The buzz around the zoonotic infection, Buruli ulcer
Carolyn O’Brien A * and Timothy P. Stinear BA
B

Carolyn O’Brien is a veterinarian with a PhD in mycobacteriology and a registered feline medicine specialist. She is currently the director of Melbourne Cat Vets, Victoria. |

Tim Stinear is a professor of microbiology at The University of Melbourne. His research interests are broad and include a long-standing interest in M. ulcerans and Buruli ulcer. |
Abstract
Here, we provide an update on the significant progress towards understanding transmission of Mycobacterium ulcerans, the causative agent of the neglected tropical skin disease, Buruli ulcer. We review the evidence that Buruli ulcer is an enzootic infection among Australian native possums, and we discuss the role of mosquitoes in transmit the infection to humans. We show how studies of M. ulcerans transmission – following the dramatic emergence of Buruli ulcer in temperate south-eastern Australia – are being supported by adherence to One Health principles, with interdisciplinary research teams uncovering connections between human health, animal health, and the environment. We also discuss some of the distinctive features of M. ulcerans revealed by genomics that should be considered when thinking about how this pathogen is spreading.
Keywords: Bairnsdale ulcer, Buruli ulcer, mosquitoes, Mycobacterium ulcerans, mycobacteria, One Health, possums, zoonosis.
Introduction
Buruli ulcer is a slowly progressing infection of subcutaneous tissue caused by Mycobacterium ulcerans. Considered by the World Health Organization (WHO) a skin-related neglected tropical disease,1 Buruli ulcer is predominantly linked with poverty and disadvantage, afflicting rural communities across west and central Africa.2
In 2004, the year Buruli ulcer become notifiable in Victoria, there were 26 human cases of this little-known condition reported across the state. Most of those 26 cases clustered around the small, Victorian seaside town of Point Lonsdale. Buruli ulcer numbers for the state stayed within that low range until 2011 when something changed, and case numbers began to swell. Now, in 2024, Buruli ulcer is a significant, endemic mycobacterial disease in Victoria, with >300 cases reported annually and cases spreading around the major metropolitan centres of Melbourne and Geelong.
Untreated, Buruli ulcer can lead to severe disfigurement and disability, but it is rarely fatal. Prompt diagnosis and administration of the correct antibiotics (WHO recommend an 8-week combination of rifampicin and clarithromycin) are key to a successful outcome with this infection.3 Fortunately, there is an excellent diagnostic polymerase chain reaction (PCR) test for M. ulcerans, and antibiotic treatment is very effective.4
Trying to address the central questions of how and why Buruli ulcer is spreading in Victoria has exercised scientists since the 1930s when local Bairnsdale GPs first noticed the disease in patients from their region in east Gippsland and Melbourne scientists discovered the causative agent, M. ulcerans.5 As often happens in science, in part because enabling technologies become available, discoveries are made contemporaneously by independent research groups. In the 1940s, Australian scientists discovered ‘Bairnsdale ulcer’ was caused by a previously undescribed mycobacterial species they called ‘Mycobacterium ulcerans’. In parallel, doctors working in the Congo and Uganda discovered that ulcerative skin infections in their patients were caused by a new species of mycobacteria they called Mycobacterium buruli, the name derived from a now-defunct county name in Uganda.6 The Australian discovery had precedence, so the accepted species designation became Mycobacterium ulcerans, but ‘Buruli ulcer’ became the WHO-endorsed name for the infection caused by this pathogen.
Although Buruli ulcer is the official name for this infection, the striking focal epidemiology of Buruli ulcer has given rise to lots of eponymous place names for this infection. These descriptions include Bairnsdale ulcer (Victoria), Daintree ulcer (FNQ), Kumusi ulcer (PNG), Kasongo ulcer (Congo) and Buruli ulcer (Uganda), among others.7 Today, Buruli ulcer has been reported in >30 countries worldwide with most cases coming from west and central Africa (~1600 cases reported in 2022) with temperate south-eastern Australia, the second most Buruli ulcer endemic region in the world.1 The increase in cases in south-eastern Australia has renewed research interest in this infection and created opportunities to address long-standing, unanswered questions. One of these questions is the incubation period. Using human cases that have only short exposure periods to known Buruli ulcer endemic areas, it has been possible to calculate an average incubation period of 4–5 months. With most cases notified in winter months, this means transmission of M. ulcerans to humans occurs in the summer months.
Readers are referred to several excellent, recent reviews on the range of issues surrounding Buruli ulcer.2,4,8–13 The focus on this current article is a summary of efforts to address the 80-year mystery of how M. ulcerans is spreading from the environment to humans in south-eastern Australia; where a One Health perspective has become so critical to help understand why there are surging local case numbers in Victoria (and beyond) and why the pathogen is spreading.
Buruli ulcer is an enzootic disease
The discovery in the mid-2000s that Australian native possums in south-eastern Australia are a major wildlife reservoir of M. ulcerans was a breakthrough in the hunt for an environmental reservoir of this pathogen.14 The common ringtail possum (Pseudocheirus peregrinus) and the common brushtail possum (Trichosurus vulpecula) are susceptible to infection with M. ulcerans (Fig. 1c, d) and they can shed high concentrations of the bacteria in their excreta into the environment. Furthermore, so close is the relationship between possums harbouring the bacteria and humans with Buruli ulcer, that possum excreta surveys can be used to predict regions of future risk for human Buruli ulcer.15 A decade of studying the interaction of M. ulcerans with native possums makes it clear that Buruli ulcer is yet another example of a zoonotic disease.14,16–19
Buruli ulcer, an infection of humans and other animals. (a, b) Typical Buruli ulcer lesions in patients from Australia and Ghana, showing central zone of necrosis with undermining on the skin. (c, d) Buruli ulcer in a common ringtail possum, with culture-confirmed lesions on the nose and tail. (e) Range of animals known to be susceptible to M. ulcerans infection, both experimentally and naturally. Image prepared by BioRender.
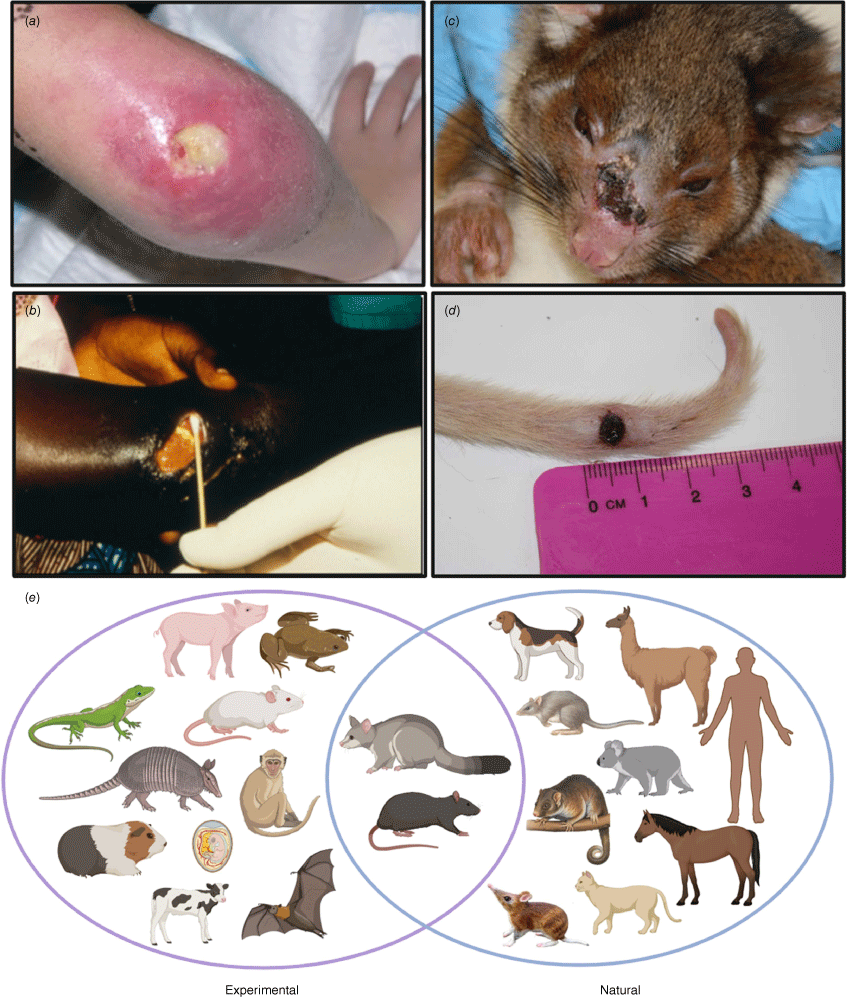
Beyond native possums, a wide range of animals in Australia are known to be naturally susceptible to M. ulcerans infection. These include native species such as the mountain brushtail possum, the long-footed potoroo, koalas, and bandicoots (Fig. 1e).12 Farm and domestic animals have also been confirmed with M. ulcerans infection, including alpacas, horses, domestic cats and dogs (Fig. 1e). The evidence for naturally infected mammalian hosts in African Buruli ulcer endemic countries is less well-developed and to date, no small mammalian reservoir analogous to the Australian native possum has been discovered. However, there is a reasonable likelihood that a similar animal reservoir might exist in Africa given the diverse range of animals that have been successfully experimentally infected with M. ulcerans. These include rodents, guinea pigs, anoles lizards, nine-banded armadillos and bats, among others (Fig. 1e). Understanding the role of animals in the ecology of Buruli ulcer is crucial. This is where a One Health approach that links veterinary sciences with public health surveillance efforts will help find effective disease control strategies.
Genomic insights on Mycobacterium ulcerans
The first complete genome of M. ulcerans revealed some striking features of this pathogen, most notably that the pathogen has a signature of reductive evolution. Genomic details of M. ulcerans have been well-described elsewhere,12 but in summary: (i) expansion of two insertion sequences to high copy-number, (ii) acquisition of a large plasmid conferring the ability to make the potent, bioactive, immunosuppressive lipid mycolactone, (iii) upregulation of a gene encoding a protein (Hsp18) that promotes biofilm formation and (iv) extensive gene-inactivation and reduced genome size relative to a closely related extant relative (Mycobacterium marinum), all pointed to a bacterial population that has passed an evolutionary bottleneck and adapted to survive in a privileged, niche environment (Fig. 2a). These genomic features are shared with niche-occupying pathogens such as Yersinia pestis (mid-gut of the flea) and Bordetella pertussis (human respiratory mucosa).
The evolution and global spread of Mycobacterium ulcerans. (a) M. ulcerans evolved from a M. marinum-like ancestor and passed an evolutionary bottleneck characterised by the acquisition of a plasmid conferring the ability to make mycolactone, upregulation of proteins involved in biofilm formation and expansion of the insertion-sequence IS2404 to high copy number. (b) Summary of the genomic population structure of M. ulcerans, showing the major lineages that have spread globally. (c) World map showing proposed evolutionary scenario for the global spread of the M. ulcerans lineage most often associated with human Buruli ulcer. Image prepared by BioRender.
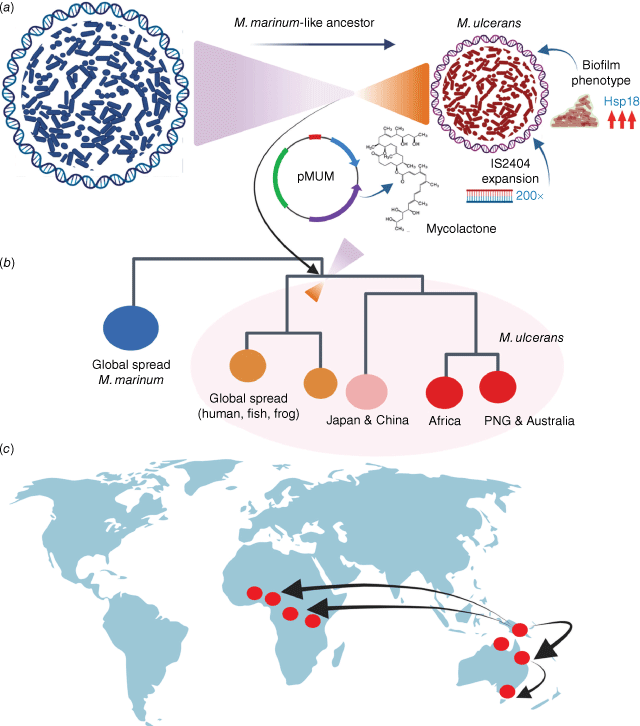
Thus, the pervasive assumption from the epidemiology of Buruli ulcer that M. ulcerans is a generalist, environmental bacterium, spread widely in soils and water, is not supported by the composition of the pathogen’s genome. Global spread of this new, niche-bacterium occurred as the niche itself spread. Of course, these inferences beg the question, what is this niche? However, that key question remains to be answered.
M. ulcerans population genomics has also shown the global dispersal of this pathogen has resulted in at least three main lineages, of which one lineage causes most of the disease reported in humans across Australia and Africa (Fig. 2b). Interestingly, it appears that this lineage may have originated from Papua New Guinea, spreading south through Australia and across into Africa twice (Fig. 2c). Assessment of the M. ulcerans molecular clock suggests the two waves of introduction to Africa occurred c. 1000 years ago and then as recently as 100 years ago.20
Genomics shows M. ulcerans behaves like an introduced, invasive weed
A detailed assessment of the pathogen genomic population structure from south-eastern Australia based on comparisons of 178 M. ulcerans clinical isolates, indicated that introduction of M. ulcerans to south-eastern Australia coincided with European colonisation of the area during the 1800s.21 The structure of the M. ulcerans phylogeny also strongly suggests there is considerable unsampled bacterial genomic diversity remaining to be discovered. One explanation is that there may be undiscovered wildlife reservoirs of the bacterium around Australia that have yet to spill over into humans. It was also possible to infer from these phylogenomic comparisons that the recent emergence of Buruli ulcer around Melbourne and Geelong is explained by introduction of the pathogen from the east of the state, once c. 40 years ago in the 1980s and then more recently in the early 2000s.21 That is, M. ulcerans is behaving like an introduced, invasive plant weed species rather than an endemic weed that expands when growth conditions permit. This is important information because it provides a potential public health intervention and control point if the bacterial reservoir can be identified and intercepted. The same phylogenomic comparisons also enabled an estimation of the time between M. ulcerans entering an area and the first human Buruli ulcer cases appearing. This time-period is ~7–9 years.21 Again, this is information that can guide potential disease control interventions. For example, sentinel wildlife surveillance by excreta surveys could detect recent introductions of the pathogen into an area well before there is a measurable risk to humans or wildlife.
Mosquitoes transmit Mycobacterium ulcerans to humans
The Australian bacteriologist Glen Buckle in his reflections on research with colleague Jean Tolhurst from the 1940s through to the 1960s to try and find the environmental reservoirs of M. ulcerans and how it was transmitted to people in south-eastern Australia, thought that bats might be a wildlife reservoir and blood-feeding insects potential vectors of the pathogen from a wildlife origin.6 These pioneering scientists’ attempts to experimentally test such ideas relied on trying to culture M. ulcerans from candidate animal and environmental sources, but the very slow growth of the pathogen stymied these efforts. In fact, all such ventures around the world that relied on culture isolation to identify environmental reservoirs of M. ulcerans failed, due to overgrowth of culture media by more rapid-growing microorganisms.
The advent of molecular methods in the 1990s and the discovery and description of the IS2404 diagnostic PCR for M. ulcerans was the technology breakthrough needed to reinvigorate efforts to understand how Buruli ulcer is spreading. There are more than 200 copies of this insertion sequence per bacterial genome making it a highly sensitive molecular target. It is also specific for M. ulcerans. Thus, the IS2404 PCR has become the gold-standard diagnostic test for clinical M. ulcerans infection, and it has been the key tool for searching for the pathogen in the environment.22
In the mid-2000s, Paul Johnson, Janet Fyfe and colleagues proposed that mosquitoes could be transmitting M. ulcerans to humans.23 Their team used the IS2404 PCR to screen thousands of mosquitoes trapped along the Bellarine Peninsula, around the seaside town of Point Lonsdale mentioned in the introduction, where many human and possum cases of Buruli ulcer had occurred. Their results revealed five different mosquito species harboured M. ulcerans at a maximum likelihood estimate (MLE) of 4.3 positive mosquitoes per 1000 trapped.23 Ten years later, an even larger mosquito surveillance study on the Mornington Peninsula revealed the same positive association between mosquitoes and M. ulcerans with a MLE of 5.88 positive insects per 1000 trapped.24 Using advanced genomic enrichment methods, this more recent study extracted near-complete bacterial genomes from IS2404-positive mosquitoes and showed that the M. ulcerans genotype between insects, possum excreta and isolates from human Buruli ulcer cases was identical. Moreover, spatial-scanning statistics revealed that the areas where human BU cases cluster overlaps areas with clusters of M. ulcerans-positive possum excreta and mosquitoes. Perhaps most telling from this study, mosquito blood-meal analysis showed individual mosquitoes had fed on both possums and humans, linking mosquitoes, possums and humans in a transmission chain.24
The nature of the interaction between M. ulcerans and mosquito is not well understood. Are mosquitoes biological or mechanical vectors of M. ulcerans? One laboratory study shows that mosquito mechanical transmission of M. ulcerans to a susceptible mammalian host during blood feeding is possible.25 This same research also confirmed a very low infectious dose, with as few as two to three bacterial colony-forming units sufficient to cause infection.25 Interestingly, the bacteria needed to be delivered below the dermis into subcutaneous tissue to establish infection. In animal models, even large concentrations of M. ulcerans applied to intact or abraded skin will not lead to a Buruli ulcer.26
There are other observations and reports that support mosquito transmission. Two case-control studies (conducted >10 years apart) found ‘use of insect repellent’ an independent protective factor against Buruli ulcer.27,28 An assessment of where on the human body Buruli ulcers occur measured a very striking non-random distribution of lesions on the lower legs and arms, areas of the body known to be subjected to mosquito attack.29 Most recently, a comparison of notification data for mosquito-borne alphavirus infections and Buruli ulcer found peak occurrence for both conditions occurred in the warmer, summer months, after adjusting the Buruli ulcer case notification data for the 5-month incubation period.30
Where to from here?
The discovery that mosquitoes are transmitting Buruli ulcer to humans is important because it leads to well-established mosquito control interventions and public messaging about mosquito bite prevention. For the first time since Buruli ulcer was first recognised in Australia over 80 years ago, we have a straightforward answer to the question of how the infection is spread, information individuals and communities can use to protect themselves.
Of course, there remain further critical questions to address about the Buruli ulcer. These include: (i) are native possums the only significant wildlife reservoir; (ii) is there a role in transmission of M. ulcerans for other wildlife species such as the grey-headed flying fox (Pteropus poliocephalus), which is also widespread in suburban Australia; (iii) what is the natural history of M. ulcerans infection in native possums; (iv) what is the nature of the interaction between M. ulcerans and mosquitoes; (v) under what conditions do mosquitoes become competent vectors for transmitting the bacterium; (vi) what spreads or introduces M. ulcerans into a new area; (vii) what is the privileged niche (inferred from genomics) occupied by M. ulcerans; (viii) are there wildlife interventions such as vaccination to control Buruli ulcer31; and (ix) how is M. ulcerans transmitted to humans in African Buruli ulcer endemic countries?
In Australia, trying to realise a One Health approach to tackle these issues will involve overcoming some significant barriers, such as the lack of joined-up surveillance systems between human and animals. Reporting of wildlife Buruli ulcer cases is ad hoc and usually reliant on volunteer wildlife carers or observant citizens who present sick or deceased animals to their local vet clinics. Wildlife Health Australia (WHA) do amazing work to try and collate these data at a national level, but with diverse stakeholder groups, a federated system of autonomous states, and a modest funding base, the task to track emerging zoonotic diseases, while linking with human health, is indeed daunting.
It is interdisciplinary research that has been critical to advance our understanding of Buruli ulcer. It will be this approach, supported by policymakers and with community involvement in eventual disease control and prevention that provides our best opportunities to combat zoonotic infectious disease threats like Buruli ulcer.
Further listening, viewing and reading: ‘Breaking Buruli, part one’32 and ‘Breaking Buruli, part two’33 in ABC’s Science Friction; the Doherty Institute on YouTube,34 and in ‘Buruli ulcer part one’35 and ‘Buruli ulcer part two’36; and WHA’s Fact Sheet: Buruli ulcer and Australian wildlife.37
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Declaration of funding
Some of the research discussed in this review was financially supported by the National Health and Medical Research Council of Australia and the Victorian Department of Health.
References
1 World Health Organization (2023) Global Health Observatory data repository: number of new reported cases: data by country (Buruli ulcer). WHO. https://apps.who.int/gho/data/node.main.A1631
2 Osei-Owusu J et al. (2023) Buruli ulcer in Africa: geographical distribution, ecology, risk factors, diagnosis, and indigenous plant treatment options – a comprehensive review. Heliyon 9, e22018.
| Crossref | Google Scholar | PubMed |
3 Van Der Werf TS et al. (2020) Pharmacologic management of Mycobacterium ulcerans infection. Expert Rev Clin Pharmacol 13, 391-401.
| Crossref | Google Scholar | PubMed |
4 Johnson PDR (2019) Buruli ulcer in Australia. In Buruli ulcer: Mycobacterium ulcerans disease (Pluschke G, Roltgen K, eds). pp. 61–76. Springer, Cham, Switzerland. 10.1007/978-3-030-11114-4_3
5 MacCullum P (1948) A new mycobacterial infection in man. J Pathol Bacteriol 60, 93-102.
| Google Scholar | PubMed |
6 Buckle G (1972) Notes on Mycobacterium ulcerans. Aust NZ J Surg 41, 320-323.
| Google Scholar | PubMed |
7 Korman TM et al. (2020) Etymologia: Buruli ulcer. Emerg Infect Dis 26, 3104.
| Crossref | Google Scholar | PubMed |
8 Singh A et al. (2018) Potential animal reservoir of Mycobacterium ulcerans: a systematic review. Trop Med Infect Dis 3, 56.
| Crossref | Google Scholar | PubMed |
9 Bolz M, Ruf MT (2019) Buruli ulcer in animals and experimental infection models. In Buruli ulcer: Mycobacterium ulcerans disease (Pluschke G, Roltgen K, eds). pp. 159–181. Springer, Cham, Switzerland. 10.1007/978-3-030-11114-4_9
10 Dhungel L et al. (2021) Linking the Mycobacterium ulcerans environment to Buruli ulcer disease: progress and challenges. One Health 13, 100311.
| Crossref | Google Scholar | PubMed |
11 Fevereiro J et al. (2021) Genetics in the host–Mycobacterium ulcerans interaction. Immunol Rev 301, 222-241.
| Crossref | Google Scholar | PubMed |
12 Muleta AJ et al. (2021) Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis 15, e0009678.
| Crossref | Google Scholar | PubMed |
13 Roltgen K et al. (2019) Laboratory diagnosis of Buruli ulcer: challenges and future perspectives. In Buruli Ulcer: Mycobacterium ulcerans disease (Pluschke G, Roltgen K, eds). pp. 183–202. Springer, Cham, Switzerland. 10.1007/978-3-030-11114-4_10
14 Fyfe JAM et al. (2010) A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis 4, e791.
| Crossref | Google Scholar | PubMed |
15 Vandelannoote K et al. (2023) Statistical modeling based on structured surveys of Australian native possum excreta harboring Mycobacterium ulcerans predicts Buruli ulcer occurrence in humans. Elife 12, e84983.
| Crossref | Google Scholar | PubMed |
16 Carson C et al. (2014) Potential wildlife sentinels for monitoring the endemic spread of human buruli ulcer in south-east Australia. PLoS Negl Trop Dis 8, e2668.
| Crossref | Google Scholar | PubMed |
17 O’Brien CR et al. (2014) Clinical, microbiological and pathological findings of Mycobacterium ulcerans infection in three Australian possum species. PLoS Negl Trop Dis 8, e2666.
| Crossref | Google Scholar | PubMed |
18 Blasdell KR et al. (2022) Environmental risk factors associated with the presence of Mycobacterium ulcerans in Victoria, Australia. PLoS One 17, e0274627.
| Crossref | Google Scholar | PubMed |
19 Xu RW et al. (2022) Possum bites man: case of Buruli ulcer following possum bite. Med J Aust 216, 452-453.
| Crossref | Google Scholar | PubMed |
20 Vandelannoote K et al. (2019) Mycobacterium ulcerans population genomics to inform on the spread of Buruli ulcer across central Africa. mSphere 4,.
| Crossref | Google Scholar | PubMed |
21 Buultjens AH et al. (2018) Comparative genomics shows that Mycobacterium ulcerans migration and expansion preceded the rise of Buruli ulcer in southeastern Australia. Appl Environ Microbiol 84,.
| Crossref | Google Scholar | PubMed |
22 Fyfe JA et al. (2007) Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl Environ Microbiol 73, 4733-4740.
| Crossref | Google Scholar | PubMed |
23 Johnson PDR et al. (2007) Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis 13, 1653-1660.
| Crossref | Google Scholar | PubMed |
24 Mee PT et al. (2024) Mosquitoes provide a transmission route between possums and humans for Buruli ulcer in southeastern Australia. Nat Microbiol 9, 377-389.
| Crossref | Google Scholar | PubMed |
25 Wallace JR et al. (2017) Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis 11, e0005553.
| Crossref | Google Scholar | PubMed |
26 Williamson HR et al. (2014) Mycobacterium ulcerans fails to infect through skin abrasions in a guinea pig infection model: implications for transmission. PLoS Negl Trop Dis 8, e2770.
| Crossref | Google Scholar | PubMed |
27 McNamara BJ et al. (2023) Comprehensive case-control study of protective and risk factors for Buruli ulcer, southeastern Australia. Emerg Infect Dis 29, 2032-2043.
| Crossref | Google Scholar | PubMed |
28 Quek TY et al. (2007) Risk factors for Mycobacterium ulcerans infection, southeastern Australia. Emerg Infect Dis 13, 1661-1666.
| Crossref | Google Scholar | PubMed |
29 Yerramilli A et al. (2017) The location of Australian Buruli ulcer lesions – implications for unravelling disease transmission. PLoS Negl Trop Dis 11, e0005800.
| Crossref | Google Scholar | PubMed |
30 Buultjens AH et al. (2023) Season of transmission of Ross River/Barmah Forest Virus and Mycobacterium ulcerans closely align in southeastern Australia, supporting mosquitoes as the vector of Buruli ulcer. bioRxiv 2023.08.07.552371. [Preprint, posted 31 August 2023].
| Crossref | Google Scholar |
31 O’Brien DP et al. (2023) Is BCG vaccination of possums the solution to the Buruli ulcer epidemic in south-eastern Australia? Med J Aust 219, 520-522.
| Crossref | Google Scholar | PubMed |
32 Kulas E (2022) Breaking Buruli, part one. In Science Friction, 27 February 2022. Australian Broadcasting Corporation. https://www.abc.net.au/listen/programs/sciencefriction/breaking-buruli-part-one/13767626
33 Kulas E (2022) Breaking Buruli, part two. In Science Friction, 6 March 2022. Australian Broadcasting Corporation. https://www.abc.net.au/listen/programs/sciencefriction/breaking-buruli-part-two/13780816
34 Doherty Institute (2024) New discovery to help stop the spread of Buruli ulcer (Mycobacterium ulcerans). In YouTube: Doherty Institute channel, 29 January 2024. https://www.youtube.com/watch?v=gkMdsAD9r7s
35 Doherty Media (2024) Buruli ulcer part one: 80 year-long transmission mystery solved. 2 February 2024. Doherty Institute. https://www.doherty.edu.au/news-events/podcast/buruli-ulcer-part-one-80-year-long-transmission-mystery-solved
36 Doherty Media (2024) Buruli ulcer part two: a diagnosis and advocating for change. 2 February 2024. Doherty Institute. https://www.doherty.edu.au/news-events/podcast/buruli-ulcer-part-two-a-diagnosis-and-advocating-for-change
37 Wildlife Health Australia (2024) Buruli ulcer and Australian wildlife. Fact Sheet, January 2024, ver. 3.8. Wildlife Health Australia. https://wildlifehealthaustralia.com.au/Portals/0/ResourceCentre/FactSheets/Mammals/Buruli_ulcer_and_Australian_wildlife.pdf
 Carolyn O’Brien is a veterinarian with a PhD in mycobacteriology and a registered feline medicine specialist. She is currently the director of Melbourne Cat Vets, Victoria. |
 Tim Stinear is a professor of microbiology at The University of Melbourne. His research interests are broad and include a long-standing interest in M. ulcerans and Buruli ulcer. |


