Cheese quality and authenticity: new technologies help solve an age-old problem
Christopher Pillidge A * , Roya Afshari A and Harsharn Gill AA School of Science, RMIT University, Bundoora, Vic. 3083, Australia.

Dr Christopher Pillidge is a Lecturer in Food Technology at RMIT University. Before joining RMIT he worked for over 18 years for the New Zealand dairy industry, including at Fonterra, where he worked on cheese lactic acid bacteria and probiotics. In 2006 he moved from New Zealand to join Dairy Innovation Australia Ltd. He took up a research position at RMIT in 2016. His research interests include food microbiology, lactic acid bacteria and use of molecular methods to solve food industry problems. |

Dr Roya Afshari is an Honorary Research Fellow at RMIT University. She was awarded her PhD from RMIT University in 2020. Her research showed that multi-omics together with data integration analysis represents a powerful new approach for gaining deeper insights into the microbiota–metabolite interactions that underpin cheese flavour and quality. In 2021, she joined a commercial pharmaceutical and food ingredients company in Melbourne. Her research interests include molecular microbiology, metabolomics and use of multi-omics technologies to study food and natural products. |

Harsharn Gill is a Professor of Food and Health Biosciences at RMIT University. He has over 25 years of experience in leading and managing food, nutrition and health R&D in the private and public sectors. Before joining RMIT, he held senior R&D leadership roles in Australia and New Zealand. He has received several major awards and has been appointed to international expert panels, including WHO/FAO, NIH and IDF. Professor Gill sits on the editorial boards for several international scientific journals and patent-protected products resulting from his research have been commercialised globally. |
Microbiology Australia 43(2) 52-56 https://doi.org/10.1071/MA22019
Submitted: 24 March 2022 Accepted: 21 April 2022 Published: 17 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Cheese represents a complex ecosystem of starter and non-starter bacteria, with populations changing over time as the cheese matures. Successive microbial communities, particularly in aged cheeses like cheddar, have a profound impact on the final cheese flavour and quality. Being able to accurately predict cheese ripening outcomes at an early stage, based on cost-effective analyses, would be of great benefit to cheesemakers. In the past, there has been a significant gap between microbiological and chemical information obtained from omics and its application to the cheese industry, but thanks to recent advances in omics analytical methods, computing programs and sensor technologies, this gap is narrowing.
Keywords: cheese authenticity, cheese quality, metabolomics, microbial profiling, multi‐omics, proteomics, sensors.
Introduction
Cheese is one of the most widely consumed dairy products. Many different varieties of cheese that vary in texture, taste, and aroma are made and consumed around the world. The number one cheese produced in Australia is cheddar,1 although other cheese types are gaining in popularity (Fig. 1). For cheddar and other low moisture cheeses that require ageing (ripening), manufacturers have a keen vested interest in getting the ripening process right. If ripening does not proceed correctly, the cheddar made in a daily production run could end up being sold off cheaply, perhaps as an ingredient for locally made processed cheese rather than being exported to an overseas customer at top dollar.
Cheesemaking begins with raw milk. After standardisation of the milk to a pre-determined protein–fat ratio and high-temperature short-time (HTST) pasteurisation, the milk is pumped into a cheese vat. The starter culture is then added, followed by addition of rennet, a mixture of milk coagulating enzymes traditionally obtained from the lining of the abomasum from young calves. Alternatively, the rennet can be plant- or microbial-derived, or it may be a highly purified form of bovine chymosin obtained through recombinant DNA technology.2 Based on the action of the rennet and starter, milk coagulation occurs. Subsequent cheesemaking steps include cutting the curd and whey drainage, heating, salting and pressing.3 In large-scale industrial cheddar cheesemaking, strains of Lactococcus lactis, a species of lactic acid bacteria (LAB), usually comprise the starter culture. Starter cultures can be added either in freeze dried form direct to the vat, known as direct vat inoculation (DVI), or grown in a separate tank then added as bulk starter. It is not uncommon for cheesemakers to also add adjunct cultures at lower levels, usually non-starter lactic acid bacteria (NSLAB) comprising strains of Lactobacillus helveticus, Lacticaseibacillus paracasei (formerly Lactobacillus paracasei),4 or other species. These NSLAB grow slowly in the young cheese during ripening to eventually reach high numbers where they modulate cheese flavour and texture development.5 Cheese ripening becomes more complicated, however, because each factory has its own distinctive resident microbiota that naturally ‘inoculate’ the cheese before ripening begins. These adventitious microbiota also contribute to the complex succession of microbes critical in determining the final cheese properties.3
How is cheese ripening monitored and controlled as it progresses? In most large cheddar-making factories, experienced cheese-graders take core samples at different ripening stages to assess and predict the final cheese flavour and texture. Some basic chemical and microbiological analyses may also be done. Alongside the skill and knowledge of the cheese grader, scientific developments in chemical analytic techniques and in omics technologies have progressed, leading to mapping of the many thousands of individual cheese components, including the microbial communities, proteolytic breakdown products, huge numbers of metabolites, as well as the fermentation primary end products. But how can all this information be applied in a meaningful way to help cheesemakers better assess and predict cheese ripening outcomes? This challenge can be tackled in part by the application of so-called omics technologies.6,7
Omics and cheese
The term omics refers to the scientific discipline of analysing the interactions and functions of large clusters of biological information molecules.8 Omics technologies include metagenomics based on high-throughput next generation sequencing (NGS) methods, metatranscriptomics, metaproteomics, and metabolomics, targeting DNA, RNA, protein and metabolites, respectively.9–11 In recent years, the application of omics technologies to study fermented food products, especially cheese, has greatly increased.7
Metagenomics encompasses amplicon sequencing and shotgun whole genome sequencing.9 In amplicon sequencing, total DNA is extracted from an environmental sample, then a targeted region (e.g. within the 16S rRNA gene for identification of bacteria) is PCR-amplified and sequenced. Due to inherent methodological errors that can occur using this approach, along with ongoing improvements in DNA sequencing and computer processing power, shotgun sequencing is gaining wider use. Here, total DNA is sequenced providing not only taxonomic identification results but also information on the total genes present in a sample and their potential corresponding protein (or enzyme) metabolic functions.12 Such DNA-based approaches have helped to identify novel microbial species not identifiable using traditional microbiological culturing techniques in many environments, including cheese. An early pioneering study to apply metagenomics in cheese involved 16S rRNA gene amplicon sequencing of 60 Irish soft cheeses.13 In addition to common LAB species, many non-LAB bacterial genera were identified, such as Prevotella and Arthrobacter. The authors found that the bacterial community composition depended upon the cheese type, the origin of the milk, production technology and the ingredients used.
Metatranscriptomics and metaproteomics involve assessing the complete gene expression and protein complement (respectively) of multi-component biological systems. Both methods are difficult to apply in fermented foods, hence relatively few studies have been published to date.7 Metatranscriptomics studies on cheeses have shown that regulation of microbial enzymes capable of impacting flavour development occurs during ripening, with one study on a Swiss-type cheese showing that regulation of central metabolism enzymes in cold ripening conditions varied depending on the species.14 Metaproteomics studies have also revealed the functional roles of microbial proteins in fermented foods, however, there have been few studies on cheese, in part due to the complexity of analysing microbial and non-microbial milk proteins and their breakdown products together.7,15 Despite these difficulties, these tools have the potential in future to provide exciting new insights into the functional aspects of the cheese microbiota.
Metabolomics consists of identification and quantification/semi quantification of all endogenous small molecules (metabolome) biosynthesised and modified in a cell, tissue or in a microbial consortium. Typically, there are two approaches: metabolite profiling (targeted analysis of specific groups of metabolites) and metabolite fingerprinting (untargeted analysis of the global metabolome profile without the need for a prior specific hypothesis on a set of metabolites). Metabolomics relies on an efficient method for metabolites extraction, followed by application of analytical instrumentation – usually gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) together with nuclear magnetic resonance (NMR) and multivariate data analysis.6,11 Other approaches may also be used; as one example, a group in the Czech Republic used matrix-assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-TOF MS) together with principal component analysis, or liquid chromatography coupled with electrospray ionisation and quadrupole time-of-flight mass spectrometry, to distinguish 27 cheeses made from milks of different animal species.16 We also showed that spatially offset RAMAN spectroscopy (SORS), a fast, inexpensive and non-invasive method, can be used with chemometrics to distinguish cheeses made from different animal species.17
Multi-omics with data integration
Individual omics approaches have shown the enormous complexity of fermented food products at a biological level. However, there has been emerging interest in developing mathematical tools that analyse high-dimensional omics datasets obtained from multiple omics platforms applied to fermented foods. For example, new insights into the cheese microbiota were obtained from the combination of strain-level metagenomics with metabolomics, highlighting that different strains of the same species may produce different metabolites in cheese.18 Samples of 55 artisanal cheeses from 27 Irish producers were analysed; the authors recovered 328 metagenome-assembled genomes, including 47 putative new species in cheese. In addition, numerous phage and bacteriocin genes were found. Most of the new species identified belonged to halophilic genera such as Psychrobacter and Halomonas, while other species belonged to genera known to be associated with cheese rinds (for example, Brevibacterium, Corynebacterium, and Arthrobacter). In another study integrated amplicon-targeted metagenomics and metabolomics provided the basis for the selection of cheese adjunct cultures for the accumulation of specific flavours in soft-type ripened cheeses.19
Multi-omics studies on Australian industrial and artisanal cheddar cheeses done by us have also revealed some interesting associations between cheese microbiota and metabolites. These studies further suggest the possibility of discovering new biomarkers for validating cheese age and brand authenticity and cheese quality. For example, some low abundant taxa such as Pediococcus spp. in artisanal cheeses correlated with the presence of 21 metabolites that may influence cheese flavour.20 Another study showed how integration of metagenomics and metabolomics datasets could enable better differentiation of ten similar mass-produced cheddar cheeses of different brands and ages (Fig. 2).21 In a further study we differentiated identical-style cheddars of the same age but of different quality manufactured by the same company.22 By integrating multi-omics datasets much better resolution was obtained, giving more confidence in the results and thus proving (or disproving) authenticity. Other associations were revealed in these studies – for example, levels of phenylalanine correlated positively with the presence of Thermus spp. which have been implicated in the pink discoloration of cheese, while cheese cholesterol showed a negative association with Streptococcus thermophilus.20 To our knowledge, this had not been previously reported. Potential cheese age- and quality-related biomarkers were also identified.
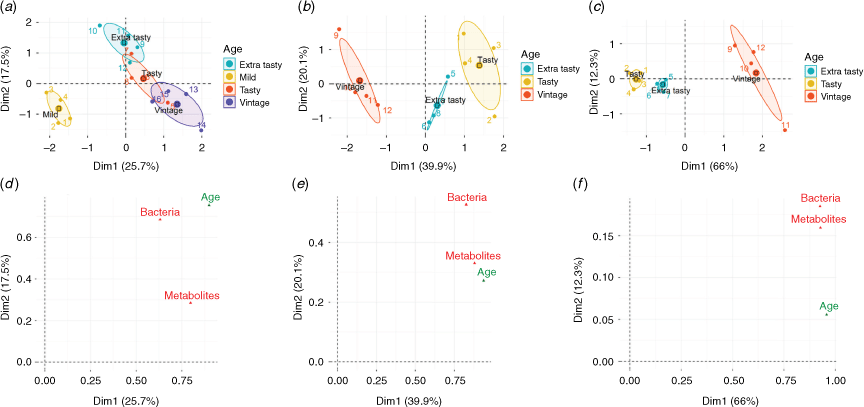
|
Challenges for multi-omics
Despite the potential of multi-omics to give new insights into cheese ripening, challenges remain. One major problem is cost. The technology is simply nowhere near the stage where it can be routinely applied. Other problems are heterogeneity across the same omics platforms, making data comparisons difficult, also challenges related to the large computational resources needed and a lack of any unified public repository where researchers can access multi-omics datasets.23 As these limitations are resolved over time, multi-omics will become a major innovation for the food industry.
Prospects for real-time monitoring
Identification of novel biomarkers to predict cheese ripening outcomes, or detailed fingerprints to prove cheese authenticity, will only be useful for the industry if analyses can be done routinely, easily and affordably. Advances in sensor and real-time monitoring technology are bringing this goal closer. Such technologies have wider applicability in terms of achieving higher process efficiency, improved product quality, ensuring food authenticity and provenance e.g. through coupling with blockchain technology, reducing food waste and improving food safety through real-time pathogen monitoring. Some recent examples include biosensors for pathogen detection in food;24 microbial potentiometric sensors (MPS) technology coupled with appropriate signal analysis tools and methodologies used to monitor kefir fermentation;25 application of an electronic nose to accurately identify and quantify four yeast species (Pichia anomala, P. kluyveri, Hanseniaspora uvarum and Debaryomyces hansenii) in fresh soft cheese;26 the development of biosensors for analysing fermentation-related parameters,27 and monitoring the microbial quality of raw milk.28 The latter is already being done in some parts of the dairy industry.
Together, these observations suggest that we can expect to have new highly sensitive tools for real-time monitoring of cheese quality in the future to complement traditional cheese grading practices. Coupled with the new insights provided by omics and multi-omics, this will lead to better prediction and management of cheese quality, as well as improvements in food safety and in ensuring product authenticity.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] In Focus (2021) The Australian Dairy Industry. https://www.dairyaustralia.com.au/[2] Olempska-Beer, ZS et al.. (2006) Food-processing enzymes from recombinant microorganisms—a review. Reg Toxicol Pharmacol 45, 144–158.
| Food-processing enzymes from recombinant microorganisms—a review.Crossref | GoogleScholarGoogle Scholar |
[3] Mayo, B et al.. (2021) Microbial interactions within the cheese ecosystem and their application to improve quality and safety. Foods 10, 602.
| Microbial interactions within the cheese ecosystem and their application to improve quality and safety.Crossref | GoogleScholarGoogle Scholar | 33809159PubMed |
[4] Zheng, J et al.. (2020) A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 70, 2782–2858.
| A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae.Crossref | GoogleScholarGoogle Scholar | 32293557PubMed |
[5] Crow, VL et al.. (2002) Raw milk flora and NSLAB as adjuncts. Austr J Dairy Technol 57, 99–105.
[6] Afshari, R et al.. (2020) Cheesomics: the future pathway to understanding cheese flavour and quality. Crit Rev Food Sci Nutr 60, 33–47.
| Cheesomics: the future pathway to understanding cheese flavour and quality.Crossref | GoogleScholarGoogle Scholar | 30285475PubMed |
[7] Anastasiou, R et al.. (2022) Omics approaches to assess flavor development in cheese. Foods 11, 188.
| Omics approaches to assess flavor development in cheese.Crossref | GoogleScholarGoogle Scholar | 35053920PubMed |
[8] Bhak J (2015) What is omics? http://omics.org/What_is_omics%3F
[9] Metzker, ML (2010) Sequencing technologies — the next generation. Nat Rev Genet 11, 31–46.
| Sequencing technologies — the next generation.Crossref | GoogleScholarGoogle Scholar | 19997069PubMed |
[10] Kleiner, M (2019) Metaproteomics: much more than measuring gene expression in microbial communities. mSystems 4, e00115-19.
| Metaproteomics: much more than measuring gene expression in microbial communities.Crossref | GoogleScholarGoogle Scholar | 31117019PubMed |
[11] Liu, X and Locasale, JW (2017) Metabolomics: A primer. Trends Biochem Sci 42, 274–284.
| Metabolomics: A primer.Crossref | GoogleScholarGoogle Scholar | 28196646PubMed |
[12] Filippis, FD et al.. (2018) Recent past, present, and future of the food microbiome. Annu Rev Food Sci Technol 9, 589–608.
| Recent past, present, and future of the food microbiome.Crossref | GoogleScholarGoogle Scholar |
[13] Quigley, L et al.. (2012) High-throughput sequencing for detection of subpopulations of bacteria not previously associated with artisanal cheeses. Appl Environ Microbiol 78, 5717–5723.
| High-throughput sequencing for detection of subpopulations of bacteria not previously associated with artisanal cheeses.Crossref | GoogleScholarGoogle Scholar | 22685131PubMed |
[14] Duru, IC et al.. (2018) Metagenomic and metatranscriptomic analysis of the microbial community in Swiss-type Maasdam cheese during ripening. Int J Food Microbiol 281, 10–22.
| Metagenomic and metatranscriptomic analysis of the microbial community in Swiss-type Maasdam cheese during ripening.Crossref | GoogleScholarGoogle Scholar | 29803134PubMed |
[15] Yang, L et al.. (2020) Metaproteomics insights into traditional fermented foods and beverages. Compr Rev Food Sci Food Saf 19, 2506–2529.
| Metaproteomics insights into traditional fermented foods and beverages.Crossref | GoogleScholarGoogle Scholar | 33336970PubMed |
[16] Kuckova, S et al.. (2019) Verification of cheeses authenticity by mass spectrometry. J Sep Sci 42, 3487–3496.
| Verification of cheeses authenticity by mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 31549465PubMed |
[17] Ostovar pour, S et al.. (2021) Spatially offset Raman spectroscopy: A convenient and rapid tool to distinguish cheese made with milks from different animal species. J Raman Spectr 52, 1705–1711.
| Spatially offset Raman spectroscopy: A convenient and rapid tool to distinguish cheese made with milks from different animal species.Crossref | GoogleScholarGoogle Scholar |
[18] Walsh, AM et al.. (2020) Meta-analysis of cheese microbiomes highlights contributions to multiple aspects of quality. Nat Food 1, 500–510.
| Meta-analysis of cheese microbiomes highlights contributions to multiple aspects of quality.Crossref | GoogleScholarGoogle Scholar |
[19] Unno, R et al.. (2021) Evaluation of the relationships between microbiota and metabolites in soft-type ripened cheese using an integrated omics approach. Front Microbiol 12, 681185.
| Evaluation of the relationships between microbiota and metabolites in soft-type ripened cheese using an integrated omics approach.Crossref | GoogleScholarGoogle Scholar | 34168634PubMed |
[20] Afshari, R et al.. (2020) New insights into cheddar cheese microbiota-metabolome relationships revealed by integrative analysis of multi-omics data. Sci Rep 10, 3164.
| New insights into cheddar cheese microbiota-metabolome relationships revealed by integrative analysis of multi-omics data.Crossref | GoogleScholarGoogle Scholar | 32081987PubMed |
[21] Afshari, R et al.. (2020) Microbiota and metabolite profiling combined with integrative analysis for differentiating cheeses of varying ripening ages. Front Microbiol 11, 592060.
| Microbiota and metabolite profiling combined with integrative analysis for differentiating cheeses of varying ripening ages.Crossref | GoogleScholarGoogle Scholar | 33324371PubMed |
[22] Afshari, R et al.. (2021) Biomarkers associated with cheese quality uncovered by integrative multi-omic analysis. Food Control 123, 107752.
| Biomarkers associated with cheese quality uncovered by integrative multi-omic analysis.Crossref | GoogleScholarGoogle Scholar |
[23] Krassowski, M et al.. (2020) State of the field in multi-omics research: from computational needs to data mining and sharing. Front Genet 11, 610798.
| State of the field in multi-omics research: from computational needs to data mining and sharing.Crossref | GoogleScholarGoogle Scholar | 33362867PubMed |
[24] Xu, L et al.. (2021) Current state of development of biosensors and their application in foodborne pathogen detection. J Food Prot 84, 1213–1227.
| Current state of development of biosensors and their application in foodborne pathogen detection.Crossref | GoogleScholarGoogle Scholar | 33710346PubMed |
[25] Hristovski, KD et al.. (2022) Real-time monitoring of kefir-facilitated milk fermentation using microbial potentiometric sensors. J Env Chem Eng 10, 107491.
| Real-time monitoring of kefir-facilitated milk fermentation using microbial potentiometric sensors.Crossref | GoogleScholarGoogle Scholar |
[26] Abu-Khalaf, N and Masoud, W (2022) Electronic nose for differentiation and quantification of yeast species in white fresh soft cheese. Sensors (Basel) 21, 8472661.
| Electronic nose for differentiation and quantification of yeast species in white fresh soft cheese.Crossref | GoogleScholarGoogle Scholar |
[27] Patra F, Duary RK (2022) Biosensors involved in fermented product. In ‘Biosensors in Food Safety and Quality’, 1st edn. p. 20. CRC Press.
[28] Poghossian, A et al.. (2019) Rapid methods and sensors for milk quality monitoring and spoilage detection. Biosens Bioelectr 140, 111272.
| Rapid methods and sensors for milk quality monitoring and spoilage detection.Crossref | GoogleScholarGoogle Scholar |



