The future of phage clinical trials in Australia
Keith PotentGriffith University
PO Box 214
Qld 4222, Australia
Tel: +61 7 5536 1133
Fax: +61 7 5594 0185
Email: k.potent@griffith.edu.au
Microbiology Australia 40(1) 16-19 https://doi.org/10.1071/MA19004
Published: 4 March 2019
Australia is well positioned to conduct clinical trials in phage-based technology. Despite challenges with translating phage therapy to mainstream medicine, our regulations are designed for safe and innovative development. Recent success indicates that Australia is ideal for conducting further phage clinical trials. There are also expert clinical research organisations and generous tax incentives.
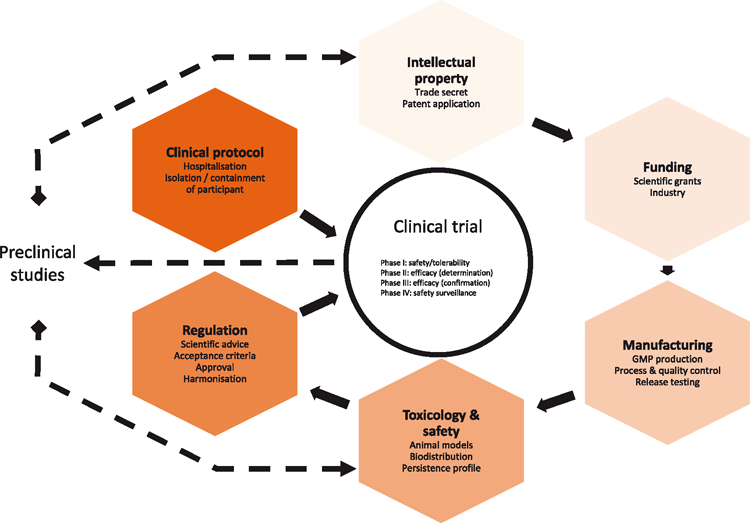
Historically, there have been barriers to translating phage therapy from ‘bench to bedside’. Some strategies for broader acceptance of phage therapy have been evaluated1–3 and the industry consensus is to gather quality clinical evidence regarding safety, tolerability, and efficacy2. As such, clinical trials (CTs) are a critical interface for translating phage therapy. The components that can influence the success of a clinical trial are depicted in Figure 1.
More broadly, the translation of phage therapy can be broken down into three main components: the phage, the CT design, and the regulations. There is ongoing debate regarding the ideal strategy for translating phage technologies. Some researchers suggest that instead of using natural phage cocktails (a mixture of numerous strains of phage targeting the same host bacterium), phage-based products such as lysins and depolymerases may be used as an alternative (and simpler) pathway to regulatory compliance4,5, while other researchers argue that, in fact, regulations should change5,6. Instead, the author proposes that the main solution to translating phage therapy is to change the CT design. This article summarises: some phage technologies; recent phage CT outcomes, the potential design for other phage-related CTs; and the process of conducting a phage CT in Australia.
Phage cocktails
Phage cocktails, rather than individual isolates, are often used to treat recalcitrant biofilms rather than individual isolates because they broaden the range of susceptible hosts and reduce the risk of replacing treated hosts with phage-resistant mutants7,8.
However, phage cocktails often raise more regulatory flags. Phage replication can result in mutant phages and there are concerns regarding potential genetic transfer of pathogenic elements, such as shiga toxin to bacteria. High-throughput sequencing may be an effective tool used during a CT to collect phage genetic data9 and enable monitoring of mutations while informing clinical researchers and regulators. In addition, this may assist with collecting pharmacokinetic and dose finding data10. Others suggest that genetically modified phages (GM-phages) may control for random genetic transfers and mutations, thus improving the chances of regulatory success.
Genetically modified phage
Over the past 30 years, phages have proven to be genetically-malleable tools11–15. New developments using genetic engineering (a.k.a. synthetic biology) to create phages12 may be relevant to the future of phage CTs. Some researchers compare GM-phages to genetically-programmable machines16 and, if gene switches are inserted, they may also reduce risks associated with uncontrolled gene transfer15. In 2018, the Nobel Prize in Chemistry for was awarded to three scientists for their work in ‘phage display of peptides and antibodies’17,18 and ‘directed evolution’19. Some researchers argue that genetic engineering of phages may secure intellectual property and in doing so increase the potential for funding CTs. Moreover, since the discovery of CRISPR/Cas9 there has been a rapid increase in the number of technologies using GM-phages20. Functions including conditional expression, conditional replication, and non-integration can be included. Although it is yet to be demonstrated, such innovation may improve the chances of regulatory approval while also providing an opportunity to create new industry for Australia.
Phage clinical trials
In the recent past, many phage therapy CTs have used traditional fixed designs and methods that have not been able to adapt their trial parameters to improve scientific precision and provide groundwork for subsequent trials. In 2009, results from a British phase I/II CT demonstrated efficacy using a single dose of a topical six-phage cocktail for antibiotic-resistant Pseudomonas aeruginosa in patients with chronic otitis. Unfortunately, many of the patients had recurrence of their infection. The authors recommended another randomised, double-blind CT with multiple doses but this trial never occurred21.
In 2017, researchers from The University of Adelaide presented promising results from a landmark phase I CT evaluating the safety and tolerability of an intranasal irrigation containing a three-phage cocktail22 targeting Staphylococcus aureus in patients with surgically-recalcitrant chronic rhinosinusitis23. Safety, tolerability, and efficacy were demonstrated, and like the otitis trial, future recommendations included longer duration of the phage therapy.
In 2019, results from the European PhagoBurn CT were published. This was the world’s first phage CT to be performed according to Good Manufacturing Practice and Good Clinical Practice. This multi-centre, randomised, controlled, double-blind phase I/II CT demonstrated tolerability of a 12-phage cocktail targeting Pseudomonas aeruginosa burn wound infections but was unable to demonstrate efficacy. Participant recruitment was slower than expected because many of the screened participants had polymicrobial infections. In addition, the bacteria isolated from participants who failed phage therapy were resistant to low phage doses. Therefore, the authors recommended increased phage dose in future to demonstrate efficacy without allowing the opportunity for phage-resistant mutants to develop24.
Adaptive design
Adaptive CT design25 may have advantages over the aforementioned traditional fixed randomised, controlled design for further development of phage technologies. Adaptive CT designs are less rigid and allow for adjustment of components such as dose, frequency, duration, allocation to different treatment arms, and sample size during the CT. The statistical modelling for adaptive CTs generally use a Bayesian inference continuous reassessment approach26, which can create updated distributions as the number of participants increases. In doing so, valid real-time data can be obtained from the smallest possible sample size without exposing participants to unnecessary risks. One drawback of this approach is that preparation of an efficient adaptive CT design may require multidisciplinary input and the use of simulation software27. Whilst this would increase lead-up costs, it often results in overall cost reduction and is an effective approach to flagging trial related issues in advance. Ultimately, adaptive designs can evolve to suit the dynamic nature of phage therapy CTs. They may also be useful in evaluating other GM-phage applications such as anticancer and gene-based therapy phage products.
Regulatory process in Australia
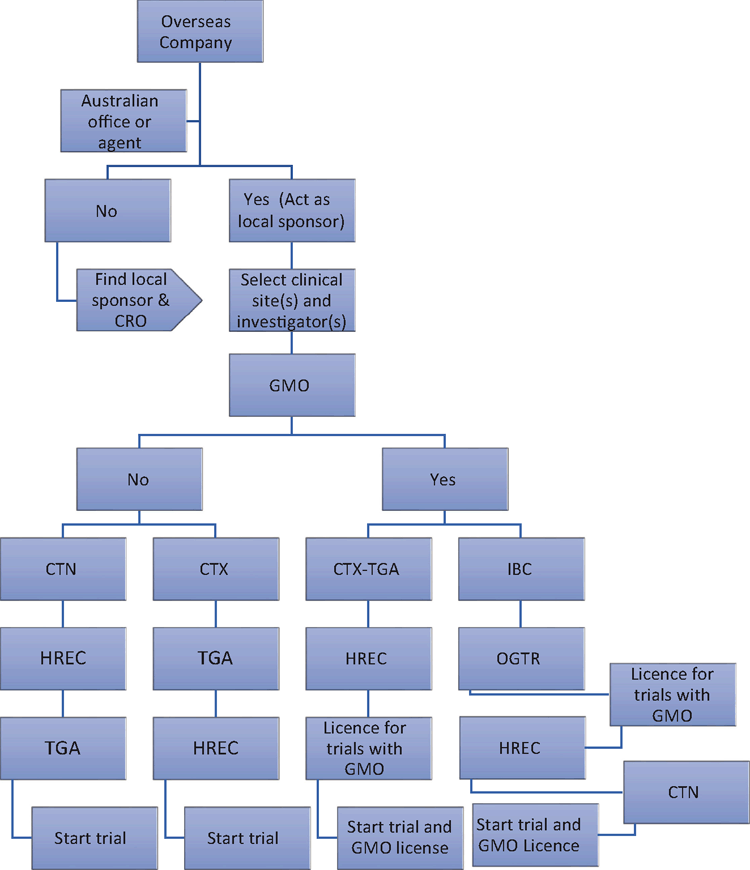
In Australia, the Therapeutic Goods Administration (TGA) has adopted many of the European Medicines Agency policies. Both natural phage and GM-phage are considered ‘gene transfer biological medicines’. The Australian regulatory process for both natural and GM-phage is shown in Figure 228.
|
There are currently no Australian-owned phage therapy companies. For CT to be undertaken in Australia, the law mandates that an overseas company must have a local representative, also known as a sponsor (a person or entity who resides in Australian and either imports, exports, or manufactures therapeutic goods). The person or entity who is in this role has primary responsibility for many of the decisions made during the planning and execution of the CT. The local sponsor will then review and select the appropriate clinical site(s) and investigator(s). The subsequent regulatory pathway will then depend on whether the phage is genetically modified. The sponsor can confirm this based on the Gene Technology Act29 and the Gene Technology Regulations30.
If the phage (product) is not a result of genetic modification, the sponsor will then decide whether it should pursue the TGA’s Clinical Trial Notification (CTN) scheme or the Clinical Trial Exemption (CTX) scheme. This should be done in collaboration with the Human Research Ethics Committee (HREC) and will depend on whether the HREC has appropriate scientific and technical expertise to assess the safety and efficacy of the product. The CTN scheme is often viewed as efficient and cost-effective compared to the comprehensive review required via the CTX pathway. However, one benefit of the CTX pathway is that once a CTX application is approved, the sponsor may conduct any number of CTs under that application without further assessment by the TGA.
For any CT, a HREC evaluates whether the risk-benefit ratio is favourable for the participant. To improve the potential benefits to the participant, the product should target a disease of unmet need and have demonstrated safety and efficacy in preclinical studies. In order to make a reasonable assessment, the HREC evaluates at least three essential documents: investigator brochure, trial protocol, and patient informed consent form. The investigator brochure should include both phage-relevant data8 and published studies that support validity (e.g. randomisation, sample size calculation, and blinded outcome assessments31,32).
Should the product be a genetically modified organism (GMO), then an alternative pathway will be required. A GMO must first be evaluated by an approved Institutional Biosafety Committee (IBC) which will determine the risk of GMO release into the environment and the suitability of the licence applicant to be accredited under the Gene Technology Act. Although this is an additional requirement, Australia’s regulators do not duplicate evaluations and the National Gene Technology Scheme33 ensures safety without stifling innovation33.
Once the Office of The Gene Technology Regulator issues a licence to the sponsor, the IBC and HREC will recommend either the CTX or CTN scheme to the TGA. This is different to the natural phage pathway and serves to eliminate regulatory duplication. Additionally, if the product is manufactured overseas, an import permit from the Department of Agriculture and Water Resources may be required. This can be evaluated through the Biosecurity Import Conditions website tool34. Participant recruitment can then commence once all appropriate approvals are obtained.
Why Australia?
Australia is considered attractive to international sponsors wishing to conduct CTs because of key financial and logistical frameworks. The research and development tax incentive scheme provides up to 43.5% reduction in a company’s income tax liability and given that the average phase I CT costs greater than $2m, the potential savings are significant. In 2013, Australia setup The National Mutual Acceptance Scheme, which is a Memorandum of Understanding between most states and territories to allow for ‘once only’ scientific and ethical review for multi-centre CTs conducted at public health organisations. This improves efficiency by reducing duplication. Readers can review the numerous clinical trial documents that the Australian government has provided online35–37.
The translation of phage therapy requires an adaptive approach. Out of the three components, CT design is easier to adjust than the phage, and much easier than the regulations. For the phage-therapy industry to reposition itself from ‘controversial’ and ‘fringe’ to mainstream, judicious use of resources in high yield trials that adapt to confounding factors should be prioritised. Nonetheless, with collaboration and experienced investigators, these hurdles can be minimised and Australia can establish itself as a good choice for phage CTs due to high quality infrastructure, efficient regulators, and the research and development tax incentive.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
The author thanks Dr Wendy Liu for her comments on the draft manuscript. This research did not receive any specific funding.
References
[1] Henein, A. (2013) What are the limitations on the wider therapeutic use of phage? Bacteriophage 3, e24872.| What are the limitations on the wider therapeutic use of phage?Crossref | GoogleScholarGoogle Scholar | 24228220PubMed |
[2] Sybesma, W. et al. (2018) Silk route to the acceptance and re-implementation of bacteriophage therapy—part II. Antibiotics (Basel) 7, 1–23.
[3] Furfaro, L.L. et al. (2018) Bacteriophage therapy: clinical trials and regulatory hurdles. Front. Cell. Infect. Microbiol. 8, 376.
| 30406049PubMed |
[4] Pires, D.P. et al. (2016) Bacteriophage-encoded depolymerases: their diversity and biotechnological applications. Appl. Microbiol. Biotechnol. 100, 2141–2151.
| Bacteriophage-encoded depolymerases: their diversity and biotechnological applications.Crossref | GoogleScholarGoogle Scholar | 26767986PubMed |
[5] Cooper, C.J. et al. (2016) Adapting drug approval pathways for bacteriophage-based therapeutics. Front. Microbiol. 7, 1209.
| Adapting drug approval pathways for bacteriophage-based therapeutics.Crossref | GoogleScholarGoogle Scholar | 27536293PubMed |
[6] Verbeken, G. et al. (2014) Call for a dedicated European Legal Framework for bacteriophage therapy. Arch. Immunol. Ther. Exp. (Warsz.) 62, 117–129.
| Call for a dedicated European Legal Framework for bacteriophage therapy.Crossref | GoogleScholarGoogle Scholar | 24500660PubMed |
[7] Fong, S.A. et al. (2017) Activity of bacteriophages in removing biofilms of Pseudomonas aeruginosa isolates from chronic rhinosinusitis patients. Front. Cell. Infect. Microbiol. 7, 418.
| Activity of bacteriophages in removing biofilms of Pseudomonas aeruginosa isolates from chronic rhinosinusitis patients.Crossref | GoogleScholarGoogle Scholar | 29018773PubMed |
[8] Abedon, S.T. (2017) Information phage therapy research should report. Pharmaceuticals 10, 43.
| Information phage therapy research should report.Crossref | GoogleScholarGoogle Scholar |
[9] Rihtman, B. et al. (2016) Assessing Illumina technology for the high-throughput sequencing of bacteriophage genomes. PeerJ 4, e2055.
| Assessing Illumina technology for the high-throughput sequencing of bacteriophage genomes.Crossref | GoogleScholarGoogle Scholar | 27280068PubMed |
[10] Parracho, H.M. et al. (2012) The role of regulated clinical trials in the development of bacteriophage therapeutics. J. Mol. Genet. Med. 6, 279–286.
| The role of regulated clinical trials in the development of bacteriophage therapeutics.Crossref | GoogleScholarGoogle Scholar | 22872803PubMed |
[11] Cooper, C.J. et al. (2018) Enhancing whole phage therapy and their derived antimicrobial enzymes through complex formulation. Pharmaceuticals 11, 34.
[12] Lemire, S. et al. (2018) Phage-based applications in synthetic biology. Annu. Rev. Virol. 5, 453–476.
| 30001182PubMed |
[13] Nicastro, J. et al. (2016) Bacteriophage Applications – Historical Perspective and Future Potential. Springer International Publishing.
[14] Nobrega, F.L. et al. (2015) Revisiting phage therapy: new applications for old resources. Trends Microbiol. 23, 185–191.
| Revisiting phage therapy: new applications for old resources.Crossref | GoogleScholarGoogle Scholar | 25708933PubMed |
[15] Pires, D.P. et al. (2016) Genetically engineered phages: a review of advances over the last decade. Microbiol. Mol. Biol. Rev. 80, 523.
| Genetically engineered phages: a review of advances over the last decade.Crossref | GoogleScholarGoogle Scholar | 27250768PubMed |
[16] Roquet, N. et al. (2016) Synthetic recombinase-based state machines in living cells. Science 353, aad8559.
| 27463678PubMed |
[17] McCafferty, J. et al. (1990) Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348, 552–554.
| Phage antibodies: filamentous phage displaying antibody variable domains.Crossref | GoogleScholarGoogle Scholar | 2247164PubMed |
[18] Smith, G.P. (1985) Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228, 1315–1317.
| Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface.Crossref | GoogleScholarGoogle Scholar | 4001944PubMed |
[19] Arnold, F.H. and Volkov, A. (1999) Directed evolution of biocatalysts. Curr. Opin. Chem. Biol. 3, 54–59.
| Directed evolution of biocatalysts.Crossref | GoogleScholarGoogle Scholar | 10021399PubMed |
[20] Sagona, A.P. et al. (2016) Genetically modified bacteriophages. Integr. Biol. (Camb) 8, 465–474.
| Genetically modified bacteriophages.Crossref | GoogleScholarGoogle Scholar | 26906932PubMed |
[21] Wright, A. et al. (2009) A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 34, 349–357.
| A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy.Crossref | GoogleScholarGoogle Scholar | 19673983PubMed |
[22] Lehman, S.M. et al. (2019) Design and preclinical development of a phage product for the treatment of antibiotic-resistant Staphylococcus aureus infections. 11, 88.
[23] Ooi, M. and Wormald, P.-J. (2017) A Phase 1 clinical trial to evaluate the safety, tolerability and prelimnary effectiveness of AB-SA01 in patients with chronic rhinosinusitis associated with Staphylococcus aureus infection, ASOHNS ASM 2017 67th Annual Scientific Meeting, The Australian Society of Otolaryngology Head and Neck Surgery, Adelaide Convention Centre, Adelaide, South Australia.
[24] Jault, P. et al. (2019) Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 19, 35–45.
| Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial.Crossref | GoogleScholarGoogle Scholar | 30292481PubMed |
[25] Zang, Y. and Lee, J.J. (2017) A robust two-stage design identifying the optimal biological dose for phase I/II clinical trials. Stat. Med. 36, 27–42.
| A robust two-stage design identifying the optimal biological dose for phase I/II clinical trials.Crossref | GoogleScholarGoogle Scholar | 27538818PubMed |
[26] Gatsonis, C. and Greenhouse, J.B. (1992) Bayesian methods for phase I clinical trials. Stat. Med. 11, 1377–1389.
| Bayesian methods for phase I clinical trials.Crossref | GoogleScholarGoogle Scholar | 1518998PubMed |
[27] Bauer, P. et al. (2016) Twenty-five years of confirmatory adaptive designs: opportunities and pitfalls. Stat. Med. 35, 325–347.
| Twenty-five years of confirmatory adaptive designs: opportunities and pitfalls.Crossref | GoogleScholarGoogle Scholar | 25778935PubMed |
[28] Pennisi, M. (2017) 2017 Life Sciences Queensland Limited Member Directory.
[29] (2000) Gene Technology Act 2000, Commonwealth of Australia.
[30] Australian Government (2016) Gene Technology Regulations 2001, Federal Register of Legislation.
[31] Wieschowski, S. et al. (2018) Preclinical efficacy studies in investigator brochures: do they enable risk–benefit assessment? PLoS Biol. 16, e2004879.
| Preclinical efficacy studies in investigator brochures: do they enable risk–benefit assessment?Crossref | GoogleScholarGoogle Scholar | 29621228PubMed |
[32] European Medicines Agency (2009) ICH Considerations: General Principles to Address Virus and Vector Shedding. European Medicines Agency, London.
[33] (2018) The Third Review of the National Gene Technology Scheme. Department of Health, Commonwealth of Australia.
[34] Department of Agriculture and Water Resources (2018) BICON: Australian biosecurity import conditions. https://bicon.agriculture.gov.au/BiconWeb4.0/ (accessed 15 December 2018).
[35] TGA (2018) Clinical trials. https://www.tga.gov.au/node/4125 (accessed 1 January 2019).
[36] TGA (2018) Australian Clinical Trial Handbook: Importing and Exporting .
[37] Department of Industry, Innovation and Science (2018) Australian clinical trials. https://www.australianclinicaltrials.gov.au/ (accessed 2 January 2019).
Biography
Dr Keith Potent is an ENT registrar (The Tweed Hospital), a Principal Investigator (Griffith University Clinical Trials Unit), and a PhD (Translational medicine) candidate (Monash University). His research interests are the translation of phage technologies and Ear, Nose, and Throat surgery.