium next to its tip, posterior view (SAM NN30829). Scale bars: (a) 2 mm; (b–e) 0.2 mm. The black arrows indicate the scape breaking points.
Carepalxis tholos sp. nov.
(Fig. 1, 2, 3d–f, 4m, n, 24a–e, 25a–d, 26a–f, 27a, b, 28, S1.)
ZooBank: urn:lsid:zoobank.org:act:BD00198D-EFF7-4FB2-8674-C52AB0F3DF4F
Holotype. Male, W of Bookabie (31°49′S, 132°32′E, South Australia, AUSTRALIA), 25 August 1998, D Hirst leg., SAM NN30842.
AUSTRALIA: Australian Capital Territory: 1 male, Cook, 23 Grylls Crescent, 35°16′S, 149°04′E, 30 October 1983, WAM T75286. New South Wales: 1 male, Hornsby, Waitara Creek, 33°42′S, 151°05′E, 12 February 2004, AM KS.91104; 1 female, Ku-ring-gai Chase National Park, path behind headquarter, 33°30′S, 151°08′E, 20 September 1998, AM KS.54010; 1 female, Rocky Hall, ‘Wonga’, 36°55′S, 149°30′E, 11 November 2004, AM KS.90418; 1 female, Royal National Park, 34°08′S, 151°04′E, 05 February 1978, AM KS.1299. Queensland: 1 female, Brisbane, 27°28′S, 153°01′E, QM W874; 1 female, Monto, 24°52′S, 151°07′E, QM S121052. South Australia: 1 female, Blackwood, 35°01′S, 138°36′E, 17–19 November 1979, SAM NN24832; 1 male, Comet Bore, N of Bunns Bore, 35°45′S, 140°48′E, 03 December 1978, SAM NN30843; 1 male, Mt Lofty, 34°59′S, 138°43′E, 05 January 1974, SAM NN30845; 1 female, Oakbank, 34°58′S, 138°51′E, June 1910, SAM NN30856; 1 female, One Tree Hill, 34°43′S, 138°39′E, 19 January 1912, SAM NN30855. Tasmania: 1 female, Kelso, 41°06′S, 146°47′E, 01 February 1963, AM KS.29077. Victoria: 1 female, Glen Waverley, ‘Black Flat’, 37°52′S, 145°09′E, 28 November 1896, NMV K-13316; 1 female, Maryborough, 37°02′S, 143°44′E, 26 November 1964, NMV K-13313. Western Australia: 1 male, Mt Ragged Campsite, Cape Arid National Park, 33°27′S, 123°27′E, 01 January 2008, WAM T81454; 1 male, Wongan Hills, 30°51′S, 116°42′E, 01 February 1949, WAM T70168.
The specific name, ‘tholos’, is a Greek noun (θόλος) in apposition that means ‘dome’ and is used in reference to the dome shaped abdomen of some females.
Males of C. tholos sp. nov. are most similar to those of C. beelzebub, both differing from C. tuberculata by the tibia of leg II having shorter pairs of macrosetae (Fig. 3a–i). Carepalxis tholos sp. nov. males differ from those of C. beelzebub by the presence of a fully formed small and rounded projection on the outer margin of the median apophysis (v. presence of the base of an unformed projection in C. beelzebub); the terminal apophysis being slightly longer and basally projected with thinner pointed tip; and the embolus being medially stronger with bent tip that is apically projected (v. medially thin with enlarged tip basally projected in C. beelzebub) (Fig. 10c, d, 11a–d, 24c, d, 25a–d). Females of C. tholos sp. nov. differ from those of all other Carepalxis by the dome-shape massive abdomen (Fig. 26a (collapsed), 27a) and an epigyne with narrow sides and thin borders, with a much wider than long atrium; the scape not protruding at its base, flattened and uniformly tapering to a rounded tip; and the central division being wide with slanted and very sclerotised borders (Fig. 26c–f).
Total length 3.3. Carapace 1.8 long, 1.7 wide, narrower at eye region, with orange–brown background, and a large light brown area apically on the carapace borders (Fig. 24a). Eye diameter AME 0.11, ALE 0.06, PME 0.11, PLE 0.06; row of eyes: AME 0.43, PME 0.42, PLE 1.16. Chelicerae orange–brown; with one promarginal tooth and one retromarginal tooth. Legs orange–brown, mottled dark mostly dorsally and ventrally on femur, joints, and centrally on tibia, metatarsus and tarsus (Fig. 24a, b). Leg tibia I clearly bent, tibia II medially with two conspicuous thick macrosetae with approximately half of the tibia’s length, followed by three slightly thinner macrosetae from where tibia clearly reduce in thickness, and ending in a pair of thick transverse macrosetae located on the border with metatarsus (Fig. 2g–i). Leg formula I > II > IV > III; length of segments: I – 2.4 + 0.9 + 1.9 + 1.4 + 0.6 = 7.2, II – 2.1 + 1.0 + 1.6 + 1.6 + 0.7 = 7.0, III – 1.1 + 0.6 + 0.7 + 0.7 + 0.5 = 3.6, IV – 1.3 + 0.7 + 1.0 + 1.1 + 0.6 = 4.7. Labium 0.21 long, 0.44 wide, and maxillae orange–brown, both beige apically (Fig. 24b). Sternum 0.8 long, 0.8 wide, orange–brown with a dusky contour (Fig. 24b). Abdomen 2.6 long, 1.6 wide, oblong, basally projected and bearing two rounded and elongated humeral humps and four short protrusions (Fig. 24a, b); dorsum pale yellow with sparse black areas, except for a longitudinal area centrally located (Fig. 24a); sides and venter pale yellow mottled dark (Fig. 24b). Pedipalp (Fig. 24c–e, 25a–d) length of segments: 0.3 + 0.2 + 0.2 + 0.8 = 1.5; paracymbium strong and curved apically; median apophysis elongated, with short and rounded projection medially, and ending in a transverse and sclerotised hook-like pointed tip; conductor lobe enlarged; terminal apophysis very conspicuous, basally with protruding prong ending in a pointed tip, and apically inflated, ending in a wide and twisted projection with pointed tip curved basally; conductor elongated with wide subquadrate distal portion ending in two lobes, the apical one more sclerotised and straight, and the basal one rounded and curved; embolus sinuous, when unexpanded only reaching the base of conductor, centrally enlarged and ending in a bent tip apically projected.
Total length 7.7. Carapace 2.9 long, 2.4 wide; wider on eye region, colour pattern as in male (Fig. 26a). Eye diameter AME 0.12, ALE 0.11, PME 0.14, PLE 0.13; row of eyes: AME 0.49, PME 0.66, PLE 1.77. Chelicerae shape and colour as in male, but with three promarginal teeth (basal largest) and three retromarginal teeth (basal largest). Leg colours as in male (Fig. 26a, b). Pedipalp length of segments: 0.9 + 0.7 + 0.8 + 1.5 = 3.9. Leg formula I > II > IV > III; length of segments: I – 5.3 + 2.2 + 4.4 + 2.6 + 1.1 = 15.6, II – 5.1 + 2.1 + 4.2 + 2.3 + 1.1 = 14.8, III – 3.2 + 1.7 + 1.8 + 1.5 + 1.0 = 9.2, IV – 4.5 + 2.2 + 2.9 + 2.2 + 1.0 = 12.8. Labium 0.45 long, 0.72 wide, and maxillae dark brown and apically beige (Fig. 26b). Sternum 1.6 long, 1.8 wide, dark brown (Fig. 26b). Abdomen 5.3 long, 5.8 wide; rounded, wrinkled, with short tubercles (Fig. 29a); dorsum olive grey mottled golden-brown (Fig. 26a); sides and venter olive grey (Fig. 26b). Epigyne wider than long with thin borders, atrium wide, slightly concave on centre (Fig. 26d, e); scape elongated, flattened, not protruding at base, much wider anteriorly and tapering into a rounded tip, reaching posteriorly beyond the epigyne area by slightly less than half of scape’s length (Fig. 26c–e); central division very wide with strongly sclerotised sides (Fig. 26e); baso-lateral flaps elongated and fleshy (Fig. 26e); spermathecae oval–round, very wide and occupying most of posterior plate (Fig. 4m, n).
Total length males 3.3–4.7 (n = 5). Total length females 6.9–9.9 (n = 5). The abdomen of females is extremely variable varying from short and wrinkled with short tubercles (Fig. 26a) to very elongated and dome shaped without tubercles (Fig. 27a).
According to the original specimen labels, C. tholos sp. nov. were collected between late August and February, possibly indicating a late winter to summer maturity for the species. No habitat information was available on labels.
Carepalxis tholos sp. nov. specimens were largely collected in southern half of the country, from the Australian Capital Territory, New South Wales, Queensland, South Australia, Tasmania, Victoria and into Western Australia (Fig. 28).
Carepalxis tholos sp. nov., male holotype (SAM NN30842). (a) Dorsal habitus; (b) ventral habitus; (c) left pedipalp, ventral view; (d) left pedipalp, retrolateral view; (e) left pedipalp, dorsal view. Scale bars: (a, b) 2 mm; (c–e) 0.2 mm. The black arrow indicates the median apophysis’ projection.
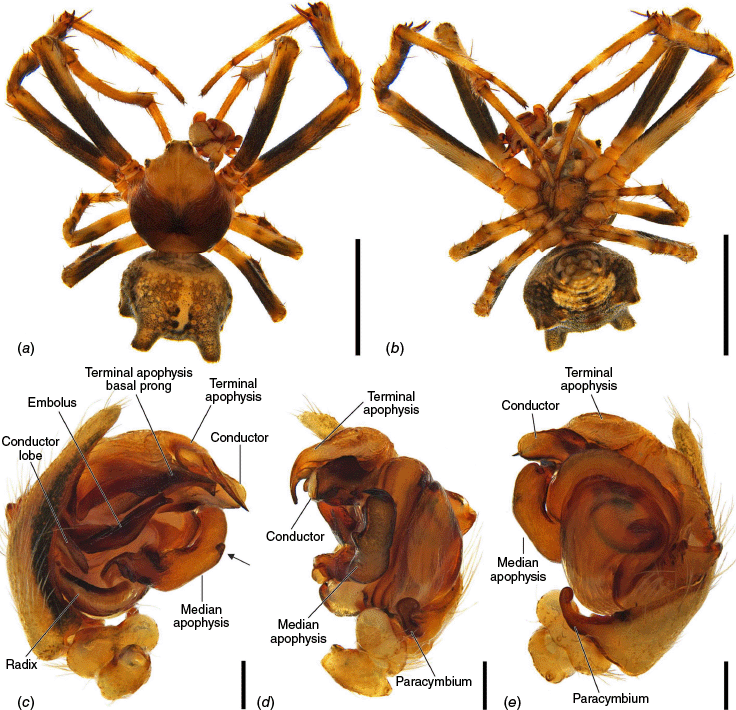
Carepalxis tholos sp. nov., male expanded left pedipalp (AM KS.91104). (a) Ventral view; (b) retrolateral view; (c) dorsal view; (d) apical view. Scale bars: 0.2 mm.
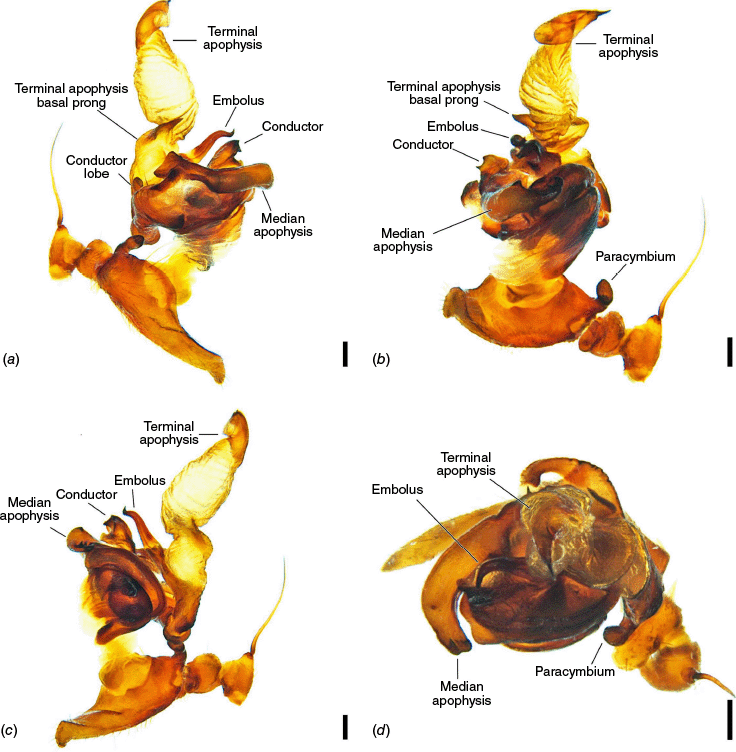
Carepalxis tholos sp. nov., female (SAM NN24832). (a) Dorsal habitus; (b) ventral habitus; (c) schematic drawing of epigyne, ventral view, highlighting atrium and scape; (d) epigyne, ventral view; (e) epigyne, lateral view; (f) epigyne, posterior view. Scale bars: (a, b) 2 mm; (c–f) 0.2 mm.
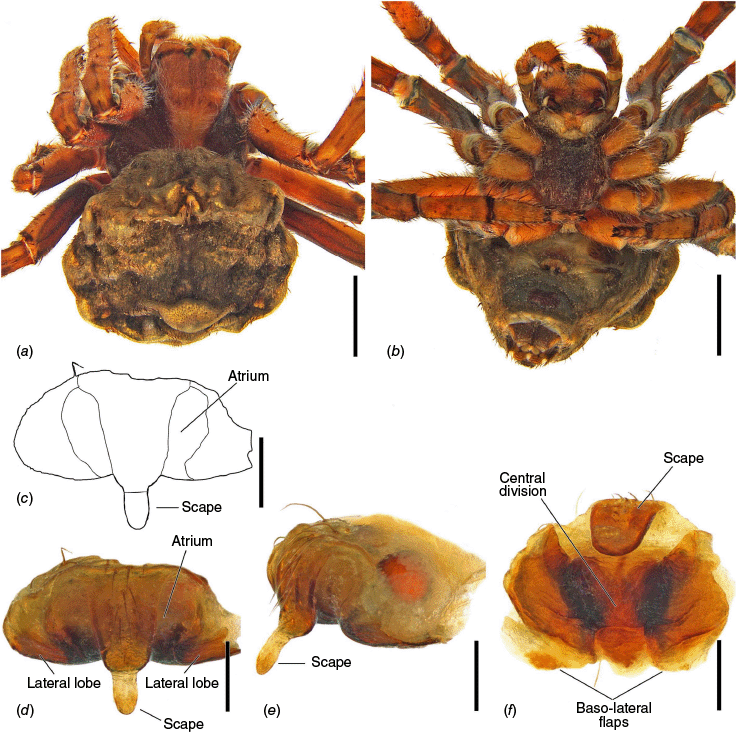
Carepalxis tuberculata Keyserling, 1886
(Fig. 1, 2, 3g–i, 4k, l, 29a–e, 30a–e, 31a–d, 32a, b, d, e, 33, S1.)
Carepalxis tuberculata Keyserling, 1886, pp. 119–121, pl. 9, fig. 5. – Simon (1896), p. 347; Rainbow (1911), p. 197; Davies (1988), p. 300, fig. 22.
Carepalxis furcula Keyserling, 1886. pp. 121, 122, pl. 9, fig. 6. – Rainbow (1911), p. 197 (synonymy by Simon 1896, p. 347).
Epeira furcifera Keyserling, 1886, pp. 144, 145, pl. 12, fig. 1. New synonymy.
Araneus furciferus (Keyserling). – Rainbow (1911), p. 186.
Verrucosa furcifera (Keyserling). – Archer (1951), p. 20 (transferred from Araneus Clerck, 1757); Lise et al. (2015), pp. 102, 104 (mentioned that the species would be transferred to Carepalxis by V. Framenau).
Carepalxis furcifera (Keyserling). – Framenau (2019), pp. 6, 8 (transferred from Verrucosa).
1 female lectotype (herein designated) of Carepalxis tuberculata, Peak Downs (22°56′S, 148°05′E, Queensland, AUSTRALIA), ZMH-A0001776, examined (photos). 8 females and 13 juveniles paralectotypes (herein designated) of Carepalxis tuberculata, Peak Downs (22°56′S, 148°05′E, Queensland, AUSTRALIA), ZMH-A00016659. 3 juveniles paralectotypes (herein designated) of Carepalxis tuberculata, Peak Downs (22°56′S, 148°05′E, Queensland, AUSTRALIA), ZMH-A00016651. 2 females misidentified syntypes of Carepalxis tuberculata, Rockhampton (23°22′S,150°30′E, Queensland, AUSTRALIA), ZMH-A00016656, examined (photos) (both are C. montifera). 2 females paralectotypes (herein designated) of Carepalxis tuberculata, Sydney (33°53′S, 151°13′E, New South Wales, AUSTRALIA), ZMH-A00016672, examined (photos of one female). 3 juveniles paralectotypes (herein designated) of Carepalxis tuberculata, Sydney (33°53′S, 151°13′E, New South Wales, AUSTRALIA), ZMH-A00016670. Holotype juvenile of Carepalxis furcula, Peak Downs (22°56′S, 148°05′E, Queensland, AUSTRALIA), ZMH-A0001730, examined (photos).
Holotype male of Carepalxis furcifera, Rockhampton (23°22′S,150°30′E, Queensland, AUSTRALIA) (ZMH-A0001768), examined (photos).
AUSTRALIA: Australian Capital Territory: 1 female, Canberra, 35°18′S, 149°08′E, QM S121053; 1 male, same data as previous, QM S121058. New South Wales: 1 female, 4 km W of West Wyalong, 33°55′S, 147°12′E, 26 January 1969, AM KS.50144; 1 female, Gunnedah, 30°59′S, 151°17′E, 15 September 1908, AM KS.33032; 1 female, Tambua Station, 31°26′S, 145°15′E, 02 October 1968, AM KS.53881; 6 females, Wheogo, near Dunedoo, 31°59′S, 149°24′E, AM KS.15806; 3 females, same locality as previous, AM KS.33037. Northern Territory: 1 male, Boggy Hole, Finke Gorge National Park, 24°09′S, 132°52′E, 14 September 1995, SAM NN30820; 1 female, same data as previous, SAM NN30821. Queensland: 1 female, Bang Bang Jump Up, 18°32′S, 140°40′E, QM S69385; 1 female, Camira, 27°38′S, 152°55′E, 04 December 1987, QM S2464; 1 female, Cooktown, 15°28′S, 145°15′E, QM S121057; 1 male, Crows Nest, 27°16′S, 152°03′E, QM S121041; 2 females, Curra, via Gympie, 26°04′S, 152°35′E, 04 September 1993, QM S25625; 1 female, Dalby, 27°11′S, 151°16′E, 27 February 1937, AM KS.117603; 3 females, Eidsvold, 25°22′S, 151°07′E, SAM NN30822–NN30824; 2 females, same data, SAM NN30827–NN30828; 1 female, El Arish, 17°49′S, 146°00′E, AM KS.51607; 1 male, Gatton, 27°34′S, 152°17′E, AM KS32636; 1 male, 5 females, 1 juvenile, Inala, Brisbane, 27°36′S, 152°58′E, 16 February 1992, QM S121051; 2 females, Happy Vale, Crownthorpe, 26°07′S, 151°56′E, QM S121059; 1 female, Miriam Vale, 24°19′S, 151°33′E, QM S121055; 1 female, Oakey, 27°26′S, 151°43′E, QM S121056; 1 female, Pentland, 20°32′S, 145°24′E, 09 June 1916, AM KS.33030; 1 female, 1 juvenile, same data as previous, AM KS.33033; 1 female, Pheasant Creek, Rockhampton area, 23°50′S, 150°07′E, 23 February 1994, QM S13958; 1 male, 30 females, 2 juveniles, same locality as previous, 04 January 1999, QM S124031; 1 female, Rossendale, via Gootchie, 25°51′S, 152°33′E, 20 February 1934, QM S42567; 1 female, same locality as previous, QM S121054; 1 male, Woodgate National Park area, 25°08′S, 152°58′E, QM S121050. South Australia: 1 female, 33 km S Thurlga Homestead, vicinity of salt lake, 32°44′S, 135°50′E, 28 February 1993, SAM NN30835; 1 female, Coongie Lakes, 27°11′S, 140°11′E, 29 October 1995, SAM NN30836; 1 male, Coopers Creek, 28°23′S, 137°41′E, SAM NN30814; 1 female, Dekina Creek, Orroroo, 32°44′S, 138°36′E, November 1915, SAM NN30830; 1 female, same data as previous, SAM NN30831; 1 male, Eden Valley, 34°39′S, 139°06′E, SAM NN30816; 1 male, Karte Conservation Park, 23 km NW Pinnaroo, 35°07′S, 140°43′E, 26 March 2000, SAM NN30846; 1 male, Mt Freeling Station, 12.1 km WNW Mt Fitton, 29°54′S, 139°26′E, 16–27 November 1998, SAM NN30841; 1 female, 1 juvenile, Mt Lindsay, WATcamp (Wartaru), 27°01′S, 129°52′E, 18 October 1996, SAM NN11572; 1 male, W of Whyalla, near lake, 33°07′S, 137°03′E, SAM NN30844. Victoria: 1 male, Barr Ck, Cohuna, 35°48′S 144°10′E, 14 December 1998, CVIC 1148; 1 female, Lake Hattah, 34°45′S, 142°20′E, NMV K-13318. Western Australia: 1 female, no exact locality, 23 January 1966, WAM T37189; 1 female, Byford, 32°13′S, 116°E, October 1987, WAM T37185; 1 female, Darlington, 31°55′S, 116°04′E, 08 April 1974, WAM T37200; 1 female, Forest River Mission, 15°13′S, 127°54′E, 26 April 1954, NMV K-13314; 1 female, Irrunytju Rockhole, 26°07′S, 128°58′E, 19– 21 January 1990, WAM T37192; 1 male, same data as previous, WAM T75745; 1 female, Kellerberrin, Moore Street, 31°37′S, 117°42′E, WAM T75881; 1 female, Kennedy Range ~28 km NE of Binthalya Stn homestead, 24°30′04.3″S, 115°01′03.4″E, 30 May 1995, WAM T159433; 1 female, Kununurra, 15°34′S, 128°29′E, 24 February 1989, WAM T37193; 1 male, North Walpole, S of Frankland River, 34°38′S, 116°37′E, 02 January 1986, WAM T70174; 1 female, Sabina River, 33°39′S, 115°24′E, 27 January 1994, WAM T37225; 1 female, same locality as previous, 28 January 1987, WAM T37233; 1 male, same locality as previous, 27 January 1984, WAM T77249; 3 males, same data as previous, WAM T77268; 1 male, same data as previous, WAM T98/1034-47; 1 male, same locality as previous, 12 January 1984, WAM T77287.
Females of C. tuberculata are most similar to C. montifera and C. kolla sp. nov. as they share rounded lateral epigyne lobes, and an elevated scape that reaches posteriorly past the epigyne area by less than 1/3 of the length of scape (Fig. 5d, e, 19c, d, 29c, d). However, although the scape of C. montifera and C. kolla sp. nov. almost cover the atrium completely, in C. tuberculata the scape only covers ~50% of the atrium (Fig. 29c, d). Males of C. tuberculata differ from other Carepalxis males by the longer pair of macrosetae on tibia II reaching beyond the metatarsus (Fig. 3g–i); and a pedipalp with a median apophysis longer than in other Carepalxis and with spatulate–triangular distal tip (Fig. 30c–e, 31a–d).
Total length 7.3. Carapace 2.2 long, 2.2 wide; reddish-brown and covered by pale yellow setae (Fig. 29a). Eye diameter AME 0.11, ALE 0.08, PME 0.11, PLE 0.06; row of eyes: AME 0.48, PME 0.59, PLE 2.15. Chelicerae reddish-brown, four promarginal teeth (basal largest) and four retromarginal teeth (second and third largest, fourth much smaller). Legs reddish-brown and mottled dark, especially ventrally on tibia, metatarsus and tarsus (Fig. 29a, b). Pedipalp length of: 0.8 + 0.3 + 0.7 + 0.9 = 2.7. Leg formula I > II > IV > III; length of segments: I – 3.2 + 1.3 + 2.1 + 1.8 + 0.9 = 9.3, II – 2.7 + 1.3 + 1.7 + 1.5 + 0.8 = 8.0, III – 1.6 + 1.1 + 1.0 + 0.9 + 0.7 = 5.3, IV – 2.4 + 1.3 + 1.6 + 1.4 + 0.7 = 7.4. Labium 0.42 long, 0.67 wide, and maxillae base reddish and apically beige (Fig. 29b). Sternum 1.1 long, 1.3 wide, reddish-brown with dusky centre (Fig. 29b). Abdomen 6.3 long, 6.3 wide; rounded, dorsum without noticeable humps or tubercles, with golden-brown background with some dark streaks (Fig. 29a); sides like dorsum (Fig. 29b); venter black with two large white spots (Fig. 29b). Epigyne approximately twice as wide as long with lateral lobes semicircular, atrium slightly oblong, almost as wide as long, with curved sides and almost flattened (Fig. 29c, e); scape elongated, base wider at the protruding portion, and with almost the same width from base to the rounded tip, which reaches posteriorly past the epigyne area by ~1/4 of the scape’s length (Fig. 29c, d); central division narrow with parallel margins (Fig. 29e); posterior plate ending in two shallow transverse slits (Fig. 29e); baso-lateral flaps absent (Fig. 29e); spermathecae relatively small and not occupying the epigyne lateral lobes (Fig. 4k, l).
Total length 4.8. Carapace 2.7 long, 2.3 wide, colour like female (Fig. 30a). Eye diameter AME 0.13, ALE 0.09, PME 0.11, PLE 0.09; row of eyes: AME 0.48, PME 0.46, PLE 1.36. Chelicerae colour as in female; with four promarginal teeth (third largest) and three retromarginal teeth of similar size. Legs colour pattern as in female with stronger dark patches on femur (Fig. 30a, b). Leg tibia II very thick with two adjoined, strong and elongated macrosetae on its distal third, with approximately half of the tibia length and overreaching the metatarsus; tibia somewhat thinner distally after the macrosetae base towards metatarsus and ending in two very short and thick transversal macrosetae (Fig. 2c–e). Leg formula I > II > IV > III; length of segments: I – 3.2 + 1.4 + 2.4 + 1.7 + 0.7 = 9.4, II – 3.1 + 1.3 + 2.3 + 1.4 + 0.7 = 8.8, III – 1.4 + 0.8 + 0.9 + 0.7 + 0.6 = 4.4, IV – 2.3 + 1.1 + 1.6 + 1.3 + 0.6 = 6.9. Labium 0.21 long, 0.42 wide, and maxillae orange–brown, both slightly lighter apically (Fig. 30b). Sternum 1.0 long, 0.8 wide, orange–brown with yellowish streaks (Fig. 30b). Abdomen 3.4 long, 2.3 wide, oblong with small humeral humps and tubercles (Fig. 30a); dorsum golden-brown with a black streak on folium (Fig. 30a); sides light brown (Fig. 30b); venter black with two large and two smaller white patches laterally (Fig. 30b). Pedipalp (Fig. 30c–e, 31a–d) length of segments: 0.4 + 0.4 + 0.2 + 0.9 = 1.9; paracymbium elongated, and slanted, apically enlarged; median apophysis elongated, curved and with a projection fused to its tip forming a spatulate–triangular apical portion; conductor lobe enlarged, canoe shaped and provided with scale-like structures; terminal apophysis basally with a strong and thick prong ending in a triangular blunt tip almost as long as the terminal apophysis apical portion; terminal apophysis apical portion inflated, and tapering to a long, thin and sclerotised projection curved downwards; conductor massive, very elongated in retrolateral view, with a large and sclerotised base followed by a median constriction and a rounded and fleshy tip; embolus elongated, with a wide base and tapering to a forked tip.
Total length females 6.1–9.2 (n = 5). Total length males 3.4–5.3 (n = 5). The colour of females can vary, with some of them having a dark-brown carapace and legs (e.g. SAM NN30827, SAM NN30828), whereas males can be slightly darker than described here. The female genitalia are variable among specimens studied, as in the case for the type series, where the epigyne varies from a scape of approximately the same width from base to tip (Fig. 29c, 32a) to a slightly wider and more elongated scape (Fig. 32b). Only few specimens had the scape broken off.
The species Epeira furcifera Keyserling, 1886 was described from Rockhampton, the same type locality of C. montifera and one of the type localities of C. tuberculata. It was recently transferred to Carepalxis by Framenau (2019), and even though the pedipalps of the male holotype of C. furcifera (lodged at the ZMH) are lost, we have identified males corresponding to the morphology of the species based on the original illustrations of the pedipalp made by Keyserling (1886, fig. 1a), and photos of the holotype’s macrosetae on tibia II. The original illustrations, but also the photos of C. furcifera we received from the ZMH, match the male assigned to C. tuberculata described by Simon (1896) and lodged at MNHN 17897 (Fig. 32d, e) and the males placed together with females we identified from scientific collections. Furthermore, our molecular analyses showed that the male previously described as C. furcifera is not conspecific with any of our sampled female specimens (see C. tuberculata in Fig. 1, 2 and S1). Therefore, we propose that C. furcifera is a junior synonym of C. tuberculata syn. nov.
Moreover, we herein also propose a lectotype for C. tuberculata in accordance with Article 74.1 of the International Commission on Zoological Nomenclature (1999). As two of the original syntypes of C. tuberculata actually belong to C. montifera (ZMH-A00016656), the selection of a lectotype as the name-bearing type of the species is deemed important to fix the species-group name of that species. We therefore chose the female specimen of registration number ZMH-A0001776 as the lectotype of C. tuberculata because, considering the array of intraspecific variations within the species, the morphology of this specific specimen matches well with the majority of the specimens we identified.
According to the original labels, most specimens of C. tuberculata were collected between September and April, with only two specimens collected in June. Therefore, the species is considered as mainly spring and summer mature. Specimens from Queensland were collected during the wet season. No habitat preference is given on labels. One specimen was collected from nests of mud wasps (genus Pison).
The distribution of C. tuberculata includes all Australian states and territories, except Tasmania (Fig. 33).
Carepalxis tuberculata Keyserling, 1886, male (SAM NN30820). (a) Dorsal habitus; (b) ventral habitus; (c) left pedipalp, ventral view; (d) left pedipalp, retrolateral view; (e) left pedipalp, dorsal view. Scale bars: (a, b) 2 mm; (c–e) 0.2 mm. The black arrow indicates the median apophysis’ apical curved and spatulate tip.
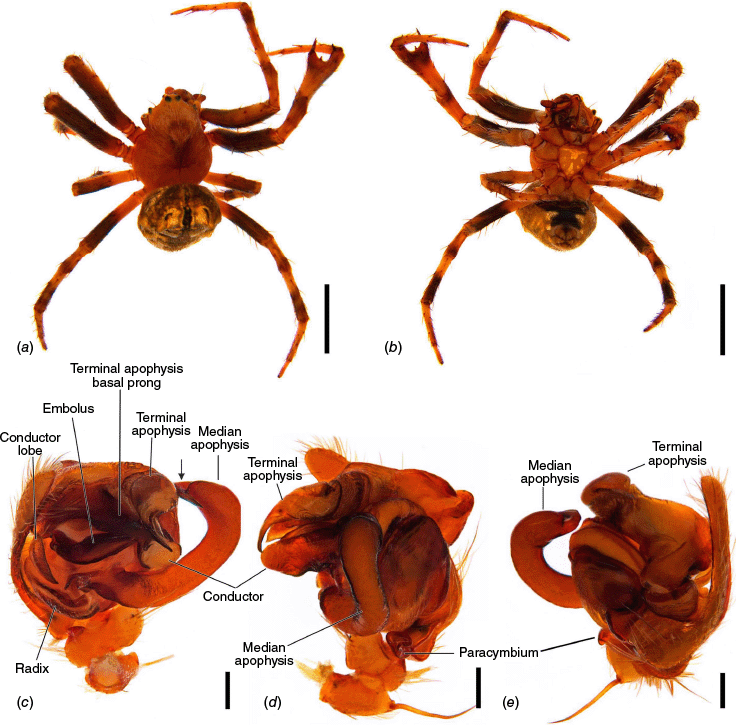
Carepalxis tuberculata Keyserling, 1886, male expanded left pedipalp (SAM NN30841). (a) Ventral view; (b) retrolateral view; (c) dorsal view; (d) apical view. Scale bars: 0.2 mm.
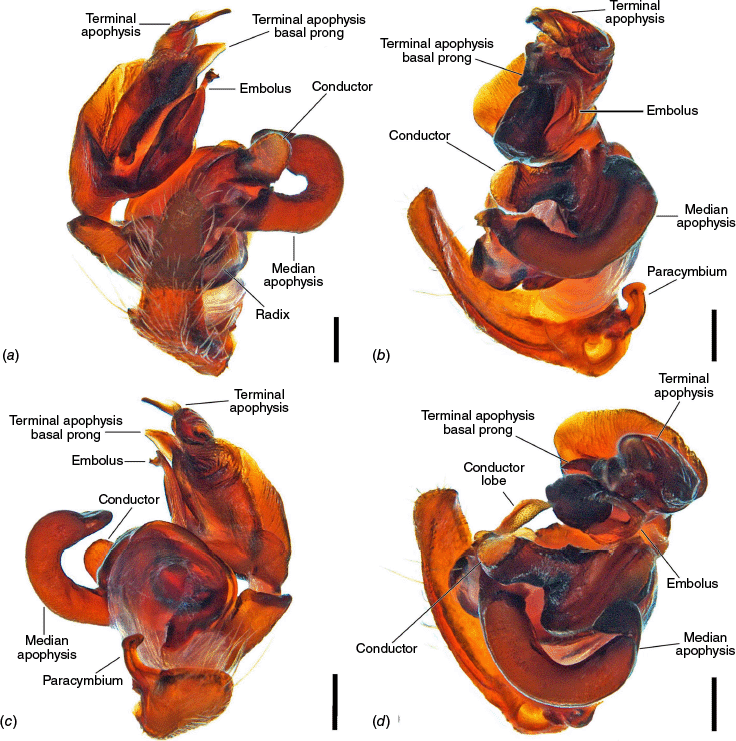
Some of Carepalxis tuberculata Keyserling, 1886 original syntypes. (a–c) Female variations, epigyne, ventral view. (a) Paralectotype specimen from Peak Downs (ZMH-A0001776); (b) paralectotype specimen from Sydney (ZMH-A00016672); (c) misidentified C. montifera specimen from Rockhampton (ZMH-A00016656). (d, e) Male described by Simon (1896) (MNHN 17897). (d) Habitus, dorsal view; (e) left pedipalp, ventral view. Scale bars: (a–c) 0.1 mm; (d) 1 mm; (e) 0.25 mm.
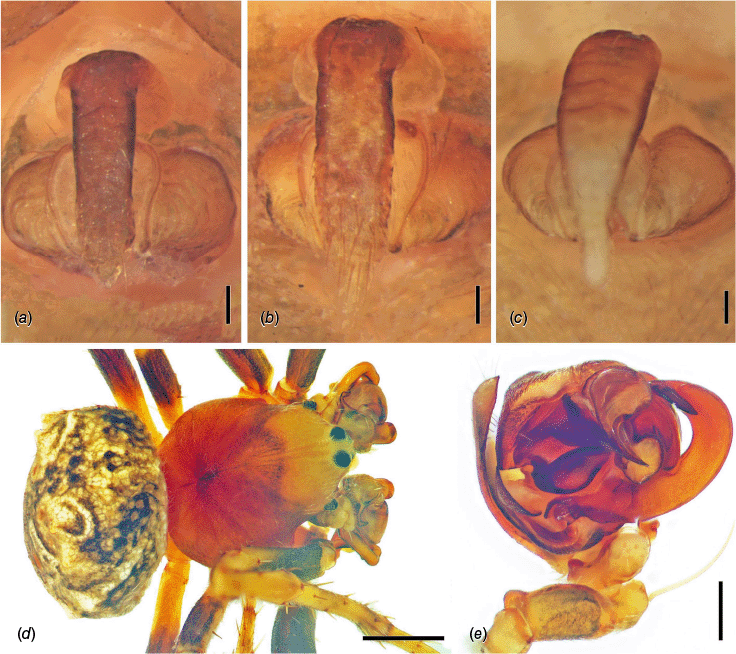
Genus Ocrepeira Marx, 1883
Type species: Epeira ectypa Walckenaer, 1841.
When Levi (1993) revised Ocrepeira, he largely diagnosed the genus against New World araneids, such as Pozonia Schenkel, 1953, Acacesia Simon, 1895, Wixia O. Pickard-Cambridge, 1882, Parawixia F.O. Pickard-Cambridge, 1904 and Wagneriana F.O. Pickard-Cambridge, 1904 based on the carapace shape (wide in eye region, low clypeus height), with two anterior humps, and attachment point of the pedicel (anterior half of the abdomen). The paramedian apophysis (=basal conductor lobe) of the male pedipalp in Ocrepeira species is L shaped, in contrast to Eriophora Simon, 1864 and Parawixia (disc shaped). Females were differentiated from Neoscona Simon, 1864 by, for example, a more sclerotised carapace and a wider cephalic region. However, Levi (1993) did not provide explicit synapomorphies for the genus. Hopfe et al. (2005) added a new Colombian species to Ocrepeira and confirmed the new species had the diagnostic characters suggested by Levi (1993). Their generic placement was also supported by COI molecular data. Ferreira-Sousa and Motta (2022) included two new species from Brazil in Carepalxis, like Levi (1992), largely based on the two humps on the female carapace. However, they for the first time questioned the diagnoses of Ocrepeira and in particular that the male of one of their new Carepalxis species shared a strong similarity with the pedipalps of Ocrepeira. Levi (1992) had previously noted characters in Neotropical Carepalxis that were similar to those in Ocrepeira, such as abdomen colouration and shape of the epigyne.
It is beyond the scope of this study to provide an updated, detailed diagnosis for Ocrepeira, but it is evident that the Neotropical representatives of Carepalxis readily confirm to Levi’s (1993) diagnosis of Ocrepeira, except for the female carapace humps. As the Neotropical C. quasimodo is not related to the Australian species of Carepalxis (Fig. 1, 2, S1), we transfer C. quasimodo to the genus Ocrepeira. No other Neotropical Carepalxis was included in our phylogenetic analyses but based on the morphology of males and females of Neotropical Carepalxis we suggest that all these species be transferred to Ocrepeira.
Ocrepeira camelus (Simon, 1895) comb. nov.
Carepalxis camelus Simon, 1895, p. 157.
Carepalxis vianai Gerschman & Schiapelli, 1948, pp. 16, 17, fig. 22–25.
Carepalxis camelus Simon. – Levi (1992), pp. 255, 258, fig. 1–5 (syn. senior of Carepalxis vianai Gerschman & Schiapelli, 1948).
Ocrepeira camelus comb. nov. is only known from two females from Paraguay and Argentina. We propose its transfer to Ocrepeira as argued above pending an examination of the unknown male. Levi (1992) detailed among the Neotropical Carepalxis this species was the most similar to the type species of Carepalxis based on female genitalia.
Ocrepeira gibbosa (O. Pickard-Cambridge, 1889) comb. nov.
Carepalxis gibbosa O. Pickard-Cambridge, 1889, pp. 48, 49, pl. 4, fig. 10.
Carepalxis gibbosa O. Pickard-Cambridge. – Keyserling (1892), pp. 54–55, pl. 2, fig. 43.
Carepalxis salobrensis Simon, 1895, p. 157.
Carepalxis rotunda O. Pickard-Cambridge, 1896, p. 224, pl. 27, fig. 7.
Carepalxis eremita Archer, 1965, p. 131, fig. 2, 3.
Carepalxis salobrensis Simon. – Levi (1992), pp. 259, 260, fig. 11–17 (syn. senior of C. eremita Archer, 1965 and C. rotunda O. Pickard-Cambridge, 1896); (syn. junior of C. gibbosa O. Pickard-Cambridge, 1889; but Levi 1992 chose the younger name as valid).
Ocrepeira gibbosa comb. nov. is the most widespread species here transferred from Carepalxis and has been reported from Mexico to northern Brazil (Levi 1992 sub C. salobrensis Simon, 1895). We propose its transfer to Ocrepeira as argued above pending an examination of the unknown male.
Ocrepeira perpera (Petrunkevitch, 1911) comb. nov.
Epeira perplexa Banks 1898, pp. 251, 252, pl. 15, fig. 1 (preoccupied by Walckenaer 1841).
Araneus perperus Petrunkevitch, 1911, p. 309 (replacement name).
Carepalxis perpera Petrunkevitch. – Levi (1991), p. 178 (transferred from Araneus).
Carepalxis perpera Petrunkevitch. – Levi (1992), pp. 258, 259, fig. 6–10.
Ocrepeira quasimodo (Ferreira-Sousa & Motta, 2022) comb. nov.
Carepalxis quasimodo Ferreira-Sousa & Motta, 2022, pp. 391–393, fig. 1–15.
Ocrepeira quasimodo comb. nov. is known from Brazil, the female holotype from Distrito Federal and three females and one male paratypes from Bahia. We propose its transfer to Ocrepeira as it was nested within a clade with other species of Ocrepeira, including the genus type species, in our molecular phylogenies (Fig. 1, 2, S1).
Ocrepeira topazio (Ferreira-Sousa & Motta, 2022) comb. nov.
Carepalxis topazio Ferreira-Sousa & Motta, 2022, pp. 394, 395, fig. 16–21.
Discussion
Our phylogenetic study on Carepalxis was designed to test the monophyly of the genus and its relationships with other backobourkiine spiders as proposed by Scharff et al. (2020). By including six Australasian Carepalxis species, we were also able to explore the evolution of some morphological characters within the genus, particularly in relation to females, as males are only known from three of the eight species.
A key outcome of our study is that Australasian and Neotropical species of Carepalxis do not form a clade (Fig. 1, 2, S1). Carepalxis quasimodo comb. nov. is nested within the two Neotropical Ocrepeira species included in our analysis, one of them the type species of that genus. We transfer all Neotropical Carepalxis species to Ocrepeira based on this phylogenetic placement but also show that this conforms to these species’ somatic and genitalic morphology. This is consistent with the distribution patterns observed in araneid genera from Australia that were previously placed in genera of the New World. For example, the Australian Hortophora biapicata (L. Koch, 1871), H. transmarina (Keyserling, 1865) and Socca pustulosa (Walckenaer, 1841) were previously included in the Neotropical genus Eriophora (e.g. Davies 1980; Court and Forster 1988), but as indicated in Scharff et al. (2020) and confirmed by recent taxonomic studies, Eriophora does not occur in Australia (Framenau et al. 2021a, 2022). Such clear biogeographic separation is also evident in recent molecular studies of Araneidae, with the backobourkiines being largely Australasian and the ‘micrathenines’ and ‘eriophorines’ restricted to North and South America (Scharff et al. 2020). This also argues for caution when interpreting araneid relationships based on limited morphological character sets alone (such as the carapace humps in Carepalxis). Based on the then known distribution, Main (1999) argued that Carepalxis was a Gondwanan taxon and within this context suggested that the occurrence of Carepalxis in Australia was anomalous.
Scharff et al. (2020) found a monophyletic backobourkiines clade with Carepalxis as a sister taxon to S. nitidula and these two as sister taxa to Hortophora (as Eriophora transmarina and Eriophora sp.). We included most of the backobourkiines sensu Scharff et al. (2020) in our 34 taxa dataset ML and BI analyses (Fig. 1, 2), except for Novakiella, Parawixia F. O. Pickard-Cambridge, 1904, and the Euro-Asian S. nitidula, especially because the latter species bears little resemblance in both somatic and genital morphology to other backobourkiines and its placement in Scharff et al.’s (2020) within the backobourkiines is debatable.
The phylogenies of our limited dataset, which were compiled to specifically test hypotheses of Carepalxis relationships within an Australian context, support the monophyly of backobourkiines with Carepalxis placed as a sister taxon to all other putative backobourkiines with maximum support (Fig. 1, 2). It also confirms for the first time the inclusion of Lariniophora within this clade, a hypothesis previously put forward based on morphology (e.g. Framenau et al. 2021a). However, when our terminals are added to the full Scharff et al. (2020) dataset with four genes (see Fig. S1), the new ML topology differs from the limited dataset topologies (Fig. 1, 2) and from the topology originally presented in Scharff et al. (2020), which included five genes (the same four we used in addition to Histone 3). Carepalxis appears as sister to Backobourkia and Parawixia dehaani, albeit with low support (see Fig. S1). By adding more Carepalxis species to the Scharff et al. (2020) topology, S. nitidula is no longer a sister to this genus, but is placed within Hortophora, rendering the latter not monophyletic. This new hypothesis is unsupported morphologically as it splits H. biapicata and H. transmarina, two apparently closely related sister species (Framenau et al. 2021a). Therefore, Singa nitidula remains an enigma within this dataset. Scharff et al.’s (2020) statement that araneid systematics remain a work in progress and that formalising their informal clades remains immature until more data (i.e. terminals and genes) are added remains as true as ever.
All our three phylogenetic hypotheses support a largely congruent topology within Australian Carepalxis consisting of two major clades. The only difference in the ML analysis of the limited dataset (Fig. 1) to the other two topologies (Fig. 2, S1) is that of C. montifera and C. ferreirasousai sp. nov. swapping their position in one of these clades. This allows for the exploration of characters of the six species included in the analyses, specifically those of females, as males are only known for three species.
Carepalxis megalostylus sp. nov. and C. tholos sp. nov. formed one of the two clades and are the only two species included in our phylogeny whose females have baso-lateral flaps on their epigynes (Fig. 21e, 22e, 26f) (C. beelzebub and C. bilobata also have this character, but we were unable to include these species in our phylogenies, see Fig. 9e, 14e). This character appears to have some phylogenetical signal, considering that the species of the other clade do not have these flaps (e.g. Fig. 5f, 18e). Therefore, as Carepalxis_sp._250 of Scharff et al.’s (2020) matrix was nested within the tholos–megastylos-clade (see Fig. S1), it may represent either C. beelzebub or C. bilobata, both species with baso-lateral flaps, or even a third and undescribed species.
Considering the clade without baso-lateral flaps, all of its species have transverse slits instead. The ML topology fits better our observations on the genital morphology of females (even though with low nodal support) (Fig. 1). The genital morphology of females of C. kolla sp. nov. and C. tuberculata is more similar to that of C. montifera (ML topology, Fig. 1) in comparison to C. ferreirasousai sp. nov. (BI topology with maximum support, Fig. 2). For example, the three species have scapes with convex bases with similar thickness from the base to their tip and similar relatively straight transverse slits forming a small subquadrate terminal portion of the posterior plate (Fig. 5d, f, 19c, e, 29c, e), whereas in C. ferreirasousai sp. nov. the scape has a straight base and strongly tapers to its tip, and the posterior plate of the epigyne has curved–convex transverse slits composing a larger terminal portion of the posterior plate (Fig. 18c, e). The short branches observed in both phylogenies for C. ferreirasousai sp. nov., C. kolla sp. nov. and C. tuberculata (Fig. 1, 2) may indicate a recent speciation event between these three species, acknowledging the low support and unstable position of C. montifera that varies between analyses.
The general somatic morphology of Carepalxis is intriguing, with one proposed synapomorphy being the presence of cephalic humps in females (e.g. Fig. 7b). Cephalic modifications in spiders are not uncommon, especially in males. Modifications are common in members of the family Linyphiidae Blackwall, 1859, as noted in Hormiga (2000) where 40 male cephalic modifications, including cephalic lobes, lateral sulci and glands opening through cuticular pores, were described across genera of the subfamily Erigoninae Emerton, 1882. One example is the dwarf spider genus Oedothorax Bertkau, 1883, whose males have a single cephalic protuberance provided with glands that produces chemical secretions that are used during courtship or copulation by females, a trait that evolved in the context of sexual selection (Michalik and Uhl 2011; Kunz et al. 2012). Another example of cephalic modification is known to occur in numerous genera of cobweb spiders (Theridiidae), such as in males of Faiditus Keyserling, 1884, Rhomphaea L. Koch, 1872 and Neospintharus Exline, 1950, among others, whose cephalic region is anteriorly modified to form a proboscis (e.g. Agnarsson 2004, fig. 30A–D). By contrast, the presence of cephalic modifications is less commonly found in females, for example, as a complete elevation of the carapace of males and females of the theridiid Dipoena Thorell, 1869 and in some Thymoites Keyserling, 1884 species (e.g. Agnarsson 2004, fig. 5D, E). In Araneidae, cephalic modifications are present in some genera as a single hump, such as in males of Scoloderus Simon, 1887, and in some females of Wixia O. Pickard-Cambridge, 1882 and Ocrepeira (Levi 1993), or appearing as tubercles in female mastophoriines sensu Scharff et al. (2020), such as Mastophora Holmberg, 1876, assisting in camouflage behaviour (e.g. Levi 2003). Nonetheless, the presence of two enlarged horn-like cephalic humps is rare and its morphological advantage still needs to be further studied, despite being possibly associated with mimicry behaviour, such as accommodating the highly elevated abdomen in resting position. This character is herein considered as a synapomorphy of Carepalxis, and its presence in different degrees in some Gasteracantha Sundevall, 1833, in the species of Ocrepeira we transferred from Carepalxis in this paper, and in Paraplectanoides Keyserling, 1886 might likely be due to convergence.
Araneids have sexual dimorphic characters on legs and the presence of two elongated macrosetae (megaspines) on tibia II of males of Carepalxis is considered a synapomorphy of the genus (Fig. 3a–i). However, macrosetae on spurs or claspers is a more common trait of males of mygalomorph spiders as observed in different families such as on tibia I of Anamidae Simon, 1889 (e.g. Aname exulans Harvey & Huey, 2020, see Harvey et al. 2020, fig. 16–18), Idiopidae Simon, 1889 (e.g. Idiops fuscus Perty, 1833, see Fonseca-Ferreira et al. 2021, fig. 7G–I), and Theraphosidae Thorell, 1869 (e.g. Catumiri argentinense (Mello-Leitão, 1941), see Guadanucci 2004, fig. 16, 17). The presence of these apophyses likely represent a sexually selected trait that is used by males fighting during their competition for females and to lift and hold females during copulation, aiding males to reach the partner’s genital opening (Frank et al. 2023).
In spiders in the infraorder Araneomorphae F. O. Pickard-Cambridge, 1902, and more specifically in many Araneidae species males have to find strategies to copulate without being eaten or killed by much larger females when extreme sexual size dimorphism occurs (Uhl et al. 2015). One example of such strategies is to copulate while moulting (e.g. Argiope bruennichi (Scopoli, 1772), see Uhl et al. 2015) or to perform a rapid and precise copulation (e.g. Nephila pilipes (Fabricius, 1793), see Zhang et al. 2022). In species where males and females have similar sizes, however, the presence of macrosetae on mega-spurs, but also coxal hooks and femoral grooves can assist males to hold females during copulation. Despite having a strong phylogenetical signal and varying among different genera, these structures still need more in-depth studies of life copulations in a variety of taxa (Robinson and Robinson 1980; Levi 1983; Scharff and Coddington 1997; Cabra-García and Hormiga 2020).
Some studies shed some light on the importance of leg structures in genera such as Neoscona Simon, 1864 (Berman and Levi 1971), Araneus Clerck, 1757 (Carmichael 1973), and Micrathena (Magalhães and Santos 2012). More recently, Cabra-García and Hormiga (2020) identified numerous leg phenotypic characters in a phylogenetic study in the genus Wagneriana, such as hooks on coxa I (e.g. Hortophora biapicata (L. Koch, 1871)), dorsal tubercles on coxa I (e.g. Wagneriana taim Levi, 1991) and macrosetae present on various articles such as in coxa IV (e.g. Ocrepeira darlingtoni (Bryant, 1945)), trochanter IV (e.g. Eriophora), all four femora, tibia I and II, and metatarsus I, II and IV. Tibial macrosetae on a mega-spur, for example, occur as a single macroseta in some species of Hortophora and in Novakiella, and in a pair in Verrucosa and Carepalxis. It remains uncertain, however, how the shape, position and location of the mega-spur and its macrosetae and other leg structures evolved in the different araneid groups and how they benefit males during courtship and mating.
The distribution patterns of some species in the genus Carepalxis are unusual among Australian Araneidae, apparently ignoring any biogeographic or climatic patterns. In particular C. beelzebub, C. montifera and C. tuberculata range from tropical regions in the north to temperate climates in the south but have also been reported from the arid and semi-arid centre of Australia (Fig. 8, 13, 33). These wide ecological tolerances are known from few Araneidae, including the wide-spread Trichonephila edulis (Labillardière, 1799) (Harvey et al. 2007) and Austracantha minax (Thorell, 1859) (VWF unpublished data). At the genus level, these distribution patterns have not been observed in Australian orb-weaving spiders. Particularly in relation to the backobourkiines, there are few species that regularly occur in the arid regions of Australia, including the monotypic Lariniophora ragnhildae (Strand, 1917) and Backobourkia collina (Keyserling, 1886) (but not the other two species of Backobourkia) (Framenau et al. 2010; Framenau 2011). However, unlike the widespread Carepalxis, both species are neither found in the tropics nor, for example, in temperate Tasmania. It is currently unknown, what traits allow such wide ecological tolerances in Carepalxis species. It is also interesting to note that Carepalxis species are clearly masters of disguise apparently avoiding predatory pressure by mimicking the gumnuts of eucalypt trees (Main 1999).
Data availability
All new molecular data that were used in this study have been deposited in GenBank and are publicly available.
Declaration of funding
Funding for revisions of the Australian Araneidae was provided by the Australian Biological Resources Study (ABRS) (grant number 205-24 [2005–2008] to Volker W. Framenau and Nikolaj Scharff and grant number 4-EHPVRMK [2021–2024] to Volker W. Framenau, Pedro de S. Castanheira, Nikolaj Scharff, Dimitar Dimitrov, Renner L. C. Baptista and A. Chopra). Dimitar Dimitrov received additional support from the Norwegian Metacentre for Computational Science (NOTUR; project NN9601K).
Acknowledgements
We thank (in no particular order) Peter Lillywhite, Catriona McPhee, Ken Walker, Richard Marchant (NMV), Michael Rix, Robert Raven, Owen Seeman, Wendy Hebron, Joseph Schubert, (QM), David Hirst (SAM), Matthew Shaw (SAM, now AM), Graham Milledge, Helen Smith (AM), Danilo Harms and Nadine Dupérré (ZMH), Hannco Bakker (RMNH), Jan Pedersen (NHMD), Mark Harvey and Julianne Waldock (WAM), Jenny Shield (CVIC), Maria Tavano (MSNG), Leonardo Ferreira Sousa (UNB, now at UFMG – Universidade Federal de Minas Gerais) and John Douglas (QVMAG) for the loan or donation of specimens in their care, assistance when visiting their respective institutions or by sending images of type material. We thank André do Prado (Murdoch University) for the assistance with the Bayesian analysis and Francisca Sâmia Martins Oliveira (Universidade Federal do Rio de Janeiro) for the assistance compiling the material analysed and with the design of the maps. We are indebted to Prof. Abha Chopra, and technicians Linda Choo, Marjan Nasseh and Melissa Emmanuel from IIID at Murdoch University for the support during DNA extractions and sequencing. Finally, we acknowledge the support from DUG technology, particularly Marie Smyth, Rowan Worth and Mark Cheeseman during the molecular analyses of this study.
References
Agnarsson I (2004) Morphological phylogeny of cobweb spiders and their relatives (Araneae, Araneoidea, Theridiidae). Zoological Journal of the Linnean Society 141(4), 447-626.
| Crossref | Google Scholar |
Archer AF (1951) Studies in the orbweaving spiders (Argiopidae). 1. American Museum Novitates 1487, 1-52.
| Google Scholar |
Archer AF (1965) Nuevos argiopidos (arañas) de las Antilles. Caribbean Journal of Science 5, 129-133 [In Spanish].
| Google Scholar |
Banks N (1898) Arachnida from Baja California and other parts of Mexico. Proceedings of the California Academy of Sciences (3) 1(7), 205-309.
| Google Scholar |
Baptista RLC, Castanheira PS, Prado AWD (2018) Notes on the orb-weaving spider genus Alpaida (Araneae, Araneidae) with description of four new species from Rio de Janeiro, Brazil. Zootaxa 4407(3), 321-345.
| Crossref | Google Scholar | PubMed |
Berman JD, Levi HW (1971) The orb weaver genus Neoscona in North America (Araneae: Araneidae). Bulletin of the Museum of Comparative Zoology 141(8), 465-500.
| Google Scholar |
Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF (2013) MITOS: improved de novo Metazoan mitochondrial genome annotation. Molecular Phylogenetics and Evolution 69(2), 313-319.
| Crossref | Google Scholar | PubMed |
Cabra-García J, Hormiga G (2020) Exploring the impact of morphology, multiple sequence alignment and choice of optimality criteria in phylogenetic inference: a case study with the Neotropical orb-weaving spider genus Wagneriana (Araneae: Araneidae). Zoological Journal of the Linnean Society 188(4), 976-1151.
| Crossref | Google Scholar |
Carmichael LD (1973) Correlation between segment length and spine counts in two spider species of Araneus (Araneae: Araneidae). Psyche 80, 62-69.
| Crossref | Google Scholar |
Castanheira PS, Framenau VW (2023) Kangaraneus, a new genus of orb-weaving spider from Australia (Araneae, Araneidae). Zoosystematics and Evolution 99(2), 307-323.
| Crossref | Google Scholar |
Chen S (2023) Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. iMeta 2, e107.
| Crossref | Google Scholar | PubMed |
Chrysanthus P (1961) Spiders from south New Guinea IV. Nova Guinea, Zoology 10, 195-214.
| Google Scholar |
Court DJ, Forster RR (1988) The spiders of New Zealand: part VI. Family Araneidae. Otago Museum Bulletin 6, 68-124.
| Google Scholar |
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9(8), 772.
| Crossref | Google Scholar | PubMed |
Davies VT (1980) Two large Australian orb-weaving spiders, Eriophora transmarina (Keyserling 1865) and Eriophora biapicata (L. Koch 1871). Memoirs of the Queensland Museum 20(1), 125-133.
| Google Scholar |
Davies VT (1988) An illustrated guide to the genera of orb-weaving spiders in Australia. Memoirs of the Queensland Museum 25, 273-332.
| Google Scholar |
Dierckxsens N, Mardulyn P, Smits G (2017) NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Research 45(4), e18.
| Crossref | Google Scholar | PubMed |
Ferreira-Sousa L, Motta PC (2022) Diagnostic notes on the spider orb-weaving genera Carepalxis and Ocrepeira (Araneae: Araneidae), with description of three new species from Central Brazil. Zootaxa 5087(2), 389-399.
| Crossref | Google Scholar | PubMed |
Fonseca-Ferreira R, Guadanucci JPL, Yamamoto FU, Brescovit AD (2021) Taxonomic revision of the Neotropical spiders of the genus Idiops Perty, 1833 (Araneae, Idiopidae), with description of four new species. European Journal of Taxonomy 780, 1-71.
| Crossref | Google Scholar |
Framenau VW (2011) Lariniophora, a new monotypic orb-weaving spider genus from Australia (Araneae: Araneidae: Araneinae). Records of the Western Australian Museum 26(2), 191-201.
| Crossref | Google Scholar |
Framenau VW (2019) Generic and family transfers, and nomina dubia for orb-weaving spiders (Araneae, Araneidae) in the Australasian, Oriental and Pacific regions. Evolutionary Systematics 3, 1-27.
| Crossref | Google Scholar |
Framenau VW, Castanheira PS (2022) Revision of the new Australasian orb-weaving spider genus Salsa (Araneae, Araneidae). ZooKeys 1102, 107-148.
| Crossref | Google Scholar | PubMed |
Framenau VW, Dupérré N, Blackledge TA, Vink CJ (2010) Systematics of the new Australasian orb-weaving spider genus Backobourkia (Araneae: Araneidae: Araneinae). Arthropod Systematics and Phylogeny 68, 79-111.
| Crossref | Google Scholar |
Framenau VW, Baptista RLC, Oliveira FSM, Castanheira PS (2021a) Taxonomic revision of the new spider genus Hortophora, the Australasian garden orb-weavers (Araneae, Araneidae). Evolutionary Systematics 5(2), 275-334.
| Crossref | Google Scholar |
Framenau VW, Vink CJ, Scharff N, Baptista RLC, Castanheira PS (2021b) Review of the Australian and New Zealand orb-weaving spider genus Novakiella (Araneae, Araneidae). Zoosystematics and Evolution 97, 393-405.
| Crossref | Google Scholar |
Framenau VW, Castanheira PS, Vink CJ (2022) Taxonomy and systematics of the new Australo-Pacific orb-weaving spider genus Socca (Araneae: Araneidae). New Zealand Journal of Zoology 49(4), 263-334.
| Crossref | Google Scholar |
Frank S-C, Christensen R, Lourenço R, Harms D, Buzatto BA (2023) Mating behavior of the Sydney funnel-web spider (Atracidae: Atrax robustus) and implications for the evolution of courtship in mygalomorph spiders. Journal of Zoology 320(3), 169-178.
| Crossref | Google Scholar |
Gerschman PBS, Schiapelli RD (1948) Arañas argentinas II. Comunicaciones del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” 4, 1-20 [In Spanish].
| Google Scholar |
Guadanucci JPL (2004) Description of Catumiri n. gen. and three new species (Theraphosidae: Ischnocolinae). Zootaxa 671, 1-14.
| Crossref | Google Scholar |
Guindon S, Gascuel O (2003) A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Systematic Biology 52, 696-704.
| Crossref | Google Scholar | PubMed |
Harvey MS, Austin AD, Adams M (2007) The systematics and biology of the spider genus Nephila (Araneae: Nephilidae) in the Australasian region. Invertebrate Systematics 21, 407-451.
| Crossref | Google Scholar |
Harvey MS, Gruber K, Hillyer MJ, Huey JA (2020) Five new species of the open-holed trapdoor spider genus Aname (Araneae: Mygalomorphae: Anamidae) from Western Australia, with a revised generic placement for Aname armigera. Records of the Western Australian Museum 35, 10-38.
| Crossref | Google Scholar |
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35, 518-522.
| Crossref | Google Scholar | PubMed |
Hopfe C, Ospina-Jara B, Scheibel T, Cabra-García J (2005) Ocrepeira klamt sp. n. (Araneae: Araneidae), a novel spider species from an Andean páramo in Colombia. PLoS One 15, e0237499.
| Crossref | Google Scholar |
Hormiga G (2000) Higher level phylogenetics of erigonine spiders (Araneae, Linyphiidae, Erigoninae). Smithsonian Contributions to Zoology 609, 1-160.
| Crossref | Google Scholar |
Hormiga G, Kulkarni S, Arnedo MA, Dimitrov D, Giribet G, Kallal RJ, Scharff N (2023) Genitalic morphology and phylogenomic placement of the Australian spider Paraplectanoides crassipes Keyserling, 1886 (Araneae, Araneidae) with a discussion on the classification of the family Araneidae. Invertebrate Systematics 37(12), 797-818.
| Crossref | Google Scholar |
Joseph MM, Framenau VW (2012) Systematic review of a new orb-weaving spider genus (Araneae: Araneidae), with special reference to the Australasian–Pacific and South-East Asian fauna. Zoological Journal of the Linnean Society 166, 279-341.
| Crossref | Google Scholar |
Kallal RJ, Hormiga G (2018) Systematics, phylogeny and biogeography of the Australasian leaf-curling orb-weaving spiders (Araneae: Araneidae: Zygiellinae), with a comparative analysis of retreat evolution. Zoological Journal of the Linnean Society 184, 1055-1141.
| Crossref | Google Scholar |
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772-780.
| Crossref | Google Scholar | PubMed |
Keyserling E (1886) ‘Die Arachniden Australiens, nach der Natur beschrieben und abgebildet. Zweiter Theil [Lieferung 33-34].’ pp. 87–152. (Bauer & Raspe: Nürnberg, German Empire) [In German] 10.5962/bhl.title.121660
Keyserling E (1892) Die Spinnen Amerikas. Epeiridae. Bauer & Raspe, Nürnberg 4, 1-208 [In German].
| Crossref | Google Scholar |
Kück P, Meusemann K (2010) FASconCAT: convenient handling of data matrices. Molecular Phylogenetics and Evolution 56, 1115-1118.
| Crossref | Google Scholar | PubMed |
Kunz K, Garbe S, Uhl G (2012) The function of the secretory cephalic hump in males of the dwarf spider Oedothorax retusus (Linyphiidae: Erigoninae). Animal Behaviour 83(2), 511-517.
| Crossref | Google Scholar |
Levi HW (1983) The orb-weaver genera Argiope, Gea, and Nanogea from the western Pacific Region (Araneae: Araneidae, Argiopinae). Bulletin of the Museum of Comparative Zoology 150, 247-338.
| Google Scholar |
Levi HW (1991) The Neotropical and Mexican species of the orb-weaver genera Araneus, Dubiepeira, and Aculepeira (Araneae: Araneidae). Bulletin of the Museum of Comparative Zoology 152(4), 167-315.
| Google Scholar |
Levi HW (1992) The American species of the orb-weaver genus Carepalxis and the new genus Rubrepeira (Araneae: Araneidae). Psyche: a Journal of Entomology 98(2–3), 026493.
| Crossref | Google Scholar |
Levi HW (1993) The Neotropical orb-weaving spiders of the genera Wixia, Pozonia, and Ocrepeira (Araneae: Araneidae). Bulletin of the Museum of Comparative Zoology 153, 47-141.
| Google Scholar |
Levi HW (2003) The bolas spiders of the genus Mastophora (Araneae: Araneidae). Bulletin of the Museum of Comparative Zoology 157(5), 309-382.
| Google Scholar |
Lise AA, Kesster CC, Silva ELC (2015) Revision of the orb-weaving spider genus Verrucosa McCook, 1888 (Araneae, Araneidae). Zootaxa 3921(1), 1-105 [Erratum 3956(4), 600.].
| Google Scholar |
Magalhães ILF, Santos AJ (2012) Phylogenetic analysis of Micrathena and Chaetacis spiders (Araneae: Araneidae) reveals multiple origins of extreme sexual size dimorphism and long abdominal spines. Zoological Journal of the Linnean Society 166(1), 14-53.
| Crossref | Google Scholar |
Main BY (1999) Notes on the biogeography and natural history of the orbweaving spider Carepalxis (Araneae, Araneidae), including a gumnut mimic from southwestern Australia. Journal of Arachnology 27, 183-188.
| Google Scholar |
Michalik P, Uhl G (2011) Cephalic modifications in dimorphic dwarf spiders of the genus Oedothorax (Erigoninae, Linyphiidae, Araneae) and their evolutionary implications. Journal of Morphology 272(7), 814-32.
| Crossref | Google Scholar | PubMed |
Petrunkevitch A (1911) A synonymic index-catalogue of spiders of North, Central and South America with all adjacent islands, Greenland, Bermuda, West Indies, Terra del Fuego, Galapagos, etc. Bulletin of the American Museum of Natural History 29, 1-791.
| Google Scholar |
Rainbow WJ (1911) A census of Australian Araneidae. Records of the Australian Museum 9(2), 107-319.
| Crossref | Google Scholar |
Rambaut AR, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67, 901-904.
| Crossref | Google Scholar | PubMed |
Robinson MH, Robinson B (1980) Comparative studies of the courtship and mating behavior of tropical araneid spiders. Pacific Insect Monographs 36, 1-218.
| Google Scholar |
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3), 539-542.
| Crossref | Google Scholar |
Scharff N, Coddington JA (1997) A phylogenetic analysis of the orb-weaver spider family Araneidae (Arachnida, Araneae). Zoological Journal of the Linnean Society 120, 355-434.
| Crossref | Google Scholar |
Scharff N, Coddington JA, Blackledge TA, Agnarsson I, Framenau VW, Szűts T, Hayashi CY, Dimitrov D (2020) Phylogeny of the orb-weaving spider family Araneidae (Araneae, Araneoidea). Cladistics 36, 1-21.
| Crossref | Google Scholar | PubMed |
Simon E (1895) Etudes arachnologiques. 26e. XLI. Descriptions d’espèces et de genres nouveaux de l’ordre des Araneae. Annales de la Société Entomologique de France 64, 131-160 [In French].
| Google Scholar |
Thorell T (1881) Studi sui Ragni Malesi e Papuani. III. Ragni dell’Austro Malesia e del Capo York, conservati nel Museo civico di storia naturale di Genova. Annali del Museo Civico di Storia Naturale di Genova 17, 1-720 [In Italian].
| Google Scholar |
Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44(W1), W232-W235.
| Crossref | Google Scholar | PubMed |
Uhl G, Zimmer S, Renner D, Schneider JM (2015) Exploiting a moment of weakness: male spiders escape sexual cannibalism by copulating with moulting females. Scientific Reports 5, 16928.
| Crossref | Google Scholar | PubMed |
van Hasselt AWM (1873) Araneae exoticae, quas collegit pro museo Lugdunensi D. A. Van Kaathoven, S. T., Novâ Hollandiâ (Melbourne). Tijdschrift voor Entomologie 16, 236-243. [In Latin].
| Google Scholar |
Zhang S, Yu L, Tan M, Tan NYL, Wong XXB, Kuntner M, Li D (2022) Male mating strategies to counter sexual conflict in spiders. Communications Biology 5(1), 534.
| Crossref | Google Scholar | PubMed |
Footnotes
1 The description is also available at https://faunaportal.org/projects/fauna-of-australia/genus/araneae-spiders/araneidae-orb-weaving-spiders/carepalxis/18/.