Are point of management assays relevant for food safety in the poultry industries?
J. M. Templeton A * , J. R. Botella B and P. J. Blackall CA Intensive Livestock & Food Safety, Department of Agriculture and Fisheries (Scopus Affiliation ID: 60028929), EcoSciences Precinct, GPO Box 267, Brisbane, Qld 4001, Australia.
B Plant Genetic Engineering Laboratory, School of Agriculture and Food Sciences, The University of Queensland, St Lucia, Qld 4067, Australia.
C Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, EcoSciences Precinct, GPO Box 267, Brisbane, Qld 4001, Australia.

Jillian Templeton is a microbiologist working for the Queensland Department of Agriculture and Fisheries. Jillian is a mid-career researcher who has led projects over the past 20 years to investigate the epidemiology of Campylobacter on Australian meat chicken farms; the development, validation and application of genotyping methods for Campylobacter; and the development of rapid diagnostic techniques for Campylobacter. |

Jimmy Botella is professor of Biotechnology at the University of Queensland. Jimmy has eleven international patents in the field of Biotechnology, has founded two biotechnology companies and is a member of the Expert Scientific Panel for the Agricultural Biotechnology Council of Australia. Jimmy’s research has been awarded with the Chinese Academy of Sciences Visiting Professorship for Senior International Scientists and the title of ‘Pathologist of Distinction’ by the International Society of Plant Pathology. |

Pat Blackall is a bacteriologist working at the Queensland Alliance for Agriculture and Food Innovation at the University of Queensland. Pat has research interests in the bacterial diseases of intensively raised animals and in the food safety pathogens that are present in those industries. |
Microbiology Australia 43(2) 67-70 https://doi.org/10.1071/MA22025
Submitted: 18 March 2022 Accepted: 13 April 2022 Published: 18 May 2022
© 2022 The Author(s) (or their employer(s)) and The State of Queensland (through the Department of Agriculture and Fisheries). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The current pandemic has ensured considerable attention has been paid to the role of the approach termed ‘Point of Care’ diagnostics. Indeed, the term ‘RAT’ (Rapid Antigen Test) and RAT hunting now have totally different meaning to that widely understood before 2020. In the veterinary field, including food safety, the term used for these types of rapid in situ assays is ‘Point of Management’ (POM) assays. In this article, we describe our recent research on low cost, low technology, in-house style POM assays in the field of food safety as applied to the poultry industries. We then discuss what are the advantages and disadvantages of these low cost, low technology POM assays.
Keywords: Campylobacter, chicken, food safety, isothermal, LAMP, point of management, poultry, Salmonella.
Key food safety pathogens for the poultry industries
As with all food production systems, the potential for food safety pathogens to enter the system and cause human illness is a key issue for the Australian poultry industries. Two genera dominate the food safety issues linked to the poultry industries – Campylobacter and Salmonella.
Over 96 million cases of food-borne illness linked to Campylobacter jejuni and C. coli are estimated to occur globally each year.1 The Australian infection rate of 146.9 cases per 100 000 population2 is one of the highest among the industrialised countries.3 Importantly, while not the only source of Campylobacter, it is recognised that in Australia, undercooked poultry are a major source.4
Salmonella is second only to Campylobacter as the most notified enteric pathogen in Australia.2 As with Campylobacter, while there are multiple sources of human infections, raw and undercooked foods, eggs, and to a lesser degree poultry meat, are often associated with Salmonella infections.5
Food-borne pathogens impose costs onto both broad society as well as the production system. While figures are not available for Australia, a recent report estimated that campylobacteriosis costs the US (population >10 times that of Australia but with 10 times lower Campylobacter infection rate3) in the range of US$1.5–US$6.9 billion per year depending on the calculation method.6 The reduced income and increased expenses associated with Salmonella in the Australian egg industry were estimated to cost $7 million annually in 2015–2016.7 The scale of these economic impacts to both broad society as well as the producers emphasises the need for improved control of food-borne pathogens in the poultry industries.
POM assays
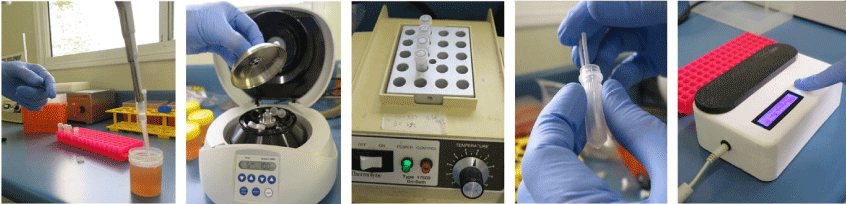
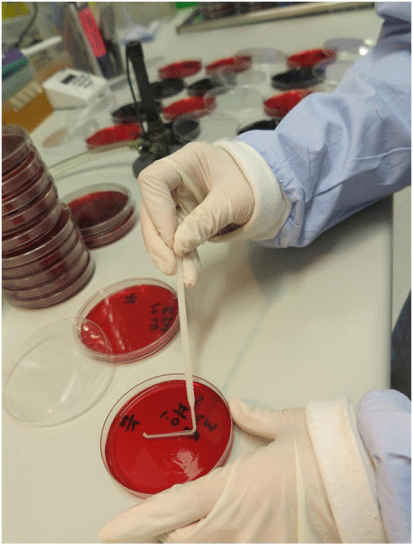
Our work in the development of POM assays arose from the finding that dipsticks made from untreated cellulose-based paper can bind nucleic acids in seconds, retain them during a rinse step that removes the contaminants and then release the nucleic acids when placed in a reaction buffer.8,9 We have combined the low-cost DNA extraction technology with isothermal amplification performed in another existing innovation – the ‘Diagnostic Droid’.10 The workflow involves a centrifugation of a 2 mL aliquot of carcass rinse, a treatment of the pellet with proteinase K and heat denaturation to release the DNA. Two dipsticks are added to bind the DNA and purify it away from the contaminants. Each dipstick is then given a single wash and the DNA eluted into a loop mediated isothermal amplification (LAMP) reaction mix for either C. jejuni or C. coli. The ‘Diagnostic Droid’ performs the amplification step and monitors the increase in turbidity associated with a positive LAMP reaction by illuminating the reaction tubes with an LED and measuring the amount of scattered light via a light sensor perpendicular to the LED light source. The results are automatically interpreted with no need for human involvement and are available within 2.5 h10 (see Fig. 1 for an illustrative workflow of our POM for Campylobacter). The Australian chicken meat industry has a self-set target of ensuring that all chicken carcasses have less than 6000 colony forming units (cfu) which is equivalent to 12 cfu/mL in the 500 mL volume used to wash the carcass. The performance of the POM assay was evaluated using 29 rinse samples that were examined by the relevant Australian Standard method11 (see Fig. 2 for an illustration of the work required by this Standard) as well as the POM assay.10 A total of 26 samples were in agreement – 16 were high in the POM assay and contained more than 12 cfu/mL and 10 were low in the POM assay and had <12 cfu/mL in the culture method. While three samples gave different results in the two assays (i.e. an 11% disagreement), only two samples (i.e. 7% of samples) were a major disagreement where the POM assay result was low and the culture result was above 12 cfu/mL. It is worth noting that these two disagreements occurred on the edge of the 12 cfu/mL cut-off – being 23 and 37 cfu/mL. All samples above 45 cfu/mL in the culture method were positive in the POM assay. Overall, this work has shown the potential for a very low-cost POM assay that uses little in the way of technology to be used to semi-quantify the level of Campylobacter in chicken carcass rinses.
|
Who pays?
In evaluating the advantages/disadvantages of a POM assay there is a critical primary issue that first needs to be addressed – who pays? In a situation where a producer has a disease problem on farm, the solution of a vaccine that controls the disease results in an extra cost but returns an increased profit via less mortalities or an improved growth rate. In these circumstances, the producer sees a direct financial benefit from a new intervention or a new management tool. In contrast, producers or processing companies that introduce an intervention to reduce the level of Campylobacter have the cost of that intervention but no direct financial return. Society would benefit from the intervention with a lower level of campylobacteriosis in the population but the producer/processor bears the costs and gains no financial benefit. Hence, any interventions to increase food safety (such as POM assays to provide near to real time information on Campylobacter levels on carcasses) must be implemented with an understanding that society will benefit but that there will be no immediate financial benefit to the individual producer or processor. Clearly, an industry providing food to the population benefits from a public perception that they provide a high-quality product that is safe and healthy. The public expects safe and healthy food but often does not understand the associated financial burdens. Hence, adoption of POM assays for food safety pathogens has to occur in a situation where no immediate benefit flows from the cost of adopting the technology.
Advantages of POM assays
In evaluating the advantages of the type of POM assay we have developed, our vision is that these assays are not seeking to replace the Australian Standard method11 but rather add to the quality and relevance of the formal testing provided by the standard methods. The POM assay we have developed is as sensitive as the gold standard of culture but gives results in a little over 2 h vs 2 days required by the standard culture method. Hence, quality assurance/quality control staff can implement routine rapid, low-cost regular monitoring of the effectiveness of the processing chain in achieving the industry set target of <6000 cfu of Campylobacter per carcass. Given that there are around 50 processing plants for chickens in Australia and that over 663 million birds were processed in 2020, the need for cost-effective, low technology, rapid assays suitable for both the large multi-state processors as well as the much smaller state and regionally based processors is clear in our view. A POM assay would allow refinements and alterations in production parameters to be rapidly monitored for their impact on Campylobacter levels. POM assays should not be seen as replacements for the nationally certified standards but rather supplementary, additional tests that support the same goal – the production of a high quality, safe food product.
Disadvantages of POM assays
POM assays suffer the same generic problem that all food safety interventions suffer as outlined above (i.e. they are an additional cost). However, it should be noted that the cost of our POM approach is far cheaper than the currently available commercial instruments and assays.
There is a general acceptance that POM assays are not as sensitive as laboratory-based methods – as clearly shown in comparisons of RATs and RT-qPCR for COVID-19.12 Our work is, at this stage, still too early to provide firm evidence about the relative sensitivity of culture and our POM assay. However, it should be noted that the three samples in disagreement all involved viable counts of less than 40 colonies on the two counting plates. As the reliable counting range for plate counts is 25–250 colonies,13,14 it is clear that the disagreements occurred in a range where both technologies (plate counts and POM assay) were struggling to detect very low numbers of Campylobacter.
Conclusion
The importance of reducing the levels of Campylobacter on chicken carcasses for public health is well established. A European study has shown that a 2-log reduction in the number of Campylobacter on carcasses would result in a 30 times reduction in the incidence of campylobacterosis.15 In our view, access to low cost, low technology, rapid POM assays is an essential requirement to ensure the level of monitoring required to achieve either the 2-log reduction suggested by the European study15 or the industry-assigned target of 6000 cfu per carcass.
While this article has focussed on Campylobacter and chicken carcasses, there are other areas where POM assays for food safety pathogens could be effective tools e.g. in detecting the presence of Salmonella on layer farms. Few layer farms currently engage in Salmonella tests due to the costs of the assay, the delay in obtaining results and the distance from the farm to the laboratory. POM assays for on-farm detection of Salmonella would remove many of those barriers and could encourage a far more proactive quality assurance program.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The research on the development of a POM assay for use in the poultry processing plant was supported by the AgriFutures Chicken Meat Program. We greatly appreciate the participation of our industry partner in that project who provided the carcass rinse samples and conventional culture results.
References
[1] Kirk, MD et al.. (2015) World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 12, e1001921.| World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis.Crossref | GoogleScholarGoogle Scholar | 26633831PubMed |
[2] (2021) Monitoring the incidence and causes of disease potentially transmitted by food in Australia: annual report of the OzFoodNet network, 2016. Commun Dis Intell 45, .
| Monitoring the incidence and causes of disease potentially transmitted by food in Australia: annual report of the OzFoodNet network, 2016.Crossref | GoogleScholarGoogle Scholar |
[3] Moffatt, CR et al.. (2017) The campylobacteriosis conundrum – examining the incidence of infection with Campylobacter sp. in Australia, 1998-2013. Epidemiol Infect 145, 839–847.
| The campylobacteriosis conundrum – examining the incidence of infection with Campylobacter sp. in Australia, 1998-2013.Crossref | GoogleScholarGoogle Scholar | 27938447PubMed |
[4] Stafford, RJ (2007) A multi-centre prospective case-control study of campylobacter infection in persons aged 5 years and older in Australia. Epidemiol Infect 135, 978–988.
| A multi-centre prospective case-control study of campylobacter infection in persons aged 5 years and older in Australia.Crossref | GoogleScholarGoogle Scholar | 17134530PubMed |
[5] Ford, L et al.. (2018) The epidemiology of Salmonella enterica outbreaks in Australia, 2001–2016. Front Sustain Food Syst 2, 86.
| The epidemiology of Salmonella enterica outbreaks in Australia, 2001–2016.Crossref | GoogleScholarGoogle Scholar |
[6] Hoffmann S, Anekwe TD (2013) Making sense of recent cost-of-foodborne-illness estimates – EIB-118. Economic Research Service US Department of Agriculture.
[7] ACIL ALLEN Consulting (2018) Economic contribution of animal medicines to Australia’ livestock industries – 2015–16. Report to Animal Medicines Australia.
[8] Mason, MG and Botella, JR (2020) Rapid (30-second), equipment-free purification of nucleic acids using easy-to-make dipsticks. Nat Protoc 15, 3663–3677.
| Rapid (30-second), equipment-free purification of nucleic acids using easy-to-make dipsticks.Crossref | GoogleScholarGoogle Scholar | 33005038PubMed |
[9] Zou, Y et al.. (2017) Nucleic acid purification from plants, animals and microbes in under 30 seconds. PLoS Biol 15, e2003916.
| Nucleic acid purification from plants, animals and microbes in under 30 seconds.Crossref | GoogleScholarGoogle Scholar | 29161268PubMed |
[10] Mason, MG et al.. (2020) An easy-to-perform, culture-free Campylobacter point-of-management assay for processing plant applications. J Appl Microbiol 128, 620–629.
| An easy-to-perform, culture-free Campylobacter point-of-management assay for processing plant applications.Crossref | GoogleScholarGoogle Scholar | 31705613PubMed |
[11] Standards Australia (2015) Food microbiology – method 6: examination of specific organisms - Campylobacter. AS 5013.6:2015.
[12] Yamayoshi, S et al.. (2020) Comparison of rapid antigen tests for COVID-19. Viruses 12, 1420.
| Comparison of rapid antigen tests for COVID-19.Crossref | GoogleScholarGoogle Scholar |
[13] Breed, RS and Dotterrer, WD (1916) The number of colonies allowable on satisfactory agar plates. J Bacteriol 1, 321–331.
| The number of colonies allowable on satisfactory agar plates.Crossref | GoogleScholarGoogle Scholar | 16558698PubMed |
[14] Tomasiewicz, DM et al.. (1980) The most suitable number of colonies on plates for counting. J Food Prot 43, 282–286.
| The most suitable number of colonies on plates for counting.Crossref | GoogleScholarGoogle Scholar | 30822862PubMed |
[15] Rosenquist, H et al.. (2003) Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int J Food Microbiol 83, 87–103.
| Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens.Crossref | GoogleScholarGoogle Scholar | 12672595PubMed |