Phylogenetic and biogeographic insights into the reproductive evolution and taxonomy of Australasian Teucrium (Lamiaceae)
Trevor C. Wilson A * and Elizabeth A. James
A * and Elizabeth A. James  B
B
A
B
Abstract
Reproductive systems in Lamiaceae typically consist of bilabiate zygomorphic flowers and dry mericarp fruit, therefore examining deviations from this strategy contributes to our understanding of evolution. Teucrium L. (Lamiaceae: Ajugoideae) is easily recognised by the unilabiate flower. The genus is cosmopolitan but most speciose in the Mediterranean region. Previous phylogenetic analysis showing three segregate genera nested within Teucrium raises questions about the taxon’s dispersal throughout the region and the distinctive morphological changes. We conducted phylogenomic analyses of nuclear genes sequenced by targeted enrichment (Angiosperms353) for all Australasian species, phrase names and outgroups (39 total). Results show high support for most species clades and unequivocally place most ‘unknowns’ in context with other described taxa. Australasian Teucrium constitute at least two distantly related clades. The most speciose, with a crown age of 10.2–14.8 Ma, is widespread across Australia and includes an arid-adapted lineage that evolved actinomorphic corollas and fleshy fruits. The second clade of three eastern Australian species has a crown age of 5.9–12.2 Ma that corresponds best with dispersal associated with the Sunda–Sahul collision. Our results highlight repeated colonisation of Teucrium in Australia and corresponding shifts towards animal dispersal that took place as early as c. 9.2 Ma.
Keywords: Ajugoideae, Angiosperms353, dispersal, GAP, Genomics for Australian Plants, HybPiper, morphological adaptation, target bait capture sequencing.
Introduction
Teucrium L. (Lamiaceae: Ajugoideae) is a diverse, widespread genus of ~250 species of herbs and shrubs, and has been an important source of remedial extracts throughout recorded history (Harley et al. 2004; Ruiters et al. 2016; Jaríc et al. 2020; Zlatić and Stanković 2020; Candela et al. 2021). The genus has a high number of species (~78%) in the general Mediterranean region yet the distribution is cosmopolitan and reaches across all continents of the southern hemisphere (Greuter et al. 1986; Navarro and El Oualidi 2000; Harley et al. 2004; Navarro 2020).
The most recent global taxonomic treatment of Teucrium was completed by Kästner (1978, 1989) and, although a later morphological analysis by Cantino (1992) shows discrepancies with this and earlier treatments, a phylogenetic analysis of chloroplast and nuclear markers shows how this and other taxonomic treatments do not correspond with evolutionary history (Salmaki et al. 2016). Teucrium is divided into two lineages referred to as the ‘core’ and ‘polium’ clades, hereafter referred to as the Teucrium core clade and Teucrium polium clade respectively. The Teucrium core clade is distinguished from the Teucrium polium clade in having a unique combination of phytochemical compounds, a radial calyx shape (cf. bilateral), a textured mericarp surface (cf. smooth) and a personate corolla rather than a non-personate corolla (Harborne et al. 1986; Kästner 1989; Marin et al. 1994; Navarro and El Oualidi 1999). The Teucrium core clade is made up of three distinct subclades, the most speciose clade consisting of taxa from the Mediterranean and adjacent areas, a second from the Americas, and a third from Australasia (i.e. Australia, New Zealand, New Guinea and Melanesia) and southern Africa. The distribution of the Teucrium polium clade overlaps somewhat with the distribution of the Teucrium core clade in the Americas, Australasia and southern Africa but has fewer species (Ryding 1998; Salmaki et al. 2016).
One of the most profound findings of the study by Salmaki et al. (2016) was that previously recognised segregate genera (i.e. Oncinocalyx F.Muell., Spartothamnella Briq. and Teucridium Hook.f.) were embedded within a clade of Australian and South African Teucrium species, and subsequently these were synonymised as Teucrium. Reproductive morphological characteristics were originally used to describe these genera, with Oncinocalyx distinguishable by hooked calyx lobes, Spartothamnella by indehiscent drupelets and Teucridium by a non-gynobasic style. Together with other species from Australasia and southern Africa, these form ‘clade 1A’ (sensu Salmaki et al. 2016) that is sister to the remainder of the Teucrium core clade. Little was interpreted about relationships within this group due to poor resolution and because only 8 of the 26 Australasian species were sampled.
Cantino (1992) speculated that Teucrium was Australian in origin due to the higher phylogenetic diversity contributed through the recognition of the genera Oncinocalyx, Spartothamnella and Teucridium. However, the nested position of these formerly segregate genera within Teucrium, alongside the determination that Schnabelia Hand.-Mazz. and Rubiteucris Kudô form a sister clade, provides more confidence that Teucrium originated from south-eastern Asia (Salmaki et al. 2016; Xiang et al. 2018). Divergence time estimation using primary and secondary calibrations (Drew and Sytsma 2012; Roy and Lindqvist 2015; Yao et al. 2016) indicated that clade 1A arose during the middle to late Miocene. The Australian flora is a mixture of vicariant lineages that have evolved in isolation along with dispersals largely during the Sunda–Sahul interchange (Crayn et al. 2015). Long distance dispersals have also occurred through other routes to or from Africa (e.g. Baum et al. 1998), South America (e.g. clusioid species, Ruhfel et al. 2016) and Asia (e.g. Holzmeyer et al. 2023) by means of bats, birds, water and wind. Although Salmaki et al. (2016) commented on the entry or dispersal and some morphological evolution of Teucrium throughout Australasia, these authors could not be absolute given sampling limitations in the study.
Knowledge about the reproductive strategy of Teucrium is scant and relies largely on examination of northern hemisphere species. Teucrium is generally considered to be pollinated by bees (Stebbins 1970; Faegri and van der Pijl 1979; Navarro and El Oualidi 1999; Westerkamp and Classen-Bockhoff 2007; Brundrett et al. 2024), although observations have only been made for a select few species showing visitation by hymenopterans (van der Pijl 1972; Petanidou 1996) and, for a single species of Teucrium, birds (Mittelbach et al. 2015; Rodríguez-Sambruno et al. 2024). Most species have the zygomorphic corolla common among Lamiaceae; however, the corolla is characteristically unilabiate (i.e. ‘one lipped’) with the abaxial corolla lobe greatly enlarged with respect to the adaxial and lateral lobes (e.g. Fig. 1e). This symmetry, in concert with the positioning of the overarching stigma and stamen, assists with dorsal pollen deposition, a mechanism that is recognised for precision and economy of resources (Westerkamp and Classen-Bockhoff 2007). Gynodioecy, another mechanism that reduces pollen wastage, is also reported for many species in the genus (Navarro and El Oualidi 1999; Merrett 2005; Walsh and O’Brien 2013). Furthermore, the reproductive system appears to be the plesiomorphic state given that the same characteristics are common throughout the rest of the Ajugoideae.
Diversity of Teucrium in Australia. (a) T.C.Wilson 979. (b) T.C.Wilson 631. (c) T.C.Wilson 365. (d) No associated voucher. (e) T.C.Wilson 908. (f) E.Wajon 3396. (g, h) No associated voucher. (i) E.Wajon 4226. (j) No associated voucher. (a) T. argutum from Camden, NSW. (b) T. corymbosum from Bungonia National Park, NSW. (c) T. fililobum subsp. fililobum from south of Norseman, WA. (d) T. sessiliflorum from Murrayville, Vic. (e) T. racemosum from east of Wilcannia, NSW. (f) T. eremaeum from Forrestania, WA. (g) T. myriocladum 33 km north of Cascade, WA. (h) T. betchei from Goulburn River National Park, NSW. (i) T. teucriiflorum from Mount Magnet, WA. (j) T. junceum in cultivation, Australian Botanic Gardens, NSW. Photographs: T. C. Wilson (a, b, c, e, j), A. Carle (CC BY-NC 4.0) (d), W. Archer (g), E. Wajon (f, i), A. N. Rodd (h).
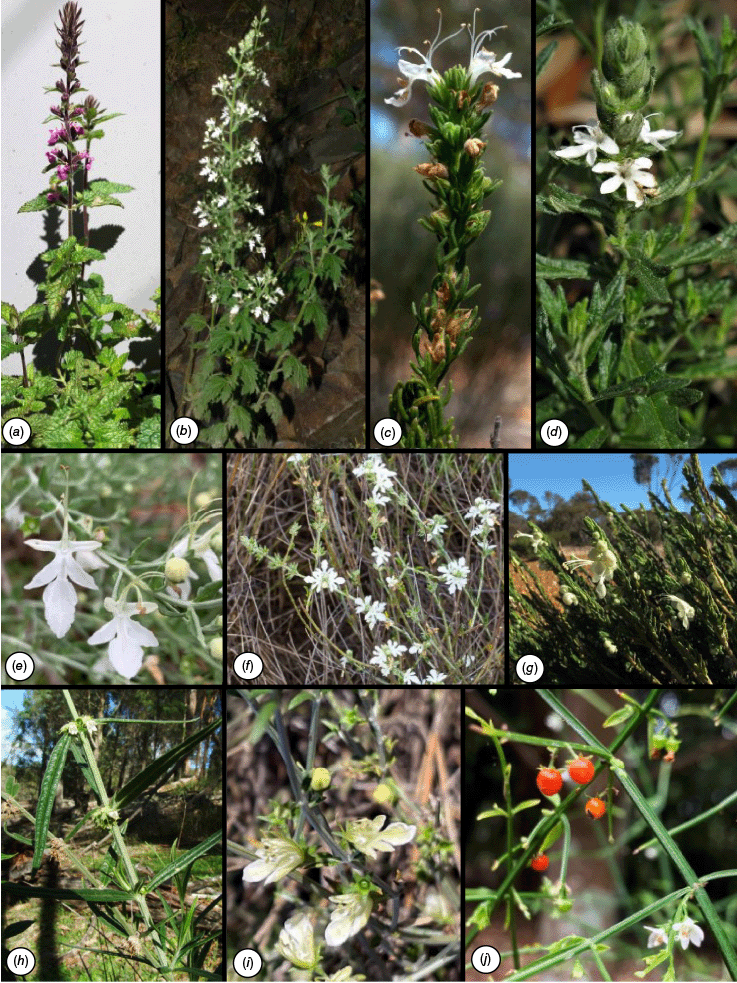
The fruit of Teucrium, typically a mericarp, is also the most common fruit type across the Lamiaceae (Bouman and Meeuse 1992; Ryding 1995; Harley et al. 2004; Zhao et al. 2021). In the absence of a more focused morphological examination across the phylogeny, the mericarp appears to be the ancestral fruit type. The mericarp is considered to be a component of a generalist dispersal syndrome, assisting a wide number of associations such as myrmecochory, barochory, hydrophily and zoochory (Blüthgen et al. 2007). Although the fruit lacks distinctive specialised structures, long-distance dispersal may be possible because the fruit has a sclerotinous layer for protection in the gut of animals (Bonn 2004; Harley et al. 2004; Vazačová and Münzbergová 2014; Zona 2017). However, the fleshy drupelets found in some Australian Teucrium also suggest specialisation towards endozoochory, likely appealing to birds due to the relatively small size (<5 mm in diameter) and usually orange–red colour (Mazer and Wheelwright 1993; Benson and McDougall 2004; Duan et al. 2014; Valenta and Nevo 2020; Lei et al. 2021). Furthermore, the Australian T. betchei (F.Muell.) Kattari & Salmaki) has hooked calyx lobes that enable adherence to animals, an epizoochorous adaptation infrequently found in other Lamiaceae (Harley et al. 2004).
Recently, consortia such as the Plant and Fungal Tree of Life (PaFToL) and Genomics for Australian Plants (GAP) have precipitated a more standardised use of target bait capture sequencing using the Angiosperms353 (A353) kit (e.g. Johnson et al. 2019; Joyce et al. 2024; Simpson et al. 2024), and have been pivotal in producing the most comprehensive angiosperm tree of life (Zuntini et al. 2024). The application of A353 sequencing towards species-level phylogenetic investigations is now on the rise (Baker et al. 2021) and has been used to resolve interspecific relationships within the Lamiaceae (e.g. Satthaphorn et al. 2023); however, the usefulness thereof at the subspecies level is less clear based on studies in other families (Slimp et al. 2021).
We sought to use target bait capture sequencing with A353 to achieve a more robust estimation of relationships for 26 recognised Teucrium species and three phrase names in Australia and New Zealand, to test species hypotheses and gain a more thorough understanding of historical dispersal and adaptive change. Including dates from a recent phylogenetic analysis of Lamiaceae (Rose et al. 2022), we also provide an estimate of divergence times to pinpoint the background behind Teucrium’s history of dispersal in Australasia coinciding with morphological evolution. Our goals were to: (1) resolve the phylogenetic relationship between all Australian Teucrium species and placement in the broader phylogeny of the genus; (2) test the validity of species and phrase names; (3) estimate divergence times for Australasian Teucrium; and (4) identify patterns of morphological evolution.
Materials and methods
Taxon sampling and data acquisition
Sampling included all 26 known Australasian Teucrium species and three Australian phrase names (Western Australian Herbarium’s Florabase, Department of Biodiversity, Conservation and Attractions, see https://florabase.dbca.wa.gov.au/, accessed 16 August 2024; Royal Botanic Gardens and Domain Trust’s PlantNET, see https://plantnet.rbgsyd.nsw.gov.au, accessed 29 January 2025). Three non-Australasian representatives from the Teucrium core (T. fruticans L. and T. laciniatum Torr.) or Teucrium polium (T. chamaedrys L.) clades were sampled to recreate the broad Teucrium phylogenetic framework reported by Salmaki et al. (2016). Multiple samples were included for most Australasian taxa to account for infraspecific morphological variation and wide geographic distribution (Fig. 2). This included within-population replicate sampling for T. junceum (A.Cunn. ex Walp.) Kattari & Heubl at the southern (Belford) and central (Berrigal) points of distribution to capture detail about within-species resolution. Sequences for 10 other species across the Teucrieae Dumort., Clerodendreae Briq. and Ajugeae Benth. were downloaded from the European Nucleotide Archive (ENA, see www.ebi.ac.uk) and Biocommons dataportal (Gustafsson et al. 2023) for use as an outgroup. The total number of samples used in this study was 115, of which 105 were newly sequenced (Supplementary Table S1).
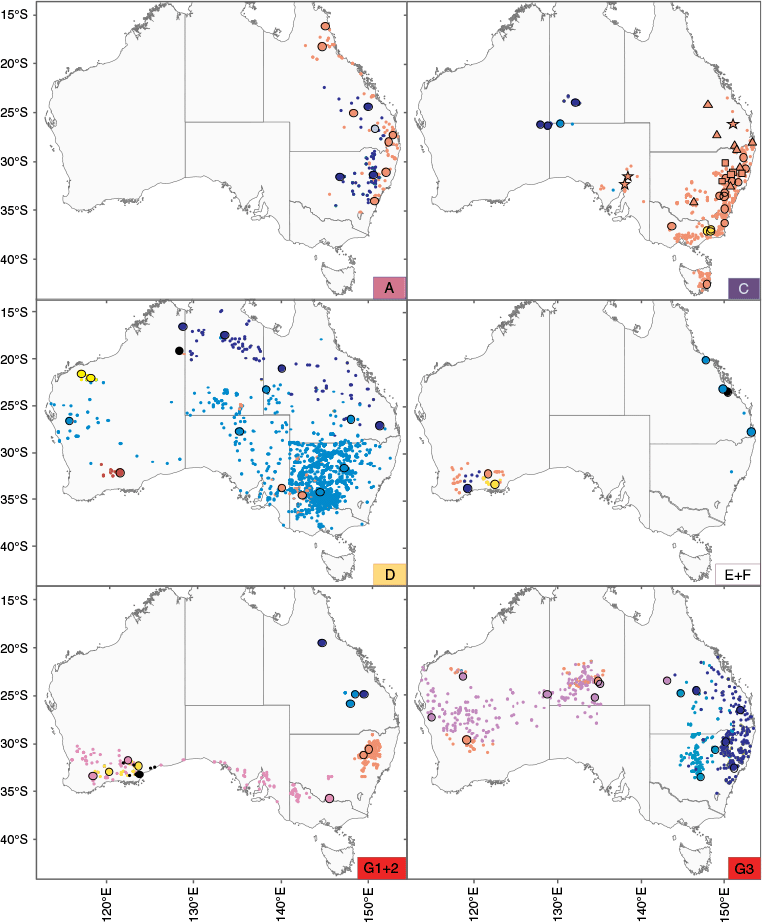
Distribution maps for Australian Teucrium species grouped by phylogenetic clades shown in Fig. 4 (corresponding clade labels provided in bottom right corner). Distributions are generated from Atlas of Living Australia data (see http://www.ala.org.au, accessed 20 May 2024), with larger outlined circles identifying the locations of specimens sampled in this study. Clade A: T. argutum, red; T. daucoides, dark blue; T. sagittatum, light blue. Clade C: T. corymbosum sens. lat., light red (T. corymbosum sens. str. Clade C2, circle; T. corymbosum Clade C1, triangle; T. corymbosum unresolved, star; T. sp. D, square); T. grandiusculum, dark blue; T. reidii, light blue; T. thieleanum, yellow. Clade D: T. albicaule, light red; T. pilbaranum, yellow; T. integrifolium, dark blue; T. diabolicum, dark red; T. racemosum, light blue; T. sp. Sturt Creek (A.A.Mitchell 5536), black. Clade E + F: T. fililobum subsp. fililobum, light red; T. fililobum subsp. glandular (W.Rogerson 233), dark blue; T. myriocladum, yellow; T. irroratum, black; T. modestum, light blue. Clade G1 + 2: T. betchei, light red; T. eremaeum, yellow; T. fallax, light blue; T. micranthum, dark blue; T. sessiliflorum, pink; T. sp. Balladonia (K.R.Newbey 7380), black. Clade G3: T. junceum, dark blue; T. disjunctum, light red; T. puberulum, light blue; T. teucriiflorum, purple.
Extraction and sequencing
DNA extraction, library enrichment and Illumina sequencing of the captured DNA libraries were outsourced to the Australian Genomic Research Facility (AGRF). DNA was extracted from specimens collected for this study at AGRF (Adelaide, Australia) using up to 30 mg of dried plant tissue (silica dried, freeze dried or destructively sampled herbarium fragment) with the NucleoSpin Plant II Mini or 96-well kit from Macherey–Nagel. Library preparation, target sequence capture and sequencing were performed at AGRF (Melbourne, Australia) using the NEBNext Ultra II FS library prep per manufacturer’s instructions, the ‘Angiosperms 353 v1’ universal probe set (ArborBioscience, Ann Arbor, MI, USA) following Johnson et al. (2019) and an Illumina HiSeq. 2500 sequencer producing 150 base pair (bp) paired-end reads.
Bioinformatics
Sequence processing and phylogenetic analysis were performed on the NCI Australia Supercomputer (Gadi) supported by the Australian BioCommons Leadership Share (Gustafsson et al. 2023). The Nextflow script hybpiper-nf (ver. 1.0.4, see https://github.com/chrisjackson-pellicle/hybpiper-nf; Jackson et al. 2021, 2023) used the HybPiper pipeline (ver. 2.1.7, see https://github.com/mossmatters/HybPiper; Johnson et al. 2016) with default settings to assemble reads against the standard Angiosperms353 (A353) target file. The script implemented Trimmomatic (ver. 0.39, see http://www.usadellab.org/cms/index.php?page=trimmomatic; Bolger et al. 2014) to first remove adapter sequences, trim and filter raw paired-end reads with options set to ILLUMINACLIP:TruSeq. 3-PE.fa:2:30:10:2:TrueLEADING:3TRAILING:3SLIDINGWINDOW:4:20MINLEN :36. Computation time and error within the assembly process were reduced by filtering using Shannon’s information index limit with a threshold of 0.8 that has been found to remove simple repeats and homopolymers (T. Allnut, pers. comm.).
The HybPiper output of supercontigs was assessed for paralogy. Paralogous loci can potentially contribute to confounding effects in downstream phylogenetic analyses and paralogy resolution methods (also known as orthology inference) such as that described by Yang and Smith (2014) have been adapted for use with target capture datasets by Morales-Briones et al. (2022). The user-friendly paralogy resolution pipeline was deployed through the Python package ParaGone (ver. 0.0.14rc, see https://github.com/chrisjackson-pellicle/ParaGone) using the default settings in the full_pipeline function. Ajuga L., Schnabelia Hand.-Maz. and members of the Clerodendreae were set as outgroup taxa using the ‘--internal_outgroups’ flag. Paralogs were initially explored using the Rooted subTrees and Rooted Ingroups (RT) and the Monophyletic Outgroups (MO) methods that are considered to be the most appropriate for datasets with high quality outgroups and probable genome duplications (Yang and Smith 2014; Jackson et al. 2021). The results were comparable between methods, therefore only analysis using MO is presented in this study. The full ParaGone pipeline outputs resolved and aligned sequences with trimmed terminals ready for further phylogenetic inference.
Phylogenetic inference and measures of discordance
Two datasets were created: the ‘total’ dataset (115 samples) was used to test species validity and phylogenetic position of the Australasian species; and the ‘reduced’ dataset (39 samples) was used to lower computational load for concordance value and divergence time estimation. The reduced dataset consisted of a single representative for each taxon present in the total dataset. Each taxon representative was selected as the one with the highest number of genes in the HybPiper output that were at least 75% assembled length relative to the target length (Supplementary Tables S1, S2).
A robust interrogation of topology and node support was sought through the comparison of the contrasting approaches of Multispecies Coalescent Modelling (MSC) and Maximum Likelihood (ML) analysis of a concatenated dataset for species phylogeny reconstruction. MSC was completed using ASTRAL-III (ver. 5.7.8, see https://github.com/smirarab/ASTRAL; Zhang et al. 2018) to recover a species tree in the presence of potentially confounding evolutionary processes such as incomplete lineage sorting (ILS). Orthologs from the ParaGone pipeline output sequence were used to infer individual gene trees. Aligned exon matrices were first used to infer individual ML gene trees with IQ-TREE (ver. 2.1.3, see http://www.iqtree.org/; Minh et al. 2020a) using default settings. Trees were subsequently used as input to infer species trees in ASTRAL that estimates support values as a local posterior probability (LPP) score – in our results we refer to LPP scores as high (1.0), moderate (0.90–0.99), low (0.80–0.89) and no (<0.80) support. The ‘-t 4’ flag was used to explore proportion of alternative quartets and the ‘-t 10’ flag was used to test the null hypothesis that a given branch in an estimated species tree should be replaced by a polytomy (Sayyari and Mirarab 2018).
A concatenated dataset of all genes was compiled for ML analysis from the ParaGone output that was first cleaned to remove any gene alignments with fewer than three samples. Analysis of the alignment using IQ-TREE (Minh et al. 2020a) inferred 1000 UltraFast bootstraps (UFB) as a measure of support (Minh et al. 2013). For this study we refer to categories of UFB values as strong (100), weak (95–99) or having no support (<95).
In addition to LPP (ASTRAL) and UFB (IQ-TREE) measures, gene conflict and concordance were examined to understand topological conflict around each branch of the species tree through two methods. The open-source software Phyparts (ver. 0.0.1, see https://bitbucket.org/blackrim/phyparts) was used to calculate concordance on the MSC tree using the individual gene trees (Smith et al. 2015). The resulting concordant and discordant proportions were displayed on nodes of the coalescent tree with the aid of the Phyparts piecharts tool (see https://github.com/mossmatters/phyloscripts/tree/master/phypartspiecharts). Gene and site concordance factors (gCF and sCF respectively) were calculated on the ML tree with IQ-TREE using the individual gene trees and cleaned alignment (Minh et al 2020b). Under an assumption of ILS, gene trees or sites that support alternative topologies should occur with approximately equal frequency. We therefore used a Chi-Square test to examine whether discordant topological frequencies at problematic nodes were significantly different, using the gCF and sCF output from IQ-TREE.
Network inference
We tested for patterns in genetic similarity and evidence of potential genetic introgression between taxa. We compiled a FASTA dataset out of a concatenation of gene alignments from the ParaGone output using Concatenator (ver. 0.3.1, see https://itaxotools.org/download.html). To infer distance between groups, a network was constructed from the concatenated alignment using the default setting of NeighborNet method with uncorrected P-distance in SplitsTree (ver. 4.14.6, see https://github.com/husonlab/splitstree4; Huson 1998). Extensive ‘cycling’ or ‘webbing’ between branches in the network reflects underlying conflict in the data that may occur for multiple reasons, including incomplete lineage sorting and hybridisation (Huson and Bryant 2006).
Divergence time estimation
Divergence times were estimated using BEAUti (ver. 2.6.7, see https://beast.community/beauti) and BEAST (ver. 2.6.7, see https://www.beast2.org/; Drummond and Rambaut 2007; Suchard and Rambaut 2009; Suchard et al. 2018) on an alignment of the reduced dataset. Given that the computational demands for Bayesian analysis of genomic datasets can be very high, we assembled a concatenated FASTA alignment with Concatenator (ver. 0.3.1) using a set of 80 gene alignments output by the ParaGone pipeline. The set included the most clock-like genes that were selected using SortaDate (see http://github.com/FePhyFoFum/SortaDate; Smith et al. 2018) that ranks genes according to root-to-tip variance using individual gene trees and the MSC tree. The files were imported into BEAUTi to create an XML input file, applying the GTR + I + G substitution model (Abadi et al. 2019), relaxed log-normal clock model and birth-death tree prior. Secondary calibration points (95% highest posterior density, HPD) for three nodes using a uniform prior were taken from the dating analysis by Rose et al. (2022) based on five fossil calibration points within the Lamiales. Calibration points were (1) 36.2–53.2 Ma for the crown of a clade consisting of Ajugeae, Clerodendreae and Teucrieae members, (2) 23.3–45.0 Ma for the crown of the Clerodendreae clade (represented by Clerodendrum + Oxera) and (3) 7.7–22.0 Ma for the crown of the Teucrium clade (represented by T. mascatense, T. polium subsp. capitatum and T. stocksianum). Our sampling was designed to match these calibration points as follows: (1) crown of the clade consisting of Ajugeae (Ajuga reptans), Clerodendreae (C. floribundum, H. linifolia, O. nerifolia, O. splendida and V. inermis) and Teucrieae (all Teucrium and Schnabelia oligophylla); (2) Clerodendreae (C. floribundum, H. linifolia, O. nerifolia, O. splendida and V. inermis); (3) Teucrium clade (Australasian and northern hemisphere members for both Teucrium core clade and Teucrium polium clade). The three Teucrium species provided by Rose et al. (2022) support the broad phylogenetic diversity of the genus based on the recognition of either molecular or morphological data. Teucrium polium subsp. capitatum and T. stocksianum were recovered as members within the Teucrium polium clade by Salmaki et al. (2016). Although T. mascatense has not been examined in a phylogenetic context, the strong phylogenetic signal corroborated by calyx morphological characteristics by Salmaki et al. (2016) provides sound support that this is a member of the Teucrium core clade based on the radially symmetric calyx (Plants of the World Online, POWO, Royal Botanic Gardens, Kew, UK, see https://powo.science.kew.org/, accessed 9 August 2024).
Six parallel BEAST runs were performed until convergence (ESS > 200), with up to 200 million Markov Chain Monte Carlo (MCMC) generations, pre-burn-in 10 million generations, and trees sampled every 10,000 generations. All runs used a starting seed tree produced by treePL ML analysis (ver. 1.0, see http://github.com/blackrim/treePL; Smith and O’Meara 2012) and the IQ-TREE tree output with the three secondary calibrations listed above. Convergence of the posterior and other parameters was assessed using Tracer (ver. 1.7.1, see https://github.com/beast-dev/tracer/releases/tag/v1.7.1; Rambaut et al. 2018) with the first 20% of MCMC discarded as burnin. Independent runs were combined after burnin using LogCombiner (ver. 2.4.7, see https://beast.community/logcombiner), summarised in TreeAnnotator (ver. 2.7.6, see https://www.beast2.org/treeannotator/; Drummond and Rambaut 2007), as a consensus tree, which was then visualised with FigTree (ver. 1.4.4, see http://github.com/rambaut/figtree; Rambaut et al. 2018). We refer to the geological time scale from the International Commission on Stratigraphy (Walker and Geissman 2022).
Results
Dataset quality and size
The recovery of targeted A353 loci was high for Teucrium and outgroup genera. Total number of raw reads ranged between 83,294 and 29,461,504 (average 8,211,976 per taxon). After trimming and filtering, the number of loci recovered ranged from 156 to 352 per taxon (average 328 loci) or 44–99% (average 93%) and number of paralog warnings ranged between 0 and 37 (average = 8, Supplementary Table S2). In 51 of 115 specimens, at least 75% of genes were recovered with at least 75% of the total target length (Supplementary Table S2, Fig. S1). Per-locus alignments ranged between 57 and 3657 bp (mean 615 bp). A 226,974-bp FASTA dataset was compiled for the SplitsTree network using the total 347 gene alignments from the ParaGone pipeline output. Following further cleaning of the output, a concatenated dataset prepared for ML analysis of the total dataset consisted of 281 genes, and for the reduced dataset, consisted of 286 genes (Table 1). An 80-gene alignment prepared from the latter dataset for divergence time estimation was 69,448 bp long.
| Dataset | Taxa | Sites (total characters) | Parsimony informative characters | Invariant characters | Number of genes | ASTRAL normalised quartet score | |
|---|---|---|---|---|---|---|---|
| Total | 115 | 277,449 | 58,166 | 183,386 | 281 | 0.641 | |
| Reduced | 39 | 265,497 | 46,493 | 181,001 | 286 | 0.711 |
Normalised quartet scores that range from 0 to 1 are derived from ASTRAL-III analysis (Zhang et al. 2018), with higher values indicative of lower discordance between gene trees.
SplitsTree network
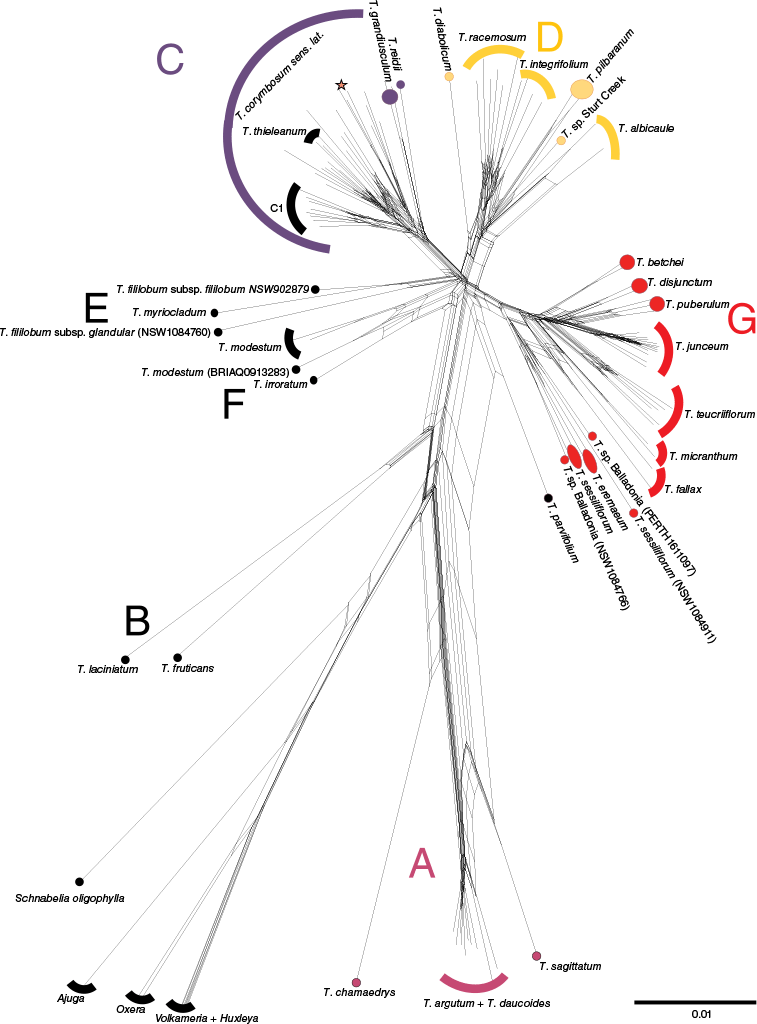
All Teucrium formed a single group with respect to the outgroup genera in the SplitsTree network (Fig. 3). Known species belonging to the Teucrium polium clade (T. argutum R.Br. and T. chamaedrys) formed a group with T. daucoides A.R.Bean and T. sagittatum A.R.Bean (Fig. 3a). All remaining Australasian Teucrium species were recovered with other representatives of the Teucrium core clade (T. fruticans and T. laciniatum) and most were organised into three subgroups C, D and G (Fig. 3). Lowest cycling occurred between either subgroups C or D and the remaining Australasian Teucrium species. Lack of structure was also prevalent throughout T. corymbosum sens. lat. in subgroup C within which T. thieleanum B.J.Conn was embedded. Within subgroup D, T. integrifolium Benth. and T. racemosum R.Br. were not resolved as distinct groups, and T. sp. Sturt Creek (A.A. Mitchell 5536) WA Herbarium was grouped with T. pilbaranum.
Relationship among species of Teucrium and outgroup genera (Ajuga, Huxleya, Oxera, Schnabelia and Volkameria) from SplitsTree network of Angiosperms353 sequence data for 115 samples (i.e. ‘total’ dataset) using the NeighborNet method with uncorrected P-distance. Teucrium forms a separate group to all other genera. Most Australasian Teucrium species grouped together with northern hemisphere relatives of the Teucrium core clade (B) and the substructure among these is similar to the topology of a corresponding Multi Species Coalescent (MSC) analysis using ASTRAL-III (Fig. 4; C, D, E, F, G). Three species of Australian Teucrium formed a group with T. chamaedrys, a northern hemisphere member of the Teucrium polium clade (A). Teucrium corymbosum sens. lat. members that formed Clade C1 of the MSC tree are identified by ‘C1’ and the three unplaced Clade C members are indicated by a red star as in Fig. 4.
Phylogenetic relationships
The MSC trees with total or reduced sampling demonstrated a near identical order of species diversification with the trees from ML analysis (Fig. 4, 5, Supplementary Fig. S2–S5). Across analyses, high LPP, concordance and UFB support were mostly associated with the same branches. Hereafter, only differences between each of these analyses are reported. Lowest discordance in gene trees, as indicated by the highest normalised quartet scores (Zhang et al. 2018; see https://github.com/smirarab/ASTRAL/blob/master/astral-tutorial.md) was found for the reduced dataset (Table 1).
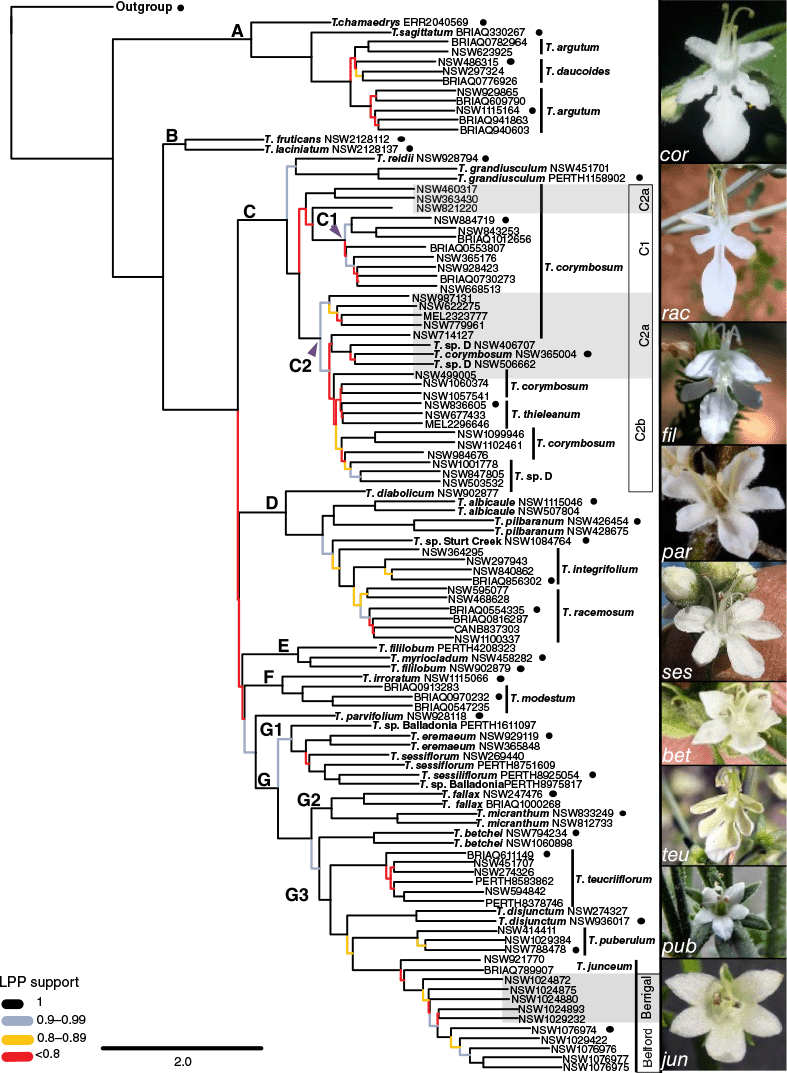
Phylogram for Teucrium recovered from Multi Species Coalescent (MSC) analysis by ASTRAL-III of the Angiosperms353 ‘total’ dataset derived from the ‘Monophyletic Outgroup’ gene tree paralogy resolution algorithm for 281 nuclear genes – the outgroup consisting of Ajugeae, Clerodendreae and Teucrieae members has been truncated for visual purposes. Nodes with ASTRAL local posterior probability (LPP) scores <1 are coloured according to legend. Internal branch lengths represent coalescent units, measuring the amount of discordance in the gene trees (branch tips have been arbitrarily drawn as a single unit since ASTRAL can only estimate lengths for branches where more than one individual has been sampled). Phylogenetic clades consistent across analyses are identified by large lettering next to the respective node. Boxes C1, C2a, C2b demonstrate the three subclades of Clade C from IQ-TREE analysis. Boxes labelled ‘Belford’ and ‘Berrigal’ indicate separate T. junceum populations consisting of five individuals each. Images on the right are representative of the floral morphological characteristics corresponding to the tree topology: bet, T. betchei from Limbri, NSW; cor, T. corymbosum from Bungonia National Park, NSW; fil, T. fililobum subsp. fililobum from south from Norseman, WA; jun, T. junceum cultivated at Australian Botanic Gardens, NSW; par, T. parvifolium cultivated material from Mount Horrible, Timaru, New Zealand; pub, T. puberulum from Kumbarilla, Qld; rac, T. racemosum from east of Wilcannia, NSW; ses, T. sessiliflorum from 24 km north-east of Cascade, WA; and teu, T. teucriiflorum from Forrestania, WA. (bet, jun, par, pub, ses, teu) no associated voucher; (cor) T.C.Wilson 631; (fil) T.C.Wilson 365; (rac) T.C.Wilson 908. Photographs: M. A. M. Renner (bet), T. C. Wilson (cor, fil, jun, rac), P. Heenan (par), M. Bennett, (CC BY-NC 4.0, pub), W. Archer (ses), E. Wajon (teu). A circle indicates representatives selected for the reduced dataset. Supplementary Table S1 provides comprehensive detail for identification, voucher numbers and sites for all specimens.
Phylogram for Teucrium recovered from Multi Species Coalescent (MSC) analysis using ASTRAL-III of the ‘reduced’-taxon Angiosperms353 dataset derived from the ‘Monophyletic Outgroup’ gene tree paralogy resolution algorithm for 286 nuclear genes. A single specimen from the total dataset was used to represent each species or notable morphological form. Nodes with local posterior probability (LPP) scores <1 are coloured according to legend. Major phylogenetic clades consistent across analyses are identified by large lettering next to the respective node. Nodes with IQ-TREE ultrafast bootstrap support <100% are indicated as an ‘X’ according to the legend. Support for relationships is based on gene tree conflict: pie charts show the fractions of supporting (above) and conflicting (below) gene trees per node calculated using Phyparts (Smith et al. 2015), with blue representing supporting gene trees, green supporting the most common alternative topology, red supporting other alternative topologies and grey representing uninformative. ASTRAL’s polytomy test P-values (Sayyari and Mirarab 2018) failing to reject a polytomy are placed to the left of the concordance values at a node.
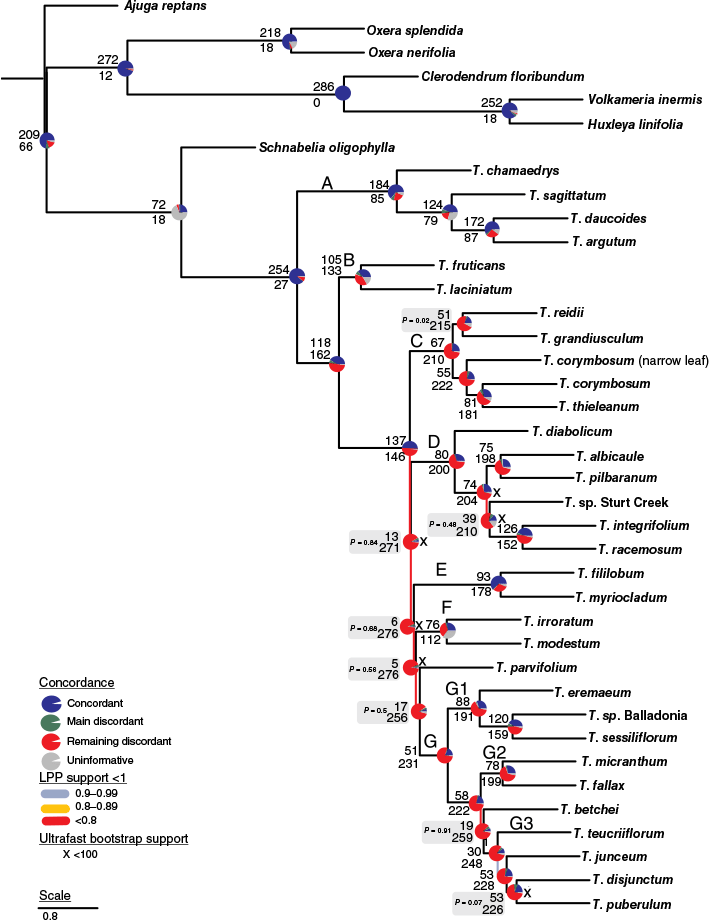
All species of Teucrium were recovered in one clade with respect to other genera (Fig. 4, 5), and formed two major clades. The first major clade (Clade A) consisted of previously recognised Teucrium polium clade members (T. argutum and T. chamaedrys), T. daucoides and T. sagittatum. The second major clade consisted of all samples belonging to the Teucrium core clade (i.e. Clades B–G), with North American and European members (T. laciniatum and T. fruticans respectively) forming a sister clade to all Australasian species (Clades C–G). Branch support and concordance values were in general highest at nodes supporting Clades C–G and lowest along the phylogenetic backbone supporting these. In the MSC tree, concordance values at the node supporting T. parvifolium and Clade G, and at nodes supporting the phylogenetic backbone of Clades D, E, F and G, were equal to or less than the main discordant topology (Fig. 5). Nodes supporting the order of these four clades and T. parvifolium were associated with a failure to reject a true polytomy and nearly equal values distributed among the main and alternative quartet topologies (Supplementary Fig. S4). A Chi-Square test using concordance factors produced by IQ-TREE failed to reject the model of independent lineage sorting for these and most other nodes (Supplementary Table S3). The most pronounced difference in phylogenetic order of these clades between MSC and ML trees was the placement of T. betchei that was recovered as sister to Clade G3 by most analyses (Fig. 4, 5, Supplementary Fig. S3) except ML analysis of the reduced dataset (Supplementary Fig. S5), where this was recovered as sister to Clade G2.
In the Teucrium polium clade (Clade A), T. sagittatum was recovered as sister to a T. argutum–T. daucoides clade, the internal structure of which varied between the MSC and ML trees with nearly all branches lacking support (Fig. 4, Supplementary Fig. S2, S3). Neither T. argutum nor T. daucoides was recovered in a single clade; however, T. argutum from south of the Carnarvon Ranges in southern Queensland was consistently recovered as a clade with high support in both analyses. In the MSC tree, this southern clade was sister to a clade consisting of T. argutum from farther north in Queensland (BRI AQ782964 and NSW 623925) and all samples of T. daucoides; however, this was provided with no LPP support.
The composition of Clade C included four recognised species of Teucrium and the phrase name T. sp. D Flora of New South Wales (S.A.Horton 4114) NSW Herbarium. The central western species T. reidii Toelken & D.Dean Cunn. and T. grandiusculum F.Muell. & Tate were recovered as sister to a clade of T. corymbosum, T. sp. D and T. thieleanum. In the ML tree (Supplementary Fig. S3), this clade branched as three discrete clades (C1, C2a and C2b). Clade C1 included T. corymbosum from the west side of the Great Dividing Range, and BRI AQ1012656 from south-east Queensland. Clade C2 included members distributed across eastern Australia and along the Great Dividing Range (Fig. 2). In the coalescent tree, Clade C2 lacked the split into Clades C2a and C2b, and three species belonging to C2a (NSW 363430 and NSW 460317 from Flinders Range, NSW 821220 from southern Queensland) were recovered with Clade C1, albeit without support (Fig. 4, Supplementary Fig. S2, S3). Branch support was mostly low for most nodes within Clade C for both analyses, including for a clade of T. thieleanum within Clade C2b. However, there was high support separating samples of Teucrium sp. D between Clades C2a and C2b, including some that shared geographic proximity with one another (e.g. NSW 406707 from the type locality of Moonbi was not recovered with other samples collected nearby, such as NSW 503532 from Duri Mountain).
Teucrium albicaule Toelken, T. diabolicum R.W.Davis & Wege, T. integrifolium, T. pilbaranum B.J.Conn, T. racemosum and T. sp. Sturt Creek were recovered as Clade D (Fig. 4, Supplementary Fig. S2, S3). A single sample of the south-western T. diabolicum was recovered as sister to the remaining samples in Clade D. Although most samples of T. integrifolium formed a single clade, the taxon was not recovered as monophyletic because a sample (NSW 364295) from the west of the Northern Territory was sister to the T. integrifolium–T. racemosum clade. A difference between analyses was that the MSC tree recovered T. sp. Sturt Creek in a clade with the T. integrifolium–T. racemosum clade with moderate LPP (Fig. 4, 5) but there was no support in the ML analysis of the concatenated dataset to identify T. sp. Sturt Creek as sister to T. pilbaranum (Supplementary Fig. S3). A test to reject a polytomy failed at the node supporting the placement of T. sp. Sturt Creek in the reduced dataset (Fig. 5).
Clade E consisted of T. fililobum Benth. and T. myriocladum Diels, both restricted to south-western Australia with the latter rendering the former as paraphyletic (Fig. 4). Clade F consisted of the eastern Australian T. modestum A.R.Bean and T. irroratum A.R.Bean, for which multiple samples of the former were recovered in one clade (Fig. 4).
Clade G was composed of T. betchei and subclades G1, G2 and G3 (Fig. 4, 5). Subclade G1 consisted of the mostly south-western distributed T. eremaeum Diels, T. sessiliflorum Benth. and T. sp. Balladonia (K.R. Newbey 7380). The latter two taxa were recovered as paraphyletic because one sample of T. sp. Balladonia (NSW 8975817) rendered T. sessiliflorum paraphyletic, whereas the second sample (PERTH 1611097) was recovered as sister to the remaining Clade G1. All constituent species in subclades G2 and G3 were recovered as monophyletic, with the former consisting of T. micranthum B.J.Conn and T. fallax A.R.Bean, and the latter branching as T. teucriiflorum (F.Muell.) Kattari & Salmaki, T. disjunctum K.R.Thiele & K.A.Sheph, and subsequently T. puberulum (F.Muell.) Kattari & Bräuchler and T. junceum. Populations of T. junceum collected from Berrigal and Belford (both New South Wales) were recovered as distinct from the samples located farther north in Queensland with high branch support yet only replicates of the Belford population formed a distinct clade with high branch support (Fig. 4, Supplementary Fig. S2, S3).
Divergence times of Teucrium
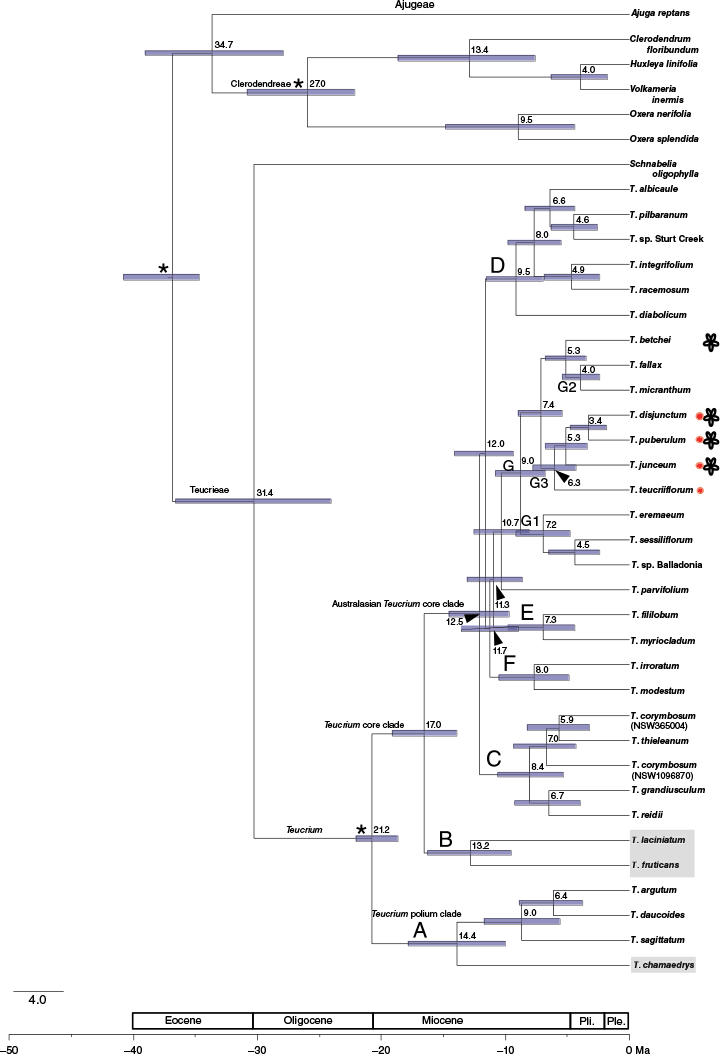
BEAST analysis of the 80-gene reduced dataset (Fig. 6, Supplementary Fig. S6) produced a similar tree topology and support values from corresponding analysis of the 286-gene dataset. Re-analysis of the 80-gene reduced dataset using ASTRAL and IQ-TREE also provided nearly similar trees to the full data counterparts, although T. irroratum was recovered with T. parvifolium, T. modestum was recovered as sister to Clade C, and the order between C, D and E was changed (results not shown). BEAST estimates from analysis including Lamiaceae and Nepetoideae constraints estimated a crown age of the Teucrium polium clade at 10.7–18.3 Ma (95% HPD) and a crown age of the clade including Australasian members at 5.9–12.2 Ma (95% HPD). The crown age of the Australasian Teucrium core clade was estimated at 10.2–14.8 Ma (95% HPD), diverging (stem) from American and Middle Eastern lineages (T. laciniatum and T. fruticans) at 14.4–19.2 Ma (95% HPD). Within the Australasian Teucrium core clade, Clade D had the oldest crown age of all named clades at 7.4–11.9 Ma (95% HPD); however, the crown node of Clade C mostly overlapped. The crown of subclade G2 + G3 and T. betchei was 5.7–9.2 Ma (95% HPD), having diverged (stem) from subclade G1 7.2–11.1 Ma (95% HPD).
BEAST chronogram of Teucrium with outgroups Ajuga, Clerodendrum, Huxleya, Oxera, Schnabelia and Volkameria. Non-Australasian Teucrium species are indicated in a grey box: T. chamaedrys is a member of the Teucrium polium clade from the Mediterranean; T. fruticans and T. laciniatum are members of the Teucrium core clade from the Mediterranean and North America respectively. Age (Ma) is indicated at all nodes above the bar (unless guided by arrow) indicating minimum to maximum age range. Red circle indicates species with a drupelet fruit, floral diagram indicates species with a corolla more radially symmetric relative to most Teucrium. Major phylogenetic clades consistent across analyses of this study are identified by large lettering next (to the left) to the respective node. Abbreviations: Ple., Pleistocene; Pli., Pliocene.
Discussion
We provide the first comprehensive molecular phylogeny of Teucrium in Australasia. The evolutionary relationships within Teucrium are complex but several taxonomic quandaries have recently become more tractable by using molecular methods in conjunction with morphology (Salmaki et al. 2016). Although the most recent study of relationships within Teucrium by Salmaki et al. (2016) provided a sound phylogenetic framework through generic realignment involving southern hemisphere taxa, only a small number of Australian species was included, limiting our understanding of the genus in this part of the world. In a bid to determine species boundaries and the evolutionary history of Teucrium in Australia, we have constructed an improved phylogeny using the A353 bait set data from a comprehensive sampling of all Australian taxa. Here we discuss the relationships and intriguing patterns revealed about the diversification, historical biogeography and evolution of reproductive processes within Teucrium in Australasia.
A major outcome of our study is the placement of all Australasian Teucrium species within the greater phylogenetic framework of Teucrium and a substantially more resolved understanding of relationships. We identify that the Teucrium core clade (sensu Salmaki et al. 2016) forms most of the diversity for Teucrium across Australasia – subsequent to the arrival and preliminary diversification, there have been shifts in pollination strategy and seed dispersal that are unique within the genus. We also confirm the somewhat understated result of at least one separate Teucrium lineage in Australia (Teucrium polium clade, sensu Salmaki et al. 2016) that is restricted to eastern Australia and represented by three extant species that appear to have arrived in Australia through a different route than the Teucrium core clade.
Australian Teucrium are two distantly related lineages
Overall, there was high support in the structuring of several large clades of Australasian Teucrium species (Fig. 4). The deepest structuring in the phylogeny provides renewed support for key morphological characters distinctive of the Teucrium core clade and Teucrium polium clade, and the breadth of morphological diversity in Teucrium across Australasia. Building on previous phylogenetic inference (Salmaki et al. 2016), our results show that most Australian and New Zealand Teucrium species belong to the Teucrium core clade. All Australasian specimens conform with two of the three previously diagnosed synapomorphies of the Teucrium core clade (i.e. actinomorphic calyx and rough-textured mericarps) but no samples of the Teucrium core clade used in this study, including non-Australian taxa, have a personate corolla (i.e. no pouch or closed labellae), a character originally reported following an exclusive examination of Mediterranean species (Navarro and El Oualidi 1999; Salmaki et al. 2016).
We show that in addition to T. argutum, two recently described Australian species (Bean 2018) also belong to the more widespread Teucrium polium clade (sensu Salmaki et al. 2016), as these form a clade with T. chamaedrys (Fig. 4), and share a subactinomorphic calyx and smooth-textured mericarps (Marin et al. 1994; Salmaki et al. 2016). Several Australian specimens of the Teucrium polium clade in our study had high levels of missing data (Supplementary Table S2) and given that our results recovered T. argutum as polyphyletic with respect to the morphologically similar T. daucoides, further investigation of species boundaries from within Clade A is warranted.
Relationships in the Teucrium core clade members of Australasia
Most phylogenetic diversity across Teucrium from Australia resides in three lineages (Clades C, D and G, Fig. 3–5). This indicates that the previous phylogeny by Salmaki et al. (2016) was able to capture the broad phylogenetic diversity of Australasian species despite restricted taxon sampling. Similar to previous results, our phylogeny demonstrated uncertainty about the branching order of these clades which, according to the combination of short branch lengths, low branch support, low concordance values, failure to reject a polytomy at several nodes and broad failure of the Chi-Square test to reject at most nodes appears to be the result of incomplete lineage sorting. The placement of Clades E and F with respect to Clades D and G was also not resolved; however, some morphological characters assist with insight about the relationship between these and Clades C, D and G. Similar morphological features in species of Clades C and F suggest they are closely related given that T. irroratum and T. modestum have leaf and reproductive (inflorescence and flower) morphological characteristics similar to those of T. corymbosum (Bean 2018). Clade E appears more closely related to Clade D because T. fililobum has floral morphological characteristics and tripartite leaves much like the deeper divergences (e.g. T. diabolicum, see Wege and Davis 2020) and more shallow divergences (e.g. T. pilbaranum, see Conn 1999) in Clade D. This character combination may also suggest a close relationship with southern African species of Teucrium that we were unable to include in this study and whose relationship with Australasian species remains unresolved (Codd 1977; Ruiters et al. 2016; Salmaki et al. 2016).
Clade C contains specimens that are currently recognised as four species, all of which are distinct from other clades based on the combination of glandular or non-glandular trichomes, simple to lobed leaves with a serrate margin, zygomorphic flowers with anthers and style exserted, and fruit a mericarp. Although T. grandiusculum has foliose inflorescences with a single pair of flowers per leaf axil, the remaining species are also distinguished from the rest of the Teucrium core clade because the flowers are clustered in foliose thyrsoids (Toelken 1985; Conn 2006; Toelken and Cunningham 2008). Based on our results, previously described similarities are correctly indicative that T. thieleanum and the phrase name T. sp. D are closely related to T. corymbosum (Conn 2006). However, the placement of T. thieleanum within a subclade of T. corymbosum identifies the diagnostic morphological characteristics as merely the result of localised variation within a broader morphological continuum rather than being indicative of a distinct evolutionary lineage. Likewise, Teucrium sp. D is not distinct from T. corymbosum because members do not form a single clade. Combined with evidence of high cycling in the SplitsTree phylogenetic network (Fig. 3), T. corymbosum, T. sp. D and T. thieleanum appear to most likely represent a composite of several closely related lineages with a reticulated history. Instead, the more compelling evidence for the recognition of a distinct taxon from the T. corymbosum complex is Clade C1 (Fig. 4). Except for a south-eastern Queensland specimen (BRI AQ1012656), plants of Clade C1 are morphologically distinct from most plants of Clade C2 by the narrowly ovate leaves, the lamina of which is at least three times as long as wide (cf. equal to or less than three times as long as wide). This is remarkably similar to the description of T. petrophilum F.Muell from the Flinders Ranges that is a synonym of T. corymbosum but was once recognised as a distinct taxon by the lanceolate (i.e. narrow-ovate) leaves and denser inflorescences (von Mueller 1853). Making this clade’s recognition as a species more tantalising is that the genetic partitioning between Clade C1 and other Clade C members occurs in an area in which the distributions overlap, including at Oxley Wild Rivers National Park where two separate clade members (NSW 1001778 and NSW 884719) are found within 15 km of one another (Fig. 2c). However, recognition of two distinct monophyletic taxa is not yet possible due to the variable placement and associated low branch support for three Clade C samples, two of which are from the Flinders Ranges (Fig. 4, Supplementary Fig. S2, S3). As these three samples were sourced from herbarium specimens of more than 30 years of age and given that the MSC or ML tree topology did not change after their removal (results not shown), low sequence capture seems to most likely be responsible for the coinciding inconsistent placement (Supplementary Fig. S1, Supplementary Table S2). Owing to the important geographic representation of these samples (i.e. Flinders Ranges), a robust taxonomic decision should await phylogenetic assessment of higher quality sequence data acquired from similar locations.
Clade D has four currently recognised species distinguished by the combination of a hoary indumentum; simple, lobed or tripartite leaves without a distinctively serrate margin; zygomorphic flowers; exserted anthers and style; fruit a mericarp; and 1–3(5) flowers organised as a botryoidal thyrse (Toelken 1985; Conn 1999; Wege and Davis 2020). This is the most widespread clade found across the arid Australian inland (Fig. 2). Given our sampling across the extent of the distribution of T. racemosum and T. integrifolium, and the consistency of the tree topology across analyses, there is mostly strong support that T. racemosum and T. integrifolium are distinct taxa. However, phylogenetic analyses recovered T. integrifolium as paraphyletic with regards to a divergent sample of T. integrifolium from the Northern Territory (NSW 364295, Fig. 4) and similarly, the SplitsTree phylogenetic network showed the same sample as distant from both T. integrifolium and T. racemosum, and associated with higher cycling with T. pilbaranum (Fig. 3). Such a pattern may be the result of genetic reticulation, as this sample of T. integrifolium (NSW 364295) is from the general area of northern Australia where both T. albicaule (closely related T. pilbaranum) and T. integrifolium occur (Fig. 2), and cycling in the SplitsTree network between most taxa in Clade D was quite high (Fig. 3). However, we have not noticed morphological differences between this specimen (NSW 364295) and other specimens of T. integrifolium used in this study. By contrast, Teucrium sp. Sturt Creek (PERTH 5421675) can be distinguished from the morphologically similar species T. integrifolium by the pedicels that are as long as three times the length of the calyx (cf. up to twice the length of the calyx) and with leaf apices that are obtuse (cf. acute), raising greater suspicion about introgression since this is placed differently between phylogenetic analyses (Fig. 4, Supplementary Fig. S3) and found less than 100 km away from populations of T. integrifolium and T. albicaule (Fig. 2). Unfortunately only a single gathering of T. sp. Sturt Creek exists, therefore determination of species status needs further examination to establish the extent of introgression and hybridisation. For both T. sp. Sturt Creek and the Northern Territory T. integrifolium sample, genomic scans at the population scale and a comparison of heterozygosity by mapping reads back to targets may help to assess hybridisation; however, collecting another sample of the former is first necessary to improve sequencing quality given that only 40 genes were assembled at over 75% (Supplementary Table S1).
The unresolved placement of T. parvifolium is suggestive of hybridity as put forward by Salmaki et al. (2016); however, we did not test for this. The association between hybridisation and polyploidy has been implicated in the success of neo- and ancient allopolyploids and in particular, in an evolutionary context (Soltis and Soltis 2009). This is because of a greater environmental amplitude than parental species (Alix et al. 2017) and may partially explain the success of T. parvifolium in New Zealand.
Clade G is the most morphologically diverse of the three main clades, consisting of nine species with a non-hoary and non-glandular indumentum, simple leaves (when present) with an entire margin, flowers either zygomorphic or actinomorphic, anthers and style either strongly exserted or included, fruit either a mericarp or drupelet and flowers always arranged as one-flowered uniflorescences (Munir 1976, 1991; Conn 2002; Thiele and Shepherd 2014; Bean 2018). A unifying character of clade G (with the exception of T. teucriiflorum) is that the corolla has an abaxial lobe nearly equal in size to the lateral and adaxial lobes (Fig. 1, 4) unlike the strongly zygomorphic, unilabiate flowers found on most other Teucrium species (Fig. 4). This feature is most pronounced in the previously described segregate genera Oncinocalyx, Spartothamnella and Teucridium and our study confirms previous results that these are closely related to T. sessiliflorum (Salmaki et al. 2016). We are able to place the previously defined segregates Oncinocalyx and Spartothamnella (Fig. 4, 5; Clade G3) in context with Australian taxa traditionally described as Teucrium (i.e. Clades G1, G2) with our increased sampling. The structure within these latter clades confirms T. fallax and T. micranthum as closely related but distinct species (clade G2) and demonstrates that T. sessiliflorum and T. sp. Balladonia are not monophyletic (clade G1). The unexpected result of the latter may be explained by low sequence capture in two samples (Supplementary Fig. S1), nonetheless T. sessiliflorum is rendered paraphyletic by a higher quality sequence of T. sp. Balladonia, suggesting that a more detailed population genomic examination is required to properly assess species boundaries between members of Clade G, the populations of which overlap (Fig. 2).
The small Clade E consists of two western Australian species, T. fililobum and T. myriocladum (sensu Western Australian Herbarium’s Florabase, see https://florabase.dbca.wa.gov.au/), and due to the strong morphological similarity between T. fililobum subsp. glandular (W.Rogerson 233) WA Herbarium (PERTH 4208323) and T. myriocladum (e.g. simple leaf, large nectar crop, porrect adaxial corolla lobes) we correctly predicted that T. fililobum is not monophyletic. However, unexpectedly (albeit with short branch lengths), T. myriocladum is sister to T. fililobum Benth. subsp. fililobum that has lobed leaves and a corolla with strongly reflexed adaxial lobes (cf. porrect), and lacks a pronounced nectar crop (Blackall and Grieve 1965; Fig. 1). High support values and high concordance for this relationship support the notion that T. fililobum subsp. fililobum and T. myriocladum are converging morphologically. Shared distance between Clade E members may be indicative of high gene flow (Fig. 3) but such a conclusion requires a more comprehensive population scale analysis between these and other sympatric (or closely located) Teucrium.
Historical diversification and dispersal of Australian Teucrium
Our study in conjunction with the broader understanding of Teucrium diversity (Salmaki et al. 2016) supports at least two separate dispersals into Australia corresponding to taxa within the Teucrium core clade or the Teucrium polium clade. Given Teucrium’s estimated origin in the Mediterranean based on distribution of diversity (Navarro and El Oualidi 2000) and dating estimates (Salmaki et al. 2016), the best candidate scenario for migration into Australasia would be the onset of the high floristic exchange between Australia and Asia beginning as early as 25 Ma (Hall 2002, 2009; Crayn et al. 2015; Kooyman et al. 2019). The stem age for the Teucrium polium clade (Clade A) ranges from late Oligocene to early Miocene (Fig. 6). Although much earlier than the previously reported period of late Miocene to early Pliocene (Salmaki et al. 2016), this timeframe nonetheless continues to show that diversification coincided with the commencement of the Australian-Asian floristic exchange. Traces of this floristic exchange have been apparent in the phylogenetic and biogeographic patterns of one of the predominantly Asian Teucrium polium subclades (‘Clade 7’ sensu Salmaki et al. 2016), the distribution of which extends to Australasia based on T. argutum and T. viscidum Blume (Navarro 2020; GBIF Secretariat 2023a, 2023b). Unfortunately, because our results lack a comprehensive sampling of the Teucrium polium clade such as that of Salmaki et al. (2016), we are unable to estimate whether all Australian Teucrium polium clade species belong to Clade 7 – all that can be ruled out is that these do not belong to ‘clade 2’ sensu Salmaki et al. 2016 (as represented by T. chamaedrys). Teucrium daucoides, T. sagittatum, T. argutum and the remainder of Clade 7 do, however, appear to be closely related by habit, leaf shape and floral shape similarities (Bean 2018; POWO, see https://powo.science.kew.org/) that suggests a single dispersal from Asia into Australia prior to continental collision. Nonetheless, without improved sampling, this is not possible to test, nor can a biogeographic origin from Asia be ruled out given that the other Clade 7 members are North American (e.g. T. canadense).
Interpretation of our results and those by Salmaki et al. (2016) suggest that, in contrast to the Teucrium polium clade described above, the Teucrium core clade may have arrived from Africa despite the genus originating in the Mediterranean or Asia (Salmaki et al. 2016). For instance, our results show multiple, broadly distributed clades (Fig. 2, Clades C, D and G) within the Teucrium core clade of Australasia (hereafter referred to as the Australasian Teucrium core clade). Furthermore, the Australasian Teucrium core clade is geographically separated from the South African relative (T. trifidum) by more distantly related Teucrium core clade and Teucrium polium clade members in Asia and northern Africa (Codd 1977; Ruiters et al. 2016; Salmaki et al. 2016). Indeed, floristic migration of many taxa between Asia and Africa has occurred during the early Miocene through an Asian land route (Zhou et al. 2012), including that of Isodon (Lamiaceae) that has an Asian centre of diversity and disjunct southern African relatives (Yu et al. 2014). However, unlike taxa considered to have dispersed along this land route, the highest phylogenetic diversity of Teucrium is found in the Mediterranean region rather than only Africa (e.g. Zhou et al. 2012; Yu et al. 2014; Rose et al. 2023). If Teucrium had dispersed via a land route through Asia, a highly specific extinction in the core clade members across Africa, Asia and the Mediterranean would be needed to explain such a high phylogenetic disjunction. This makes long-distance dispersal between Africa and Australia somewhat plausible given that mericarps (of Teucrium and other Lamiaceae) can travel within birds (Vazačová and Münzbergová 2014) and evidence that supports a Miocene floristic exchange directly between Australia and Africa (Raven and Axelrod 1974; Les et al. 2003; Yuan et al. 2005; Li et al. 2009; Ladiges et al. 2011; Yao et al. 2016; Li et al. 2020; Žerdoner Čalasan et al. 2022). The capacity for dispersal over oceans is also supported by the presence of T. parvifolium in New Zealand because the separation of New Zealand from Australia c. 80 Ma ago (Trewick et al. 2007; Hall 2009) is far older than the stem age of the Australasian Teucrium core clade 14.4–19.2 Ma. Divergence of T. parvifolium from Clade G c. 8.7–12.8 Ma overlaps with the drier late Miocene climate in New Zealand that would have been favourable for Teucrium (Pole 2014). Naturally, dispersal from Australia still cannot be ruled out and the sampling of more South African taxa will be key to further reconciliation regarding biogeographic origin.
Our date estimate of 10.2–14.8 Ma for the ancestor of the Australasian Teucrium core clade is older than the range reported by Salmaki et al. (2016) although both ranges overlap slightly in the late Miocene. Even though Australia was warm and wet throughout the early Miocene, the considerable climatic change known as the Hill Gap was underway by the late Miocene (Byrne et al. 2008) where our range estimate of the Australasian core clade ancestor lies (Fig. 6). Ensuing aridification during this time may have opened suitable habitat of woodlands and shrublands where Teucrium could have undergone complex lineage diversification. This may be one reason for short branch lengths and low topological support along the backbone of this clade (Flower and Kennett 1994; Byrne et al. 2008; Knorr and Lohmann 2014; Gillespie et al. 2020).
Evolution of alternative reproductive strategies
We argue that the core Teucrium lineage has evolved different pollination strategies in Australasia where a shift to a more generalist pollination syndrome coincides with evolutionary shifts towards more specialised fruit dispersal syndromes. Two members of Clade E have flowers with corollas bearing a nectar crop, a strongly reflexed abaxial lobe and a somewhat narrow tube; these characteristics in combination are generally indicative of ornithophily (Raven 1972; Faegri and van der Pijl 1979; Proctor et al. 1996; Schemske and Bradshaw 1999; Temeles and Rankin 2000; Castellanos et al. 2003; Cronk and Ojeda 2008; Johnson 2013; Zung et al. 2015; Wilson et al. 2017). Evolution of bird pollination in Teucrium is not unexpected given that eight other Australian Labiate genera have ornithophilous species although ornithophily has not previously been reported for Australian Teucrium (Brundrett et al. 2024). If ornithophily is validated for Clade E, this would demonstrate an independent evolution of bird pollination in the genus (Valido et al. 2004; Rodríguez-Sambruno et al. 2024). A second, more prevalent pollination strategy appears to be a more generalist insect pollination syndrome in T. betchei and most of Clade G3. Compared to other Teucrium, these taxa have a more radially shaped corolla due to the equal arrangement of the relatively similar-sized corolla lobes, and these lack nototribic pollination because the stamens and pistil are included within the corolla and closer to the nectaries (Munir 1976, 1991; Merrett 2005; Fig. 1, 4). Such a combination is expected to broaden pollinator diversity because this presents less restriction to floral rewards and the lack of specific pollen placement shows less dependence on hiding pollen from potential thieves accessing nectar or assistance towards transferring pollen onto a broader range of body types (Webb and Pearson 1993; Gong and Huang 2009). Although more field studies are necessary to confirm these putative pollination syndromes, a shift in pollinator diversity and visitation frequency with respect to floral morphological characteristics appears to have occurred in another Australian Labiate genus with similar compartmentalised floral morphological characteristics (Wilson et al. 2017). Assuming these morphological characteristics are the result of selective forces elicited by the effectiveness and diversity of pollinators, shifts to new pollination strategies, coinciding with the recent (c. <5 Ma) progressive aridification and climatic instability (Sniderman et al. 2007; Byrne et al. 2008) are associated with shallowly divergent clades (or recent origins according to the dating analysis). However, not all species with these distinctive floral morphological characteristics were recovered together because T. teucriiflorum with a strongly zygomorphic flower (Fig. 4, 5) was recovered in Clade G3 and the placement of T. betchei without a strongly zygomorphic flower remains uncertain (compare Fig. 5, 6). These ambiguities suggest that transition between states is nuanced. Given that most other Clade G species have equal-sized corolla lobes unlike other Teucrium species, the evolution of a more radially shaped corolla appears to have been gradual throughout the diversification of Clade G (e.g. T. sessiliflorum, Fig. 4).
Our results demonstrate that drupelets are a synapomorphy of Clade G3 and suggest that evolutionary shifts away from a mericarp fruit type are phylogenetically restricted. A close relationship to T. betchei, which has mericarps yet is also zoochorous due to the specialised hooked calyx lobes, indicates two independent yet closely timed shifts towards zoochory (i.e. endozoochory, epizoochory). This implicates strong selection favouring specific modes of animal dispersal rather than a more generalist dispersal approach (i.e. with a mericarp and no specialised calyx lobes) at the crown of Clade G (7.2–11.1 Ma). Furthermore, this appears to approximately coincide with the evolutionary path from a specific pollination syndrome (i.e. melittophily) towards a putative generalised insect pollination syndrome (as inferred above), raising the question about whether placing a premium on one mode of genetic dispersal (i.e. a specific suite of animal vectors) can influence a shift on another (i.e. a specific suite of pollinators). Little information is available about the interactive influence between pollination and seed dispersal, and testing for any relationship first requires broader, foundational knowledge to account for exceptions and assumptions (Green et al. 2022).
Conclusions
The comprehensive phylogenetic analysis of Australian Teucrium presented in this study has revealed timing and dispersal pathways incorporating evolutionary history, dispersal and diversification throughout Australasia. Two distantly related clades of Teucrium have entered Australia in succession through different channels. One lineage, potentially the oldest lineage, has had sufficiently greater opportunity to exploit disparate niches, switch modes of pollination and possibly prioritise more specific modes of fruit dispersal over pollination strategy. However, a difference in age between lineages is not shown unequivocally by our results as there is an overlap in age range between the crown of the core clade (10.2–14.8 Ma) and Australian members of the polium clade (5.9–12.2 Ma).
The A353 bait set has enabled insights into the diversification of Teucrium in Australasia, and despite the hurdles of limited sampling and poor resolution in parts of the tree, the flexibility to use herbarium samples has overcome some of the barriers associated with specimen acquisition. However, the corollary problem was that a high number of herbarium samples had relatively high amounts of missing data that may have inaccurately boosted unrelatedness estimates between some taxa and introduced erroneous paraphyly in the phylogeny. This study has demonstrated limitations in the current gene set in resolving closely related species, as shown with the highly sampled species (e.g. T. corymbosum). Replicate sampling of two T. junceum populations may further illustrate this point, as only one of two populations was confidently resolved as a distinct clade. This may make it appear that the technique offers less promise than originally anticipated for population genomic studies (Slimp et al. 2021), but genetic connectivity between populations of the same species may also be responsible for low phylogenetic structure. A more targeted bait set may improve resolution of closely related taxa and the reusability of the A353 dataset may improve the scope to rectify current phylogenetic shortcomings in the future. Expanded sampling, especially of taxa across Africa, is a priority to improve our perspective on how the Teucrium core clade diversified across the globe, particularly between Australia and Africa. Regarding phrase name taxa and the morphologically similar species included in our study (e.g. T. daucoides and T. argutum), a better understanding of the close relatives has confidently been achieved but the results are not sufficient to assist with formalising their distinctiveness. Our phylogenetic tree furthermore provides a robust framework to progress with understanding species dynamics and reproductive evolution, such as by enabling the design of new studies to better understand pollination (e.g. T. fililobum and T. myriocladum) and dispersal mechanisms (e.g. T. betchei and T. teucriiflorum) at population or clade-specific levels.
Data availability
All raw DNA sequence data generated for this study are deposited in the European Nucleotide Archive under the following bioprojects: PRJEB49212; PREJEB90362.
Declaration of funding
This study was supported by the following: Australian Biological Resources Study (RG19-17 to T. C. Wilson and E. A. James); Genomics for Australian Plants (GAP): Australian Angiosperm Tree of Life (AAToL) Stage 2; Marlies Eichler Postdoctoral Fellowship grant (Australasian Systematic Botany Society to T. C. Wilson); and Bush Blitz 2021–2022 Taxonomy Research Project (DNP-BCK-2021-007 to T. C. Wilson).
Acknowledgements
The authors acknowledge the Traditional Custodians of the land across Australia and New Zealand on which the study, in all facets, was completed and pay respects to Elders past and present. Provision of computing and data resources: Australian BioCommons Leadership Share (ABLeS) program, co-funded by Bioplatforms Australia, enabled by NCRIS (National Collaborative Research Infrastructure Strategy), the National Computational Infrastructure and Pawsey Supercomputing Research Centre. Infrastructure support and data: Genomics for Australian Plants Consortium, with the assistance of Mabel Lum (BioCommons) and Lalita Simpson (then Genomics for Australian Plants). Contribution of collections in field: Fiona Murdoch, Guy Taseski (University of New South Wales), Joel Cohen (then Botanic Gardens of Sydney), Ryan O’Donnell (then University of New England); in herbarium: staff at NSW, BRI, MEL, PERTH. Assistance with analyses and data: Chris Jackson and Theo Allnut (both Royal Botanic Gardens Victoria), Francis Nge and Eilish McMaster (both Botanic Gardens of Sydney) and James Clugston (then Botanic Gardens of Sydney). Contributions of photography: Eddy Wajon, William Archer, Tony Rodd, Annabel Carle, Matt A. M. Renner, Peter Heenan and Landcare Research (New Zealand). We sincerely appreciate Ben Anderson and another anonymous reviewer for comments that improved this paper.
References
Abadi S, Azouri D, Pupko T, Mayrose I (2019) Model selection may not be a mandatory step for phylogeny reconstruction. Nature Communications 10, 934.
| Crossref | Google Scholar | PubMed |
Alix K, Gérard PR, Schwarzacher T, Heslop-Harrison JS (2017) Polyploidy and interspecific hybridization: partners for adaptation, speciation and evolution in plants. Annals of Botany 120, 183-194.
| Crossref | Google Scholar | PubMed |
Baker WJ, Dodsworth S, Forest F, Graham SW, Johnson MG, McDonnell A, Pokorny L, Tate JA, Wicke S, Wickett NJ (2021) Exploring Angiosperms353: an open, community toolkit for collaborative phylogenomic research on flowering plants. American Journal of Botany 108, 1059-1065.
| Crossref | Google Scholar | PubMed |
Baum DA, Small RL, Wendel JF (1998) Biogeography and floral evolution of baobabs (Adansonia, Bombacaceae) as inferred from multiple data sets. Systematic Biology 47, 181-207.
| Crossref | Google Scholar | PubMed |
Bean AR (2018) A conspectus of Teucrium (Lamiaceae) in Queensland. Austrobaileya 37, 3-18.
| Crossref | Google Scholar |
Benson D, McDougall L (2004) Ecology of Sydney plant species: part 3: dicotyledon families Cabombaceae to Eupomatiaceae. Cunninghamia 4, 217-431.
| Google Scholar |
Blüthgen N, Menzel F, Hovestadt T, Fiala B, Blüthgen N (2007) Specialization, constraints, and conflicting interests in mutualistic networks. Current Biology 17, 341-346.
| Crossref | Google Scholar | PubMed |
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114-2120.
| Crossref | Google Scholar | PubMed |
Brundrett MC, Ladd PG, Keighery GJ (2024) Pollination strategies are exceptionally complex in southwestern Australia – a globally significant ancient biodiversity hotspot. Australian Journal of Botany 72, BT23007.
| Crossref | Google Scholar |
Byrne M, Yeates DK, Kearney JL, Bowler J, Williams MAJ, Cooper S, Donnellan SC, Keogh JS, Leys R, Melville J, Murphy DJ, Porch N, Wyrwoll K-H (2008) Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398-4417.
| Crossref | Google Scholar | PubMed |
Candela RG, Rosselli S, Bruno M, Fontana G (2021) A review of the phytochemistry, traditional uses and biological activities of the essential oils of genus Teucrium. Planta Medica 87, 432-479.
| Crossref | Google Scholar | PubMed |
Cantino PD (1992) Evidence for a polyphyletic origin of the Labiatae. Annals of the Missouri Botanic Gardens 79, 361-379.
| Crossref | Google Scholar |
Castellanos MC, Wilson P, Thomson JD (2003) Pollen transfer by hummingbirds and bumblebees, and the divergence of pollination modes in Penstemon. Evolution 57, 2742-2752.
| Crossref | Google Scholar | PubMed |
Codd LE (1977) The South African species of Teucrium (Lamiaceae). Bothalia 12, 177-179.
| Crossref | Google Scholar |
Conn BJ (1999) Teucrium pilbaranum (Labiatae), a new species from the Pilbara, Western Australia. Telopea 8, 299-303.
| Crossref | Google Scholar |
Conn BJ (2002) Teucrium micranthum (Labiatae), a new species from Queensland, Australia. Telopea 9, 803-807.
| Crossref | Google Scholar |
Conn BJ (2006) Teucrium thieleanum (Labiatae), a new species from Victoria, Australia. Telopea 11, 135-140.
| Crossref | Google Scholar |
Crayn DM, Costion C, Harrington MG (2015) The Sahul–Sunda floristic exchange: dated molecular phylogenies document Cenozoic intercontinental dispersal dynamics. Journal of Biogeography 42, 11-24.
| Crossref | Google Scholar |
Cronk Q, Ojeda I (2008) Bird-pollinated flowers in an evolutionary and molecular context. Journal of Experimental Biology 59, 715-727.
| Crossref | Google Scholar | PubMed |
Drew BT, Sytsma KJ (2012) Phylogenetics, biogeography, and staminal evolution in the tribe Mentheae (Lamiaceae). American Journal of Botany 99, 933-953.
| Crossref | Google Scholar | PubMed |
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7, 214.
| Crossref | Google Scholar | PubMed |
Duan Q, Goodale E, Quan RC (2014) Bird fruit preferences match the frequency of fruit colours in tropical Asia. Scientific Reports 4, 5627.
| Crossref | Google Scholar | PubMed |
Flower BP, Kennett JP (1994) The middle Miocene climatic transition: east Antarctic ice sheet development, deep ocean circulation and global carbon cycling. Palaeogeography, Palaeoclimatology, Palaeoecology 108, 537-555.
| Crossref | Google Scholar |
GBIF Secretariat (2023a) Teucrium corymbosum R.Br. In ‘GBIF Backbone Taxonomy’. (GBIF Secretariat: Copenhagen, Denmark) Available at https://www.gbif.org/species/7308603 [Verified 13 January 2025]
GBIF Secretariat (2023b) Teucrium viscidum Blume. In ‘GBIF Backbone Taxonomy’. (GBIF Secretariat: Copenhagen, Denmark) Available at https://www.gbif.org/species/7307951 [Verified 13 January 2025]
Gillespie RG, Bennett GM, De Meester L, Feder JL, Fleischer RC, Harmon LJ, Hendry AP, Knope ML, Mallet J, Martin C, Parent CE, Patton AH, Pfennig KS, Rubinoff D, Schluter D, Seehausen O, Shaw KL, Stacy E, Stervander M, Stroud JT, Wagner C, Wogan GOU (2020) Comparing adaptive radiations across space, time, and taxa. Journal of Heredity 111, 1-20.
| Crossref | Google Scholar | PubMed |
Gong Y-B, Huang S-Q (2009) Floral symmetry: pollinator-mediated stabilizing selection on flower size in bilateral species. Philosophical Transactions of the Royal Society – B. Biological Sciences 276, 4013-4020.
| Crossref | Google Scholar | PubMed |
Green AJ, Baltzinger C, Lovas-Kiss Á (2022) Plant dispersal syndromes are unreliable, especially for predicting zoochory and long-distance dispersal. Oikos 2022, e08327.
| Crossref | Google Scholar |
Greuter W, Burdet HM, Long G (1986) Med-Checklist Conservatoire et Jardin Botanique de la Ville de Genève. Genéve 3, 366-380.
| Google Scholar |
Gustafsson OJR, Al Bkhetan Z, Francis R, Manos S (2023) Enabling national step changes in bioinformatics through ABLeS, the Australian BioCommons Leadership Share. Zenodo 2023, ver. 3.0 [Program details, published 15 November 2023].
| Crossref | Google Scholar |
Hall R (2002) Cenozoic geological and plate tectonic evolution of Southeast Asia and the Southwest Pacific: computer-based reconstructions, model and animations. Journal of Asian Earth Sciences 20, 353-434.
| Crossref | Google Scholar |
Hall R (2009) Southeast Asia’s changing palaeogeography. Blumea 54, 148-161.
| Crossref | Google Scholar |
Harborne JB, Tomás-Barberán FA, Williams CA, Gil MI (1986) A chemotaxonomic study of flavonoids from European Teucrium species. Phytochemistry 25, 2811-2816.
| Crossref | Google Scholar |
Holzmeyer L, Hauenschild F, Muellner‐Riehl AN (2023) Sunda–Sahul floristic exchange and pathways into the Southwest Pacific: new insights from wet tropical forest trees. Journal of Biogeography 50, 1257-1270.
| Crossref | Google Scholar |
Huson DH (1998) SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14, 68-73.
| Crossref | Google Scholar |
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23, 254-267.
| Crossref | Google Scholar | PubMed |
Jackson C, McLay T, Schmidt-Lebuhn AN (2021) hybpiper-rbgv and yang-and-smith-rbgv: containerization and additional options for assembly and paralog detection in target enrichment data. BioRxiv 2021, 2021.11.08.467817 [Preprint, published 10 November 2021].
| Crossref | Google Scholar |
Jackson C, McLay T, Schmidt-Lebuhn AN (2023) hybpiper‐nf and paragone‐nf: containerization and additional options for target capture assembly and paralog resolution. Applications in Plant Sciences 11, e11532.
| Crossref | Google Scholar | PubMed |
Johnson KA (2013) Are there pollination syndromes in the Australian epacrids (Ericaceae: Styphelioideae)? A novel statistical method to identify floral traits per syndrome. Annals of Botany 112, 141-149.
| Crossref | Google Scholar | PubMed |
Johnson MG, Gardner EM, Liu Y, Medina R, Goffinet B, Shaw AJ, Zerega NJC, Wickett NJ (2016) HybPiper: extracting coding sequence and introns for phylogenetics from high-throughput sequencing reads using target enrichment. Applications in Plant Sciences 4, 1600016.
| Crossref | Google Scholar | PubMed |
Johnson MG, Pokorny L, Dodsworth S, Botigué LR, Cowan RS, Devault A, Eiserhardt WL, Epitawalage N, Forest F, Kim JT, Leebens-Mack JH, Leitch IJ, Maurin O, Soltis DE, Soltis PS, Wong GK, Baker WJ, Wickett NJ (2019) A universal probe set for targeted sequencing of 353 nuclear genes from any flowering plant designed using k-medoids clustering. Systematic Biology 68, 594-606.
| Crossref | Google Scholar | PubMed |
Joyce EM, Schmidt-Lebuhn AN, Orel HK, Nge FJ, Anderson BM, Hammer TA, McLay TGB (2024) Navigating phylogenetic conflict and evolutionary inference in plants with target capture data. Australian Systematic Botany 38, SB24011.
| Crossref | Google Scholar |
Kästner A (1978) Beiträge zur Wuchsformenanalyse und systematischen Gliederung von Teucrium L. – I. Die Infloreszenzen und Blüten. Flora 167, 485-514 [In German].
| Crossref | Google Scholar |
Kästner A (1989) Übersicht zur systematischen Gliederung der Gattung Teucrium L. Biocosme Mésogéen 6, 63-77 [In German].
| Google Scholar |
Knorr G, Lohmann G (2014) Climate warming during Antarctic ice sheet expansion at the Middle Miocene transition. Nature Geoscience 7, 376-381.
| Crossref | Google Scholar |
Kooyman RM, Morley RJ, Crayn DM, Joyce EM, Rossetto M, Slik JF, Strijk JS, Su T, Yap JYS, Wilf P (2019) Origins and assembly of Malesian rainforests. Annual Review of Ecology, Evolution, and Systematics 50, 119-143.
| Crossref | Google Scholar |
Ladiges PY, Marks CE, Nelson G (2011) Biogeography of Nicotiana section Suaveolentes (Solanaceae) reveals geographical tracks in arid Australia. Journal of Biogeography 38, 2066-2077.
| Crossref | Google Scholar |
Lei B, Cui J, Newman C, Buesching CD, Xie Z, Macdonald DW, Zhou Y (2021) Seed dispersers shape the pulp nutrients of fleshy-fruited plants. Proceedings of the Royal Society of London – B. Biological Sciences 288, 20210817.
| Crossref | Google Scholar | PubMed |
Les DH, Crawford DJ, Kimball RT, Moody ML, Landolt E (2003) Biogeography of discontinuously distributed hydrophytes: a molecular appraisal of intercontinental disjunctions. International Journal of Plant Science 164, 917-932.
| Crossref | Google Scholar |
Li YQ, Dressler S, Zhang DX, Renner SS (2009) More Miocene dispersal between Africa and Asia – the case of Bridelia (Phyllanthaceae). Systematic Botany 34, 521-529.
| Crossref | Google Scholar |
Li ZZ, Lehtonen S, Martins K, Gichira AW, Wu S, Li W, Hu GW, Liu Y, Zou CY, Wang QF, Chen JM (2020) Phylogenomics of the aquatic plant genus Ottelia (Hydrocharitaceae): implications for historical biogeography. Molecular Phylogenetics and Evolution 152, 106939.
| Crossref | Google Scholar | PubMed |
Marin PD, Petković B, Duletić S (1994) Nutlet sculpturing of selected Teucrium species (Lamiaceae): a character of taxonomic significance. Plant Systematics and Evolution 192, 199-214.
| Crossref | Google Scholar |
Mazer SJ, Wheelwright NT (1993) Fruit size and shape: Allometry at different taxonomic levels in bird-dispersed plants. Evolutionary Ecology 7, 556-575.
| Crossref | Google Scholar |
Merrett MF (2005) Gynodioecy in Teucridium parvifolium (Verbenaceae), a threatened, small-leaved shrub from New Zealand. New Zealand Journal of Botany 43, 613-617.
| Crossref | Google Scholar |
Minh BQ, Nguyen MAT, von Haeseler A (2013) Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution 30, 1188-1195.
| Crossref | Google Scholar | PubMed |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, Von Haeseler A, Lanfear R (2020a) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530-1534.
| Crossref | Google Scholar |
Minh BQ, Hahn MW, Lanfear R (2020b) New methods to calculate concordance factors for phylogenomic datasets. Molecular Biology and Evolution 37, 2727-2733.
| Crossref | Google Scholar |
Mittelbach M, Yurkov AM, Nocentini D, Nepi M, Weigend M, Begerow D (2015) Nectar sugars and bird visitation define a floral niche for basidiomycetous yeast on the Canary Islands. BMC Ecology 15, 2.
| Crossref | Google Scholar | PubMed |
Morales-Briones DF, Gehrke B, Huang CH, Liston A, Ma H, Marx HE, Tank DC, Yang Y (2022) Analysis of paralogs in target enrichment data pinpoints multiple ancient polyploidy events in Alchemilla s.l. (Rosaceae). Systematic Biology 71, 190-207.
| Crossref | Google Scholar |
Munir AA (1976) A taxonomic revision of the genus Spartothamnella (Chloanthaceae). Journal of the Adelaide Botanic Gardens 1, 3-25.
| Google Scholar |
Munir AA (1991) A taxonomic revision of the genus Oncinocalyx F.Muell. (Verbenaceae). Journal of the Adelaide Botanic Gardens 14, 77-84.
| Google Scholar |
Navarro T, El Oualidi J (1999) Flower and life strategy diversity in Teucrium L. (Lamiaceae). Acta Botanica Malacitana 24, 63-75.
| Crossref | Google Scholar |
Navarro T, El Oualidi J (2000) Synopsis of Teucrium L. (Labiatae) in the Mediterranean region and surrounding areas. Flora Mediterranea 10, 349-363.
| Google Scholar |
Petanidou TH (1996) Labiatae: a key family for wild bees and the pollination ecology in Mediterranean phryganic communities. Lamiales Newsletter 4, 4-6.
| Google Scholar |
Pole M (2014) The Miocene climate in New Zealand: Estimates from paleobotanical data. Palaeontologia Electronica 17, 1-79.
| Crossref | Google Scholar |
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67, 901-904.
| Crossref | Google Scholar | PubMed |
Raven PH (1972) Why are bird-visited flowers predominantly red? Evolution 26, 674.
| Crossref | Google Scholar | PubMed |
Raven PH, Axelrod DI (1974) Angiosperm biogeography and past continental movements. Annals of the Missouri Botanical Garden 61, 539-673.
| Crossref | Google Scholar |
Rodríguez-Sambruno C, Narbona E, del Valle JC, Valido A (2024) Bird–flower colour on islands supports the bee-avoidance hypothesis. Functional Ecology 38, 600-611.
| Crossref | Google Scholar |
Rose JP, Xiang CL, Sytsma KJ, Drew BT (2022) A timeframe for mint evolution: towards a better understanding of trait evolution and historical biogeography in Lamiaceae. Botanical Journal of the Linnean Society 200, 15-38.
| Crossref | Google Scholar |
Rose JP, Wiese J, Pauley N, Dirmenci T, Celep F, Xiang C-L, Drew BT (2023) East Asian–North American disjunctions and phylogenetic relationships within subtribe Nepetinae (Lamiaceae). Molecular Systematics and Evolution 187, 107873.
| Crossref | Google Scholar | PubMed |
Roy T, Lindqvist C (2015) New insights into evolutionary relationships within the subfamily Lamioideae (Lamiaceae) based on pentatricopeptide repeat (PPR) nuclear DNA sequences. American Journal of Botany 102, 1721-1735.
| Crossref | Google Scholar | PubMed |
Ruhfel BR, Bove CP, Philbrick CT, Davis CC (2016) Dispersal largely explains the Gondwanan distribution of the ancient tropical clusioid plant clade. American Journal of Botany 103, 1117-1128.
| Crossref | Google Scholar | PubMed |
Ruiters AK, Tilney PM, van Vuurenb SF, Viljoen AM, Kamatou GPP, van Wyk BE (2016) The anatomy, ethnobotany, antimicrobial activity and essential oil composition of southern African species of Teucrium (Lamiaceae). South African Journal of Botany 102, 175-185.
| Crossref | Google Scholar |
Ryding O (1995) Pericarp structure and phylogeny of the Lamiaceae-Verbenaceae-complex. Plant Systematics and Evolution 198, 101-141.
| Crossref | Google Scholar |
Ryding O (1998) Teucrium (Lamiaceae) in NE tropical Africa. Edinburgh Journal of Botany 55, 209-220.
| Crossref | Google Scholar |
Salmaki Y, Kattari S, Heubl G, Bräuchler C (2016) Phylogeny of non-monophyletic Teucrium (Lamiaceae: Ajugoideae): implications for character evolution and taxonomy. Taxon 65, 805-822.
| Crossref | Google Scholar |
Satthaphorn J, Paton AJ, Zuntini AR, Cowan RS, Leeratiwong C (2023) Phylogeny and infrageneric classification of Clerodendrum (Lamiaceae). Botanical Journal of the Linnean Society 204, 103-136.
| Crossref | Google Scholar |
Sayyari E, Mirarab S (2018) Testing for polytomies in phylogenetic species trees using quartet frequencies. Genes 9, 132.
| Crossref | Google Scholar | PubMed |
Schemske DW, Bradshaw HD, Jr (1999) Pollinator preference and the evolution of floral traits in monkey flowers (Mimulus). Proceeding of the National Academy of Sciences 96, 11910-11915.
| Crossref | Google Scholar | PubMed |
Simpson L, Cantrill D, Byrne M, Allnutt TR, King GJ, Lum M, Al Bkhetan Z, Andrew RL, Baker WJ, Barrett MD, Batley J, Berry O, Binks RM, Bragg J, Broadhurst L, Brown G, Bruhl JJ, Edwards RJ, Ferguson S, Forest F, Gustafsson J, Hammer TA, Holmes GD, Jackson CJ, James EA, Jones A, Kersey PJ, Leitch IJ, Maurin O, McLay TJB, Murphy DJ, Nargar K, Nauheimer L, Sauquet H, Schmidt-Lebuhn AN, Shepherd KA, Syme AE, Waycott M, Wilson TC, Crayn DM (2024) The Genomics for Australian Plants (GAP) framework initiative – developing genomic resources for understanding the evolution and conservation of the Australian flora. Australian Systematic Botany 38, SB24022.
| Crossref | Google Scholar |
Slimp M, Williams LD, Hale H, Johnson MG (2021) On the potential of Angiosperms353 for population genomic studies. Applications in Plant Sciences 9, e11419.
| Crossref | Google Scholar | PubMed |
Smith SA, O’Meara BC (2012) treePL: divergence time estimation using penalized likelihood for large phylogenies. Bioinformatics 28, 2689-2690.
| Crossref | Google Scholar | PubMed |
Smith SA, Moore MJ, Brown JW, Yang Y (2015) Analysis of phylogenomic datasets reveals conflict, concordance, and gene duplications with examples from animals and plants. BMC Evolutionary Biology 15, 150.
| Crossref | Google Scholar | PubMed |
Smith SA, Brown JW, Walker JF (2018) So many genes, so little time: a practical approach to divergence-time estimation in the genomic era. PLoS One 13, e0197433.
| Crossref | Google Scholar | PubMed |
Sniderman JMK, Pillans B, O’Sullivan PB, Kershaw AP (2007) Climate and vegetation in southeastern Australia respond to Southern Hemisphere insolation forcing in the late Pliocene-early Pleistocene. Geology 35, 41-44.
| Crossref | Google Scholar |
Soltis PS, Soltis DE (2009) The role of hybridization in plant speciation. Annual Review of Plant Biology 60, 561-588.
| Crossref | Google Scholar | PubMed |
Stebbins GL (1970) Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics 1, 307-326.
| Crossref | Google Scholar |
Suchard MA, Rambaut A (2009) Many-core algorithms for statistical phylogenetics. Bioinformatics 25, 1370-1376.
| Crossref | Google Scholar | PubMed |
Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A (2018) Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evolution 4, vey016.
| Crossref | Google Scholar |
Temeles EJ, Rankin AG (2000) Effect of the lower lip of Monarda didyma on pollen removal by hummingbirds. Canadian Journal of Botany 78, 1164-1168.
| Crossref | Google Scholar |
Thiele KR, Shepherd KA (2014) Spartothamnella canescens (Lamiaceae, Chloantheae), a new species from Western and Central Australia, with notes on the status of S. sp. Helena & Aurora Range. Nuytsia 24, 177-185.
| Google Scholar |
Toelken HR (1985) Notes on Teucrium (Labiatae). Journal of the Adelaide Botanic Gardens 7, 295-300.
| Google Scholar |
Toelken HR, Cunningham DD (2008) Teucrium reidii (Labiatae): a new species from north-western South Australia. Journal of the Adelaide Botanic Gardens 22, 97-100.
| Google Scholar |
Trewick SA, Paterson AM, Campbell HJ (2007) Hello New Zealand. Journal of Biogeography 34, 1-6.
| Crossref | Google Scholar |
Valenta K, Nevo O (2020) The dispersal syndrome hypothesis: how animals shaped fruit traits, and how they did not. Functional Ecology 34, 1158-1169.
| Crossref | Google Scholar |
Valido A, Dupont YL, Olesen JM (2004) Bird–flower interactions in the Macaronesian islands. Journal of Biogeography 31, 1945-1953.
| Crossref | Google Scholar |
van der Pijl L (1972) Functional consideration and observation on the flowers of some Lamiaceae. Blumea 20, 93-103.
| Google Scholar |
Vazačová K, Münzbergová Z (2014) Dispersal ability of island endemic plants: What can we learn using multiple dispersal traits? Flora 209, 530-539.
| Crossref | Google Scholar |
von Mueller F (1853) Diagnoses et descriptiones plantarum novarum, quas in Nova Hollandia australi praecipue in regionibus interioribus. Linnaea: ein Journal für die Botanik in ihrem ganzen Umfange, oder Beiträge zur Pflanzenkunde 25, 426.
| Google Scholar |
Walker JD, Geissman JW (2022) Geologic Time Scale, ver. 6.0, updated October 2022. (Geological Society of America) 10.1130/2022.CTS006C
Walsh NG, O’Brien E (2013) Gynodioecy in Teucrium racemosum (Lamiaceae). Muelleria 31, 77-80.
| Crossref | Google Scholar |
Webb CJ, Pearson PE (1993) The evolution of approach herkogamy from protandry in New Zealand Gentiana (Gentianaceae). Plant Systematics and Evolution 186, 187-191.
| Crossref | Google Scholar |
Wege J, Davis R (2020) Better the devil you know: Teucrium diabolicum (Lamiaceae), a new species from mining tenements in the Coolgardie bioregion. Nuytsia 31, 129-133.
| Crossref | Google Scholar |
Westerkamp C, Classen-Bockhoff R (2007) Bilabiate flowers: the ultimate response to bees? Annals of Botany 100, 361-374.
| Crossref | Google Scholar | PubMed |
Wilson TC, Conn BJ, Henwood MJ (2017) Great expectations: correlations between pollinator assemblages and floral characters in Lamiaceae. International Journal of Plant Sciences 178, 170-187.
| Crossref | Google Scholar |
Xiang C-L, Zhao F, Cantino PD, Drew BT, Li B, Liu E-D, Soltis DE, Soltis PS, Peng H (2018) Molecular systematics of Caryopteris (Lamiaceae) and its allies with reference to the molecular phylogeny of subfamily Ajugoideae. Taxon 67, 376-394.
| Crossref | Google Scholar |
Yang Y, Smith SA (2014) Orthology inference in nonmodel organisms using transcriptomes and low-coverage genomes: improving accuracy and matrix occupancy for phylogenomics. Molecular Biology and Evolution 31, 3081-3092.
| Crossref | Google Scholar | PubMed |
Yao G, Drew BT, Yi TS, Yan HF, Yuan YM, Ge XJ (2016) Phylogenetic relationships, character evolution and biogeographic diversification of Pogostemon s.l. (Lamiaceae). Molecular Phylogenetics and Evolution 98, 184-200.
| Crossref | Google Scholar | PubMed |
Yu X-Q, Maki M, Drew BT, Paton AJ, Li H-W, Zhao J-L, Conran JG, Li J (2014) Phylogeny and historical biogeography of Isodon (Lamiaceae): rapid radiation in south-west China and Miocene overland dispersal into Africa. Molecular Phylogenetics and Evolution 77, 183-194.
| Crossref | Google Scholar | PubMed |
Yuan YM, Wohlhauser S, Möller M, Klackenberg J, Callmander MW, Küpfer P (2005) Phylogeny and biogeography of Exacum (Gentianaceae): a disjunctive distribution in the Indian Ocean Basin resulted from long distance dispersal and extensive radiation. Systematic Biology 54, 21-34.
| Crossref | Google Scholar | PubMed |
Žerdoner Čalasan A, Hammen S, Sukhorukov AP, McDonald JT, Brignone NF, Böhnert T, Kadereit G (2022) From continental Asia into the world: global historical biogeography of the saltbush genus Atriplex (Chenopodieae, Chenopodioideae, Amaranthaceae). Perspectives in Plant Ecology Evolution and Systematics 54, 125660.
| Crossref | Google Scholar |
Zhang C, Rabiee M, Sayyari E, Mirarab S (2018) ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19, 153.
| Crossref | Google Scholar | PubMed |
Zhao F, Chen Y-P, Salmaki Y, Drew BT, Wilson TC, Scheen A-C, Celep F, Bräuchler C, Bendiksby M, Wang Q, Min D-Z, Peng H, Olmstead RG, Li B, Xiang C-L (2021) An updated tribal classification of Lamiaceae based on plastome phylogenomics. BMC Biology 19, 2.
| Crossref | Google Scholar | PubMed |
Zhou L, Su YCF, Thomas DC, Saunders RMK (2012) Out-of-Africa’ dispersal of tropical floras during the Miocene climatic optimum: evidence from Uvaria (Annonaceae). Journal of Biogeography 39, 322-335.
| Crossref | Google Scholar |
Zona S (2017) Fruit and seed dispersal of Salvia L. (Lamiaceae): a review of the evidence. Botanical Review 83, 195-212.
| Crossref | Google Scholar |
Zung JL, Forrest JRK, Castellanos MC, Thomson JD (2015) Bee- to bird-pollination shifts in Penstemon: effects of floral-lip removal and corolla constriction on the preferences of free-foraging bumble bees. Evolutionary Ecology 29, 341-354.
| Crossref | Google Scholar |
Zuntini AR, Carruthers T, Maurin O, Bailey PC, Leempoel K, Brewer GE, Epitawalage N, et al. (2024) Phylogenomics and the rise of the angiosperms. Nature 629, 843-850.
| Crossref | Google Scholar | PubMed |


