Antimicrobial susceptibility testing guidelines as a necessary tool to guide chemotherapeutic interventions in aquaculture
Ron A MillerFood and Drug Administration
Center for Veterinary Medicine
Office of New Animal Drug Evaluation
7500 Standish Place
Rockville, MD 20855, USA
Tel: +1 240 402 0795
Email: ron.miller@fda.hhs.gov
Microbiology Australia 37(3) 104-107 https://doi.org/10.1071/MA16036
Published: 10 August 2016
The selection of chemotherapy in aquatic animal medicine is not as straightforward as one might believe. A multitude of factors can impact effectiveness in situ. Some of these factors include the pathogen(s) present and their antimicrobial susceptibility, site(s) of infection, timing of treatment, host health/disease status, dose and regimen, water salinity, and water temperature. This article will focus on the first of these factors, and how susceptibility testing of target pathogen(s) can be used to both inform therapy decisions and assist in compliance with principles of prudent and judicious use.
Antimicrobials have long been used to relieve pain and suffering and to control infections in food-producing animals, including fish. The safe and prudent prescription of effective antimicrobials by veterinarians to treat aquatic animals has contributed immensely to the increased food production capacity of aquaculture worldwide. However, the use of antimicrobials in aquaculture is not without risk. The American Veterinary Medical Association has published educational materials for veterinarians which describe prudent and judicious use guidelines for antimicrobials in aquaculture1. Antimicrobial-resistant bacteria, pathogenic to animals and humans, have been found in and near fish and shellfish farms where medicated feed has been administered2–7. In addition, fish have been implicated as potential reservoirs of zoonotic pathogens8, some of which may carry resistance genes including extended-spectrum beta-lactamases9. Cabello suggested the unrestricted use of antimicrobials in aquaculture in any country has the potential to affect human and animal health on a global scale, and that this problem should be dealt with through unified local and global preventive strategies10.
One mitigation step that can be used to minimise the dissemination of antimicrobial resistance is to make every attempt to ensure that chemotherapeutic intervention is necessary. Part of this decision process should be to determine the pathogen’s susceptibility to antimicrobials approved for use in the target animal species. Whenever possible it is important to use internationally standardised antimicrobial susceptibility testing (AST) methods. The most widely used AST methods are those published by the Clinical and Laboratory Standards Institute (CLSI). The CLSI has published two guidelines: VET03-A – Methods for Antimicrobial Disk Susceptibility Testing of Bacteria Isolated from Aquatic Animals11, and VET04-A2 – Methods for Broth Dilution Susceptibility Testing of Bacteria Isolated from Aquatic Animals12, as well as an informational supplement, the VET03/VET04-S2 – Performance Standards for Antimicrobial Susceptibility Testing of Bacteria Isolated from Aquatic Animals13. These documents provide detailed standardised AST methods (and recommended non-standard modifications for fastidious pathogens), quality control parameters, and interpretive categories for aquatic animal pathogens at incubation temperatures of 18°C, 22°C, 28°C and 35°C. As global consensus guidelines, the quality control parameters included allow for harmonisation of AST data on an international scale. These standardised AST methods also permit performance monitoring within a lab, and development of lab-specific interpretive categories (see below) when limited data are available in the literature for a given pathogen and antimicrobial combination.
Antimicrobial susceptibility testing
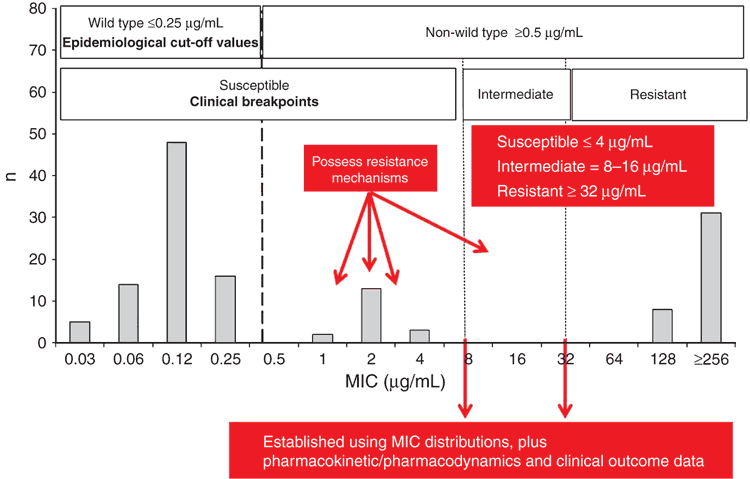
The disk diffusion method is the most commonly used AST method in aquatic animal disease diagnostic laboratories. The diameter of zone of inhibition produced by a disk impregnated with an antimicrobial and placed on an agar plate is measured to the nearest millimeter. Another very popular AST method is the broth microdilution method. This method determines minimal inhibitory concentrations (MICs) for each antimicrobial, typically in a 96-well plate format. Based on an AST result, an interpretive category is attributed to the antimicrobial and specific isolate tested. Different interpretive categories are used by diagnostic laboratories dependent upon whether results are needed to inform treatment, in which case clinical breakpoints should be used; if results are being used for antimicrobial resistance (genotype) surveillance, epidemiological cut-off values (ECVs) should be used (Figure 1).
|
Clinical breakpoint interpretive categories include susceptible (S), intermediate (I) and resistant (R). Susceptible is a category based on a breakpoint that implies that isolates are inhibited by usually achievable concentrations of antimicrobial when the dosage recommended to treat the site of infection is used. Intermediate is a category based on a breakpoint that includes isolates with corresponding MICs that approach usually attainable blood and tissue levels, and for which response rates may be lower than for susceptible isolates. Resistant is a category based on a breakpoint that implies that isolates are not inhibited by usually achievable concentrations of the antimicrobial with normal dosage schedules, and/or that demonstrate MICs that fall in the range in which specific microbial resistance mechanisms are likely, and clinical effectiveness of the antimicrobial against the isolate has not been reliably shown in treatment studies. When detection of an emerging genotype is the primary goal, an ECV which is a zone diameter or MIC that separates microbial populations into those with and without acquired and/or mutational resistance genes is used. Wild type (WT) is an interpretive category based on an ECV that describes isolates with no mechanisms of acquired resistance for the antimicrobial. Non-wild type (NWT) is a category based on an ECV that describes isolates with presumed or known mechanisms of acquired resistance and/or decreased susceptibility for the antimicrobial14.
Incorporation of antimicrobial susceptibility testing into the diagnostic process
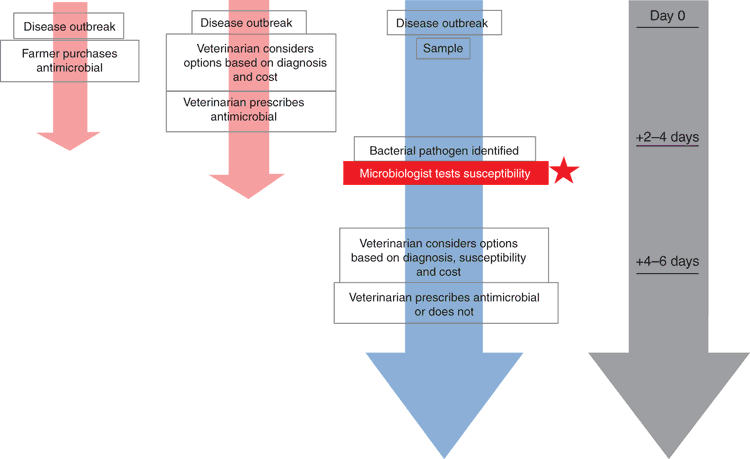
The OIE’s Aquatic Animal Health Code emphasised the importance of clinical assessment, pathogen identification, and susceptibility testing before initiation of therapy15. Too often, particularly in developing countries, antimicrobials are obtained over-the-counter by fish farmers before a proper diagnosis can be made. In many countries where a veterinarian–client–patient relationship does exist, a veterinarian may choose to prescribe an antimicrobial without a complete knowledge of the pathogen(s) present and their susceptibility (or lack thereof) to the prescribed antimicrobial. It is understood that the pressure placed on veterinarians to intervene quickly is critical, particularly in aquaculture where losses can be sudden and devastating. However, ideally, to be in full compliance with the prudent and judicious use principles outlined by the AVMA and the OIE, additional steps should be taken prior to prescribing a medicated feed to an entire animal population (Figure 2). These include isolation and identification of the pathogen, assessment of susceptibility to antimicrobials of interest, and prescription of an approved antimicrobial at a dose appropriate for a given situation. Alternatively, improved husbandry practices may supplant the need for intervention with a medicated feed. To illustrate this approach and demonstrate how AST can be used as a helpful tool in intervention decisions, two scenarios are provided below.
A rainbow trout case from a large farm in Europe is found through culture and identification, to have succumbed to furunculosis caused by Aeromonas salmonicida. One scenario may include a need to quickly find the best course of action to prevent further losses on the farm. In the country of origin, oxolinic acid is approved by the regulatory body to treat furunculosis in trout. Upon completion of AST testing in accordance with CLSI guidelines VET03-A11 and VET04-A212, the isolate has an oxolinic acid zone diameter of 30 mm and MIC of 0.12 µg/mL. What does this mean? After consulting the clinical breakpoint table (table 12) in CLSI’s VET03/VET04-S2 supplement, we find this isolate is considered susceptible to oxolinic acid. The veterinarian should consider administration of oxolinic acid in feed to the affected population. Additional improved husbandry interventions may also be warranted.
Consider a second scenario where an Atlantic salmon is determined through culture and identification to have died from enteric redmouth disease caused by Yersinia ruckeri. After conducting standardised AST the isolate is found to have a florfenicol zone diameter of 29 mm, and an MIC of 4 µg/mL. The VET03/VET04-S2 informational supplement13 does not provide clinical breakpoints or ECVs for Y. ruckeri. The veterinarian is forced to consult available literature on Y. ruckeri susceptibility to florfenicol, and prior experience to determine the best course of action.
In the latter scenario, there is an opportunity to develop laboratory-specific interpretive categories that may be reliable as long as the AST methods used do not change and quality control parameters are maintained. However, when laboratory-independent interpretive categories are available13 these should be used whenever appropriate.
Conclusions
Understanding how AST can be used as a tool to guide clinical decision making in aquatic animal medicine is an emerging issue. Knowledge of and use of available standardised AST methods and interpretive categories will improve data quality, international harmonisation, and encourage prudent prescribing practices. Application of a suitable interpretive category (i.e. clinical breakpoint or ECV) for a given situation (i.e. clinical intervention or antimicrobial resistance surveillance) is also critical to minimise antimicrobial resistance development and maintain therapeutic effectiveness now and in the future.
References
[1] AVMA (2006) Judicious use of antimicrobials for aquatic veterinarians. American Veterinary Medical Association. https://www.avma.org/KB/Policies/Pages/Judicious-Use-of-Antimicrobials-for-Treatment-of-Aquatic-Animals-by-Veterinarians.aspx[2] Damir, K. et al. (2013) Occurrence, characterization and antimicrobial susceptibility of Vibrio alginolyticus in the Eastern Adriatic Sea. Mar. Pollut. Bull. 75, 46–52.
| Occurrence, characterization and antimicrobial susceptibility of Vibrio alginolyticus in the Eastern Adriatic Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVSnsLnO&md5=afe6f895e853f487e6995b96609a5c6eCAS | 24012112PubMed |
[3] Miranda, C.D. et al. (2013) Role of shellfish hatchery as a reservoir of antimicrobial resistant bacteria. Mar. Pollut. Bull. 74, 334–343.
| Role of shellfish hatchery as a reservoir of antimicrobial resistant bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFChu7nP&md5=f71dcc7f607701bc64749150b9c3f324CAS | 23880028PubMed |
[4] Huys, G. et al. (2001) Comparison of the antimicrobial tolerance of oxytetracycline-resistant heterotrophic bacteria isolated from hospital sewage and freshwater fishfarm water in Belgium. Syst. Appl. Microbiol. 24, 122–130.
| Comparison of the antimicrobial tolerance of oxytetracycline-resistant heterotrophic bacteria isolated from hospital sewage and freshwater fishfarm water in Belgium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltFertL8%3D&md5=f1fdb5eadf4d752639462d9f7415f6e4CAS | 11403391PubMed |
[5] Guardabassi, L. et al. (2000) Increase in the prevalence of oxolinic acid resistant Acinetobacter spp. observed in a stream receiving the effluent from a freshwater trout farm following the treatment with oxolinic acid-medicated feed. Aquaculture 188, 205–218.
| Increase in the prevalence of oxolinic acid resistant Acinetobacter spp. observed in a stream receiving the effluent from a freshwater trout farm following the treatment with oxolinic acid-medicated feed.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktV2ksbk%3D&md5=b2c89c8bf3c53928194940e1a823f6acCAS |
[6] Sathiyamurthy, K. et al. (1997) Antibiotic-resistant Vibrio cholerae in Parangipettai coastal environs, south east India. Microb. Drug Resist. 3, 267–270.
| Antibiotic-resistant Vibrio cholerae in Parangipettai coastal environs, south east India.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXls1yltr4%3D&md5=9aebc9cc93e373709cd2665158ddfebaCAS | 9270997PubMed |
[7] Husevag, B. and Lunestad, B.T. (1995) Presence of the fish pathogen Aeromonas salmonicida and bacteria resistant to antimicrobial agents in sediments from Norwegian fish farms. Bull. Eur. Assoc. Fish Pathol. 15, 17–19.
[8] Haenen, O.L.M. et al. (2013) Bacterial infections from aquatic species: potential for and prevention of contact zoonoses. Rev. Sci. Tech. 32, 497–507.
| 1:STN:280:DC%2BC2cvmtFOjtg%3D%3D&md5=823ffb474e5a6ce52a5ff9945943cb33CAS |
[9] Sousa, M. et al. (2011) Gilthead seabream (Sparus aurata) as carriers of SHV-12 and TEM-52 extended –spectrum beta-lactamases-containing Escherichia coli isolates. Foodborne Pathog. Dis. 8, 1139–1141.
| Gilthead seabream (Sparus aurata) as carriers of SHV-12 and TEM-52 extended –spectrum beta-lactamases-containing Escherichia coli isolates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1ajsbjK&md5=3f561bc3cf5aa281b8b3d40fce190efbCAS | 21657938PubMed |
[10] Cabello, F.C. (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ. Microbiol. 8, 1137–1144.
| Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnsVCks7Y%3D&md5=441d12ce160392d9ac2e8b2f5ccdc74cCAS | 16817922PubMed |
[11] CLSI (2006) Methods for antimicrobial disk susceptibility testing of bacteria isolated from aquatic animals; Approved Guideline. CLSI document VET03-A. Wayne, PA.
[12] CLSI (2014) Methods for broth dilution susceptibility testing of bacteria Isolated from aquatic animals; Approved Guideline, 2nd edn. CLSI document VET04-A2. Wayne, PA.
[13] CLSI (2014) Performance standards for antimicrobial susceptibility testing of bacteria isolated from aquatic animals; Approved Supplement, 2nd edn. CLSI document VET03/VET04-S2. Wayne, PA.
[14] CLSI (2011) Generation, presentation, and application of antimicrobial susceptibility test data for bacteria of animal origin; a report. CLSI document VET05-R. Wayne, PA.
[15] World Organisation for Animal Health (2015) Aquatic Animal Health Code. http://www.oie.int/en/international-standard-setting/aquatic-code/access-online/
Biography
Dr Ron Miller is a regulatory review microbiologist in the U.S. FDA – Center for Veterinary Medicine’s Office of New Animal Drug Evaluation. He is the Chair of the Clinical and Laboratory Standards Institute (CLSI) – Veterinary Antimicrobial Susceptibility Testing Subcommittee’s Working Group on Aquatic Animals. His interests include susceptibility testing method development, antimicrobial resistance surveillance tools, and aquatic microbiology.