Targeting tuberculosis with LNP-mRNA vaccines: opportunities, challenges and future directions
Hannah Lukeman A B # , Elizabeth Chan A B # and James Triccas A B *A
B

Hannah Lukeman is a third year PhD candidate at The University of Sydney. She is researching the mechanisms and efficacy of next-generation vaccine platforms against Mycobacterium tuberculosis. |

Elizabeth Chan is undertaking her PhD candidature at The University of Sydney. Her research focuses on characterising next-generation vaccine platforms, such as mRNA and protein in adjuvant vaccines and comparing their immune mechanisms to explore their application in protecting against lung pathogens. |

James Triccas is a professor of medical microbiology and the deputy director of the Sydney Institute for Infectious Diseases (Sydney ID) at The University of Sydney. His research uses a multidisciplinary approach to understand immunity to medically important lung pathogens and develop new treatments to control infection, with a long-standing focus on tuberculosis and more recently COVID-19. |
Abstract
Tuberculosis (TB) remains one of the leading causes of infectious disease mortality, despite widespread use of the Bacillus Calmette–Guérin (BCG) vaccine. The COVID-19 pandemic demonstrated the potential of mRNA vaccines to induce rapid and robust immunity, leading to interest in their application against TB. However, developing an effective mRNA vaccine for TB presents unique challenges, including the need for strong cellular immune responses and lung-targeted immunity. This review explores the potential of lipid nanoparticle (LNP)-encapsulated mRNA vaccines to generate protective immune responses against Mycobacterium tuberculosis. We discuss recent advances in the field, including promising preclinical studies, and highlight key knowledge gaps that must be addressed before mRNA vaccines can be considered a viable option for TB control.
Keywords: cellular immunity, lipid nanoparticle, mRNA vaccine, mucosal immunity, Mycobacterium tuberculosis, preclinical studies, TB, tuberculosis, vaccination.
The need for novel tuberculosis vaccines
Five years after the start of the COVID-19 pandemic, one of humanities oldest pathogens, Mycobacterium tuberculosis, is once again the leading cause of death globally from a single infectious agent. In 2023 alone, there were an estimated 10.8 million new cases of tuberculosis (TB) and 1.25 million deaths worldwide.1 A major challenge for TB control is the absence of an effective vaccine. Bacillus Calmette–Guérin (BCG) is the only licensed vaccine against TB and, although it is protective in young children, efficacy against pulmonary infection in adults is inconsistent, ranging between 0 and 80%.2 A more reliable and effective vaccine would offer significant benefits to global public health and the economy.3,4 However, the TB vaccine development pipeline remains limited, with only a few candidates progressing to late-stage clinical trials despite more than a century of research (Table 1). Notably, all advanced candidates rely on traditional vaccine platforms, such as protein subunits, live attenuated strains or inactivated bacteria.
| Platform | Vaccine | Developer | Description | |
|---|---|---|---|---|
| Protein subunit | GamTBvac | Gamaleya research centre, Ministry of Health of the Russian Federation | Three M. tuberculosis antigens: Ag85a and a ESAT6-CFP10 fusion protein, adjuvanted with dextran 500 kDa, diethylaminoethyl (DEAE)-Dextran 500 kDa and CpG oligodeoxynucleotide (ODN) | |
| M72/AS01E | Bill & Melinda Gates Medical Research Institute, Wellcome, GSK | M72 fusion protein of M. tuberculosis antigens Mtb32a and Mtb39a, formulated with AS01E adjuvant | ||
| Live attenuated | MTBVAC | Biofabri S.L. | Attenuated form of M. tuberculosis, with deletion of phoP and fadD26 virulence genes | |
| VPM1002 | Serum Institute of India Pvt Ltd (SIIPL), Serum Life Science Europe GmbH (SLS Europe) | Recombinant version of the Bacille Calmette–Guérin (BCG) vaccine, with the gene for urease C replaced with the Listeria monocytogenes listeriolysin O (LLO) gene | ||
| Inactivated | Immuvac (MIP) | Indian Council of Medical Research (ICMR), Cadila | Heat-killed Mycobacterium indicus pranii |
Potential of the LNP-mRNA vaccine platform against tuberculosis
The mRNA vaccine platform is attractive for use against TB for several reasons. Firstly, although still a highly technological process, the cell free method of production allows for rapid, scalable and cost-effective development.5 This not only facilitates increased speed of screening target antigens, which has been a major historical bottleneck in TB vaccine development,3 but makes mass distribution of these vaccines in low-income countries, who have the greatest burden of TB, economically viable.4 Efforts are also being made with the support of the World Health Organization (WHO) to establish facilities in these countries to enable local production of mRNA vaccines.6 Furthermore, mRNA vaccines are non-infectious, meaning they are safe for use in immunocompromised individuals, such as those infected with the human immunodeficiency virus (HIV), for whom TB is the leading cause of death.1
To date, all mRNA vaccines licenced for use in humans target viral infections, including Pfizer’s COMIRNATY (BNT162b) and Moderna’s SPIKEVAX (mRNA-1273) against SARS-CoV-2, and Moderna’s mRESVIA (mRNA-1345) for respiratory syncytial virus (RSV).7 For these infections, protection is primarily associated with the generation of high-titre, vaccine-specific neutralising antibodies that prevent entry of the virus into host cells.8 In addition to this humoral immunity, mRNA vaccines also elicit strong Th1 CD4+ T cell responses, producing IFN-γ, IL-2 and TNF cytokines that mediate further innate and adaptive immune responses targeted against intracellular pathogens.9 This is particularly relevant for TB, where Th1 immunity is considered essential for host protection.10,11
Encouragingly, recent preclinical studies – including our own – have demonstrated the potential of mRNA vaccines to induce protective immune responses against M. tuberculosis.12–16 Our group developed an mRNA vaccine encoding for CysVac2, a fusion protein of M. tuberculosis antigens Ag85B and CysD, and demonstrated robust generation of vaccine-specific CD4+ T cells producing multiple cytokines (e.g. IFN-γ, IL-2 and TNF).12 This was associated with significant protection against M. tuberculosis in mice. Similar protective outcomes have been reported by others using different mRNA constructs.13,15
However, these findings come with caveats. In our extended infection model, the CysVac2 mRNA vaccine failed to provide sustained protection beyond the acute phase of infection.12 This suggests that current mRNA formulations may be suboptimal for long-term control of TB. Moreover, the field still lacks a validated correlate of protection (CoP) for TB,3 complicating the interpretation of immune readouts in animal models and limiting predictive value for human efficacy. Although mRNA platforms show promise, significant gaps remain in translating preclinical findings into durable, clinically relevant protection against TB.
mRNA vaccines for tuberculosis: additional challenges
Ongoing efforts to define CoPs against TB has garnered interest in lung mucosal immunity, particularly the role of resident memory T (TRM) cells. TRM cells are antigen-experienced memory T cells located in peripheral tissues, and are therefore well posed to rapidly respond to pathogens at their site of infection.17 The induction of vaccine-specific CD4+ TRM cells in the lung has correlated with protection against M. tuberculosis challenge across diverse vaccine platforms. For example, Flórido et al.18 used an intranasal influenza vector vaccine to induce polyfunctional, Th1-skewed (IFN-γ+, TNF+, IL-2+) CD4+ TRM cells in the lung parenchyma and demonstrated that protection was independent of circulating T cell populations. Intranasal administration of BCG also induced protective lung TRM cells expressing markers of Th-17 polarisation.19 Similarly, intratracheal delivery of the CysVac2 protein with adjuvant led to the formation of functionally analogous TRM cells in the lung.20 Therefore, the induction of lung TRM cells is thought to be essential for robust protection against TB.
By contrast, mRNA vaccines appear to have limited capacity to induce lung-resident TRM cells (Fig. 1). This has been observed consistently across both human and animal studies. For instance, following vaccination with the widely used BNT162b COVID-19 mRNA vaccine, TRM cells were either absent, or present at very low frequencies in the lungs of vaccinated individuals and mice.9,21 Furthermore, in one study where lung TRM cells were detected post-mRNA vaccination, their frequencies were significantly lower than in individuals who had recovered from infection, and polyfunctional CD4+ and CD8+ T cells were virtually absent.22 These findings suggest that in their current form, mRNA vaccines display a limited capacity to establish lung-localised T cell populations considered critical for effective TB protection, and may explain the lack of sustained protection against the bacterium.
The ability of mRNA vaccines to induce protective immune responses against TB. mRNA vaccines can induce robust systemic responses including polyfunctional Th1 (IL-2+, TNF+, IFN-γ+) and Th17 (IL-17+) effector memory T (TEM) cells. These circulating T cells can traffic through the lung and contribute to anti-TB immunity. Lung-resident T (TRM) cells, positioned at the site of infection, are also thought to play a critical role in protection against TB. It remains unclear, however, whether current mRNA vaccine platforms are capable of inducing TRM cells with a phenotype associated with durable TB immunity.
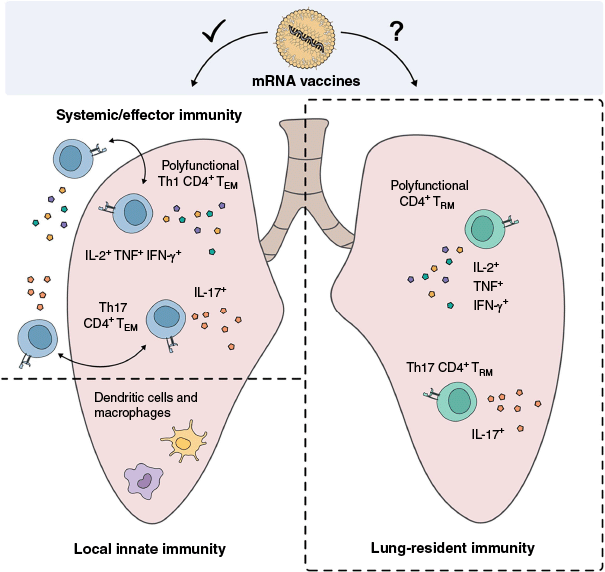
Prospects for mRNA-based tuberculosis vaccines
mRNA vaccines are well established to induce robust systemic immune responses when delivered intramuscularly. However, their inability to induce TRM cells in the lung restricts immunity against TB, where localised mucosal immunity is considered essential. Thus, new strategies are needed to improve the ability of mRNA vaccines to establish protective lung immunity.
Preclinical vaccines have shown that vaccines capable of inducing lung TRM cells typically use intrapulmonary delivery routes, such as intranasal and intratracheal administration. Attempts to apply these methods to mRNA vaccines have been made; however, lipid nanoparticle (LNP) formulations are highly inflammatory when delivered into the lung, leading to significant morbidity and mortality in animal models.23 Thus, there is interest in the development of new LNP formulations that are safe for mucosal delivery.24,25 Although a subset of LNP formulations have successfully elicited mucosal antibody responses,26,27 reports of robust TRM cells induction have been limited. Additionally, intrapulmonary vaccination alone often results in limited systemic immunity, which may be insufficient for protection against extrapulmonary or disseminated TB.
An alternative approach involves alerting the physicochemical properties (i.e. size or surface charge) of mRNA vaccine formulations to achieve tissue-specific targeting following intramuscular vaccination. Cationic LNPs, for example, have been shown to promote concentrated mRNA expression in the lung following systemic administration.28,29 However, it is unclear if this strategy enhances vaccine-specific immune responses and protective lung-resident memory. Additionally, there are concerns regarding the toxicity and reactogenicity of some cationic LNPs, including associations with clotting and other adverse event.30 Therefore, safety profiling of mRNA vaccines must be a priority in the development of next-generation LNP formulations.
Künzli and colleagues have shown that these challenges can, at least in part, be addressed.31 Using a self-amplifying mRNA platform and a novel, low reactogenicity dendron based nanoparticle, they were able to induce lung TRM cells in mice by intramuscular and intranasal vaccination. In addition, they demonstrated the potential for combined vaccine strategies, using an initial intramuscular dose followed by an intranasal boost to first induce systemic memory then draw cells into the lung. This study provides proof-of-concept that rational design of both the mRNA platform and delivery system can support mucosal T cell immunity, while avoiding the toxicity seen with earlier formulations.
Conclusion
Although mRNA vaccines offer speed, scalability and strong systemic immunity, their current formulations fall short in eliciting the lung-resident T cell responses considered essential for durable TB protection. Overcoming this will require innovation in both delivery routes and lipid formulations, efforts that are already showing promise and will be critical to realising the potential of mRNA vaccines in TB control.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Declaration of funding
The authors acknowledge the National Health and Medical Research Council (NHMRC) Centre of Research Excellence in Tuberculosis Control (2035369) for supporting their TB vaccine studies.
Acknowledgements
We thank Dr Claudio Counoupas (Centenary Institute) and Dr Megan Steain (The University of Sydney) for helpful discussions.
References
1 World Health Organisation (2024) Global tuberculosis report 2024. WHO, Geneva, Switzerland. https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-health/tb-reports/global-tuberculosis-report-2024
2 Mangtani P et al. (2014) Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 58, 470-480.
| Crossref | Google Scholar | PubMed |
3 Looney MM et al. (2023) Conference report: WHO meeting summary on mRNA-based tuberculosis vaccine development. Vaccine 41, 7060-7066.
| Crossref | Google Scholar | PubMed |
4 Portnoy A et al. (2023) The potential impact of novel tuberculosis vaccine introduction on economic growth in low- and middle-income countries: a modeling study. PLoS Med 20, e1004252.
| Crossref | Google Scholar | PubMed |
5 Kis Z et al. (2020) Rapid development and deployment of high-volume vaccines for pandemic response. J Adv Manuf Process 2, e10060.
| Crossref | Google Scholar | PubMed |
6 Council on the Economics of Health For All (2023) The mRNA Vaccine Technology Transfer Hub: a pilot for transformative change for the common good? World Health Organization. https://www.who.int/publications/m/item/the-mrna-vaccine-technology-transfer-hub--a-pilot-for-transformative-change-for-the-common-good
7 US Food and Drug Administration (2025) Vaccines Licensed for Use in the United States. USFDA. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
8 Khoury DS et al. (2021) Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27, 1205-1211.
| Crossref | Google Scholar | PubMed |
9 Li C et al. (2022) Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat Immunol 23, 543-555.
| Crossref | Google Scholar | PubMed |
10 Darrah PA et al. (2007) Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13, 843-850.
| Crossref | Google Scholar | PubMed |
11 Derrick SC et al. (2011) Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine 29, 2902-2909.
| Crossref | Google Scholar | PubMed |
12 Lukeman H et al. (2025) An LNP-mRNA vaccine modulates innate cell trafficking and promotes polyfunctional Th1 CD4+ T cell responses to enhance BCG-induced protective immunity against Mycobacterium tuberculosis. eBioMedicine 113, 105599.
| Crossref | Google Scholar | PubMed |
13 De Voss CJ et al. (2025) Novel mRNA vaccines induce potent immunogenicity and afford protection against tuberculosis. Front Immunol 16, 1540359.
| Crossref | Google Scholar | PubMed |
14 Reshetnikov V et al. (2024) Untranslated region sequences and the efficacy of mRNA vaccines against tuberculosis. Int J Mol Sci 25, 888.
| Crossref | Google Scholar | PubMed |
15 Larsen SE et al. (2023) An RNA-based vaccine platform for use against Mycobacterium tuberculosis. Vaccines 11, S47-S51.
| Crossref | Google Scholar | PubMed |
16 Xue T et al. (2004) RNA encoding the MPT83 antigen induces protective immune responses against Mycobacterium tuberculosis infection. Infect Immun 72, 6324-6329.
| Crossref | Google Scholar | PubMed |
17 Ogongo P et al. (2019) Lung tissue resident memory T-cells in the immune response to Mycobacterium tuberculosis. Front Immunol 10, 992.
| Crossref | Google Scholar | PubMed |
18 Flórido M et al. (2018) Pulmonary immunization with a recombinant influenza A virus vaccine induces lung-resident CD4+ memory T cells that are associated with protection against tuberculosis. Mucosal Immunol 11, 1743-1752.
| Crossref | Google Scholar | PubMed |
19 Dijkman K et al. (2019) Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med 25, 255-262.
| Crossref | Google Scholar | PubMed |
20 Counoupas C et al. (2020) Mucosal delivery of a multistage subunit vaccine promotes development of lung-resident memory T cells and affords interleukin-17-dependent protection against pulmonary tuberculosis. NPJ Vaccines 5, 105.
| Crossref | Google Scholar | PubMed |
21 Israelow B et al. (2021) Adaptive immune determinants of viral clearance and protection in mouse models of SARS-CoV-2. Sci Immunol 6, eabl4509.
| Crossref | Google Scholar | PubMed |
22 Pieren DKJ et al. (2023) Limited induction of polyfunctional lung-resident memory T cells against SARS-CoV-2 by mRNA vaccination compared to infection. Nat Commun 14, 1887.
| Crossref | Google Scholar | PubMed |
23 Ndeupen S et al. (2021) The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 24, 103479.
| Crossref | Google Scholar | PubMed |
24 Ongun M et al. (2024) Lipid nanoparticles for local delivery of mRNA to the respiratory tract: effect of PEG-lipid content and administration route. Eur J Pharm Biopharm 198, 114266.
| Crossref | Google Scholar | PubMed |
25 Maniyamgama N et al. (2025) Muco-penetrating lipid nanoparticles having a liquid core for enhanced intranasal mRNA delivery. Adv Sci 12, 2407383.
| Crossref | Google Scholar | PubMed |
26 Vaca G et al. Intranasal mRNA-LNP vaccination protects hamsters from SARS-CoV-2 infection. Sci Adv 9, eadh1655.
| Crossref | Google Scholar | PubMed |
27 Leekha A et al. (2024) Multi-antigen intranasal vaccine protects against challenge with sarbecoviruses and prevents transmission in hamsters. Nat Commun 15, 6193.
| Crossref | Google Scholar | PubMed |
28 LoPresti ST et al. (2022) The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs. J Control Release 345, 819-831.
| Crossref | Google Scholar | PubMed |
29 Qiu M et al. (2022) Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA 119, e2116271119.
| Crossref | Google Scholar | PubMed |
30 Omo-Lamai S et al. (2024) Physicochemical targeting of lipid nanoparticles to the lungs induces clotting: mechanisms and solutions. Adv Mat 36, 2312026.
| Crossref | Google Scholar | PubMed |
31 Künzli M et al. (2022) Route of self-amplifying mRNA vaccination modulates the establishment of pulmonary resident memory CD8 and CD4 T cells. Sci Immunol 7, eadd3075.
| Crossref | Google Scholar | PubMed |
 Hannah Lukeman is a third year PhD candidate at The University of Sydney. She is researching the mechanisms and efficacy of next-generation vaccine platforms against Mycobacterium tuberculosis. |
 Elizabeth Chan is undertaking her PhD candidature at The University of Sydney. Her research focuses on characterising next-generation vaccine platforms, such as mRNA and protein in adjuvant vaccines and comparing their immune mechanisms to explore their application in protecting against lung pathogens. |
 James Triccas is a professor of medical microbiology and the deputy director of the Sydney Institute for Infectious Diseases (Sydney ID) at The University of Sydney. His research uses a multidisciplinary approach to understand immunity to medically important lung pathogens and develop new treatments to control infection, with a long-standing focus on tuberculosis and more recently COVID-19. |


