The direct medical cost of operatively managing forearm fractures at Port Moresby General Hospital, Papua New Guinea: a retrospective cost-of-illness study
Kila Garo A , Ian Umo B * , Jackson Nuli A , Richard Kulau A , Henao Asa A and Ikau Kevau CA
B
C
Abstract
Forearm fractures are common and account for 31% of all orthopaedic admissions to Port Moresby General Hospital (PMGH). As such, the primary aim of this study was to calculate the direct medical cost of operatively managing forearm fractures at PMGH.
A retrospective cost-of-illness study was carried out over a 6-month period involving 43 patients with forearm fractures at PMGH. Direct medical costs were calculated using a bottom-up approach to estimate expenses from both patient and hospital perspectives.
The total direct medical cost over 6 months was K972,384.60 (US$238,569.24), and on average, the direct medical cost was K22,613.60 (US$5548.12) per patient (s.d. = 274.32). Salaries accounted for 87% of hospital costs. Hospital costs represented 99.1% of the total direct medical cost in this study.
This study underscores the substantial economic burden of operatively managing forearm fractures at PMGH. The study findings highlight the need for policy reforms aimed at improving cost-efficiency, strengthening patient financial protection, and enhancing the sustainability of trauma care services.
Keywords: direct medical cost, forearm fracture, health economics, low to middle income country, operative management, orthopaedics, orthopedics, Papua New Guinea, Port Moresby General Hospital.
Introduction
Trauma continues to be a major global public health issue, contributing significantly to both societal and economic burdens. Globally, injuries are responsible for approximately 4.4 million deaths annually, with many more individuals surviving with long-term disabilities that require costly medical, surgical, and rehabilitative care.1,2 The financial implications of trauma are immense, especially in low- and middle-income countries (LMICs), where the strain on already limited healthcare resources is exacerbated by the need for surgical interventions.
Forearm fractures are among the most common trauma-related injuries, representing more than 20% of all injury-related fractures globally.3 In LMICs, the incidence of forearm fractures is estimated at 799 per 100,000 population.4 At Port Moresby General Hospital (PMGH), the main tertiary referral hospital in Papua New Guinea (PNG), forearm fractures comprise approximately 31% of all orthopaedic admissions, making them a major cause of surgical intervention and hospitalisation.5
Management of forearm fractures can be conservative or operative, depending on the type and severity of the fracture. At PMGH, the majority (83%) of these fractures are treated operatively using open reduction and internal fixation (ORIF), often employing plates and screws, rush pins, or Kirschner wires (K-wires).5 These surgical methods are indicated particularly for displaced fractures with more than 50% displacement or where alignment cannot be maintained non-operatively through casting. In cases of open fractures, management typically includes debridement, reduction, and stabilisation with an external fixator; however, because of limited availability and cost constraints, K-wires are often used as substitutes.
Globally, the direct cost of orthopaedic procedures exceeds US$350 billion annually.6 These costs include hospital resources, surgical supplies, implants, staff salaries, and postoperative care. Despite the high burden of orthopaedic injuries in PNG, there is a complete absence of published data quantifying the direct medical costs associated with operative fracture management in the country. Costing studies are vital for understanding the economic impact of trauma care, identifying inefficiencies, and guiding policy to optimise resource allocation.
Given the lack of local data and the economic implications of trauma management, this study aims to fill a critical gap by estimating the direct medical costs associated with operatively managing forearm fractures at PMGH.
Materials and methods
Study setting
The study was conducted at PMGH. The hospital is the only national referral hospital of PNG and provides surgical services for the National Capital District, Central Province, and Gulf Province. The hospital has an orthopaedic ward with a 52-bed capacity and is managed by three orthopaedic specialists and rotating general surgery registrars.
Costing perspective and analysis
A bottom-up approach of direct medical costing was used in this study.7,8 Direct medical costing refers to costs associated with resources involved in patient care for an illness.9 The patient and hospital perspectives of direct medical costing were calculated.
Patient perspective of costing
The patient perspective of costing involved hospital fees. The hospital fees were obtained from PMGH as shown in Supplementary Appendix S1. Patient fees were inclusive of admission fee; operation fee; full blood count fee; urea, electrolyte and creatinine fee; liver function test fee; and crossmatch fee. Formula 2 in Table 1 was used to calculate hospital fees per person.
Hospital perspective of costing
The hospital perspective includes costs of drugs, consumables, orthopaedic implants, and orthopaedic drill, as listed in the survey form shown in Supplementary Appendix S2. These are fixed costs per unit or item that were obtained from the National Department of Health Medical and Dental Catalogue.11 The costs of drugs, consumables, and orthopaedic implants were calculated using Formula 1, as detailed in Table 1. Workforce costs were also included in this study and were based on base salaries, excluding additional allowances such as clinical, housing, and other benefits. The exclusion of these allowances is not expected to affect the study outcomes, as human resources represent the largest proportion of total costs. Workforce costs were estimated using the average number of healthcare workers attending to patients. Each worker’s fortnightly salary was calculated using salary data sourced from PMGH position records, as shown in Supplementary Appendix S3. Formula 3 in Table 1 was applied to derive the fortnightly salary figures.
Costs are expressed in Kina, with conversion to US dollars using the 2024 average exchange rate from the Bank of PNG (1 K = 0.25 USD). Direct medical costing followed the approach described by Umo and James.10 Although this method captures the bulk of health-system expenditures, it may not fully account for all overheads or indirect costs. Total direct medical cost per patient was calculated using Formula 3 in Table 1.
In calculating the direct medical costs, the following assumptions were included:
A1. Use of standardised unit costs: unit costs for drugs, consumables, and implants are based on prices listed in the national medical and dental catalogue, assuming these represent economic (opportunity) costs and are valid proxies for resource valuation.
A2. Fixed quantities of inputs: the number of units used per patient is assumed to be constant and clinically appropriate, without variation for individual patient differences. This implies a uniform clinical pathway, consistent with the ‘average patient’ approach in cost analysis.
A3. Uniform tariff-based hospital fees: hospital service costs (admission, laboratory tests, surgery) are assumed to be tariff-based and fixed per patient, reflecting average fees charged and not necessarily resource use variations.
A4. Exclusion of indirect and intangible costs: the analysis includes only direct medical costs, in line with a health system perspective, and excludes indirect costs (e.g. patient transport, productivity losses) and intangible costs (e.g. pain, suffering), as commonly practised in cost-minimisation and cost-analysis studies.
A5. Base salary use for workforce costs: human resource costs are calculated using base salaries only, excluding allowances (e.g. housing, risk, overtime) and assuming these do not materially affect comparative cost results. This aligns with the principle of using actual economic costs where feasible.
A6. Proportional attribution of staff time: staff salary costs are proportionally allocated to patient care using the mean number of healthcare workers per patient. This assumes that time and effort can be averaged across cases, consistent with macro-costing principles.
A7. No time discounting or inflation adjustment: costs are calculated for a short-term episode of care without discounting for time or inflation adjustment, assuming a 1-year time horizon where such adjustments are not materially significant.
A8. Constant resource utilisation across cases: the model assumes a standard treatment protocol and does not capture heterogeneity in patient care (e.g. complications, extended stay), which is common in average cost estimations for budgetary planning.
A9. Full cost recovery assumed from prices: it is implicitly assumed that listed costs and fees reflect full cost recovery (i.e. include overheads and infrastructure), although this may not be true unless explicitly verified.
Participants
Patients of any age with both open and closed forearm fractures who required surgery for open reduction were included in this study. Patients who had undergone closed reduction or had bilateral forearm fractures were excluded from the study. Implant costs were not paid for by the patients.
Data collection
Charts of patients admitted to the orthopaedic ward from January to June 2024 with forearm fractures were identified, and data were retrieved using a standard survey form (Supplementary Appendix S2). Patient data collected included age, sex, type of injury, cause of the injury, and operative management. Data were entered into Microsoft Access and analysed using Microsoft Excel.
Results
A total of 43 patients were included in this study. The male-to-female ratio was 4:1. The mean age of patients was 21.5 years, with a minimum age of 9 years and maximum age of 60 years. In terms of region of origin, 39% (n = 17) of patients were from the Southern region. The majority of patients were unemployed (53.5%, n = 23). There were no deaths, and the mean length of hospital stay was 11.2 (s.d. = 2.1) days. Patient demographics are shown in Table 2.
| Characteristic | n (%) | |
|---|---|---|
| Sex | ||
| Male | 35 (81.4) | |
| Female | 8 (18.6) | |
| Age group (years) | ||
| 0–5 | 0 | |
| 6–15 | 9 (20.9) | |
| 16–25 | 11 (25.6) | |
| 26–35 | 13 (30.2) | |
| 36–45 | 8 (18.6) | |
| 46–55 | 1 (2.3) | |
| >55 | 1 (2.3) | |
| Region of origin | ||
| Southern | 17 (39.5) | |
| Momase | 5 (11.6) | |
| Highlands | 19 (44.2) | |
| Islands | 2 (4.7) | |
| Occupation of patients | ||
| Primary school student | 11 (25.6) | |
| Tertiary student | 1 (2.3) | |
| Subsistence farmer | 4 (9.3) | |
| Unemployed | 23 (53.5) | |
| Teacher | 1 (2.3) | |
| House girl | 1 (2.3) | |
| Waste officer | 1 (2.3) | |
| Heavy machine operator | 1 (2.3) | |
Falls were responsible for 37.2% (n = 16) of forearm fractures. Alcohol-related violence accounted for 30.2% (n = 13) of cases. It was found that 76.7% (n = 33) of cases were closed forearm fractures, with 72.1% (n = 31) of fractures involving both radius and ulna. Table 3 shows injury characteristics.
| Fracture type | Treatment | Mean cost (PGK) | Mean cost (USD) | Min–Max (PGK) | Min–Max (USD) | |
|---|---|---|---|---|---|---|
| Open radius ulna fracture | Debridement + K-wire insertion | 10,129.01 | 2532.25 | 9800–10,500 | 2450–2625 | |
| Open Galeazzi fracture | Debridement + K-wire insertion | 10,129.01 | 2532.25 | 9800–10,500 | 2450–2625 | |
| Closed olecranon fracture | ORIF + K-wire + tension band wiring | 11,129.01 | 2782.25 | 10,800–11,500 | 2700–2875 | |
| Closed Galeazzi fracture | ORIF + rush pin insertion | 20,258.02 | 5064.50 | 19,800–21,000 | 4950–5250 | |
| ORIF with plate and screws | 30,387.03 | 7596.75 | 29,000–32,000 | 7250–8000 | ||
| Closed radius ulna fracture | ORIF with plate and screws | 30,387.03 | 7596.75 | 29,000–32,000 | 7250–8000 |
Patient costs
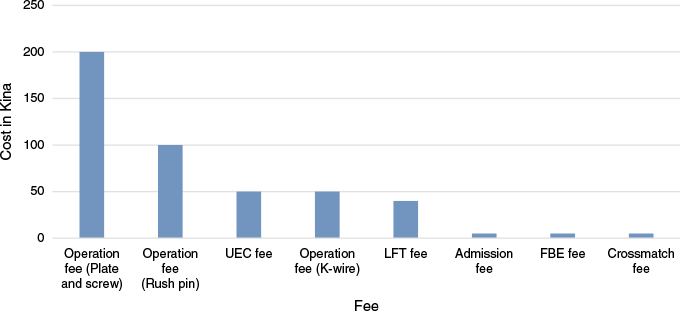
The total patient cost was K8,995.00 (US$2206.87). Operation fees were the highest of patient costs, as shown in Fig. 1.
Hospital costs
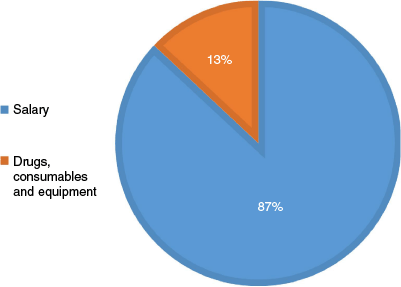
The total hospital cost was K963,389.60 (US$236,362.22) and represented 99.1% of the total direct medical cost. Salaries accounted for 87% of hospital costs, and 13% of the costs were due to consumables such as drugs and equipment/implants etc. (see Fig. 2 and Table 4).
Breakdown of percentage of hospital costs among patients admitted for open reduction at PMGH from 1 January to 30 June 2024.
| Item | Total cost (K) | |
|---|---|---|
| Plate and screws/K-wires (set) | 21,500.00 | |
| Suction machine use | 8600.00 | |
| Halothane (bottle) | 6450.00 | |
| Oxygen (bottle) | 4300.00 | |
| Rush pin | 4300.00 | |
| Nitrous oxide | 3440.00 | |
| Diathermy tip | 3010.00 | |
| Diathermy pad | 2580.00 | |
| LFT test | 1290.00 | |
| UEC test | 1075.00 | |
| K-wire | 860.00 | |
| Vicryl 2 Suture | 860.00 | |
| Laryngeal mask airway | 860.00 | |
| X-match (crossmatch) | 860.00 | |
| Vicryl 1 suture | 774.00 | |
| Vicryl 0 suture | 645.00 | |
| FBE test | 645.00 | |
| Ceftriaxone 1 g (vial) | 430.00 | |
| Chromic suture | 430.00 | |
| Rapid diagnostic test | 430.00 | |
| Blood giving set | 344.00 | |
| Endotracheal tube | 344.00 | |
| Normal saline (500 mL) | 258.00 | |
| Hartmann’s solution (500 mL) | 258.00 | |
| 4.3% Dextrose/Saline | 258.00 | |
| Burette IV line | 258.00 | |
| Morphine (10 mg/mL) | 215.00 | |
| IV line | 215.00 | |
| Iodine (bottle) | 215.00 | |
| Nylon suture | 215.00 | |
| Flagyl (Metronidazole) | 172.00 | |
| IDC | 172.00 | |
| Gentamicin | 129.00 | |
| Pethidine | 129.00 | |
| Black IVC | 129.00 | |
| IDC bag | 129.00 | |
| Green IVC | 107.50 | |
| CMP | 86.00 | |
| Sterile water (for injection) | 86.00 | |
| Pink IVC | 86.00 | |
| Yellow IVC | 86.00 | |
| Surgical blade (No. 15) | 86.00 | |
| Maxalon (Metoclopramide) | 64.50 | |
| 20 mL syringe | 64.50 | |
| Large abdominal sponge | 64.50 | |
| Gloves (pair) | 61.92 | |
| 10 mL syringe | 51.60 | |
| Amoxyl | 43.00 | |
| Buscopan | 43.00 | |
| 5 mL syringe | 43.00 | |
| Small abdominal sponge | 43.00 | |
| Gel (lubricating) | 43.00 | |
| 2 mL syringe | 38.70 | |
| Pink needle | 21.50 | |
| Yellow needle | 21.50 | |
| Black needle | 21.50 | |
| Purple needle | 21.50 | |
| Elastoplast (strip) | 21.50 | |
| Masks (surgical) | 21.50 | |
| Panadol 500 mg PR | 17.20 | |
| Diclofenac 50 mg oral | 12.90 | |
| Clear plaster (strip) | 12.90 | |
| Raytec gauze | 12.90 | |
| Panadol 500 mg oral | 8.60 | |
| Swab (gauze) | 8.60 |
Abbreviations: LFT, liver function test; UEC, urea electrolytes and creatinine; FBE, full blood examination; IV, intravenous; IDC, indwelling catheter; IVC, intravenous cannula; CMP, chloramphenicol; PR, per rectal.
The total direct medical cost over the 6-month period was K972,384.60 (US$238,569.24), and on average, the direct medical cost was K22,613.60 (US$5548.12) per patient (s.d. = 274.32). Sensitivity analysis using salary as the variable is presented in Table 5. A reduction in salary costs to 70% would increase consumable costs to K289,016.88. An increase in salary costs to 95% would drop consumable costs to K48,169.48.
| Salary % of hospital cost | Salary cost (K) | Consumable cost (K) | Total hospital cost (K) | Total direct cost (K) | Average cost per patient (K) | |
|---|---|---|---|---|---|---|
| 70 | 674,372.72 | 289,016.88 | 963,389.60 | 972,384.60 | 22,613.60 | |
| 75 | 722,542.20 | 240,847.40 | 963,389.60 | 972,384.60 | 22,613.60 | |
| 80 | 770,711.68 | 192,677.92 | 963,389.60 | 972,384.60 | 22,613.60 | |
| 85 | 818,881.16 | 144,508.44 | 963,389.60 | 972,384.60 | 22,613.60 | |
| 87 (BASE CASE) | 837,148.95 | 126,240.65 | 963,389.60 | 972,384.60 | 22,613.60 | |
| 90 | 867,050.64 | 96,338.96 | 963,389.60 | 972,384.60 | 22,613.60 | |
| 95 | 915,220.12 | 48,169.48 | 963,389.60 | 972,384.60 | 22,613.60 |
Discussion
This study aimed to quantify the direct medical costs associated with operatively managing forearm fractures at PMGH. With an average cost of K22,613.60 (US$5548.12) per patient and a total expenditure of K972,384.60 (US$238,569.24) over 6 months, the findings demonstrate a significant financial burden on the hospital system in PNG.
The average direct medical cost per patient in this study represents a substantial cost in PNG, especially when considering the country’s limited health budget and high unemployment rate (53.5% of patients in this study). This average cost aligns closely with similar studies in other LMICs. For instance, Umo et al. reported a comparable cost structure for trauma-related surgeries in PNG, suggesting that operative fracture care may be one of the more expensive interventions in trauma care because of its reliance on specialised implants and surgical teams.10,12 In contrast, Schade et al. report significantly higher direct costs for open tibia fractures in high-income countries, largely driven by prolonged hospitalisation, complex fixation, and postoperative complications.13 This difference illustrates the limited availability and use of advanced surgical techniques and postoperative rehabilitation in PNG, which, while lowering costs, may also affect clinical outcomes.
In this study, 99.1% of the total direct medical cost was borne by the hospital, with hospital workforce salaries accounting for 87% of this cost. This proportion is considerably higher than that reported in orthopaedic costing studies from other LMICs. In Malawi, for example, Twea et al. found that salaries accounted for 48–55% of the cost in orthopaedic service delivery, with higher proportional expenditure on drugs and implants.14 The discrepancy is largely attributable to the heavily subsidised healthcare model at PMGH, where surgical implants are provided free of charge to certain patients and where tariff-based patient fees remain fixed and minimal. Additionally, the exclusion of overhead and indirect costs from this study may have resulted in underestimation of non-salary expenses. Moreover, the use of base salaries in this study, excluding allowances (e.g. housing or risk), further consolidates the apparent dominance of salary components.
From the patient perspective, the total patient out-of-pocket cost was K8,995.00 (US$2206.87), a figure that must be considered against the economic vulnerability of patients, over half of whom were unemployed. In PNG, where out-of-pocket expenditure for health services contributes significantly to household financial distress, this cost can be considered catastrophic, especially when benchmarked against the World Health Organization’s catastrophic health expenditure threshold (i.e. >10% of household income).15 Globally, O’Hara et al. have shown that long bone fractures can result in prolonged income loss and financial instability even in high-income settings, suggesting that the economic ripple effect of such injuries may be more severe in PNG’s underinsured population.16
A key observation from this study was the high average hospital stay of 11.2 days, which significantly contributes to workforce costs. Although prolonged hospitalisation may reflect postoperative care practices in PNG, it contrasts with enhanced recovery protocols in high-income countries where patients are often discharged within 3–5 days after forearm ORIF.6,17 This difference may be due to systemic limitations such as delay in transfer to orthopaedic ward, backlog of patients requiring surgery, delayed access to physiotherapy, monitoring of slow wound healing, and the absence of structured outpatient rehabilitation in PNG. As James et al. observed, surgical services in PNG suffer from delays and infrastructure limitations that hinder timely discharges and efficient bed turnover, further exacerbating the resource strain.18
The implant-related cost structure in this study presents a notable deviation from the global norm. Although implants were provided at no cost to certain patients, they still represented a measurable hospital expense, especially when considering substitute use of K-wires over more advanced fixation devices because of availability and affordability. Globally, implant costs are often one of the most significant contributors to orthopaedic surgery expenditures.19 In this context, the reduced financial burden on patients in PNG is commendable but raises sustainability questions. If external funding or government subsidies were to be withdrawn, the cost would likely shift to patients, dramatically increasing financial barriers to care.
Lastly, sensitivity analysis of workforce costs demonstrated their overwhelming influence on overall expenditure. When salaries were adjusted, the proportional cost of consumables shifted markedly. This result underscores the rigidity of hospital labour costs, a trend similarly observed in other health system costing studies in PNG and LMICs.14,20 The dominance of fixed salary costs also supports calls for optimising hospital workflows, minimising inpatient days, and potentially integrating task-sharing to reduce per-patient resource allocation.
This study is not without its limitations. Firstly, this was a retrospective study, and the data collected from records might not be accurate. The costing did not factor in power, water consumption, theatre time, and building infrastructure. Furthermore, the study did not include post-discharge fees and rehabilitation costs. Although this underestimates the total cost of forearm fracture management, it helps model a clinical cost that can help inform the scalability of services at PMGH.
Conclusion
This study highlights the significant economic burden of operative forearm fracture management at PMGH, with hospital costs, particularly salaries, dominating total expenditures. Although patient fees remain low, the true cost borne by the health system is substantial. Compared with the international literature, PNG’s cost structure reflects both the strengths of subsidised care and the challenges of limited infrastructure. These findings support the need for policy reforms focused on cost-efficiency, patient financial protection, and long-term sustainability in trauma care delivery.
Acknowledgements
The authors would like to thank Dr Jedda Resis for assisting in data collection and analysis.
References
1 Goddard SD, Jarman MP, Hashmi ZG. Societal burden of trauma and disparities in trauma care. Surg Clin North Am 2024; 104(2): 255-266.
| Crossref | Google Scholar | PubMed |
2 Velopulos CG, Enwerem NY, Obirieze A, et al. National cost of trauma care by payer status. J Surg Res 2013; 184: 444-449.
| Crossref | Google Scholar | PubMed |
3 Hedström EM, Svensson O, Bergström U, Michno P. Epidemiology of fractures in children and adolescents. Acta Orthop 2010; 81(1): 148-153.
| Crossref | Google Scholar | PubMed |
4 Cordero DM, Miclau TA, Paul AV, Morshed S, Miclau T 3rd, Martin C, et al. The global burden of musculoskeletal injury in low and lower-middle-income countries. OTA Int 2020; 3(2): e062.
| Crossref | Google Scholar | PubMed |
5 PMGH Surgical Audit. Orthopaedics. 2022-2023. Available at https://devpolicy.org/2019-PNG-Update/Presentations/Parallel-2C-Road-traffic-injuries-at-the-orthopaedic-unit-Henao-Asa.pdf
6 Healthcare. Improving US orthopedic care via patient-centric pathways. 2023. Available at https://www.mckinsey.com
8 Shrime MG. Getting costs right in global surgery. World J Surg 2021; 45: 3565-3566.
| Crossref | Google Scholar | PubMed |
9 Fautrel B, Boonen A, de Wit M, et al. Cost assessment of health interventions and diseases. RMD Open 2020; 6: e001287.
| Crossref | Google Scholar | PubMed |
10 Umo I, James K. The direct medical cost of acute appendicitis surgery in a resource-limited setting of Papua New Guinea. World J Surg 2021; 45: 3558-3564.
| Crossref | Google Scholar | PubMed |
11 Papua New Guinea Medical and Dental Health Catalogue. National Department of Health, 10th edn. 2012. Available at https://drive.google.com/uc?export=download&id=1OIJFZvSQ8STYTrP1UBSUDQ2NREdYFvBK [cited 2024].
12 Umo I, James K, Didilemu F, Sinen B, Borchem I, Inaido D, Ikasa R. The direct medical cost of trauma aetiologies and injuries in a resource limited setting of Papua New Guinea: A prospective cost of illness study. Lancet Reg Health West Pac 2022; 20: 100379.
| Crossref | Google Scholar | PubMed |
13 Schade AT, Khatri C, Nwankwo H, Carlos W, Harrison WJ, Metcalfe AJ. The economic burden of open tibia fractures: a systematic review. Injury 2021; 52(6): 1251-1259.
| Crossref | Google Scholar | PubMed |
14 Twea P, Watkins D, Norheim OF, Munthali B, Young S, Chiwaula L, et al. The economic costs of orthopaedic services: a health system cost analysis of tertiary hospitals in a low-income country. Health Econ Rev 2024; 14(1): 13.
| Crossref | Google Scholar | PubMed |
15 World Health Organization. Catastrophic out-of-pocket health spending (SDG indicator 3.8.2 and regional indicators where available). The Global Health Observatory. 2024. Available at https://www.who.int/data/gho/data/themes/topics/indicator-groups/indicator-group-details/GHO/catastrophic-out-of-pocket-health-spending-sdg-indicator-3-8-2-
16 O’Hara NN, Slobogean GP, Klazinga NS, Kringos DS. Analysis of Patient Income in the 5 Years Following a Fracture Treated Surgically. JAMA Netw Open 2021; 4(2): e2034898.
| Crossref | Google Scholar | PubMed |
17 Bonafede M, Espindle D, Bower AG. The direct and indirect costs of long bone fractures in a working-age US population. J Med Econ 2013; 16(1): 169-178.
| Crossref | Google Scholar | PubMed |
18 James K, Borchem I, Talo R, Aihi S, Baru H, Didilemu F, et al. Universal access to safe, affordable, timely surgical and anaesthetic care in Papua New Guinea: the six global health indicators. ANZ J Surg 2020; 90(10): 1903-1909.
| Crossref | Google Scholar | PubMed |
19 Logan C, Hess A, Kwon JY. Damage control orthopaedics: Variability of construct design for external fixation of the lower extremity and implications on cost. Injury 2015; 46(8): 1533-1538.
| Crossref | Google Scholar | PubMed |
20 Saweri OPM, Batura N, Pomat W, et al. What does it cost to deliver antenatal care in Papua New Guinea? Results from a health system costing and budget impact analysis using cross-sectional data. BMJ Open 2024; 14(11): e080574.
| Crossref | Google Scholar | PubMed |