Use of bacteriophages as biological control agents in horticulture
Rhianna O’Regan A , Annaleise Wilson A and İpek Kurtböke A AA University of the Sunshine Coast, School of Science and Engineering and the GeneCology Research Centre, Maroochydore DC, Qld 4558, Australia
B Email: ikurtbok@usc.edu.au
Microbiology Australia 40(1) 47-50 https://doi.org/10.1071/MA19008
Published: 26 February 2019
Bacterial diseases in horticultural settings or infestation of fresh produce with human pathogenic bacteria can constitute a serious public health risk. To control horticultural bacterial diseases, chemical control strategies have traditionally been used, such as the application of bactericides and copper-based products, which resulted in development of resistance in bacteria against these agents. Moreover, the use of such chemical preventative measures on fresh produce can detrimentally affect human, animal and ecosystem health. Bacteriophages have been used to control pathogenic bacteria since the 1920s due to their specificity against host bacteria, as well as their ability to survive and infect their host without detrimental effects to the surrounding environments. As a result, their targeted host specific applications in horticultural settings can be of interest to growers as well as to the consumers. In this laboratory report, the efficacy of a bacteriophage cocktail when applied to fresh herbs inoculated with Escherichia coli was determined. Significant (P ≤ 0.001) reductions in E. coli colony forming units were observed in phage treated herb samples compared to counts in the control. These findings suggest that bacteriophage present as an alternative biocontrol for E. coli in horticulture.
Xanthomonas campesteris pv campesteris, the cause of black rot in brassicas, was one of the first bacteria to be challenged by a phage battery in 19241,2, followed in 1925 by isolation of phage active against Pectobacterium carotovarum subsp atrosepticum1,3 that resulted in the prevention of potato tuber rot. Field trials date back to the mid-1930s when corn seeds were treated with bacteriophages specific to Pantoea stewartia that resulted in significant reduction in Stewart’s wilt disease incidence1,4. In the late 1960s a laboratory trial demonstrated that the use of bacteriophage significantly reduced bacterial spot infection in the leaves of peach seedlings caused by Xanthomonas pruni1,5. More recently, Kurtböke et al.6 demonstrated effective elimination of human pathogenic Enterobacteriaceae species contaminating strawberries post-harvest using bacteriophage suspensions containing multiple polyvalent phages targeting the members of this bacterial family. In another study jointly conducted by Terragen Biotech Pty Ltd and the University of the Sunshine Coast (USC) in Queensland, Australia, Ashfield-Crook et al.7 investigated the control of potato scab causing streptomycetes using streptophages. Again, another recent study conducted at the USC targeted the control of E. coli test strains using locally grown herbs. This laboratory report will present some of the preliminary findings of this study.
E. coli is a facultatively anaerobic bacterium that can survive outside of the host in fecal matter and soil8. Although most strains of E. coli are harmless, a few pathogenic strains such as serotype O157:H7 can cause serious infections in humans such as haemorrhagic enteritis9, with some rare cases leading to bowel necrosis, septicemia and haemolytic uraemic syndrome10. Many strains of E. coli can contaminate fresh produce including herbs11, lettuce12, spinach10, vegetables13,14, and herbs like coriander that has been reported to be contaminated by E. coli 0157:H715. To control such pathogenic bacteria, antimicrobial treatments have traditionally been used16. However, due to the rise in antibiotic resistance among human pathogenic bacteria17, alternative biocontrol agents and strategies are needed.
Bacteriophages can be effectively used as a control method on fresh produce contaminated with pathogenic bacteria including E. coli6,18 and as their application is less destructive to the natural habitat they can also be used on edible food16,19. Examples include Listeria monocytogenes specific phage (P100) that has been rated as GRAS by the US FDA, the EU EFSA and Australian FSANZ, and commercially available as Listex™ to control this pathogen in RTE foods20.
The objective of this study was to use a bacteriophage cocktail composed of eight different phages as a biocontrol agent against E. coli (JM109), used under laboratory settings to deliberately infect five different locally grown herb samples. Additionally, the effectiveness of the phage cocktail against the same E. coli under natural settings was tested using pot parsley plants.
Eight different bacteriophages were obtained from the Microbial Library of the USC and their characteristics were previously described6. Each phage sample was propagated on E. coli (JM109) (https://www.atcc.org/Products/All/69905.aspx) with a titer of ~1010pfu/mL. A bacteriophage cocktail was then prepared using each individual phage sample in equal volumes and used to treat herbs contaminated with the test strain.
Herb samples; parsley, coriander, mint, Vietnamese mint and rosemary were obtained from a local supplier and were surface sterilised6 to ensure removal of any microbial contaminants that might be originating from the environment. Each herb leaf was then inoculated with JM109 and left to stand for 10 minutes to allow absorption of the bacterium into the plant tissue. Serial dilutions of the infected leaf samples were performed and from selected dilutions inoculations were made onto Tryptic Soy Agar (TSA) (OXOID, Australia) in triplicate. The phage-treated group of leaf samples were submerged into the bacteriophage cocktail solution for 1 hour. The phage treated herb leaves were then subjected to 10-fold serial dilutions and plated out in the same way as the control samples.
In the second phase of the study, potted parsley plants were obtained from a local supplier and they were divided into four different treatment groups: (1) a control with neither E. coli nor phage cocktail; (2) a control treated with E. coli only; (3) a treatment group exposed to both E. coli and phage cocktail; and (4) a third control treated with phage cocktail only.
In contrast to the two different controls (one with no JM109 or phage cocktail exposure, the other one exposed to phage cocktail only), two of the potted parsley plants were deliberately infected with ~5 mL of JM109 by gently rubbing the strain onto the plant using sterile gloves. The two pots containing infected parsley samples were first incubated at room temperature for 10 min. Parsley samples from one of the JM109 treated pot plants were cut and soaked in sterile distilled water. The samples from the second pot were first exposed to the phage cocktail for 1 h and then cut and soaked in sterile distilled water. The first potted parsley plant served as a control without any JM109 or phage cocktail application. All parsley samples from all of the treatments were shaken on an orbital shaker for 15 min at 110 rpm in 37°C and subsequently subjected to serial dilutions. Aliquots (200 µL) from selected dilutions for all potted parsley samples were finally inoculated in triplicate onto both TSA plates for general bacterial counts and MacConkey (OXOID, Australia) for its selectivity toward E. coli. Results were analysed using Student’s t-test21.
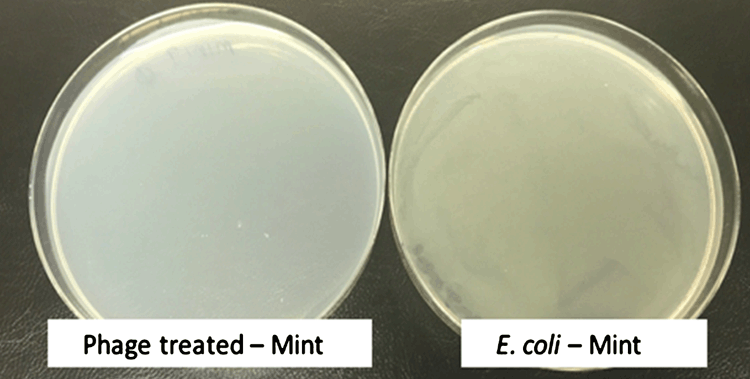
Use of the bacteriophage cocktail reduced the JM109 colony counts on all of the tested herbs with a high degree of significance (P ≤ 0.001), resulting in complete lysis. An example of full plate clearance is illustrated in Figure 1.
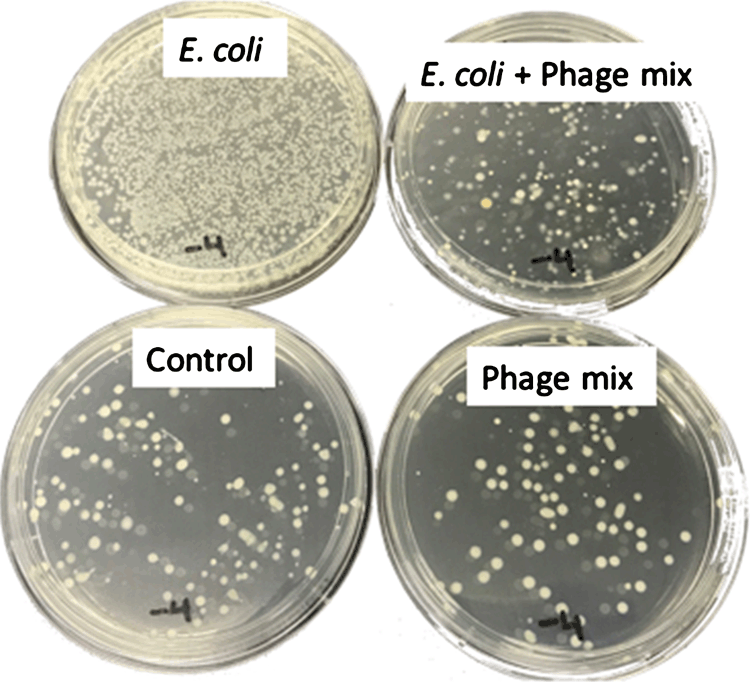
When aliquots were taken from potted parsley sample suspensions and inoculated onto either the TSA or the selective MacConkey agar plates, JM109 numbers were again found to be significantly reduced if the parsley samples were treated with the phage cocktail compared to the untreated control treatments (P ≤ 0.001) (Figure 2). The phage cocktail did not demonstrate lytic activity against the resident microflora present on the parsley prior to inoculation with JM109 (Figure 2, bottom plates).
The bacteriophage cocktail successfully reduced the numbers of JM109 on each different surface-sterilised herb indicating that surface structure or chemical compositions of the herb plant did not display significantly different interference with the phage activity. Moreover, bacteriophage activity was also persistent on non-surface-sterilised potted parsley samples when they were deliberately contaminated with the E. coli. Since the JM109 is a highly engineered strain of E. coli, the technique was also tested using other E. coli species (ATCC 25922 and ATCC BAA-196: ESBL +ve, as well as using local isolates listed in Kurtböke et al.6 using only parsley as the test herb. Again, significant reduction in the numbers of the tested different E. coli strains were achieved (P ≤ 0.001) (İ Kurtböke, 2015, unpublished data). All these findings were in line with other studies where successful bacteriophage applications were reported6,18 and suggest that bacteriophage biocontrol strategies might be an alternative to chemical controls used in horticultural settings. However, as stated by Jones et al.1, a number of factors should be considered during phyllospheric applications of the phages such as establishment of high-density phage populations in close proximity to the pathogen targeted for control at critical times in its disease cycle. Environmental factors may impact phage survival and persistence; such as inactivation by UV22 that would impact phage survival and persistence. Accordingly, the design of phage protective delivery methods is of importance as well as careful monitoring of the phages during field use to minimise development of resistance by the targeted host bacteria. Recently, Ashfield-Crook et al.7 also demonstrated that polyvalent phages might also have unintended consequences in field applications by simultaneously removing beneficial microflora and resulting in increased risk of secondary infections. Although bacteriophages have significant potential to be utilised as biocontrol agents in agricultural and horticultural settings, the generation of further data and careful observations in the field have critical importance for their acceptance as reliable disease control agents.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] Jones, J.B. et al . (2012) Considerations for using bacteriophages for plant disease control. Bacteriophage 2, 208–214.| Considerations for using bacteriophages for plant disease control.Crossref | GoogleScholarGoogle Scholar | 23531902PubMed |
[2] Mallmann, W.L. and Hemstreet, C.J. (1924) Isolation of an inhibitory substance from plants. Agric. Res. 28, 599–602.
[3] Kotila, J.E. and Coons, G.H. (1925) Investigations on the blackleg disease of potato. Mich. Aes. Res. Rep. 67, 3–29.
[4] Thomas, R.C. (1935) A bacteriophage in relation to Stewart’s disease of corn. Phytopathology 25, 371–372.
[5] Civerolo, E.L. and Keil, H.L. (1969) Inhibition of bacterial spot of peach foliage by Xanthomonas pruni bacteriophage. Phytopathology 59, 1966–1967.
[6] Kurtböke, D.I. et al . (2016) Isolation and characterization of Enterobacteriaceae species infesting post-harvest strawberries and their biological control using bacteriophages. Appl. Microbiol. Biotechnol. 100, 8593–8606.
| Isolation and characterization of Enterobacteriaceae species infesting post-harvest strawberries and their biological control using bacteriophages.Crossref | GoogleScholarGoogle Scholar | 27357225PubMed |
[7] Ashfield-Crook, N.R. et al . (2018) Assessment of the detrimental impact of polyvalent streptophages intended to be used as biological control agents on beneficial soil streptoflora. Curr. Microbiol. 75, 1589–1601.
| Assessment of the detrimental impact of polyvalent streptophages intended to be used as biological control agents on beneficial soil streptoflora.Crossref | GoogleScholarGoogle Scholar | 30242439PubMed |
[8] Rogers, M. and Peterson, N. (2011) E. coli Infections: Causes, Treatment and Prevention. 1st edition. Nova Science Publishers Inc.
[9] Clark, M. (2015) About E. coli . http://www.about-ecoli.com (accessed 2 January 2019).
[10] O157:H7 Investigation Team, CDC (2006) Ongoing multistate outbreak of Escherichia coli serotype O157:H7 infections associated with consumption of fresh spinach-United States MMWR 55, 1045–1046.
| 17008868PubMed |
[11] Levy, D.J. et al . (2015) Microbial safety and quality of fresh herbs from Los Angeles, Orange County and Seatle farmers’ markets. J. Sci. Food Agric. 95, 2641–2645.
| Microbial safety and quality of fresh herbs from Los Angeles, Orange County and Seatle farmers’ markets.Crossref | GoogleScholarGoogle Scholar | 25382560PubMed |
[12] Uhlich, G.A. et al . (2008) Characterization of Shiga toxin-producing Escherichia coli isolates associated with two multistate food-borne outbreaks that occurred in 2006. Appl. Environ. Microbiol. 74, 1268–1272.
| Characterization of Shiga toxin-producing Escherichia coli isolates associated with two multistate food-borne outbreaks that occurred in 2006.Crossref | GoogleScholarGoogle Scholar | 18083883PubMed |
[13] Beauchat, L. (1998) Surface decontamination of fruits and vegetables eaten raw: a review. Geneva: WHO/FSF/FOS/98.2, Food Safety Unit, World Health Organization.
[14] Beuchat, L. (1996) Pathogenic microorganisms associated with fresh produce. J. Food Prot. 59, 204–216.
| Pathogenic microorganisms associated with fresh produce.Crossref | GoogleScholarGoogle Scholar |
[15] Yoshitomi, K.J. et al . (2012) Detection and isolation of low levels of E. coli 0157:H7 in cilantro real-time PCR, immunomagnetic separation, and culture methods with and without an acid treatment. J. Food Sci. 77, M481–M489.
| Detection and isolation of low levels of E. coli 0157:H7 in cilantro real-time PCR, immunomagnetic separation, and culture methods with and without an acid treatment.Crossref | GoogleScholarGoogle Scholar | 22860597PubMed |
[16] Snyder, A.B. et al . (2016) Developing and optimizing bacteriophage treatment to control enterohemorrhagic Escherichia coli on fresh produce. Int. J. Food Microbiol. 236, 90–97.
| Developing and optimizing bacteriophage treatment to control enterohemorrhagic Escherichia coli on fresh produce.Crossref | GoogleScholarGoogle Scholar | 27454784PubMed |
[17] WHO (2015) Worldwide country situation analysis: response to antimicrobial resistance. Bull. World Health Organ. 93, 363–364.
| 26454900PubMed |
[18] Boyacioglu, O. et al . (2013) Biocontrol of Escherichia coli O157:H7 on fresh-cut leafy greens: using a bacteriophage cocktail in combination with modied atmosphere packaging. Bacteriophage 3, e24620.
| Biocontrol of Escherichia coli O157:H7 on fresh-cut leafy greens: using a bacteriophage cocktail in combination with modied atmosphere packaging.Crossref | GoogleScholarGoogle Scholar | 23819107PubMed |
[19] Kazi, M. and Annapure, U. (2016) Bacteriophage biocontrol of foodborne pathogens. J. Food Sci. Technol. 53, 1355–1362.
| Bacteriophage biocontrol of foodborne pathogens.Crossref | GoogleScholarGoogle Scholar | 27570260PubMed |
[20] Jordan, K. et al . (2018) Listeria monocytogenes in the food processing environment. Curr. Clin. Microbiol. Rep. 5, 106–119.
| Listeria monocytogenes in the food processing environment.Crossref | GoogleScholarGoogle Scholar |
[21] Bailey, N.T.J. (1959) Statistical Methods in Biology. The English Universities Press Ltd, London.
[22] Sinton, L.W. et al . (1999) Sunlight inactivation of fecal bacteriophages and bacteria in sewage-polluted seawater. Appl. Environ. Microbiol. 65, 3605–3613.
| 10427056PubMed |
Biographies
Rhianna O’Regan is a graduate of the University of the Sunshine Coast (USC). She completed a Special Research Project course under the supervision of Dr İpek Kurtböke working on the bacteriophage control of herb infesting E. coli pathogen. Following her graduation, she worked at Q-Pharm in Brisbane QLD, as a Clinical Laboratory Officer for 2 years and currently is returning to the USC to conduct her MSc studies under the supervision of Dr İpek Kurtböke on fungal diseases associated with corals to be investigated jointly with the co-supervision of Dr David Bourne at JCU.
Annaleise Wilson is also a graduate of the USC. She completed a Special Research Project course under the supervision of Dr İpek Kurtböke working on the bacteriophage control of milk contaminating E. coli pathogen. Following her graduation, she continued with her Honours studies under the supervision of Dr İpek Kurtböke in a project linked with the CSIRO Food Safety and Stability research group led by Dr Narelle Fegan in Melbourne and recently graduated with first class Honours. She is now a PhD student at the UQ studying molecular pathogenesis of food borne pathogens under the supervision of Prof Mark Turner.
Dr Ipek Kurtböke has been working in the field of biodiscovery and has been an active member of the international actinomycete research community since 1982. She currently conducts research and teaches in the field of applied microbiology and biotechnology and is senior lecturer at the USC. She has also been an active member of the World Federation of Culture Collections (WFCC) including serving as the Vice-President of the Federation (2010–2013) and is currently the President of the Federation (2017–2020).