Gonorrhoea gone wild: rising incidence of gonorrhoea and associated risk factors among gay and bisexual men attending Australian sexual health clinics
Denton Callander A K , Rebecca Guy A , Christopher K. Fairley B C , Hamish McManus A , Garrett Prestage A , Eric P. F. Chow B C , Marcus Chen B D , Catherine C. O Connor A E F , Andrew E. Grulich A , Christopher Bourne A G , Margaret Hellard H , Mark Stoové H I , Basil Donovan A J and on behalf of the ACCESS CollaborationA The Kirby Institute, UNSW Sydney, Sydney, NSW 2052, Australia.
B Melbourne Sexual Health Centre, 580 Swanston Street, Melbourne, Vic. 3000, Australia.
C Central Clinical School, Monash University, Melbourne, Vic. 3800, Australia.
D Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Vic. 3000, Australia.
E RPA Sexual Health Clinic, Sydney Local Health District, 16 Marsden Road, Sydney, NSW 2050, Australia.
F Central Clinical School, University of Sydney, Sydney, NSW 2006, Australia.
G STI Programs Unit, NSW Ministry of Health, 150 Albion Street, Sydney, NSW 2060, Australia.
H Burnet Institute, 85 Commercial Road, Melbourne, Vic. 3000, Australia.
I School of Public Health and Preventative Medicine, Monash University, Melbourne, Vic. 3800, Australia.
J Sydney Sexual Health Centre, Sydney Hospital, 8 Macquarie Street, Sydney, NSW 2000, Australia.
K Corresponding author. Email: d.callander@unsw.edu.au
Sexual Health 16(5) 457-463 https://doi.org/10.1071/SH18097
Submitted: 14 May 2018 Accepted: 17 July 2018 Published: 9 November 2018
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Background: Gonorrhoea notifications continue to rise among gay and bisexual men in Australia and around the world. More information is needed on infection trends, accounting for testing and complimented by demographics and risk practices. Methods: A retrospective cohort analysis was undertaken using repeat gonorrhoea testing data among gay and bisexual men from 2010 to 2017, which was extracted from a network of 47 sexual health clinics across Australia. Poisson and Cox regression analyses were used to determine temporal trends in gonorrhoea incidence rates, as well as associated demographic and behavioural factors. Results: The present analysis included 46 904 gay and bisexual men. Gonorrhoea incidence at any anatomical site increased from 14.1/100 person years (PY) in 2010 to 24.6/100 PY in 2017 (P < 0.001), with the greatest increase in infections of the pharynx (5.6-15.9/100 PY, P < 0.001) and rectum (6.6–14.8/100 PY, P < 0.001). After adjusting for symptomatic and contact-driven presentations, the strongest predictors of infection were having more than 20 sexual partners in a year (hazard ratio (HR) = 1.9, 95% confidence interval (CI): 1.7–2.2), using injecting drugs (HR = 1.7, 95%CI: 1.4–2.0), being HIV positive (HR = 1.4, 95%CI: 1.2–1.6) and being aged less than 30 years old (HR = 1.4, 95%CI: 1.2–1.6). Conclusions: Gonorrhoea has increased dramatically among gay and bisexual men in Australia. Enhanced prevention efforts, as well as more detailed, network-driven research are required to combat gonorrhoea among young men, those with HIV and those who use injecting drugs.
Introduction
In recent years, surveillance systems and epidemiological studies from several countries have shown substantial increases in Neisseria gonorrhoea diagnoses among gay, bisexual and other men who have sex with men.1–4 In Australia, a reliance on passive notification data challenges our ability to report on trends of gonorrhoea by exposure category, risk practice or HIV status, precluding a detailed understanding of the populations and subpopulations most affected by these observed changes. Systems of case reporting are also strongly influenced by changes in diagnostic testing, which is particularly relevant for gonorrhoea given increases in comprehensive screening among gay and bisexual men,5 the introduction of more sensitive nucleic acid amplification testing (NAAT)6 and implementation of dual chlamydia–gonorrhoea testing in the microbiology departments of many Australian laboratories.
One way to assess if rising passive notifications reflect a changing rate of infection is by calculating the incidence of new infections. While not yet possible at the level of an entire population, incidence can be calculated in Australia through the use of data on repeat gonorrhoea testing available from sexual health clinics, which also routinely collect detailed information on patient demographics and behaviours. Collectively, these data can be used to evaluate trends in epidemiology that account for changes in testing among clinic attendees and assess associated risks, both of which have implications for prevention and management. This paper presents a longitudinal analysis of gonorrhoea incidence among gay and bisexual men attending Australian sexual health clinics.
Methods
A retrospective cohort was constructed using routine testing data from 47 publicly funded sexual health clinics in Australia for the period 1 January 2010 to 31 December 2017. Consultation, demographic, behavioural and pathology data were extracted from sites participating in a sentinel surveillance network known as ACCESS (Australian Collaboration for Coordinated Enhanced Sentinel Surveillance), which has been previously described in more detail.7 Patients were linked between and within services using anonymous identifiers. The cohort consisted of male patients aged 16 years and older who reported sexual contact with other men at any point during the 8-year study period.
Gonorrhoea incidence was calculated using repeat testing methods, which limited our analysis to only those patients with at least two gonorrhoea tests during the study period. An incident infection was defined as a negative test followed by a positive test, and time at risk was defined as the time between each patients’ first test and their last test within the study period; more detail on this method of calculating incidence for bacterial sexually transmissible infections (STIs) has been published previously.8 Time at risk was censored for 6 weeks following an incident infection to allow for treatment.
We stratified overall and annual incidence of gonorrhoea by anatomical site (any/all, urogenital, anorectal and pharyngeal), patient place of residence (urban or non-urban9), self-reported injecting drug use in the 12 months before a consultation (yes/no), condom use during anal sex in the 12 months prior (always/sometimes or never) and number of sexual partners in the 12 months prior (<20/≥ 20 partners). Partner numbers were stratified in accordance with definitions of ‘high risk’ used to guide STI testing among gay men in Australia.10 Wilcoxon rank sum tests were used to also assess temporal changes in the proportion of patients annually reporting injecting drug use, inconsistent condom use and a high number of sexual partners. Prescription data were used to stratify gonorrhoea incidence on the basis of HIV pre-exposure prophylaxis (PrEP) among HIV-negative men.
Changes in incidence over time were assessed by fitting year as an independent variable in Poisson regression analyses. To account for uneven time at risk in the first and last years of this analysis, we extended calculations back to 2009 and into the first quarter of 2018. The number of tests per patient per year was controlled for in the regression analysis, in order to account for the possibility of changes in incidence due to increasing or decreasing test frequency over time. Further, we compared changes over time between clinics that utilised NAAT for gonorrhoea over the study duration and those that switched from culture at some point between 2010 and 2017. Patient factors (excluding PrEP) were included in a Cox proportional hazards model, with incident gonorrhoea at any anatomical site as the dependent variable, stratified by clinic to account for potential clustering. The model also controlled for patient age (stratified at the median for our sample: <30/≥ 30 years) and if patients were symptomatic or attended as the result of contact tracing.
ACCESS received ethical approval from the human research ethics committee of the Alfred Hospital (248/17) and from two committees representing the interests of gay and bisexual men (AIDS Council of New South Wales and the Victorian AIDS Council).
Results
Participants
From 2010 to 2017, a total of 75 723 individual gay and bisexual men attended a participating clinic; of those 46 904 (62%) had at least two tests for gonorrhoea. Our sample comprised 14% men who were HIV positive and 61% born in Australia. At their first visit during the study period, the median age of HIV-positive clinic attendees was 37 years (interquartile range (IQR): 30–46) and 29 years for HIV-negative men (IQR: 24–38, P < 0.001). Most participants lived in urban areas (78% of HIV-positive men and 88% of HIV-negative men, P < 0.001).
During the study period, 13% of HIV-positive men and 5% of HIV-negative men reported injecting drug use at least once (P < 0.001), which increased over time among both groups: 6% of HIV-positive and 3% of HIV-negative men reported injecting drug use in the 12 months before their consultation in 2010, which increased to 9% and 4% respectively in 2017 (P’s <0.001). Overall, 50% of HIV-positive and 44% of HIV-negative men reported at least one instance of 20 or more sexual partners in the 12 months before a consultation (P < 0.001) while, by year, the proportion of HIV-negative men reporting more than 20 partners increased from 28% in 2010 to 38% in 2017 (P < 0.001), but remained stable among HIV-positive men (36% in 2010 and 30% in 2017, P = 0.1). In total, 5292 men were identified as receiving PrEP from a participating clinic at least once between 2014 and 2017, representing 15% of the HIV-negative men in our sample.
Overall gonorrhoea incidence rates
The median time between gonorrhoea tests at any anatomical site was 190 days among HIV-negative men and 270 days among HIV-positive men (P < 0.001). The overall incidence rate for infections at any anatomical site was 20.0/100 PY (95%CI: 19.7–20.3). Among HIV-negative men, the rate was 18.7/100 PY (18.4–19.0) and 29.1/100 PY among HIV-positive men (95%CI: 28.2–30.1, P < 0.001). Men who attended for testing on account of symptoms had an incidence rate at any anatomical site of 40.7/100 PY (95%CI: 39.0–42.7), while those tested asymptomatically had a rate of 19.0/100 PY (95%CI: 18.7–19.3, P < 0.001). Table 1 details the total number of incident gonorrhoea and time at risk among gay and bisexual men from 2010 to 2017, stratified by year, anatomical site and HIV status.
Annual gonorrhoea incidence rates
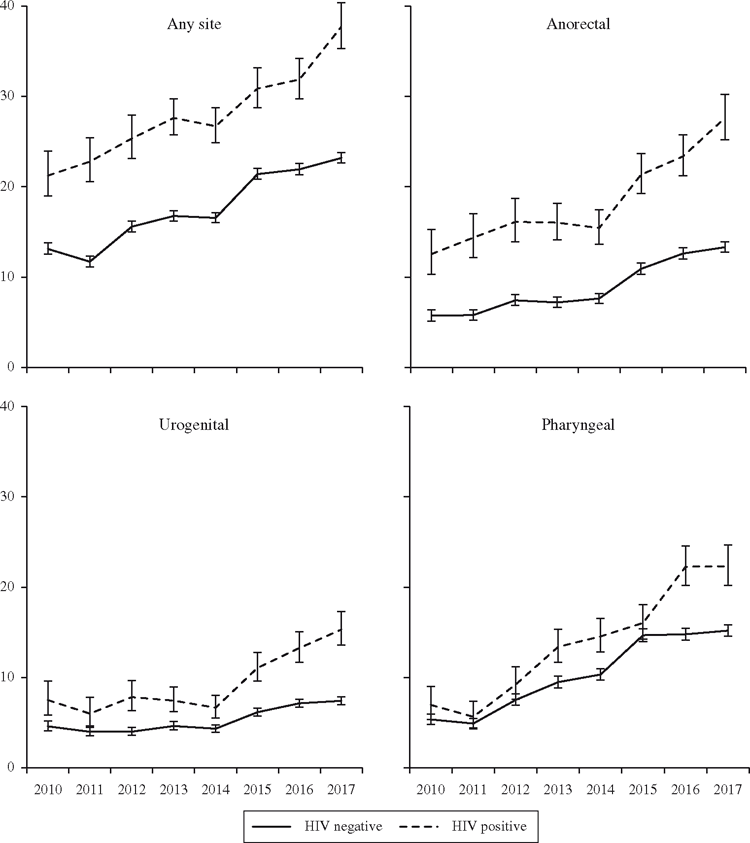
Overall, the incidence of gonorrhoea at any anatomical site increased from 14.1/100 PY in 2010 to 24.6/100 PY in 2017 (P < 0.001; Table 1), an increase that was significant even after controlling for the frequency with which men tested. The incidence rate of anorectal gonorrhoea more than doubled during the study period (6.6/100 PY in 2010 to 14.8/100 PY in 2017, P < 0.001) while the incidence of pharyngeal gonorrhoea nearly tripled (5.6–15.9/100 PY, P < 0.001). These dramatic changes contrast with urogenital incidence over the 8 study years, which increased but to a lesser degree (5.0 to 8.2/100 PY, P < 0.001). Figure 1 shows the changes to incidence over time by anatomical site. Increases in incidence were observed across sites that used NAAT for the entirety of the study period (14.5–26.3/100 PY, P < 0.001) and those that switched from culture testing (13.3–22.1/100 PY, P < 0.001), suggesting that testing technology was not primarily responsible for the temporal changes observed in this analysis.
From 2010 to 2017, gonorrhoea incidence at any anatomical site increased from 13.1/100 PY to 23.2/100 PY among HIV-negative men (P < 0.001) and from 21.2/100 PY to 37.7/100 PY among HIV-positive men (P < 0.001). Overall, men living in urban areas had a higher and increasing rate of infection (14.7/100 PY in 2010 to 26.2/100 PY in 2017, P < 0.001) when compared with men living in non-urban areas (13.1/100 PY in 2010 and 14.3/100 PY in 2017, P = 0.1). And while incidence of gonorrhoea increased across age groups, it was higher overall among gay and bisexual men younger than 30 years.
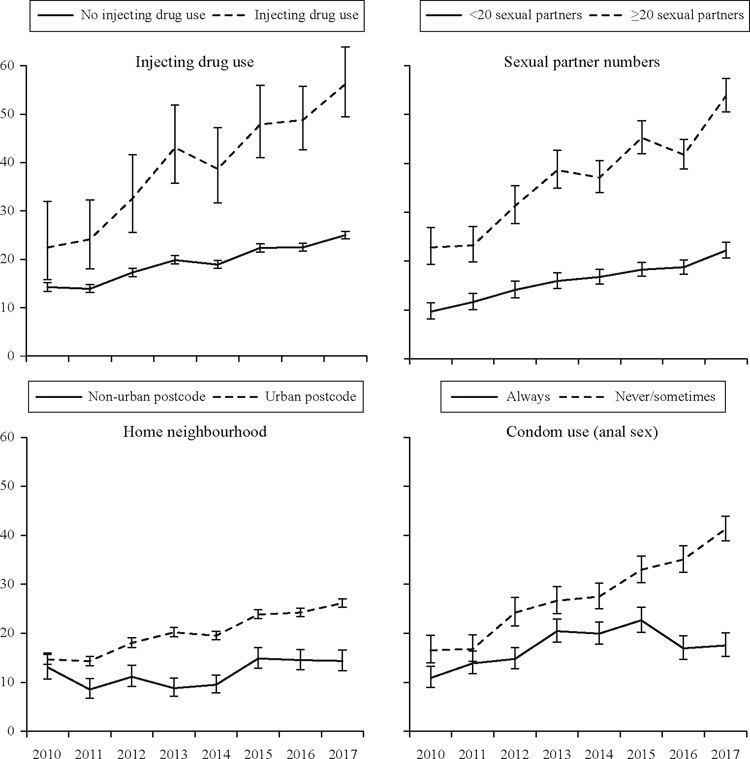
There were notable differences in annual gonorrhoea incidence on the basis of self-reported risk behaviour. From 2010 to 2017, gonorrhoea incidence increased among men who used condoms inconsistently during anal sex (16.5/100 PY to 41.3/100 PY, P < 0.001) but also, although to a lesser degree, among those who reported always using condoms (10.9/100 PY to 17.5/100 PY, P < 0.001). Gonorrhoea incidence among men who reported more than 20 sexual partners in 12 months was 53.9/100 PY in 2017, up from 22.8/100 PY in 2010 (P < 0.001). Notably, although lower overall, the incidence of gonorrhoea also rose significantly among men reporting fewer than 20 partners in a year: from 9.6/100 PY in 2010 to 22.2/100 PY in 2017 (P < 0.001). And for men who reported injecting drug use, gonorrhoea incidence increased from 22.4/100 PY to 56.2/100 PY (P < 0.001), while increasing from 14.3/100 PY to 25.0/100 PY (P < 0.001) among men who did not report injecting. These changes are shown in Figure 2. From 2014 to 2017, the incident rate of gonorrhoea among men receiving PrEP from a sexual health clinic was 36.9/100 PY (95%CI: 35.5–38.4), which compared with 18.6/100 PY among HIV-negative men not receiving PrEP (95%CI: 18.2–19.0, P < 0.001).
In the multivariate analysis, after adjusting for symptom status and self-report STI contact, the following factors were independently associated with incident gonorrhoea at any anatomical site: being HIV positive (hazard ratio (HR) = 1.4, 95%CI: 1.2–1.6, P < 0.001), being under 30 years (HR = 1.4, 95%CI: 1.2–1.5, P < 0.001), being born in Australia (HR = 1.2, 95%CI: 1.1–1.4, P = 0.001) and, in the 12 months before an infection, injecting drug use (HR = 1.7, 95%CI: 1.4–2.0, P < 0.001), inconsistent condom use during anal sex (HR = 1.4, 95%CI: 1.3–1.6, P < 0.001) and having 20 or more sexual partners (HR = 1.9, 95%CI: 1.7–2.2, P < 0.001). It is worth noting that significant differences in gonorrhoea incidence between men in urban and non-urban areas became inconsequential after controlling for sexual partner numbers.
Discussion
The incidence of gonorrhoea among gay and bisexual men attending Australian sexual health clinics has increased dramatically over time, most prolifically in the throat. We identified several associated and unsurprising behavioural covariates – condom (non) use and having many sexual partners – but also found that injecting drugs was a significant predictor of infection. Younger men, those born in Australia, and those with HIV were all more likely than their peers to be diagnosed with gonorrhoea, and men who received PrEP were considerably more likely to become infected during the study period.
Although gonorrhoea incidence increased across anatomical sites, by far the greatest change was in infection of the throat. Modelling research has previously highlighted oral sex as key in sustaining endemic gonorrhoea among gay and bisexual men,11 and it is likely that it is, at least partly, driving the overall rise in gonorrhoea documented by our analysis. With emerging evidence suggesting that it may even be possible to transmit infection via kissing,12 it is clear that greater attention needs to be paid to the throat and its role in rising rates of gonorrhoea in Australia. The use of mouthwash may hold some promise here, but more research is required to understand the degree to which such an intervention can stem infection rates.13,14 Renewed attention to collecting throat swabs for gay men is also important. While pharyngeal samples were commonly collected from men attending sexual health clinics included in this analysis, other research has found that they may be missed in other settings, notably in general practice clinics where so much Australian screening takes place.15
Injecting drug use was a significant predictor of incident gonorrhoea even though not itself a route for infection. In Australia, gay men most commonly inject crystal methamphetamine, which has strong associations with sexual subcultures of intensive sex partying or what is sometimes known as ‘chemsex’16. Previous research has documented that for some men, these subcultures are associated with increased sexual and non-sexual risk taking,17 but it is notable that even after accounting for condom use and partner numbers, injecting drugs remained significantly associated with incident gonorrhoea. This finding echoes similar findings from a smaller Melbourne-based study, which in 2016 found that individual-level factors of condom use and partner numbers only explained part of the risk for infection among gay and bisexual men.18 Collectively, these findings suggest that a more nuanced understanding of the sexual behaviours associated with injecting drug use and the sexual networks of men who use drugs in this way is needed, including the clustering of gonorrhoea defined (or not) by these networks.
Gonorrhoea incidence was much higher among men receiving PrEP from sexual health clinics than other men, which is consistent with earlier research.19 The use of PrEP in lieu of condoms to prevent HIV is the most likely explanation for the dramatic difference in gonorrhoea incidence, including the increase in anorectal infections in the period following the introduction of several large PrEP implementation trials in Australia. It is worth noting, however, that gonorrhoea rates were increasing in the years before PrEP was even introduced, so while its effect on behaviour is likely to exacerbate an already significant problem, the issue of gonorrhoea requires broader consideration for risk and prevention that moves beyond a sole focus on PrEP, condoms or anal sex.
As noted, several other Western, English-speaking countries have observed increases in gonorrhoea diagnoses among men in recent years. Population-level estimates of gonorrhoea incidence among gay men in the USA found a 151% increase from 2010 to 2015, suggesting an even greater increase than observed in Australia. In Canada, data from one sentinel surveillance system in British Columbia found that incident gonorrhoea among gay men had been increasing steadily since 2008, with – as observed here – higher rates among younger men and those living with HIV,2 trends and relationships that have been observed in England as well.4 Overall, the epidemiological similarities between Australia and these countries suggest the need for greater collaboration and connection between respective health departments and researchers, including any attempt to implement or evaluate gonorrhoea-focused interventions.
There are a few considerations when interpreting these data. First, our sample consisted of men attending sexual health clinics, services that tend to attract individuals with higher infection risk profiles than community samples.20 Second, trends in incidence calculated from clinical populations may be biased if the characteristics of the population changes over time. To minimise this potential, we controlled for several demographic and behavioural risk factors. Importantly, our assessment of these factors was limited to those routinely collected by participating services, which serve clinical and not research purposes. More factors than were assessed here and, in some cases greater nuance of existing factors (e.g. condom use with casual vs regular partners), are likely contributors to gonorrhoea diagnoses among gay men. Indeed, the reliance on routinely collected and the retrospective nature of the data used for this analysis should be recognised as a potential limitation. And finally, it is feasible that changes in testing frequency might bias incidence calculated through repeat testing, but we accounted for this potential by controlling for test frequency in our regression analyses of incidence trends over time.
Overall, this analysis suggests that there has been a major increase in gonorrhoea among gay and bisexual men in Australia, one that is not driven (at least not exclusively) by increases in testing or the introduction of PrEP. Targeted interventions and nuanced policy and research are required to meet the spread of gonorrhoea among these men and reduce its overall burden.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
ACCESS is funded by the Australian Department of Health. The authors acknowledge those who supported the provision of data for this analysis, including Dougle Boyle, Afrizal, Heng Lui and Tobias Vickers. We also acknowledge the contributions of investigators from the clinics involved with this analysis, namely Debbie Allen, Katherine Brown, Christopher Carmody, Manoji Gunathilake, Eva Jackson, David Lewis, Josephine Lusk, Lewis Marshall, Arun Menon, Alison Nikitas, Maree O’Sullivan, Cheryn Palmer, Phillip Read, Darren Russell, Nathan Ryder, David Templeton and Emanuel Vhalkis.
References
[1] The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2017. Sydney, NSW: UNSW Sydney; 2016.[2] Ling DI, Janjua NZ, Wong S, Krajden M, Hoang L, Morshed M, Achen M, Murti M, Lester RT, Wong J, Ogilvie G, Gilbert M. Sexually transmitted infection trends among gay or bisexual men from a clinic-based sentinel surveillance system in British Columbia, Canada. Sex Transm Dis 2015; 42 153–9.
| Sexually transmitted infection trends among gay or bisexual men from a clinic-based sentinel surveillance system in British Columbia, Canada.Crossref | GoogleScholarGoogle Scholar |
[3] Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2016. Atlanta, GA: Department of Health and Human Services; 2017.
[4] Mohammed H, Mitchell H, Sile B, Duffell S, Nardone A, Hughes G. Increase in sexually transmitted infections among men who have sex with men, England. Emerg Infect Dis 2016; 22 88–91.
| Increase in sexually transmitted infections among men who have sex with men, England.Crossref | GoogleScholarGoogle Scholar |
[5] Hull P, Mao L, Lea T, Lee E, Kolstee J, Duck T, Feeney L, Prestage G, Zablotska I, de Wit J, Holt M. Gay community periodic survey, Sydney 2017. Sydney, NSW: UNSW Sydney; 2017.
[6] Cornelisse VJ, Chow EPF, Huffam S, Fairley CK, Bissessor M, de Petra V, Howden BP, Denham I, Bradshaw CS, Williamson D, Chen MY. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44 114–7.
| Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing.Crossref | GoogleScholarGoogle Scholar |
[7] Callander D, Moriera C, El-Hayek C, Asselin J, van Gemert C, Watchirs Smith L, Nguyen L, Dimech W, Boyle DI, Donovan B, Stoové M, Hellard M, Guy R. Australian Collaboration for Coordinated Enhanced Sentinel Surveillance (ACCESS): a protocol for monitoring the control of sexually transmissible infections and blood borne viruses. JMIR Res Protoc 2018;
| Australian Collaboration for Coordinated Enhanced Sentinel Surveillance (ACCESS): a protocol for monitoring the control of sexually transmissible infections and blood borne viruses.Crossref | GoogleScholarGoogle Scholar |
[8] Callander D, McManus H, Guy R, Hellard M, O’Connor CC, Fairley CK, Chow EPF, McNulty A, Lewis DA, Carmody C, Schmidt H-MA, Kim J, Donovan B. Rising chlamydia and gonorrhoea incidence and associated risk factors among female sex workers in Australia: a retrospective cohort study. Sex Transm Dis 2018; 45 199–206.
| Rising chlamydia and gonorrhoea incidence and associated risk factors among female sex workers in Australia: a retrospective cohort study.Crossref | GoogleScholarGoogle Scholar |
[9] Australian Bureau of Statitics. Australian Statistical Geography Standard (ASGS). Canberra, ACT: Australian Bureau of Statistics; 2011.
[10] Templeton DJ, Read P, Varma R, Bourne C. Australian sexually transmissible infection and HIV testing guidelines for asymptomatic men who have sex with men 2014: a review of the evidence. Sex Health 2014; 11 217–29.
| Australian sexually transmissible infection and HIV testing guidelines for asymptomatic men who have sex with men 2014: a review of the evidence.Crossref | GoogleScholarGoogle Scholar |
[11] Hui B, Fairley CK, Chen M, Grulich A, Hocking J, Prestage G, Walker S, Law M, Regan D. Oral and anal sex are key to sustaining gonorrhoea at endemic levels in MSM populations: a mathematical model. Sex Transm Infect 2015; 91 365–9.
| Oral and anal sex are key to sustaining gonorrhoea at endemic levels in MSM populations: a mathematical model.Crossref | GoogleScholarGoogle Scholar |
[12] Chow EPF, Lee D, Tabrizi SN, Phillips S, Snow A, Cook S, Howden BP, Petalotis I, Bradshaw CS, Chen MY, Fairley CK. Detection of Neisseria gonorrhoeae in the pharynx and saliva: implications for gonorrhoea transmission. Sex Transm Infect 2016; 92 347–9.
| Detection of Neisseria gonorrhoeae in the pharynx and saliva: implications for gonorrhoea transmission.Crossref | GoogleScholarGoogle Scholar |
[13] Fairley CK, Zhang L, Chow EP. New thinking on gonorrhoea control in MSM: are antiseptic mouthwashes the answer? Curr Opin Infect Dis 2018; 31 45–9.
| New thinking on gonorrhoea control in MSM: are antiseptic mouthwashes the answer?Crossref | GoogleScholarGoogle Scholar |
[14] Chow EPF, Walker S, Hocking JS, Bradshaw CS, Chen MY, Tabrizi SN, Howden BP, Law MG, Maddaford K, Read TRH, Lewis DA, Whiley DM, Zhang L, Grulich AE, Kaldor JM, Cornelisse VJ, Phillips S, Donovan B, McNulty AM, Templeton DJ, Roth N, Moore R, Fairley CK. A multicentre double-blind randomised controlled trial evaluating the efficacy of daily use of antibacterial mouthwash against oropharyngeal gonorrhoea among men who have sex with men: the OMEGA (Oral Mouthwash use to Eradicate GonorrhoeA) study protocol. BMC Infect Dis 2017; 17 456–72.
| A multicentre double-blind randomised controlled trial evaluating the efficacy of daily use of antibacterial mouthwash against oropharyngeal gonorrhoea among men who have sex with men: the OMEGA (Oral Mouthwash use to Eradicate GonorrhoeA) study protocol.Crossref | GoogleScholarGoogle Scholar |
[15] Holt M, Hull P, Lea T, Guy R, Bourne C, Prestage G, Zablotska I, de Wit J, Mao L. Comprehensive testing for, and diagnosis of, sexually transmissible infections among Australian gay and bisexual men: findings from repeated, cross-sectional behavioural surveillance, 2003–2012. Sex Transm Infect 2014; 90 208–15.
| Comprehensive testing for, and diagnosis of, sexually transmissible infections among Australian gay and bisexual men: findings from repeated, cross-sectional behavioural surveillance, 2003–2012.Crossref | GoogleScholarGoogle Scholar |
[16] Bui H, Zablotska-Manos I, Hammoud M, Jin F, Lea T, Bourne A, Iversen J, Bath N, Grierson J, Degenhardt L, Prestage G, Maher L. Prevalence and correlates of recent injecting drug use among gay and bisexual men in Australia: results from the FLUX study. International Journal of Drug Policy 2018; 55 222–30.
| Prevalence and correlates of recent injecting drug use among gay and bisexual men in Australia: results from the FLUX study.Crossref | GoogleScholarGoogle Scholar |
[17] Bourne A, Reid D, Hickson F, Torres-Rueda S, Weatherburn P. Illicit drug use in sexual settings (‘chemsex’) and HIV/STI transmission risk behaviour among gay men in South London: findings from a qualitative study. Sex Transm Infect 2015; 91 564–8.
| Illicit drug use in sexual settings (‘chemsex’) and HIV/STI transmission risk behaviour among gay men in South London: findings from a qualitative study.Crossref | GoogleScholarGoogle Scholar |
[18] Chow EPF, Tomnay J, Fehler G, Whiley D, Read TRH, Denham I, Bradshaw CS, Chen MY, Fairley CK. Substantial increases in chlamydia and gonorrhea positivity unexplained by changes in individual-level sexual behaviors among men who have sex with men in an Australian sexual health service from 2007 to 2013. Sex Transm Dis 2015; 42 81–7.
| Substantial increases in chlamydia and gonorrhea positivity unexplained by changes in individual-level sexual behaviors among men who have sex with men in an Australian sexual health service from 2007 to 2013.Crossref | GoogleScholarGoogle Scholar |
[19] Kojima N, Davey DJ, Klausner JD. Pre-exposure prophylaxis or HIV infection and new sexually transmitted infections among men who have sex with men. AIDS 2016; 30 2251–2.
| Pre-exposure prophylaxis or HIV infection and new sexually transmitted infections among men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[20] Ali H, Donovan B, Fairley CK, Ryder N, McNulty A, Chen MY, Marshall L, O’Connor CC, Dickson . Ali H, Donovan B, Fairley CK, Ryder N, McNulty A, Chen MY, Marshall L, O’Connor CC, Dickson . Are Australian sexual health clinics attracting priority populations? Sex Health 2013; 10 456–9.
| Are Australian sexual health clinics attracting priority populations?Crossref | GoogleScholarGoogle Scholar |