Foodborne pathogenic E. coli (focus on STEC)
Robert S Barlow and Glen E MellorFood Safety and Stability
CSIRO Animal, Food and Health Sciences
PO Box 745, Archerfield BC, Qld 4108, Australia
Tel: +61 7 3214 2035
Fax: +61 7 3214 2050
Email: robert.barlow@csiro.au
Microbiology Australia 34(2) 80-83 https://doi.org/10.1071/MA13028
Published: 13 May 2013
Foodborne pathogenic E. coli continue to emerge and evolve as significant human pathogens. With cattle and other ruminants acting as natural reservoirs, they contaminate food directly via contamination of animals at slaughter or indirectly via the use of contaminated manure or water during food production. E. coli O157 remains the predominant disease causing serotype although additional serotypes such as O26 and O111, along with E. coli possessing novel combinations of virulence genes, highlight the increasing complexity associated with reducing the prevalence of foodborne pathogenic E. coli. Variability in the severity of disease caused by different E. coli provides insight into the significance of virulence factors thereby enabling the design of possible control methods such as vaccines. The continuing burden of foodborne pathogenic E. coli presents a challenge for food producers and researchers to overcome to ensure an ongoing supply of safe and healthy food.
What is STEC?
Escherichia coli is a member of the gut microbiome of the majority of warm blooded animals, including humans. E. coli generally exist harmlessly within the gut performing physiological activities that benefit themselves, their host, and the associated microbiome. However, E. coli is perhaps most well known for its ability to cause disease in humans. Shiga toxin-producing E. coli (STEC) were first identified as a foodborne pathogen in 1982 and are characterised by the ability to produce Shiga toxin1. STEC are an important public health concern as they have caused large foodborne outbreaks, are particularly dangerous to small children where acute renal failure is often observed, which can sometimes lead to death.
How does E. coli become pathogenic?
Enterohaemorrhagic E. coli (EHEC) are a subset of STEC that have acquired additional virulence traits, such as the locus of enterocyte effacement (LEE), that enhances the capacity to attach to intestinal cells and subsequently cause disease. Conversely there are many STEC that are considered non-pathogenic to humans because they lack specific additional virulence traits2. Even within the disease causing STEC or EHEC variability in virulence between different serotypes may be attributed to specific virulence factors encoded on large, horizontally acquired gene cassettes3. Furthermore, differences in the types of Shiga toxins present and the point at which the Shiga toxin-bearing phage insert in the chromosome have also been associated with isolates that cause varying disease symptoms in humans4.
How does food become contaminated with pathogenic E. coli?
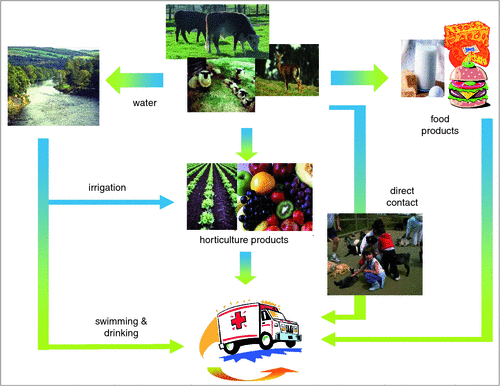
Cattle and other ruminants (Figure 1) are able to harbor and shed populations of STEC and EHEC often without deleterious effects to the animal’s health. Transmission of pathogenic E. coli to foods can occur directly, particularly when beef or dairy cattle are slaughtered and the exterior surface of the carcase becomes contaminated, or indirectly through the use of contaminated cattle manure or water during horticulture production. The contamination of beef carcases is an issue when ground beef is produced. In this scenario, contamination that is on the surface of the meat is mixed and distributed throughout the product and thorough cooking to the centre of the product then becomes the only point of control. Similarly, contamination of horticulture products is a risk to human health as pathogenic E. coli can adhere to the surface of these products and can be extremely difficult to remove. Moreover, many horticulture products are consumed raw and consequently emphasis must be placed on ensuring contamination events during production are eliminated. There are many other ways in which food can become contaminated with pathogenic E. coli (Figure 2) and producers should therefore be aware of the inputs to their production system and, where possible, implement strategies to reduce the risk of contamination.

|
|
Outbreaks – changing paradigm?
Since the first description of STEC in 1982 E. coli O157 has been the most commonly implicated serotype in disease outbreaks. Consequently, STEC-related research initially progressed with absolute focus on the O157 serotype to the exclusion of most other serotypes. Between 1982 and 2002 the USA recorded 350 outbreaks of E. coli O157 resulting in 8,598 cases5. While in Australia sporadic cases of E. coli O157 infection are more likely to occur than outbreaks, there were at least four outbreaks attributed to the serotype between 2001 and 20096. Analysis of the USA outbreaks indicated that food remains the predominant transmission route accounting for 52% of outbreaks with ground beef and produce accounting for 41% and 21% of foodborne outbreaks, respectively5. Similar trends have been observed in Europe, Japan and South America.
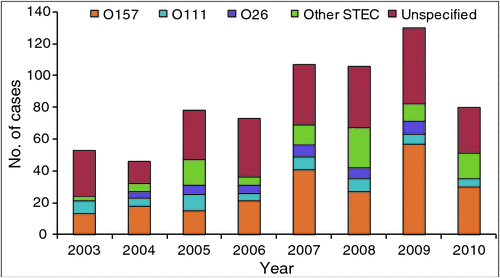
Despite E. coli O157 remaining the dominant serotype implicated in sporadic cases and outbreaks of STEC-associated disease, it has become apparent over the last 10-15 years that a group of additional serotypes of STEC are responsible for a much greater proportion of disease than originally estimated. Australian STEC outbreak data from 2001 to 2009 determined that seven of the 11 outbreaks that occurred during that time were attributable to serotypes other than O1576. STEC notifications in Australia between 2001 and 2008 revealed that non-O157 serotypes were substantial contributors to the overall incidence of STEC-associated infections observed in the Australian health system (Figure 37). In the USA, STEC surveillance data analysis determined that the ratio of non-O157 to O157 infections is approaching two to one with 63,000 E. coli O157 and 110,000 non-O157 infections estimated to occur annually8. Although the occurrence of serious diseases and death remains more common with E. coli O157 infections than with non-O157, the burden of non-O157 infections on health systems is evident. As surveillance and methods for non-O157 isolation improve we can expect greater implication of non-O157 STEC in outbreaks and sporadic cases of human disease.
|
In addition to the materialisation of non-O157 STEC serotypes as major human pathogens, 2011 saw the emergence of a novel pathotype of E. coli that was ultimately responsible for the most devastating E. coli outbreak to date. The three month long outbreak was caused by a hybrid E. coli of serotype O104:H4 that harbored virulence traits common to STEC as well as enteroaggregative E. coli. A total of 4321 cases were recorded with 852 cases progressing to haemolytic uraemic syndrome and 50 deaths were reported9. Investigation of the outbreak concluded that contaminated fenugreek sprouts was the food vehicle responsible. The novel nature of the E. coli strain involved in this outbreak highlights the complexity associated with producing safe food and the difficulty in preparing for the emergence of novel E. coli pathotype. At the time of the outbreak the O104:H4 strain would not have been detected using conventional testing approaches for O157 and non-O157 STEC.
How can we prevent contaminated foods entering commerce?
There are generally two ways we can prevent foods contaminated with pathogenic E. coli from entering commerce: put hurdles in place to restrict food becoming contaminated or find ways to identify and treat foods that have become contaminated. Researchers have demonstrated that vaccines and probiotics such as Lactobacillus species can be effective in reducing pathogenic E. coli loads in cattle10,11, however constraints relating to overall efficacy as well as timing and cost of application have limited their implementation at a commercial level. Consequently, the current focus for preventing STEC-related foodborne illness revolves around implementing practices that reduce the likelihood of releasing contaminated food products into commerce. Examples of practices include: carcase decontamination during cattle slaughter, the use of properly composted manure in agriculture, and mechanical interventions like pasteurisation and irradiation to name a few. At the consumer level, cooking remains the only effective control mechanism, however, it is of little use to foods destined to be consumed raw. Washing of raw fruits and vegetables may be useful in reducing microbial loads but it can be problematic and it will have no effect if the organisms have become internalised during growth12. Testing product prior to its release into commerce can assist in reducing the likelihood of exposure to consumers. Interestingly, despite a surge in produce-related STEC outbreaks in the last decade there is not a concerted push to use testing as a way to improve the safety of these products. However, as the STEC paradigm shifts, both in terms of the food vehicles involved and the range of E. coli pathotypes involved, incorporation of testing or more substantial control measures may be required.
References
[1] Karmali, M.A. et al. (1983) Sporadic cases of haemolytic uraemic syndrome associated with faecal cytotoxin-producing Escherichia coli in stools. Lancet 1, 619–620.| Sporadic cases of haemolytic uraemic syndrome associated with faecal cytotoxin-producing Escherichia coli in stools.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3s7ktVersg%3D%3D&md5=b426811b8babaef9857bca9465fbb8c3CAS | 6131302PubMed |
[2] Hussein, H.S. (2007) Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J. Anim. Sci. 85, E63–E72.
| Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2s7itlWmsA%3D%3D&md5=143effb4da141358fe5fa24de9e5e5aaCAS | 17060419PubMed |
[3] Bolton, D.J. (2011) Verocytotoxigenic (Shiga toxin-producing) Escherichia coli: virulence factors and pathogenicity in the farm to fork paradigm. Foodborne Pathog. Dis. 8, 357–365.
| Verocytotoxigenic (Shiga toxin-producing) Escherichia coli: virulence factors and pathogenicity in the farm to fork paradigm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXivVGisLo%3D&md5=030a8bca4c6c5609b14cd28a45a68d06CAS | 21114423PubMed |
[4] Mellor, G.E. et al. (2012) Phylogenetically related Argentinean and Australian Escherichia coli O157 are distinguished by virulence clades and alternative Shiga toxin 1 and 2 prophages. Appl. Environ. Microbiol. 78, 4724–4731.
| Phylogenetically related Argentinean and Australian Escherichia coli O157 are distinguished by virulence clades and alternative Shiga toxin 1 and 2 prophages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XptFemt7Y%3D&md5=ccf691752472c69ff50af61af23b49e7CAS | 22544241PubMed |
[5] Rangel, J.M. et al. (2005) Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 11, 603–609.
| Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002.Crossref | GoogleScholarGoogle Scholar | 15829201PubMed |
[6] Vally, H. et al. (2012) Epidemiology of Shiga toxin producing Escherichia coli in Australia, 2000–2010. BMC Public Health 12, 63.
| Epidemiology of Shiga toxin producing Escherichia coli in Australia, 2000–2010.Crossref | GoogleScholarGoogle Scholar | 22264221PubMed |
[7] OzFoodNet Working Group (2008) Monitoring the incidence and causes of disases potentially transmitted by food in Australia: annual report of the OzFoodNet Network, 2007. Commun. Dis. Intell. 32, 400–424.
[8] Scallan, E. et al. (2011) Foodborne illness acquired in the United States – major pathogens. Emerg. Infect. Dis. 17, 7–15.
| 21192848PubMed |
[9] Soon, J.M. et al. (2012) Escherichia coli O104:H4 outbreak from sprouted seeds. Int. J. Hyg. Environ. Health , .
| Escherichia coli O104:H4 outbreak from sprouted seeds.Crossref | GoogleScholarGoogle Scholar | 22898546PubMed |
[10] Brashears, M.M. et al. (2003) Prevalence of Escherichia coli O157:H7 and performance by beef feedlot cattle given Lactobacillus direct-fed microbials. J. Food Prot. 66, 748–754.
| 1:STN:280:DC%2BD3s3isFOmuw%3D%3D&md5=57659c1a8fffb7664c35f5f3e6278009CAS | 12747680PubMed |
[11] Smith, D.R. et al. (2009) A two-dose regimen of a vaccine against type III secreted proteins reduced Escherichia coli O157:H7 colonization of the terminal rectum in beef cattle in commercial feedlots. Foodborne Pathog. Dis. 6, 155–161.
| A two-dose regimen of a vaccine against type III secreted proteins reduced Escherichia coli O157:H7 colonization of the terminal rectum in beef cattle in commercial feedlots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisVSmur4%3D&md5=399499b6d4339b27839fc4559016df3bCAS | 19105625PubMed |
[12] Olaimat, A.N. and Holley, R.A. (2012) Factors influencing the microbial safety of fresh produce: a review. Food Microbiol. 32, 1–19.
| Factors influencing the microbial safety of fresh produce: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XnvVSiurc%3D&md5=b2a4bfc4649ac254f9f413e472ba998fCAS | 22850369PubMed |
Biographies
Robert Barlow is a research microbiologist with CSIRO Animal, Food & Sciences. He did his PhD on integron-associated antimicrobial resistance in beef production systems and has spent over 15 years conducting research into foodborne pathogenic E. coli and antimicrobial resistant bacteria in beef destined for export. Robert routinely assists food production industries and SMEs by providing advice and guidance on matters relating to food safety.
Glen Mellor is a research microbiologist with CSIRO Animal, Food & Health Sciences. Glen has extensive knowledge relating to the typing and virulence of pathogenic E. coli strains. In recent years, he has investigated the global genotypic diversity of E. coli O157 from Australia, Argentina and the USA and has been a key researcher in large-scale surveys within the red meat and poultry industries.


