The AMR Hub: a public–private partnership to overcome barriers to commercialisation and deliver antimicrobial stewardship innovations
Michelle Bonello A # , Tanya L. Applegate A # * , Steve Badman B , Catriona S. Bradshaw C D E , Alex Broom F , Paul Field G , Jane S. Hocking E , Wilhelmina M. Huston H , Fabian Kong E , Gerald L. Murray I J K , Elisa Mokany L , Shivani Pasricha M , Alison V. Todd L , David M. Whiley N O , Virginia Wiseman A P , Rebecca Guy A and on behalf of investigators and partner investigators of the AMR HubA
B
C
D
E
F
G
H
I
J
K
L
M
N
O
P

Dr Michelle Bonello (BSc(Hons), MPH, PhD) is Business Manager of the Industrial Transformation Research Hub to Combat AMR. She has a background is molecular biology and public health research. |

Assoc. Prof. Tanya Applegate is Lead of the Diagnostics Innovations Group in the Surveillance Evaluation and Research Program at the Kirby Institute, UNSW Sydney. Tanya leads a multistakeholder, multidisciplinary diagnostics research program spanning all stages of the pipeline with over 20 years working in regulatory, hospital, private industry and academic environments. Her translational research aims to provide equitable access to healthcare for people who are marginalised or living in remote or resource limited settings. |

Dr Steven Badman is Director of Medical and Scientific Affairs, Cepheid, for the Australia, New Zealand, Papua New Guinea and western Pacific region. Steve is a clinically trained public health researcher with 20+ years of international infectious disease research and public health implementation in several countries, including remote Australia, Indonesia, Malaysia, Thailand, Vanuatu, Papua New Guinea, Tuvalu, Kiribati and Myanmar. |

Catriona S. Bradshaw is a professor at Monash University, Melbourne. Catriona heads a translational research programme to improve the diagnosis, treatment and control of drug-resistant and refractory sexually transmitted infections (STIs), with a focus on improving antimicrobial stewardship and AMR in STIs. Her programme includes interventions to achieve a vaginal microbiota associated with optimal reproductive health outcomes. |

Alex Broom is Professor of Sociology and Director of the Sydney Centre for Healthy Societies, Faculty of Arts and Social Sciences, The University of Sydney. |

Paul Field is the representative for the Global Antibiotic Research & Development Partnership (GARDP) in Australia. GARDP is developing and delivering new treatments for drug-resistant bacterial infections including zoliflodacin a first-in-class antibiotic against Neisseria gonorrohoeae. |

Prof. Jane Hocking is an epidemiologist and implementation researcher whose research interests include the epidemiology and control of STIs, sexual health and the implementation and evaluation of primary care interventions. She is a Dame Kate Campbell Fellow, holds a NHMRC Investigator Grant (L2) and is the head of the Sexual Health Unit at the Melbourne School of Population and Global Health, University of Melbourne. |

Prof. Wilhelmina Huston is a molecular microbiologist in the field of STIs based at the University of Technology—Sydney. Her research is focussed on chlamydial pathogenesis, but also host responses and interplay with microbiome factors in the context of both infection and disease pathologies. She is also a committed educator with research and academic development outcomes in higher education teaching and learning in Science. |

Dr Fabian Kong is a senior fellow and Deputy Head of the Sexual Health Unit at The University of Melbourne. Fabian comes from a clinical pharmacy background with expertise in evidence medicine and international health and has worked for the WHO, UNDP and The World Bank. He currently works as an epidemiologist to optimise treatments for STIs using pharmacokinetics and novel culture models, with a focus on multi-drug resistant gonorrhoea in the mouth. |

Gerald Murray is a senior research officer in the department of Obstetrics and Gynaecology at The University of Melbourne, based at the Royal Women’s Hospital. His research interests include investigating mechanisms of AMR, studying the human microbiota in health and disease, and human papillomavirus (HPV). |

Dr Elisa Mokany is a co-founder and the Chief Technology Officer at SpeeDx. Elisa is a strong advocate of SpeeDx’s goal of pioneering solutions to address the global problem of antibiotic resistance with the company’s lead first commercial test world-wide for detection of a STI that detects both the infectious agent together with markers of antibiotic resistance. |

Shivani Pasricha is a microbiologist and laboratory head in the Department of Infectious Diseases, University of Melbourne. Using molecular and genomic approaches, her research aims to improve the detection, prevention and surveillance of STIs. Her current research includes developing cutting-edge CRISPR-diagnostics for the point-of-care detection of STIs and AMR. |

Adj. Prof. Alison Todd is the Chief Scientific Officer and co-founder of SpeeDx. She is an elected Fellow of the Australian Academy of Technology and Engineering and awarded Member (AM) in the general division of the Order of Australia. Her commitment to deliver innovative clinical diagnostics to improve patient outcomes has contributed to the development of over 30 products, utilising her patented inventions from a portfolio of over 20 patent families. |

Assoc. Prof. David Whiley is a principal research fellow at The University of Queensland Centre for Clinical Research (UQCCR) and a research scientist at Pathology Queensland. Antibiotic-resistant Neisseria gonorrhoeae is a particular research interest of his, including enhancing gonorrhoea AMR surveillance and treatment. |

Virginia Wiseman is Professor of Health Economics (BEc(Hons), PhD) and Theme Director of Health Economics & Health Systems Research at the Kirby Institute, UNSW Sydney, with a dual appointment in the Department of Global Health and Development at the London School of Hygiene & Tropical Medicine. She has 24 years’ experience in translational research, evaluating the economic and health impacts of public health interventions in Australia, Africa and the Asia-Pacific. Virginia leads the Australian Centre for Stronger Investments in Infectious Diseases (STRIDE). |

Prof. Rebecca Guy (BAppSc, MAppEpid, PhD) is a public health epidemiologist and Head of the Surveillance and Evaluation Research Program at the Kirby Institute, UNSW Sydney. Her research focuses on the epidemiology and prevention of infectious diseases, including HIV, STIs and respiratory tract infections. She has led national surveillance, cohort and data linkage studies, and cluster randomised trials of large-scale public initiatives, particularly among marginalised populations. This research has translated into policy and practice, including national strategies, national guidelines and WHO guidelines. |
Abstract
Antimicrobial resistance (AMR) is recognised as one of the greatest scientific challenges of the 21st century, disproportionately affecting people living in low- and middle-income countries. With bacterial pathogens becoming increasingly resistant to antibiotics, there is an urgent need for innovative approaches to combat this growing threat. The World Health Organization has recognised this need and prioritised further research to enhance diagnostics, surveillance and our understanding the epidemiology and drivers of AMR. The Industrial Transformation Research Hub to Combat AMR, or the AMR Hub, is an Australian collaborative private–public research partnership involving over 20 organisations. It aims to foster multidisciplinary collaborations across sectors and develop wholistic solutions that address barriers to the commercialisation of tools to minimise the risks of AMR. The AMR Hub’s research is focusing on sexually transmitted infections, which are increasingly resistant to antibiotics and have few alternative candidates in the pipeline. Investigators are together developing novel diagnostics, optimising treatment, identifying tools to detect active bacterial infections, and engaging stakeholders to optimise AMR innovation. Through a multidisciplinary ecosystem across sectors, the AMR Hub seeks to fast-track the development of adaptable technologies, new antibiotics and stewardship innovations for prevention, while also addressing societal, economic and commercial aspects of AMR solutions.
Keywords: antimicrobial resistance, antimicrobial stewardship, antimicrobials, diagnostics, multidisciplinary, Mycoplasma genitalium, Neisseria gonorrhoeae, pre-commercialisation barriers, private–public partnership, sexually transmitted infections.
Background
Antimicrobial resistance (AMR) poses a significant threat to global health and its economy, necessitating urgent action and innovative antimicrobial stewardship solutions.1–3 As AMR is driven largely by the complex challenges of poverty, with people living in economically disadvantaged communities bearing the greatest burden.4 Many bacterial pathogens are now resistant to first-line antibiotics, so with limited classes remaining and disincentives for industry to invest in their ongoing development – the current drug pipeline is alarmingly insufficient to meet the ongoing threat of AMR.5
To mitigate the impact of AMR on human health, the World Health Organization (WHO) set a global research agenda that extends beyond prioritising technical remedies such as enhanced diagnostics, surveillance and novel drug development, but also seeks to improve our understanding of the epidemiology, burden and drivers of AMR.6 To effectively inform global policy, this requires a comprehensive understanding of the true value of antimicrobial stewardship and necessitates transformative shifts in societal norms, behaviours, and healthcare practices, alongside the restructuring of healthcare financing mechanisms.
The AMR Hub – an industry-led, private–public partnership model
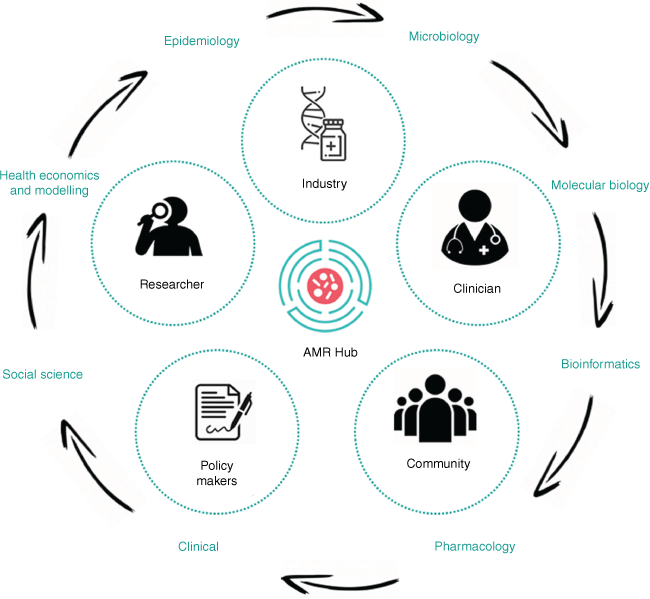
The Industrial Transformation Research Hub to Combat AMR (see https://amrhub.org.au/; the ‘AMR Hub’ or the ‘Hub’ herein) is a leading, collaborative platform to address industry-identified, commercially relevant research priorities. The Hub has over 20 partner organisations and brings together researchers, industry, clinicians, non-for-profit organisations and stakeholders who aim develop innovative solutions to not only overcome barriers to diagnostic and pharmaceutical commercialisation, but also optimise their delivery in context of a broader spectrum of social, political and cultural interventions (Fig. 1). The multi-disciplinary capabilities within the Hub include microbiology, molecular biology, bioinformatics, pharmacology, clinical, social science, health economics, disease modelling and epidemiology – existing across organisations and career stages. The inclusion and capacity building of early- to mid-career researchers not only secures a range of perspectives and future-facing innovations, but strategically builds the next generation of science and innovation workforce with the skills to meaningfully engage and partner with industry. This holistic approach offers a highly integrated ecosystem that fosters an enhanced pre-commercialisation environment to fast-track the development of novel diagnostic technology, new antimicrobial compounds and stewardship innovations.
The exemplar problem of sexually transmitted infections as priority pathogens
The Hub focuses on sexually transmitted infections (STIs) as an exemplar of the wider problem of AMR. STIs are a critical area of concern as AMR is increasing the challenges of treatment for infections such as Neisseria gonorrhoeae and Mycoplasma genitalium.7,8 N. gonorrhoeae has been listed as a high priority pathogen by WHO with increasing resistance to common antibiotics, such as azithromycin and ceftriaxone.9,10 Similarly, M. genitalium has developed resistance to macrolide and fluoroquinolone antibiotics, with clinicians increasingly encountering infections that are difficult or impossible to treat.11–14
To tackle the multifaceted problem of STI AMR, the Hub has four synergistic, cross-cutting research themes.
Theme 1. Developing novel diagnostics to determine AMR status and improve treatment
Theme 1 focuses on the development of novel, molecular ‘second-generation’ resistance guided therapy tests to rapidly determine the susceptibility and resistance profiles of pathogens and inform judicious antibiotic use.15–18 Second-generation diagnostic tests represent a global paradigm shift, enabling effective antibiotics, both old and new, to be prescribed precisely, to safeguard their efficacy and to combat the spread of AMR.19,20 Researchers in the Hub are applying a combination of bacterial culture and genomics analysis, including machine learning tools, to identify new biomarkers. The application of multiplexing technologies facilitates the simultaneous detection of resistance to two or more antimicrobials in one assay.
The Hub’s partnership with international not-for-profit, FIND (the Foundation of Innovative New Diagnostics), also facilitates the identification of low-cost, point-of-care diagnostic applications. Point-of-care diagnostics are necessary for antimicrobial stewardship to rapidly inform antibiotic choices, minimise AMR and preserve the lifespan of antibiotics.21–23 Working together with SpeeDx, who specialise in molecular diagnostics, and Cepheid, who specialise in molecular point-of-care testing, the Hub is establishing an innovative pathway from discovery to manufacture. This includes optimising the use of new medicines like Zoliflodacin, a novel first-in-class oral antibiotic for the treatment of uncomplicated gonorrhoea developed by the Hub partner the Global Antibiotic Research and Development Partnership (GARDP). The engagement of end-users and policymakers early in the design process ensures outcomes are aligned with community needs (see Theme 4).
Theme 2. Optimising STI treatment
Driven by the imperative to restore the antibiotic pipeline, Theme 2 expedites STI antibiotic discovery and optimisation through the development of new, tailored protocols to facilitate faster and more comprehensive assessments of compound suitability. Recognising the variability in treatment efficacy across anatomical sites (e.g. oropharyngeal, anorectal and urogenital sites), this research focuses on exploring the pharmacokinetics of current and new antimicrobials in various infection sites, particularly at non-genital sites of concern such as the oropharynx.24 Oral N. gonorrhoeae infections are a significant public health issue and present unique challenges, for several reasons. Firstly, cure rates are lower than those for anogenital infections,25 secondly, they play a substantial role in spreading the disease through oral sex and saliva contact, and finally, they harbour a propensity to acquire resistance genes from commensal Neisseria sp. microorganisms.24,26,27 The current lack of pharmacokinetic data specific to oropharyngeal infections complicates treatment optimisation efforts.
To overcome these challenges, Theme 2 is pioneering innovative methodologies to measure pharmacokinetics at different infection sites and has developed the world’s first in vitro model of N. gonorrhoeae infection or colonisation in the human oropharynx.28,29 This model enables screening of novel compounds targeting oral N. gonorrhoeae infections, offering hope for more effective therapies and the evaluation of new compounds in development.
Theme 3. Developing novel diagnostics to determine the presence of active bacterial infections
This theme develops revolutionary methods to detect active bacterial infections, also known as viability assays, as an alternative to current nucleic acid amplification methods to determine if the bacterium is alive or dead. This novel diagnostic approach will be a leap forward in modern healthcare and empower healthcare providers to swiftly make informed therapeutic decisions, thereby mitigating the guesswork when prescribing antibiotics and effectively reducing unnecessary treatments for people whose infections are no longer viable. These viability tests can also serve as a clinical tool to enhance our understanding of antibiotic efficacy during clinical trials or during routine ‘test of cure’ visits.
Spearheading this initiative is the InSignia assay, a groundbreaking molecular technology owned by Hub partner, SpeeDx. Unlike traditional diagnostic methods, the InSignia assay distinguishes between living and dead bacteria by measuring and normalising the value of two nucleic acids, reflective of active and inactive bacterial transcription. With InSignia development already completed for C. trachomatis, the Hub is focusing initially on N. gonorrhoea and M. genitalium. Through rigorous testing and validation protocols, the reliability and accuracy of the assay will be established, paving the way for its implementation in clinical settings.
One of the advantages of the InSignia assay is its ability to provide both molecular and phenotypic information, enabling antimicrobial susceptibility testing (AST).30 Integration of molecular AST into high-throughput formats enables rapid screening of large numbers of antimicrobial compounds, overcoming barriers encountered in traditional culture-based methods and accelerating the identification of new antibiotics. The integration of AST into point-of-care tests would further revolutionise precision medicine by facilitating real-time selection of patient-specific, effective antibiotics.
Theme 4. Engaging stakeholders to optimise AMR innovation
The final Hub Theme adopts a comprehensive strategy to engage the perspective of diverse, multidisciplinary stakeholders – inclusive of the community, health professionals, policy makers, epidemiologists, scientists and innovators. The involvement of this spectrum of stakeholders from inception of ideas, to roll-out of change aims to bridge the gap between industry innovation and end-user adoption. This approach acknowledges the importance of understanding market needs and addressing professional and institutional barriers to innovation uptake.
To ensure that innovation is market-sensitive and human-centred, Theme 4 uses social science methods to develop a sophisticated understanding of the priorities and needs in different settings to guide innovation and address barriers to adoption.31,32 This involves conducting scoping studies across diverse settings, such as sexual health services and primary care, and in low- and middle-income countries. It also tackles the development of novel surveillance tools and strategies to track emerging AMR, informing new technologies and investment opportunities.
Modelling and health economics evaluations aim to understand the true value of AMR solutions, financial drivers and new models. Low economic returns from investment in new antibiotics and AMR innovations is a long-standing challenge, identifying the need for sustainable financing and incentivisation models. Theme 4 applies advanced models to comprehensively value these products by incorporating population-wide health impacts (e.g. effects on transmission, population immunity, emergence of new variants, B. B. A. Hui et al. 2023, unpubl. data), equity impacts (e.g. unequal burden and access to interventions borne by vulnerable subgroups) and economy-wide impacts (e.g. output, employment, investment, consumption, trade and education).
Conclusion
We can no longer only rely on the development of new drugs or indeed diagnostic or stewardship innovations alone to solve the multidimensional problem of AMR. We require a range of low-cost, implementable and rapidly adaptable technologies to support broader social, economic and commercial outcomes. AMR solutions must be addressed from the perspective of awareness and prevention, requiring highly collaborative private–public partnerships across sectors to secure predictable market opportunities and solutions. To address this need, the AMR Hub fosters an ecosystem of innovative science, technology, engineering and antimicrobial stewardship to mitigate AMR now and provide sustainable solutions an into the future.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
T. L. Applegate received in-kind cartridges and loaned devices from Cepheid Pty Ltd for unrelated investigator-driven research. C. S. Bradshaw received diagnostic kits and platforms for investigator-led studies over the past 5 years from SpeeDx Pty Ltd and Cepheid Pty Ltd. W. M. Huston conducted contractual research activities from diagnostic companies, including a diagnostic company with AMR-related products, SpeeDx Pty Ltd. G. L. Murray received testing kits form SpeeDx Pty Ltd and TIB MOLBIOL for unrelated investigator-driven research. A. V. Todd is co-inventor of VITA-PCR (known as InSignia), which is the intellectual property owned by SpeeDx Pty Ltd. All other authors (M. Bonello, A. Broom, P. Field, J. S. Hocking, F. Kong, S. Pasricha, V. Wiseman and R. Guy) declare that they have no conflicts of interest.
Declaration of funding
S. Badman is a paid employee of Cepheid Pty Ltd, USA. E. Mokany and A. V. Todd are paid employees and shareholders of SpeeDx Pty Ltd. This paper was supported by the Australian Research Council Industrial Transformation Research Hub to Combat Antimicrobial Resistance (ARC ITRP; IH190100021). The ARC ITRP grant includes cash support from AMR Hub Partner organisations, SpeeDx Pty Ltd, Cepheid Pty Ltd and GARDP Foundation. D. M. Whiley received investigator-driven research funding from SpeeDx Pty Ltd.
References
1 Tacconelli E, et al. (2017) Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland. https://remed.org/wp-content/uploads/2017/03/lobal-priority-list-of-antibiotic-resistant-bacteria-2017.pdf
2 Murray C et al. (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629-655.
| Crossref | Google Scholar |
3 MTPConnect (2022) Australian Antimicrobial Resistance Network (AAMRNet): 2022–2023 Pre-Budget Submission. January 2022. Report for the Australian Government Department of Industry, Science, Energy and Resources. MTPConnect, Melbourne, Vic., Australia. https://treasury.gov.au/sites/default/files/2022-03/258735_mtpconnect.pdf
4 Alividza V et al. (2018) Investigating the impact of poverty on colonization and infection with drug-resistant organisms in humans: a systematic review. Infect Dis Poverty 7, 76.
| Crossref | Google Scholar |
5 Australian Medical Association (2022) Antimicrobial resistance: the silent global pandemic. AMA Ltd. https://www.ama.com.au/antimicrobial-resistance
6 World Health Organization (2023) Global research agenda for antimicrobial resistance in human health. Policy brief. WHO, Geneva, Switzerland. https://www.who.int/publications/m/item/global-research-agenda-for-antimicrobial-resistance-in-human-health
7 Jensen JS, Unemo M (2024) Antimicrobial treatment and resistance in sexually transmitted bacterial infections. Nat Rev Microbiol
| Crossref | Google Scholar |
8 Trembizki E et al. (2021) Enhanced molecular surveillance in response to the detection of extensively resistant gonorrhoea in Australia. J Antimicrob Chemother 76, 270-271.
| Crossref | Google Scholar | PubMed |
9 Kong FYS et al. (2020) Treatment efficacy for pharyngeal Neisseria gonorrhoeae: a systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 75, 3109-3119.
| Crossref | Google Scholar | PubMed |
10 Kong FYS, Hocking JS (2022) Treating pharyngeal gonorrhoea continues to remain a challenge. Lancet Infect Dis 22, 573-574.
| Crossref | Google Scholar | PubMed |
11 Ertl NG et al. (2024) Concurrent parC and gyrA fluoroquinolone resistance mutations and associated strains in Mycoplasma genitalium in Queensland, Australia. J Antimicrob Chemother 79, 467-469.
| Crossref | Google Scholar | PubMed |
12 Murray GL et al. (2023) gyrA Mutations in Mycoplasma genitalium and their contribution to moxifloxacin failure: time for the next generation of resistance-guided therapy. Clin Infect Dis 76, 2187-2195.
| Crossref | Google Scholar | PubMed |
13 Murray GL et al. (2022) parC Variants in Mycoplasma genitalium: trends over time and association with moxifloxacin failure. Antimicrob Agents Chemother 66, e0027822.
| Crossref | Google Scholar | PubMed |
14 Chua TP et al. (2022) Impact of 16S rRNA single nucleotide polymorphisms on Mycoplasma genitalium organism load with doxycycline treatment. Antimicrob Agents Chemother 66, e0024322.
| Crossref | Google Scholar | PubMed |
15 Sweeney EL et al. (2022) Mycoplasma genitalium: enhanced management using expanded resistance-guided treatment strategies. Sex Health 19, 248-254.
| Crossref | Google Scholar | PubMed |
16 Tickner JA et al. (2022) Novel probe-based melting curve assays for the characterization of fluoroquinolone resistance in Mycoplasma genitalium. J Antimicrob Chemother 77, 1592-1599.
| Crossref | Google Scholar | PubMed |
17 Sweeney EL et al. (2022) Individualised treatment of Mycoplasma genitalium infection-incorporation of fluoroquinolone resistance testing into clinical care. Lancet Infect Dis 22, e267-e270.
| Crossref | Google Scholar | PubMed |
18 Vodstrcil LA et al. (2022) Combination therapy for Mycoplasma genitalium, and new insights into the utility of parC mutant detection to improve cure. Clin Infect Dis 75, 813-823.
| Crossref | Google Scholar | PubMed |
19 Tickner JA et al. (2022) The need for a commercial test using the penA60 allele to identify ceftriaxone-resistant Neisseria gonorrhoeae. Lancet Infect Dis 22, 1271-1272.
| Crossref | Google Scholar | PubMed |
20 Bell SFE et al. (2023) Antimicrobial susceptibility assays for Neisseria gonorrhoeae: a proof-of-principle population-based retrospective analysis. Lancet Microbe 4, 544-551.
| Crossref | Google Scholar | PubMed |
21 Lee DYJ et al. (2021) Reflex detection of ciprofloxacin resistance in Neisseria gonorrhoeae by use of the SpeeDx ResistancePlus GC assay. J Clin Microbiol 59, 10.1128/jcm.00089-21.
| Crossref | Google Scholar | PubMed |
22 Read TRH et al. (2019) Outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: a prospective evaluation. Clin Infect Dis 68, 554-560.
| Crossref | Google Scholar | PubMed |
23 Vodstrcil LA et al. (2024) Near-to-patient-testing to inform targeted antibiotic use for sexually transmitted infections in a public sexual health clinic: the NEPTUNE cohort study. Lancet Reg Health West Pac 44, 101005.
| Crossref | Google Scholar | PubMed |
24 Fairley CK et al. (2019) Models of gonorrhoea transmission from the mouth and saliva. Lancet Infect Dis 19, e360-e366.
| Crossref | Google Scholar |
25 Moran JS (1995) Treating uncomplicated Neisseria gonorrhoeae infections: is the anatomic site of infection important? Sex Transm Dis 22, 39-47.
| Crossref | Google Scholar | PubMed |
26 Goytia M et al. (2021) Antimicrobial resistance profiles of human commensal Neisseria species. Antibiotics 10, 538.
| Crossref | Google Scholar | PubMed |
27 Gaspari V et al. (2023) Non-pathogenic Neisseria species of the oropharynx as a reservoir of antimicrobial resistance: a cross-sectional study. Front Cell Infect Microbiol 13, 1308550.
| Crossref | Google Scholar | PubMed |
28 Kong FYS et al. (2022) Optimisation of treatments for oral Neisseria gonorrhoeae infection: Pharmacokinetics Study (STI-PK project) – study protocol for non-randomised clinical trial. BMJ Open 12, e064782.
| Crossref | Google Scholar | PubMed |
29 Kong FYS et al. (2017) Pharmacokinetics of a single 1 g dose of azithromycin in rectal tissue in men. PLoS ONE 12, e0174372.
| Crossref | Google Scholar |
30 Lima N et al. (2019) P070. Determination of antibiotic susceptibility and efficacy by VITA-PCR. Sex Transm Infect 95, A106 [Poster presentation abstract].
| Crossref | Google Scholar |
31 Broom A et al. (2023) Sex, drugs and superbugs: the rise of drug resistant STIs. SSM Qual Res Health 4, 100310.
| Crossref | Google Scholar |
32 Broom AP et al. (2022) Vulnerability and antimicrobial resistance. Crit Public Health 308-317.
| Crossref | Google Scholar |
 Dr Michelle Bonello (BSc(Hons), MPH, PhD) is Business Manager of the Industrial Transformation Research Hub to Combat AMR. She has a background is molecular biology and public health research. |
 Assoc. Prof. Tanya Applegate is Lead of the Diagnostics Innovations Group in the Surveillance Evaluation and Research Program at the Kirby Institute, UNSW Sydney. Tanya leads a multistakeholder, multidisciplinary diagnostics research program spanning all stages of the pipeline with over 20 years working in regulatory, hospital, private industry and academic environments. Her translational research aims to provide equitable access to healthcare for people who are marginalised or living in remote or resource limited settings. |
 Dr Steven Badman is Director of Medical and Scientific Affairs, Cepheid, for the Australia, New Zealand, Papua New Guinea and western Pacific region. Steve is a clinically trained public health researcher with 20+ years of international infectious disease research and public health implementation in several countries, including remote Australia, Indonesia, Malaysia, Thailand, Vanuatu, Papua New Guinea, Tuvalu, Kiribati and Myanmar. |
 Catriona S. Bradshaw is a professor at Monash University, Melbourne. Catriona heads a translational research programme to improve the diagnosis, treatment and control of drug-resistant and refractory sexually transmitted infections (STIs), with a focus on improving antimicrobial stewardship and AMR in STIs. Her programme includes interventions to achieve a vaginal microbiota associated with optimal reproductive health outcomes. |
 Alex Broom is Professor of Sociology and Director of the Sydney Centre for Healthy Societies, Faculty of Arts and Social Sciences, The University of Sydney. |
 Paul Field is the representative for the Global Antibiotic Research & Development Partnership (GARDP) in Australia. GARDP is developing and delivering new treatments for drug-resistant bacterial infections including zoliflodacin a first-in-class antibiotic against Neisseria gonorrohoeae. |
 Prof. Jane Hocking is an epidemiologist and implementation researcher whose research interests include the epidemiology and control of STIs, sexual health and the implementation and evaluation of primary care interventions. She is a Dame Kate Campbell Fellow, holds a NHMRC Investigator Grant (L2) and is the head of the Sexual Health Unit at the Melbourne School of Population and Global Health, University of Melbourne. |
 Prof. Wilhelmina Huston is a molecular microbiologist in the field of STIs based at the University of Technology—Sydney. Her research is focussed on chlamydial pathogenesis, but also host responses and interplay with microbiome factors in the context of both infection and disease pathologies. She is also a committed educator with research and academic development outcomes in higher education teaching and learning in Science. |
 Dr Fabian Kong is a senior fellow and Deputy Head of the Sexual Health Unit at The University of Melbourne. Fabian comes from a clinical pharmacy background with expertise in evidence medicine and international health and has worked for the WHO, UNDP and The World Bank. He currently works as an epidemiologist to optimise treatments for STIs using pharmacokinetics and novel culture models, with a focus on multi-drug resistant gonorrhoea in the mouth. |
 Gerald Murray is a senior research officer in the department of Obstetrics and Gynaecology at The University of Melbourne, based at the Royal Women’s Hospital. His research interests include investigating mechanisms of AMR, studying the human microbiota in health and disease, and human papillomavirus (HPV). |
 Dr Elisa Mokany is a co-founder and the Chief Technology Officer at SpeeDx. Elisa is a strong advocate of SpeeDx’s goal of pioneering solutions to address the global problem of antibiotic resistance with the company’s lead first commercial test world-wide for detection of a STI that detects both the infectious agent together with markers of antibiotic resistance. |
 Shivani Pasricha is a microbiologist and laboratory head in the Department of Infectious Diseases, University of Melbourne. Using molecular and genomic approaches, her research aims to improve the detection, prevention and surveillance of STIs. Her current research includes developing cutting-edge CRISPR-diagnostics for the point-of-care detection of STIs and AMR. |
 Adj. Prof. Alison Todd is the Chief Scientific Officer and co-founder of SpeeDx. She is an elected Fellow of the Australian Academy of Technology and Engineering and awarded Member (AM) in the general division of the Order of Australia. Her commitment to deliver innovative clinical diagnostics to improve patient outcomes has contributed to the development of over 30 products, utilising her patented inventions from a portfolio of over 20 patent families. |
 Assoc. Prof. David Whiley is a principal research fellow at The University of Queensland Centre for Clinical Research (UQCCR) and a research scientist at Pathology Queensland. Antibiotic-resistant Neisseria gonorrhoeae is a particular research interest of his, including enhancing gonorrhoea AMR surveillance and treatment. |
 Virginia Wiseman is Professor of Health Economics (BEc(Hons), PhD) and Theme Director of Health Economics & Health Systems Research at the Kirby Institute, UNSW Sydney, with a dual appointment in the Department of Global Health and Development at the London School of Hygiene & Tropical Medicine. She has 24 years’ experience in translational research, evaluating the economic and health impacts of public health interventions in Australia, Africa and the Asia-Pacific. Virginia leads the Australian Centre for Stronger Investments in Infectious Diseases (STRIDE). |
 Prof. Rebecca Guy (BAppSc, MAppEpid, PhD) is a public health epidemiologist and Head of the Surveillance and Evaluation Research Program at the Kirby Institute, UNSW Sydney. Her research focuses on the epidemiology and prevention of infectious diseases, including HIV, STIs and respiratory tract infections. She has led national surveillance, cohort and data linkage studies, and cluster randomised trials of large-scale public initiatives, particularly among marginalised populations. This research has translated into policy and practice, including national strategies, national guidelines and WHO guidelines. |