Movement of arboviruses between Indonesia and Western Australia
Harapan Harapan A B C D and Allison Imrie D EA Medical Research Unit, School of Medicine, Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia
B Tropical Disease Centre, School of Medicine, Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia
C Depertment of Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia
D School of Biomedical Sciences, University of Western Australia, Nedlands, WA 6009, Australia
E Tel.: +61 8 6457 1377; Fax: +61 8 6457 4519; Email: allison.imrie@uwa.edu.au
Microbiology Australia 42(4) 165-169 https://doi.org/10.1071/MA21047
Submitted: 30 August 2021 Accepted: 20 October 2021 Published: 8 November 2021
Journal Compilation © The Authors 2021 Open Access CC BY-NC-ND, published (by CSIRO Publishing) on behalf of the ASM
Abstract
Dengue virus (DENV) and chikungunya virus (DENV) are arboviruses of major public health importance. Monitoring circulation of medically important mosquito-borne viruses in the Indo Pacific region allows countries to predict disease outbreaks and prepare mitigation and control strategies. We have monitored long-term molecular epidemiology of DENV and CHIKV in Indonesia and Western Australia (WA), with febrile Western Australian travellers returning from Indonesia as sentinels. Our findings provide insights into the transmission dynamics of CHIKV genotypes and DENV serotypes, genotypes and lineages in the region and virus importation to WA. Our ongoing studies provide valuable and timely information on transmission of emerging and re-emerging arboviruses in the Indo Pacific region and furthermore provide detailed genomic data that inform our understanding of viral and epidemic virulence.
Dengue in Australia
Dengue is the most medically important mosquito-borne viral disease in humans, associated with significant morbidity, mortality, and economic cost1. The disease is caused by infection with any of four dengue virus (DENV-1 to DENV-4) serotypes. Since the first cases were documented in the Indonesian capital city of Jakarta in 19682, dengue has become prevalent in all provinces and nearly 60% of the Indonesian population of 270 million people live in areas where DENV is known to be circulating. In Australia transmission of DENV is currently restricted to urban areas of northern Queensland and the Torres Strait where Aedes aegypti, the main DENV vector, is well established. Modernisation of transportation systems has enhanced introduction of DENV from endemic countries and led to regular outbreaks in Australia where the number of imported dengue cases increased by 298% and 155%, in 2010 and 2011 respectively, compared with the 5-year mean3. More than half of all dengue cases imported to Australia are acquired in Indonesia3–6.
In Western Australia (WA), dengue outbreaks were reported in the north of the state up until the mid-1940s. Ae. aegypti mosquitoes were eradicated by the end of the 1960s7 and currently, there are no endemic mosquito species with potential to vector DENV in WA8. Although autochthonous DENV transmission does not occur in WA, the state has reported the highest number of dengue cases in the country in recent years9; approximately 80% were acquired in Bali10, one of the most popular tourist destinations for WA residents especially after new budget airlines began offering affordable package holidays in the early 2000s. Dengue notification in WA increased from an annual average of 14 cases in 2006 to 558 cases in 201510.
We have monitored long-term molecular epidemiology of DENV in WA and in Indonesia to understand virus movement from the Asia Pacific region to the state. Our analysis of DENV imported by travellers returning to WA identified a DENV-2 (Cosmopolitan genotype) lineage that emerged during a major dengue outbreak in Bali during 2011–20126. The virus was exported to other Asian and European countries in subsequent years11,12. As introduction of new lineages to unaffected regions may be associated with more severe forms of dengue disease13–16, circulation of this lineage and other newly emergent lineages should be monitored. DENV genomic surveillance among returning travellers provides valuable and timely information to understand DENV transmission in Indonesia and neighbouring countries; monitor emergence and transmission dynamics of virulent DENV lineages in the Asia Pacific region, and also to identify genetic and biological features of DENV introduced to WA.
DENV movement from Indonesia to Western Australia
Data from the WA Notifiable Infectious Diseases Database, Communicable Disease Control Directorate, Department of Health, indicate that between 1 January 2016 and 31 December 2017 there were 739 imported dengue fever (DF) notifications17. In this surveillance system both confirmed and probable cases are included. Case definitions for confirmed and probable cases, based on WHO criteria, have been published elsewhere by WA Department of Health18.
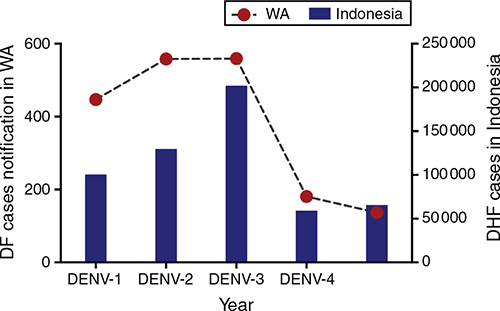
There was a sharp decrease in DF notifications in WA in 2017 (181 cases) compared with 2016 (558 cases) and case numbers continued to decrease in 2018 (137 cases). DF case notifications in WA mirrored the number of dengue haemorrhagic fever (DHF) cases reported by Ministry of Health (MoH) of Indonesia (Figure 1). In Indonesia, DHF cases decreased from 201 885 cases in 2016 to 59,047 cases in 2017, with incidence rate 77.9 and 22.5 per 100 000 population, respectively19,20. In 2018, there were 65 602 DHF cases and 467 deaths21.
|
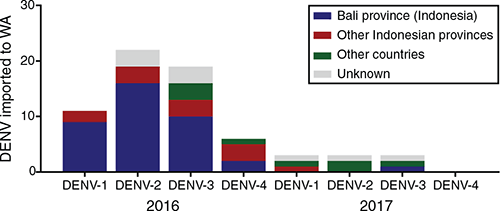
During 2016–2017 we isolated and/or sequenced 67 DENV (58 in 2016 and 9 in 2017). In 2016, 48 of 58 DENV (82.7%) originated in Indonesia, of which 37 (77.1%) were derived from travellers returning from Bali. One virus was imported to WA from Solomon Islands and one from an unspecified Pacific nation; one each from Thailand and an unspecific Southeast Asian country, in addition to six DENV of unknown origin (Figure 2). In 2017, a low number of dengue cases was reported in WA. Nine DENVs were successfully sequenced and only two viruses (serotypes DENV-1 and DENV-3) were isolated from travellers known to have returned from Bali; six samples originated in East Timor, Vietnam, Solomon Island, Thailand, Malaysia and India, and travel history was unknown for the remaining DENV.
|
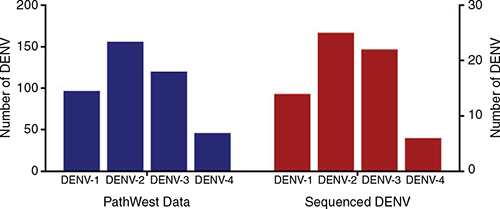
Of the 67, 2016–2017 DENV sequenced the predominant serotype was DENV-2 (25; 37.3%) followed by DENV-3 (22; 32.8%), DENV-1 (14; 20.9%) and DENV-4 (6; 8.9%) (Figure 3). This serotype distribution was similar to diagnostic RT-PCR serotyping data obtained from PathWest Laboratory. Among 419 DENV diagnostic serotype reports from 2016–2017, DENV-2 accounted for 37.2% followed by DENV-3 (28.6%), DENV-1 (23.1%) and DENV-2 (10.9%) (Figure 3).
|
All four serotypes were identified in WA travellers during 2016 with DENV-2 at highest frequency. As is often reported globally, DENV-4 circulated less frequently than the other three DENV serotypes.
Movement of chikungunya from Indonesia to Western Australia
Chikungunya virus (CHIKV) is endemic in Indonesia. CHIKV is vectored by Ae. aegypti and Ae. albopictus mosquitoes, the same species that transmit DENV. The first chikungunya outbreak reported in Indonesia occurred in June 1982 in Jambi province of Sumatra Island, followed by multiple outbreaks between 1983 and 198422 in Yogyakarta on the island of Java. Cases were then rarely reported in the country, until CHIKV re-emerged throughout Indonesia in early 2001. Multiple outbreaks have been reported subsequently, of which two were nation-wide, during 2009–2010 and in 2013, with 137 655 and 15 324 cases respectively23,24.
Modernisation of transportation systems has also contributed to the global expansion of CHIKV. CHIKV-infected travellers have been documented in more than twenty countries throughout Asia, Europe, and North America23. In Australia, data from the National Arbovirus and Malaria Advisory Committee indicate that between 2008 and 2014, there were 327 notified chikungunya cases in the country and almost 40% originated from Indonesia24–29. There was a significant increase in cases during 2012–2013 from 20 cases in the previous year26 to 96 cases in 2012–201325. Exported chikungunya cases that originated from Indonesia have been reported, of which most are travellers returning to Australia22.
Chikungunya was included as notifiable infectious disease in WA from May 2008. Since that time, the average annual incidence was six cases until 2013 when the case count increased significantly to 51 cases for the period January to September30. The majority (92%) originated from Indonesia, of which most (94%) were travellers returning from Bali30. In Indonesia, during the same year, there was a nationwide chikungunya outbreak in which 15 324 cases were reported to MoH31. The increase in imported chikungunya cases in WA, and Australia, reflected the increased incidence in Indonesia identified by national chikungunya surveillance during 2012–2013. Phylogenetic analysis of CHIKV E gene sequences provides important information on CHIKV movements to Australia, and our studies have identified importation of CHIKV associated with the nation-wide chikungunya outbreak in Indonesia in 201322.
The risk of dengue and chikungunya emergence in Australia
Dengue outbreaks have been reported in Australia since 1879. International travel plays an important role in introduction of dengue and chikungunya although autochthonous transmission is dependent on presence of the mosquito vectors locally. Aedes mosquito species known to vector DENV, CHIKV, and other medically important arboviruses were previously present in WA, New South Wales, Northern Territory and Queensland and in WA dengue outbreaks were reported in the north of the state up until the mid-1940s. Improvements in public health infrastructure led to eradication of the vectors in many areas and they have not been present in WA since the 1960s. DENV introduced to Australia by travellers is associated with regularly reported outbreaks6. During 1990–2009, 31 dengue outbreaks were recorded in northern Queensland alone. During 2004–2013, there were 5943 imported dengue cases nationally with an average incidence of 7.78 per 100 000 travellers over the 9-year period4, ranging between 1.65 to 17.75 per 100 000 travellers in 2005 and 2013, respectively. The number of affected areas across the country also increased significantly4. More than half of all imported dengue cases were acquired in Indonesia3,4 and Indonesia is the main source of dengue and chikungunya importation cases9,22–32.
WA demonstrates the highest relative risk of imported dengue compared to other states3, associated with high rates of travel to endemic countries in the Southeast Asian region such as Indonesia. More than 80% of imported dengue cases in WA were acquired in Bali3. A modelling study using the assumption that Aedes mosquitoes are introduced solely by human transport, indicates that the risks of dengue outbreak are higher in northern part of WA but can extend to the Perth metropolitan area during summer33. In 2013, the first acquired DENV infection in WA after 1960 was reported8, thought to be caused by a bite from a single infected mosquito vector that was transiently introduced to the region. This case highlights the public health importance of maintaining surveillance efforts to ensure that any incursions of dengue vectors into WA are promptly identified and do not become established, particularly given the large numbers of viraemic travellers returning from dengue-endemic regions.
Currently, although autochthonous transmission of dengue in Australia is restricted to urban areas of north Queensland and the Torres Strait where Ae. aegypti remains well established, large parts of Australia including WA are climatically suitable for dengue vector species7. A modelling study using the current distribution of Ae. aegypti and climate change scenarios for 2030 and 2050 suggested that WA is one of the regions at high risk for Ae. aegypti expansion. These data indicate there is a risk of both mosquito vectors and DENV becoming established in WA. In addition, Australia has several other mosquito species potentially capable of facilitating an outbreak of CHIKV should the virus be introduced. Aedes spp. were highly competent laboratory vectors of CHIKV, particularly Ae. vigilax, Ae. procax, as well as other species including Culex linealis34,35. Therefore, case and vector surveillance as well as population health responses are crucial for minimising any potential impact of DENV and CHIKV establishment in Australia. In summary, our molecular epidemiology studies indicate that continuous surveillance using WA travellers as sentinels provides timely information on transmission dynamics of major arboviruses in the Asia Pacific region. These data provide important indicators that support and strengthen preparedness for emerging epidemic threats, including mosquito-borne diseases, in Australia and the region.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] Harapan, H. et al. (2020) Dengue: A Minireview. Viruses 12, .| Dengue: A Minireview.Crossref | GoogleScholarGoogle Scholar | 32751561PubMed |
[2] Kho, L.K. et al. (1969) Dengue hemorrhagic fever in Djakarta. J Indones Med Assoc 19, 417.
[3] Knope, K. and Giele, C. (2013) Increasing notifications of dengue in Australia related to overseas travel, 1991 to 2012. Commun. Dis. Intell. Q. Rep. 37, E55–E59.
| 23692160PubMed |
[4] Huang, X.D. et al. (2016) Dynamic spatiotemporal trends of imported dengue fever in Australia. Scientific Reports 6, 30360.
| Dynamic spatiotemporal trends of imported dengue fever in Australia.Crossref | GoogleScholarGoogle Scholar |
[5] Warrilow, D. et al. (2012) Sources of dengue viruses imported into Queensland, Australia, 2002-2010. Emerg. Infect. Dis. 18, 1850–1857.
| Sources of dengue viruses imported into Queensland, Australia, 2002-2010.Crossref | GoogleScholarGoogle Scholar | 23092682PubMed |
[6] Ernst, T. et al. (2015) Emergence of a new lineage of dengue virus type 2 identified in travelers entering Western Australia from Indonesia, 2010-2012. PLoS Negl. Trop. Dis. 9, .
| Emergence of a new lineage of dengue virus type 2 identified in travelers entering Western Australia from Indonesia, 2010-2012.Crossref | GoogleScholarGoogle Scholar | 25635775PubMed |
[7] Beebe, N.W. et al. (2009) Australia’s dengue risk driven by human adaptation to climate change. PLoS Negl. Trop. Dis. 3, .
| Australia’s dengue risk driven by human adaptation to climate change.Crossref | GoogleScholarGoogle Scholar | 19415109PubMed |
[8] Lindsay, M.D. et al. (2015) Investigation of the first case of dengue virus infection acquired in Western Australia in seven decades: evidence of importation of infected mosquitoes? PLoS Negl. Trop. Dis. 9, .
| Investigation of the first case of dengue virus infection acquired in Western Australia in seven decades: evidence of importation of infected mosquitoes?Crossref | GoogleScholarGoogle Scholar | 26406471PubMed |
[9] NNDSS Annual Report Working Group (2016) Australia’s notifiable disease status, 2014: Annual report of the National Notifiable Diseases Surveillance System. Commun. Dis. Intell Q. Rep. 40, E48–145.
| 27080029PubMed |
[10] WA Department of Health (2016) Notifiable infectious disease reports, WA Department of Health Dengue fever notifications in Western Australia. Perth Government of Western Australia, Department of Health. http://www.public.health.wa.gov.au/3/1547/3/dengue_fever.pm (accessed 16 November 2016).
[11] Chang, S.F. et al. (2016) Laboratory-based surveillance and molecular characterization of dengue viruses in Taiwan, 2014. Am. J. Trop. Med. Hyg. 94, 804–811.
| Laboratory-based surveillance and molecular characterization of dengue viruses in Taiwan, 2014.Crossref | GoogleScholarGoogle Scholar | 26880779PubMed |
[12] Shihada, S. et al. (2017) Genetic diversity and new lineages of dengue virus serotypes 3 and 4 in returning travelers, Germany, 2006–2015. Emerg. Infect. Dis. 23, 272–275.
| Genetic diversity and new lineages of dengue virus serotypes 3 and 4 in returning travelers, Germany, 2006–2015.Crossref | GoogleScholarGoogle Scholar | 28098525PubMed |
[13] Rico-Hesse, R. et al. (1997) Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology 230, 244–251.
| Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas.Crossref | GoogleScholarGoogle Scholar | 9143280PubMed |
[14] Ty Hang, V.T. et al. (2010) Emergence of the Asian 1 genotype of dengue virus serotype 2 in Viet Nam: in vivo fitness advantage and lineage replacement in South-East Asia. PLoS Negl. Trop. Dis. 4, .
| Emergence of the Asian 1 genotype of dengue virus serotype 2 in Viet Nam: in vivo fitness advantage and lineage replacement in South-East Asia.Crossref | GoogleScholarGoogle Scholar |
[15] Williams, M. et al. (2014) Lineage II of Southeast Asian/American DENV-2 is associated with a severe dengue outbreak in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 91, 611–620.
| Lineage II of Southeast Asian/American DENV-2 is associated with a severe dengue outbreak in the Peruvian Amazon.Crossref | GoogleScholarGoogle Scholar | 25002298PubMed |
[16] Shrivastava, A. et al. (2015) Lineage shift of dengue virus in Eastern India: an increased implication for DHF/DSS. Epidemiol. Infect. 143, 1599–1605.
| Lineage shift of dengue virus in Eastern India: an increased implication for DHF/DSS.Crossref | GoogleScholarGoogle Scholar | 25314901PubMed |
[17] Communicable Disease Control Directorate Department of Health Western Australia (2017) Notifiable Infectious Disease Reports. Perth Department of Health Western Australia. www.public.health.wa.gov.au/2/243/3/infectious_diseases_az_for_health_professionals.pm (accessed 23 September 2021).
[18] Department of Health WA (2019) Dengue fever. Department of Health of Western Australia: Perth. https://ww2.health.wa.gov.au/Articles/A_E/Dengue-Fever (accessed 3 September 2021).
[19] Kemenkes, R.I. (2018) Profil kesehatan Indonesia tahun 2017. Jakarta: Kementerian Kesehatan RI.
[20] Kemenkes, R.I. (2017) Profil kesehatan Indonesia tahun 2016. Jakarta: Kementerian Kesehatan RI.
[21] Kemenkes, R.I. (2019) Profil kesehatan Indonesia tahun 2018. Jakarta: Kementerian Kesehatan RI.
[22] Porter, K.R. et al. (2004) A serological study of Chikungunya virus transmission in Yogyakarta, Indonesia: evidence for the first outbreak since 1982. Southeast Asian J. Trop. Med. Public Health 35, 408–415.
| 15691147PubMed |
[23] Harapan, H. et al. (2019) Chikungunya virus infection in Indonesia: a systematic review and evolutionary analysis. BMC Infect. Dis. 19, 243.
| Chikungunya virus infection in Indonesia: a systematic review and evolutionary analysis.Crossref | GoogleScholarGoogle Scholar | 30866835PubMed |
[24] Nasci, R.S. (2014) Movement of Chikungunya virus into the Western Hemisphere. Emerg. Infect. Dis. 20, 1394–1395.
| Movement of Chikungunya virus into the Western Hemisphere.Crossref | GoogleScholarGoogle Scholar | 25061832PubMed |
[25] Knope, K.E. et al. (2016) Arboviral diseases and malaria in Australia, 2013–14: annual report of the National Arbovirus and Malaria Advisory Committee. Commun. Dis. Intell. Q. Rep. 40, E400–E436.
| 28278416PubMed |
[26] Knope, K.E. et al. (2016) Arboviral diseases and malaria in Australia, 2012–13: annual report of the National Arbovirus and Malaria Advisory Committee. Commun. Dis. Intell. Q. Rep. 40, E17–E47.
| 27080023PubMed |
[27] Knope, K.E. et al. (2014) Arboviral diseases and malaria in Australia, 2011–12: annual report of the National Arbovirus and Malaria Advisory Committee. Commun. Dis. Intell. Q. Rep. 38, E122–E142.
| 25222207PubMed |
[28] Knope, K. et al. (2013) Arboviral diseases and malaria in Australia, 2010–11: annual report of the National Arbovirus and Malaria Advisory Committee. Commun. Dis. Intell. Q. Rep. 37, E1–E20.
| 23692155PubMed |
[29] Wright, P. et al. (2012) Arboviral diseases and malaria in Australia, 2009–10: annual report of the National Arbovirus and Malaria Advisory Committee. Commun. Dis. Intell. Q. Rep. 36, 70–81.
| 23153083PubMed |
[30] Fitzsimmons, G.J. et al. (2010) Arboviral diseases and malaria in Australia, 2008–09: annual report of the National Arbovirus and Malaria Advisory Committee. Commun. Dis. Intell. Q. Rep. 34, 225–240.
| 21090179PubMed |
[31] Department of Health WA (2013) Returned travellers from Bali behind spike in chikungunya virus infections. Diseases WAtch 17.
[32] Kemenkes, R.I. (2014) Profil kesehatan Indonesia tahun 2013. Jakarta: Kementerian Kesehatan RI.
[33] Viennet, E. et al. (2013) Assessing the threat of chikungunya virus emergence in Australia. Commun. Dis. Intell. Q. Rep. 37, E136–E143.
| 24168087PubMed |
[34] Ho, S.H. et al. (2017) Predicting arboviral disease emergence using Bayesian networks: a case study of dengue virus in Western Australia. Epidemiol. Infect. 145, 54–66.
| Predicting arboviral disease emergence using Bayesian networks: a case study of dengue virus in Western Australia.Crossref | GoogleScholarGoogle Scholar | 27620510PubMed |
[35] van den Hurk, A.F. et al. (2010) Vector competence of Australian mosquitoes for chikungunya virus. Vector Borne Zoonotic Dis. 10, 489–495.
| Vector competence of Australian mosquitoes for chikungunya virus.Crossref | GoogleScholarGoogle Scholar | 19877822PubMed |
Biographies
Dr Harapan is an Assistant Professor in School of Medicine, Unversitas Syiah Kuala Indonesia. He completed his PhD at University of Western Australia School of Biomedical Sciences. His PhD project focused on molecular epidemiology and public health investigations of major arboviruses in Indonesia with focus on dengue, chikungunya and Zika virus. He received his Diploma of Tropical Medicine and Hygiene from Mahidol University Bangkok, Faculty of Tropical Medicine. He has published more than 150 scientific articles. He is Editor-in-Chief of Narra J; Southeast Asia Editor for Reviews in Medical Virology; Associate Editor for BMC Public Health; and Topic Editor in Frontiers in Public Health. He is a member of American Society for Virology, American Society for Microbiology, The American Society of Tropical Medicine and Hygiene, and Australian Society for Microbiology. His fields of interest include public health and tropical diseases. He is also currently working in COVID-19-related research.
Dr Allison Imrie is an Associate Professor in School of Biomedical Sciences, University of Western Australia. Her early work with human immunodeficiency virus (HIV) focussed on virus transmission between transmitter and recipient pairs and immune responses in early HIV infection. She works with colleagues in the Asia Pacific region to investigate neglected tropical diseases including dengue, Zika, chikungunya and leptospirosis, and in Australia to investigate endemic mosquito-borne viruses and she collaborates with colleagues in Australia and the US to identify novel mosquito-borne viruses. She has served as a consultant with the World Health Organization and Department of Foreign Affairs and Trade to develop prevention and control strategies for emerging infectious diseases. Most recently she has been funded to investigate immune responses in people with recent coronavirus infection.


