Necrotic disease in bivalve larval cultures
Tzu Nin Kwan A , Christopher Bolch B and John Bowman CA Institute for Marine and Antarctic Studies, University of Tasmania, 21 Nubeena Crescent, Taroona, Tas. 7053, Australia. Tel: +61 3 6226 8270, Email: Tzu.Kwan@utas.edu.au
B Institute for Marine and Antarctic Studies, University of Tasmania, Science Building, Newham, Tas. 7248, Australia. Tel: +61 3 6324 3815, Email: chris.bolch@utas.edu.au
C Tasmanian Institute of Agriculture, University of Tasmania, Life Sciences Building, College Road, Sandy Bay, Tas. 7005, Australia. Tel: +61 3 6226 6380, Email: john.bowman@utas.edu.au
Microbiology Australia 38(3) 131-133 https://doi.org/10.1071/MA17048
Published: 9 August 2017
The health of marine bivalve larvae is greatly affected by bacteria in the environment particularly when reared in marine hatcheries. This is generally because high stocking densities resulting in high organic loads of both food and faeces, can support increased bacterial growth and biomass levels. Increased bacterial load can lead to larval disease referred to as bacillary necrosis (BN) leading in turn to rapid larval mortality and loss of production. Despite more than 50 years since the first detailed description of BN, we still do not fully understand its causes and mechanisms. Through the manipulation of a model larval culture of the Australian blue mussels (Mytilus galloprovincialis), we determined that BN is linked with rapid and systematic changes in the bacterial community.
Early investigation of larval mortality in bivalve larval cultures in the 1950s reported mortality associated with infection by gram negative bacilli that necrotised larval tissues, leading to the descriptive term bacillary necrosis (BN)1. The disease is capable of causing total collapse of larval cultures (larval crash) in a period of 24–48 hours and is today, the most prevalent hatchery disease worldwide, affecting more than 20 bivalve species. Whilst it is difficult to quantify the impact of BN in shellfish hatcheries, frequent recurrence can severely impact hatchery production with repercussions often felt throughout the supply chains.
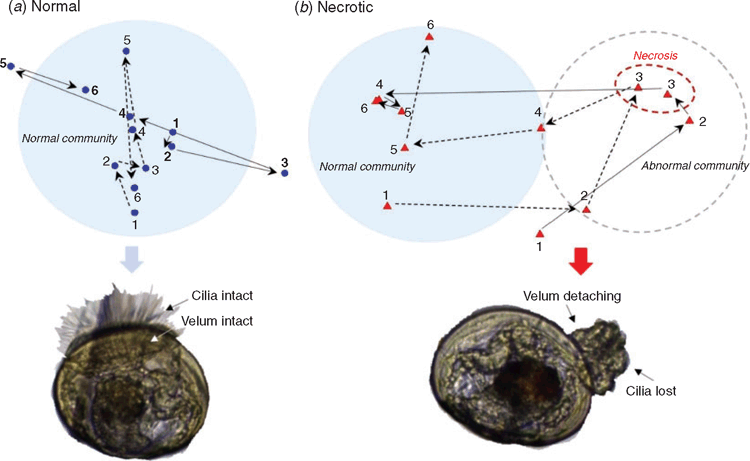
The prevailing view that BN is an opportunistic disease leads to the emphasis on sound husbandry practices primarily to reduce excess build-up of organic matter. However, whether and how enriched organic conditions are linked with development of BN is unclear. Efforts to study BN have also been complicated by the unpredictable nature of the outbreaks. To address this problem, we deliberately overfed a series of identical small-scale larval cultures with microalgae to create an environment that would increase the incidence of the disease. In cultures that developed mass mortalities, automated ribosomal intergenic spaces analysis (ARISA) demonstrated that BN involves rapid and systematic changes in the bacterial community, firstly in the seawater, then rapidly proceeding to the larvae as the disease progresses and necrosis occurs (Figure 1). This study shows that, at least within the system analysed here, BN is a condition of abnormal changes in seawater-associated communities that are capable of affecting the larvae, suggestive of seawater-to-larvae infectivity. The similarity of bacterial communities in seawater and larvae at the onset of mortality suggest swamping by outgrowth of particular bacteria. Bacterial diversity examination using Illumina MiSeq sequencing of 16S rRNA amplicons showed that mortality in the model systems was linked with a bacterial community increasingly dominated by Psychroserpens, Polaribacter, Marinomonas, and members of the Candidatus phylum Gracilibacteria.
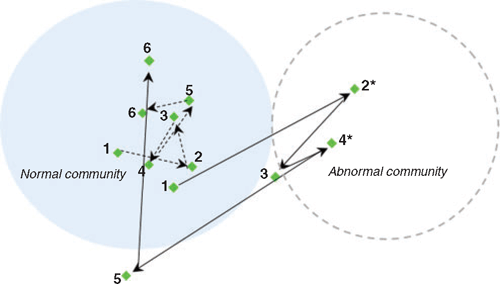
The observation that BN did not occur in all overfed cultures suggests variability in causation made it difficult to detect and control in small-scale larval cultures. In one instance, a replicate culture in which the initial development of BN was observed, actually had the first rearing day of normal seawater community as starting inoculum, but rapidly deviated to abnormal community in the next 24 h period (Figure 2). This suggests the seawater community characterised using ARISA fingerprinting technique may have missed the low concentration of genotypes that later emerge and drive the community to become abnormal. It appears that seawater is an important reservoir of diverse bacteria that play a critical role in the variability of BN. The link between bacterial diversity and the sporadic nature of BN is not easily established, partly due to technical limitations in resolving strain-level variation occurring in low concentration.
|
Currently, commercial hatchery operators still lack specific and effective means to mitigate bacillary necrosis because of the overall lack of understanding of its development process. Water changes are an important part of larval rearing activity, mainly because they help reduce organic matter build up in cultures therefore controlling the bacterial concentrations. However, there is no direct demonstration of how water changes can help mitigate BN. This study shows 48 h interval water changes, currently regarded as a common practice for static culture systems, can be effective if carried out before abnormal community changes are detected in the larvae (Figure 2). In cultures that suffered more rapid BN (such as Figure 1b), water changes did not alter the trajectory of abnormal communities.
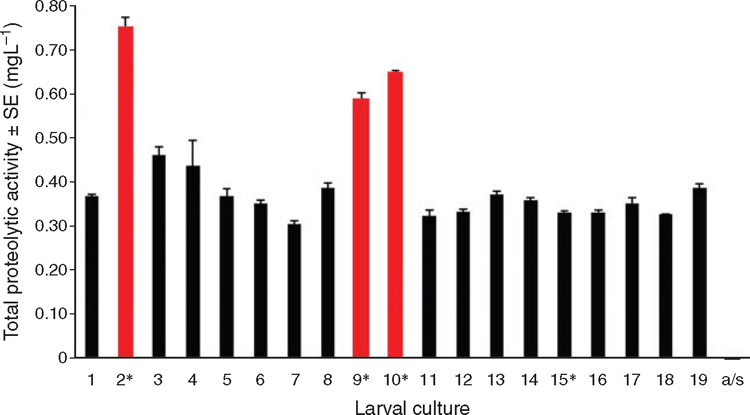
Challenge bioassay studies demonstrate that necrotic properties of BN can be attributed to proteolytic activity of bacteria2. However, it is unclear how proteolytic activity is involved with BN in larval culture. A different larval culture experiment observed high microbial proteolytic activity in 3 of 4 larval cultures suffering mass mortalities (Figure 3). This suggests microbial proteolytic activity is an important disease mechanisms given that protease production in bacterial extracellular products (ECPs) is common in some seawater bacteria (such as Polaribacter and Marinomonas detected in the earlier study) and has been demonstrated to be the major virulence factors of multiple pathogenic Vibrio strains associated with BN3. However, the lack of association of proteolytic activity in one of the BN affected larval cultures (i.e. culture 15 of Figure 3) is interesting and may suggest diversity in bacterial community and in the mechanisms that lead to mortality. More work is necessary to confirm this preliminary association, particularly monitoring how seawater proteolytic activity changes with bacterial community shifts and leads/does not lead to mortality. Such work may demonstrate the potential of using protease assays as a method to assess the risk of bacillary necrosis. Once validated, protease assays could be a useful detection method to complement molecular and culturing techniques.
Based on the model larval cultures, we were able to demonstrate that BN has links with systematic bacterial community changes suggestive of a seawater-to-larvae infection. However, how this model understanding translates to commercial scale cultures requires more research. This study also described the potential of protease monitoring to aid future BN studies.
References
[1] Tubiash, H.S. et al. (1965) Bacillary necrosis a disease of larval and juvenile bivalve mollusks. 1. etiology and epizootiology. J. Bacteriol. 90, 1036.| 1:STN:280:DyaF28%2FkvVyisg%3D%3D&md5=cb9e3a4e3185fd72bf5c29c700c4f4bfCAS |
[2] Hasegawa, H. et al. (2009) Virulence of metalloproteases produced by Vibrio species on Pacific oyster Crassostrea gigas larvae. Dis. Aquat. Organ. 85, 123–131.
| Virulence of metalloproteases produced by Vibrio species on Pacific oyster Crassostrea gigas larvae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpslOqt7s%3D&md5=fac218cc9784bc1b33bbbf54700d2befCAS |
[3] Saulnier, D. et al. (2010) A large-scale epidemiological study to identify bacteria pathogenic to Pacific oyster Crassostrea gigas and correlation between virulence and metalloprotease-like activity. Microb. Ecol. 59, 787–798.
| A large-scale epidemiological study to identify bacteria pathogenic to Pacific oyster Crassostrea gigas and correlation between virulence and metalloprotease-like activity.Crossref | GoogleScholarGoogle Scholar |
Biographies
Tzu Nin Kwan is a PhD student and research assistant at the Institute of Marine and Antarctic Studies at the University of Tasmania. His research includes molecular microbiology of bivalve hatchery with interests in infection and pathogenesis.
Christopher Bolch is a Senior Lecturer at the Launceston laboratories of the Institute of Marine and Antarctic Studies. His research focuses on molecular detection, diversity of toxic and harmful algae and bacteria in marine and freshwater systems and managing their impact on marine and freshwater industries.
John Bowman is an Associate Professor at the Tasmanian Institute of Agriculture of University of Tasmania. His focused interests are on cold adapted bacteria, the food-borne pathogens, aquaculture associated microbiology, soil microbiology relevant to agricultural, and microbial systematics in general.