Anal sex and associated HIV-related sexual risk factors among female sex workers in Andhra Pradesh, India
Rajesh Kumar Patra A , Bidhubhusan Mahapatra B C , Dolly Kovvali A , Laxminarayana Proddutoor A and Niranjan Saggurti BA Hindustan Latex Family Planning Promotion Trust, Veena Dhari Apt. 3-5-816, 3rd Floor, King Koti Road, Hyderguda, Hyderabad 500 029, India.
B Population Council, 142, Golf Links, New Delhi 110 003, India.
C Corresponding author. Email: bbmahapatra@popcouncil.org
Sexual Health 9(5) 430-437 https://doi.org/10.1071/SH11155
Submitted: 8 November 2011 Accepted: 8 May 2012 Published: 28 September 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Abstract
Background: This study aims to understand the correlates of anal sex practices among female sex workers (FSWs) and examine the association of anal sex with HIV-related sexual risk factors in Andhra Pradesh, India. Methods: A cross-sectional behavioural survey was conducted in 2011 among 795 FSWs aged 18 years or older. Probability-based cluster sampling was used to select respondents from sex work hotspots. Results: One-quarter (23%) of FSWs had practiced anal sex in the last year. The odds of practicing anal sex were higher among FSWs aged 35 years or more than in those aged less than 25 years (adjusted odds ratio (AOR): 2.05, P < 0.05), in those formerly married compared to those currently married (AOR: 1.88, P < 0.01), in those having an income only from sex work compared to those having additional sources of income (AOR: 1.54, P < 0.05), those reporting heavy alcohol consumption compared to those who did not (AOR: 2.80, P < 0.01) and those who experienced violence compared to those who had not (AOR: 2.80, P < 0.01). FSWs practicing anal sex were more likely to experience sexually transmissible infection (STI) related symptoms than those practicing only vaginal sex. There was no association between anal sex practice and condom use. Conclusions: Anal sex is associated with STI symptoms, a factor for HIV risk. HIV intervention programmes need to educate FSWs about the risks associated with anal sex.
Additional keywords: prostitution, sexual practices, sexually transmissible infections.
Introduction
Studies across the globe examining HIV risk transmission dynamics among female sex workers (FSWs) have primarily focussed on risk from peno–vaginal intercourse in the past, paying limited attention to the significance of penile–anal intercourse.1,2 Since the early 1990s, researchers have suggested that anal sex can also increase the risk of HIV and other sexually transmissible infections (STI).1,3–9 Empirical researches have subsequently demonstrated that anal intercourse carries a higher risk burden than vaginal intercourse even when practiced with a condom.5,10–13 Since condoms are generally manufactured for use in vaginal intercourse, the chances of condom breakage are higher if used in anal sex, thus resulting in an increased risk of exposure to HIV and other STIs.13,14 Furthermore, a growing body of research also suggests that the high rates of anal cancer could be attributable to the practice of anal intercourse.15–17 However, researchers often neglect the importance of anal sex as a determinant of STIs or as one of the outcomes in HIV prevention interventions.18
Surveys have documented widely varying estimates of anal sex prevalence among sex workers. One study conducted in Rwanda and Kenya showed that ~5% of FSWs reported ever practicing anal sex,2 but many other studies in Kenya indicate higher prevalence levels of anal sex among FSWs ranging between 14% and 40%.19–23 A study conducted in South Africa suggests that more than 40% of the FSWs soliciting at truck stops had engaged in anal sex.24 Other research in South Africa suggests that FSWs, on average, engage in five anal sex acts per week.25 These wide variations in the prevalence estimates of anal sex may be due to differences in study inclusion criteria, the recruitment strategy or the reference period for occurrence of the event.
Parallel to global estimates, varying estimates of anal sex prevalence have been reported in studies conducted among FSWs in India. One study conducted in Karnataka, India, estimated that 13% of the FSWs had engaged in anal sex,26 and another small-scale study in a similar setting estimated it to be at 27%.27 In contrast, focus group discussions conducted among 50 sex workers in Andhra Pradesh, India, suggests that ~80% of the FSWs practiced anal sex regularly.28 Empirical research suggests that the practice of anal sex among FSWs is independently associated with age, duration of sex work, number of sexual partners and alcohol use.2
Extensive research on predictors of heterosexual anal sex in the general population is available outside India in other cultural and geographical settings, but it is limited in the context of sex work. In India, although substantial research has focussed on understanding how vaginal sex practices relate to HIV risk, the relationship between anal sex and HIV-related sexual risk factors has not been researched extensively. Also, the Indian studies examining anal sex practices among FSWs are constrained by several limitations,27,28 including the failure to identify subgroups of sex workers who were more likely to practice anal sex. The study by Mondal and colleagues27 was limited to examining the prevalence of anal sex among sex workers in Karnataka and lacked advanced analyses to draw statistical inferences on the predictors of anal intercourse. The limitation in analysis could be due to the data collection approach used (the Polling Booth Survey method was used, where responses are collected from a group of individuals and hence cannot be analysed at an individual level). The second study, conducted in Andhra Pradesh by Matheou,28 used the focus group discussion approach with a small sample size of 50 FSWs, which limited the scope for any scientific analysis. As anal sex has been recognised as a marker to vulnerability towards HIV and associated risk factors, it is important to examine the subgroups of sex workers engaging in anal sex and determine whether such practices influence their HIV-related sexual risk behaviours. Therefore, this study has two objectives: (i) to examine the correlates of anal sex practice among FSWs in India and (ii) examine the relationship between practice of anal sex and HIV-related sexual risk factors.
Methods
Study context
This study was based in Krishna and Vizianagaram, two coastal districts of Andhra Pradesh, which is identified as one of the high HIV epidemic states in India29 and where previous studies have reported a high prevalence of anal sex among sex workers.28 The study districts were intentionally selected to include areas where the HIV prevention programmes funded by Avahan, the India AIDS initiative,30 were implemented.
According to census of India, in 2011, Krishna District had a population of 4 529 009, 68% of which is rural.31 The population density is 519 individuals per square kilometre and the literacy rate is 74%.31 Developmental indicators suggest that 95% of the households in the district have access to electricity, 93% have access to drinking water, 60% have toilet facilities, 46% live in a pucca (concrete) house, 21% of girls wed before the age of 18 years and 77% households have a below poverty line (BPL) card.32 Mapping estimates suggest that in 2009, the district had ~8000 FSWs accounting for 7% of the total sex worker population in the state.33 The HIV sentinel survey in 2008 estimated an HIV prevalence of 0.7% among women attending antenatal care clinics.33
The district of Vizianagaram had a population of 2 342 868, 82% of which is rural.34 It has a population density of 358 individuals per square kilometre and literacy rate of 59%.34 Developmental indicators suggest that 79% households have access to electricity, 84% have access to drinking water, 19% have toilet facilities, 34% live in a pucca (concrete) house, 29% of girls wed before the age of 18 years and 87% had a BPL card at the time of the survey.32 In 2009, 1038 sex workers were mapped in the district, comprising nearly 1% of the state’s FSW population.35 In 2008, the HIV prevalence among antenatal clinic attendees was 0.9% and ~12% among walk-in females at Integrated Counselling and Testing Centres. 35
Study design
This study utilises data from the Behavioural Tracking Survey (BTS), a cross-sectional behavioural survey conducted among FSWs in 2011 to monitor the key components of the HIV prevention programme: safer sex behaviour, STI treatment-seeking behaviours and community mobilisation. In the BTS, FSWs were defined as females aged 18 years or more who had engaged in sex in exchange for cash in the month before the survey. A sample size of 400 FSWs was estimated for each district, allowing for detection of an absolute difference of 15% or more from the assumed value of 50% for consistent condom use with all clients, with 95% confidence, 90% power and a design effect of 1.7.
Hotspots where FSWs congregate to solicit clients such as brothels, streets, parks, cinema halls and homes were designated to be the primary sampling units (PSUs) in the BTS. A list of hotspots that served as a sampling frame of PSUs was produced by a rapid mapping exercise conducted using key informant interviews with community members and key local stakeholders such as the police and social workers. Each hotspot was mapped to validate the existing list of hotspots developed by nongovernmental organisations (NGOs) for implementing the programme, which helped in validating FSW size estimates and identifying active hotspots with the data provided by NGOs implementing the programme. For each hotspot, data were gathered on the number of FSWs present, segregated by the time slot when sex work was undertaken (e.g. 0900–1500 hours, 1500–19 000 hours, etc.) and by type of sex work. The data collected in the mapping exercise were consolidated and finalised after discussions with the NGOs, thus ensuring all the sex work sites in the districts were covered by the mapping team.
Hotspots located in the area covered by an NGO outreach worker for programme implementation were grouped to form a stratum of 200–250 FSWs. The number of interviews to be conducted in each stratum was allocated proportionately according to its size and was further disaggregated within each stratum by type of sex work. In each stratum, FSWs were recruited through a two-stage sampling procedure. In the first stage, a fixed number of PSUs were selected within each stratum using the proportion to population size procedure. The number of interviews to be conducted in each PSU was allocated proportionally. For FSWs based in nonpublic places (brothels, hotels, lodges, roadside eating establishments and homes), the conventional cluster sampling approach was used by selecting hotspots. For FSWs based in public places (streets, market areas, highways and cinema halls), time–location cluster sampling was used, where a hotspot was replicated multiple times to form a cluster for each time slot when FSWs congregate at the hotspot.36 In the second stage, respondents were selected within each selected hotspot.
A total of 1062 FSWs were approached, of which 267 refused participation, resulting in a total analytical sample of 795 FSWs with a response rate of 75%. Sample weights were calculated to account for the unequal selection probability of respondents and nonresponse rates within each PSU. The survey instrument was developed in English and translated into Telugu, the local language of Andhra Pradesh. The translated forms were reviewed by study investigators fluent in both English and Telugu. Trained investigators with verbal and written skills in Telugu conducted interviews.
Ethical considerations
The BTS procedures was reviewed and approved by the institutional review boards of Family Health International and Karnataka Health Promotion Trust. A comprehensive informed consent process was followed, and no names or identifying information were recorded. Interviews took place in locations where women were comfortable and their privacy was assured.
Measures
Sociodemographic and sex work related variables
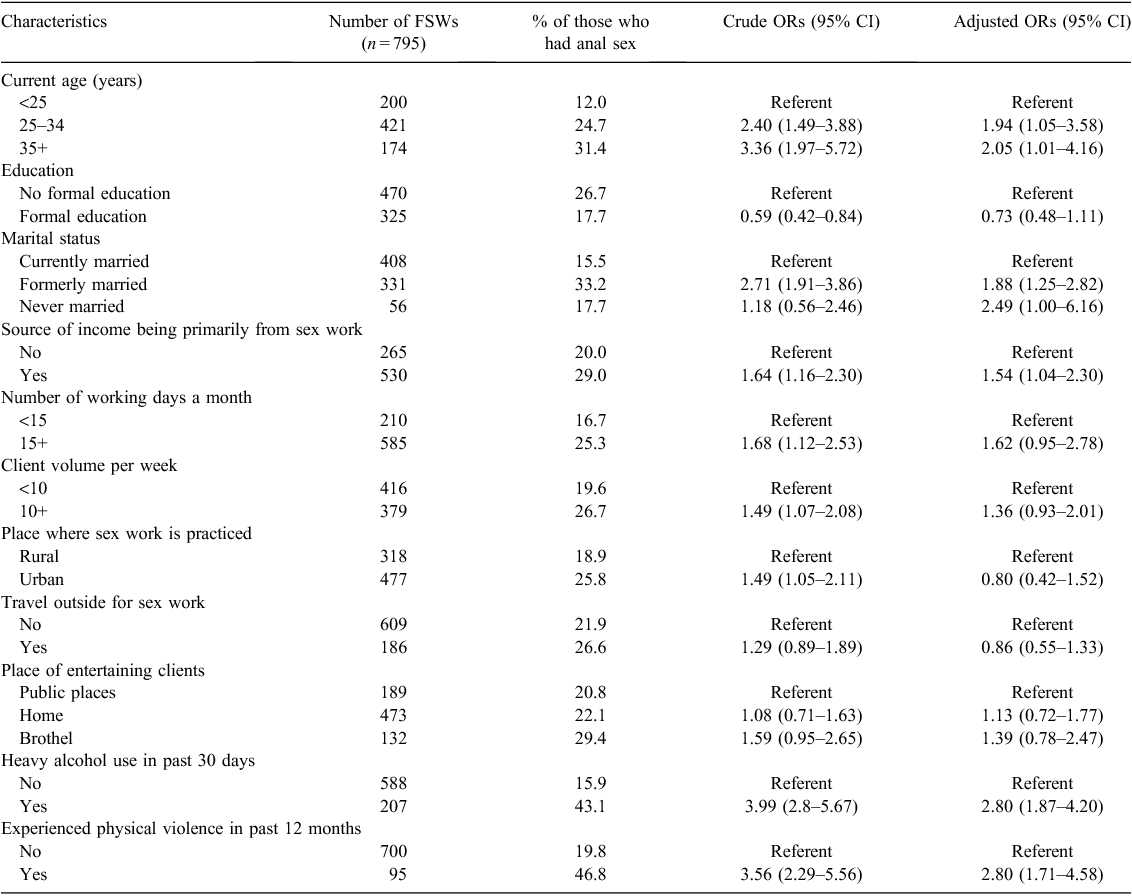
Single item questions were used to collect sociodemographic and sex work related information. They included age (grouped into three categories: <25 years, 25–34 years and 35+ years), education (recoded into two categories: no formal education and formal education), marital status (recoded into three categories: currently married, formerly married and never married), place where sex work is practiced (rural or urban), source of income being primarily from sex work (recoded into two categories: no and yes), number of working days in a month (recoded into two groups: <15 days and 15+ days), client volume per week (grouped into two categories: <10 v. 10+); place of entertaining clients (grouped into public place-based, home-based and brothel-based); travel outside for sex work (no or yes), heavy alcohol use (consumption of four or more drinks on a single occasion at least once) in past 30 days (no or yes) and experience of physical violence from someone including clients, regular partners and goondas (abusive men) in past 12 months (no or yes) were considered. These variables were used as independent variables when examining the associations with anal sex practices.
Anal sex in the last 12 months
All respondents were asked a single item question on whether they had practiced anal sex with any sexual partner in last 12 months, with response categories of ‘no’ and ‘yes’. This variable was used as a dependent variable in the first multivariate model where we explored the correlates of anal sex practice, and as a key independent variable in the series of multivariate models when examining its association with HIV-related sexual risk factors.
HIV risk behaviours
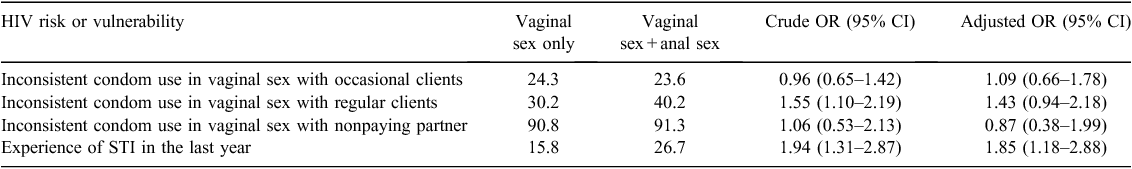
HIV risk was assessed in terms of inconsistent condom use in vaginal sex. Inconsistent condom use in vaginal sex was measured for each of the following three types of sex partners: occasional clients, regular clients, and nonpaying partners. FSWs were asked about the frequency of condom use for each type of partners with response options of ‘always’, ‘sometimes’ or ‘never’. FSWs who reported having always used a condom were considered as consistent condom users; others were categorised as inconsistent condom users.
Self-reported STI
Participants were asked if they experienced any genital ulcers or sores or vaginal discharge during the year before the survey. Those who answered affirmatively to any of these symptoms were considered to have suffered from some STI in the year preceding the survey.
Statistical analyses
Univariate, bivariate and multivariate analyses were performed. Multivariate logistic regression analyses were done in two stages: the first was to identify the correlates of anal sex, with the practice of anal sex in last 12 months as the dependent variable; the second was to examine the association of anal sex with HIV-related sexual risk factors, where anal sex has been considered as the key independent variable. We fitted independent logistic regression models for different measures of HIV-related sexual risk factors to predict the effect of anal sex on these risk behaviours. Each multivariate model was controlled for age, education, marital status, source of income being primarily from sex work, number of working days in a month, client volume per week, place of entertaining clients, heavy alcohol use in the past 30 days, experience of physical violence in the past 12 months and travel outside for sex work. The results from logistic regression were presented in the form of odds ratios and their corresponding 95% confidence intervals (CI). All the analyses were performed using STATA ver. 11.1 (StataCorp., College Station, TX, USA).
Results
Sample characteristics
The majority of the survey participants were young women, with a mean age of 29 years (s.d. = 6.2) and had been practicing sex work for 4.5 years on average (s.d. = 3.3). About one-fifth (21%) of FSWs were 35 years or older, and more than half (51%) were currently married. Two-thirds (67%) reported sex work as the only source of income and three-quarters (74%) had been practicing sex work for more than 15 days a month. Three-fifths (60%) were engaged in sex work in urban areas. About 23% of FSWs had travelled outside the district for sex work and 60% reported sex with clients in home-based settings (Table 1).
Anal sex practices
Nearly four-fifths (79%) of FSWs reported that their clients had demanded anal sex, with the average number of clients being 3 of their last 10 clients. One-quarter (23%) of FSWs reported that they had anal sex in the last year. Only 4% of FSWs who had anal sex in last 12 months reported not having used a condom at the last instance of anal sex. The majority of FSWs reported the reason for engaging in anal sex to be for more money (94%), followed by risk of losing clients (22%) and the perception that men finish more quickly (19%) (Table 2).

|
Bivariate analysis suggests that anal sex in the last 12 months was higher among older FSWs (35+ years) than in younger FSWs (<25 years) (31% v. 12%, P < 0.001), in those with no formal education than in those with some education (27% v. 18%, P = 0.003), in those formerly married FSWs than in those currently married ones (33% v. 16%, P < 0.001) and in those with sex work as only source of income than in those who had other sources of income (29% v. 20%, P = 0.004). The practice of anal sex also varied considerably by sex work related characteristics. Anal sex in the last 12 months was higher among FSWs working for 15 days or more in a month than among those working for fewer days (25% v. 17%, P = 0.012), those with 10 or more clients a week than among those with fewer clients (27% v. 20%, P = 0.018), those had sex in brothels than among those who had sex in public places (29% v. 21%, P = 0.077) and in those practicing sex work in urban areas compared to rural areas (26% v. 19%, P = 0.023). Anal sex was also higher in those FSWs who reported heavy consumption of alcohol in the past 30 days compared to those who did not (43% v. 16%, P < 0.001) and among those who had experienced violence compared with those who had not (47% v. 20%, P < 0.001).
The multivariate analysis confirms the findings of the bivariate analysis and suggests that older FSWs were more likely than younger ones to have practiced anal sex (adjusted odds ratio (AOR): 2.05; 95% CI: 1.01–4.16), and formerly married FSWs were more likely to practice anal sex than currently married FSWs (AOR: 1.88; 95% CI: 1.25–2.82). The odds of anal sex practice were also higher among FSWs whose primary source of income was sex work (AOR: 1.54; 95% CI: 1.04–2.30), those who reported heavy alcohol consumption (AOR: 2.80; 95% CI: 1.87–4.20) and those who had experienced violence (AOR: 2.80; 95% CI: 1.71–4.58) than their counterparts.
Association between anal sex and HIV risk behaviours
After adjusting for different demographics and sex work related factors, the data showed that FSWs practicing anal sex were more likely to report experiencing STI-related symptoms in the last 12 months before the survey than those who did not engage in anal sex (27% v. 16%; AOR: 1.85, 95% CI: 1.18–2.88). However, anal sex was not associated with the rate of condom use with different types of sexual partners (Table 3).
Discussion
This cross-sectional study of FSWs in two coastal districts of Andhra Pradesh is one of the first to document the correlates of anal sex practice among sex workers in India and found that a substantial proportion of FSWs practicing anal sex with their clients. The study found that anal sex was more likely to be practiced among FSWs who were more than 25 years old, had been formerly married, gained their income primarily from sex work, had experienced physical violence in last 12 months and reported heavy alcohol use in last 12 months compared with their counterparts. The research also documented positive associations between anal sex and experiencing STI-related symptoms; however, no statistical association was noted between anal sex practice and inconsistent condom use in vaginal sex.
The study findings on the association between age and anal sex practices corroborates the findings of other studies linking older age to increased anal sex practice.1,37 This positive association may be due to fact that old sex workers get fewer clients to entertain than younger sex workers and may fear for their survival in the situation of a reducing number of clients. Hence, such FSWs tend to agree to the demands made by clients, which can range from the type of sex – particularly anal sex – the place of sex or alcohol consumption. This explanation is supported by the findings of the current research with 40% of older sex workers reporting that they had practiced anal sex for fear of losing their clients and that ~75% of the older sex workers reported sex work to be the only source of income. In addition, FSWs who gained income only from sex work were twice as likely to practice anal sex as who had income from other sources as well.
This study’s findings of the higher likelihood of anal sex among formerly married FSWs are supported by similar findings from the study by Leynaert et al.10 Though it is not possible to determine whether such FSWs transitioned from currently married to formerly married in last 12 months, it is highly likely that most of these FSWs would have gone through this transition much earlier than 12 months preceding the survey. Post hoc analysis suggests that 79% of the formerly married FSWs were either living alone or staying with their children, indicating greater dependence of family members on their income. In such situation, sex workers need to earn more money to contribute to the family income38 either by entertaining more clients or charging more money per client while surrendering to clients’ demands. As a large proportion of the formerly married FSWs tend to be in their late 30s, their ability to attract clients may not be that encouraging compared with younger sex workers and hence, these sex workers may agree to clients’ demands for anal sex. Further in-depth studies are needed to confirm these assumptions.
The importance of alcohol consumption in the practice of risky sexual behaviour has been well documented by many research studies among sex workers as well as in the general population.2,39,40 This study also noted a strong association between heavy consumption of alcohol and anal sex. Although information on whether FSWs consumed alcohol before having anal sex was not collected, there is evidence to suggest that they may consume alcohol before sex in general to numb themselves from the pain of anal sex.41 The research also noted that experience of physical violence was one of the strongest correlates of anal sex, which corroborates the findings of other studies in India and Moscow.26,42 Though it is difficult to establish a one-to-one relationship between experiencing violence and anal sex based on a cross-sectional survey, future research should examine the context of this relationship. However, it is clear from the study findings that experiencing violence can create an unsafe environment for a FSW, which can hamper her negotiation power for safer sex; engagement in anal sex may be one of the outcomes of that weak negotiation.
The research findings documented a similar rate of inconsistent condom use among FSWs who reported anal sex versus who did not report anal sex in last 12 months, which is contrary to the other research among sex workers in Rwanda and Kenya.2 Furthermore, our study findings suggests that a significantly higher proportion of FSWs engaging in anal sex were more likely to report experiencing STI-related symptoms in the last 12 months. Given no difference in condom use levels, the high rates of STIs may be occurring due to practice of anal sex. Interestingly, majority of FSWs who practiced anal sex reported using a condom at last anal sex. Post hoc analysis suggests that ~80% of FSWs practicing anal sex either reported no use of lubricant or used oil-based lubricants like coconut oil or Vaseline in anal sex, which would have increased the likelihood of condom breakage. This is supported by empirical research showing higher chances of condom breakage when a condom is used in anal sex.13,14,43 Hence, even though many FSWs used condoms, the protection from STIs may be very minimal and resulting in STIs.
Although the study findings offer important evidences for programme intervention, certain limitations need to be kept in mind while interpreting the results. First, data were collected in a cross-sectional survey and although associations between some of the behavioural measures and anal sex are evident, the cause–effect relationship between them is difficult to establish. Second, previous research suggests that the prevalence of anal sex may be under-reported, as the information was self-reported and the stigma associated with reporting such sensitive experiences is recognised.3,44 Third, biological samples were not collected in the survey; instead, self-reported STI symptoms were used as marker for HIV risk. Future research should collect biological samples and attempt to establish the association of anal sex with HIV and other STIs. Next, information on inconsistent condom use in anal sex was not collected in this study; however, it is recommended that future research studies among sex workers in India collect such information to examine the correlates of unprotected anal sex. Further, we suggest that information on risk perception specific to anal sex and anal sex practices by type of partners should be collected to better understand the context of anal sex practices and help programme planners in strategising interventions that are more effective.
The findings of this study have important policy implications. The high rates of STI-related symptoms, particularly anal sores and ulcers, reported by FSWs engaging in anal sex highlight the need for inclusion of communication messages related to the need for a safer sex environment even during anal sex. The finding that a substantial proportion of FSWs are practicing anal sex in a setting where HIV prevention programmes have been implemented for some time suggests that such interventions have not provided adequate information on the risks of anal sex and the need to abstain from such practices. HIV prevention programmes should educate FSWs on risks associated with anal sex practices in their routine visits to the STI clinics as well as during one-to-one counselling sessions. Special attention is needed in such interventions at clinics to build the skills of FSWs on safer sex negotiation with clients demanding anal sex. From the data, it is evident that FSWs who are in disadvantaged life situations, such as FSWs who are older, formerly married and working in brothels reported anal sex more than their counterparts. In order to make anal sex safer, the peer educators or community collectives needs to work together to educate these disadvantaged sex workers on the HIV risks associated with anal sex and the need for safer sex practices. Furthermore, FSWs who reported anal sex were also the ones reporting heavy alcohol consumption and who had experienced violence, vulnerability factors that need special attention from the programme using structural interventions. Structural interventions such as community collectivisation initiatives need to undertake education campaigns within sex work settings to educate both clients and FSWs on the need for safer sex practice even in anal sex. Further, STI clinics treating both FSWs and their clients could be the place for focussed communication on anal sex and its associated STI risk. In summary, anal sex is practiced by specific subgroups of FSWs, who may be in disadvantaged life situations in India and elsewhere, and may need increased attention from HIV prevention interventions.
Conflicts of interest
None declared.
Acknowledgements
This paper was written as part of the Knowledge Network project of the Population Council, which is a grantee of the Bill & Melinda Gates Foundation through Avahan, the Indian AIDS Initiative. The views expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Bill & Melinda Gates Foundation and Avahan. RKP led the study design and conception and assisted in manuscript preparation. BM led the analyses, manuscript preparation, interpretation of the findings, and assisted in conceptualization. DK assisted in study design and data collection. LP assisted in study design, data collection and interpretation of the study findings. NS provided overall guidance with analytical approach and interpretation of study findings. All authors read and approved the final manuscript.
References
[1] Baldwin JI, Baldwin JD. Heterosexual anal intercourse: an understudied, high-risk sexual behavior. Arch Sex Behav 2000; 29 357–73.| Heterosexual anal intercourse: an understudied, high-risk sexual behavior.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2Flsl2rsg%3D%3D&md5=f5efc10733f0cc401860f1be9c57921fCAS |
[2] Veldhuijzen NJ, Ingabire C, Luchters S, Bosire W, Braunstein S, Chersich M, et al Anal intercourse among female sex workers in East Africa is associated with other high-risk behaviours for HIV. Sex Health 2011; 8 251–4.
| Anal intercourse among female sex workers in East Africa is associated with other high-risk behaviours for HIV.Crossref | GoogleScholarGoogle Scholar |
[3] Halperin DT. Heterosexual anal intercourse: prevalence, cultural factors, and HIV infection and other health risks, part I. AIDS Patient Care STDS 1999; 13 717–30.
| Heterosexual anal intercourse: prevalence, cultural factors, and HIV infection and other health risks, part I.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c3gsl2gug%3D%3D&md5=d6bd73f6ee7123e461f2277462d519a1CAS |
[4] Misegades L, Page-Shafer K, Halperin D, McFarland W. Anal intercourse among young low-income women in California: an overlooked risk factor for HIV? AIDS 2001; 15 534–5.
| Anal intercourse among young low-income women in California: an overlooked risk factor for HIV?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mzptl2iug%3D%3D&md5=ec56cad6223b3ff0daf28a00bc7d18ebCAS |
[5] Lane T, Pettifor A, Pascoe S, Fiamma A, Rees H. Heterosexual anal intercourse increases risk of HIV infection among young South African men. AIDS 2006; 20 123–5.
| Heterosexual anal intercourse increases risk of HIV infection among young South African men.Crossref | GoogleScholarGoogle Scholar |
[6] Erickson PI, Bastani R, Maxwell AE, Marcus AC, Capell FJ, Yan KX. Prevalence of anal sex among heterosexuals in California and its relationship to other AIDS risk behaviors. AIDS Educ Prev 1995; 7 477–93.
| 1:STN:280:DyaK28vgtlWisg%3D%3D&md5=136acab5c90f21a143ab4897232d40daCAS |
[7] Buchacz K, van der Straten A, Saul J, Shiboski SC, Gomez CA, Padian N. Sociodemographic, behavioral, and clinical correlates of inconsistent condom use in HIV-serodiscordant heterosexual couples. J Acquir Immune Defic Syndr 2001; 28 289–97.
| 1:STN:280:DC%2BD3MnltFaguw%3D%3D&md5=d7750ccc2c9dd860eea407393c53cefdCAS |
[8] Padian N, Marquis L, Francis DP, Anderson RE, Rutherford GW, O’Malley PM, et al Male-to-female transmission of human immunodeficiency virus. JAMA 1987; 258 788–90.
| Male-to-female transmission of human immunodeficiency virus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s3osVantA%3D%3D&md5=84eafdb2e7a29605f121b7dbc9d144a8CAS |
[9] Skurnick JH, Kennedy CA, Perez G, Abrams J, Vermund SH, Denny T, et al Behavioral and demographic risk factors for transmission of human immunodeficiency virus type 1 in heterosexual couples: report from the Heterosexual HIV Transmission Study. Clin Infect Dis. 1998; 26 855–64.
| Behavioral and demographic risk factors for transmission of human immunodeficiency virus type 1 in heterosexual couples: report from the Heterosexual HIV Transmission Study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3isFKmsw%3D%3D&md5=980bdd36e309c275b3c7a85f0b0db7f5CAS |
[10] Leynaert B, Downs AM, de Vincenzi I. Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV. Am J Epidemiol 1998; 148 88–96.
| Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1czis1Gnsw%3D%3D&md5=100399e1d6ed26d0f51b34cb033ff862CAS |
[11] Padian NS, Shiboski SC, Glass SO, Vittinghoff E. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study. Am J Epidemiol 1997; 146 350–7.
| Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2svhs1Cltg%3D%3D&md5=46c5046482552bb11677a413c55329d2CAS |
[12] Voeller B. AIDS and heterosexual anal intercourse. Arch Sex Behav 1991; 20 233–76.
| AIDS and heterosexual anal intercourse.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M3ot1KmtA%3D%3D&md5=8e116fb0930ac1c0842f4e6e5901272eCAS |
[13] Silverman BG, Gross TP. Use and effectiveness of condoms during anal intercourse: a review. Sex Transm Dis 1997; 24 11–7.
| Use and effectiveness of condoms during anal intercourse: a review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s7ntFGlsg%3D%3D&md5=9e5478596881bb5f5c0b939b66f5ae86CAS |
[14] Priddy FH, Wakasiaka S, Hoang TD, Smith DJ, Farah B, Del Rio C, et al Anal sex, vaginal practices, and HIV incidence in female sex workers in urban Kenya: implications for the development of intravaginal HIV prevention methods. AIDS Res Hum Retroviruses 2011; 27 1067–1072.
| Anal sex, vaginal practices, and HIV incidence in female sex workers in urban Kenya: implications for the development of intravaginal HIV prevention methods.Crossref | GoogleScholarGoogle Scholar |
[15] Eng C. Anal cancer: current and future methodology. Cancer Invest 2006; 24 535–44.
| Anal cancer: current and future methodology.Crossref | GoogleScholarGoogle Scholar |
[16] Scott H, Khoury J, Moore BA, Weissman S. Routine anal cytology screening for anal squamous intraepithelial lesions in an urban HIV clinic. Sex Transm Dis 2008; 35 197–202.
| Routine anal cytology screening for anal squamous intraepithelial lesions in an urban HIV clinic.Crossref | GoogleScholarGoogle Scholar |
[17] Frisch M, Glimelius B, van den Brule AJ, Wohlfahrt J, Meijer CJ, Walboomers JM, et al Sexually transmitted infection as a cause of anal cancer. N Engl J Med 1997; 337 1350–8.
| Sexually transmitted infection as a cause of anal cancer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c%2FgtVSjtg%3D%3D&md5=85b48368c0091b3841291d9bd7f8096cCAS |
[18] Leichliter JS. Heterosexual anal sex: part of an expanding sexual repertoire? Sex Transm Dis 2008; 35 910–1.
| Heterosexual anal sex: part of an expanding sexual repertoire?Crossref | GoogleScholarGoogle Scholar |
[19] Schwandt M, Morris C, Ferguson A, Ngugi E, Moses S. Anal and dry sex in commercial sex work, and relation to risk for sexually transmitted infections and HIV in Meru, Kenya. Sex Transm Infect 2006; 82 392–6.
| Anal and dry sex in commercial sex work, and relation to risk for sexually transmitted infections and HIV in Meru, Kenya.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28rotlKktQ%3D%3D&md5=402322c14979834a68f66132f5567b9bCAS |
[20] Bogart LM, Kral AH, Scott A, Anderson R, Flynn N, Gilbert ML, et al Sexual risk among injection drug users recruited from syringe exchange programs in California. Sex Transm Dis 2005; 32 27–34.
| Sexual risk among injection drug users recruited from syringe exchange programs in California.Crossref | GoogleScholarGoogle Scholar |
[21] Fonck K, Kaul R, Keli F, Bwayo JJ, Ngugi EN, Moses S, et al Sexually transmitted infections and vaginal douching in a population of female sex workers in Nairobi, Kenya. Sex Transm Infect 2001; 77 271–5.
| Sexually transmitted infections and vaginal douching in a population of female sex workers in Nairobi, Kenya.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MvltVynsQ%3D%3D&md5=6c004856cc3d547a93c8abc7d642b99aCAS |
[22] Ferguson A, Morris C. Assessing the role of anal intercourse in the epidemiology of AIDS in Africa. Int J STD AIDS 2003; 14 856
| Assessing the role of anal intercourse in the epidemiology of AIDS in Africa.Crossref | GoogleScholarGoogle Scholar |
[23] Gross M, Holte SE, Marmor M, Mwatha A, Koblin BA, Mayer KH. Anal sex among HIV-seronegative women at high risk of HIV exposure. The HIVNET Vaccine Preparedness Study 2 Protocol Team. J Acquir Immune Defic Syndr 2000; 24 393–8.
| Anal sex among HIV-seronegative women at high risk of HIV exposure. The HIVNET Vaccine Preparedness Study 2 Protocol Team.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3cvmsVWjsw%3D%3D&md5=3f4efdf0b3df480101eb67a33825e33fCAS |
[24] Karim SS, Ramjee G. Anal sex and HIV transmission in women. Am J Public Health 1998; 88 1265–6.
| Anal sex and HIV transmission in women.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1czmslCqsw%3D%3D&md5=66a82058199bab8b48379f6e6f3587d1CAS |
[25] Ramjee G, Weber AE, Morar NS. Recording sexual behavior: comparison of recall questionnaires with a coital diary. Sex Transm Dis 1999; 26 374–80.
| Recording sexual behavior: comparison of recall questionnaires with a coital diary.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MzosFyisw%3D%3D&md5=a4a2a2e4ccbaa078dc6137f07c0b5f1aCAS |
[26] Beattie TS, Bhattacharjee P, Ramesh BM, Gurnani V, Anthony J, Isac S, et al Violence against female sex workers in Karnataka state, south India: impact on health, and reductions in violence following an intervention program. BMC Public Health 2010; 10 476–487.
| Violence against female sex workers in Karnataka state, south India: impact on health, and reductions in violence following an intervention program.Crossref | GoogleScholarGoogle Scholar |
[27] Mondal S, Ramesh B, Blanchard JF, Moses S, eds. Condom use and experience of violence: evidence from polling booth surveys among female sex workers in Karnataka, India. XVI International AIDS Conference; 2006; Toronto, Canada.
[28] Matheou A. A blind spot in HIV prevention – female anal sex in India. New Delhi: International HIV/AIDS Alliance in India; 2010.
[29] National AIDS Control Organization (NACO). Targeted interventions among core groups under NACP III: operational guidelines. New Delhi: NACO; 2007. Available online at: http://www.nacoonline.org/upload/Publication/NGOs%20and%20targetted%20Intervations/NACP-III.pdf [verified 7 November 2011].
[30] Bill & Melinda Gates Foundation (BMGF). Avahan – the India AIDS initiative: the business of HIV prevention at scale. New Delhi, India: BMGF; 2008.
[31] Registrar General of India. Krishna: census 2011. New Delhi: Office of the Registrar General & Census Commissioner; 2011. Available online at: http://www.census2011.co.in/census/district/133-krishna.html [verified March 2012].
[32] International Institute for Population Sciences (IIPS). District level household and facility survey (DLHS-3), 2007–08: India: key indicators: states and districts. Mumbai: IIPS, Ministry of Health and Family Welfare Government of India; 2010.
[33] Indian Institute of Public Health (IIPH). Epidemiological appraisal of HIV situation in District Krishna using data triangulation. Hyderabad: IIPH; 2010.
[34] Registrar General of India. Vizianagaram: census 2011. New Delhi: Office of the Registrar General & Census Commissioner; 2011. Available online at: http://www.census2011.co.in/census/district/129-vizianagaram.html [verified March 2012].
[35] Indian Institute of Public Health (IIPH). HIV situation and response in Vizianagaram District: epidemiological appraisal using data triangulation. Hyderabad: IIPH; 2010.
[36] Saidel T, Adhikary R, Mainkar M, Dale J, Loo V, Rahman M, et al Baseline integrated behavioural and biological assessment among most at-risk populations in six high-prevalence states of India: design and implementation challenges. AIDS 2008; 22 S17–34.
| Baseline integrated behavioural and biological assessment among most at-risk populations in six high-prevalence states of India: design and implementation challenges.Crossref | GoogleScholarGoogle Scholar |
[37] Leichliter JS, Chandra A, Liddon N, Fenton KA, Aral SO. Prevalence and correlates of heterosexual anal and oral sex in adolescents and adults in the United States. J Infect Dis 2007; 196 1852–9.
| Prevalence and correlates of heterosexual anal and oral sex in adolescents and adults in the United States.Crossref | GoogleScholarGoogle Scholar |
[38] Dandona R, Dandona L, Kumar GA, Gutierrez J, McPherson S, Samuels F, et al Demography and sex work characteristics of female sex workers in India. BMC Int Health Hum Rights 2006; 6 6–16.
| Demography and sex work characteristics of female sex workers in India.Crossref | GoogleScholarGoogle Scholar |
[39] Hutton HE, McCaul ME, Santora PB, Erbelding EJ. The relationship between recent alcohol use and sexual behaviors: gender differences among sexually transmitted disease clinic patients. Alcohol Clin Exp Res 2008; 32 2008–15.
| The relationship between recent alcohol use and sexual behaviors: gender differences among sexually transmitted disease clinic patients.Crossref | GoogleScholarGoogle Scholar |
[40] Cook RL, Comer DM, Wiesenfeld HC, Chang CC, Tarter R, Lave JR, et al Alcohol and drug use and related disorders: an underrecognized health issue among adolescents and young adults attending sexually transmitted disease clinics. Sex Transm Dis 2006; 33 565–70.
| Alcohol and drug use and related disorders: an underrecognized health issue among adolescents and young adults attending sexually transmitted disease clinics.Crossref | GoogleScholarGoogle Scholar |
[41] Kalichman SC, Simbayi LC, Jooste S, Cain D, Cherry C. Sensation seeking, alcohol use, and sexual behaviors among sexually transmitted infection clinic patients in Cape Town, South Africa. Psychol Addict Behav 2006; 20 298–304.
| Sensation seeking, alcohol use, and sexual behaviors among sexually transmitted infection clinic patients in Cape Town, South Africa.Crossref | GoogleScholarGoogle Scholar |
[42] Decker MR, Wirtz AL, Baral SD, Peryshkina A, Mogilnyi V, Weber RA, et al Injection drug use, sexual risk, violence and STI/HIV among Moscow female sex workers. Sex Transm Infect 2012; 88 278–283.
| Injection drug use, sexual risk, violence and STI/HIV among Moscow female sex workers.Crossref | GoogleScholarGoogle Scholar |
[43] Bradley J, Rajaram S, Alary M, Isac S, Washington R, Moses S, et al Determinants of condom breakage among female sex workers in Karnataka, India. BMC Public Health 2011; 11 S14
| Determinants of condom breakage among female sex workers in Karnataka, India.Crossref | GoogleScholarGoogle Scholar |
[44] Smith LB, Adler NE, Tschann JM. Underreporting sensitive behaviors: the case of young women’s willingness to report abortion. Health Psychol 1999; 18 37–43.
| Underreporting sensitive behaviors: the case of young women’s willingness to report abortion.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M7it1CgsA%3D%3D&md5=31a5e3629f2876416b288941e8cfe04eCAS |