Bioinnovation and drug discovery at ANSTO’s Australian Synchrotron
Ashish Sethi A * , Rachel M. Williamson A , Emily G. Finch A , Daniel Häusermann A , Helen E. A. Brand A and Danielle E. Martin A *A

Dr Ashish Sethi is the scattering group manager at ANSTO’s Australian Synchrotron. His research expertise includes the use of integrated structural biology methods to characterise biomolecular structure and function. |

Dr Rachel Williamson is the crystallography group manager at ANSTO’s Australian Synchrotron. She specialises in chemical crystallography and structural biology, with research interests in organic electronics and biosensors. |

Dr Emily Finch is the microscopy group manager at ANSTO’s Australian Synchrotron. Her research interests lie in geoscience, particularly metal mobility in Earth’s crust to support green technologies. |

Dr Daniel Häusermann is the imaging group manager at ANSTO’s Australian Synchrotron. His research interests lie in supporting cancer detection and diagnosis, understanding biological functioning and assessing engineering structures. |

Dr Helen Brand is currently the acting science operations manager at ANSTO’s Australian Synchrotron. Her research focuses on the thermoelastic properties and crystal chemistry of minerals relevant to environmental, planetary geology and industrial applications. |

Dr Danielle Martin is currently the acting senior principal scientist (head of science), who oversees strategic developments, managing, prioritising and facilitating team and stakeholder interactions, capital and asset management programs and leading key science initiatives across ANSTO’s Australian Synchrotron. |
Abstract
ANSTO’s Australian Synchrotron (AS) is a premier national research facility providing Australia, New Zealand and the broader region with access to world-class instrumentation and advanced analytical techniques. Synchrotrons worldwide have established themselves as invaluable tools for drug discovery and biological innovation, and the AS is no different. The Australian Synchrotron’s capabilities provide significant data regarding the molecular and structural dynamics of complex biological systems. These enable insights from mapping drug-target interactions at the atomic level to visualising physiological responses within tissues and organisms. The following article outlines these capabilities and their application to drug discovery in more detail.
Keywords: bioinnovation, biomedical imaging, CFS, crystallographic fragment screening, microbeam radiation therapy, microspectroscopy, MRT, small-angle X-ray scattering, synchrotron, synchrotron-based drug discovery, structural biology.
Introduction
As one of Australia’s major research facilities, the Australian Nuclear Science & Technology Organisation’s (ANSTO) Australian Synchrotron (AS) is a national institute that drives scientific development for both academic and industrial research applications (Fig. 1, 2). It supports a broad range of studies, from physics, chemistry, materials, medicine, biology, biochemistry and more, providing Australia, New Zealand, Singapore and the surrounding region with access to world-class instrumentation and cutting-edge analytical techniques. Synchrotrons have positioned themselves as invaluable tools for drug discovery and biological innovation. By using the high-intensity radiation produced by a synchrotron source, the AS enables the study of the atomic and molecular composition of any substance with high precision. The facility supports a number of techniques or ‘beamlines’, including scattering, crystallography, microscopy and imaging beamlines, able to provide significant data regarding the molecular and structural dynamics of complex biological systems (Fig. 3). The following sections highlight how these powerful tools are accelerating breakthroughs in drug discovery and bioinnovation.
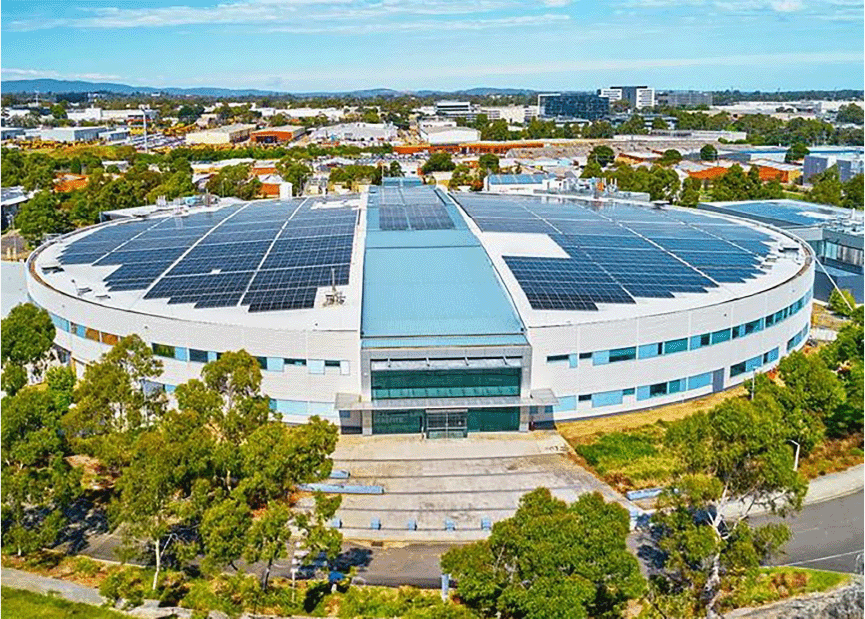
Top view of the Australian Synchrotron, showcasing the oval-shaped synchrotron ring, the onsite guest house (top LH corner), and the solar panels on the roof, highlighting the integration of national research infrastructure with sustainable energy solutions.
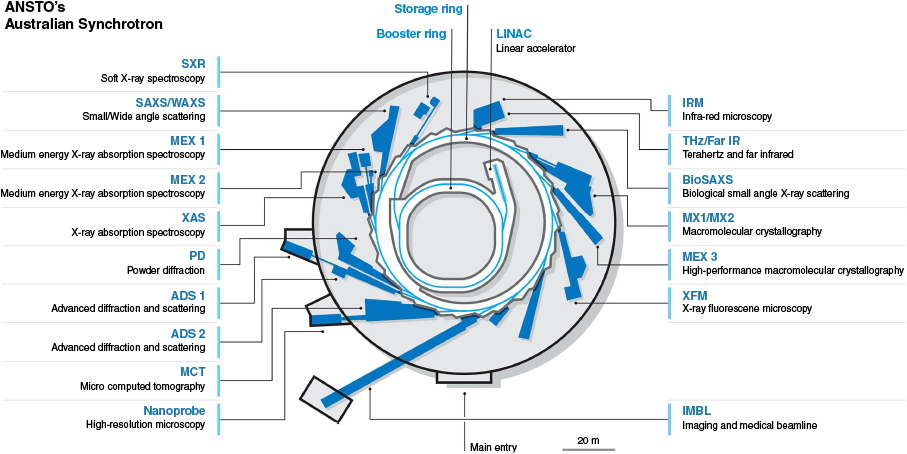
Floorplan of the Australian Synchrotron, showcasing all 18 beamlines, including 15 operational and 3 under construction at the time of writing (ADS1, ADS2, Nanoprobe). This highlights our broad infrastructure supporting advanced research in a wide range of applications including bioinnovation and drug discovery.
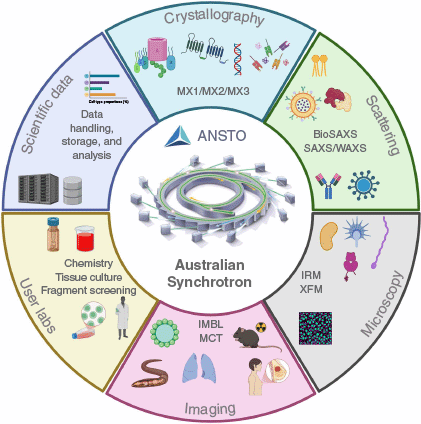
Schematic representation of the Australian Synchrotron’s integrated capabilities for advancing bioinnovation and drug discovery. It highlights our key beamline capabilities, including crystallography, scattering, imaging and microscopy, as well as user laboratories and data handling systems, supporting cutting-edge research towards therapeutic development. Created in BioRender.
Crystallography: unlocking the 3-D structure of drug targets
X-Ray crystallography has long been one of the most reliable and widely used methods for determining the three-dimensional (3-D) structure of biomolecules, and X-ray crystallography beamlines at the Australian Synchrotron (AS) are playing a central role in structure-based drug discovery and design.1 The ability to determine the precise atomic arrangement or coordinates of molecules allows scientists to visualise drug targets in unparalleled detail, providing critical information for the design of small molecules that can bind to specific sites on these targets. Crystallography therefore supports a wide range of therapeutic categories, such as infectious,2–4 autoimmune5 and neurodegenerative disease.6 For example, synchrotron researchers have elucidated the structures of bacterial enzymes that are involved in antibiotic resistance, allowing the design of new antibiotics that can evade resistance mechanisms.7,8 In neuroscience research, crystallography has been instrumental in uncovering the structural characteristics of amyloid proteins involved in Alzheimer’s disease, providing essential information for drug design.9–11 Research into the SARS-CoV-2 virus and others has also used synchrotron crystallography to examine aspects of the virus, from vaccine and possible drug targets to the immune response of infected individuals.12–14
The crystallographic beamlines at the AS include the Macromolecular (MX1)15 and Microfocus (MX2)16 beamlines, and the High Performance Macromolecular Crystallographic (MX3) beamline (Fig. 2, 3). With its microfocus and high flux beam, MX3 is particularly well suited for studying microcrystals, which are often weakly diffracting. These challenging proteins and protein-complexes are often highly flexible representing the native biological state. MX3 as a new state-of-the-art beamline at the facility, will enable significant advancements in techniques such as serial crystallography, time-resolved crystallography and tray screening. These transformative technologies will enable researchers to capture biomolecular interactions and conformational changes in real time, providing a level of detail that was previously unattainable. Such innovations are crucial, allowing the timely unravelling the complexities of drug targets, accelerating the development of targeted therapies and improving the efficacy of new treatments.
The AS is also supporting the development of a Crystallographic Fragment Screening (CFS) pipeline. CFS using synchrotrons is a very effective approach for identifying potential drug candidates.17 Traditionally, it has been a slow and labour-intensive process, even when using a synchrotron, and primary screening has remained outside of the repertoire of most laboratories. Attempts are now underway in collaboration with key Australian researchers, to take advantage of advances in technologies to establish a standard CFS pipeline for the design of high-affinity molecules for protein interactions. Together with the AS MX suite, this will significantly enhance Australia’s capability for structure-based drug design, enabling the design of new drugs and therapies more specific, effective and efficient.
Scattering methods: studying biological interactions in solution
One of the major techniques at the AS is Small Angle X-ray Scattering (SAXS). SAXS allows researchers to study the shape and structure of macromolecules such as proteins, nucleic acids and lipids and their interactions in solution (Fig. 2, 3). This technique is especially valuable for drug discovery as it provides crucial insights into how potential drug candidates interact with target proteins, enzymes and other biomolecules. SAXS also allows for the real-time tracking of protein structure and conformational changes, aiding in the design of small molecules that target modified or pathogenic forms of disease-associated proteins. The Biological SAXS (BioSAXS) beamline specifically offers high flux radiation to support structural biology research at low concentrations, providing a streamlined and highly automated data collection and processing experience. This is enhanced when used with the ‘Coflow’ sample autoloader, which supports in-line size exclusion chromatography (SEC) coupled with SAXS (SEC-SAXS)18 and can batch-run up to 96 pre-equilibrated samples. This setup is particularly useful for the high-throughput studies of poorly stable, low concentration protein–protein and protein–ligand interactions in solution. Such studies can identify potential drug binding sites, including any induced conformational changes. These findings are highly relevant for targeting proteins associated with infectious diseases,19–23 phase separation,24,25 enzymes functions26,27 and cancer.28
SAXS also plays a crucial role in optimising lipid nanoparticle (LNP) formulation for enhanced drug delivery. Using the temperature-controlled sample environments, researchers can investigate biomolecular–lipid interactions at different temperatures, providing insights into the dynamics and formulations of LNPs.29–31 This can be applied, for example, to examine mRNA delivery systems,32 to assess their efficiency or immunogenic response generation. Additionally, a Flowthrough Capillary setup allows for dynamic measurements that involve probing biomolecular or LNP interactions tracked in real time. This is particularly important for measuring LNP delivery and behaviours within biological systems, contributing again to the development of improved systems for vaccine and drug delivery.33,34 Together these advanced SAXS-focused methodologies are crucial for vaccine and drug development by providing essential information about biomolecular dynamics, molecular interactions and the optimisation of different drug delivery systems.
Microscopy: examining cellular processes in drug development
The Infrared Microspectroscopy (IRM) beamline is another powerful tool for enhancing drug discovery research at the AS (Fig. 2, 3). IRM utilises infrared radiation to examine the molecular components of cells and tissues, allowing researchers to map the chemistry of a system and trace the dynamics of drug candidates on cellular processes in close to real time.35 As infrared radiation is non-ionising, it is particularly useful for determining the effects of pharmaceutical drugs on living cells, with specialised IRM techniques allowing for the sub-cellular chemical mapping of single eukaryotic cells.36 As a non-destructive technique,37 IRM provides a clear picture of a cell’s response to external stimuli, including protein expression modifications, lipid profiles and capturing the chemistry of metabolic processes.38 IRM as a key biomedical tool has therefore been applied to the study of many different disease pathologies, including neurotoxicity,39 Alzheimer’s disease,40 diabetes mellitus and various blood diseases including malaria and leishmania.41,42
IRM has also proven a valuable technique in cancer research for the investigation of drug–tumour cell interactions. By comparing molecular changes in cells prior to and following pharmacological intervention, investigators can derive valuable information regarding drug action and drug resistance mechanisms. This information is important in the identification of compounds that can selectively kill cancer cells without significant causing side effects to healthy tissue.43
Imaging: advanced high-resolution techniques in pharmaceutical development
The Imaging group at the AS, with its Imaging and Medical beamline (IMBL) and the Micro-Computed Tomography (MCT) beamline,44 provides essential facilities for the investigation of drug behaviour within biological organs and tissues,45 and also supports broader studies in organ46 and tissue morphology (Fig. 2, 3).47 The Imaging beamlines enable the physiology of disease or the activity of pharmaceutical drug candidates to be captured in situ and at high spatial resolution.48 Beamline capabilities include high-resolution whole-organ imaging, small- and large-animal scans such as whole-pig CT, and live-animal lung imaging using phase contrast.49,50 These capabilities are crucial to the development and validation of realistic preclinical models and to the understanding of complex disease environments, both of which underpin bioinnovation and therapeutic developments.
IMBL also allows researchers to examine the distribution and physiological effect of drug treatment within tissues such as tumours and surrounding organs. Preclinical studies conducted at IMBL have provided valuable insights into the effects of radiation therapy on tumour growth, interaction with adjacent tissues, and overall distribution within the host organism. One particularly promising technique being investigated at IMBL is Microbeam Radiation Therapy (MRT), an experimental cancer treatment using synchrotron-generated, spatially fractionated arrays of micrometre-scale radiation fields referred to as microbeams. MRT has demonstrated advanced tumour control ability while minimising damage to healthy tissues. MRT exhibits a post-treatment window of transient localised increased capillary permeability, which offers promising avenues for drug delivery studies, especially in evaluating how therapeutic agents interact with tumour environments under conditions of high-dose radiation. Such techniques are crucial in assessing treatment efficacy and refining therapeutic approaches at the preclinical stage, as demonstrated in recent studies involving glioma-bearing animal models.51–53 Imaging is therefore a key aspect of drug discovery; to define the distribution of drugs within an organism, map their transit across biological barriers and also their delivery to the targeted tissues of interest.
The IMBL has also been used for high-resolution visualisation and characterisation of breast cancer tissues, including the detection of microcalcifications, tumour vasculature and tissue boundaries, offering new insights into tumour heterogeneity and aiding in the development of more targeted therapies.54–57 The MCT beamline can be similarly applied but for higher-resolution imaging of smaller samples and is thus perfectly positioned to analyse the complex architectures of cells and tissues, before, during and after drug treatments. In this way the imaging capabilities at the AS are essential to aid in the design of drug delivery systems for the targeted delivery of drugs with limited potential for unwanted off-target effects.
Conclusion
ANSTO’s AS is a cutting-edge bioinnovation facility, providing crucial infrastructure to underpin pharmaceutical development and discovery in Australia. Its comprehensive array of scattering, crystallography, microscopy and imaging beamlines holds unprecedented potential to track the dynamics of molecular and structural biology and thereby enabling scientists to design better and more targeted therapeutic drugs. Through the capacity to facilitate protein and ligand interaction examination, drug target 3-D structure determination, cellular processes exploration, and observation of interactions between drugs within living organisms, the synchrotron holds potential to determine future directions for medical innovation. Owing to ongoing development in synchrotron technologies, coupled with intense focus in research driven by user demand and collaborations, the AS has the potential to be one of the top providers of national and international collaborations, in the effort to achieve novel treatments of disease. With capabilities that are the latest in innovation and backed by the collaborative model of research, the facility is a utility of the pharmaceutical world and a main collaborator towards achieving improved human health.
Data availability
This article does not contain any original data. All data discussed are from previously published sources, which are cited throughout the text.
Declaration of funding
All research featured was supported by various Australian, New Zealand and international funding sources.
Acknowledgements
Part or all of this research was conducted at ANSTO’s Australian Synchrotron. We acknowledge ANSTO and the invaluable support of our academic research community, alongside our staff scientists, whose expertise in experimental design, data analysis and beamline operation were essential in the successful completion of the research highlighted within this article.
References
1 Jose J et al. (2023) Fragment-based and structure-guided discovery of perforin inhibitors. Eur J Med Chem 261, 115786.
| Crossref | Google Scholar | PubMed |
2 Haywood J et al. (2022) A fungal tolerance trait and selective inhibitors proffer HMG-CoA reductase as a herbicide mode-of-action. Nat Commun 13(1), 5563.
| Crossref | Google Scholar | PubMed |
3 Cheong EZK et al. (2022) Crystal structure of the Rubella virus protease reveals a unique papain-like protease fold. J Biol Chem 298(8), 102250.
| Crossref | Google Scholar | PubMed |
4 Zhan J et al. (2022) Molecular basis of functional effects of phosphorylation of the C-terminal domain of the rabies virus P protein. J Virol 96(9), e0011122.
| Crossref | Google Scholar | PubMed |
5 Chang YG et al. (2022) Structure of the metastatic factor P-Rex1 reveals a two-layered autoinhibitory mechanism. Nat Struct Mol Biol 29(8), 767-773.
| Crossref | Google Scholar | PubMed |
6 Nematollahi A et al. (2016) Structure of the PLP-form of the human kynurenine aminotransferase II in a novel spacegroup at 1.83 Å resolution. Int J Mol Sci 17(4), 446.
| Crossref | Google Scholar | PubMed |
7 Hansen MH et al. (2023) Resurrecting ancestral antibiotics: unveiling the origins of modern lipid II targeting glycopeptides. Nat Commun 14(1), 7842.
| Crossref | Google Scholar | PubMed |
8 Brazel EB et al. (2022) Dysregulation of Streptococcus pneumoniae zinc homeostasis breaks ampicillin resistance in a pneumonia infection model. Cell Rep 38(2), 110202.
| Crossref | Google Scholar | PubMed |
9 Zhang S et al. (2025) Global analysis of endogenous protein disorder in cells. Nat Methods 22(1), 124-134.
| Crossref | Google Scholar | PubMed |
10 Crespi GA et al. (2015) Molecular basis for mid-region amyloid-β capture by leading Alzheimer’s disease immunotherapies. Sci Rep 5, 9649.
| Crossref | Google Scholar | PubMed |
11 Ishii K et al. (2024) Crystal structure of Alzheimer’s disease phospholipase D3 provides a molecular basis for understanding its normal and pathological functions. FEBS J 291(24), 5398-5419.
| Crossref | Google Scholar | PubMed |
12 Calleja DJ et al. (2022) Insights into drug repurposing, as well as specificity and compound properties of piperidine-based SARS-CoV-2 PLpro inhibitors. Front Chem 10, 861209.
| Google Scholar |
13 Ku Z et al. (2022) Engineering SARS-CoV-2 specific cocktail antibodies into a bispecific format improves neutralizing potency and breadth. Nat Commun 13(1), 5552.
| Crossref | Google Scholar | PubMed |
14 Rouet R et al. (2023) Broadly neutralizing SARS-CoV-2 antibodies through epitope-based selection from convalescent patients. Nat Commun 14(1), 687.
| Crossref | Google Scholar | PubMed |
15 Cowieson NP et al. (2015) MX1: a bending-magnet crystallography beamline serving both chemical and macromolecular crystallography communities at the Australian Synchrotron. J Synchrotron Radiat 22(1), 187-190.
| Crossref | Google Scholar | PubMed |
16 Aragão D et al. (2018) MX2: a high-flux undulator microfocus beamline serving both the chemical and macromolecular crystallography communities at the Australian Synchrotron. J Synchrotron Radiat 25(Pt 3), 885-891.
| Crossref | Google Scholar | PubMed |
17 Whitehouse RL et al. (2023) Fragment screening libraries for the identification of protein hot spots and their minimal binding pharmacophores. RSC Med Chem 14(1), 135-143.
| Crossref | Google Scholar | PubMed |
18 Kirby N et al. (2016) Improved radiation dose efficiency in solution SAXS using a sheath flow sample environment. Acta Crystallogr D Struct Biol 72(Pt 12), 1254-1266.
| Crossref | Google Scholar | PubMed |
19 Busby JN et al. (2024) The ABC toxin complex from Yersinia entomophaga can package three different cytotoxic components expressed from distinct genetic loci in an unfolded state: the structures of both shell and cargo. IUCrJ 11(Pt 3), 299-308.
| Crossref | Google Scholar | PubMed |
20 Sethi A et al. (2023) Structural insights into the multifunctionality of rabies virus P3 protein. Proc Natl Acad Sci USA 120(14), e2217066120.
| Crossref | Google Scholar | PubMed |
21 Fellner M et al. (2025) Similar but distinct-biochemical characterization of the Staphylococcus aureus serine hydrolases FphH and FphI. Proteins 93(5), 1009-1021.
| Crossref | Google Scholar | PubMed |
22 Newton-Vesty MC et al. (2024) On the function of TRAP substrate-binding proteins: the isethionate-specific binding protein IseP. Biochem J 481(24), 1901-1920.
| Crossref | Google Scholar | PubMed |
23 Oldham KEA et al. (2022) Serine acetyltransferase from Neisseria gonorrhoeae; structural and biochemical basis of inhibition. Biochem J 479(1), 57-74.
| Crossref | Google Scholar | PubMed |
24 Hewage TW et al. (2019) A new crystal structure and small-angle X-ray scattering analysis of the homodimer of human SFPQ. Acta Crystallogr F Struct Biol Commun 75(Pt 6), 439-449.
| Crossref | Google Scholar | PubMed |
25 Knott GJ et al. (2022) Structural basis of dimerization and nucleic acid binding of human DBHS proteins NONO and PSPC1. Nucleic Acids Res 50(1), 522-535.
| Crossref | Google Scholar | PubMed |
26 Meng Y et al. (2023) Phosphorylation-dependent pseudokinase domain dimerization drives full-length MLKL oligomerization. Nat Commun 14(1), 6804.
| Google Scholar |
27 Sethi A et al. (2021) Structural insights into the unique modes of relaxin-binding and tethered-agonist mediated activation of RXFP1 and RXFP2. J Mol Biol 433(21), 167217.
| Crossref | Google Scholar | PubMed |
28 Metcalfe RD et al. (2023) Structures of the interleukin 11 signalling complex reveal gp130 dynamics and the inhibitory mechanism of a cytokine variant. Nat Commun 14(1), 7543.
| Google Scholar |
29 Yu H et al. (2022) Polyphenol-functionalized cubosomes as thrombolytic drug carriers. Adv Healthc Mater 11(21), e2201151.
| Crossref | Google Scholar | PubMed |
30 Rajesh S et al. (2022) Application of fluconazole-loaded pH-sensitive lipid nanoparticles for enhanced antifungal therapy. ACS Appl Mater Interfaces 14,.
| Crossref | Google Scholar | PubMed |
31 Faisal KS et al. (2022) Microstructure-thermal property relationships of poly (ethylene glycol-b-caprolactone) copolymers and their micelles. Polymers 14(20), 4365.
| Crossref | Google Scholar | PubMed |
32 Yu H et al. (2024) pH-Dependent lyotropic liquid crystalline mesophase and ionization behavior of phytantriol-based ionizable lipid nanoparticles. Small 20(20), e2309200.
| Crossref | Google Scholar | PubMed |
33 Yu H et al. (2023) Real-time pH-dependent self-assembly of ionisable lipids from COVID-19 vaccines and in situ nucleic acid complexation. Angew Chem Int Ed Engl 62(35), e202304977.
| Google Scholar |
34 Awad M et al. (2023) Lyophilized lipid liquid crystalline nanoparticles as an antimicrobial delivery system. Antibiotics 12(9), 1405.
| Crossref | Google Scholar | PubMed |
35 Karagiannis TC et al. (2023) Characterization of K562 cells: uncovering novel chromosomes, assessing transferrin receptor expression, and probing pharmacological therapies. Cell Mol Life Sci 80(9), 248.
| Crossref | Google Scholar | PubMed |
36 Vongsvivut J et al. (2019) Synchrotron macro ATR-FTIR microspectroscopy for high-resolution chemical mapping of single cells. Analyst 144(10), 3226-3238.
| Crossref | Google Scholar | PubMed |
37 Zohdi V et al. (2015) Importance of tissue preparation methods in FTIR micro-spectroscopical analysis of biological tissues: ‘traps for new users’. PLoS ONE 10(2), e0116491.
| Crossref | Google Scholar | PubMed |
38 Vongsvivut J et al. (2015) Synchrotron-FTIR microspectroscopy enables the distinction of lipid accumulation in thraustochytrid strains through analysis of individual live cells. Protist 166(1), 106-121.
| Crossref | Google Scholar | PubMed |
39 Sanislav O et al. (2024) Cell invasive amyloid assemblies from SARS-CoV-2 peptides can form multiple polymorphs with varying neurotoxicity. Nanoscale 16(42), 19814-19827.
| Crossref | Google Scholar | PubMed |
40 Summers KL et al. (2017) A multimodal spectroscopic imaging method to characterize the metal and macromolecular content of proteinaceous aggregates (“amyloid plaques”). Biochemistry 56(32), 4107-4116.
| Crossref | Google Scholar | PubMed |
41 Chakkumpulakkal Puthan Veettil T et al. (2023) Synchrotron-Infrared Microspectroscopy of Live Leishmania major Infected Macrophages and Isolated Promastigotes and Amastigotes. Anal Chem 95(8), 3986-3995.
| Crossref | Google Scholar | PubMed |
42 Blank M et al. (2022) The effect of carbamazepine on bone structure and strength in control and osteogenesis imperfecta (Col1a2 (+/p.G610C)) mice. J Cell Mol Med 26(14), 4021-4031.
| Crossref | Google Scholar | PubMed |
43 Chen W et al. (2024) Size-dependent penetration of nanoparticles in tumor spheroids: a multidimensional and quantitative study of transcellular and paracellular pathways. Small 20(8), e2304693.
| Google Scholar |
44 Arhatari BD et al. (2025) Micro-computed tomography beamline of the Australian Synchrotron: density measurements. Rev Sci Instrum 96(2), 023707.
| Crossref | Google Scholar | PubMed |
45 Mentzel F et al. (2022) Fast and accurate dose predictions for novel radiotherapy treatments in heterogeneous phantoms using conditional 3D-UNet generative adversarial networks. Med Phys 49(5), 3389-3404.
| Crossref | Google Scholar | PubMed |
46 O’Connell DW et al. (2022) Accurate measures of changes in regional lung air volumes from chest X-rays of small animals. Phys Med Biol 67(20), 205002.
| Crossref | Google Scholar | PubMed |
47 Stainsby AV et al. (2025) Effect of prenatal diaphragmatic hernia on pulmonary arterial morphology. Anat Rec 308(4), 1082-1093.
| Crossref | Google Scholar | PubMed |
48 Harker SA et al. (2024) Using X-ray velocimetry to measure lung function and assess the efficacy of a pseudomonas aeruginosa bacteriophage therapy for cystic fibrosis. Sci Rep 14(1), 29727.
| Crossref | Google Scholar | PubMed |
49 Murrie RP et al. (2015) Live small-animal X-ray lung velocimetry and lung micro-tomography at the Australian Synchrotron imaging and medical beamline. J Synchrotron Radiat 22(4), 1049-1055.
| Crossref | Google Scholar | PubMed |
50 Donnelley M et al. (2019) Live-pig-airway surface imaging and whole-pig CT at the Australian Synchrotron imaging and medical beamline. J Synchrotron Radiat 26(Pt 1), 175-183.
| Crossref | Google Scholar | PubMed |
51 Livingstone J et al. (2017) Preclinical radiotherapy at the Australian Synchrotron’s imaging and medical beamline: instrumentation, dosimetry and a small-animal feasibility study. J Synchrotron Radiat 24(Pt 4), 854-865.
| Crossref | Google Scholar | PubMed |
52 Yang Y et al. (2014) In vitro study of genes and molecular pathways differentially regulated by synchrotron microbeam radiotherapy. Radiat Res 182(6), 626-639.
| Crossref | Google Scholar | PubMed |
53 Dipuglia A et al. (2019) Validation of a Monte Carlo simulation for microbeam radiation therapy on the imaging and medical beamline at the Australian Synchrotron. Sci Rep 9(1), 17696.
| Crossref | Google Scholar | PubMed |
54 Tavakoli Taba S et al. (2021) Propagation-based phase-contrast CT of the breast demonstrates higher quality than conventional absorption-based CT even at lower radiation dose. Acad Radiol 28(1), e20-e26.
| Crossref | Google Scholar | PubMed |
55 Pacilè S et al. (2018) Advantages of breast cancer visualization and characterization using synchrotron radiation phase-contrast tomography. J Synchrotron Radiat 25(Pt 5), 1460-1466.
| Crossref | Google Scholar | PubMed |
56 Gureyev TE et al. (2019) Propagation-based X-ray phase-contrast tomography of mastectomy samples using synchrotron radiation. Med Phys 46(12), 5478-5487.
| Crossref | Google Scholar | PubMed |
57 Crosbie JC et al. (2013) Reference dosimetry at the Australian Synchrotron’s imaging and medical beamline using free-air ionization chamber measurements and theoretical predictions of air kerma rate and half value layer. Med Phys 40(6), 062103.
| Crossref | Google Scholar | PubMed |
 Dr Ashish Sethi is the scattering group manager at ANSTO’s Australian Synchrotron. His research expertise includes the use of integrated structural biology methods to characterise biomolecular structure and function. |
 Dr Rachel Williamson is the crystallography group manager at ANSTO’s Australian Synchrotron. She specialises in chemical crystallography and structural biology, with research interests in organic electronics and biosensors. |
 Dr Emily Finch is the microscopy group manager at ANSTO’s Australian Synchrotron. Her research interests lie in geoscience, particularly metal mobility in Earth’s crust to support green technologies. |
 Dr Daniel Häusermann is the imaging group manager at ANSTO’s Australian Synchrotron. His research interests lie in supporting cancer detection and diagnosis, understanding biological functioning and assessing engineering structures. |
 Dr Helen Brand is currently the acting science operations manager at ANSTO’s Australian Synchrotron. Her research focuses on the thermoelastic properties and crystal chemistry of minerals relevant to environmental, planetary geology and industrial applications. |
 Dr Danielle Martin is currently the acting senior principal scientist (head of science), who oversees strategic developments, managing, prioritising and facilitating team and stakeholder interactions, capital and asset management programs and leading key science initiatives across ANSTO’s Australian Synchrotron. |


