Marine microbes in the Plastic Age
A Mark Osborn and Slobodanka StojkovicSchool of Applied Sciences
RMIT University
Bundoora, Vic. 3083, Australia
Tel: +61 3 9925 7126
Email: mark.osborn@rmit.edu.au
Microbiology Australia 35(4) 207-210 https://doi.org/10.1071/MA14066
Published: 30 October 2014
We are living in the ‘Plastic Age’, but unfortunately our non-human relatives with whom we share our planet are not adapted to cope with the thousands of tons of plastic waste entering rivers, seas and oceans each year. Plastic poses both physical and chemical threats to aquatic life. It leads to damage or death of animals following plastic entanglement or ingestion and/or can lead to bioaccumulation of co-pollutants absorbed on plastic surfaces. Once ingested, co-pollutants can be absorbed into tissues and accumulated in the food chain. As nature’s biodegraders and recyclers, microorganisms may play a role in mitigating the impact of our disposable plastic lifestyle, or alternatively, plastic may serve as a vector for transport of pathogenic microorganisms into marine fauna. Here, we review current understanding of the microbiology of marine plastics and highlight future challenges for this emerging research discipline.
The dominance of plastic across human society is a recent phenomenon, with petroleum oil-derived synthetic plastic polymers only finding widespread usage during the second half of the last century. We, alongside all other organisms, now live in the ‘Plastic Age’, with plastic infrastructure and industrial and consumer products now prevalent and playing a critically important role across every aspect of our lives1. However, the essential qualities of plastics, namely, resilience, durability, light weight, flexibility and resistance to degradation that have driven the adoption of plastics as materials of choice has also lead to the cosmopolitan distribution of plastic waste across the planet, and especially within marine environments. Initially, environmental plastic litter was considered primarily as an aesthetic issue, but the United Nations Environment Programme (UNEP) has now identified plastic pollution as a global environmental threat2 with a proposal that plastic be designated as a hazardous waste product3.
Our plastic world
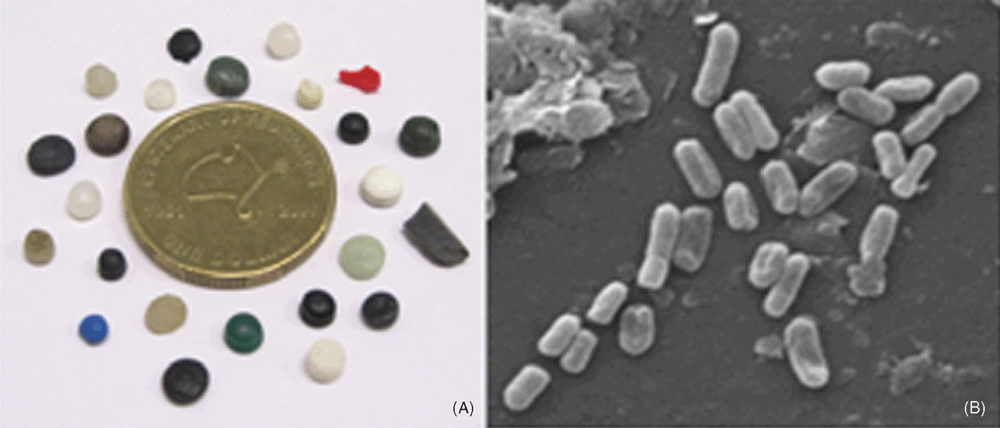
Global plastic production increased from 1.7 million tons in 1950 to 288 million tons in 20124, representing an 8.7% year-on-year increase5. Plastic consumption in Australia alone exceeded an average of over 1.5 million tons per annum between 2007 and 20126. The five major classes of plastic polymers, comprising ~90% of polymer production, are: polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), and polyethylene terephthalate (PET)1. The ever-increasing production of industrial and consumer plastics, the latter which includes a substantial proportion of single-use disposable plastics, results in a plastic deluge into marine environments. A short walk along the tide-line of any beach quickly highlights the pervasive presence of plastic litter in our marine environments, but a closer look also reveals the abundance of so-called microplastics (defined as plastics ≤5 mm in diameter; Figure 1A).
|
The presence of microplastics in our oceans was first reported in the Sargasso Sea in 19728 with initial estimates of particle distribution of 50–12,000 per km2. Later that year, PS spherules carrying adsorbed co-pollutants (polychlorinated biphenyl) were reported in coastal American waters9, foreshadowing the subsequent identification of a much greater problem of adsorbed co-pollutants on plastic surfaces, now threatening marine fauna10. Intriguingly, both of these pioneer studies also noted the presence of microorganisms on the surface of plastics, with diatoms (and also hydroids) identified on pellets from the Sargasso Sea8 and of rod-shaped Gram negative bacteria on PS spherules9. A subsequent study of American offshore waters demonstrated the widespread distribution of plastic fragments in oceanic waters11, but for many years, interest in the environmental distribution and ecological impact of plastic particles in marine environments remained limited. In 2004, Richard Thompson and colleagues published a paper in Science (Lost at Sea: Where is all the Plastic?)12 revisiting the issue of microplastics in marine environments. They demonstrated both widespread occurrence of microplastic fragments and fibres in both pelagic and benthic systems and highlighted increasing accumulation of microplastics between the 1960/70s and 1980/90s. The accumulation and fragmentation of plastics into microplastics13 has now led to the global dispersal of plastic across marine environments14, and in particular within the gyres (or ‘Garbage Patches’) of the Atlantic15 and Pacific16 (but also Indian) oceans14. Estimates of surface plastics, alone, are as high as 5 × 105 pieces per km2 of ocean15, with plastic identified as the most abundant component of litter within marine environments17. A recent study18 of Australian coastal waters also reported high average sea surface plastic concentrations exceeding 4,000 plastic pieces per km2.
The microbial ‘plastisphere’
As nature’s biodegraders, microorganisms may already be ameliorating the accumulation of plastic and/or their associated co-pollutants within marine environments. However, hard evidence for biodegradation, especially over ecologically-relevant timescales is lacking. Indeed, our understanding of the marine microbial plastisphere is still in its infancy, with initial studies just beginning to characterise the structure and taxonomic diversity of plastisphere microbial communities.
Following the initial reports of microorganisms on plastic fragments in the North Atlantic in the early 1970s8,9, a 25 year hiatus followed until Dang and Lovell19 explored initial stages of biofilm formation (24–72 hours) on plastic plates in marine waters. These biofilms were dominated by alphaproteobacteria, in particular Roseobacter spp. Similar short-term exposure experiments (up to 36 hours) were then undertaken in Korean harbour waters20, comparing communities present on acryl with those on glass and steel coupons. Molecular analysis of bacterial 16S rRNA genes suggested successional changes in community structure, with some taxa common across multiple surfaces, whilst some taxa were found only on one substrate. A third exposure experiment in surface waters in the China Sea21, compared differences in microbial communities on PVC with those on glass and Plexiglass after 24- and 72-hour exposures. Sequencing of bacterial 16S rRNA genes showed primary clustering of communities with time rather than surface type, and identified seven bacterial phyla, with alphaproteobacteria (including Roseobacteria) and gammaproteobacteria most abundant. These three early studies highlighted that plastic, as with any other available substrate, in the marine environment will be colonised by diverse bacterial taxa. Furthermore, they suggest that plastic biofilm communities will not solely be comprised of bacteria (and other microorganisms) that are specific to plastic alone.
Two exposure experiments explored colonisation of environmentally-abundant plastics, namely PET (synonymous with plastic bottled drinks) and with the most abundant marine plastic: PE (used for production of plastic bags and food packaging). A six-month exposure experiment using PET in seawater22 yielded biofilms up to 90 μm thick and demonstrated a capacity for longer-term microbial survival on marine plastics. Culture-based analysis of PE-food bags submerged for 3 weeks below the seawater surface23 showed significant increases in heterotrophic bacterial numbers on PE bags over time, accompanied by corresponding decreases in PE buoyancy. This study suggests that microbial colonisation (biofouling) of PE could contribute towards transport of previously buoyant plastic from surface into deeper waters. As microbial colonisation of plastics will be widespread in marine environments, this mechanism may partly explain the recent and perhaps surprising finding that global loads of buoyant plastic (especially PE, PP and PS) currently present at the ocean surface are estimated to be ten of thousands of tons lower than expected from estimates of plastic loads released into open oceans14. This raises a number of intriguing questions concerning plastic-microbial interactions in marine systems, in particular, as to whether microbial biofouling contributes to plastic transport to deeper waters and sediments, analogous to the concept of marine snow24, in addition, as to whether microorganisms may degrade either the plastics and/or plastic-adsorbed co-pollutants, as we have hypothesised previously25.
Following these earlier studies, there is now considerable interest in characterising the microbial communities present on marine plastic surfaces. In the first study exploring microbial community composition on plastic fragments recovered from the open ocean, Zettler and colleagues26 coined the term ‘plastisphere’ to define communities of microorganisms colonising plastic in the environment. They used 454-pyrosequencing of bacterial 16S rRNA genes amplified from plastic fragments from the Atlantic Ocean and showed that the plastisphere of just six different fragments (3 each of PE and PP) were comprised of over 1,000 different operational taxonomic units (OTU, analogous to species). Comparing these communities with those in the seawater from which the plastics were recovered, identified a number of species detected only on the plastic surface, including the cyanobacterium Phormidium; Pseudoalteromonas spp., often associated with marine algae and also members of the Hyphomonodaceae, which possess prosthecate filaments facilitating surficial attachment. It is unknown whether abundance of these taxa reflects a ‘preference’ for plastic as a substrate, or alternatively whether they would colonise other substrates in marine waters. Intriguingly, the authors highlighted the presence of cells in ‘pits’ in the plastic, using electron microscopy speculating this is suggestive of microbial degradation of plastic surfaces.
Two other recent studies have utilised electron microscopy to investigate microbial diversity on marine plastics. Firstly, rod-shaped bacteria and pennate diatoms were shown to be most prevalent on plastic fragments from the North Pacific gyre27. Analysis of plastic fragments recovered from seawater around Australia28 similarly revealed a morphologically diverse array of microorganisms, especially of diatoms, but also of other microbial eukaryotes, including coccolithophores, dinoflagellates and fungi. Assorted marine invertebrates were also identified suggesting plastics may serve as a ‘raft’ for complex multitrophic communities. This study also identified the presence of ‘pits’ and ‘grooves’ in plastic surfaces, again highlighting an urgent need for research to provide definitive evidence of marine plastic biodegradation.
We recently identified several further challenges as we investigate plastisphere microbial ecology. Firstly, we showed that the structure and composition of plastisphere microbial communities varies both seasonally and with geographical location29. In this research, PET drinking water bottles were attached onto buoys at three locations in the North Sea in winter, spring and summer. Seasonal differences in plastisphere communities were observed, with higher relative abundance of photosynthetic brown algae and cyanobacteria on bottles exposed during summer months, while winter communities were dominated by heterotrophic bacteria, including Bacteroidetes and gammaproteobacteria, in addition to photosynthetic diatoms (Synedra spp). Comparison of communities on plastic fragments from offshore waters around Northern Europe additionally demonstrated that plastisphere communities varied both with polymer type and the geographical location from which fragments were recovered. We also explored early stage microbial biofilm formation on PE microplastics (Figure 1B) within sediment (rather than pelagic) systems across sediment types7. These experiments revealed rapid successional changes in bacterial community structure on microplastics, with communities at 14 days dominated by Arcobacter and Colwellia spp. Interestingly, we observed convergence in the structure and composition of these plastisphere communities, while the structure of the communities in the different sediment types remained different, suggesting possible selection for these two genera in the PE plastisphere. While both Arcobacter and Colwellia have been associated with hydrocarbon degradation, we can, at this stage, only speculate on whether these bacteria are involved in PE biodegradation.
Much of the research undertaken thus far has been partly motivated by an interest in identifying evidence of biodegradation of marine plastics or, at least, has discussed its potential. However, an alternative impact of microbial plastic colonisation has also been highlighted by the observation of a high relative abundance of Vibrio spp. on plastic fragments recovered from the North Atlantic26. This observation, together with a report of Escherichia coli on plastic (and also seaweed) in beach waters suggests that plastic could serve as a vector for the transport of pathogenic microorganisms into marine fauna30.
Outlook
To understand the diversity and ecology of the microbial plastisphere, we will need to consider the likelihood that each individual plastic fragment present within the marine environment will have been subject to complex dynamic changes in its biofilm community structure and ecology, during the myriad of divergent routes, transitioning across and between the terrestrial, freshwater and marine environment. Along that journey, each plastic fragment may develop into a unique environmental microhabitat, shaped by both travel through differing physical–chemical environments, but additionally, due to adsorption of organic and inorganic chemicals and by the colonisation of diverse microorganisms. We conclude by highlighting five key questions and challenges for this emergent research topic:
-
Do plastic surfaces select specifically for particular microbial species and/or alternatively, are plastic surfaces just primarily a convenient substrate for colonisation of microbial phototrophs driving development of multi-trophic complex biofilm assemblages?
-
Does microbial biofilm formation (biofouling) drive reductions in plastic buoyancy leading to plastic transport to the deeper ocean and into sediments?
-
How do the structure and function of plastisphere microbial communities change during transport from terrestrial environments, via freshwater, into marine waters and additionally into benthic environments?
-
Does microbial degradation of plastic (and bioplastic) and of adsorbed co-pollutants occur in marine environments and if so over what timescales? What are the ecological constraints upon plastic and co-pollutant degradation?
-
Are plastic surfaces a potential site for accumulation of pathogenic microorganisms that can be ingested by and impact upon marine fauna?
References
[1] Andrady, A.L. and Neal, M.A. (2009) Applications and societal benefits of plastic. Phil Trans R Soc B 364, 1977–1984.| Applications and societal benefits of plastic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpt1Skt78%3D&md5=f683cb29bff2bcd25539ff3a5a7b7a7cCAS | 19528050PubMed |
[2] UNEP (2011) Plastic debris in the ocean. In: Year Book: Emerging issues in our global environment. Chapter 3. Nairobi, Kenya, United Nations Environment Programme.
[3] Rochman, C.M. et al. (2013) Policy: classify plastic waste as hazardous. Nature 494, 169–171.
| Policy: classify plastic waste as hazardous.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXis1ajt7k%3D&md5=1852f3991c41164d4ce30778a154c9d3CAS | 23407523PubMed |
[4] Plastics Europe (2013) Plastics – the facts 2013. An analysis of European latest plastics production, demand and waste data. http://www.plasticseurope.org/Document/plastics-the-facts-2013.aspx
[5] UNEP (2014) Valuing plastics: the business case for measuring, managing and disclosing plastic use in the consumer goods industry. United Nations Environment Programme, Nairobi, Kenya.
[6] Plastics and Chemical Industries Association (2012) 2011–12 National Plastics Recycling Survey final report. http://www.pacia.org.au/reports/plasticsrecyclingsurvey
[7] Harrison, J.P. et al. (2014) Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 14, 232.
| Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms.Crossref | GoogleScholarGoogle Scholar | 25245856PubMed |
[8] Carpenter, E.J. and Smith, K.L. (1972) Plastics on the Sargasso Sea surface. Science 175, 1240–1241.
| Plastics on the Sargasso Sea surface.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE387hvVaqsQ%3D%3D&md5=8d216bb2218afd9daa2e425b59170b54CAS | 5061243PubMed |
[9] Carpenter, E.J. et al. (1972) Polystyrene spherules in coastal waters. Science 178, 749–750.
| Polystyrene spherules in coastal waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXkslGjsQ%3D%3D&md5=2925cc1f06ea1d60f833431754af42e9CAS | 4628343PubMed |
[10] Teuten, E.L. et al. (2009) Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2027–2045.
| Transport and release of chemicals from plastics to the environment and to wildlife.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpt1Skt7o%3D&md5=ff1e7b2117c596f8d6a6800e20d3cb82CAS | 19528054PubMed |
[11] Colton, J.B. et al. (1974) Plastic particles in surface water of the Northwestern Atlantic. Science 185, 491–497.
| Plastic particles in surface water of the Northwestern Atlantic.Crossref | GoogleScholarGoogle Scholar | 17830390PubMed |
[12] Thompson, R.C. et al. (2004) Lost at sea: where is all the plastic? Science 304, 838.
| Lost at sea: where is all the plastic?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVSntbg%3D&md5=fbc13ee468f0b829d2180a4dc592a454CAS | 15131299PubMed |
[13] Barnes, D.K.A. et al. (2009) Accumulation and fragmentation of plastic debris in global environments. Phil Trans R Soc B 364, 1985–1998.
| Accumulation and fragmentation of plastic debris in global environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpt1Skt7w%3D&md5=f394d8e26b10026991b71f224e8f1eb2CAS |
[14] Cózar, A. et al. (2014) Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 111, 10239–10244.
| Plastic debris in the open ocean.Crossref | GoogleScholarGoogle Scholar | 24982135PubMed |
[15] Law, K.L. et al. (2010) Plastic accumulation in the North Atlantic subtropical gyre. Science 329, 1185–1188.
| Plastic accumulation in the North Atlantic subtropical gyre.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtV2hsr7J&md5=a4d0b566d453938d5386b2d1d2fb7065CAS | 20724586PubMed |
[16] Law, K.L. et al. (2014) Distribution of surface plastic debris in the eastern Pacific ocean from an 11-year data set. Environ. Sci. Technol. 48, 4732–4738.
| Distribution of surface plastic debris in the eastern Pacific ocean from an 11-year data set.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXls1Krtbg%3D&md5=d0eb031a0af2c72422dc82dd11daf704CAS | 24708264PubMed |
[17] Derraik, J.G.B. (2002) The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull. 44, 842–852.
| The pollution of the marine environment by plastic debris: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmvVWns78%3D&md5=706b0bb33b1a5fb465e1d946d50c6bcfCAS |
[18] Reisser, J. et al. (2013) Marine plastic pollution in waters around Australia: characteristics, concentrations, and pathways. PLoS ONE 8, e80466.
| Marine plastic pollution in waters around Australia: characteristics, concentrations, and pathways.Crossref | GoogleScholarGoogle Scholar | 24312224PubMed |
[19] Dang, H. and Lovell, C.R. (2000) Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Appl. Environ. Microbiol. 66, 467–475.
| Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhtFertbY%3D&md5=5e3b2464709e47071ddeb3ff746b098cCAS | 10653705PubMed |
[20] Lee, J.-W. et al. (2008) Bacterial communities in the initial stage of marine biofilm formation on artificial surfaces. J. Microbiol. 46, 174–182.
| Bacterial communities in the initial stage of marine biofilm formation on artificial surfaces.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovV2qsb4%3D&md5=d7243f06b0f9f344b36bd0d69dccee27CAS | 18545967PubMed |
[21] Dang, H. et al. (2008) Cross-ocean distribution of Rhodobacterales bacteria as primary surface colonizers in temperate coastal marine waters. Appl. Environ. Microbiol. 74, 52–60.
| Cross-ocean distribution of Rhodobacterales bacteria as primary surface colonizers in temperate coastal marine waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsVGlsQ%3D%3D&md5=6f1b7027ffd3d80a789931894827c91cCAS | 17965206PubMed |
[22] Webb, H.K. et al. (2009) Poly(ethylene terephthalate) polymer surfaces as a substrate for bacterial attachment and biofilm formation. Microbes Environ. 24, 39–42.
| Poly(ethylene terephthalate) polymer surfaces as a substrate for bacterial attachment and biofilm formation.Crossref | GoogleScholarGoogle Scholar | 21566352PubMed |
[23] Lobelle, D. and Cunliffe, M. (2011) Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 62, 197–200.
| Early microbial biofilm formation on marine plastic debris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVSqsbg%3D&md5=0608db18753d18e55fd2ef0da179a26aCAS | 21093883PubMed |
[24] Alldredge, A.L. and Silver, M.W. (1988) Characteristics, dynamics and significance of marine snow. Prog. Oceanogr. 20, 41–82.
| Characteristics, dynamics and significance of marine snow.Crossref | GoogleScholarGoogle Scholar |
[25] Harrison, J.P. et al. (2011) Interactions between microplastics and microorganisms: a call for research. Mar. Technol. Soc. J. 45, 12–20.
| Interactions between microplastics and microorganisms: a call for research.Crossref | GoogleScholarGoogle Scholar |
[26] Zettler, E.R. et al. (2013) Life in the ‘plastisphere’: microbial communities on plastic marine debris. Environ. Sci. Technol. 47, 7137–7146.
| 1:CAS:528:DC%2BC3sXptFeht7w%3D&md5=00b75d274f084c1ee54541671e6297dfCAS | 23745679PubMed |
[27] Carson, H.S. et al. (2013) The plastic-associated microorganisms of the North Pacific Gyre. Mar. Pollut. Bull. 75, 126–132.
| The plastic-associated microorganisms of the North Pacific Gyre.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlClu7zN&md5=3f356d3b70a0fafaac491b18716959ffCAS | 23993070PubMed |
[28] Reisser, J. et al. (2014) Millimeter-sized marine plastics: a new pelagic habitat for microorganisms and invertebrates. PLoS ONE 9, e100289.
| Millimeter-sized marine plastics: a new pelagic habitat for microorganisms and invertebrates.Crossref | GoogleScholarGoogle Scholar | 24941218PubMed |
[29] Oberbeckmann, S. et al. (2014) Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microbiol. Ecol. , .
| Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters.Crossref | GoogleScholarGoogle Scholar | 25109340PubMed |
[30] Quilliam, R.S. et al. (2014) Seaweeds and plastic debris can influence the survival of faecal indicator organisms in beach environments. Mar. Pollut. Bull. 84, 201–207.
| Seaweeds and plastic debris can influence the survival of faecal indicator organisms in beach environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXovVKgsb4%3D&md5=5d566b78c561f87e29758273d7fac616CAS | 24878304PubMed |
Biographies
Mark Osborn is an Environmental Microbiologist and Associate Professor in the School of Applied Sciences at RMIT University, Melbourne, Australia studying the ecology, diversity, function and biotechnological capabilities of microorganisms within natural and urban environments. His research investigates how microorganisms respond to and mitigate against the impact of anthropogenically sourced environmental pollutants, including hydrocarbons, plastics, heavy metals, nitrogenous fertilisers and greenhouse gases. Mark has published over 70 peer-reviewed articles and reviews and is the editor of a textbook entitled Molecular Microbial Ecology. He is the Section Editor (for Microbiology) of the Open Access journal Cogent Biology and a member of the editorial board of the ISME Journal. Mark also tweets (@MicrobialLife) on eclectic aspects of current microbiology research.
Slobodanka Stojkovic is an Algal Ecophysiologist, Microbial Ecologist and Postdoctoral Fellow in the School of Applied Sciences at RMIT University. Her research studies the ecology and functioning of aquatic microorganisms. Her research focuses on impacts of pollutants and environmental factors especially in relation to climate change impacts on the performance of aquatic organisms, and phototrophs, in particular. Slobodanka has worked on a range of aquatic ecosystems, both nationally and internationally, resulting in over 15 peer-reviewed publications, and a number of industry reports.


