Surfaces and fomites as a source of healthcare-associated infections
Jen KokCentre for Infectious Diseases and Microbiology Laboratory Services
Institute of Clinical Pathology and Medical Research
Marie Bashir Institute
Centre for Research Excellence in Critical Infections
University of Sydney, Westmead Hospital
Westmead, NSW, Australia
Tel: +61 2 9845 6255
Fax: +61 2 9893 8659
Email: jen.kok@swahs.health.nsw.gov.au
Microbiology Australia 35(1) 24-25 https://doi.org/10.1071/MA14007
Published: 17 February 2014
Fomites are inanimate objects in the environment that can become contaminated with pathogenic microorganisms, facilitating their transfer from one patient or surface to another. Understanding how pathogens are spread in the environment and terminating the spread is important for controlling nosocomial outbreaks.
Healthcare-associated infections (HAI) are associated with excess morbidity, mortality and healthcare costs1. The quality of infection control practices, environmental cleaning, understaffing, overcrowding and poor healthcare facility infrastructure are all factors that can affect HAI rates. The true prevalence of HAI may be underestimated by the presence of unrecognised infections.
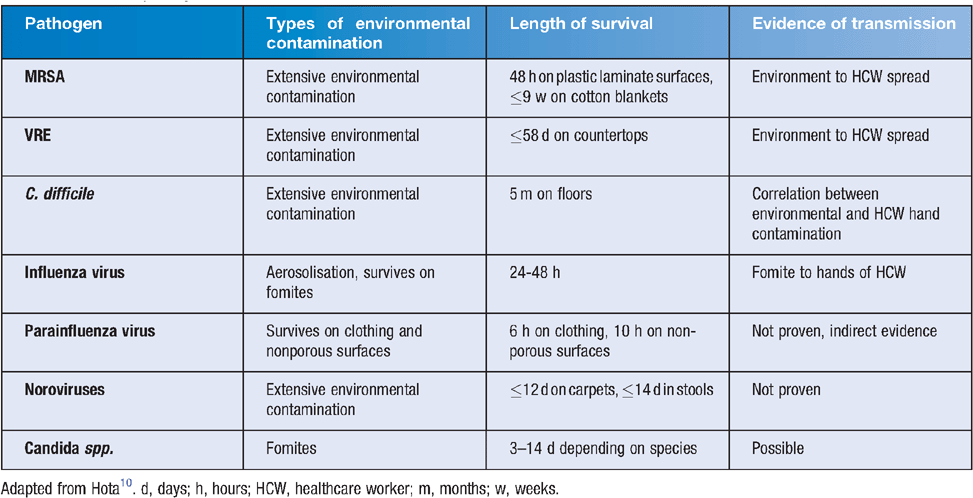
Within any nosocomial environment, surfaces and fomites serve as reservoirs of infection, with healthcare workers acting as vectors for transmission2. Although bacterial species are the best-studied examples, there is mounting evidence that surfaces can serve as reservoirs for viruses and fungi3. Microorganism survival on fomites is influenced by intrinsic properties such as the surface porosity and the pathogen’s characteristics (for example, non-enveloped viruses remain viable longer on surfaces compared with enveloped viruses) and by extrinsic factors such as environmental temperature and humidity. A person’s age, personal habits, type of activities, personal mobility and the level of cleanliness within the surroundings can influence the nature and frequency of contact with contaminated surfaces, and can affect the transfer of pathogens to and from fomites4. Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE) and Clostridium difficile are examples of some pathogens that have been recovered from contaminated surfaces. Table 1 outlines the environmental contamination, length of survival and evidence of transmission of common nosocomial pathogens.
|
The methodology and quality of evidence in published studies examining environmental contamination rates and their effects on pathogen transmission are highly variable. When interpreting the results of such studies, it is important to address several important issues. First, contamination rates do not necessarily reflect transmission rates. The degree of contamination by specific pathogens warrants careful scrutiny, as colonising organisms may not necessarily be pathogenic. Second, temporality should also be assessed, that is, whether evidence of environmental and/or patient contamination before and/or after contact is clearly assessed. Last, confounding factors including hand hygiene, wearing of personal protective equipment, the cleaning of fomites and/or other interventions should also be examined.
In a study of skin and environmental contamination by patients with MRSA, Chang et al. noted that 15 (18%) and 29 (35%) of 83 patients contaminated their surrounding environment with MRSA within 25 and 33 hours, respectively5. However, the study did not address several key issues including no measurement of baseline contamination rate within rooms prior to patient admission, failure to type MRSA isolates collected from surveillance swabs of the nose, skin and environment in order to assess clonality, and failure to perform surveillance swabs on roommates already colonised with MRSA that may have contaminated the shared room.
In contrast, an outbreak of MRSA was successfully terminated by interrupting fomite colonisation through the extensive closure of wards and institution of a cleaning program that included targeted removal of dust from ward surfaces, furniture, floors and medical equipment6. There were also significant cost benefits by terminating the outbreak: reduced hospital bed-days and unnecessary treatment costs and increased efficiency within the hospital system.
Although disinfection of reservoirs should be prioritised in any hospital environment7, infection control ‘bundles’ to reduce HAI may be more effective than any single measure alone, and the efficacy of each intervention may not be easily determined8. In addition, current cleaning and disinfection methods vary across different health institutions, are operator dependent and may not adequately disinfect heavily contaminated surfaces. There is also limited data comparing the efficacy of different methods such as neutral detergents, sodium hypochlorite and hydrogen peroxide9. Hydrogen peroxide may be more effective in disinfecting difficult-to-reach areas and has significant sporicidal activity. However, it may not be compatible with all types of medical equipment, and biological soiling of surfaces reduces the efficacy of hydrogen peroxide disinfection.
In summary, pathogens responsible for HAI can survive for hours to months on environmental surfaces, and transmission may occur upon contact with contaminated surfaces. The transmission cycle is established when new reservoirs are created, and HCW and/or patients act as vectors for transmission. Enhanced cleaning and disinfection of environmental surfaces, along with other infection control measures, can reduce the transmission of such pathogens.
References
[1] Turnidge, J.D. et al. (2009) Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med. J. Aust. 191, 368–373.| 19807625PubMed |
[2] Boyce, J.M. (2007) Environmental contamination makes an important contribution to hospital infection. J. Hosp. Infect. 65, 50–54.
| Environmental contamination makes an important contribution to hospital infection.Crossref | GoogleScholarGoogle Scholar | 17540242PubMed |
[3] Barker, J. et al. (2001) Spread and prevention of some common virla infections in community facilities and domestic homes. J. Appl. Microbiol. 91, 7–21.
| Spread and prevention of some common virla infections in community facilities and domestic homes.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MzovV2rug%3D%3D&md5=6936e5b34726aad1f85a25421c60c269CAS | 11442709PubMed |
[4] Boone, S.A. and Gerba, C.P. (2007) Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 73, 1687–1696.
| Significance of fomites in the spread of respiratory and enteric viral disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjs1ahtro%3D&md5=da968ed7f089348e0be5e503f6307466CAS | 17220247PubMed |
[5] Chang, S. et al. (2010) Occurrence of skin and environmental contamination with methicillin-resistant Staphylococcus aureus before results of polymerase chain reaction at hospital admission become available. Infect. Control Hosp. Epidemiol. 31, 607–612.
| Occurrence of skin and environmental contamination with methicillin-resistant Staphylococcus aureus before results of polymerase chain reaction at hospital admission become available.Crossref | GoogleScholarGoogle Scholar | 20397963PubMed |
[6] Rampling, A. et al. (2001) Evidence that hospital hygiene is important in the control of methicillin resistant Staphylococcus aureus. J. Hosp. Infect. 49, 109–116.
| Evidence that hospital hygiene is important in the control of methicillin resistant Staphylococcus aureus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mrit1aksg%3D%3D&md5=78991e99849880eea8b58727b4934bc2CAS | 11567555PubMed |
[7] Dancer, S.J. (2009) The role of environmental cleaning in the control of hospital-acquired infection. J. Hosp. Infect. 73, 378–385.
| The role of environmental cleaning in the control of hospital-acquired infection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MjmsFOqsQ%3D%3D&md5=5d01bc6a86afcfc2c1e96c47a18e493aCAS | 19726106PubMed |
[8] Jain, R. et al. (2011) Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N. Engl. J. Med. 364, 1419–1430.
| Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlsVGqur8%3D&md5=11bd87fbe34a566ff26ff0ed23c8ec7dCAS | 21488764PubMed |
[9] White, L.F. et al. (2007) A microbiological evaluation of hospital cleaning methods. Int. J. Environ. Health Res. 17, 285–295.
| A microbiological evaluation of hospital cleaning methods.Crossref | GoogleScholarGoogle Scholar | 17613092PubMed |
[10] Hota, B. (2004) Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin. Infect. Dis. 39, 1182–1189.
| Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection?Crossref | GoogleScholarGoogle Scholar | 15486843PubMed |


