Nine new species of Australian Nicotiana (Solanaceae)
Mark W. Chase A B * , Maarten J. M. Christenhusz
A B * , Maarten J. M. Christenhusz  B , Luiz A. Cauz-Santos
B , Luiz A. Cauz-Santos  C , Felipe Nollet
C , Felipe Nollet  D , Jeremy J. Bruhl
D , Jeremy J. Bruhl  E , Damien D. Andrew
E , Damien D. Andrew  E , Ruth Palsson
E , Ruth Palsson  E , Richard W. Jobson
E , Richard W. Jobson  F , Guy M. Taseski
F , Guy M. Taseski  G and Rosabelle Samuel
G and Rosabelle Samuel  C
C
A Department of Environment and Agriculture, Curtin University, Bentley, WA 6102, Australia.
B Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3DS, UK.
C Department of Botany and Biodiversity Research, University of Vienna, Rennweg 14, A-1030 Vienna, Austria.
D Departamento de Biologia, Universidade Federal Rural de Pernambuco, 52171-900 Recife, Pernambuco, Brazil.
E Department of Botany and N.C.W. Beadle Herbarium, University of New England, Armidale, NSW 2351, Australia.
F National Herbarium of New South Wales, Narellan Road, Mount Annan, NSW 2567, Australia.
G School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia.
Australian Systematic Botany 36(3) 167-205 https://doi.org/10.1071/SB23001
Submitted: 24 January 2023 Accepted: 1 June 2023 Published: 7 July 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Nine new species in Australian Nicotiana section Suaveolentes are described, including N. clarksonii M.W.Chase & Christenh., N. erytheia M.W.Chase & Christenh., N. latifolia M.W.Chase & Christenh., N. latzii M.W.Chase, R.W.Jobson & Christenh., N. gibbosa M.W.Chase, D.D.Andrew & J.J.Bruhl, N. olens M.W.Chase & Christenh., N. praecipitis M.W.Chase & K.Durham, N. karara M.W.Chase & Christenh. and N. bungonia M.W.Chase & Taseski. Some have been known from herbarium specimens for a long time, but their distinction from other species concepts was unsuspected until DNA studies showed their divergent nature. Others are known from one or only a few new localities. All are morphologically and genetically distinct from their close relatives. Increased sampling of populations in our molecular studies has led us to the conclusion that the widespread, recently described species N. insecticida is a species complex, comprising as many as six genetically distinct taxa, one of which includes material from the type locality of N. hesperis. Here, we describe a new name, N. erytheia, for all material we previously assigned to N. hesperis that is phylogenetically distinct from that of the type locality. To make the relationships of the new species clearer, we provide a tree produced by analysis of nuclear (RADseq) DNA data.
Keywords: cryptic species, Nicotiana hesperis, Nicotiana megalosiphon, Nicotiana rosulata, Nicotiana simulans, Nicotiana suaveolens, river drainage basins, wild tobaccos.
Introduction
The genus Nicotiana is a common sight in the arid regions of Australia, where many species can be found in abundance during seasons when conditions are conducive to their growth and flowering. In years with little rain, they either do not germinate or abort their development early and produce only a few cleistogamous flowers, after which they produce seeds and senesce. All native Australian species are members of N. section Suaveolentes, a clade of allotetraploid species that originated 5–6 million years ago in South America (Clarkson et al. 2017; Schiavinato et al. 2020; Cauz-Santos et al. 2022). Previous taxonomic studies by American botanists, Wheeler (1935) and Goodspeed (1954), and Australian botanists, Burbidge (1960), Horton (1981) and Symon and colleagues (Symon 1984, 1998; Clarkson and Symon 1991; Symon and Kenneally 1994; Symon and Lepschi 2007) enumerated 21 species and taxa in Australia. A PhD project focused mostly on the Australian species (Marks et al. 2011), but this did not include extensive fieldwork and accepted the 16 species as delimited by Horton (1981) plus five species described later (cited above).
In 2018, Chase and Christenhusz (2018a, 2018b) and Chase et al. (2018a, 2018b) described four new species and resurrected one name from synonymy (Chase and Christenhusz 2018c). Another new species, N. paulineana Newbigin & P.M.Waterh., was recently added by Bally et al. (2021) for the entity listed in the Australian Plant Census (CHAH, see https://biodiversity.org.au/nsl/services/search/taxonomy, accessed 21 June 2023) as Nicotiana sp. Corunna (Symon 17088). Chase and Christenhusz (2021a, 2021b) and Chase et al. (2021a, 2021b, 2021c, 2021d, 2021e) added another seven new species and elevated four subspecies to specific rank (Chase and Christenhusz 2021c, 2021d; Chase et al. 2021e, 2021f). Chase et al. (2022a) added four species from the N. benthamiana complex, which were demonstrated to be genetically distinct by Cauz-Santos et al. (2022). We here describe an additional nine new species, which brings the total number of named Australian species to 51. This is not the last of the new species we have discovered, but there are aspects of the others that still need further investigation before we describe them. Several of these are shown in the RADseq tree (Fig. 1), labelled as ‘sp. nov. XXX’.
Phylogenetic tree for the species of Nicotiana section Suaveolentes, based on the RADseq (nuclear) data matrix of Chase et al. (2021, 2022b), to which we have added the accessions for the new species reported in this paper. Vouchers for these added accessions are indicated under the additional specimens examined for each of the new species. All nodes for which there are no bootstrap percentages indicated are 100. (a) The basal nodes of the RADseq tree. (b) The more derived clades in the same RADseq tree as in (a).
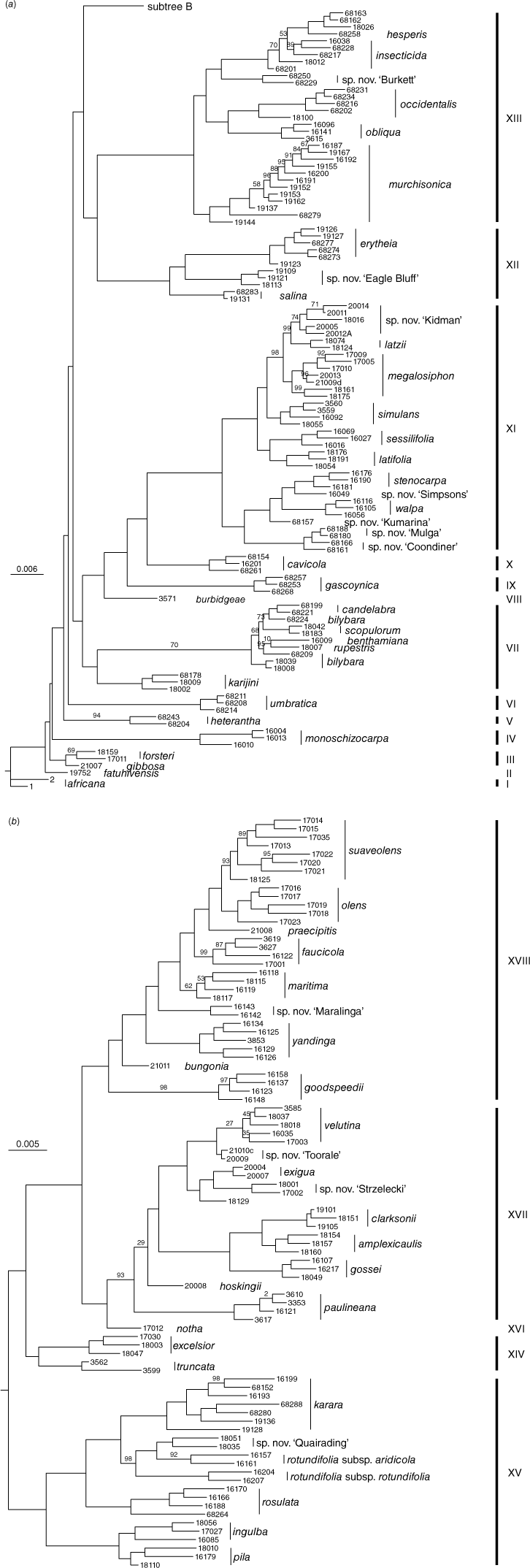
Phylogenetic relationships for the previously published new species are covered in Chase et al. (2021g). We provide a phylogenetic tree for the nine new species included here (Fig. 1a, b), which is based on the RADseq matrix from Chase et al. (2021g), to which we have added additional accessions for this set of new species. The additional new accessions included in this matrix are cited under each new species below. Details of the methods and analyses are provided in Chase et al. (2021g, 2022b).
We provide distribution maps for most species. For widespread species with many previous collections available via the Australasian Virtual Herbarium database (see https://avh.chah.org.au/), we downloaded locality data from the website and corrected them manually so that they correspond to the species as we delimit them. For example, Nicotiana megalosiphon Van Heurck & Müll.Arg. was previously considered to comprise two subspecies (Horton 1981), N. megalosiphon subsp. megalosiphon and N. megalosiphon subsp. sessilifolia P.Horton. Chase and Christenhusz (2021d) elevated the latter to species status as N. sessilifolia (P.Horton) M.W.Chase & Christenh. Here, we downloaded the AVH records and corrected these to map the three species, namely, N. megalosiphon, N. sessilifolia and N. latifolia, that we now recognise from the original collections (all recorded in AVH as N. megalosiphon s.l.). In another case, specimens of N. karara M.W.Chase & Christenh. (also described here as new) were previously considered N. rosulata (S.Moore) Domin, and to make it clear how we now circumscribe the latter, we include both in the map. Nicotiana rosulata was previously considered by Horton (1981) to range from north-western Western Australia to the Northern Territory and South Australia, but in the circumscription we use here, it is restricted to Western Australia, narrowly distributed from the Goldfields region to the North West Coastal Highway. All other collections referred to N. rosulata sensu Horton (1981) are other species, of which Nicotiana karara is one.
We have not previously provided conservation assessments for the new species we described and refrain from doing so here. We have extensively studied herbarium collections at AD, BM, CANB, CNS, K, NSW, NT and PERTH, but even for these, further study is required; we have visited BRI, but did not exhaustively examine their holdings of Nicotiana; and we have not visited DNA or MEL. Thus, we cannot say with great confidence that we know all species well enough to provide accurate assessments of their conservation status. For example, at the time we described N. salina M.W.Chase & Christenh. (Chase et al. 2021b), we knew it only from the type locality at Lake Weelhamby in Western Australia, but during fieldwork in mid-November 2022 focused on investigating nearby salt lakes, we found N. salina at five additional sites. Furthermore, our more informed herbarium studies at PERTH and CANB (later in November 2022) turned up eight previous collections of N. salina labelled variously as N. hesperis N.T.Burb., N. occidentalis H.-M.Wheeler, N. rosulata and N. rotundifolia Lindl. If we had evaluated the conservation status of this species at the time we described it, when it was known from a single locality, that assessment would now be highly inaccurate. It is almost certainly true that we could do comprehensive assessments on some of these new species described here (e.g. N. karara, N. latifolia M.W.Chase & Christenh., N. olens M.W.Chase & Christenh.), but we believe that doing these without also assessing their close relatives, which have been circumscribed in our studies differently from Horton (1981), would not be appropriate. Our intention is to fill the gaps in our herbarium studies in the next 2 years and conduct at least two additional field trips before we assess the conservation status of all Australian species in N. section Suaveolentes.
Taxonomy
Nicotiana clarksonii M.W.Chase & Christenh., sp. nov.
Type: Queensland: Belgravia, 17.1°S, 144.4°E, 23 Apr 1998, Hyland 15763 (holo: CNS QRS115635!; iso: CNS QRS116337!, CNS QRS115635!).
Nicotiana clarksonii is closely related (Fig. 1b) and morphologically similar to N. amplexicaulis N.T.Burb. (Fig. 7–9), but it has flowers twice the size and a simpler (less branched) inflorescence structure and grows in limestone caves and outcrops instead of sandstone, sandstone-derived soils and clay.
Erect, herbaceous, annual to short-lived perennial herbs, forming a rosette, but with numerous large leaves in the basal portion of the stems, the main stem with major branches from near the base and fewer, shorter branches in the upper portion. Leaves with broadly winged petioles making the leaves appear sessile, the wing 1.5–3.0 cm across, blades 5.4–21.5 × 1.9–11.5 cm, ovate, widest near the base, the apex acute in the basal leaves to acuminate in those higher up, upper leaves as wide at their base as in the middle, auriculate, margins entire, sometimes toothed topped with a glandular hair, undulating, ciliate, often bullate, especially on the base, upper leaves sessile, often with a somewhat auriculate base. Vestiture composed of dense pubescence all over, short- to long-haired, all with small glands, and longest hairs somewhat twisted with swollen bases but these not glandular, leaf surfaces also densely pubescent with short glandular hairs that make leaves feel wet to the touch, calyx and peduncle similar, with long hairs and short glandular hairs. Inflorescence bracts sessile, linear lanceolate, ~0.5–2.3 cm long, the apex acuminate. Calyx 1.20–1.40 × 0.15–0.20 cm, one lobe slightly longer and one shorter than the others, the tips acuminate, slightly flaring, 0.3–0.5 cm longer than and persistent in the fruit, the calyx enlarged at maturity. Corolla tube 2.4–2.8 cm long (from tip of the calyx), 0.35–0.40 cm in diameter, with no throat cup and smaller in diameter at the point it meets the calyx; in some plants the floral tube is curved rather than straight, the limb 2.4–2.8 cm across, the lobes slightly cleft, cleft 0.2 cm deep, sinus 0.4 cm deep, lobes 0.8–1.0 cm long; four stamens of the same length at the throat of the floral tube and the fifth ~5 mm deeper in the tube, all with filaments 0.2–0.3 cm long. Fruit a capsule splitting in four lobes, 0.8–1.2 cm long at maturity.
Known only from northern Queensland at the Mungana Caves and on Christmas Creek Station near Greenvale. In contrast, N. amplexicaulis has a range that is disjunct from N. clarksonii, occurring further south in Queensland, generally in transitional rainforest vegetation. Both species occur at sheltered sites.
Limestone outcrops and caves in vine thickets and forests that are wet in the monsoon season but arid otherwise. These plants can grow deep inside limestone caves (Fig. 6), in places with so little light that the only companion plants are ferns and mosses.
Collected in flower April–December. We suspect that it could be in flower at any time of the year, depending on rainfall.
Named for John R. Clarkson, Parks and Wildlife Service, Atherton, Queensland, who facilitated access to and accompanied us when we visited the Mungana Caves. He has a special interest in Nicotiana and described one species, namely, N. wuttkei J.R.Clarkson & Symon (Clarkson and Symon 1991). He is also the acclaimed, long-time Treasurer of the Australasian Systematic Botany Society.
This species has the same chromosome number as does N. amplexicaulis, n = 18 (Chase et al. 2022b).
Nicotiana clarksonii was first collected at the Christmas Creek Station in 1975 by Thorsborne (BRI AQ0102628) and then at the Mungana Caves in 1982 by Goodwin (BRI AQ0338801). In many cases, specimens of this species have been previously identified as N. forsteri Roem. & Schult., because of their shared sessile leaves with an auriculate leaf base, but the latter is a distantly related species (Chase et al. 2022b; Fig. 1) with a different floral morphology (see illustration of N. forsteri below in the section on N. gibbosa M.W.Chase, D.D.Andrew & J.J.Bruhl). It has also been associated with N. amplexicaulis (Fig. 7–9), to which it is sister (Fig. 1b), but it has flowers twice the size of the latter and grows on limestone- rather than sandstone-derived or clay soils. The Christmas Creek Station site is 650 km north of the main range of N. amplexicaulis in the areas near the Carnarvon Range, Queensland. Nicotiana clarksonii and N. amplexicaulis are highly similar, even down to their vestiture and chromosome number, but their ecology and distribution are different, which, combined with the morphological differences, justifies recognition of N. clarksonii as a distinct species. At the Mungana Caves, we observed one accession that was different from the others (Fig. 4, 5), different enough to make us think that there might in fact be two species at Mungana (they differ mostly in the shape of the floral tube and leaf width to length ratio). However, genetically, there seems to be no differences between the two forms. We illustrate both here (Fig. 2–5), but all other material we have examined has been like that in Fig. 2 and 3.
QUEENSLAND. Mungana Caves, Archway Cave, 0.90 km north of Burke Developmental Road, 325 m, 17°5′34″S, 144°23′46″E, 6 Sep. 2018, Chase & Christenhusz 18151 (BRI, CANB); Mungana Caves, Carpentaria Cave 1, 335 m, 17°5′41″S, 144°23′42″E, 2 May 2019, Chase & Christenhusz 19101 (BRI); Mungana Caves, Carpentaria Cave 2, 335 m, 17°5′42″S, 144°23′38″E, 2 May 2019, Chase & Christenhusz 19102 (BRI); Mungana Caves, outside Archway Cave, 0.9 km north of Burke Developmental Road, 360 m, 17°5′34″S, 144°23′47″E, 2 May 2019, Chase & Christenhusz 19103 (BRI); Mungana Caves, Ryan Imperial Cave, 345 m, 17°5′25″S, 144°23′30″E, 2 May 2019, Chase & Christenhusz 19104 (BRI); Mungana Caves, Hair Cave, Echidna’s Rest, 380 m, 17°6′10″S, 144°24′43″E, 2 May 2019, Chase & Christenhusz 19105 (BRI); NPR 98, Royal Archway Cave, Mungana, 80 m, 17.5°S, 144.23°E, 17 May 1997, Ford 1933 (QRS 112344); Fern Cave (Spring Tower) Chillagoe, 17.13277778°S, 144.43472222°E, 11 July 1982, Godwin C2317 (BRI AQ0338801); Burdekin, Belgravia, 3 km west of Mungana, 340 m, 17.075°S, 144.375°E, 20 May 1999, Gray 7554 (QRS 107535.3, BRI AQ0845080); Burke Developmental Road, 1 km E of Chillagoe, 380 m, 17.6°S, 144.25°E, 26 July 1995, Gray 6273 (QRS 107535.1, QRS 107536.4, QRS 107535.3); Archway Cave, Mungana National Park, 17.10°S, 144.38°E, 21 Oct 1990, Hind 6088 (NSW 234670); Mungana Fern Cave, 20 miles [~32.2 km] west of Chillagoe, 17.0818053°S, 144.4177893°E, 30 Dec. 1982, Jacobsen 3 (BRI AQ0339113, BRI AQ0339515); Christmas Creek Station, 600 m, 15 June 1975, 19.08192453°S, 145.2508554°E, Thorsborne 76 (BRI AQ0102628).
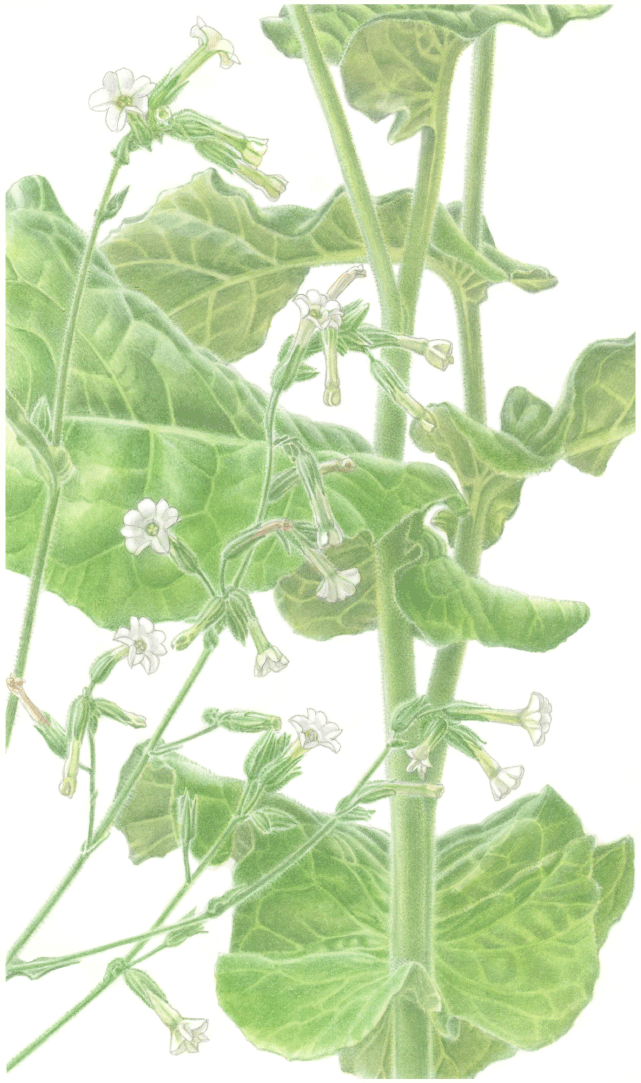
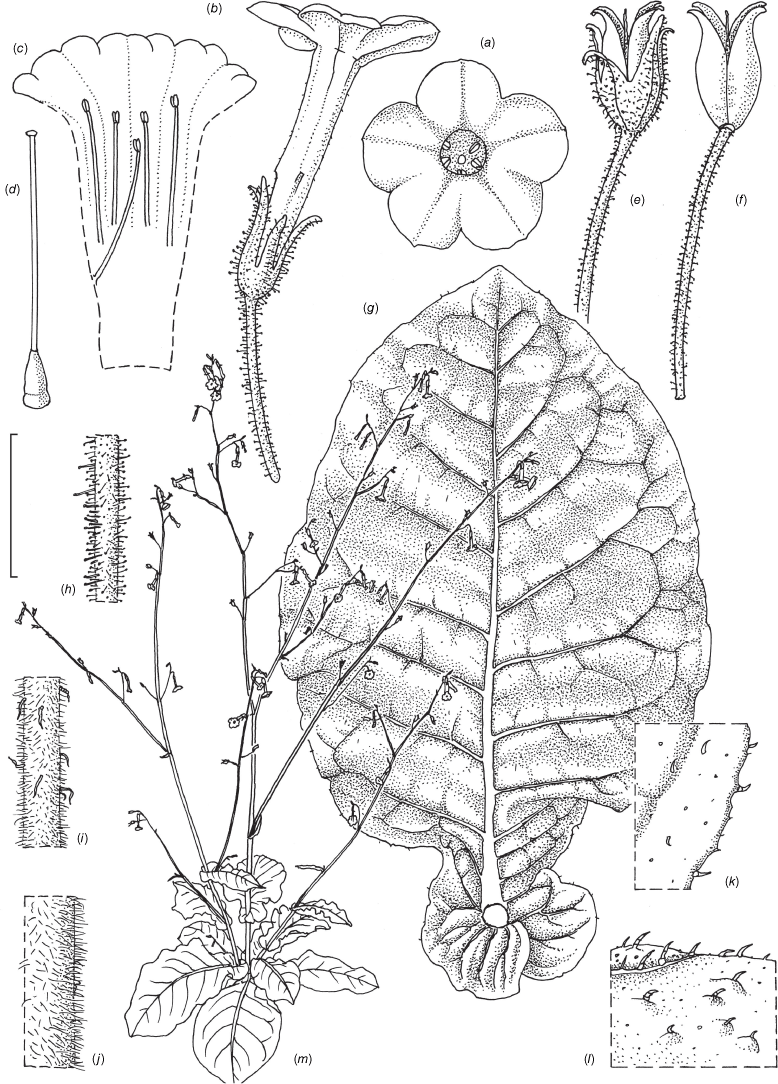
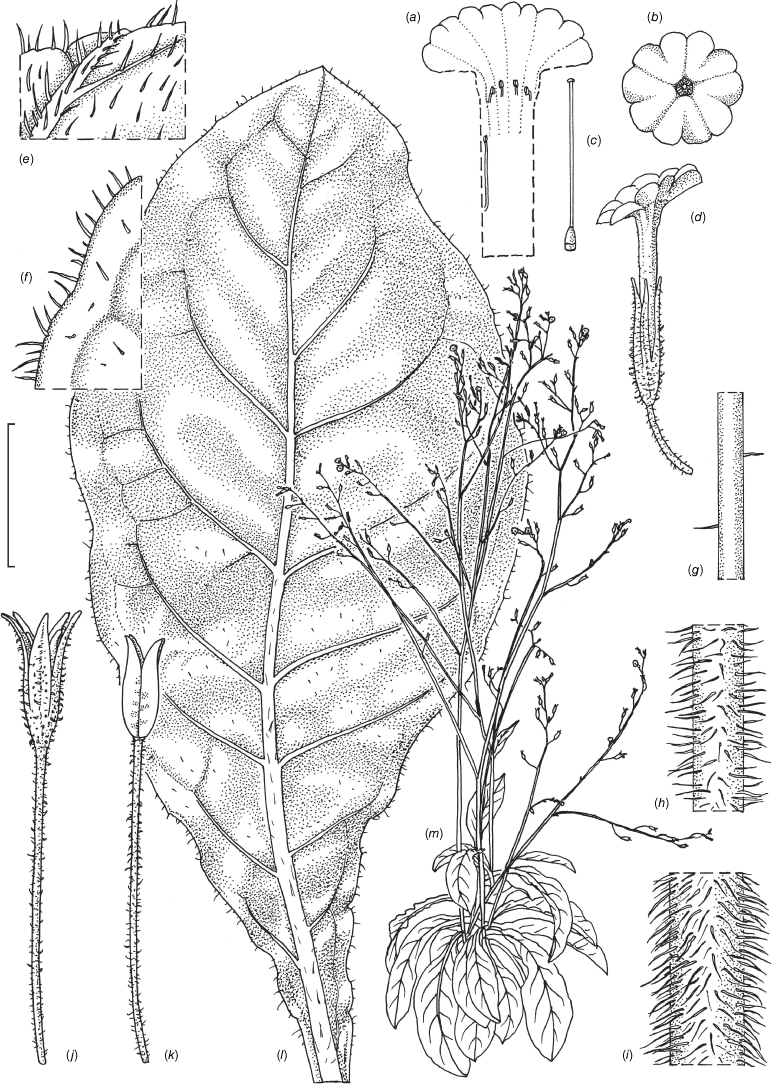
Nicotiana clarksonii based on living plants cultivated at the Royal Botanic Gardens, Kew, from seeds associated with Chase & Christenhusz 18151, Archway Cave, Mungana Caves, Queensland. Painted by Deborah Lambkin.

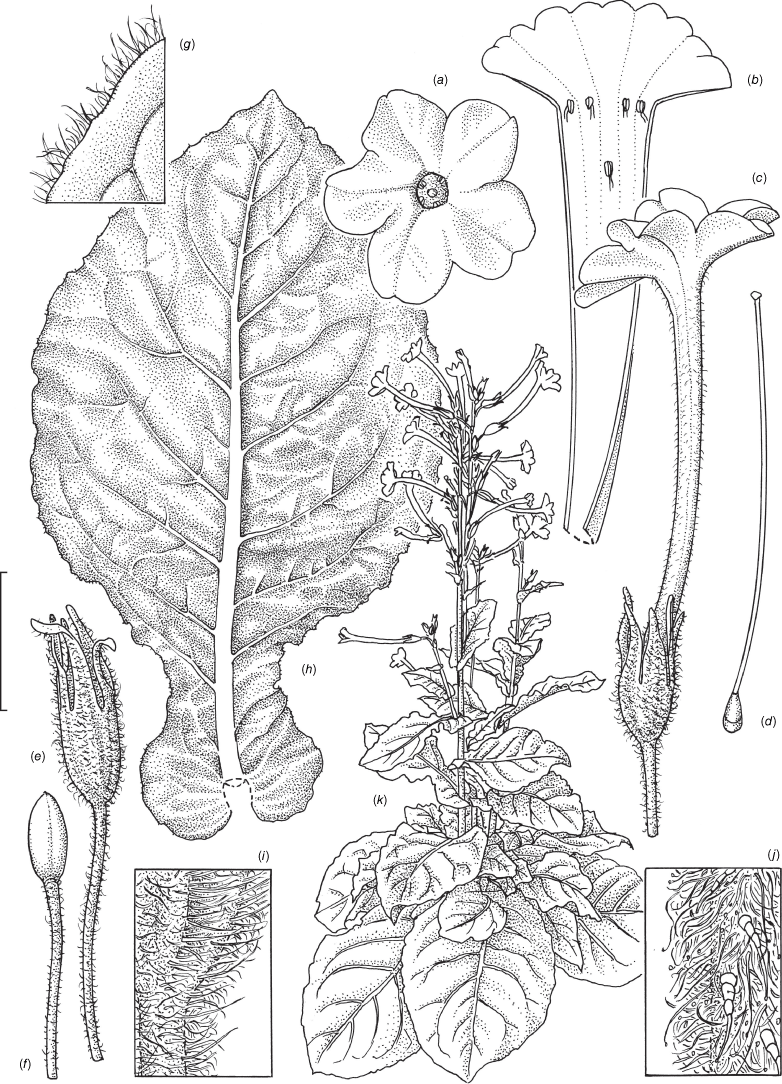
Nicotiana clarksonii based on living plants cultivated at the Royal Botanic Gardens, Kew, from seeds associated with Chase & Christenhusz 18151, Archway Cave, Mungana Caves, Queensland. Drawn by Deborah Lambkin. (a) Carpel, style and stigma. (b) Corolla, split open to show positions of stamens. (c) Flower, side view. (d) Floral limb, face-on. (e) Pubescence on leaf margin. (f) Pubescence on lower portion of stem. (g) Pubescence on calyx. (h) Pubescence on mid-vein. (i) Stem leaf. (j) Mature capsule. (k) Capsule without calyx. (l) Habit. Scale bars: 1.5 cm (a–d, j, k); 3.3 mm (e–h); 3.0 cm (i); plant in (l) is 64 cm tall.
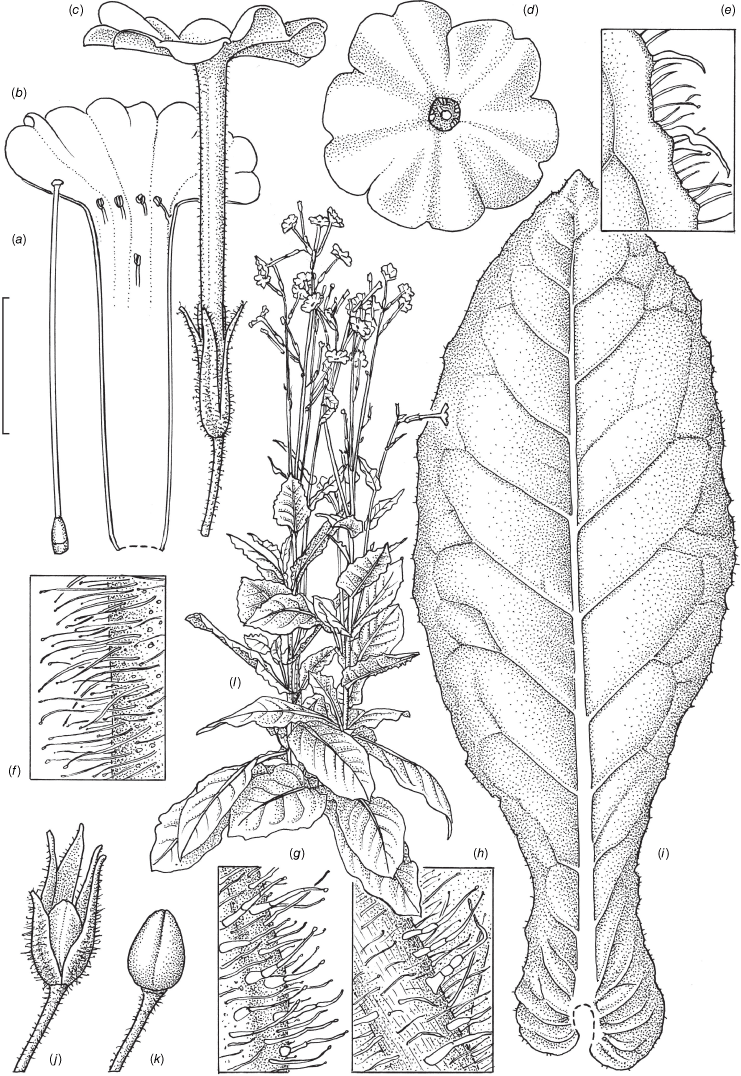
Nicotiana clarksonii based on living plants cultivated at the Royal Botanic Gardens, Kew, from seeds associated with Chase & Christenhusz 18152, Hair Cave, Mungana Caves, Queensland. Painted by Deborah Lambkin.

Nicotiana clarksonii based on living plants cultivated at the Royal Botanic Gardens, Kew, from seeds associated with Chase & Christenhusz 18152, Hair Cave, Mungana Caves, Queensland. Drawn by Deborah Lambkin. (a) Floral limb, face-on. (b) Corolla split open to show positions of stamens. (c) Flower, side view. (d) Carpel, style and stigma. (e) Mature capsule. (f) Capsule with calyx removed. (g) Pubescence on leaf margin. (h) Stem leaf. (i) Pubescence on base of calyx. (j) Pubescence on base of stem. (k) Habit. Scale bars: 1.5 cm (a–f); 3.3 mm (g); 3.0 cm (h); 3.3 mm (i, j); plant in (k) is 48 cm tall.
Small plant of N. clarksonii growing deep in Hair Cave, Mungana Caves, Queensland. Photograph by Maarten Christenhusz.

Nicotiana amplexicaulis based on living plants cultivated at the Royal Botanic Gardens, Kew, from seeds associated with Chase & Christenhusz 18160, Riverside Station, Nogoa River, Queensland. Painted by Deborah Lambkin.
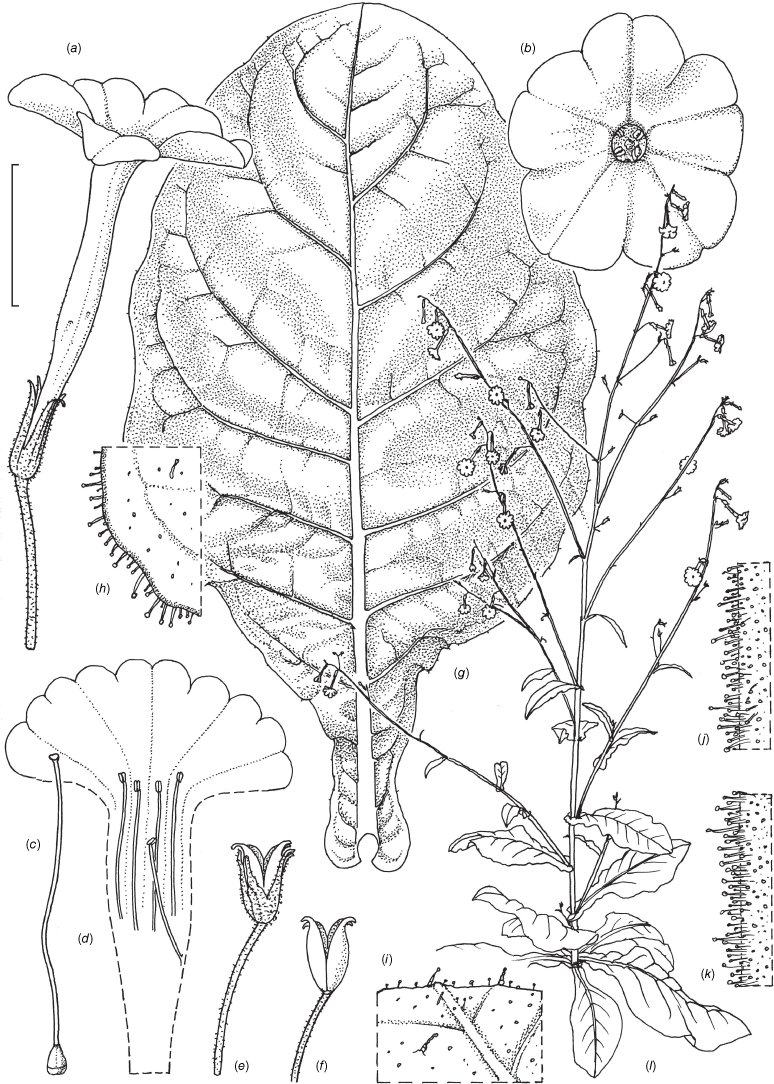
Nicotiana amplexicaulis based on living plants cultivated at the Royal Botanic Gardens, Kew, from seeds associated with Chase & Christenhusz 18160, Riverside Station, Nogoa River, Queensland. Drawn by Deborah Lambkin. (a) Floral limb, face-on. (b) Flower, side view. (c) Corolla split open to show stamen positions. (d) Carpel, style and stigma. (e) Capsule without calyx. (f) Mature fruit. (g) Stem leaf. (h) Pubescence on upper stem. (i) Pubescence on lower stem. (j) Pubescence on leaf margin. (k) Habit. Scale bars: flower parts, 1.0 cm (a–f); 4.0 cm (g); 3.3 mm (h–j); plant in (k) is 116 cm tall.
Nicotiana gibbosa M.W.Chase, D.D.Andrew & J.J.Bruhl, sp. nov.
Type: New South Wales. Oxley Wild Rivers National Park, ~170 m from the end of Long Point Picnic Area Rd along Chandler View Circuit, 1018 m, 30°39′53.7″S,151°56′4.4″E,18 Feb. 2021, Andrew 240 & Bruhl (holo: NSW; iso: K, NE 111501).
Nicotiana gibbosa is sister (Fig. 1a) and morphologically similar to N. forsteri (Fig. 13), but it has a flower three times the size, a different inflorescence structure with a single main axis and limited basal branching (v. extensive basal branches), narrower petiole wings and reduced auriculate bases. Both have protrusions on the exterior of the floral tube where the filaments are attached to the tube and pustules on the leaves with a single terminal hair, although these are much more obvious and frequent in the latter.
Erect, herbaceous, annual herbs, forming a minimal rosette, but with numerous large leaves in the basal portion of the stems, the main stem with major branches in the upper half of the inflorescence, but only a few small ones in the lower half. Leaves with narrowly winged petioles, the wing 1.6–4.0 cm wide, bullate, blades 20.0–36.5 × 3.2–13.4 cm (including petiole), broadly ovate, the apex blunt to acute in the basal leaves, becoming acuminate in those higher up; upper leaves, base gently attenuate, weakly auriculate, margins entire, undulate, often basally bullate, uppermost leaves sessile often with a somewhat auriculate base. Vestiture composed of dense, short-haired glands on all surfaces, leaf laminas with a few raised pustules with a terminal, twisted longer hair. Inflorescence bracts sessile, linear lanceolate, ~0.5–2.3 cm long, the apex acuminate. Calyx 1.2–1.4 × 0.3–0.4 cm, one lobe slightly longer and one shorter than the others, the tips acuminate, slightly flaring, 0.3–0.5 cm longer than and surrounding the fruit, the calyx persistent and enlarged at maturity. Flowers white, pendent to slightly out-facing, lower part of the floral tube longer than the upper and projecting outward, the upper part reflexed backward. Corolla tube 3.2–3.6 cm long (from tip of the calyx), 0.3 cm in diameter at the apex of calyx and 0.5 cm in diameter in the middle, with a weak throat cup and prominent bulges where the filaments connect to the floral tube, the limb 3.1–3.5 cm across, the lobes slightly cleft, cleft 0.1 cm deep, sinus 0.4 cm deep, lobes 1.4–1.6 cm long; four stamens near throat of the floral tube, lower pair 0.2 cm longer than the upper pair, and the fifth ~0.5 cm deeper in the tube, all with the filaments 0.4–0.6 cm long and attached to the floral tube in pockets. Fruit a capsule splitting in four lobes, 0.8–1.2 cm long at maturity.
Known thus far only from a few collections on the Northern Tablelands of New South Wales at Long Point, Curricabark Creek and Wollomombi Falls and the Macleay River on the New South Wales North coast (Fig. 14).
Growing in the transition between eucalypt grassy to layered open forest and low closed forest (dry rainforest) as attested by the range of closed forest elements at the known sites (Fig. 14). From the available fire records, it seems that three of the known four sites were some distance from recent fires. The type locality had suffered severe drought followed by above-average summer rainfall in February 2021. Rainfall records for the Williams collection indicated that it was also collected in a season with above-average rainfall.
Collected in flower in February–March and October. We suspect that it could flower at any time of the year, depending on disturbance (including fires) and rainfall.
Named for the pouches in the middle of the floral tube inside which the filaments are attached, from the Latin gibbosus (pouched).
Observations and collections of Nicotiana that are or are likely to be N. gibbosa have been referred to N. forsteri (Fig. 13), which is frequent in rainforest, dry rainforests and vine scrub vegetation all along the eastern coast of Australia and on Lord Howe Island and New Caledonia (Marks 2010). Thus far, N. gibbosa appears to have been collected only a few times and only in seasons of above-average rainfall following disturbance events (drought or fire). The type locality will be monitored for its occurrence. Nictotiana gibbosa is recorded generally at higher elevations than is N. forsteri.
NEW SOUTH WALES. 1.7 km due N of ‘Kunderang East’ Station, Mackay [Macleay] River, plants in dense regeneration from moderately intense wildfire near the edge of dry rainforest, 220 m, 15 Oct. 1991, 30.8033°S, 152.144°E, Pollock s.n. (NSW 269628); Curricabark Wildlife Refuge, Curricabark Creek in the Pigna Barney River catchment, 785 m, 1996, 31°47′30″S, 151°37′20″E, Robinson s.n. (NSW 447376); Wollomombi Falls, in wet scrub with Alectryon forsythii, Notolaea microcarpa, Mar. 1962, 30°32′S, 152°3′E, Williams s.n. (NE 22881).
Nicotiana gibbosa based on living plants cultivated at the Royal Botanic Gardens, Kew, grown from seeds associated with the holotype Andrew 240 & Bruhl, Long Point area past Hillgrove, Oxley Wild Rivers NP, New South Wales, vouchered as Chase & Christenhusz 21007 (K). Painted by Deborah Lambkin.

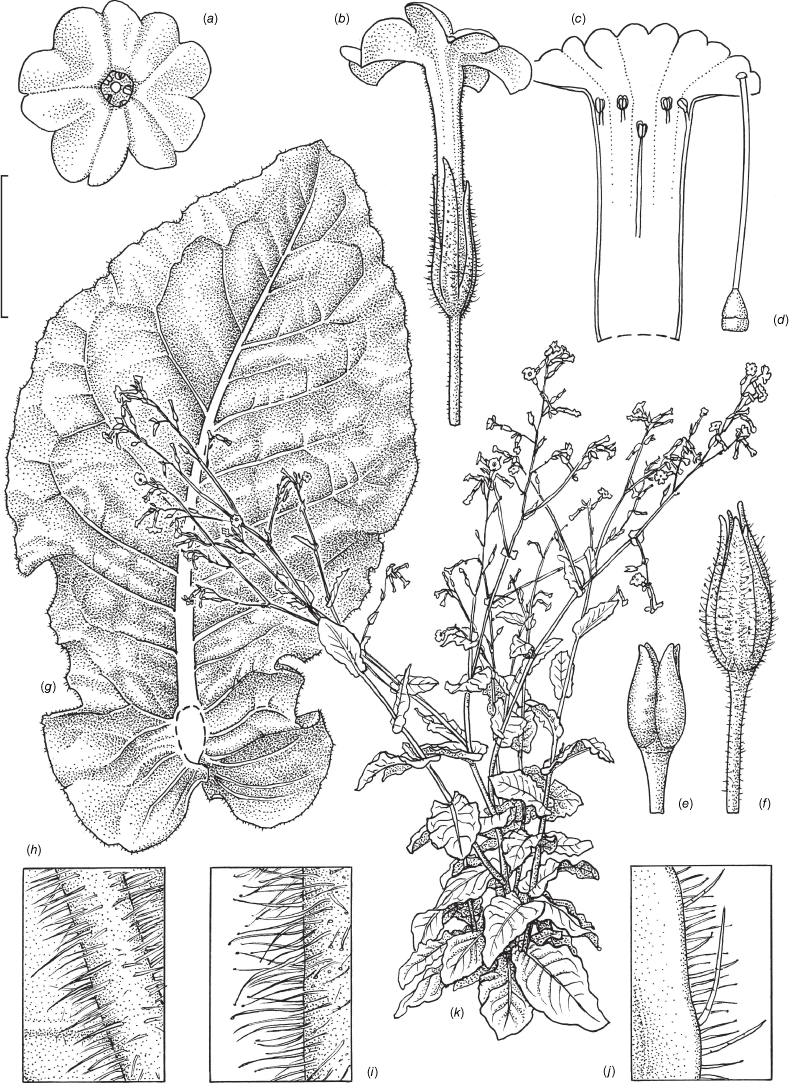
Nicotiana gibbosa based on living plants cultivated at the Royal Botanic Gardens, Kew, grown from seeds associated with the holotype Andrew 240 & Bruhl (NSW), Long Point area past Hillgrove, Oxley Wild Rivers NP, New South Wales. Drawn by Deborah Lambkin from living plants vouchered as Chase & Christenhusz 21007 (K). (a) Flower, side view. (b) Floral limb, face-on. (c) Carpel, style and stigma. (d) Corolla split open to show stamen positions. (e) Capsule at maturity. (f) Capsule with calyx removed. (g) Stem leaf. (h) Pubescence on leaf margin. (i) Pubescence leaf surface, showing pustulate hairs. (j) Pubescence on upper stem. (k) Pubescence on lower stem. (l) Habit. Scale bars: 2.0 cm (a–f); 6.0 cm (g); 5.0 mm (h–k); plant in (l) is 148 cm tall.
Habitat of Nicotiana gibbosa at Long Point, New South Wales, eucalypt shrubby to layered open forest, low closed forest ecotone after heavy rain following a fire the previous year. Photograph by Jeremy Bruhl.

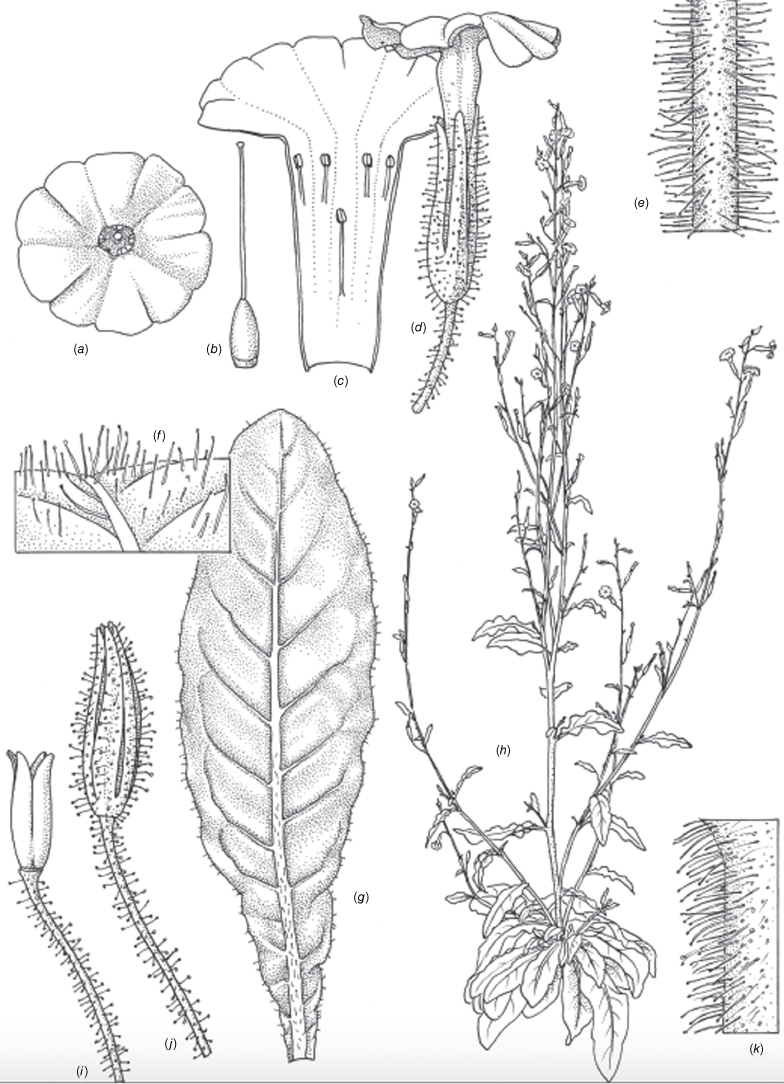
Nicotiana forsteri based on living plants cultivated at the Royal Botanic Gardens, Kew, grown from seeds associated with Forster 41986 (BRI AQ0837934), Woroon National Park, west-south-west of Windera, Queensland. Drawn by Deborah Lambkin, vouchered as Chase & Christenhusz 18030 (K). (a) Floral limb, face-on. (b) Flower, side view. (c) Corolla split open to show positions of stamens. (d) Carpel, style and stigma. (e) Mature capsule. (f) Mature capsule with calyx removed. (g) Stem leaf. (h) Pubescence on pedicel. (i) Pubescence on upper stem. (j) Pubescence on lower stem. (k) Pubescence on leaf margin. (l) Pubescence on leaf surface showing pustulate hairs. (m) Habit. Scale bars: 1.0 cm (a–f, h–l); 6.0 cm; plant in (m) is 123 cm tall.
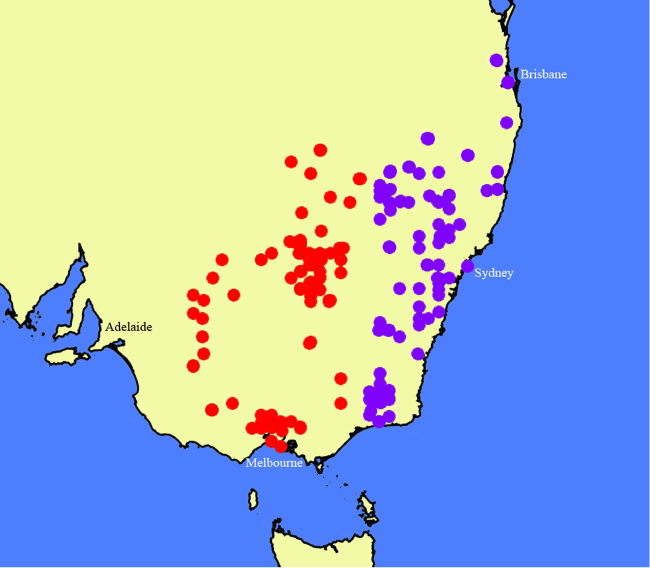
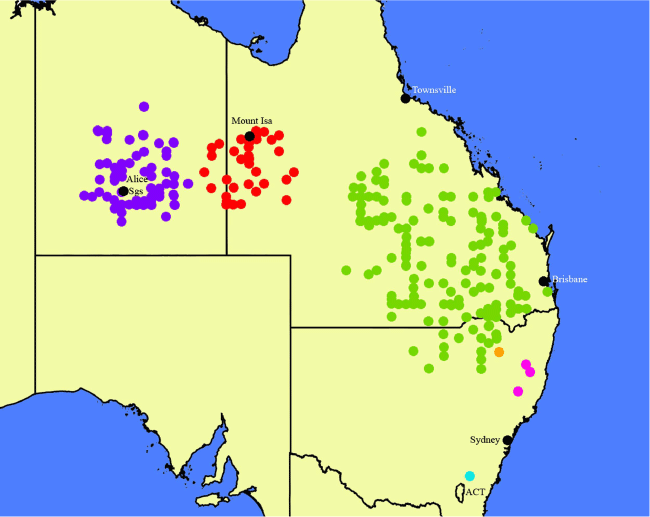
Map of the distributions of Nicotiana megalosiphon (green circles), N. latifolia (red circles), N. sessilifolia (blue circles), N. gibbosa (rose-purple circles), N. praecipitis (orange circle) and N. bungonia (aqua circle). Created by Maarten Christenhusz from data downloaded from the Australasian Virtual Herbarium website.
Nicotiana latzii M.W.Chase, R.W.Jobson & Christenh., sp. nov.
(Fig. 15.)
Type: Queensland: 5 km east of Ethabuka Station Homestead, 85 m, 23°51′151″S, 138°30′50″E, 14 Oct. 2005, Latz 21442 (holo: NT A0110082!).
Nicotiana latzii is closely related (Fig. 1a) and in morphology similar to N. latifolia and N. sessilifolia, but it lacks the broadly winged petiole and auriculate leaf base and has a shorter floral tube than do these two species. It is also similar to N. simulans N.T.Burb. in floral features, but has a much shorter petiole or wing.
Erect, herbaceous, annual herbs, forming a minimal rosette, but with numerous large leaves in the basal portion of the stems, the main stem with major branches in the lower half of the inflorescence but only a few small ones in the upper half. Leaves with narrowly winged petioles to nearly sessile, the wing 1.0–1.5 cm wide, bullate, blades 8.2–12.8 × 1.5–4.8 cm (including petiole), broadly ovate to lanceolate, the apex blunt to acute in the basal leaves, becoming acuminate in those higher up; upper leaf base gently attenuate, many slightly auriculate basally, margins entire, undulate, often basally bullate, uppermost leaves sessile often with a narrowly auriculate base. Vestiture composed of dense, gland-topped short hairs and longer multicellular, non-glandular hairs on all surfaces, in some cases the longer multicellular hairs on leaf margins with 1–2 branches. Inflorescence bracts sessile, linear lanceolate, ~0.5–2.3 cm long, the apex acuminate. Calyx 1.4–1.6 × 0.2 cm, one lobe slightly longer and one shorter than the others, the tips acuminate, slightly flaring to clasping, slightly wider and longer in fruit, extending 0.5 cm beyond and surrounding the capsule; the calyx slightly enlarging at maturity. Flowers white, outwardly to upward facing, upper part of the floral tube 0.2 cm longer than the lower. Corolla tube 2.0–2.5 cm long (from tip of the calyx), slightly longer on the upper side of the flower, 0.2 cm in diameter, with no throat cup, the limb 1.6–1.8 cm across, the lobes slightly cleft, cleft 0.1 cm deep, sinus 0.7 cm deep, lobes 0.6 cm long; four stamens near throat of the floral tube in two pairs, didynamous, the lower pair 0.1 cm longer than the upper pair, and the fifth ~0.7 cm deeper in the tube. Fruit a capsule splitting in four lobes, 0.9–1.1 cm long at maturity.
Thus far known only from north-central western Queensland between Ethabuka and Glenormiston Stations.
Named for Peter K. Latz, who has extensively studied the flora of the central portion of Australia for more than 40 years, contributing his voluminous collections to the Northern Territory Herbarium (NT) in Alice Springs and elsewhere. He collected the holotype and another specimen of this species on one of his trips to the Ethabuka Station in the far west of Queensland.
Unlike the other members of the N. simulans complex, which are all n = 20, N. latzii is n = 18 (M. W. Chase and F. Nollet, unpubl. data).
Collections of this species could be confused with N. simulans, N. latifolia or N. sessilifolia. The type specimen was originally identified by Latz as N. megalosiphon subsp. sessilifolia, from which it clearly differs in leaf shape and smaller flower (2.0–2.5 v. 3.8–5.0 cm). In addition to the collections at the Ethabuka Station by Latz, this species has been collected by R. W. Jobson east of the Glenormiston Homestead ~100 km north-east of Ethabuka. The holotype is represented in the phylogenetic analysis (Fig. 1a) by a secondary specimen grown from viable seeds removed from Latz 21442 and vouchered as Chase & Christenhusz 18074 (BRI). The other accession (Fig. 1a) is Chase & Christenhusz 18124 (NSW; BRI), a secondary voucher grown from seeds associated with Jobson 3235 (NSW).
QUEENSLAND. 14 km east of Ethabuka Homestead, Gypsum Hill, 23.8492°S, 138.6128°E, 15 Oct. 2005, Latz 21474 (NT A0110202!); anabranch of the Georgina River, Donohue Highway, 24 km east of Glenormiston Homestead, swampy roadside vegetation, 160 m, 22°53′43″S, 139°2′21″E, 30 Sep. 2016, Jobson 3235 (NSW).
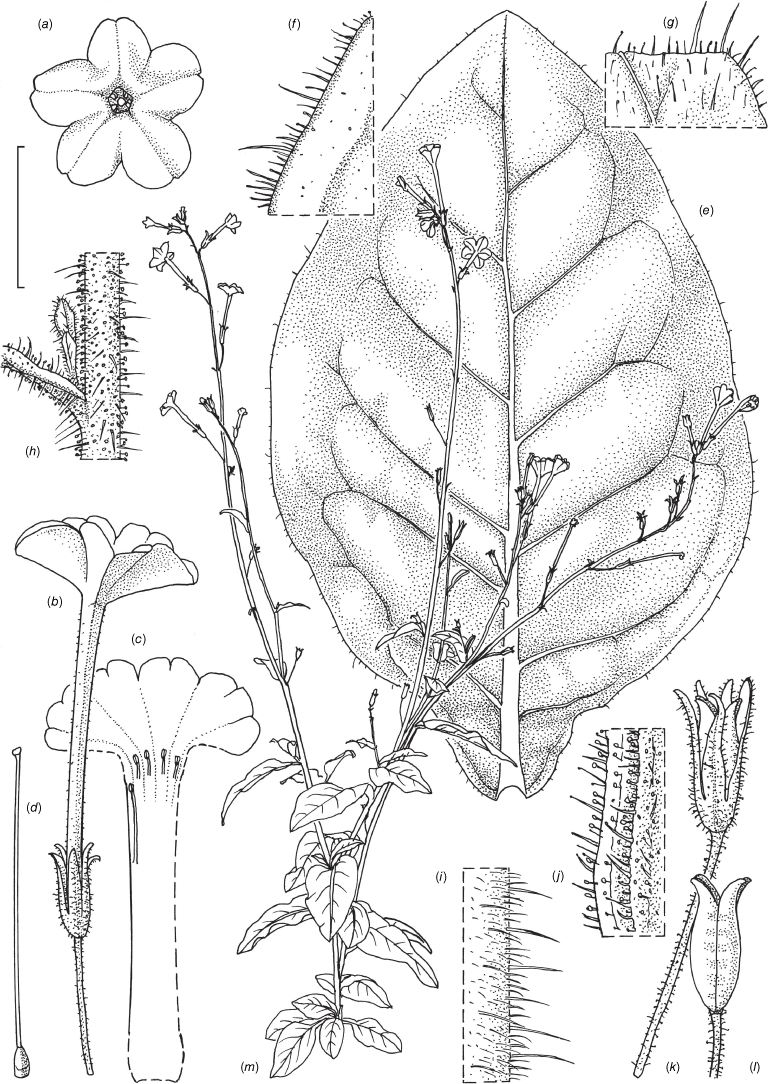
Nicotiana latzii based on living plants cultivated at the Royal Botanic Gardens, Kew, grown from seeds associated with Latz 21442 (NT A0110082), Ethabuka Station, Queensland. Drawn by Deborah Lambkin, vouchered as Chase & Christenhusz 18074 (K). (a) Floral limb, face-on. (b) Flower, side view. (c) Corolla split open to show positions of the stamens. (d) Carpel, style and stigma. (e) Stem leaf. (f) Pubescence on leaf margin. (g) Pubescence on leaf surface. (h) Pubescence on upper stem with leaf base and axillary shoot. (i) Pubescence on lower stem. (j) Pubescence on calyx. (k) Mature fruit. (l) Capsule with calyx removed. (m) Habit. Scale bars: 2.0 cm (a–e); 5.0 mm (f–j); 1.0 cm (k, l); plant in (m) is 77 cm tall.
Nicotiana latifolia M.W.Chase and Christenh., sp. nov.
Type: Queensland. Mount Isa, Mondarra Drive, Leichhardt River crossing, 335 m, 20°40′57″S, 139°29′45″E, 27 Sep. 2018, Chase & Christenhusz 18191 (holo. BRI; iso. CANB).
Nicotiana latifolia is closely related and morphologically similar to N. sessilifolia, from which it differs in its broadly winged petiole and auriculate leaf base v. a more narrowly winged petiole and a much narrower auriculate base. It is also similar to N. latzii, which has a shorter petiole and floral tube and lacks the broadly auriculate leaf base of N. latifolia.
Erect, herbaceous, annual to short-lived perennial herbs, up to 2.2 m tall, not forming a rosette, numerous large leaves in the basal portion of the stems, the main stem with major branches from near the base and few branches in the upper portion. Leaves with such broadly winged petioles they appear sessile, the wing 2.0–3.5 cm wide, blades 6.2–25.8 × 2.3–13.2 cm, ovate, widest near the base, the apex acute in the basal leaves to acuminate in those higher up; upper leaves as wide at their base as in the middle, auriculate, margins entire, undulating, ciliate, often bullate, especially on the base, upper leaves sessile often with an auriculate base. Vestiture of dense pubescence all over, short to long-haired, the shorter ones with glands predominating on upper stems and on leaf surface, longest hairs often with swollen bases on the upper stems, calyx and peduncle similar, with some long hairs but shorter glandular hairs predominate. Inflorescence bracts sessile, linear lanceolate, ~0.5–2.3 cm long, the apex acuminate. Calyx 1.2–1.4 × 0.3 cm, one lobe slightly longer and one shorter than the others, the tips acuminate, flaring to reflexed, especially in fruit, 0.5–0.6 cm longer than and surrounding the mature fruit, the calyx persistent and enlarged at maturity. Corolla tube pale green, 4.6–5.0 cm long (from tip of the calyx), 0.2–0.3 cm in diameter, with no throat cup, the limb white, 2.7–3.5 cm across, the lobes minutely cleft, cleft 0.1 cm deep, sinus 0.5–0.9 cm deep, lobes 1.1–1.4 cm long; four stamens of the same length 0.1–0.2 cm inside throat of the floral tube and the fifth ~0.7–0.9 cm deeper in the tube, all with the filaments 0.2–0.3 cm long. Fruit a capsule splitting in four lobes, 0.8–1.1 cm long at maturity.
Known from Queensland west of Middleton to the Northern Territory east of Tarlton Downs Station (Fig. 14).
In open to slightly shaded river courses, sometimes in riverbeds in red sand near woodland of Eucalyptus camaldulensis and E. coolabah. It is frequent under road and rail bridges over watercourses and floodways (Fig. 17).
Named in reference to the broad, sessile leaves of this species (from the Latin, latus, wide, and folium, leaf).
Specimens of Nicotiana latifolia have long been considered to belong to N. megalosiphon subsp. sessilifolia, now N. sessilifolia (Chase and Christenhusz 2021d; Fig. 18). The new species is sister to this (Fig. 1a) but easily distinguished by its sessile leaves with a broadly auriculate base (i.e. petioles so broadly winged that the leaves appear sessile). The two species are disjunct (Fig. 14), N. latifolia in the east, in western Queensland and the adjacent Northern Territory, in the Lake Eyre drainage, and N. sessilifolia in the southern half of the Northern Territory, particularly in the central ranges. A voucher grown from seeds removed from Forster 37332 (BRI AQ0816103) will be deposited at BRI and K, as Chase & Christenhusz 18054 and was used for Fig. 1a.
QUEENSLAND. Cravens Peak, near Salty Bore; Simpson Desert, west of Boulia, 200 m, 23°1′21″S, 138°13′12″E, 18 June 2010, Forster 37332 (BRI AQ0816103); Ramsay Street (Barkly Highway), Cloncurry, bridge over the anabranch of the Cloncurry River, 180 m, 20°42′16″S, 140°29′47″E, 21 Sep. 2018, Chase & Christenhusz 18176 (BRI, CANB); Hemsley Drive, Cloncurry River, west of the bridge, 180 m, 20°40′53″S, 140°29′40″E, 21 Sep. 2018, Chase & Christenhusz 18177 (BRI, CANB); Duchess–Cloncurry road, Malbon River, 65 m, 21°4′6″S, 140°17′13″E, 22 Sep. 2018, Chase & Christenhusz 18179 (BRI, CANB); Duchess–Cloncurry road, Spring Creek, 280 m, 21°13′33″S, 140°10′23″E, 22 Sep. 2018, Chase & Christenhusz 18180 (BRI, CANB); Duchess–Mount Isa road, north-west of Duchess, Wills River, near Bushy Park airstrip, 380 m, 21°16′10″S, 139°43′39″E, 22 Sep. 2018, Chase & Christenhusz 18182 (BRI, CANB); Mount Isa, Twenty Third Avenue, under high-voltage pylon near Leichhardt River, high on banks near river, 350 m, 20°44′42″S, 139°29′36″E, 22 Sep. 2018, Chase & Christenhusz 18184 (BRI, CANB); Barkly Highway, Spear River Bridge, near Mount Isa airport, 335 m, 20°39′6″S, 139°29′28″E, 23 Sep. 2018, Chase & Christenhusz 18186 (BRI, CANB); Wills Developmental Road, Leichhardt River bridge, 40 m, 18°48′27″S, 139°47′13″E, 26 Sep. 2018, Chase & Christenhusz 18187 (BRI, CANB); Kajibbi–Kamilaroi road, near Coolullah Station, 100 m, 19°49′33″S, 140°9′9″E, 26 Sep. 2018, Chase & Christenhusz 18188 (BRI, CANB); Burke Developmental Road, Corella River Bridge, north-west of Cloncurry, northern bank on rocks, 165 m, 20°26′24″S, 140°19′11″E, 26 Sep. 2018, Chase & Christenhusz 18189 (BRI, CANB).
Nicotiana latifolia based on living plants cultivated at the Royal Botanic Gardens, Kew, grown from seeds associated with Chase & Christenhusz 18177 (BRI), Cloncurry, on the banks of the Cloncurry River, Queensland. Drawn by Deborah Lambkin. (a) Floral limb, face-on. (b) Flower, side view. (c) Corolla split open to show positions of stamens. (d) Carpel, style and stigma. (e) Mature fruit. (f) Capsule with calyx removed. (g) Pubescence on leaf margin. (h) Pubescence on leaf lamina. (i) Pubescence on lower stem. (j) Pubescence on upper stem. (k) Stem leaf. (l) habit. Scale bars: 2.0 cm (a–f); 1.0 cm (g–j); 3.0 cm (k); plant in (l) is 90 cm tall.
Habitats of Nicotiana latifolia. (a) Wills Developmental Road, Leichhardt River Bridge, Queensland. (b) Barkly Highway, Spear River Bridge, near Mount Isa Airport, Queensland. Photographs by Maarten Christenhusz.

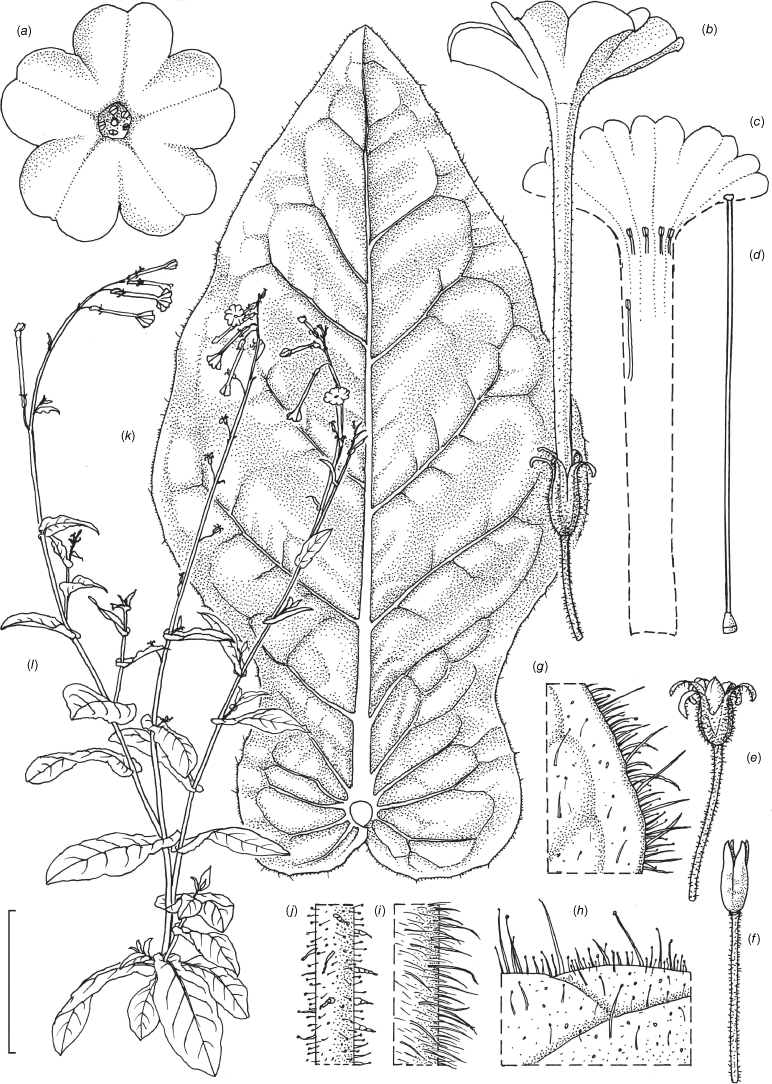
Nicotiana sessilifolia based on living plants cultivated at the Royal Botanic Gardens, Kew, grown from seeds associated with Chase & Christenhusz 16046 (NT), the Old Telegraph Station, Alice Springs, Northern Territory. Drawn by Deborah Lambkin. (a) Floral limb, face-on. (b) Corolla spit open to show positions of the stamens. (c) Flower, side view. (d) Carpel, style and stigma. (e) Pubescence on leaf surface near midvein. (f) Pubescence on leaf margin. (g) Pubescence on upper stem and pedicel. (h) Pubescence on lower stem. (i) Upper stem leaf. (j) Lower stem leaf. (k) Mature capsule. (l) Fruit with calyx removed. (m) Habit. Scale bars: 2.0 cm (a–d, k, l); 7.0 mm (e–h), 4.0 cm (i, j); plant in (m) is 110 cm tall.
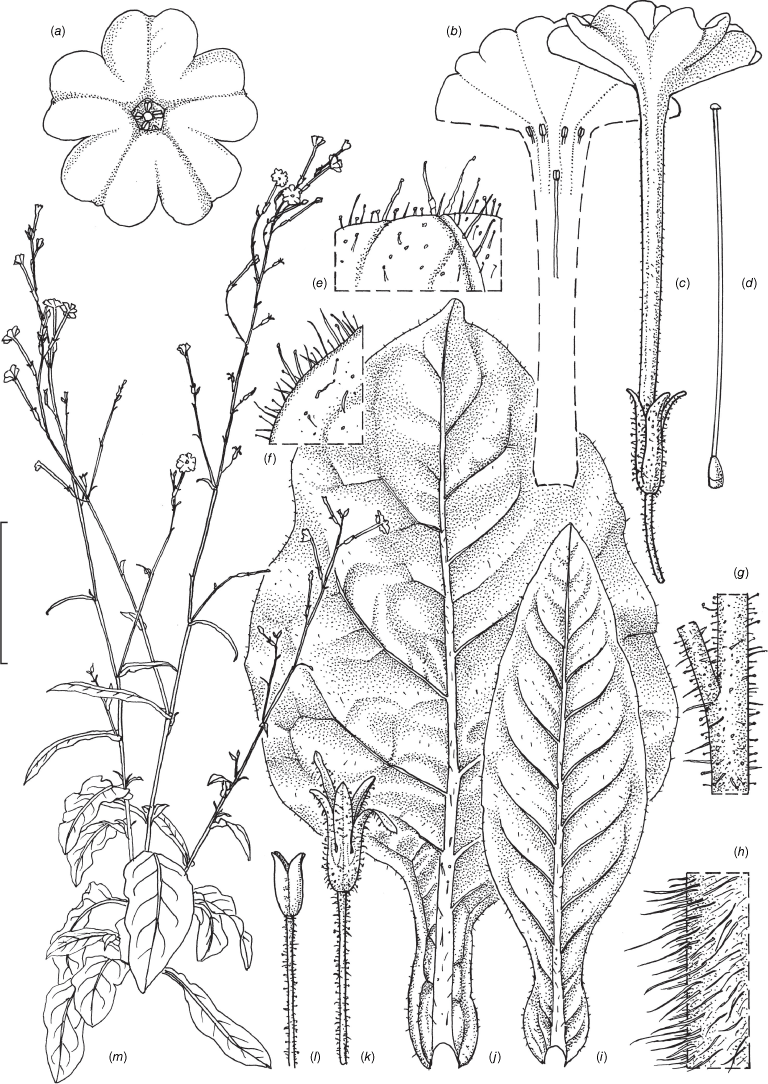
Nicotiana praecipitis M.W.Chase & K.Durham, sp. nov.
Type: New South Wales. Mount Kaputar NP, Mount Coryah Track, 1353 m, 30°16′50.96″S, 150°7′21.41″E, 29 Mar. 2021, Durham 97 & Palsson (holo: NSW; iso: NE 111470).
Nicotiana praecipitis is morphologically similar to N. olens and N. suaveolens, but it has differently shaped leaves with prominent hair-tipped teeth; the inflorescence structure is more upright and branched basally, with a larger flower in N. praeciptis (5.4–5.7 v. 1.8–4.2 cm) with the upper tube longer than the lower and slightly didynamous stamens. Nicotiana suaveolens has a mat of thick hairs on the bases of its stems and more stem leaves that rarely bear teeth.
Erect, herbaceous, short-lived perennial herbs, forming a small rosette, but with numerous large leaves in the basal portion of the stems, the main stem with major branches in the lower half of the inflorescence and a few larger ones in the upper half. Leaves with narrowly winged petioles, the wing 0.3–0.5 cm wide, with the base so gradually attenuate that the length of the petiole is arbitrarily determined, blades 9.5–30.5 × 2.8–15.5 cm (including petiole), ovate to lanceolate, the apex blunt to acute in the basal leaves, becoming acuminate in those higher up, margins undulate, with teeth topped by a multicellular, often bent and sometimes branched hair, base gently attenuate and often bullate; upper leaves initially petiolate, becoming narrow and almost sessile above. Vestiture composed of sparse, long, multicellular non-glandular hairs on the basal stems, leaf veins and margins and floral tubes, becoming glabrous on upper stems and leaves, pedicels and calyces with short glandular hairs and broadly based longer hairs with a smaller gland. Inflorescence bracts sessile, linear lanceolate, ~0.5–2.3 cm long, the apex acuminate. Calyx 1.4–1.6 × 0.2 cm, one lobe slightly longer and one shorter than the others, the tips acuminate and reflexed, 0.3–0.5 cm longer than and surrounding the fruit, the calyx persistent and enlarged slightly at maturity. Flowers white, out-facing, not pendent, upper part of the floral tube longer than the lower and projecting outward. Corolla tube 5.4–5.7 cm long (from tip of the calyx), 0.3 cm in diameter with a throat cup, upper part of tube slightly longer than the lower, the limb 3.0–3.8 cm across, the lobes slightly cleft, cleft 0.1 cm deep, sinus 0.8 cm deep, lobe 1.2–1.5 cm long; stamens five, upper two 0.15 cm longer than the lower two, all four attached near throat of the floral tube, the fifth 1.5 cm deeper in the tube, all with the filaments 0.1–0.2 cm long. Fruit a capsule splitting in four lobes, 0.9–1.2 cm long at maturity.
Known thus far only from the type collection on the Mount Coryah Walking Track, Kaputar National Park, New South Wales (Fig. 14).
On high ledges near the cliff top and the base of rock faces with an east-south-eastern aspect, in dark gravelly soil between basalt boulders (Fig. 21) of the Nandewar Volcanic Complex (Abbott 1969). It grows in an area that occasionally experiences snow in the austral winter.
Named for the rocky ledges below the summit on which it perches, from the Latin for precipitous.
This species would most likely be confused with N. suaveolens Lehm., but no previous collections of the new species have been located. It was discovered by Kay Durham and Ruth Palsson while hiking the Mount Coryah Walking Trail, not looking specifically for Nicotiana. Ruth had walked past when Kay noticed it, remarking to Ruth, ‘Isn’t that a Nicotiana?’
Its habit and habitat are unusual for the genus. There are no records of Nicotiana collected in Mount Kaputar National Park on AVH or Bionet apart from Constable s.n. (NSW 48734!), which is N. forsteri. However, this specimen was mistakenly attributed to Mount Lindsay in Mount Kaputar National Park. The location details are in the process of being corrected (R. Palssen, pers. comm.). On the same day, 25 May 1949, Constable appears to have collected ~40 specimens at Mount Lindesay, north-west of Kyogle, New South Wales, and various other mountains with the same or similar name in eastern Australia. In each case, he misspelt Lindesay as Lindsay, which is the name of the mountain in Mount Kaputar National Park but unlikely to have been visited on the same day as Mount Lindesay. Palsson and Durham have sighted Nicotiana forsteri at several locations in Mount Kaputar National Park at much lower elevations, ~500 m, but did not have a permit to collect on those instances. The material illustrated here (Fig. 19, 20) and used in the RADseq analysis was grown from seeds of the original gathering and has been given a secondary number, Chase & Christenhusz 21008 (CANB, K).
Nicotiana praecipitis, based on living plants cultivated at the Royal Botanic Gardens, Kew, grown from seeds associated with the holotype Durham 97 & Palsson (NSW). Painted by Deborah Lambkin.
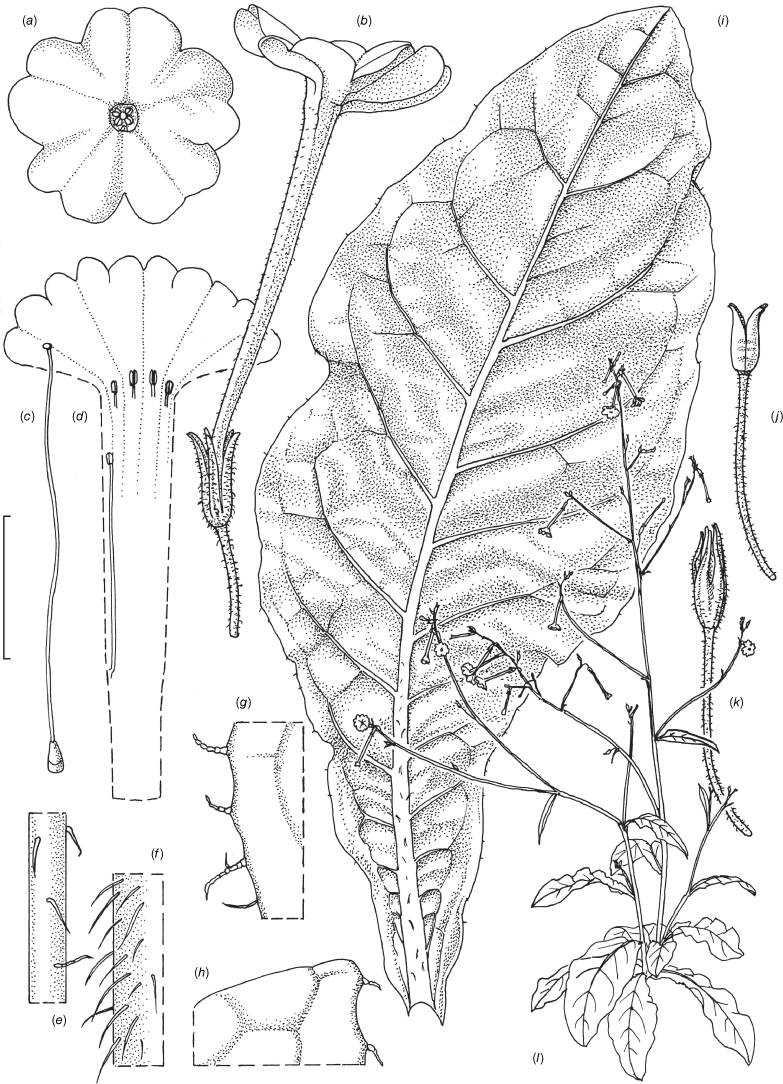
Nicotiana praecipitis based on living plants cultivated at the Royal Botanic Gardens, Kew, Chase & Christenhusz 21008 (K), grown from seeds associated with the holotype Durham 97 & Palsson (NSW). Drawn by Deborah Lambkin. (a) Floral limb, face-on. (b) Flower, side view. (c) Carpel, style and stigma. (d) Floral tube split to show positions of the anthers. (e) Pubescence on upper stem. (f) Pubescence on lower stem. (g) Pubescence on leaf margin. (h) Pubescence on leaf lamina. (i) Stem leaf. (j) Capsule with calyx removed. (k) Mature fruit. (l) Habit. Scale bars: 2.0 cm (a–d); 1.0 cm (e–h); 4.0 cm (i); 2.0 cm (j, k); plant in (l) is 121 cm tall.
Nicotiana olens M.W.Chase & Christenhusz, sp. nov.
Type: New South Wales. Cocoparra National Park, Woolshed Flat Trail, Pines Picnic Grounds, 240 m, 34°4″57′S, 146°12″58.3′E, 10 Oct. 2017, Chase & Christenhusz 17019 (holo: NSW; iso: CANB).
Nicotiana olens is closely related and morphologically similar to N. suaveolens, in which it was previously included. The main morphological differences are in its habit; the former has several large inflorescences produced from the base of the plant (v. a single main axis with side branches). Nicotiana olens is nearly glabrous (except for a few hairs at the base of the stems v. some hairs on nearly all parts). The flowers of N. olens (1.9–2.3 cm long) are typically shorter than those of N. suaveolens (2.2–4.2 cm long), but there is a slight overlap. Nicotiana suaveolens grows on limestone, often associated with caves, whereas N. olens is found on clay and sandstone in open sites.
Erect to arching, herbaceous, annual to short-lived perennial herbs, up to 2.0 m tall, forming a loose rosette to no rosette, numerous large leaves in the basal portion of the stems and few in the upper portions, the major branches from near the base with few side branches. Leaves with narrowly winged petioles, up to 1.5 cm wide and 7.5 cm long, blades 3.7–25.8 × 1.5–15.5 cm (including petiole), ovate-lanceolate, the apex acute in the basal leaves to acuminate in those higher up, the apical portion curled in some cases, base gradually to abruptly attenuate, uppermost leaves sessile, bract-like, margins entire, undulate, ciliate, often minutely dentate and basally bullate. Vestiture composed of long, somewhat curly, non-glandular hairs on the leaf margins and main veins and basal stems, but becoming nearly glabrous on the upper stems, ultimate stems, calyces and peduncles with short glandular hairs and a few longer, curly, non-glandular, multicellular hairs with a swollen base. Calyx 1.0–1.6 × 0.2–0.3 cm, one lobe slightly longer and one shorter than the others, the tips acuminate, clasping to reflexed, 0.5–0.6 cm longer than and surrounding the mature fruit, the calyx persistent and enlarged at maturity. Corolla tube white to pale green, 1.9–2.3 cm long (from tip of the calyx), 0.2–0.4 cm in diameter, often slightly curved downward, with a small throat cup, the upper side slightly longer than the lower, the limb white, 1.9–3.1 cm across, the lobes minutely cleft, cleft 0.2 cm deep, sinus 0.3–0.4 cm deep, lobe 0.7–1.7 cm long; stamens didynamous, 0.1–0.2 cm inside throat of the floral tube and the fifth 0.5–0.6 cm deeper in the tube, all with the filaments 0.2–0.3 cm long. Fruit a capsule splitting into four lobes, 0.8–1.2 cm long at maturity; only some plants are self-pollinating.
Distributed in central-western New South Wales west of the Great Dividing Range and in Victoria from Melbourne west to near the Grampians (Fig. 24), but not approaching the border of South Australia. The species might extend into southern Queensland, but no specimens have been seen from that state.
In open to slightly shaded sites, sometimes in riverbeds and open woodlands, on clay and sandstone substrates (Fig. 22, 23a).
This species is n = 15 with some autotetraploids recorded, whereas N. suaveolens is n = 16 (Chase et al. 2022b).
Specimens of Nicotiana olens have long been identified as N. suaveolens, the type of which was cultivated in France, grown from seeds collected by Joseph Banks at Port Jackson in the 18th century. The new species is sister to this (Fig. 1b), but it is easily distinguished by its habit, distribution and ecology. The two species are disjunct (Fig. 24), with the western edge of the Great Dividing Range being the transition point, N. olens to the west and N. suaveolens in the mountains of the Great Dividing Range and east-ward, often associated with limestone caves and gorges (e.g. Abercrombie, Jenolan, Wombeyan Caves; Fig. 25). It seems likely to us that N. olens as circumscribed here is a species complex; however, without doing a great deal more sampling, we cannot determine how many taxa should be recognised. A specimen identified as N. goodspeedii H.-M.Wheeler (Purdie 7721; CANB 789872) from Yathong Nature Reserve in New South Wales has flowers much too large for that species. It yielded viable seeds, which were secondarily vouchered as Chase & Christenhusz 18060 (to be deposited at NSW); these were studied by L. A. Cauz-Santos and M. W. Chase (unpubl. data) and appear to be N. olens introgressed with N. velutina. Plants grown from seeds associated with Walsh 8382 (MEL 2396268A) are vouchered as Chase & Christenhusz 17023 (MEL) and included in the phylogenetic analysis (Fig. 1b).
NEW SOUTH WALES. Near Mount Hope, 32.85°S, 145.87°E, 8 Nov. 1922, Patterson 18 (NSW 48706); Yenda–Rankin Springs Stock Route, north-west of Yenda, 34°S, 146.1667°E, 30 July 1959, Burbidge 6421 (AD 96129155; CANB 79280.1; NSW 55822; PERTH 3685349); 2.6 km from Goolgowie towards Hillston, 33.9167°S, 145.66679°E, Nov. 1992, Butler 1693 (CBG 9216072.1); Whitton Stock Route Road, north of Yenda, Steamboat Creek, 200 m, 34°6′16.9″S, 146°11′52.4″E, 10 Oct. 2017, Chase & Christenhusz 17016 (NSW, CANB); Whitton Stock Route Road, north of Yenda, just south of road into Woolshed Campground, 200 m, 34°6′5″S, 146°11′43.4″E, 10 Oct. 2017, Chase & Christenhusz 17017 (NSW, CANB); Cocoparra NP, Woolshed Flat Trail, 40 m, 34°4′57″S, 146°13′8.8″E, 10 Oct. 2017, Chase & Christenhusz 17018 (NSW, CANB). VICTORIA. Little River area, Ford Motor Co. proving ground; 37.9167°S, 144.4167°E, 24 Sep. 1998, Walsh 4782 (MEL 2051415A); Mornington Peninsula National Park, Fingal walking track, 90 m, 38°28′40″S, 144°53′14″E, 11 Dec. 2015, Walsh 8382 (MEL 2396268A); Melbourne, Kew, Galatea Point, Yarrabend Park, 30 m, 37°47′57″S, 145°0′21″E, 18 Oct. 2017, Walsh 8229 (MEL 2418211A).
Habitat of Nicotiana olens (Chase & Christenhusz 17018) in Cocoparra National Park, Woolshed Flat Trail, New South Wales. Photo by Maarten Christenhusz.

(a) Habit of Nicotiana olens (Chase & Christenhusz 17017), Whitton Stock Route Road, north of Yenda, New South Wales. (b) Close-up of inflorescence of N. olens (Chase & Christenhusz 17017).

Nicotiana bungonia M.W.Chase & Taseski, sp. nov.
(Fig. 26.)
Type: New South Wales. Bungonia Gorge, Bungonia National Park, along Mount Ayre Track, ~250 m east of Mount Ayre, 478 m, 34°48′9.71″S, 150°1′44.64″E, 30 Dec. 2020, Taseski 1398 (holo: NSW; iso K).
Nicotiana bungonia is morphologically similar to N. suaveolens, to which specimens were previously assigned, despite their atypically small flowers (0.5–0.6 v. 2.2–4.2 cm long respectively). It also differs in its highly branched inflorescences. It could also be mistaken for N. goodspeedii, to which it is similar in flower size and shape and branched inflorescence, but it differs in its wider leaves and sparser pubescenc; N. goodspeedii has only a few hairs on the bases of its stems and leaves to none.
Erect herbaceous, annual to short-lived perennial herbs, up to 1.0 m tall, almost no rosette, numerous large leaves in the basal portion of the stems and few in the upper portions, the major branches from near the base with many side branches. Leaves with narrowly winged petioles, up to 0.8 cm wide and 4.5 cm long, blades 3.7–15.8 × 1.5–10.5 cm (including petiole), ovate-lanceolate, the apex acute in the basal leaves to acuminate in those higher up, base gradually to abruptly attenuate, uppermost leaves sessile, bract-like, margins entire, undulate, ciliate, often minutely dentate and basally bullate. Vestiture composed of long, somewhat curly, non-glandular hairs on the leaf margins and main veins and basal stems, but becoming nearly glabrous on the upper stems, ultimate stems, calyces and peduncles, with short glandular hairs and a few longer, curly, non-glandular, multicellular hairs with a swollen base. Calyx 1.0–1.2 × 0.1–0.2 cm, one lobe slightly longer and one shorter than the others, the tips acuminate, clasping to reflexed, slightly longer than and surrounding the mature fruit, the calyx enlarged at maturity. Corolla tube white to pale green, 0.5–0.6 cm long (from tip of the calyx), 0.2–0.3 cm in diameter, without a throat cup, the limb white, 0.5–0.8 cm across, the lobes minutely cleft, cleft 0.1 cm deep, sinus 0.1–0.2 cm deep, lobe 0.3–0.4 cm long; four stamens of the same length 0.1 cm inside throat of the floral tube and the fifth ~0.2–0.3 cm deeper in the tube, all with the filaments 0.2–0.3 cm long. Fruit a capsule, 0.6–0.8 cm long, splitting in four lobes.
Known thus far only from Bungonia Gorge, south-eastern New South Wales (Fig. 14).
In open Eucalyptus agglomerata woodland on steep valley walls and ridgetops, growing in a variety of substrates, including siltstone, mudstone, shale transition areas and limestone.
Named for the Bungonia Caves, a name of Aboriginal origin, meaning ‘sandy creek’ in the Ngunnawal language (Geographical Names Board of New South Wales, https://www.gnb.nsw.gov.au). The epithet is a noun in apposition.
The habit of Nicotiana bungonia is similar to that of N. suaveolens, but the flowers are much smaller than those of the latter. Both occur on or are associated with limestone, often around caves. One of the other two specimens cited below was annotated by Horton in 1979 as N. suaveolens, but she noted on the label that the corolla was ‘unusually short’. The species falls within the larger N. suaveolens clade, but not close to the latter (Fig. 1b). We thought at first that it might be a hybrid between N. suaveolens and perhaps N. goodspeedii, but it has been collected in Bungonia Gorge for over 50 years and sets abundant seeds, which would be unlikely if it were a hybrid between these two species (n = 16 and n = 20; Chase et al. 2022b). Chromosomes have yet to be studied, but its genome size is similar to others in the N. suaveolens clade (1C = 3.6 pg; S. Mian, RBG, Kew, unpubl. data), so it is not a neo-allopolyploid such as N. notha M.W.Chase & Christenh. (Chase et al. 2021c). Genetically, it is distinct (Fig. 1b) and does not exhibit an admixture of these two species (L. A. Cauz-Santos and M. W. Chase, unpubl. data). Secondary vouchers grown from seeds associated with the type collection, Chase & Christenhusz 2011 (NSW, CANB) were included in the phylogenetic analysis (Fig. 1b).
NEW SOUTH WALES. South edge of Bungonia Gorge, ~0.25 miles [400 m] north-west of main Bungonia Lookdown, extensively outcropping limestone forming a series of low cliffs with intervening ledges, Dec. 1965, 34.8°S, 150.02°E, Rodd s.n. (NSW 101138!); Bungonia Gorge, south of Marulan, Southern Highlands, 34.8°S, 150.02°E, 10 Nov. 1966, Pullen 4173A (CANB 162437!; NSW 99788!).
Nicotiana bungonia based on living plants cultivated at the Royal Botanic Gardens, Kew, grown from seeds associated with the holotype Taseski 1398 (NSW), vouchered as Chase & Christenhusz 21011 (K). Drawn by Deborah Lambkin from a plant grown from seeds of the type collection. (a) Floral limb, face-on. (b) Flower, side view. (c) Floral tube split to show positions of anthers. (d) Carpel, style and stigma. (e) Mature capsule. (f) Fruit with calyx removed. (g) Pubescence on leaf lamina near midvein. (h) Pubescence on leaf margin. (i) Pubescence on calyx. (j) Pubescence on upper stem. (k) Stem leaf. (l) Habit. Scale bars: 1.0 cm (a–f); 3.3 mm (g–i); 1.0 cm (j); 3.0 cm (k); the plant in (l) is 112 cm tall.
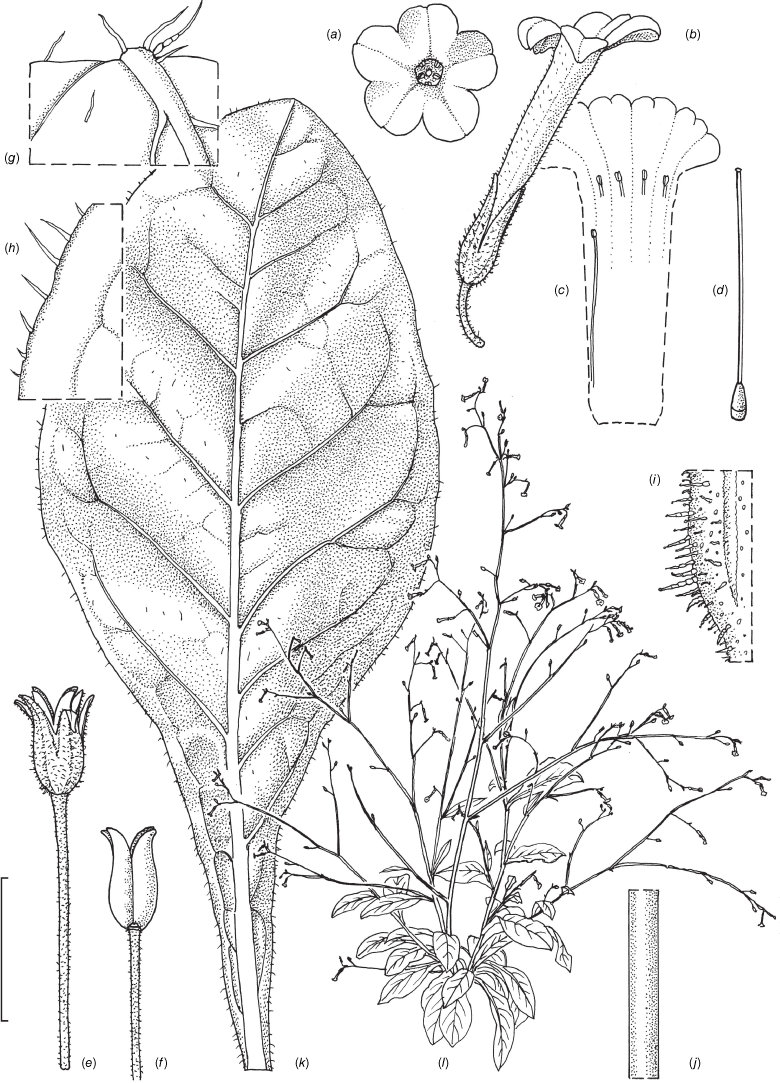
Nicotiana karara M.W.Chase & Christenhusz, sp. nov.
Type: Western Australia, Mungada Road to the Karara Mine, near junction with Lochada Road, 280 m, 29°11′42″S, 116°23′16″E, 23 Aug. 2015, Chase & Christenhusz 68280 (holo: PERTH; iso: CANB).
Nicotiana karara is morphologically similar to and related to N. rosulata and the N. rotundifolia species complex (Fig. 1b). Like N. rosulata, it has an inflorescence that is pubescent basally but becomes glabrous in the upper portions. Nicotiana karara differs in its more highly branched inflorescences and smaller flowers with didynamous stamens and generally leafy stems (no rosette, but small plants of nearly all species of N. section Suaveolentes, including this one, can form strict rosettes if the plants are small at the time of flowering or grow in full sun; Fig. 28a).
Erect herbaceous, annual to short-lived perennial herbs, up to 1.0 m tall, generally with no rosette and numerous large leaves in the basal portion of the stems with few in the upper portions, the major branches from near the base with many side branches in the upper half of the stem. Leaves with narrowly winged petioles, up to 1.0 cm wide and 3.5 cm long, blades 3.4–19.8 × 1.1–7.8 cm (including petiole), ovate-oblanceolate, the apex acute in the basal leaves to acuminate in those higher up, base gradually attenuate, uppermost leaves nearly sessile, bract-like, margins entire, undulate, ciliate, often basally bullate. Vestiture composed of long, somewhat curly, non-glandular hairs on the leaf margins and main veins, dense on the bases of stems, but becoming nearly glabrous on the upper stems, calyces and peduncles densely covered with short, often broadly based glandular hairs. Calyx 0.8–1.0 × 0.10–0.15 cm, one lobe slightly longer and one shorter than the others, the tips acuminate, clasping the floral tube, slightly longer than and surrounding the mature fruit with the apices reflexed. Corolla tube white to pale green, 0.6–0.8 cm long (from tip of the calyx), 0.2 cm in diameter, with a throat cup, the limb white, 0.7–1.0 cm across, the lobes cleft, sometimes with a small mucro, cleft 0.1 cm deep, sinus 0.1–0.2 cm deep, lobe 0.4–0.5 cm long; stamens mostly didynamous, 0.1 cm inside throat of the floral tube and the fifth ~0.2–0.3 cm deeper in the tube, all with the filaments 0.2–0.3 cm long. Fruit a capsule, 0.5–0.8 cm long, splitting in four lobes.
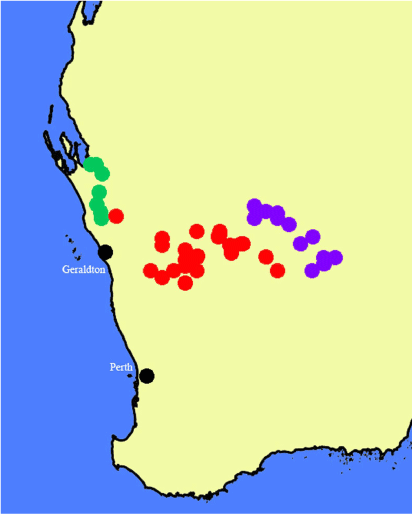
Found in the eastern edge of the wheatbelt from the Galatea bridge over the Murchison River south to Wubin and nearly as far east as the Weld Range and Lake Barlee in Western Australia (Fig. 29).
In lightly shaded sites under mulga, occasionally in the open or in vegetation on the margins of salt lakes but not in the salt vegetation. In the eastern part of its range, it often occurs near N. salina M.W.Chase, M.F.Fay & Christenh., but not close to the salt vegetation.
Named for the former Karara Station in the Shire of Perenjori, where the type was collected. The name is of Aboriginal origin, perhaps referring to Acacia bushland (Acacia tetragonophylla is common in this region and has the common name, kurara; http://www.flora.sa.gov.au/cgi-bin/speciesfacts_display.cgi?form=speciesfacts&name=Acacia_tetragonophylla). The species epithet is a noun in apposition.
Specimens of Nicotiana karara resemble those of N. rosulata and have been confused with the latter, but the flowers are generally smaller. Plants of N. karara are not rosulate with several to many leaves in the lower half of the stems, unless they are small and growing in full sun, in which case the two species are almost impossible to distinguish because they are both likely to be strictly rosulate (Fig. 28a). Larger plants of N. rosulata retain their rosulate habit, whereas those of N. karara develop sometimes numerous stem leaves (Fig. 28b). Both occur in similar sites and have nearly glabrous upper stems, which distinguishes them from the species of the N. rotundifolia complex, to which they are both closely related (Fig. 1b). Nicotiana rosulata is mainly found in the Goldfields area from Leonora as far west as Dalgaranga Station just to the north-west of Mount Magnet, whereas N. karara is slightly disjunct and occurs in the Salt River and Murchison River drainages.
WESTERN AUSTRALIA. Great Northern Highway (95), north of Jibberding, 315 m, 29°28′0″S, 117°11′56″E, 5 Aug. 2015, Chase & Christenhusz 68152 (PERTH); Mungada Road to the Karara Mine, Boiada Camp, 300 m, 29°11′37″S, 116°30′35″E, 23 Aug. 2015, Chase & Christenhusz 68285 (PERTH); Bowgada–Mullewa road to Wubin, 340 m, 29°20′22″S, 116°10′2″E, 23 Aug. 2015, Chase & Christenhusz 68288 (PERTH); Great Northern Highway (95), ~5 km north of Mount Magnet, in the Granites, 465 m, 28°0′25″S, 117°51′27″E, 18 Sep. 2016, Chase & Christenhusz 16185 (PERTH); Yalgoo–Paynes Find road, south-east of Yalgoo, 335 m, 28°28′52″S, 116°49′21″E, 19 Sep. 2016, Chase & Christenhusz 16193 (PERTH); Yalgoo–Paynes Find road, south-east of junction with track to Thundelarra Station, 320 m, 28°51′8″S, 117°4′39″E, 19 Sep. 2016, Chase & Christenhusz 16197 (PERTH); Yalgoo–Paynes Find road, north-west of Paynes Find, abundant on verges, 330 m, 29°1′21″S, 117°14′27″E, 19 Sep. 2016, Chase & Christenhusz 16199 (PERTH); Great Northern Highway (95), near salt ponds north of Jibberding, 290 m, 29°50′55″S, 116°56′19 117°11′56″E, 19 Sep. 2016, Chase & Christenhusz 16203 (PERTH); 1.9 km north of Galatea Bridge on Highway 1, eastern side of road, 200 m, 27°48′39″S, 114°41′25″E, 21 Sep. 2019, Chase & Christenhusz 19136 (PERTH); Morawa–Yalgoo road, Barnong Station, western side of road, ~47 km south-west of Yalgoo, 290 m, 28°38′2″S, 116°19′1″E, 22 Sep. 2019, Chase & Christenhusz 19136 (PERTH).
Nicotiana karara, based on living plants cultivated at the Royal Botanic Gardens, Kew, grown from seeds associated with the holotype, Chase & Christenhusz 68280 (PERTH), Mungada Road to the Karara Mine, Western Australia. Drawn by Deborah Lambkin. (a) Corolla split to show positions of didynamous anthers. (b) Floral limb, face-on. (c) Carpel, style and stigma. (d) Flower, side view. (e) Pubescence, leaf lamina. (f) Pubescence, leaf margin. (g) Pubescence, upper stem. (h) Pubescence on middle stem. (i) Pubescence on lower stem. (j) Mature capsule. (k) Capsule with calyx removed. (l) Stem leaf. (m) Habit. Scale bars: 1.0 cm (a–d, e–k); 3.0 cm (l); plant in (m) 88 cm tall.
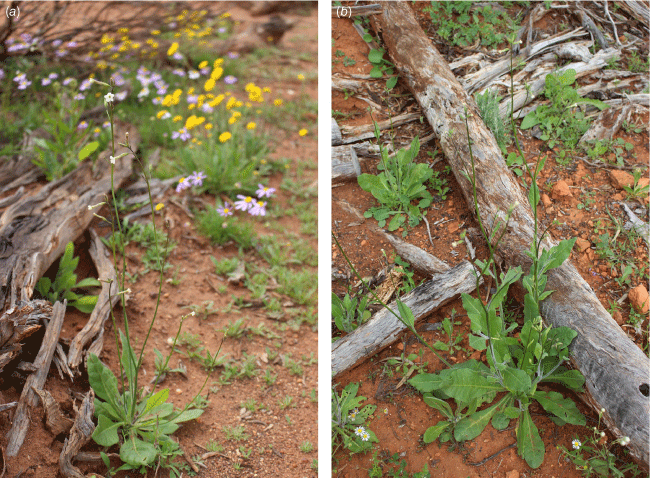
Variation in habits of Nicotiana karara. (a) Habit of Nicotiana karara, at the type locality, Mungada Road to the Karara Mine, Western Australia, Chase & Christenhusz 28280. (b) Habit of Nicotiana karara, Bowgada–Mullewa road, Western Australia, Chase & Christenhusz 28288. Note the strict rosette in this small plant in nature; the same accession has stem leaves in cultivation (Fig. 27). The plant in (b) has stem leaves in nature. Photographs by Maarten Christenhusz.
Nicotiana erytheia M.W.Chase & Christenh., sp. nov.
Type: Western Australia. North West Coastal Highway (1), ~8 km north of entrance to Eurardy Station, on both sides of road, 165 m, 27°30′20″S, 114°43′9″E, 21 Sep. 2019, Chase & Christenhusz 19126 (holo: PERTH; iso: CANB).
Nicotiana erytheia is closely related to N. salina and an undescribed species we have preliminarily been calling N. sp. nov. Eagle Bluff (Fig. 1a). These three species are similar in their relatively short habit with many branches from the base of the plant, but the calyx in N. erytheia does not reflex at maturity and its leaves are narrower for their length than those of N. salina (1:3 v. 1:2). The ecology and ranges of these three species distinguish them most readily (see below).
Erect, herbaceous, annual herbs, up to 80 cm tall, forming a loose rosette with many leaves on the stems, producing several thin, wiry stems simultaneously. Leaves with petioles up to 3.0 cm long with a wing up to 1.8 cm wide, blades ovate-lanceolate, 3.8–22.5 × 1.6–9.2 cm including petiole, becoming sessile on the stems, gently attenuate, the apex obtuse to acute, margins entire, ciliate, undulating. Vestiture on stem bases and major leaf veins composed of dense long, hairs that are often slightly twisted and bearing a minute gland, leaf surface densely covered with mostly shorter, gland-tipped hairs, stems progressively less woolly and more predominantly covered with shorter hairs with a prominent ellipsoidal gland and a few longer, sometimes slightly bent with a small peduncle with some longer hairs, but more commonly moderate length and shorter hairs, all with a gland, calyx with short hairs, all gland-tipped, corolla tube exterior densely covered with short, straight to slightly recurving, small gland-tipped hairs. Inflorescence bracts sessile, linear, ~0.5–1.0 cm long, the apex acuminate. Calyx 0.9–1.4 × 0.1–0.2 cm, one lobe slightly longer and one shorter, the tips acuminate, appressed to ovary to slightly flaring, 0.3–0.4 cm longer than and surrounding the fruit, slightly wider, persistent and clasping to slightly reflexed in the fruit. Corolla tube 0.3–0.8 cm long (from end of the calyx), 0.15–0.20 cm in diameter, with a slight throat cup with a pinched apex, the limb 1.1–1.5 cm across, the lobes minutely cleft, sinus 0.4 cm, deep, four stamens in stamen cup, 0.15–0.20 cm deeper than tube entrance, the fifth 0.3–0.4 cm deeper in tube. Fruit a capsule, 0.7–1.0 cm long, splitting in four lobes.
Restricted to Western Australia in the narrow transition zone from the more humid areas along the coast to the dry interior from just south of the Wooramel River to the Murchison River along the North West Coastal Highway (Fig. 19).
Named for Erytheia, one of the Hesperid nymphs of Greek mythology, who were the ‘daughters of the evening’ or ‘nymphs of the west’, a reference to the occurrence of this species in the far west of Western Australia. The species epithet is a noun in apposition.
Specimens of Nicotiana erytheia are similar to those of N. hesperis and are likely to have been confused with the latter. Both have small flowers, but they are not closely related, although both are members of the N. occidentalis clade and bear the characteristic short, gland-tipped hairs covering all parts.
Chase and Christenhusz (2021a) described N. insecticida M.W.Chase & Christenh. and noted that its geographical range was especially large, stretching from the western coast of the Pilbara Craton in Western Australia to the central Northern Territory. There are probably only a few species of N. section Suaveolentes (e.g. N. heterantha Symon & Kenneally) with such a large range. Given their generally highly inbred nature (Cauz-Santos et al. 2022), maintenance of genetic cohesion over such large distances is unlikely, and when we added many more accessions to the matrix, we saw that there is a clear geographic structure in N. insecticida that is consistent with approximately six genetically distinct species (L. A. Cauz-Santos and M. W. Chase, unpubl. data), among them material collected at the type locality of N. hesperis (Chase & Christenhusz 68258, PERTH; Fig. 1a).
In September 1959, Nancy Burbidge visited Rocky Pool, ~40 km west of Carnavon in Western Australia and collected what she designated as the type specimen of N. hesperis (Burbidge 6494A!, CANB 236744). In 2015, we visited this location and found several species of Nicotiana growing there, one of which was similar to the Burbidge’s specimen of N. hesperis. This accession (Chase & Christenhusz 68258, PERTH; Fig. 1a) is clearly distantly related to those that Chase and Christenhusz (2021c) ascribed to N. hesperis from sites further south along the North West Coastal Highway. Furthermore, this accession appears to be highly introgressed with another species of the N. insecticida species complex, and if this is the case then the type material of N. hesperis collected by Burbidge in 1959 may also have been a hybrid or introgressed individual. In 2023, M. W. Chase, M. J. M. Christenhusz and L. A. Cauz-Santos made a return trip to Rocky Pool and collected another plant similar to the type specimen of N. hesperis, which we plan to examine genetically. We also travelled further east in the Gascoyne River basin, as far east as the Great Northern Highway, and we collected several other small-flowered accessions that are like those at Rocky Pool. We think these additional accessions also correspond to N. hesperis, which would make this a widespread species, but confined to this river basin further north (120 km) of the area where N. erytheia occurs (Fig. 29), starting at the Wooramel River. We have found no material of N. erytheia further inland than the transition zone along the coastal plain and the inland scrub between the Murchison and Wooramel rivers.
A study of the species limits in the larger N. occidentalis clade is underway (L. A. Cauz-Santos and M. W. Chase, unpubl. data), and these more detailed analyses will include greater sampling of accessions. In this paper, we have shown that the accessions of Chase and Christenhusz (2021c) included in N. hesperis, except for that from the Rocky Pool type locality, are members of what we describe here as N. erytheia.
The three closely related species of the N. occidentalis complex, including N. erytheia (Fig. 1a), are similar morphologically and most readily distinguished by their ecologies and distribution. These three are Nicotiana sp. nov. Eagle Bluff, which is found in exposed sites in the Tamala eolianite limestone heathlands on the offshore Abrolhos Islands and the coast from just north of Geraldton north to Lake Macleod, N. salina, associated with salt lakes and rivers in the interior from Lake Weelhamby south to Jibberding and east to the Goldfields, where it grows near the salt vegetation but not directly in it, and N. erytheia, found under mulga and other acacias in a narrow, near-coastal range from the Murchison River just to the south of the Wooramel River. Specimens of N. erytheia collected near the Shark Bay Road (Chase & Christenhusz 68273, 68274) are much laxer in habit, with primarily cleistogamous flowers (at least in cultivation).
WESTERN AUSTRALIA. Hamelin Pool Road off Shark Bay Road, 10 m, 26°24′11″S, 114°9′59″E, 22 Aug. 2015, Chase & Christenhusz 68273 (PERTH); Hamelin Pool Road off Shark Bay Road, 10 m, 26°24′11″S, 114°9′59″E, 22 Aug. 2015, Chase & Christenhusz 68274 (PERTH); North West Coastal Highway, 2 km south of Billabong Roadhouse, 150 m, 26°50′37″S, 114°37′29″E, 22 Aug. 2015, Chase & Christenhusz 68276 (PERTH); North West Coastal Highway, south of Nerren Nerren,190 m, 27°8′46″S, 114°37′11″E, 22 Aug. 2015, Chase & Christenhusz 68277 (PERTH); North West Coastal Highway, bridge over the Murchison River, south-eastern side of bridge, 180 m, 27°49′40″S, 114°41′19″E, 22 Aug. 2015, Chase & Christenhusz 68278 (PERTH); North West Coastal Highway, opposite road to Eurardy Station, verge on eastern side of road, 245 m, 27°34′31″S, 114°42′3″E, 19 Sep. 2019, Chase & Christenhusz 19117 (PERTH); ~0.6 km south of turnoff to Coburn Station, verge on eastern side of road, 150 m, 26°42′20″S, 114°34′24″E, 20 Sep. 2019, Chase & Christenhusz 19123 (PERTH); ~1.9 km north of Galatea Bridge on North West Coastal Highway, eastern side of road, 200 m, 27°48′39″S, 114°41′25″E, 21 Sep. 2019, Chase & Christenhusz 19127 (PERTH).
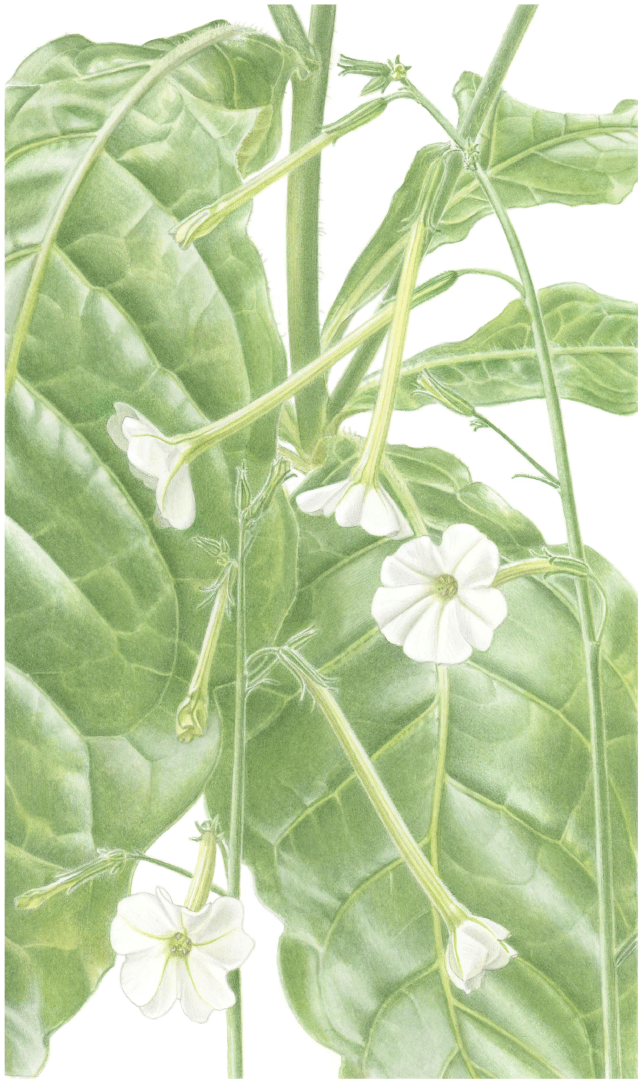
Nicotiana erytheia based on living plants cultivated at the Royal Botanic Gardens, Kew, grown from seeds associated with Chase & Christenhusz 19119, North West Coastal Highway, north of the Billabong Roadhouse, Western Australia. Painted by Deborah Lambkin (reproduced from Curtis’ Botanical Magazine with permission).

Nicotiana erytheia based on living plants cultivated at the Royal Botanic Gardens, Kew, grown from seeds associated with Chase & Christenhusz 19119, Highway 1, ~14 km north of the Billabong Roadhouse, Western Australia. (a) Corolla limb, face-on. (b) Ovary, style and stigma. (c) Corolla, split to show positions of anthers. (d) Flower, side view. (e) Upper stem. (f) Pubescence on leaf lamina. (g) Stem leaf. (h) Habit. (i) Fruit, calyx removed. (j) Mature capsule. (k) Pubescence on lower stem. Drawn by Deborah Lambkin (reproduced from Curtis’ Botanical Magazine with permission). Scale bars: 1 cm (a–d, i, j); 3 cm (g); 5 mm (e, f, k). Plant in (h) is 78 cm tall.
Conflicts of interest
Jeremy Bruhl is an Associate Editor of Australian Systematic Botany but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages its editors to publish in the journal, and they are kept totally separate from the decision-making processes for their manuscripts. The other authors declare that they have no conflicts of interest.
Declaration of funding
Financial support to prepare the RAD library and analyse data was provided by research grants P26548-B22 and P33028-B of the Austrian Science Fund (FWF) to Rose Samuel. Fieldwork was funded by honorary and visiting professorships from The University of Western Australia and Curtin University to M. W. Chase.
Acknowledgements
We thank the agencies and rangers responsible for the national parks and reserves of Western Australia, the Northern Territory, Queensland and New South Wales for issuing collecting permits in the years 2015–2019, to M. W. Chase, and for providing assistance on the ground, especially. Leaf samples of N. fatuhivensis were provided by Kenneth Wood, National Tropical Botanical Garden, Hawai’i. For removal of still viable seeds from herbarium specimens, we thank the curators of the following herbaria: AD, BRI, CANB, CNS, NSW, NT, PERTH and K. Seeds were imported to the UK and living material grown at the Royal Botanic Gardens, Kew, in the Quarantine House, with the help and advice of Sara Redstone, who is gratefully acknowledged. Herbarium specimens made from these cultivated specimens have been returned to Australian herbaria. We thank Kingsley Dixon and Lionel Johnston for hosting us in Perth. Sahr Mian in the Cytology Laboratory at the Royal Botanic Gardens, Kew, is thanked for estimating the genome size of Nicotiana bungonia. We also thank Russell Barrett and an anonymous reviewer for helpful comments that improved the quality of the paper.
References
Abbott MJ (1969) Petrology of the Nandewar Volcano, N.S.W., Australia. Contributions to Mineralogy and Petrology 20, 115-134.
| Crossref | Google Scholar |
Bally J, Marks CE, Jung H, Jia F, Roden S, Cooper T, Newbigin E, Waterhouse PM (2021) Nicotiana paulineana, a new Australian species in Nicotiana section Suaveolentes. Australian Systematic Botany 34, 477-484.
| Crossref | Google Scholar |
Burbidge NT (1960) The Australian species of Nicotiana L. (Solanaceae). Australian Journal of Botany 8, 342-380.
| Crossref | Google Scholar |
Cauz-Santos LA, Dodsworth S, Samuel R, Christenhusz MJM, Patel D, Shittu T, Jakob A, Paun O, Chase MW (2022) Genomic insights into recent species divergence in Nicotiana benthamiana and natural variation in Rdr1 gene controlling viral susceptibility. The Plant Journal 111, 7-18.
| Crossref | Google Scholar |
Chase MW, Christenhusz MJM (2018a) Nicotiana karijini. Curtis’s Botanical Magazine 35, 228-236.
| Crossref | Google Scholar |
Chase MW, Christenhusz MJM (2018b) Nicotiana gascoynica. Curtis’s Botanical Magazine 35, 245-252.
| Crossref | Google Scholar |
Chase MW, Christenhusz MJM (2018c) Nicotiana stenocarpa. Curtis’s Botanical Magazine 35, 318-327.
| Crossref | Google Scholar |
Chase MW, Christenhusz MJM (2021a) Nicotiana insecticida. Curtis’s Botanical Magazine 38, 350-364.
| Crossref | Google Scholar |
Chase MW, Christenhusz MJM (2021b) Nicotiana pila. Curtis’s Botanical Magazine 38, 394-404.
| Crossref | Google Scholar |
Chase MW, Christenhusz MJM (2021c) Nicotiana hesperis. Curtis’s Botanical Magazine 38, 405-415.
| Crossref | Google Scholar |
Chase MW, Christenhusz MJM (2021d) Nicotiana megalosiphon. Curtis’s Botanical Magazine 38, 425-434.
| Crossref | Google Scholar |
Chase MW, Conran JG, Christenhusz MJM (2018a) Nicotiana yandinga. Curtis’s Botanical Magazine 35, 237-244.
| Crossref | Google Scholar |
Chase MW, Conran JG, Christenhusz MJM (2018b) Nicotiana faucicola. Curtis’s Botanical Magazine 35, 253-260.
| Crossref | Google Scholar |
Chase MW, Dodsworth S, Christenhusz MJM (2021a) Nicotiana walpa. Curtis’s Botanical Magazine 38, 298-308.
| Crossref | Google Scholar |
Chase MW, Fay MF, Christenhusz MJM (2021b) Nicotiana salina. Curtis’s Botanical Magazine 38, 416-424.
| Crossref | Google Scholar |
Chase MW, Fay MF, Nollet F, Christenhusz MJM (2021c) Nicotiana notha. Curtis’s Botanical Magazine 38, 340-349.
| Crossref | Google Scholar |
Chase MW, Palsson RL, Christenhusz MJM (2021d) Nicotiana hoskingii. Curtis’s Botanical Magazine 38, 365-373.
| Crossref | Google Scholar |
Chase MW, Przeslawski RA, Falvey LE, Fay MF, Christenhusz MJM (2021e) Nicotiana murchisonica. Curtis’s Botanical Magazine 38, 383-393.
| Crossref | Google Scholar |
Chase MW, Dodsworth S, Christenhusz MJM (2021f) Nicotiana ingulba. Curtis’s Botanical Magazine 38, 309-318.
| Crossref | Google Scholar |
Chase MW, Christenhusz MJ, Palsson RL, Fay MF, Dodsworth S, Conran JG, Cauz‐Santos LA, Nollet F, Samuel R, Paun O (2021g) Species delimitation in Nicotiana sect. Suaveolentes (Solanaceae): reciprocal illumination leads to recognition of many new species. Curtis’s Botanical Magazine 38, 266-86.
| Crossref | Google Scholar |
Chase MW, Cauz-Santos LA, Dodsworth S, Christenhusz MJM (2022a) Taxonomy of the Australian Nicotiana benthamiana complex (Nicotiana section Suaveolentes; Solanaceae): five species, four newly described, with distinct ranges and morphologies. Australian Systematic Botany 35, 345-363.
| Crossref | Google Scholar |
Chase MW, Samuel R, Leitch AR, Guignard MS, Conran JG, Nollet F, Fletcher P, Jakob A, Cauz-Santos LA, Vignolle G, Dodsworth S (2022b) Down, then up: non-parallel genome size changes and a descending chromosome series in a recent radiation of Australian allotetraploid plant species, Nicotiana section Suaveolentes (Solanaceae). Annals of Botany 131, 123-142.
| Crossref | Google Scholar |
Clarkson JR, Symon DE (1991) Nicotiana wuttkei (Solanaceae), a new species from north-eastern Queensland with an unusual chromosome number. Austrobaileya 3, 389-392 Available at https://www.jstor.org/stable/41738779.
| Google Scholar |
Clarkson JJ, Dodsworth S, Chase MW (2017) Time-calibrated phylogenetic trees establish a lag between polyploidisation and diversification in Nicotiana (Solanaceae). Plant Systematics and Evolution 303, 1001-1012.
| Crossref | Google Scholar |
Horton P (1981) A taxonomic revision of Nicotiana (Solanaceae) in Australia. Journal of the Adelaide Botanical Garden 3, 1-56 Available at https://www.jstor.org/stable/23872354.
| Google Scholar |
Marks CE (2010) Definition of South Pacific taxa of Nicotiana section Suaveolentes (Solanaceae). Muelleria 28, 74-84.
| Crossref | Google Scholar |
Marks CE, Newbigin E, Ladiges PY (2011) Comparative morphology and phylogeny of Nicotiana section Suaveolentes (Solanaceae) in Australia and the South Pacific. Australian Systematic Botany 24, 61-86.
| Crossref | Google Scholar |
Schiavinato M, Marcet-Houben M, Dohm JC, Gabaldón T, Himmelbauer H (2020) Parental origin of the allotetraploid tobacco Nicotiana benthamiana. The Plant Journal 102, 541-554.
| Crossref | Google Scholar |
Symon DE (1984) A new species of Nicotiana (Solanaceae) from Dalhousie Springs, South Australia. Journal of the Adelaide Botanical Garden 7, 117-121 Available at https://www.jstor.org/stable/23874136.
| Google Scholar |
Symon DE (1998) A new Nicotiana (Solanaceae) from near Coober Pedy, South Australia. Journal of the Adelaide Botanical Garden 18, 1-4 Available at https://www.jstor.org/stable/23874102.
| Google Scholar |
Symon DE, Kenneally KF (1994) A new species of Nicotiana (Solanaceae) from near Broome. Western Australia. Nuytsia 9, 421-425.
| Crossref | Google Scholar |
Symon DE, Lepschi BJ (2007) A new status in Nicotiana (Solanaceae): N. monoschizocarpa (P.Horton) Symon & Lepschi. Journal of the Adelaide Botanical Garden 21, 92.
| Google Scholar |
Wheeler HM (1935) Studies in Nicotiana. II. A taxonomic survey of the Australasian species. University of California Publications in Botany 18, 45-68.
| Google Scholar |