Therapeutics for COVID-19: established and in development
Kasha P Singh A B , Joe Sasadeusz A B C , Sharon R Lewin A B C D and Jennifer Audsley AA The Peter Doherty Institute for infection and Immunity, The University of Melbourne and Royal Melbourne Hospital, Melbourne, Vic., Australia
B Victorian Infectious Diseases Service, Royal Melbourne Hospital, Melbourne, Vic., Australia
C Department of Infectious Diseases, Alfred Health and Monash University, Melbourne, Vic., Australia
D Tel.: +61 3 8344 3159, Email: sharon.lewin@unimelb.edu.au
Microbiology Australia 41(4) 217-223 https://doi.org/10.1071/MA20058
Published: 10 November 2020
Abstract
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first recognised in late 2019, with over 30 000 000 cases and over 1 000 000 deaths reported by the end of September 2020. SARS-CoV-2 infection is usually associated with fever, cough, coryza, dyspnoea, anosmia, headache and fatigue and may cause pneumonia and hypoxemia. An excessive/dysregulated inflammatory response may lead to lung damage including acute respiratory distress syndrome (ARDS), coagulopathy and other complications. Mortality amongst hospitalised patients is higher in those needing intensive care. In Australia over 27 000 cases with 882 deaths had been reported by 30 September, most in Victoria. Two therapies have proven beneficial in treatment of hospitalised patients in expedited randomised placebo-controlled trials and are now in widespread use. Dexamethasone improved survival of those requiring respiratory support and the antiviral agent remdesivir decreased time to recovery in mild-moderate disease. Remdesivir was authorised by the Australian Therapeutic Goods Administration in July 2020. Over 200 other therapeutics are being tested for COVID-19 in more than 2000 clinical trials, and many more agents are in preclinical development. We review the evidence for some of the candidates for therapy in COVID-19.
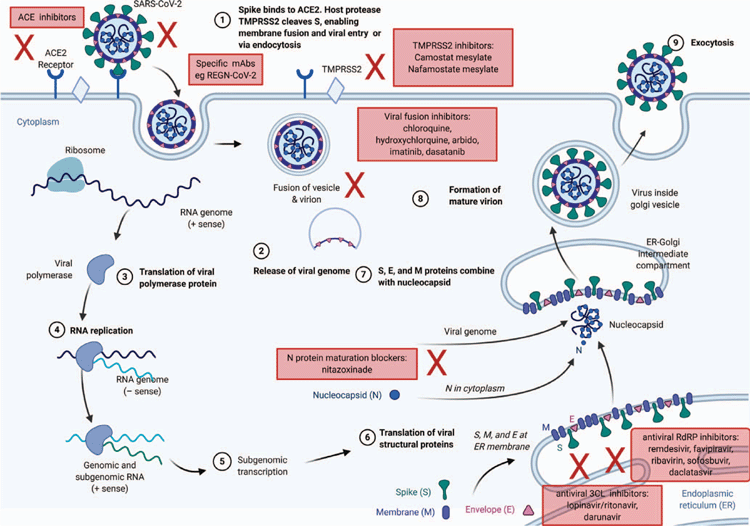
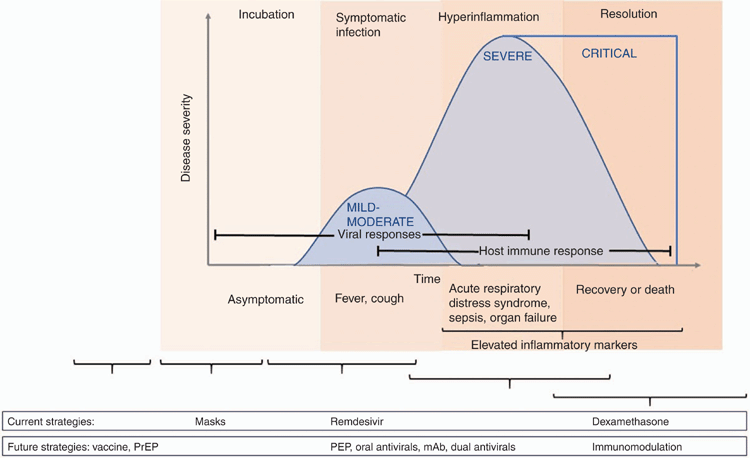
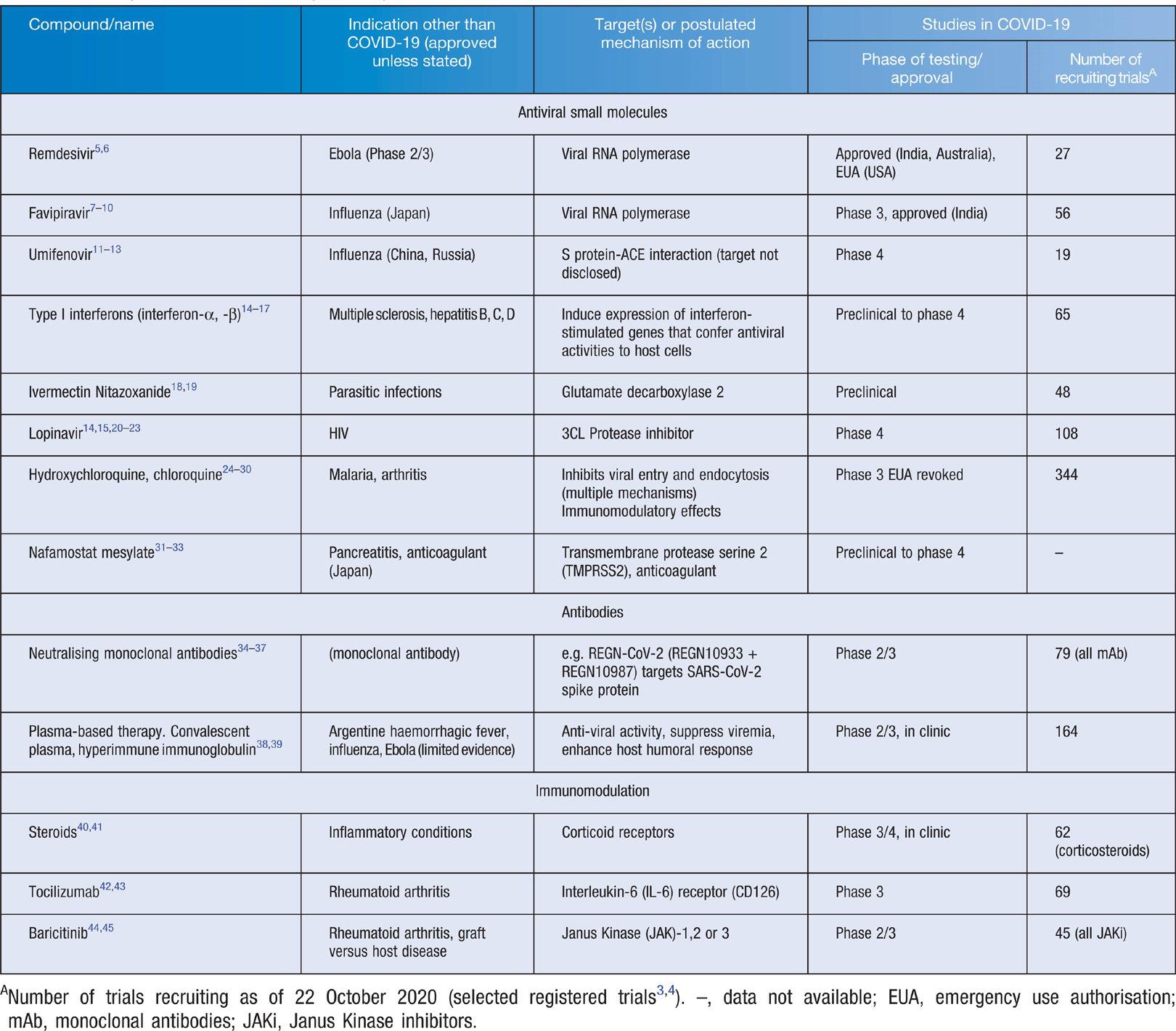
The aim of treatment for COVID-19 is to reduce disease severity and prevent mortality. Therapeutics may also be used to prevent or abort infection (pre- or post-exposure prophylaxis) and reduce post-infectious complications (Figure 1). In Australia, the National COVID-19 Clinical Evidence Taskforce has been established to review rapidly emerging evidence in real-time and maintain a ‘living document’ for COVID-19 management guidelines2, available at https://covid19evidence.net.au/#living-guidelines. Interventions may be grouped into those targeting the virus and those targeting the immune response (Figure 2, Table 1). Adjunctive therapies including anticoagulation and optimising oxygenation are also critical elements of management but are beyond the scope of this review.
|
|
Therapeutic approaches
Antiviral small molecules
Repurposed drugs for Ebola: remdesivir
In a Phase III randomised placebo-controlled trial (ACTT-1, NCT04280705) including 1063 adults with COVID-19 with lower respiratory tract infection, participants receiving up to 10 days of remdesivir had shorter time to recovery than those in the placebo group (median 11 and 15 days, rate ratio for recovery 1.32; 95% CI 1.12–1.55, P < 0.001)5. Stratification by severity showed improved time to recovery for patients receiving oxygen at baseline, but not for those receiving no oxygen nor for those receiving high-flow oxygen or mechanical ventilation. There was no significant difference in mortality rates, although further follow-up data is awaited.
Questions remain about the optimal time and duration of administration of remdesivir. In one study (n = 397), 10 days treatment was associated with better outcomes than 5 in those going on to need mechanical ventilation; however, no control arm was included (mortality 17% with 10 days (n = 41), 40% with 5 days (n = 25))6. Whether there is incremental benefit above treatment with dexamethasone is not yet clear. Studies are ongoing including use of inhaled remdesivir (NCT04480333) and use in combination with other agents.
Repurposed drugs for influenza: favipiravir and umfenovir
Favipiravir is an oral RNA-dependent RNA polymerase inhibitor with in vitro antiviral activity at high concentrations7. An open-label study in mild-moderate COVID-19 (n = 80) in combination with inhaled interferon-α reported positive results, and favipiravir was approved for marketing in China for COVID-19 in March 20208. Multiple phase 2 trials are active including in Australia9. Concerns exist about the pharmacokinetics of favipiravir, with low trough levels in critically ill patients and potential for the emergence of resistance10.
Umfenovir (Arbidol) is a non-nucleoside antiviral targeting the viral spike (S-)protein-ACE2 host receptor interactions, inhibiting membrane fusion of the viral envelope10. Umfenovir may also promote interferon synthesis. Published results so far are inconclusive12,13, with randomised studies in progress.
Non-specific immune enhancement with antiviral activity: interferons
Type I interferons have broad antiviral activities and recombinant IFN-I proteins (parenteral and inhaled) are being trialled in COVID-1914,15. Use of interferons in acute infection needs to be explored carefully, as type I interferons have been associated with exacerbation of inflammation in progression to severe COVID-19, with potential to worsen disease16,46. Timing may be critical. In a retrospective study in COVID-19 (n = 446), late use of IFN-α led to increased mortality and delayed recovery, whilst earlier use was associated with reduced mortality17.
Repurposed anti-parasitic agents: ivermectin and nitazoxanide
Ivermectin (used to treat infections such as strongyloidiasis, scabies and onchocerciasis), and nitazoxanide (used in giardia and cryptosporidium) have in vitro activity against SARS-CoV-2 and are in preclinical early clinical trials against COVID-1918. Nitazoxanide also has reported immunomodulatory activity, suppressing murine IL-6 levels18,19.
Repurposed anti-HIV drugs: lopinavir/ritonavir and other anti-HIV proteases
The HIV protease inhibitor lopinavir has in vitro activity against SARS-CoV-1 and MERS, and possible activity in vivo20. In vitro activity was demonstrated against SARS-CoV-221, but no benefit in treatment of COVID-19 was reported in randomised published studies or in press release of results from the large UK ‘RECOVERY’ (Randomised Evaluation of COVID-19 Therapy) and WHO ‘Solidarity’ Trials13,22–24. Other studies are ongoing with lopinavir/ritonavir and other antiretrovirals including nelfinavir, tenofovir, lamivudine and others, either alone or in combination.
Repurposed drugs for malaria: hydroxychloroquine and chloroquine.
Hydroxychloroquine (licensed as an antimalarial and anti-arthritis agent) and chloroquine were widely used in the early days of the COVID-19 pandemic, due to their potential to block viral entry, immunomodulatory impact and in vitro activity against SARS-CoV-225. Potential toxicities include prolonged QT interval, lowered convulsive threshold, retinopathy and cardiac myopathy. Randomised trials in mild, moderate and severe disease show no benefit in COVID-1926–29 and the WHO have discontinued the hydroxychloroquine arm in the ‘Solidarity’ trial. A US federal drug administration (FDA) emergency use authorisation (EUA) for hydroxychloroquine in COVID-19 issued in March was revoked in June. Hydroxychloroquine has also been shown not effective for SARS-CoV-2 post-exposure prophylaxis28. Studies investigating its utility for prevention of COVID-19 in healthcare workers are continuing, including in Australia30.
Repurposed drugs with cellular targets used for viral entry: ACE2 and TMRPSS2 inhibitors
SARS-CoV-2 uses the acetylcholinesterase (ACE)-2 receptor for cell entry, and serine protease TMPRSS2 for S-protein priming, both potential targets for antiviral intervention31. Agents that block these interactions are in clinical trials, including the serine protease inhibitor nafamostat mesylate which also has anticoagulant activity and is approved in Japan for treatment of pancreatitis32,33.
The upregulation of ACE-2 receptors with use of the common anti-hypertensive agents ACE-inhibitors and angiotensin receptor blockers (ARB) was theorised to potentially lead to poorer outcomes in COVID-19 by enhancing viral entry; however, this has not been borne out by clinical data47. Conversely, ARBs could provide benefit via receptor blockade, impairing viral cell entry48.
Antibodies
SARS-CoV-2 specific neutralising antibodies
In Ebola and HIV, pathogen-targeting antibodies have been identified and cloned for therapeutic use34. Regeneron Pharmaceuticals (USA) published their discovery of several antibodies highly potent in suppressing SARS-CoV-2 replication in mouse models35. REGN-CoV-2 (includes both REGN10933 and REGN10987) is designed to bind to two points on the SARS-CoV-2 S-protein to prevent it entering healthy cells. It is being assessed for safety and efficacy in preventing secondary infection or symptom onset amongst 2000 household contacts of people with SARS-CoV-236.
Another investigational monoclonal antibody LY-CoV555, developed after identification from blood from a patient who had recovered from COVID-19, is being studied in COVID-19 under an adaptive trial master protocol (‘ACTIV-2’ (outpatients) and ‘ACTIV-3’ (inpatients)) designed to enable phase 2 testing of investigational agents with the capacity to expand smoothly to phase 337.
Convalescent plasma
In SARS, improved outcomes with convalescent plasma were reported in small retrospective case series38. A randomised trial in severe COVID-19 suggests no benefit, although the study was stopped early due to insufficient numbers, and plasma was given late (median 30 days from symptoms onset)39. Many other randomised studies are in progress. Meanwhile, over 50 000 patients have received convalescent plasma for treatment of COVID-19, mainly outside of clinical trial settings and an FDA EUA was issued on 23 August for its use in COVID-19.
Immunomodulation: blocking the pathogenic host immune response
Steroids
The ‘RECOVERY’ trial showed a reduction in 28-day mortality in patients hospitalised with COVID-19 receiving oxygen or invasive mechanical ventilation treated with dexamethasone40. 2104 patients receiving IV/oral dexamethasone (6 mg/day up to 10 days, median 6 days) had lower mortality compared to 4321 receiving usual care (22.9% vs 25.7%, age adjusted rate ratio 0.83 (0.75–0.93, P < 0.0001). Mortality was reduced by 35% among those receiving invasive mechanical ventilation, 20% in those on supplemental oxygen only and no impact was seen amongst those not receiving any respiratory support at randomisation. These results are consistent with respiratory compromise being driven by an overactive inflammatory response. Patients with symptoms for over 7 days had greater mortality benefit in response to dexamethasone treatment, compared to those with more recent onset. A recent meta-analysis combines results from ‘RECOVERY’ with six other studies to demonstrate a mortality benefit of steroids (including dexamethasone and hydrocortisone) in severe COVID-1941.
Anti-cytokine therapies
IL6 plays a key role in driving the dysregulated inflammatory response in COVID-19 with higher levels associated with greater disease severity49. IL-6 receptor antagonist tocilizumab is used to treat rheumatoid arthritis and is FDA-approved to treat cytokine release syndrome associated with CAR T-cell immunotherapy. It has beneficial effects in vitro and in animal models of sepsis and influenza42. Thousands of patients are reported to have been treated with tocilizumab; however, assessment of efficacy is impaired by lack of control groups and follow up.
A non-randomised study of tocilizumab in patients requiring mechanical ventilation reported a 45% reduction in hazard of death (hazard ratio 0.55 (95% CI 0.33–0.90)) in patients receiving tocilizumab (n = 78) compared to those who did not (n = 76) with follow up 47 days (median), although superinfections were more common (54% vs 26%, P < 0.001)43. Another IL6 receptor blocking agent, sarilumab and IL6 antagonist siltuximab are also in phase 3 trials.
The Janus kinase (JAK) 1/2 inhibitor, baricitinib, licensed for rheumatoid arthritis, has been identified as a candidate for therapy against SARS-CoV-2 due to potent anti-inflammatory effects and possible off-target antiviral effects44. A case series describing use in patients with moderate-severe COVID-19 reported safety and suggests improved outcomes45. Randomised studies are in progress and will be imperative in ascertaining the benefit of these agents, particularly in combination with direct acting antivirals, and the balance with immunocompromise and adverse events.
Other considerations
Personalisation
Different factors drive disease manifestations at different stages of SARS-CoV2 infection, and optimal management may depend on stage of illness at time of presentation. Furthermore, the course of COVID-19 differs significantly amongst individuals, with higher risk of progression to severe disease seen in the elderly. Individualised therapy using informatics strategies based on stage and prediction of disease progression have been associated with improved patient outcomes50. Therapy based on immunophenotyping has also been suggested, based on readily identifiable immunological signatures associated with different disease trajectories51,52.
Combination therapy
The benefit of therapeutic antiviral combinations is also being explored. In a randomised trial in early COVID-19 (<7 days onset), the combination of lopinavir/ritonavir, interferonβ-1B and ribavirin resulted in greater reduction of virus in nasopharyngeal swabs and quicker time to recovery compared to lopinavir/ritonavir alone14. Combining antiviral and immunomodulatory agents may be important in management of COVID-19 given the role of the inflammatory response in disease evolution severity. Timing of different therapeutics should be considered carefully in trial design.
Conclusions
Repurposing existing therapies will be the quickest way to find effective intervention and improve outcomes in COVID-19; however, bigger gains are likely through the development of new agents specific to SARS-CoV2. These are being rapidly engineered using high-throughput screening platforms. Websites tracking COVID-19 clinical trials and the development of new agents show over 300 novel agents are in pre-clinical testing. Their diverse actions reflect the range of pathology seen in COVID-19 that is a consequence of both viral action as well as immune response. Their introduction into clinical trials will be the next exciting phase of therapeutics.
Conflicts of interest
JA, JS and SRL have received investigator-initiated funding for research from Gilead Sciences for work unrelated to COVID-19. SRL has received honoraria for education activities supported by Gilead Sciences and Viiv Healthcare. SRL has received research support from Gilead Sciences, Viiv Healthcare and Merck. SRL is a member of scientific advisory boards to Merck Gilead Sciences and Biotron.
Acknowledgements
This research did not receive any specific funding.
References
[1] Oberfeld, B. et al. (2020) SnapShot: COVID-19. Cell 181, 954–954e.1[2] National COVID-19 Clinical Evidence Taskforce (2020) Caring for people with COVID-19. Living Guidelines. https://covid19evidence.net.au/#living-guidelines (accessed 11 August 2020).
[3] BioCentury (2020) COVID-19 Clinical Trial Dashboard. https://www. biocentury.com/reports/covid-19-clinical-trial-dashboard (accessed 16 August 2020).
[4] Thorlund, K. et al. (2020) A real-time dashboard of clinical trials for COVID-19. The Lancet Digital Health 2, e286–e287.
| 32363333PubMed |
[5] Beigel, J.H. et al. (2020) Remdesivir for the treatment of Covid-19 – preliminary report. N. Engl. J. Med. , .
| Remdesivir for the treatment of Covid-19 – preliminary report.Crossref | GoogleScholarGoogle Scholar | 32991794PubMed |
[6] Goldman, J.D. et al. (2020) Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. , .
| Remdesivir for 5 or 10 days in patients with severe Covid-19.Crossref | GoogleScholarGoogle Scholar | 32459919PubMed |
[7] Furuta, Y. et al. (2017) Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 93, 449–463.
| Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase.Crossref | GoogleScholarGoogle Scholar |
[8] Cai, Q. et al. (2020) Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering (Beijing) , .
| Experimental treatment with Favipiravir for COVID-19: an open-label control study.Crossref | GoogleScholarGoogle Scholar | 33145303PubMed |
[9] ClinicalTrials.gov. An adaptive clinical trial of antivirals for COVID-19 infection (VIRCO). Identifier NCT04445467, 2020 Jun 24, Bethesda, MD: National Library of Medicine (US). http://clinicaltrials.gov/ct/show/NCT04445467 (accessed 26 August 2020).
[10] Irie, K. et al. (2020) Pharmacokinetics of favipiravir in critically ill patients with COVID-19. Clin. Transl. Sci. , .
| Pharmacokinetics of favipiravir in critically ill patients with COVID-19.Crossref | GoogleScholarGoogle Scholar | 32475019PubMed |
[11] Wang, X. et al. (2020) The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 6, 28.
| The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro.Crossref | GoogleScholarGoogle Scholar | 32373347PubMed |
[12] Lian, N. et al. (2020) Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin. Microbiol. Infect. 26, 917–921.
| Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study.Crossref | GoogleScholarGoogle Scholar | 32344167PubMed |
[13] Li, Y. et al. (2020) Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial. Med (NY). , .
| Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial.Crossref | GoogleScholarGoogle Scholar | 33136711PubMed |
[14] Hung, I.F. et al. (2020) Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395, 1695–1704.
| Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial.Crossref | GoogleScholarGoogle Scholar | 32401715PubMed |
[15] Shalhoub, S. (2020) Interferon beta-1b for COVID-19. Lancet 395, 1670–1671.
| Interferon beta-1b for COVID-19.Crossref | GoogleScholarGoogle Scholar | 32401712PubMed |
[16] Lee, J.S. and Shin, E.C. (2020) The type I interferon response in COVID-19: implications for treatment. Nat. Rev. Immunol. , .
| The type I interferon response in COVID-19: implications for treatment.Crossref | GoogleScholarGoogle Scholar | 32788708PubMed |
[17] Wang, N. et al. (2020) Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe , .
| Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients.Crossref | GoogleScholarGoogle Scholar | 32707096PubMed |
[18] Caly, L. et al. (2020) The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 178, 104787.
| The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro.Crossref | GoogleScholarGoogle Scholar | 32251768PubMed |
[19] Hong, S.K. et al. (2012) Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice. Int. Immunopharmacol. 13, 23–27.
| Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice.Crossref | GoogleScholarGoogle Scholar | 22430099PubMed |
[20] Chu, C.M. et al. (2004) Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 59, 252–256.
| Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings.Crossref | GoogleScholarGoogle Scholar | 14985565PubMed |
[21] Choy, K.T. et al. (2020) Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 178, 104786.
| Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro.Crossref | GoogleScholarGoogle Scholar | 32251767PubMed |
[22] Cao, B. et al. (2020) A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 382, 1787–1799.
| A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19.Crossref | GoogleScholarGoogle Scholar | 32187464PubMed |
[23] (2020) Recovery, Statement from the Chief Investigators: no clinical benefit from use of lopinavir-ritonavir in hospitalised COVID-19 patients studied in RECOVERY (Randomised Evaluation of COVID-19 Therapy), Lopinavir-Ritonavir results.
[24] WHO (2020) WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19.
[25] Yao, X. et al. (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 71, 732–739.
| In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).Crossref | GoogleScholarGoogle Scholar | 32150618PubMed |
[26] Hernandez, A.V. et al. (2020) Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann. Intern. Med. , .
| Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review.Crossref | GoogleScholarGoogle Scholar | 33085507PubMed |
[27] Skipper, C.P. et al. (2020) Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann. Intern. Med. , .
| Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial.Crossref | GoogleScholarGoogle Scholar | 32673060PubMed |
[28] Boulware, D.R. et al. (2020) A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N. Engl. J. Med. 383, 517–525.
| A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19.Crossref | GoogleScholarGoogle Scholar | 32492293PubMed |
[29] Horby, P. et al. (2020) Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv. , .
| Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial.Crossref | GoogleScholarGoogle Scholar |
[30] (2020) COVID-SHIELD clinical trial. https://www.covidshieldtrial.com.au/#!/ (accessed 17 August 2020).
[31] Hoffmann, M. et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8.
| SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor.Crossref | GoogleScholarGoogle Scholar | 32142651PubMed |
[32] Hoffmann, M. et al. (2020) Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob. Agents Chemother. 64, e00754-20.
| Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19.Crossref | GoogleScholarGoogle Scholar | 32312781PubMed |
[33] Doi, K. et al. (2020) Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: a case series. Crit. Care 24, 392.
| Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: a case series.Crossref | GoogleScholarGoogle Scholar | 32620147PubMed |
[34] Salazar, G. et al. (2017) Antibody therapies for the prevention and treatment of viral infections. NPJ Vaccines 2, 19.
| Antibody therapies for the prevention and treatment of viral infections.Crossref | GoogleScholarGoogle Scholar | 29263875PubMed |
[35] Baum, A. et al. (2020) Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 369, 1014–1018.
| Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies.Crossref | GoogleScholarGoogle Scholar | 32540904PubMed |
[36] ClinicalTrials.gov. Study assessing the efficacy and safety of anti-spike SARS-CoV2 Monoclonal Antibodies for Prevention of SARS CoV-2 Infection in Healthy Adults Who are Household Contacts to an Individual With a Positive SARS-CoV-2 RT-PCR Assay. Identifier NCT04452318, 2020 Jun 30, Bethesda, MD: National Library of Medicine (US). http://clinicaltrials.gov/ct/show/NCT04452318 (accessed 30 September 2020).
[37] NIH (2020) Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV). https://www.nih.gov/research-training/medical-research-initiatives/activ/covid-19-therapeutics-prioritized-testing-clinical-trials (accessed 27 August 2020).
[38] Cheng, Y. et al. (2005) Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 24, 44–46.
| Use of convalescent plasma therapy in SARS patients in Hong Kong.Crossref | GoogleScholarGoogle Scholar | 15616839PubMed |
[39] Li, L. et al. (2020) Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA , .
| Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial.Crossref | GoogleScholarGoogle Scholar | 32492084PubMed |
[40] Horby, P. et al. (2020) Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N. Engl. J. Med. , .
| Dexamethasone in hospitalized patients with Covid-19 – preliminary report.Crossref | GoogleScholarGoogle Scholar | 33031652PubMed |
[41] Sterne, J.A.C. et al. (2020) Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA , e2017023..
[42] Cortegiani, A. et al. (2020) Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. , .
| Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review.Crossref | GoogleScholarGoogle Scholar | 32828727PubMed |
[43] Somers, E.C. et al. (2020) Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. , ciaa954.
| Tocilizumab for treatment of mechanically ventilated patients with COVID-19.Crossref | GoogleScholarGoogle Scholar | 32651997PubMed |
[44] Richardson, P. et al. (2020) Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395, e30–e31.
| Baricitinib as potential treatment for 2019-nCoV acute respiratory disease.Crossref | GoogleScholarGoogle Scholar | 32032529PubMed |
[45] Titanji, B.K. et al. (2020) Use of baricitinib in patients with moderate to severe COVID-19. Clin. Infect. Dis. , ciaa879..
| Use of baricitinib in patients with moderate to severe COVID-19.Crossref | GoogleScholarGoogle Scholar | 32926165PubMed |
[46] Lucas, C. et al. (2020) Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584, 463–469.
| Longitudinal analyses reveal immunological misfiring in severe COVID-19.Crossref | GoogleScholarGoogle Scholar | 32717743PubMed |
[47] de Abajo, F.J. et al. (2020) Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet 395, 1705–1714.
| Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study.Crossref | GoogleScholarGoogle Scholar | 32416785PubMed |
[48] Gurwitz, D. (2020) Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 81, 537–540.
| Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics.Crossref | GoogleScholarGoogle Scholar | 32129518PubMed |
[49] Zhou, F. et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062.
| Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study.Crossref | GoogleScholarGoogle Scholar | 32171076PubMed |
[50] Garcia-Vidal, C. et al. (2020) Personalized therapy approach for hospitalized patients with COVID-19. Clin. Infect. Dis. , ciaa964.
| Personalized therapy approach for hospitalized patients with COVID-19.Crossref | GoogleScholarGoogle Scholar | 32649747PubMed |
[51] Kuri-Cervantes, L. et al. (2020) Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 5, eabd7114.
| Comprehensive mapping of immune perturbations associated with severe COVID-19.Crossref | GoogleScholarGoogle Scholar | 32669287PubMed |
[52] Giamarellos-Bourboulis, E.J. et al. (2020) Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27, 992–1000.e3.
| Complex immune dysregulation in COVID-19 patients with severe respiratory failure.Crossref | GoogleScholarGoogle Scholar | 32320677PubMed |
Biographies
Dr Kasha Singh is an infectious diseases physician with a wide range of interests including public and refugee health and translational research. Dr Singh worked in the UK for 10 years, completing a HIV fellowship at Chelsea and Westminster Foundation Trust in London. While based in London, Dr Singh was also involved in running international clinical trials of tuberculosis treatment with the MRCP/UCL, including capacity development and education. Dr Singh is interested in persistent viral infections and the public health impact and management of infectious diseases, particularly HIV, hepatitis B, tuberculosis and now also COVID-19.
Associate Professor Joe Sasadeusz is an infectious diseases physician who subspecializes in medical virology. He has particular interests in viral hepatitis in both HIV uninfected as well as coinfected individuals. He also works in infections in immunocompromised hosts, especially patients who have undergone a haematopoietic stem cell transplant. He is heavily involved in the education of general practitioners via the VHITTAL program. He also conducts clinical trials and has multiple research projects in the above areas.
Professor Sharon Lewin, is the inaugural Director of the Doherty Institute. She is also a Melbourne Laureate Professor, The University of Melbourne and a National Health and Medical Research Council (NHMRC) Practitioner Fellow. As an infectious diseases physician and basic scientist, her laboratory focuses on basic, translational and clinical research aimed at finding a cure for HIV and understanding the interaction between HIV and hepatitis B virus. Her laboratory is funded by the NHMRC, the National Institutes of Health, The Wellcome Trust, the American Foundation for AIDS Research and multiple commercial partnerships. She is also the Chief Investigator of a NHMRC Centre of Research Excellence (CRE), The Australian Partnership for Preparedness Research on Infectious Diseases Emergencies (APPRISE) that aims to bring together Australia’s leading experts in clinical, laboratory and public health research to address the key components required for a rapid and effective emergency response to infectious diseases.
Dr Jennifer Audsley is a Clinical Research Fellow based at the Doherty Institute. She has specific research interests in HIV-hepatitis co-infection and PrEP (Pre-exposure prophylaxis) for HIV prevention. Her research in HIV focuses on long-term treatment in people living with HIV and hepatitis B virus (HBV), liver disease pathogenesis in people living with HIV and HBV, and HBV cure in the setting of HIV-HBV co-infection. She leads the HIV-Hepatitis group within Professor Lewin's research program.