Seafood-borne parasites in Australia: human health risks, fact or fiction?
Shokoofeh ShamsiSchool of Animal and Veterinary
Sciences and Graham Centre for
Agricultural Innovation
Charles Sturt University
Australia
Email: sshamsi@csu.edu.au
Microbiology Australia 41(1) 33-37 https://doi.org/10.1071/MA20009
Published: 3 March 2020
Seafood is an increasingly popular source of healthy protein. Since 1961, the average annual increase in global food fish consumption has been twice as high as population growth and exceeds the consumption of meat from all terrestrial animals combined1. The following overview of seafood safety concerns is intended to help readers to understand potential risks associated with parasites in seafood products and the need for a national approach to reduce or minimise them. It is important to note that parasite infections are not limited to seafood: all other types of foods, including vegetables and red meat can also be infected with a broad range of parasites, some of which are more dangerous than parasites in seafood. The main issue is lack of science based contemporaneous safety protocols which focus on seafood-borne parasites. As a result, in Australia regulatory control of parasites in seafood lags far behind other food sectors. Seafood safety is a broad topic. The focus of this article is on an understudied field in Australia, seafood-borne parasitic diseases. The word ‘seafood’ in this context encompasses fish and shellfish products from marine and freshwater ecosystems that are, directly or indirectly, meant for human consumption.
Parasites and seafood
The knowns
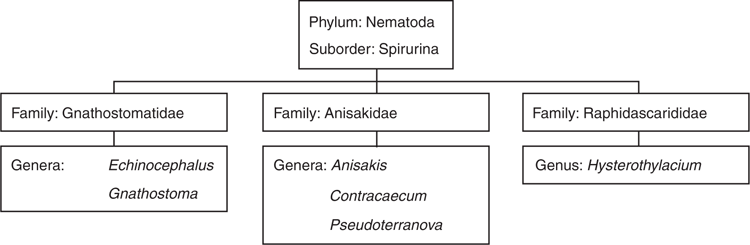
Occurrence in seafood: A wide range of parasites transmissible to humans can be found in seafood products. Their life cycle, pathogenicity and significance has been reviewed previously2,3. Of over 40 transmissible parasites from seafood reported worldwide2, this article will focus more on nematodes belonging to families Anisakidae, Raphidascarididae and Gnathostomatidae (Figure 1), which seem to be of most concern in Australia due to their common occurrence, infecting seafood products in local and global food supply chains. Recent studies showed that 86% of tiger flathead4, 56% of anchovy5 and 100% of pilchard sold in a fish market in Australian east coast were infected with at least one type of infective stage of potentially zoonotic anisakid/raphidascarid nematodes. These nematodes included Anisakis, Contracaecum, Hysterothylacium and Terranova larval types, all of these genera known to have species infecting humans. All of these parasites are now known4,6 to migrate from the internal organs of the fish into other parts, including the flesh, after the fish is caught/dead, hence increasing the risk they may pose to public health. Migration of parasites into fish flesh can be minimised by evisceration of fish immediately after capture and appropriate cold storage at all times preceding consumption. Pre-freezing is recommended if fish are intended to be consumed raw or partially cooked. While a diverse range of potentially zoonotic nematodes have been found in Australian wild caught marine fish, little is known about their diversity and abundance in the freshwater fish species in Australia.
|
Occurrence in humans: With the frequent occurrence and abundance of these parasites, the popularity of seafood, the multicultural cuisine enjoyed in Australia and the ready availability of a variety of seafood, it is not surprising that human infections can occur regularly. In Australia, since the early 20th century, there have been numerous documented cases and many anecdotal cases of human infection7 with a broad range of parasites acquired from seafood. This not only includes nematodes, such as Contracaecum sp.8, Gnathostoma sp.9,10 (possibly a misdiagnosed case, see Shamsi and Sheorey7) and Dioctophyme renale11, but also other parasites including tapeworms, such as Adenocephalus pacificus12, Spairometra spp.13, and Diphyllobothrium latum (now accepted as Dibothriocephalus latus)14, flukes such as Clonorchis sinensis14, and Paragonimus westermani15, and Myxosporidians such as Myxobolus plectroplites16. The reported parasites include both marine (e.g. Adenocephalus pacificus) and freshwater (e.g. Clonorchis sinensis and Paragonimus westermani) parasites. However, due to a decline in parasitology teaching in the Australian medical schools, many of these species of zoonotic parasites have fallen into obscurity7,17.
Clinical signs: Diseases due to some seafood-borne parasites can be severe to life threatening. However, many are mild, perhaps underdiagnosed, and hence not well documented. Clinical signs due to seafood-borne parasites of concern in Australia (anisakids/raphidascarids and gnathostomids) are broad mainly due to the wide range of species implicated in infections, each parasite species having unique characteristics. Clinical signs, particularly those caused by anisakid/raphidascarid nematodes, are varied and include: (1) mild vague symptoms that mimic food poisoning; and (2) acute gastrointestinal symptoms that mimic appendicitis. In addition, (3) infection/exposure with anisakid nematodes, even those killed in well-cooked seafood, can also cause allergic reactions, including anaphylactic shock18 in sensitive consumers and workers handling seafood products. Gnathostomid nematodes are also migratory parasites which cause highly variable clinical signs. They invade a variety of tissues, including subcutaneous, pulmonary, gastrointestinal, genitourinary, auricular or ocular tissues, or the central nervous system with migratory lesions in these tissues.
Imported seafood: Some of the countries Australia imports from are known to harbour highly pathogenic parasites in their seafood products. Fresh and frozen imports are predominantly from Thailand, China and Vietnam19 where parasites such as Clonorchis sinensis, the Chinese liver fluke, and Opisthorchis viverrini, the south Asian liver fluke are common. The safety of these products for human consumption in this country relies on Australian legislation which is reflected in the latest imported food control order. At present no additional tests are applied to detect zoonotic parasites in seafood on entry to Australia (Imported Food Control Order 2019 in force 17 September 2019; https://www.legislation.gov.au/Details/F2019L01233). Australia relies on equivalency of the exporting countries testing procedures to provide documentary evidence of their adherence to internationally recognised food safety controls (Imported Food Control Order 2019 in force 17 September 2019; https://www.legislation.gov.au/Details/F2019L01233) such as parasite control detailed in the Codex Code of practice for fishery products (Codex Code of practice for fishery products; http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXC%2B52-2003%252FCXP_052e.pdf). Freezing and cooking are effective in killing the parasites.
Human movement: Australia is a popular destination for both tourists and migrants from both developed and developing countries. Seafood-borne parasites are also common in both developing and developed countries across the globe and, unlike many other parasites, are not limited to low income communities. Several seafood-borne parasites, such as C. sinensis, Heterophyes heterophyes, Opisthorchis spp. and Dibothriocephalus spp. reach adult stage in humans, producing thousands of eggs a day. Several species of other parasites from the same family, and some from the same genus, as the aforementioned zoonotic parasites are endemic in Australia. The possibility therefore exists that their intermediate hosts may be successfully exploited by exotic zoonotic species as part of their life cycle. Australia is also home to potential gastropod intermediate hosts such as native thiarid species and Gabbia australis, which may make establishment of a parasite life cycle viable20. Currently there is no requirement to screen travellers and immigrants for infection with these parasites at the time of the entry to the country and this is seen as a viable entry point14,21,22.
The unknowns
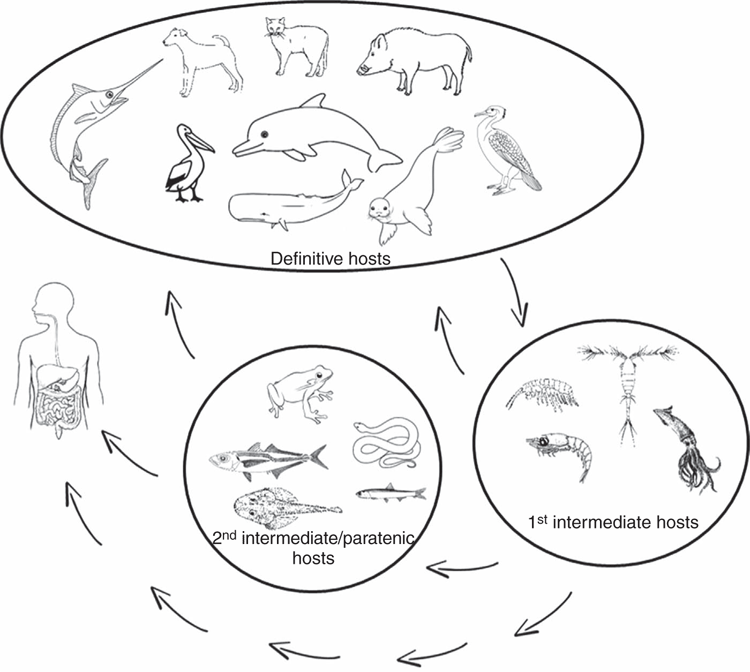
Transmission patterns and ecology: The detailed life cycle of zoonotic nematodes, in particular developmental stages which occur between the egg of the parasite and the infective stage (third stage larva, L3) are not fully known in Australia. These life stages may take place in a broad range of invertebrates and smaller fish (Figure 2)24. Also little understood are the major drivers that influence parasite distribution in these animals. Understanding life cycles of these parasites is central to determining the potential infection source, to designing effective educational campaigns for various stakeholders and to developing practical control and prevention programs.
|
Another emerging concern is understanding the effects of climate change on fish-parasite systems. Climate change unquestionably alters the prerequisites for parasite transfer, most likely to favour zoonotic parasites25. Increased water temperature, for example, usually results in parasites spreading more rapidly and higher infection rates in fish26.
Clinical knowledge and diagnostics: Correct diagnosis relies on effective communication between the patient, the clinician and the laboratory personnel and their awareness of pathogens. In Australia accurate diagnosis of seafood-borne parasitic diseases is very challenging. In the author’s experience one of the main reasons for this is lack of awareness and low diagnostic suspicion regarding seafood-borne parasites among key stakeholders, clinicians and laboratory personnel. As an example, when a patient presents with acute gastrointestinal signs inclusive of a history of raw seafood consumption a differential diagnosis of seafood-borne parasitism is not routinely considered27. Anisakids/raphidascarids nematodes are also initiators of allergic reactions in humans. In many countries it has been confirmed that many previously diagnosed seafood allergies are an allergy to the parasite rather than the seafood itself18. Although fish and shellfish allergy is one of the most common allergy in adulthood (https://www.health.harvard.edu/staying-healthy/adult-food-allergies) in many parts of the world28, in Australia nothing is known of the percentage of the ‘allergic population’ that may be attributable to parasites of seafood.
Should clinicians consider seafood-borne parasitic diseases in their action plans, there is no standard diagnostic test for these parasites in Australia. Reliance is being placed on the diagnostic tests developed overseas which, quite often, are not suitable to diagnose cases acquired in Australia and have variable/low specificity and sensitivity. For example, the recent human cases in Western Australia9 and Queensland29 were diagnosed as Gnathostoma based on a serologic test developed in Thailand, specificity and sensitivity of this test being uncertain. Interestingly, there is no report of this parasite in any Australian fish and none of the previous reports of Gnathostoma in other animals (such as cat, dog, bandicoot, quoll and pig)10,30–33 provides a description or justification for their identification. A closely related parasite from the same family as Gnathostoma is Echinocephalus and both share a high degree of morphological similarity. Parasites belonging to the genus Echinocephalus are commonly found in Australian fish and molluscs34 but has not been considered as zoonotic in government risk assessment studies35,36 due to lack of reports of human illness. In some Asian countries, following successful experimental infection of kittens and primates with Echinocephalus larvae, and due to popularity of consuming raw seafood, this parasite has been recommended to be considered of public health importance37,38. Although presence of Gnathostoma spp. cannot be fully ruled out in Australia because there has never been a comprehensive study on seafood-borne parasites in this country, the fundamental concern is in the utilisation of serologic tests developed overseas to accurately identify Australian endemic parasites. Hence, it is likely that seafood-borne parasitic diseases are an underrecognised/underdiagnosed and underreported condition in Australia.
Recommendations
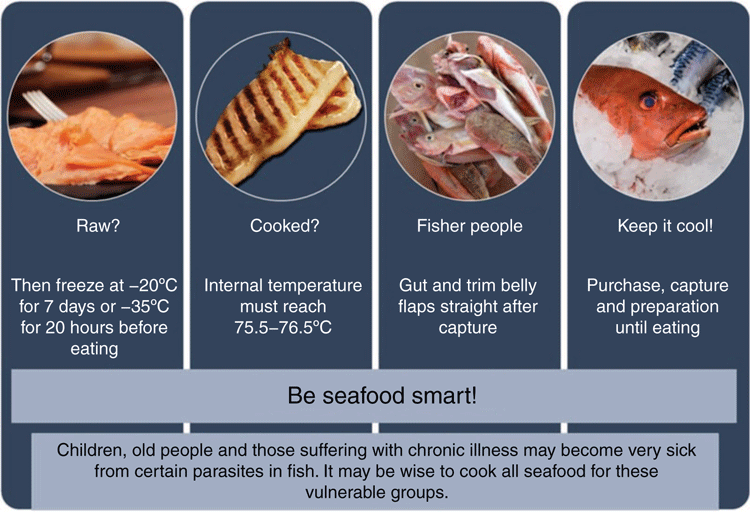
As long as there is awareness, prevention of infection with seafood-borne parasites is simple and easily doable (Figure 3). As rightly recommended by the National Health and Medical Research Council, Australia’s leading health research body, Australians should eat more fish. However, this recommendation should be supported by a strong body of research and education toward seafood safety to ensure the risk due to parasites is minimal.
Research: Some of the urgent areas for research would be:
-
Determining the occurrence and the life cycle (including transmission patterns) of seafood-borne parasites in Australian marine and freshwater systems.
-
Developing reliable tests specifically to detect infections both in humans and infected seafood for parasites prevalent in Australia.
-
Determining factors contributing to the low awareness of these important parasites and diseases caused across multiple stakeholders.
Translation of research outcomes including education: In Australia the gap between scientists and other stakeholders involved in seafood safety is significant2. In the recent risk assessments35,36 the frequent presence of zoonotic parasites reported in commonly wild caught fish4,5,39 has been fully overlooked and the number of human infections in the country7 has been significantly underestimated with two documented reports only! A preliminary study also shows that the lack of awareness among medical doctors in Australia is significant27. Another challenge is the increasing decline in expertise in parasitology7,17 and reduction in the parasitology curriculum in Australian medical and veterinary schools7. Research based education, communication and decision making of all stakeholders is central to seafood safety in this country. This includes fisher people, fish farmers, importers of seafood product, chefs, aquatic veterinarians, public health experts, clinicians, diagnostic laboratory staff, general and at‐risk communities, and jurisdictional and federal agencies.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgement
The author thanks Ms Michelle Williams (Charles Sturt University), who assisted with the design of Figure 3.
References
[1] FAO (2018) The State of World Fisheries and Aquaculture 2018 – meeting the sustainable development goals. Rome.[2] Shamsi, S. (2019) Seafood-borne parasitic diseases: a ‘One-Health’ approach is needed. Fishes 4, 9.
[3] Shamsi, S. (2016) Seafood-borne parasitic diseases in Australia: how much do we know about them? Microbiol. Aust. 37, 27–29.
| Seafood-borne parasitic diseases in Australia: how much do we know about them?Crossref | GoogleScholarGoogle Scholar |
[4] Shamsi, S. and Suthar, J. (2016) A revised method of examining fish for infection with zoonotic nematode larvae. Int. J. Food Microbiol. 227, 13–16.
| A revised method of examining fish for infection with zoonotic nematode larvae.Crossref | GoogleScholarGoogle Scholar | 27043384PubMed |
[5] Hossen, M.S. and Shamsi, S. (2019) Zoonotic nematode parasites infecting selected edible fish in New South Wales, Australia. Int. J. Food Microbiol. 308, 108306.
| Zoonotic nematode parasites infecting selected edible fish in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar | 31442713PubMed |
[6] Shamsi, S. et al. (2018) Description and genetic characterization of a new Contracaecum larval type (Nematoda: Anisakidae) from Australia. J. Helminthol. 92, 216–222.
| Description and genetic characterization of a new Contracaecum larval type (Nematoda: Anisakidae) from Australia.Crossref | GoogleScholarGoogle Scholar | 28473011PubMed |
[7] Shamsi, S. and Sheorey, H. (2018) Seafood‐borne parasitic diseases in Australia: are they rare or underdiagnosed? Intern. Med. J. 48, 591–596.
| Seafood‐borne parasitic diseases in Australia: are they rare or underdiagnosed?Crossref | GoogleScholarGoogle Scholar | 29722196PubMed |
[8] Shamsi, S. and Butcher, A.R. (2011) First report of human anisakidosis in Australia. Med. J. Aust. 194, 199–200.
| First report of human anisakidosis in Australia.Crossref | GoogleScholarGoogle Scholar | 21401462PubMed |
[9] Jeremiah, C.J. et al. (2011) Gnathostomiasis in remote northern Western Australia: the first confirmed cases acquired in Australia. Med. J. Aust. 195, 42–44.
| Gnathostomiasis in remote northern Western Australia: the first confirmed cases acquired in Australia.Crossref | GoogleScholarGoogle Scholar | 21728942PubMed |
[10] Heydon, G.M. (1929) Creeping eruption or Larva Migrans in North Queensland and a note on the worm Gnathostoma spinigerum (Owen). Med. J. Aust. 1, 583–591.
| Creeping eruption or Larva Migrans in North Queensland and a note on the worm Gnathostoma spinigerum (Owen).Crossref | GoogleScholarGoogle Scholar |
[11] Fernando, S.S. (1983) The giant kidney worm (Dioctophyma renale) infection in man in Australia. Am. J. Surg. Pathol. 7, 281–284.
| The giant kidney worm (Dioctophyma renale) infection in man in Australia.Crossref | GoogleScholarGoogle Scholar | 6220617PubMed |
[12] Moore, C.V. et al. (2016) Rare human infection with pacific broad tapeworm Adenocephalus pacificus, Australia. Emerg. Infect. Dis. 22, 1510–1512.
| Rare human infection with pacific broad tapeworm Adenocephalus pacificus, Australia.Crossref | GoogleScholarGoogle Scholar | 27433924PubMed |
[13] Hughes, A.J. and Biggs, B.A. (2002) Parasitic worms of the central nervous system: an Australian perspective. Intern. Med. J. 32, 541–553.
| Parasitic worms of the central nervous system: an Australian perspective.Crossref | GoogleScholarGoogle Scholar | 12412938PubMed |
[14] Sweet, W.C. (1924) The intestinal parasites of man in Australia and its dependencies as found by the Australian hookworm campaign. Med. J. Aust. 1, 405–407.
| The intestinal parasites of man in Australia and its dependencies as found by the Australian hookworm campaign.Crossref | GoogleScholarGoogle Scholar |
[15] Mukerjee, C.M. et al. (1992) Pleuropulmonary paragonimiasis in a Laotian immigrant to Australia. Chest 101, 849–851.
| Pleuropulmonary paragonimiasis in a Laotian immigrant to Australia.Crossref | GoogleScholarGoogle Scholar | 1541157PubMed |
[16] Boreham, R.E. et al. (1998) Incidental finding of Myxobolus spores (Protozoa: Myxozoa) in stool samples from patients with gastrointestinal symptoms. J. Clin. Microbiol. 36, 3728–3730.
| Incidental finding of Myxobolus spores (Protozoa: Myxozoa) in stool samples from patients with gastrointestinal symptoms.Crossref | GoogleScholarGoogle Scholar | 9817910PubMed |
[17] Sandeman, M. and Warner, L. (2002) An investment in human and animal health: parasitology in Australia. Deakin West, ACT: FASTS occasional paper series; no. 4.
[18] Audicana, M.T. et al. (2002) Anisakis simplex: dangerous – dead and alive? Trends Parasitol. 18, 20–25.
| Anisakis simplex: dangerous – dead and alive?Crossref | GoogleScholarGoogle Scholar | 11850010PubMed |
[19] Anonymous (2015) Australia’s seafood trade. Department of Agriculture, Canberra.
[20] Beesley, P.L. (1998) Mollusca: the southern synthesis. Australian Government Publishing Service; CSIRO.
[21] Ryan, N. et al. (1988) Parasitic infections of refugees. Med. J. Aust. 148, 491–494.
| Parasitic infections of refugees.Crossref | GoogleScholarGoogle Scholar | 3367816PubMed |
[22] Sandars, D.F. (1953) Another record of a human diphyllobothriid in Australia. Med. J. Aust. 2, 55–58.
| Another record of a human diphyllobothriid in Australia.Crossref | GoogleScholarGoogle Scholar | 13070965PubMed |
[23] Yagi, K. et al. (1996) Female worm Hysterothylacium aduncum excreted from human: a case report. Kiseichugaku Zasshi 45, 12–23.
[24] Anderson, R.C. (2000) Nematode parasites of vertebrates: their development and transmission. CABI Publishing.
[25] Lõhmus, M. and Björklund, M. (2015) Climate change: what will it do to fish—parasite interactions? Biol. J. Linn. Soc. Lond. 116, 397–411.
| Climate change: what will it do to fish—parasite interactions?Crossref | GoogleScholarGoogle Scholar |
[26] Dziekońska-Rynko, J. and Rokicki, J. (2007) Life cycle of the nematode Contracaecum rudolphii Hartwig, 1964 (sensu lato) from northern Poland under laboratory conditions. Helminthologia 44, 95–102.
| Life cycle of the nematode Contracaecum rudolphii Hartwig, 1964 (sensu lato) from northern Poland under laboratory conditions.Crossref | GoogleScholarGoogle Scholar |
[27] Seal, A. et al. (2020) A preliminary report on the awareness and knowledge of seafood-borne parasitic diseases among medical doctors in Australia. Parasitol. Int. 74, 101993.
| 31521766PubMed |
[28] Woo, C.K. and Bahna, S.L. (2011) Not all shellfish "allergy" is allergy! Clin. Transl. Allergy 1, 3.
| Not all shellfish "allergy" is allergy!Crossref | GoogleScholarGoogle Scholar | 22410209PubMed |
[29] Lin, Y.d. and Anstey, N. (2017) An autochthonous case of gnathostomiasis acquired in Queensland, Australia. Am. J. Trop. Med. Hyg. 95, 353–354.
[30] Olds, R.J. (1952) Gnathostoma spinigerum (Owen, 1836) in cats in north Queensland. Aust. Vet. J. 28, 124–126.
| Gnathostoma spinigerum (Owen, 1836) in cats in north Queensland.Crossref | GoogleScholarGoogle Scholar |
[31] Trueman, K.F. and Ferris, P.B.C. (1977) Gnathostomiasis in three cats. Aust. Vet. J. 53, 498–499.
| Gnathostomiasis in three cats.Crossref | GoogleScholarGoogle Scholar | 612327PubMed |
[32] Vogelnest, L. and Woods, R. (2008) Medicine of Australian Mammals. CSIRO Publishing, Melbourne.
[33] Smales, L.R. (1999) Species of Gnathostoma (Nematoda : Spiruroidea) from bandicoots and dasyurids (marsupialia) from Australia. Trans. R. Soc. S. Aust. 123, 83–84.
[34] Beveridge, I. (1987) Echinocephalus overstreeti Deardorff & Ko, 1983 (Nematoda: Gnathistomatoidea) from elasmobranchs and molluscs in South Australia. Trans. R. Soc. S. Aust. III, 79–92.
[35] Anantanawat, S. et al. (2012) A semi-quantitative risk assessment of harmful parasites in Australian finfish. South Australian Research & Development Institute.
[36] NSW Department of Primary Industries Food Authority (2017) Risk assessment of the seafood safety scheme. http://www.foodauthority.nsw.gov.au/aboutus/science/risk-assessment-of-food-safety-schemes/seafood-safety-scheme
[37] Ko, R.C. (1977) Effects of temperature-acclimation on infection of Echinocephalus sinensis (Nematoda Gnathostomatidae) from oysters to kittens. Can. J. Zool. 55, 1129–1132.
| Effects of temperature-acclimation on infection of Echinocephalus sinensis (Nematoda Gnathostomatidae) from oysters to kittens.Crossref | GoogleScholarGoogle Scholar | 884639PubMed |
[38] Ko, R.C. et al. (1975) Prevalence and histopathology of Echinocephalus sinensis (Nematoda Gnathostomatidae) in natural and experimental hosts. Can. J. Zool. 53, 550–559.
| Prevalence and histopathology of Echinocephalus sinensis (Nematoda Gnathostomatidae) in natural and experimental hosts.Crossref | GoogleScholarGoogle Scholar | 1131750PubMed |
[39] Shamsi, S. et al. (2018) New and known zoonotic nematode larvae within selected fish species from Queensland waters in Australia. Int. J. Food Microbiol. 272, 73–82.
| New and known zoonotic nematode larvae within selected fish species from Queensland waters in Australia.Crossref | GoogleScholarGoogle Scholar | 29550686PubMed |
Biography
Associate Professor Shokoofeh Shamsi has completed a Master degree in Medical Sciences and a PhD in Veterinary Sciences. She is currently based at School of Animal and Veterinary Sciences, Charles Sturt University. She leads a research group mainly working on seafood safety, aquatic animal health and diseases, wildlife parasitology, and emerging zoonotic diseases.