Hidden in plain sight: Lindauera gen. nov.: a new genus of Dictyotales from New Zealand
Wendy A. Nelson A B * and Judy E. Sutherland A
A B * and Judy E. Sutherland A
A National Institute of Water and Atmospheric Research Ltd, Private Bag 14-901, Wellington 6241, New Zealand.
B School of Biological Sciences, University of Auckland, Auckland 1142, New Zealand.
Australian Systematic Botany 36(2) 157-166 https://doi.org/10.1071/SB22032
Submitted: 8 December 2022 Accepted: 27 March 2023 Published: 26 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
A new genus in the Dictyotales, Lindauera, is described based on the species Dictyota papenfussii Lindauer from Taranaki, North Island, New Zealand. Lindauera is distinguished from Dictyota by both molecular data and by the possession of primary, prostrate dichotomously branched axes that attach thalli to substrates. We present data extending the known distribution of this species within New Zealand and confirm its occurrence in Australia.
Keywords: Australia, Dictyota papenfussii, Dictyotales, Lindauera gen. nov., Lobospira bicuspidata, New Zealand, Phaeophyceae, phylogenetic analyses.
Introduction
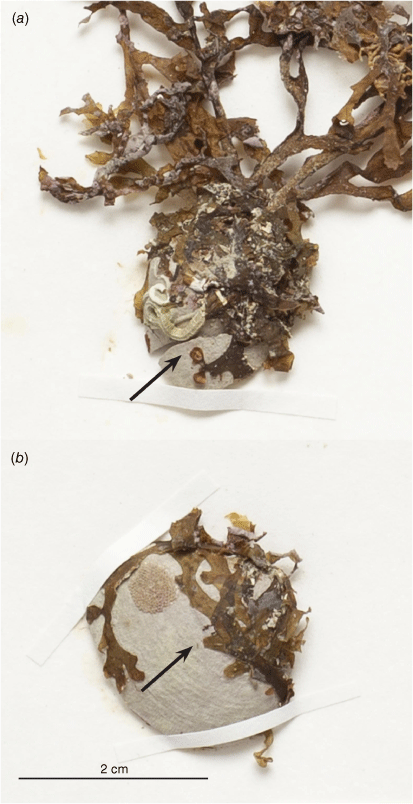
The brown alga Dictyota papenfussii Lindauer was described in 1949, with additional anatomical and morphological information provided in the later flora treatment (Lindauer 1949; Lindauer et al. 1961). The type material was collected from Pihama, Taranaki (North Island, New Zealand). Lindauer designated specimen VWL 4110 (AK295737) as the ‘Type’ (Fig. 1), and distributed specimens of D. papenfussii labelled ‘Co-types’, as specimen number 187, in Fascicle VIII, of the Algae Nova-Zelandicae Exsiccatae (Nelson and Phillips 1996).
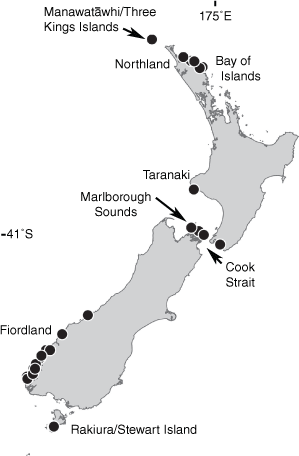
The species was reported to occur sporadically in shallow water in the upper subtidal, growing in dense tufts on stones. Lindauer reported that it had a seasonal distribution from late summer through autumn (February to May). Adams (1994) gave the distribution of D. papenfussii as Taranaki, the Cook Strait region, the southern South Island in Fiordland, and Stewart Island (Fig. 2). In both the original description and the flora treatment (Lindauer 1949; Lindauer et al. 1961), there is reference to ‘an identical specimen’ reported by Valerie May Jones from Sydney. However, in their treatment of brown algae from New South Wales, Millar and Kraft (1994) considered the record of Dictyota papenfussii from Sydney to be Dictyota dichotoma (Huds.) J.V.Lamour.
|
Dictyota papenfussii was described as having prostrate primary fronds, with dark brown, flat, and linear erect fronds 7–19 cm in height, a percurrent main axis, sometimes forked below, and axes up to 4 mm wide, a decompound holdfast with a stupose stipe, and basally stupose axes. The axes were reported to be spirally twisted, narrowing upward, with subdichotomous-pinnately decompound alternate branches, and very short, regularly alternate ultimate tooth-like ramuli.
The order Dictyotales is diverse and speciose (Bringloe et al. 2020), with peak diversity found in the tropical Indo-Pacific, and Australasia (Küpper et al. 2019). There is a single family and two tribes recognised in the order; members of the Dictyoteae have a single apical cell, whereas members of the Zonarieae are characterised by having many localised or marginal apical cells. As noted by Bogaert et al. (2020), the morphology of species of Dictyota J.V.Lamour. is highly variable, and many species also display considerable intra-specific morphological variability. Bogaert et al. (2020, p. 4) noted that given this morphological plasticity, ‘molecular techniques are indispensable for species identification’. The most common markers used include psaA, psbA, rbcL and cox1.
As part of a wider survey of the Dictyotales, sequence data have been obtained from specimens collected throughout the New Zealand region. Analyses have shown that specimens with the morphology and anatomy of Dictyota papenfussii form a clade that is distinct from Dictyota, both from New Zealand and elsewhere. In these analyses, a psbA sequence in GenBank attributed to Lobospira bicuspidata Aresch., was also placed in this clade. This result contrasts with the analyses conducted by Lee and Bae (2002) using rbcL and 18S rDNA data, in which L. bicuspidata was found to group with other members of the Zonarieae. Lobospira is a monotypic genus endemic to Australia, with a very distinctive thallus apex. As observed by Edelstein and Womersley (1975) the occurrence of a marginal row of apical cells in both the adult axes and juvenile stages indicates that Lobospira is correctly placed in Zonarieae and not the Dictyoteae. Dictyota papenfussii from New Zealand has a single apical cell typical of the Dictyoteae.
In this paper, we describe a new genus for Dictyota papenfussii and provide new data on its geographic, seasonal and ecological distribution, and compare this taxon with sequence data from newly collected material of Lobospira bicuspidata from Australia.
Materials and methods
Specimens of Dictyota papenfussii have been collected from a wide variety of habitats throughout the New Zealand region over the past 15 years, pressed as herbarium specimens and subsampled at the time of collection, with material being placed in silica gel for genetic analyses. This study is based on both this recent material, as well as observations of additional specimens in the herbaria of Tāmaki Paenga Hira Auckland Museum (AK) and Te Papa Tongarewa Museum of New Zealand (WELT) (herbarium codes follow Index Herbariorum, see http://sweetgum.nybg.org/ih/, accessed January 2022).
There are six psbA sequences in GenBank ascribed to L. bicuspidata, four (KU249198.1, MW225728.1 MW225729.1, MW225731.1) collected at Jervis Bay, New South Wales (NSW), Australia and two (MW225732.1, MW225733.1) collected at Port MacDonnell in South Australia (Vieira et al. 2016, 2021). The vouchers of material for these sequences were unable to be located in either Australia or Belgium. Two specimens of Lobospira bicuspidata collected from Cape Peron, Western Australia, were borrowed from the Western Australian Herbarium (PERTH 09316523, J.Huisman, 15 February 2021; PERTH 09316639, J.Huisman, 16 February 2021) (Fig. 3), including subsamples dried in silica gel for molecular analysis.

|
DNA sequence data and phylogenetic analyses
DNA was extracted using the CTAB-Proteinase K method of Zuccarello and Lokhorst (2005). Collection locations and voucher numbers are given in Table 1. We attempted to amplify the following three markers from each specimen: psaA, psbA and rbcL as in Nelson et al. (2018). Polymerase chain-reaction (PCR) products were purified using ExoSAP-IT (Affymetrix, Santa Clara, CA USA), and sequenced at Macrogen Inc. (Seoul, South Korea). Sequences were trimmed and aligned using Geneious Prime (ver. 2023.0.4, Biomatters Ltd, Auckland, New Zealand, see https://www.geneious.com). All attempts to sequence the holotype and isotype material of D. papenfussii were unsuccessful.
|
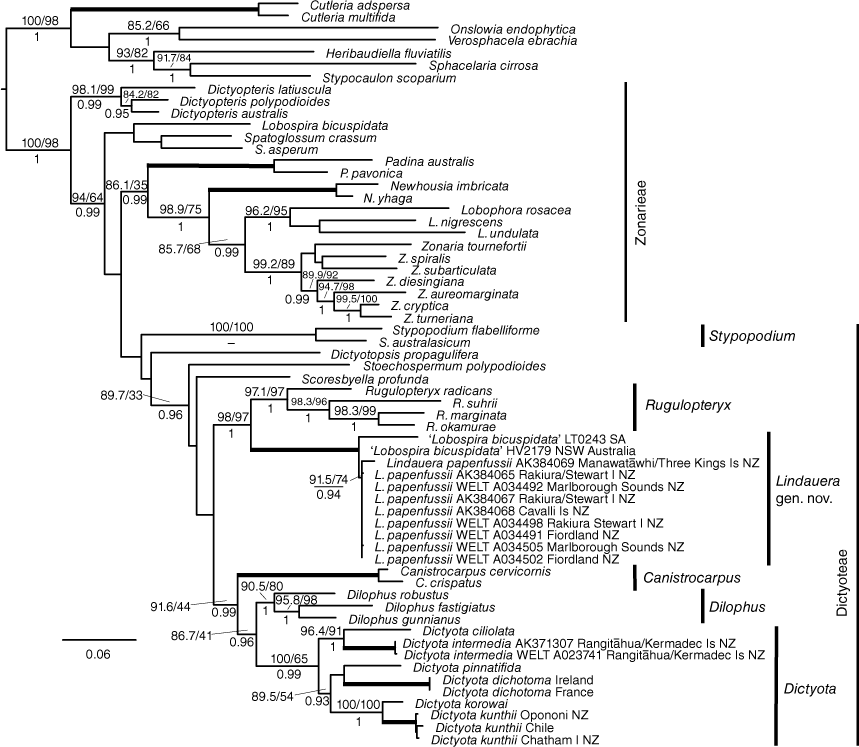
We used BLAST searches to identify GenBank sequences with high homology to ours, confirming the placement of our specimens within Dictyotales, and assembled a concatenated alignment of our data with sequences from representative Dictyotales taxa from GenBank, including including Canistrocarpus De Paula & De Clerck, Dictyota, Dictyotopsis Sond., Dictyopteris J.V.Lamour., Dilophus J.Agardh, Lobophora J.Agardh, Newhousia Kraft, G.W.Saunders, I.A.Abbott & Haroun, Padina Adans., Rugulopteryx De Clerck & Coppejans, Scoresbyella Womersley, Spatoglossum Kütz., Stoechospermum Kütz., Stypopodium Kütz., and Zonaria C.Agardh (Table 1). We included sequences from Cutleria adspersa (Mert. Ex Roth) De Not., and C. multifida (Turner) Grev. (Tilopteridales), Onslowia endophytica Searles and Verosphacela ebrachia E.C.Henry (Onslowiales), Heribaudiella fluviatilis (Aresch.) Sved., Sphacelaria cirrosa (Roth) C.Agardh and Stypocaulon scoparium (L.) Kütz. (Sphacelariales) as outgroups. GenBank accession numbers of taxa in the phylogenetic dataset are given in Table 1.
The dataset was initially partitioned by marker and by codon (i.e. 9 partitions) and the most appropriate partitioning strategy and models of sequence evolution were identified using IQ-TREE2 (ver. 2.1.2, see https://github.com/iqtree/iqtree2; Kalyaanamoorthy et al. 2017; Minh et al. 2020). We used IQ-TREE2 to estimate the maximum likelihood (ML) tree under this partitioning strategy and models, along with support under both the approximate likelihood ration test (aLRT, Anisimova and Gascuel 2006) and 1000 ML bootstrap replicates.
We used MrBAYES (ver. 3.2.6, see https://github.com/NBISweden/MrBayes/; Ronquist et al. 2012) to implement a Bayesian analysis. Because MrBAYES uses a more restricted suite of models than does IQ-TREE2, we used a second IQ-TREE2 analysis to identify appropriate models and an appropriate partitioning strategy for the Bayesian analysis. Partitioning strategies and models for all analyses are listed in Table 2. For the Bayesian analysis we ran four MCMC chains for 5 million generations, sampling every 1000 generations. Burn-in values were assessed through inspection of log-likelihood plots and average parameter values using Tracer (ver. 1.7.2, see https://github.com/beast-dev/tracer/releases/tag/v1.7.2; Rambaut et al. 2018) and were confirmed by inspection of potential scale reduction factor (PSRF) values calculated in MrBAYES. Trees were visualised using FigTree (ver. 1.4.4, see http://tree.bio.ed.uk/software/figtree).

|
Results
Sequence data from specimens identified as Dictyota papenfussii were distinctly different from those of other Dictyota taxa; percentage homology to sequence data from any Dictyota taxon was less than 88% for psaA, 93% for psbA and 92% for rbcL. The phylogenetic matrix contained 62 taxa and 2558 characters. In the phylogenetic analysis (Fig. 4), the clade containing specimens identified as Dictyota papenfussii was clearly distinct from Dictyota, and was resolved in a well-supported monophyletic clade with Rugulopteryx taxa (98/97/1). Maximum pairwise percentage sequence identities between Dictyota papenfussii and Rugulopteryx sequences were 93% (psbA) and 93.5% (rbcL).
Two psbA sequences from Australia, ascribed to Lobospira bicuspidata, were resolved with the Dictyota papenfussii sequences. The sequence of specimen HV2179 (MW225728.1), from NSW, differed from that of WELT A034505 by one substitution over 766 bp. The sequence of LT0243 from South Australia differed from the NSW sequence by 16 substitutions over 708 bp, and differed from that of WELT A034505 by 17 substitutions over 732 bp. Four further sequences of Australian material ascribed to Lobospira bicuspidata from NSW and one from South Australia were identical to that of HV2179.
We obtained two rbcL sequences from the specimens of Lobospira bicuspidata collected from Western Australia but were unable to sequence either psaA or psbA from these specimens. The two rbcL sequences were identical, and this sequence was resolved with Zonarieae taxa in our analysis, in an unsupported (90.9/58/0.72) clade with Spatoglossum crassum and S. asperum.
On the basis of the level of differentiation of ‘Dictyota’ papenfussii specimens from Dictyota and support for this clade, we propose a new genus.
Taxonomy
Lindauera W.A.Nelson & J.E.Sutherl., gen. nov.
Dark brown, flat, erect fronds, narrowing upwards, with subdichotomous to alternate branching. Axes frequently spirally twisted. Attached to solid substrates by primary, prostrate, dichotomously branched axes.
Etymology
The genus is named to recognise the contributions of Victor W. Lindauer (1888–1964), a pioneering New Zealand phycologist, and, in particular, his work documenting the brown algal flora.
Lindauera papenfussii (Lindauer) W.A.Nelson & J.E.Sutherl., comb. nov.
(Fig. 1.)

|
|
Illustrations
N. M. Adams, Seaweeds of New Zealand 105 (1994); W. A. Nelson, New Zealand Seaweeds 61 (2020).
Specimens examined
NEW ZEALAND: Manawatāwhi/Three Kings Islands: Princes Islands, Rosemary Island, Cattons Cave, 13 Apr. 2013, R.D’Archino, AK384069. North Island: Bay of Islands, Deep Water Cove, 30 Nov. 2009, R.D’Archino, WELT A030593; Bay of Islands Te Miko Reef, 10 Feb. 2010, S.Miller & R.D’Archino, WELT A032190; Bay of Islands, Te Miko Reef, 11 Feb. 2010, R.Stewart & B.Crocker, WELT A032191; Cavalli islands, Motokawanui, 9 Apr. 2013, R.D’Archino, AK384068. South Island: Marlborough Sounds, Queen Charlotte Sound mouth, White Rocks, 20 Nov. 2008, R.D’Archino, WELT A034492; Queen Charlotte Sound mouth, D’Urville I., 27 Mar. 2017, beam trawl, WELT A034505; Arnott Point, 10 Dec. 200, N.Shears, WELT A026837; Fiordland, Doubtful Sound, Secretary Island, 22 Jan. 2000, C.Duffy, WELT A026094; Shelter Islands, 27 Jan. 2008, F.Thomas, C.Hepburn, & D.Richards, WELT A034491; Fiordland, Breaksea Sound, 19 Dec. 2019, R.D’Archino, WELT A034502; Fiordland, Dusky Sound, Five-Fingers Peninsula, 14 Nov. 1984, P.Brotherson, WELT A016265. Rakiura/Stewart Island: Port Pegasus, Hell’s Gates, 4 Mar. 2009, C.Hepburn, AK384065; Port Pegasus, Pearl Island outer, 6 Mar. 2009, R.D’Archino, AK384067, and Pearl Island, 3 Dec. 2018, M.Desmond & A.Kluibenschedl, WELT A034498.
Distribution
On the basis of the results of phylogenetic analyses and examination of herbarium specimens, the distribution of Lindauera papenfussii is now understood to extend in New Zealand from Manawatāwhi/Three Kings Islands, and the northern North Island through to the southern South Island (particularly Fiordland), and Stewart Island (Fig. 2), and in Australia it has been found in both New South Wales and South Australia.
Habitat and ecology
Lindauera papenfussii is found from the upper subtidal zone through to depths of at least 20 m, usually in areas of moderate exposure. It is found growing on rocky reefs as well as on stable shell hash and cobbles. It can be locally abundant, forming dense patches.
Phenology
Specimens have been collected throughout the year, with fertile material recorded from November through to February (late spring through summer).
Conservation status
Nelson et al. (2019) categorised Dictyota papenfussii as ‘data deficient’ on the basis of the information available at the time of assessment.
Notes
The morphology of L. papenfussii is quite variable, having pointed to rounded tips, and in the height that thalli attain. In northern areas, thalli are typically smaller than those found in the south and generally have more rounded and diverging apices than specimens collected from Taranaki south.
Discussion
Despite sharing many features with Dictyota, Lindauera differs from other genera in the Dictyoteae in that it does not have the rhizoidal attachment typical of the tribe, but rather possesses prostrate, primary fronds. In the emended description of Dictyota (De Clerck et al. 2006), and as summarised by Bogaert et al. (2020), most Dictyota species attach to substrates by way of multicellular, uniseriate, basal or marginal branching rhizoids, although a few species attach by stoloniferous holdfasts. Rugulopteryx, the most closely related genus to Lindauera in our analyses, possesses both basal rhizoids, and stoloniferous holdfasts, whereas in Canistrocarpus attachment to substrates is by basal or marginal rhizoids and for Dilophus by rhizoids or stolons (Womersley 1987; De Clerck et al. 2006).
De Clerck et al. (2006) described Rugulopteryx and Canistrocarpus by using a combination of sequence data and anatomical and morphological features (e.g. number of medullary cell layers, undulate thalli, presence of uni- or multi-cellular antheridial paraphyses, number of sporangial stalk cells, presence or absence on sporangial involucre). Although sporangial material of Lindauera has been found, no recent material has been collected displaying antheridia or oogonia, nor any herbarium specimens that have been examined. In the original description Lindauer (1949) stated ‘sexual organs not seen’, but in Lindauer et al. (1961) there is a description of pyriform oogonia and antheridial sori, neither of which were illustrated. The predominance of sporophytic thalli is not unusual in the Dictyoteae (e.g. Phillips et al. 1990; Hwang et al. 2009; García-Gómez et al. 2021). Although research on gametogenesis in members of the order is limited, diurnal and lunar periodicity have been shown to influence gametogenesis and release of gametes (Müller 1962; Phillips et al. 1990; Bogaert et al. 2020). Further work is required to understand reproduction in Lindauera, including the influence of seasonality, and whether diurnal and lunar periodicity influence the production and release of gametes and spores.
We consider that the sequences in GenBank from specimens collected in Jervis Bay, New South Wales, and Port MacDonnell, South Australia, have been incorrectly assigned to Lobospira bicuspidata Aresch. These sequence data confirm the presence of Lindauera in Australia and support Lindauer’s observation about material collected from Sydney being ‘identical to D. papenfussii’. On the basis of morphology, Lobospira belongs in the Zonarieae, possessing a distinctive row of 8–12 apical cells in a terminal invagination. The sequence data from newly collected specimens of Lobospira bicuspidata from Western Australia, that displayed the very distinctive apex of this genus, were placed in our analyses within the Zonarieae. Further work and field investigations are required to understand the geographic and seasonal distribution of Lindauera in Australia.
We have documented a more extensive geographic range in New Zealand for L. papenfussii than formerly understood, but there remain large gaps in its recorded distribution that are likely to reflect collection gaps. In addition, further work is required in New Zealand to understand the seasonality and ecology of this species.
Data availability
The data that support this study are available in the article, specimens accessible in two publicly accessible herbaria (AK, WELT), and sequence data submitted in GenBank.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Research was supported by NIWA SSIF funding in the Oceans National Centre.
Acknowledgements
We thank the many people who have assisted with field work and contributed to collections of Dictyotales from New Zealand, particularly Roberta D’Archino, Kate Neill, Tracy Farr, and colleagues at NIWA, and Chris Hepburn and the team at the University of Otago. We are very grateful to the skipper and crew of the R/V Polaris for field work in Fiordland and Rakiura. We thank John Huisman for advice and the collections of Lobospira from Western Australia that were critical for this study, and Lara Shepherd (Te Papa) for attempting sequencing of type material of Lindauera. We very much appreciate the assistance of herbarium staff at Tāmaki Paenga Hira Auckland Museum, Te Papa Tongarewa Museum of New Zealand, and the Western Australian Herbarium with specimens and specimen images; and Erika Mackay and Kate Neill (NIWA) for assistance with preparation of plates.
References
Adams NM (1994) ‘Seaweeds of New Zealand.’ (Canterbury University Press)Anisimova M, Gascuel O (2006) Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Systematic Biology 55, 539–552.
| Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative.Crossref | GoogleScholarGoogle Scholar |
Bogaert KA, Delva S, De Clerck O (2020) Concise review of the genus Dictyota J.V. Lamouroux. Journal of Applied Phycology 32, 1521–1543.
| Concise review of the genus Dictyota J.V. Lamouroux.Crossref | GoogleScholarGoogle Scholar |
Bringloe TT, Starko S, Wade RM, Vieira C, Kawai H, De Clerck O, Cock JM, Coelho SM, Destombe C, Valero M, Neiva J, Pearson GA, Faugeron S, Serrão EA, Verbruggen H (2020) Phylogeny and evolution of the brown algae. Critical Reviews in Plant Sciences 39, 281–321.
| Phylogeny and evolution of the brown algae.Crossref | GoogleScholarGoogle Scholar |
De Clerck O, Leliaert F, Verbruggen H, Lane CE, De Paula JC, Payo DA, Coppejans E (2006) A revised classification of the Dictyoteae (Dictyotales, Phaeophyceae) based on rbcL and 26S ribosomal DNA sequence data analyses. Journal of Phycology 42, 1271–1288.
| A revised classification of the Dictyoteae (Dictyotales, Phaeophyceae) based on rbcL and 26S ribosomal DNA sequence data analyses.Crossref | GoogleScholarGoogle Scholar |
Edelstein T, Womersley HBS (1975) The thallus and spore development of Lobospira bicuspidata Areschoug (Dictyotales: Phaeophyta). Transactions of the Royal Society of South Australia 99, 149–156.
García-Gómez JC, Florido M, Olaya-Ponzone L, Rey Díaz de Rada J, Donázar-Aramendía I, Chacón M, Quintero JJ, Magariño S, Megina C (2021) Monitoring extreme impacts of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in El Estrecho Natural Park (Biosphere Reserve). Showing radical changes in the underwater seascape. Frontiers in Ecology and Evolution 9, 639161
| Monitoring extreme impacts of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in El Estrecho Natural Park (Biosphere Reserve). Showing radical changes in the underwater seascape.Crossref | GoogleScholarGoogle Scholar |
Hwang I-K, Lee WJ, Kim H-S, De Clerck O (2009) Taxonomic reappraisal of Dilophus okamurae (Dictyotales, Phaeophyta) from the western Pacific Ocean. Phycologia 48, 1–12.
| Taxonomic reappraisal of Dilophus okamurae (Dictyotales, Phaeophyta) from the western Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587–589.
| ModelFinder: fast model selection for accurate phylogenetic estimates.Crossref | GoogleScholarGoogle Scholar |
Küpper FC, Peters AF, Kytinou E, Asensi AO, Vieira C, Macaya EC, De Clerck O (2019) Dictyota falklandica sp.nov. (Dictyotales, Phaeophyceae) from the Falkland Islands and southernmost South America. Phycologia 58, 640–647.
| Dictyota falklandica sp.nov. (Dictyotales, Phaeophyceae) from the Falkland Islands and southernmost South America.Crossref | GoogleScholarGoogle Scholar |
Lee W, Bae K (2002) Phylogenetic relationship among several genera of Dictyotaceae (Dictyotales, Phaeophyceae) based on 18S rRNA and partial rbcL gene sequences. Marine Biology 140, 1107–1115.
| Phylogenetic relationship among several genera of Dictyotaceae (Dictyotales, Phaeophyceae) based on 18S rRNA and partial rbcL gene sequences.Crossref | GoogleScholarGoogle Scholar |
Lindauer VW (1949) Additions to the marine algae of New Zealand. Transactions of the Royal Society of New Zealand 77, 390–393.
Lindauer VW, Chapman VJ, Aiken M (1961) The marine algae of New Zealand 11: Phaeophyceae. Nova Hedwigia 3, 129–350, plates 57(1)–97(41).
Millar AJK, Kraft GT (1994) Catalogue of marine brown algae (Phaeophyta) of New South Wales, including Lord Howe Island, south-western Pacific. Australian Systematic Botany 7, 1–46.
| Catalogue of marine brown algae (Phaeophyta) of New South Wales, including Lord Howe Island, south-western Pacific.Crossref | GoogleScholarGoogle Scholar |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530–1534.
| IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era.Crossref | GoogleScholarGoogle Scholar |
Müller D (1962) Über jahres- und lunarperiodische Erscheinungen bei einigen Braunalgen. [On annual and lunar periodic phenomena in some brown algae.] Botanica Marina 4, 140–155.
| Über jahres- und lunarperiodische Erscheinungen bei einigen Braunalgen. [On annual and lunar periodic phenomena in some brown algae.]Crossref | GoogleScholarGoogle Scholar | [In German]
Nelson WA, Phillips L (1996) The Lindauer legacy — current names for the Algae Nova-Zelandicae Exsiccatae. New Zealand Journal of Botany 34, 553–582.
| The Lindauer legacy — current names for the Algae Nova-Zelandicae Exsiccatae.Crossref | GoogleScholarGoogle Scholar |
Nelson WA, Bilewitch JP, Sutherland JE (2018) Distribution of the genus Zonaria (Dictyotales: Phaeophyceae) in New Zealand, and description of Zonaria cryptica sp. nov from Stewart Island. New Zealand Journal of Botany 56, 264–275.
| Distribution of the genus Zonaria (Dictyotales: Phaeophyceae) in New Zealand, and description of Zonaria cryptica sp. nov from Stewart Island.Crossref | GoogleScholarGoogle Scholar |
Nelson WA, Neill K, D’Archino R, Rolfe JR (2019) ‘Conservation status of New Zealand macroalgae, 2019.’ New Zealand Threat Classification Series 30. (Department of Conservation: Wellington, New Zealand)
Phillips JA, Clayton MN, Maier I, Boland W, Müller DG (1990) Sexual reproduction in Dictyota diemensis (Dictyotales, Phaeophyta). Phycologia 29, 367–379.
| Sexual reproduction in Dictyota diemensis (Dictyotales, Phaeophyta).Crossref | GoogleScholarGoogle Scholar |
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67, 901–904.
| Posterior summarization in Bayesian phylogenetics using Tracer 1.7.Crossref | GoogleScholarGoogle Scholar |
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBAYES 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61, 539–542.
| MrBAYES 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space.Crossref | GoogleScholarGoogle Scholar |
Vieira C, De Clerck O, Payri CE (2016) First report of the Hawaiian genus Newhousia (Dictyotales, Phaeophyceae) from Madang, Papua New Guinea and description of the new species N. yhaga sp. nov. Botanica Marina 59, 31–37.
| First report of the Hawaiian genus Newhousia (Dictyotales, Phaeophyceae) from Madang, Papua New Guinea and description of the new species N. yhaga sp. nov.Crossref | GoogleScholarGoogle Scholar |
Vieira C, Steen F, D’hondt S, Bafort Q, Tyberghein L, Fernandez-García C, Wysor B, Tronholm A, Mattio L, Payri C, Kawai H, Saunders G, Leliaert F, Verbruggen H, De Clerck O (2021) Global biogeography and diversification of a group of brown seaweeds (Phaeophyceae) driven by clade-specific evolutionary processes. Journal of Biogeography 48, 703–715.
| Global biogeography and diversification of a group of brown seaweeds (Phaeophyceae) driven by clade-specific evolutionary processes.Crossref | GoogleScholarGoogle Scholar |
Womersley HBS (1987) Dictyotales. In ‘The Marine Benthic Flora of Southern Australia Part II. Phaeophyta’. pp. 187–259. (South Australian Government Printing Division: Adelaide, SA, Australia)
Zuccarello GC, Lokhorst GM (2005) Molecular phylogeny of the genus Tribonema (Xanthophyceae) using rbcL gene sequence data: monophyly of morphologically simple algal species. Phycologia 44, 384–392.
| Molecular phylogeny of the genus Tribonema (Xanthophyceae) using rbcL gene sequence data: monophyly of morphologically simple algal species.Crossref | GoogleScholarGoogle Scholar |