Physicochemical properties and bacterial diversity of pristine and stressed Malaysian mangrove ecosystems
Getha Krishnasamy A * , Jeyanny Vijayanathan B , Nur Nabilah Alias B and Norlia Basherudin BA
B

Getha Krishnasamy is a Senior Research Officer at the Biomolecules Research Laboratory, Forest Research Institute Malaysia (FRIM). She is the curator of the FRIM Microbial Culture Collection, and an elected Executive Committee member of the Malaysian Society for Microbiology. She has extensive expertise in microbial research that focuses on actinomycetes, endophytic bacteria and fungi isolated from various environments. Getha’s main interest is in investigating how microbes interact with each other in the environment, and harnessing these microbes for agricultural and industrial uses. Her current research involves innovative fermentation methods for production of bioactive compounds from basidiomycetes. |

Jeyanny Vijayanathan is a Senior Research Officer and Head of the Soil Management Branch in the Forestry Biotechnology Division. She has been elected as the global soil expert for the Intergovernmental Technical Panel on Soils by the FAO. She has extensive expertise in soil chemistry, fertility, plant nutrition and soil carbon dynamics across forestry, agriculture, environmental and degraded ecosystems. Her current research involves assessment on soil health and plant productivity in the changing environment. |

Nur Nabilah Alias is a researcher at the Genetics Laboratory, Forest Research Institute Malaysia (FRIM). Her research focuses on next-generation sequencing (NGS) and multi-omics approaches, including genomics, transcriptomics, metabolomics and metagenomics. She has expertise in bioinformatics, particularly in the processing and interpretation of high-throughput sequencing data for multi-omics studies. Her research also involves plant DNA profiling, DNA barcoding and the development of SSR markers to support genetic and evolutionary studies. Currently, she focuses on population genomics to investigate population structure and adaptive variation, especially in response to climate change. |

Norlia Basherudin is a Senior Research Officer at the Genetics Laboratory, Department of Forestry Biotechnology, Forest Research Institute Malaysia (FRIM). Her research specialises in plant molecular biology, with a focus on genetic engineering, gene function analysis and the detection of living modified organisms (LMOs). Her current projects include the production of hairy root cultures expressing proinsulin, the development of detection methods for genetically modified eucalyptus, and the characterisation of genes involved in quassinoid biosynthesis. |
Abstract
Recent microbiome research highlights the complexity and specialisation of microbial communities living in mangrove ecosystems. Distinct prokaryotic community structures reflect the unique environmental conditions and ecological processes in these habitats, emphasising their key roles in biogeochemical transformation and nutrient cycling. Here we highlight the changes in bacterial diversity and community structures in sediments from two contrasting Malaysian mangrove ecosystems, Tanjung Piai mangroves as a degraded pollution-affected site and Matang Mangrove Forest as a pristine well-preserved site, using 16S rRNA gene amplicon sequencing approach. Higher organic carbon and organic matter contents in Matang, in contrast to the higher available phosphorus content in Tanjung Piai sediment, clearly showed differences in the bacterial diversity of both sites. We also observed higher species richness and community diversity in Tanjung Piai, suggesting a more heterogeneous bacterial population potentially influenced by external nutrient inputs and other confounding factors due to anthropogenic activities. Collectively, the findings suggested a possible relationship between specific anthropogenic activity-related variables in mangrove sediment and individual bacterial taxa. Thus, understanding the complex interactions of soil sediment microbiome within the habitat could facilitate the assessment of impact from stress factors and identify mangrove ecosystems needing rehabilitation, as part of the mangrove conservation strategies.
Keywords: amplicon sequencing, anthropogenic activity, bacterial diversity, sediment microbiome, soil physicochemical, tropical mangrove.
Coastal mangroves: role, threats and associated microbiomes
Tropical coastal mangrove forests are biogeochemically active zones susceptible to tidal change and salinity, enabling exchange of sediments and organic matter between water and land.1 Besides serving to protect coastlines and impede erosion, these coastal wetlands support various key functions which includes biogeochemical cycling, ecosystem remediation, carbon sequestration, flood mitigation, breeding and nursing grounds for aquatic species, and providing bioresources for local community, industries and tourism.2 Malaysia has among the largest extents of mangrove forests in South East Asia. Despite having significant impact on environment protection, biodiversity conservation and socio-economic benefits, this unique ecosystem is constantly threatened by a multitude of stress factors such as intensive harvesting, pollution from excessive anthropogenic activities, rise in sea level, sediment salinisation and other natural disasters.3 Over the years, reports showed a drastic decline in Malaysian coastal mangrove areas from approximately 700,000 ha in 1975 to 572,000 ha in 2000.4 This raised an urgent need for close monitoring of soil quality status of microsites within affected stands in the country, for establishing soil restoration, rehabilitation and sustainable management strategies in coastal mangrove forests.5
Mangrove forests harbour a highly dynamic and diverse microbiome composition and structure, including bacteria, archaea, viruses, fungi and protists, due to the environmental conditions and nutrient contents in these ecosystems.6 With high organic matter as well as anaerobic and reduced condition of soil, mangrove sediments have high capacity to accumulate pollutants discharged from anthropogenic activities to the near shore marine environment. These constant changes have repercussions, as alterations to the mangrove sediment physicochemical properties can influence microbiome diversity and composition within the ecosystem.7 Therefore, it is likely that there will be a difference in the mangrove microbiome structure influenced by anthropogenic pressures, and knowing the indicator species is useful for monitoring the impact of these stresses over time.8
Metagenomics research using high throughput next-generation sequencing (NGS) to identify microbial taxa from environmental samples, have expanded our understanding of the mangrove microbiome, its diversity, abundance and potential functional roles.9 Prokaryotic communities associated with mangrove sediments and the rhizosphere, which includes bacteria and archaea, play crucial roles in nutrient cycling to maintain soil fertility and ecological balance in the productive mangrove ecosystems.10 While the relevance of mangrove prokaryotic processes to carbon, nitrogen and phosphorus cycling are known, information on the influence of stress factors on the changes in bacterial diversity and community structure is still lacking in Malaysia.11 Here, we present a study which examined bacterial communities in two contrasting Malaysian coastal mangrove areas, Tanjung Piai and Matang mangroves, using 16S rRNA gene amplicon sequencing. The idea was to generate a baseline data set on mangrove habitat specificity to changes in the microbiome of both ecosystems, with regards to interaction with different sediment physicochemical properties.
Sediment physicochemical properties in stressed and pristine mangrove ecosystems
Mangrove forests act as important carbon sinks, providing a niche for bacterial communities crucial in regulating nutrient cycles.12 Variables such as erosion, flooding, agricultural activities, industrial and sewage effluents, and urban development can heavily impact the physicochemical properties of mangrove sediments.13 Tanjung Piai National Park is located in the Johor State land comprising of coastal mangroves and intertidal mudflats, at the southernmost tip of continental Asia. Studies in Tanjung Piai mangroves outlined its importance in biodiversity as a Bruguiera-Rhizophora dominated forest.14 Although being awarded as a Ramsar site (wetlands of international importance), this coastal mangrove area of 526 ha has been impacted by various factors hampering the efforts in conservation strategies and eco-tourism.14 An oil slick contamination incident reported previously15 highlights its geographical location as an important waterway of the Straits of Malacca for cargo ships. Thus presents potential hydrocarbon and heavy metal contamination risks, and shoreline retreat caused by hydrodynamic conditions and regular ship traffic, which further impacts mangrove health.11
The Matang Mangrove Forest Reserve (MMFR), located in the districts of Krian, Larut Matang and Manjung in the State of Perak on the northwest coast of Peninsular Malaysia, is known worldwide as an exemplary sustainably managed tropical mangrove forest with an area of 40,288 ha. Most areas are covered by pure stands of the economically valuable Bakau Forest (Rhizophora apiculata and R. mucronata). The MMFR provides ecosystem services such as coastal protection, biodiversity conservation, ecotourism, wood provision for charcoal production, fishery maintenance and mangrove propagule production. For 115 years, the mangroves in the productive zones have been harvested under a 30-year rotation cycle, indicating a long-standing sustainable management regime.16
Systematic sampling of sediments at 5–20 cm depth was carried out by establishing five transects at each mangrove site. Sediment samples from Tanjung Piai were collected from transects facing seawards on the intertidal mudflats with sparse vegetation (Fig. 1), and also facing landwards in an old growth forest parallel to the intertidal mudflats. In Matang, samples were collected from undisturbed pristine mangrove forest patches or the Virgin Jungle Reserve areas (Fig. 2). Overall, soil physicochemical analysis showed a significantly higher organic carbon (OC) content which correlates with organic matter (OM) value of 2.9-fold higher in the pristine Matang site compared to Tanjung Piai. We found that despite having comparative levels of nitrogen (N), the available phosphorus (Av. P) level was significantly higher in Tanjung Piai (Table 1).
Mangrove sediment collected at 5–20 cm depth using augers in one of the five transects established for sampling in Tanjung Piai mangrove; figure shows one of the transect area facing seawards on the intertidal mudflats with sparse vegetation (source: Forest Research Institute Malaysia).

Pristine mangrove forest patches within Matang Mangroves are showcases of the rich biological diversity of this unique ecosystem (source: Forest Research Institute Malaysia).

| Properties | Matang | Tanjung Piai | |
|---|---|---|---|
| Dry pH | 5.67 ± 0.10 | 5.70 ± 0.20 | |
| EC (mS cm−1) | 9.26 ± 0.45a | 14.29 ± 0.51b | |
| OC (%) | 7.16 ± 0.48a | 5.75 ± 0.29b | |
| OM (%) | 71.33 ± 0.12a | 24.82 ± 0.17b | |
| Av. P (ppm) | 21.01 ± 13.07a | 41.55 ± 4.12b | |
| N (%) | 0.29 ± 0.01 | 0.30 ± 0.01 | |
| CEC (cmol kg−1) | 28.04 ± 2.34 | 30.56 ± 0.59 |
EC, electrical conductivity; OC, organic carbon; OM, organic matter; Av. P, available phosphorus; N, nitrogen; CEC, cation exchange capacity. Different lowercase letters beside values in rows denotes significant differences (P < 0.01).
Most mangrove ecosystems are known to be rich in C and OM but poor in nutrients which includes N and P. The inputs from litterfall alters the OC, OM and N values as microbes utilise these elements for various processes such as decomposition, nitrification, denitrification and transformation.17 Our findings indicated that there are several aquaculture farming activities upstream of the Tanjung Piai mangroves. The effluent from these aquaculture farms flows downstream and resides in the intertidal enclave of Tanjung Piai, presenting a potential source of the high Av. P observed in the sediments. Effluents from shrimp and aquaculture farming are known to contain high P.18 Similar studies on mangroves in South America and Asia showed that areas impacted by anthropogenic activities may result in up to 17-fold higher N and P accumulation rates in comparison with values under conserved conditions. While others reported that these differences may by up to 5-fold higher in impacted as compared to the conserved mangrove ecosystems.19 Thus, there is a high possibility that besides influx of sediment rich nutrients from the open sea facing the Straits of Malacca, upstream influx may have also altered the nutrient dynamics of Tanjung Piai mangroves.
Mangrove–microbiome interactions in different ecosystems
Outcomes of the amplicon sequencing analysis showed higher number of unique amplicon sequence variants (ASVs) in the Tanjung Piai mangroves. Unique site-specific ASVs may serve as indicators of core microbial community components shaping mangrove sediment microbiomes.12 Additionally, alpha diversity indices dataset measured significant differences in bacterial communities between Matang and Tanjung Piai, with the latter exhibiting higher species richness and diversity. Tanjung Piai experiences higher levels of external nutrient inputs from human settlements, shrimp farming activities and the waterways of the Straits of Malacca. Oil spills and illegal water ballast dumping,20 and high lead and zinc contents,21 have also been reported. Increased nutrient levels such as Av. P and other confounding factors due to anthropogenic activities, may have favoured a more diverse bacterial population and higher unique ASVs in Tanjung Piai. External nutrient inputs from anthropogenic disturbances are known to shift the ecosystem equilibrium forcing microorganisms to adapt to the new habitat. This indirectly causes disturbed mangrove areas to have higher unique bacterial taxa, promoting a variety of microbes to thrive and survive in the ecosystem.12 Conversely, Matang mangroves appears to support a more stable and less variable bacterial community structure. This is likely due to minimal human and tidal disturbances, and ecological stability where well-established bacterial populations dominate, leading to lower species turnover. For example, areas closer to seafront with continuous tidal buffering and sediment turnover tend to have higher microbial diversity compared to more stable areas such as Matang, with less frequent water exchange and salinity variations.22
Distribution and dominance of specific bacterial taxa varied significantly between the pristine Matang and anthropogenically-disturbed Tanjung Piai mangroves (Fig. 3). Proteobacteria and Gammaproteobacteria emerged as the dominant phylum and class, respectively, across both sites. This is consistent with previous studies on mangrove ecosystems worldwide.23 Proteobacteria is linked to several processes, notably carbon, nitrogen and sulfur cycling,24 and thrives in high nutrient conditions.25 Gammaproteobacteria are well known for their role in sulfur oxidation, particularly in mangrove environments where anaerobic respiration is crucial for organic matter decomposition.26 Notable variations were observed with Alphaproteobacteria, another major class of Proteobacteria. A significantly higher abundance in Matang, aligns with previous reports of Alphaproteobacteria as a key bacterial group in well-preserved mangrove ecosystems.27 Its lower abundance in Tanjung Piai could be linked to higher sediment Av. P level. Negative correlation between Alphaproteobacteria abundance and Av. P level was observed by others,28 explaining its lower prevalence in the P-enriched environment in Tanjung Piai. Conversely, Chloroflexi (class Dehalococcoidia) and Bacteroidota (class Bacteroidia), which were positively correlated with Av. P,28 were significantly abundant in Tanjung Piai.
Bacterial phyla composition in composite replicate samples of Matang (M1–M5) and Tanjung Piai (P1–P5) mangrove sediments.
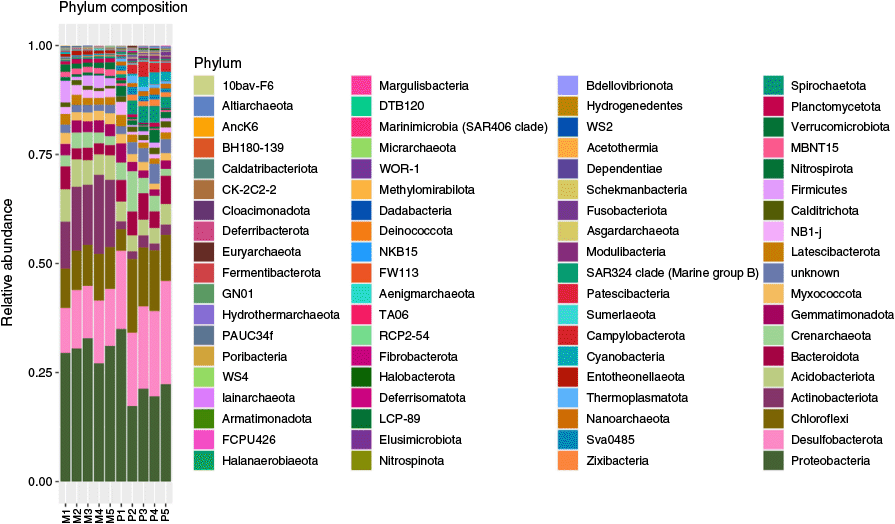
Actinomycetota (mostly Acidimicrobiia and Actinomycetes) were significantly dominant in the pristine mangrove ecosystem. Genomic data mining of uncultured Acidimicrobiia species suggested that they display metabolic versatility indicating their important role in biogeochemical cycling.29 High abundance of actinomycetes in the Matang sediments suggest a potential role in enhancing mangrove health through phytohormone production, nitrogen fixation and mineral solubilisation.30 The significantly lower abundance of this taxa in Tanjung Piai suggests potential ecological stressors or anthropogenic disturbances affecting their survival. Members of the phylum Desulfobacterota, including Desulfobacteria, are known for sulfate and nitrate reduction under anaerobic conditions and play a key role in anoxic organic matter mineralisation. The elevated abundance of Desulfobacteria in Tanjung Piai may be linked to contamination, as they are also often associated with the degradation of anthropogenic hydrocarbons and pollutants.31
Conclusion
Although the interactions between environmental settings and sediment microbes have not been fully studied yet, the present study suggested that increased bacterial diversity in Tanjung Piai most likely reflects anthropogenic stress-induced opportunism in the mangrove ecosystem. The significant reduction in beneficial bacterial taxa such as Actinomycetes and Alphaproteobacteria, along with the increased abundance of pollution-associated groups such as Desulfobacteria and Bacteroidia, provides evidence that disturbances in Tanjung Piai may impact soil microbial health. These findings support the idea that microbial diversity can serve as a bioindicator of ecosystem health, providing valuable insights into the extent of environmental degradation. Despite the knowledge gained from this work, there are several considerations to be addressed in future studies. Sampling done in both areas at different times is important to enrich our understanding of the relationship between changing environmental inputs, such as seasonal or tidal variability, and the mangrove microbial communities. Functional pathway analysis using bioinformatics tools such as PICRUSt2 for predicting bacterial metabolic specialisations, will also be included in future studies to validate ecological roles of key taxa in the microbiomes.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This work was supported by a joint funding from the Department of Forestry Malaysia and the Ministry of Water, Land and Natural Resources Malaysia (Grant No. 23410207001).
References
1 Biber PD (2006) Measuring the effects of salinity stress in the red mangrove, Rhizophora mangle L. Afr J Agric Res 1, 1-4.
| Crossref | Google Scholar |
2 Shiau YJ, Chiu CY (2020) Biogeochemical processes of C and N in the soil of mangrove forest ecosystems. Forests 11, 492.
| Crossref | Google Scholar |
3 Allard SM, Costa MT, Bulseco AN, Helfer V, Wilkins L, Hassenrück C, Zengler K, Zimmer M, Erazo N, Mazza Rodrigues JL, Duke N, Melo V, Vanwonterghem I, Junca H, Makonde HM, Jiménez DJ, Tavares T, Fusi M, Daffonchio D, Duarte CM, Peixoto RS, Rosado AS, Gilbert JA, Bowman J (2020) Introducing the mangrove microbiome initiative: Identifying microbial research priorities and approaches to better understand, protect and rehabilitate mangrove ecosystems. mSystems 5, e00658-20.
| Crossref | Google Scholar | PubMed |
4 Hamdan O, Muhamad Afizzul M, Ismail P. Assessment of mangrove changes in Malaysia using remote sensing. In: Tariq Mubarak H, Wan Mohd Shukri WA, Wan Tarmeze WA, Rosazlin A, Ismail P, editors. Proceedings of the National Seminar on Mangrove and Coastal Forest 2019, 10–11 September 2019, Sungai Petani; 2020. pp 50–58.
5 Jeyanny V, Ne’ryez SR, MI F, Daljit SK, Maisarah MZ, Wan Rasidah K, Husni MH (2018) Assessing soil quality of a regenerating mangrove forest using geospatial modelling approach. Malays J Soil Sci 22, 161-173.
| Google Scholar |
6 Alsharif SM, Ismaeil M, Saeed AM, El-Sayed WS (2024) Metagenomic 16S rRNA analysis and predictive functional profiling revealed intrinsic organohalides respiration and bioremediation potential in mangrove sediment. BMC Microbiol 24, 176.
| Crossref | Google Scholar | PubMed |
7 Sampaio CJS, de Souza J, Damião AO, Bahiense TC, Roque M (2019) Biodegradation of polycyclic aromatic hydrocarbons (PAHs) in a diesel oil-contaminated mangrove by plant growth-promoting rhizobacteria. 3 Biotech 9, 155.
| Crossref | Google Scholar | PubMed |
8 de Araujo JE, Taketani RG, Pylro VS, Leite LR, de Cássia Pereira e Silva M, Lemos LN, de Mello Lourenço MV, Andreote FD (2021) Genomic analysis reveals the potential for hydrocarbon degradation of Rhodopirellula sp. MGV isolated from a polluted Brazilian mangrove. Braz J Microbiol 52, 1397-1404.
| Crossref | Google Scholar | PubMed |
9 Gong B, Cao H, Peng C, Perčulija V, Tong G, Fang H, Wei X, Ouyang S (2019) High-throughput sequencing and analysis of microbial communities in the mangrove swamps along the coast of Beibu Gulf in Guangxi, China. Sci Rep 9, 9377.
| Crossref | Google Scholar | PubMed |
10 Muwawa EM, Obieze CC, Makonde HM, Jefwa JM, Kahindi J, Khasa DP (2021) 16S rRNA gene amplicon-based metagenomic analysis of bacterial communities in the rhizospheres of selected mangrove species from Mida Creek and Gazi Bay, Kenya. PLoS One 16, e0248485.
| Crossref | Google Scholar | PubMed |
11 Jeyanny V, NurNabilah A, Norlia B, Krishnasamy G, Lee SL, Singh NR, Muhammad Amiruddin Z (2021) Metagenomic insights on soil microbiome biodiversity from an eroding coastline of Tanjung Piai, Johor State Park, Malaysia. J Trop For Sci 33, 414-424.
| Crossref | Google Scholar |
12 Lai J, Cheah W, Palaniveloo K, Suwa R, Sharma S (2022) A systematic review of the physicochemical and microbial diversity of well-preserved, restored, and disturbed mangrove forests: what is known and what is the way forward? Forests 13, 2160.
| Crossref | Google Scholar |
13 Sahoo K, Dhal NK (2009) Potential microbial diversity in mangrove ecosystems: a review. Indian J Mar Sci 38, 249-256.
| Google Scholar |
14 Tan DD, Wan Juliana WA, Maimon A (2012) Community structure and productivity of mangrove forests in two National Parks of West Malaysia. Malays For 75, 165-176.
| Google Scholar |
15 Nor Aslinda A, Wan Hasliza WJ, Mohd Radzi AH (2014) Coastal erosion at Tanjong Piai, Johor, Malaysia. J Coast Res 71, 122-130.
| Crossref | Google Scholar |
16 Chen D, Satyanarayana B, Wolswijk G, Abd Rahim NH, Amir AA, Hugé J, Dahdouh-Guebas F (2024) Historical ecological monitoring and appraisal for extractive uses and other values in Malaysia unveils consequences of regime shifts in 120 years of mangrove management. J Nat Conserv 79, 126582.
| Crossref | Google Scholar |
17 Alongi DM (2014) Carbon cycling and storage in mangrove forests. Ann Rev Mar Sci 6, 195-219.
| Crossref | Google Scholar | PubMed |
18 Marins RV, Lacerda LD, AraÚjo I, Fonseca LV, Silva F (2020) Phosphorus and suspended matter retention in mangroves affected by shrimp farm effluents in NE Brazil. An Acad Bras Cienc 92, e20200758.
| Crossref | Google Scholar | PubMed |
19 Pérez A, Machado W, Sanders CJ (2021) Anthropogenic and environmental influences on nutrient accumulation in mangrove sediments. Mar Poll Bull 165, 112174.
| Crossref | Google Scholar |
20 Kunasekaran P, Rozak NIN, Adam SM, Shuib A (2018) Perception of local communities on the indicators of governance in Tanjung Piai National Park. Int J Bus Soc 19, 79-87.
| Google Scholar |
21 Halim NHA, Abdullah R, Kadir WR, Ajeng AA, Zawawi NZB (2022) Heavy metals distribution and fractionation in mangrove sediments linked to organic deposits vis-à-vis accumulation in Rhizophora spp. at Tanjung Piai, Johor, Malaysia. Appl Ecol Environ Res 20, 4011-4030.
| Crossref | Google Scholar |
22 Capdeville C, Pommier T, Gervaix J, Fromard F, Rols JL, Leflaive J (2019) Mangrove facies drive resistance and resilience of sediment microbes exposed to anthropic disturbance. Front Microbiol 9, 3337.
| Crossref | Google Scholar | PubMed |
23 Costa GMD, Costa SS, Baraúna RA, Castilho BP, Pinheiro IC, Silva A, Schaan AP, Ribeiro-Dos-Santos Â, Graças D (2023) Effects of degradation on microbial communities of an Amazonian mangrove. Microorganisms 11, 1389.
| Crossref | Google Scholar | PubMed |
24 Mhete M, Eze PN, Rahube TO, Akinyemi FO (2020) Soil properties influence bacterial abundance and diversity under different land-use regimes in semi-arid environments. Sci Afr 7, e00246.
| Crossref | Google Scholar |
25 Gumiere T, Gumiere SJ, Matteau J-P, Constant P, Létourneau G, Rousseau AN (2019) Soil bacterial community associated with high potato production and minimal water use. Front Environ Sci 6, 161.
| Crossref | Google Scholar |
26 Ullah R, Yasir M, Khan I, Bibi F, Sohrab SS, Al-Ansari A, Al-Abbasi F, Al-Sofyani AA, Daur I, Lee SW, Azhar EI (2017) Comparative bacterial community analysis in relatively pristine and anthropogenically influenced mangrove ecosystems on the Red Sea. Can J Microbiol 63, 649-660.
| Crossref | Google Scholar | PubMed |
27 Dias AC, Andreote FD, Rigonato J, Fiore MF, Melo IS, Araújo WL (2010) The bacterial diversity in Brazilian non disturbed mangrove sediment. Antonie Van Leeuwenhoek 98, 541-51.
| Crossref | Google Scholar | PubMed |
28 Hermans SM, Buckley HL, Case BS, Curran-Cournane F, Taylor M, Lear G (2017) Bacteria as emerging indicators of soil condition. Appl Environ Microbiol 83, e02826-16.
| Crossref | Google Scholar | PubMed |
29 Hu D, Cha G, Gao B (2018) A phylogenomic and molecular markers-based analysis of the class Acidimicrobiia. Front Microbiol 9, 987.
| Crossref | Google Scholar | PubMed |
30 Boubekri K, Soumare A, Mardad I, Lyamlouli K, Ouhdouch Y, Hafidi M, Kouisni L (2022) Multifunctional role of Actinobacteria in agricultural production sustainability: a review. Microbiol Res 261, 127059.
| Crossref | Google Scholar | PubMed |
31 Novair SB, Biglari Z, Asgari Lajaye B, Shu W, Price GW (2024) The role of sulphate-reducing bacteria (SRB) in bioremediation of sulphate-rich wastewater: focus on the source of electron donors. Process Saf Environ Prot 184, 190-207.
| Crossref | Google Scholar |
 Getha Krishnasamy is a Senior Research Officer at the Biomolecules Research Laboratory, Forest Research Institute Malaysia (FRIM). She is the curator of the FRIM Microbial Culture Collection, and an elected Executive Committee member of the Malaysian Society for Microbiology. She has extensive expertise in microbial research that focuses on actinomycetes, endophytic bacteria and fungi isolated from various environments. Getha’s main interest is in investigating how microbes interact with each other in the environment, and harnessing these microbes for agricultural and industrial uses. Her current research involves innovative fermentation methods for production of bioactive compounds from basidiomycetes. |
 Jeyanny Vijayanathan is a Senior Research Officer and Head of the Soil Management Branch in the Forestry Biotechnology Division. She has been elected as the global soil expert for the Intergovernmental Technical Panel on Soils by the FAO. She has extensive expertise in soil chemistry, fertility, plant nutrition and soil carbon dynamics across forestry, agriculture, environmental and degraded ecosystems. Her current research involves assessment on soil health and plant productivity in the changing environment. |
 Nur Nabilah Alias is a researcher at the Genetics Laboratory, Forest Research Institute Malaysia (FRIM). Her research focuses on next-generation sequencing (NGS) and multi-omics approaches, including genomics, transcriptomics, metabolomics and metagenomics. She has expertise in bioinformatics, particularly in the processing and interpretation of high-throughput sequencing data for multi-omics studies. Her research also involves plant DNA profiling, DNA barcoding and the development of SSR markers to support genetic and evolutionary studies. Currently, she focuses on population genomics to investigate population structure and adaptive variation, especially in response to climate change. |
 Norlia Basherudin is a Senior Research Officer at the Genetics Laboratory, Department of Forestry Biotechnology, Forest Research Institute Malaysia (FRIM). Her research specialises in plant molecular biology, with a focus on genetic engineering, gene function analysis and the detection of living modified organisms (LMOs). Her current projects include the production of hairy root cultures expressing proinsulin, the development of detection methods for genetically modified eucalyptus, and the characterisation of genes involved in quassinoid biosynthesis. |


