Using the genetic characteristics of Neisseria gonorrhoeae strains with decreased susceptibility to cefixime to develop a molecular assay to predict cefixime susceptibility
Xiaomeng Deng A F , Lao-Tzu Allan-Blitz B C and Jeffrey D. Klausner A D E
A F , Lao-Tzu Allan-Blitz B C and Jeffrey D. Klausner A D E
A David Geffen School of Medicine, University of California Los Angeles, 10833 Le Conte Avenue, Los Angeles, CA 90095, USA.
B Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
C Department of Medicine, Children’s Hospital of Boston, 300 Longwood Avenue, Boston, MA 02115, USA.
D Division of Infectious Disease, Department of Medicine, David Geffen School of Medicine, University of California Los Angeles, Center for Health Sciences, 37–121, 10833 Le Conte Avenue, Los Angeles, CA 90095, USA.
E Department of Epidemiology, Fielding School of Public Health, University of California Los Angeles, 650 Charles E. Young Drive S., Los Angeles, CA 90095, USA.
F Corresponding author. Email: dengxiaomeng0416@gmail.com
Sexual Health 16(5) 488-499 https://doi.org/10.1071/SH18227
Submitted: 4 December 2018 Accepted: 1 April 2019 Published: 24 June 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Background: In the last two decades, gonococcal strains with decreased cefixime susceptibility and cases of clinical treatment failure have been reported worldwide. Gonococcal strains with a cefixime minimum inhibitory concentration (MIC) ≥0.12 µg mL−1 are significantly more likely to fail cefixime treatment than strains with an MIC <0.12 µg mL−1. Various researchers have described the molecular characteristics of gonococcal strains with reduced cefixime susceptibility, and many have proposed critical molecular alterations that contribute to this decreased susceptibility. Methods: A systematic review of all published articles in PubMed through 1 November 2018 was conducted that report findings on the molecular characteristics and potential mechanisms of resistance for gonococcal strains with decreased cefixime susceptibility. The findings were summarised and suggestions were made for the development of a molecular-based cefixime susceptibility assay. Results: The penicillin-binding protein 2 (PBP2) encoded by the penA gene is the primary target of cefixime antimicrobial activity. Decreased cefixime susceptibility is conferred by altered penA genes with mosaic substitute sequences from other Neisseria (N.) species (identifiable by alterations at amino acid position 375–377) or by non-mosaic penA genes with at least one of the critical amino acid substitutions at positions 501, 542 and 551. Based on this review of 415 international cefixime decreased susceptible N. gonorrhoeae isolates, the estimated sensitivity for an assay detecting the aforementioned amino acid alterations would be 99.5% (413/415). Conclusions: Targeting mosaic penA and critical amino acid substitutions in non-mosaic penA are necessary and may be sufficient to produce a robust, universal molecular assay to predict cefixime susceptibility.
Additional keywords: antimicrobial resistance, antimicrobial stewardship.
Introduction
Neisseria (N.) gonorrhoeae, is the world’s second most prevalent sexually transmissible bacterial infection.1 However, N. gonorrhoeae is a naturally competent organism that can acquire new genes from the microbial environment.2 Those newly acquired genetic variations can render the organism less susceptible, or even resistant, to antimicrobial therapy.2
As the organism has developed resistance to multiple classes of antibiotics such as sulfas, penicillins, tetracyclines, fluoroquinolones and macrolides, the third-generation extended-spectrum cephalosporins, like cefixime, are among the few reliable efficacious treatment options left.3 Cefixime is a highly useful antibiotic used for the treatment of gonorrhoea. N. gonorrhoeae remains susceptible to cefixime in most but not all countries.4 Currently, the World Health Organization (WHO) recommends cefixime, in combination with azithromycin, as dual therapy for oropharyngeal, genital and anorectal gonococcal infection.5 In settings where local resistance data confirm cefixime susceptibility, the WHO recommends cefixime in a single dose for genital and anorectal gonococcal infection. In the USA, the Centers for Disease Control and Prevention also recommends cefixime, in combination with azithromycin, as an alternative regimen where ceftriaxone is not available.6 In the UK, oral cefixime in combination with azithromycin is also recommended in penicillin-allergic patients for whom intramuscular injection is contraindicated or refused.7 Cefixime has a serum half-life of 3–4 h in patients with normal renal function, high bioavailability after a single oral dose and is very well tolerated even in penicillin-allergic patients.6 However, in the last two decades, various investigators have reported cases of N. gonorrhoeae infection with strains that have decreased susceptibility to cefixime.8–30 Furthermore, in the past 10 years, cases of N. gonorrhoeae treatment failure in patients treated with cefixime have also been reported in Japan,31 Norway,32 UK,33,34 South Africa,28 France,9 Australia35 and Canada.24 Various research teams and governmental institutions have expressed the need for more research on the mechanisms of cefixime resistance and the development of new tools to predict cefixime susceptibility.36–38
One target protein that is critical for the antimicrobial activity of many β-lactam antibiotics, including cefixime, is the penicillin-binding protein 2 (PBP2), which is a protein essential for the development of bacterial cell walls.39 PBP2 is a transpeptidase encoded by the penA gene. Mosaicism of the penA gene in N. gonorrhoeae was first described by Ameyama et al.40 who found that some penA nucleotide sequences of N. gonorrhoeae contained portions that highly resemble those of other non-pathogenic or commensal Neisseria species, such as N. perflava, N. cinerea, N. flavescens and N. meningitidis. penA mosaicism, along with other point mutations in penA, helps N. gonorrhoeae to develop resistance against extended-spectrum cephalosporins like cefixime.14–27,41–43
In this review, we describe molecular mechanisms of cefixime-decreased susceptibility in N. gonorrhoeae, summarise findings from published reports of various gene mutations contributing to that decreased susceptibility and make suggestions on how to develop a molecular-based cefixime susceptibility assay.
Methods
Definition of cefixime-decreased susceptibility
Historically, defining decreased susceptibility or resistance of N. gonorrhoeae to cefixime has been challenging due to the scarcity of treatment failure cases.37 The most up-to-date recommendations of antimicrobial minimum inhibitory concentration (MIC) breakpoints by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI) in the USA are as follows:
EUCAST: susceptible ≤0.125 µg mL−1; resistant >0.125 µg mL−1.44
CLSI: susceptible ≤ 0.25 µg mL−1; intermediate: not applicable; resistant: not applicable.45
However, those recommendations might be too liberal when we compare those breakpoints to reported cases of clinical treatment failure. Allen et al. reported that the frequency of treatment failure with cefixime for gonococcal infections with an MIC ≥0.12 µg mL−1 was 25%, compared with 1.9% among those with an MIC <0.12 µg mL−1.24 For this review, we have described the molecular characteristics of all N. gonorrhoeae strains reported, to date, with a MIC ≥0.12 µg mL−1 as having a ‘decreased susceptibility’ to cefixime.
Literature and sequence database review
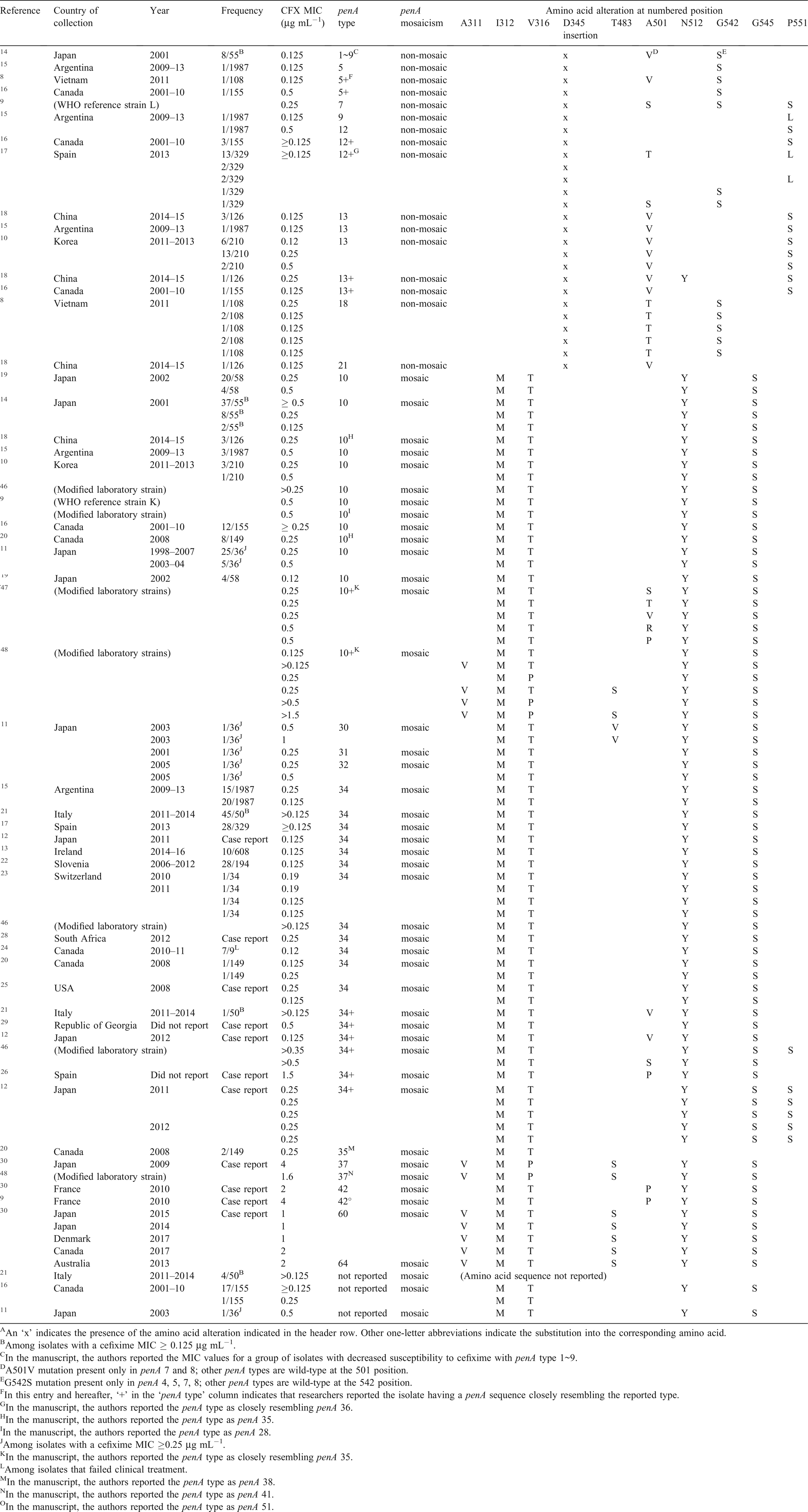
One author (X. Deng) searched all articles published on PubMed from 1 January 1995 to 1 November 2018 under the search terms ‘Neisseria gonorrhoeae’, ‘cefixime’ and ‘molecular’ and reviewed relevant articles cited as references. We identified a total of 74 articles from that search. We included articles that presented epidemiological or experimental evidence of certain molecular alterations contributing to cefixime-decreased susceptibility in N. gonorrhoeae; there was a total of 25 reports. All N. gonorrhoeae strains with a reported MIC ≥0.12 µg mL−1 and specific penA alterations associated with cefixime decreased susceptibility were included in Table 1, along with their specific MIC plus the time and location of collection.
|
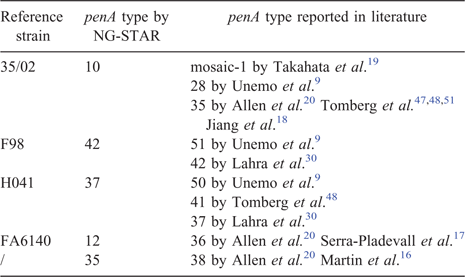
The nomenclature of penA reflects the differences in amino acid sequence rather than nucleotide sequence. Historically, each new amino acid sequence gets a sequential whole number, and is classified into mosaic, semi-mosaic (alterations of either the first or second half of the penA gene only27), non-mosaic (point mutations only) and wild-type (penA peptide sequence identical to that of the N. gonorrhoeae reference strain, M3209149,50). Each different DNA sequence of an existing amino acid sequence gets a decimal number;27,28 for example, the eighth DNA sequence reported for penA allele type 2 is assigned allele number penA2.008. Our review has exposed conflicting nomenclature for the same penA peptide sequence (Table 2); likely due to the lack of a single, centralised database requiring new submissions of sequences to be compared with existing entries and subsequently given appropriate designations. We also noticed a general lack of consensus in the standard style of nucleotide or amino acid sequence reporting. Many sequences reported were truncated, leading to incomplete information that hindered data interpretation. We highly recommend future researchers to submit complete gene sequencing data to GenBank (https://www.ncbi.nlm.nih.gov/genbank/), which is supported by the National Center for Biotechnology Information (NCBI) and N. gonorrhoeae Sequence Typing for Antimicrobial Resistance (NG-STAR, https://ngstar.canada.ca/), which is supported by the Government of Canada, for easy reference. To prevent misclassification in this manuscript, we further verified penA sequences from each research article against the penA profiles in NG-STAR, a centralised, comprehensive and publicly accessible database for standardised characterisation of molecular alterations in N. gonorrhoeae worldwide.27 One author (X. Deng) conducted all the multiple peptide sequence alignment using the Multiple Sequence Alignment tool by Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/).
|
Estimation of assay sensitivity
We proposed a parsimonious group of penA amino acid locations to predict decreased susceptibility to cefixime in N. gonorrhoeae strains. We estimated the sensitivity of a hypothetical assay for predicting decreased susceptibility using those locations by calculating the number of isolates with genotypic mutations in those locations divided by the number of phenotypically decreased susceptible isolates using the data summarised in Table 1.
Results
Overview of the molecular mechanisms of cefixime-decreased susceptibility
Table 3 shows a list of penA types from the NG-STAR database and the presence or absence of amino acid alterations associated with cefixime-decreased susceptibility. Decreased susceptibility to cefixime has been associated with many genetic alterations in the penA gene. There is strong laboratory and epidemiological research supporting mosaicism14,40 and other point mutations19,47,48,51 of the penA gene as mechanisms for cefixime-decreased susceptibility by means of PBP2 target alteration. Evidence supporting the importance of alterations in other genes are not as compelling. However, there is evidence that isolates with identical penA alleles could have different MIC levels (susceptible with an MIC <0.125 µg mL−1 vs highly resistant with an MIC ≥ 0.5 µg mL−1), indicating the involvement of other genes in decreasing susceptibility to cefixime.27 Several candidate genes contributing to cefixime decreased susceptibility include mtrR, the transcriptional regulator for the mtrCDE efflux pump system;52 ponA, encoding for penicillin-binding protein 1 (PBP1);53 pilQ, encoding for the type IV pilus secretin;54 and penB (alias: porB1b), encoding for a major outer membrane protein, porB, a porin.55
penA – penA mosaicism
Mosaicism in penA was found to be the primary determinant of cefixime-decreased susceptibility by many studies.14–27,41–43 From an aggregate of 415 N. gonorrhoeae isolates with a MIC ≥0.12 µg mL−1 from 25 reports representing 22 countries, 83.1% (345 out of 415) were found to have mosaic penA genes (Table 1). Among all the different mosaic patterns, penA1010,56,57 and penA3415,17,21–23,27,42,56,58 were the most frequently reported mosaic penA patterns of all isolates with mosaic penA gene mutations among N. gonorrhoeae strains with reduced cefixime susceptibility [accounting for 39.1% (135 out of 345) and 49.9% (172 out of 345) respectively]. While penA34 was found worldwide, penA10 was mostly found in Asia, and was also associated with resistance to, and treatment failure by, another third-generation cephalosporin, ceftibuten.59
The I312M, V316T, N512Y and G545S amino acid substitutions are frequently seen in mosaic penA patterns. Researchers have found that amino acid substitutions, I312M,19,25 V316T/P19,25,48 and G54519,25,60,61 are associated with reduced cefixime susceptibility. However, a gene transformation study by Tomberg et al.51 in 2010 showed that when introduced into a wild-type penA, I312M, V316T and G545S together only minimally elevated the cefixime MIC. The reversion of the triple mutation in a cefixime-resistant strain 35/02 (with penA10) back into wild-type returned its MIC to that similar to the wild-type penA MIC, indicating that those three mutations are important to cefixime resistance only in the context of other mutations found in mosaic penA10 alleles.
In the same study by Tomberg et al.,51 chimeric penA genes were created by replacing sequential portions of penA10 with the corresponding regions of a wild-type penA gene. Reversion of amino acid regions 309–353 (containing I312M and V316T and 13 other substitutions), 489–528 (containing N512Y and two other mutations) and 528–581 (containing G545S and nine other substitutions), showed significant decrease in MIC. Among the three, the reversion of region 528–581 decreased the MIC to such a significant degree that the chimera could not be selected despite multiple attempts, indicating mutations in this region may be critical factors in influencing cefixime susceptibility. When the G545S substitution was re-introduced to the 528–581-wild-type penA10, the resulting strain’s cefixime MIC only rose to 0.05 µg mL−1, suggesting that other mutations in the 528–581 region were necessary to significantly elevate the cefixime MIC to >0.125 µg mL−1.
The reversion of region 489–528 (containing N512Y and two other mutations) decreased the MIC from ~0.125 to ~0.05 µg mL−1. Y512N reversion alone presented a decreased MIC from ~0.125 to ~0.06 µg mL−1. Accounting for most of the effect on cefixime resistance by mutations in the 489–528 region, N512Y showed major importance in conferring cefixime resistance in the context of other mosaic changes.
In summary, I312M, V316T, N512Y and G545S were found to be important to cefixime-decreased susceptibility or resistance only in the context of other mutations found in mosaic penA alterations. All of them were associated with, but none was necessary or sufficient for, cefixime-decreased susceptibility or resistance.
penA – non-mosaic penA with point amino acid alterations – A501 + G542 + P551
Although the penA34 mosaicism was present in 98% of all isolates with a MIC ≥0.25 µg mL−1 in the study by Grad et al. involving more than 1100 N. gonorrhoeae isolates collected in the USA, the percentage lowered to 91% when a lower and more clinically relevant MIC breakpoint (≥0.125 µg mL−1) was used.27 That indicates the role of other non-penA34 mutations, most notably, other amino acid substitutions in the penA gene. In the last decade, more than a dozen cefixime-resistant N. gonorrhoeae strains reported in Asia8,10,14,18 and Europe17 were found to have non-mosaic penA alleles (Table 1). Among reports from Asia, over 20% of N. gonorrhoeae strains with decreased susceptibility to cefixime had non-mosaic penA mutations. Gene transformation experiments have identified multiple, important amino acid substitutions in non-mosaic penA that significantly decrease cefixime susceptibility.47
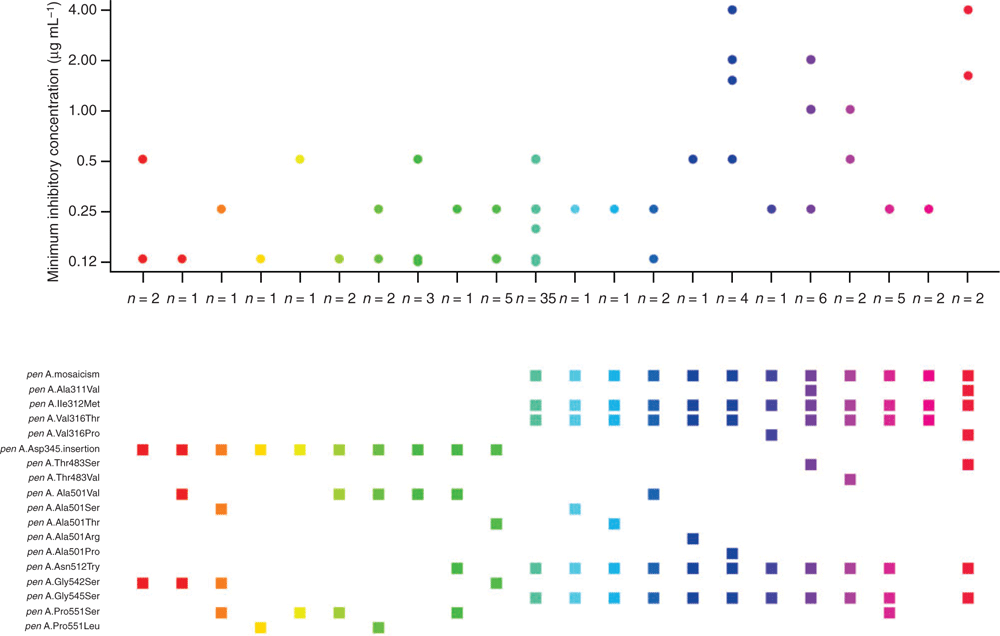
Three amino acid substitutions, A501S/V/T/P, G542S and P551S/L/P, have been associated with cefixime-decreased susceptibility independent from penA mosaicism.16,21 The study by Tomberg et al. demonstrated that an independent A501 substitution potentially decreased cefixime susceptibility by increasing the rigidity of the PBP2 active site.47
In Table 1, we have summarised the cefixime-decreased susceptible related penA amino acid alterations in all N. gonorrhoeae strains with a cefixime MIC ≥0.12 µg mL−1. One important finding is that 68 out of 70 (97.1%) non-mosaic penA strains with reduced susceptibility to cefixime have a point mutation in at least one of the three codons, A501, G542 and P551. Additionally, decreased susceptible N. gonorrhoeae strains with non-mosaic penA have mostly been reported in Asia and Europe.
In Figure 1, we have summarised the MIC levels of all N. gonorrhoeae strains with cefixime MIC ≥ 0.12 µg mL−1 by different combinations of decreased susceptible-related penA amino acid alterations (n = 240). Strains lacking a specific cefixime MIC value or penA alteration records (n = 175) were excluded.
mtrR
mtrR is a repressor gene that regulates the expression of the mtrCDE efflux pump system, an important mechanism in transporting antimicrobial agents out of the bacterial cell.52 Changes in the promoter or coding sequence of the mtrR gene can potentially decrease antimicrobial susceptibility by increased efflux.62 There are conflicting reports on the importance of the mtrR gene in cefixime-decreased susceptibility. Mutations frequently found in strains with cefixime-decreased susceptibility include a –35A deletion in the promoter region,13,22 plus A39T and G45D22 in the coding region. We did not find gene transformation studies that looked into mtrR alterations’ contribution to cefixime resistance independent from penA changes. Nonetheless, other studies report that mutations in the mtrR gene have little or no association with cefixime susceptibility.58,62
penB (porB1b)
penB, also known as porB1b, encodes for an outer membrane porin and is thought to increase penicillin resistance by changing the bacterial membrane permeability to certain antibiotics when penA and mtrR mutations are also present, although its role in cefixime resistance is unclear.58,63 Alterations found in strains with cefixime-decreased susceptibility include G120K and A121N/D substitutions.42 While the study by Grad et al. in the USA suggested no correlation between cefixime-decreased susceptibility and G120K or G120D/A121D, the lack of G120K and A121N mutations strongly predicted susceptibility.27
Two other mutations in the penB gene commonly found in cefixime-decreased susceptible strains are G101K/D and A102D/N/S. Combinations of mutations at those two sites were found in all 48 strains with a cefixime MIC >0.125 µg mL−1 out of the total 329 N. gonorrhoeae strains in the Serra-Pladevall et al. study17,22 and among all 127 strains with a cefixime MIC ≥0.125 µg mL−1 out of the total 194 N. gonorrhoeae strains in the study by Jeverica et al.22
We have found no evidence that alterations in the penB gene alone can confer decreased susceptibility to cefixime.
ponA
ponA encodes for penicillin-binding protein 1 (PBP1), an additional cell wall protein important in β-lactam antibiotic antimicrobial activity. Alterations in the ponA gene were associated with penicillin resistance by PBP1 target mutation, although its role in cefixime-decreased susceptibility is unclear.19,37,58 One mutation, L421P,17,42,64 was frequently found in cefixime-decreased susceptible strains, but a gene transformation study showed that a ponA L421P substitution does not contribute additional decreased susceptibility to cefixime without a mosaic penA gene.62
pilQ
pilQ encodes for a type IV pili secretin.54 Mutations in the pilQ gene are thought to increase penicillin resistance by changing the bacterial membrane permeability when penA, mtrR or penB mutations are also present, although its role in cefixime resistance is also unclear.58,63
While the study by Whiley et al.63 concluded that changes in the pilQ gene are unlikely associated with cefixime-decreased susceptibility, the study by Grad et al.27 found that a 176–183 deletion (vs full length), N341S, D526N/G or N648S each strongly predicted N. gonorrhoeae susceptibility to cefixime. Notably, among those four mutations, N648S was the only mutation that was found to be relatively common (in 23.7% of all isolates compared with ≤10% for any of the other three).
Discussion
Prediction of cefixime susceptibility using molecular markers
Antimicrobial stewardship is important in the control of antimicrobial resistance in infectious diseases.65 Antimicrobial use results in selective pressure, favouring the development of resistant organisms.65 Therefore, the use of molecular assays to predict antimicrobial susceptibility at the time of treatment allows for targeted therapy, enabling the use of antimicrobials shown to be highly effective and rapidly bactericidal, as well as the use of older medications previously deemed non-effective because of prevalent resistance.66,67 Such molecular assays to predict susceptibility could be a valuable complement to antimicrobial stewardship and novel drug development in slowing the emergence of antimicrobial resistance.38
Previously, we developed a polymerase chain reaction-based assay using high resolution melt analysis to predict N. gonorrhoeae susceptibility to ceftriaxone and cefixime by targeting the penA34 type.68 While that assay showed greater than 98% sensitivity in predicting cefixime-decreased susceptibility in North America, where the penA34 is the most common penA type,27,50 the assay is limited by its inability to identify cefixime-decreased susceptibility as a result of N. gonorrhoeae strains with non-mosaic penA or other non-34 mosaic penA patterns. As N. gonorrhoeae strains with various non-34 penA types account for many of the cefixime-resistant strains reported in Asia and Europe, it is likely only a matter of time for such strains to emerge in other parts of the world, including in the USA.
Additional amino acid alterations should be considered when developing a molecular assay to predict cefixime susceptibility, especially when a lower and more clinically relevant MIC breakpoint (<0.12 µg mL−1) is used.
For the penA gene, either of the following patterns of alterations could lead to elevated cefixime MIC levels: (1) mosaic penA types with characteristic polymorphisms I312M, V316T, N512Y, G545S; or (2) non-mosaic penA types with mutations at any combinations of the three amino acid positions: A501, G542 or P551. A multiple peptide sequence alignment showed that a wild-type sequence of amino acid region 375–377 can reliably distinguish 32 out of 33 types of wild-type and non-mosaic penA types (with the only exception being the non-mosaic penA49 type possessing a A377V substitution) from all 21 reported mosaic penA types (data not shown). Other researchers have targeted other regions or used the characteristic amino acid polymorphisms in mosaic penA types such as I312M, V316T and G545S.19,69
Non-mosaic penA types with critical point mutations can be identified by the absence of changes at amino acid region 375–377, and the presence of one of the three critical point mutations A501, G542 or P551. A molecular assay that detects any penA alterations at amino acid positions 375–377, 501, 542 or 551 would be the most parsimonious assay to predict decreased susceptibility to cefixime. That modified assay would be more effective in predicting cefixime susceptibility than the assay we previously developed,68 with greater sensitivity for diverse international isolates. Based on the molecular characteristics of all reported strains with decreased susceptibility to cefixime as recorded in Table 1, the estimated sensitivity for the proposed assay would be 99.5% (413 out of 415).
Other genes, such as mtrR, ponA, penB and pilQ, have a less clear role in cefixime resistance and seem to further increase cefixime MIC values only when penA alterations are present. Although certain alterations in these genes were found to be highly associated with full susceptibility as mentioned above, detecting these changes appears to be unnecessary as N. gonorrhoeae will constantly undergo selection by antimicrobial agents and gradually gain mutations that eventually lead to cefixime resistance.
Conclusion
Because of the continued challenge in treating antimicrobial-resistant N. gonorrhoeae infections, there have been many reports published regarding the genetic alterations associated with decreased susceptibility to third-generation cephalosporins. Researchers have provided epidemiological and molecular evidence that alterations in the penA gene are the primary determinants of cefixime-decreased susceptibility, while other genes in the presence of an altered penA gene further contribute in reducing cefixime susceptibility but are neither necessary nor sufficient in independently conferring decreased susceptibility. Based on those data, we proposed the optimal targets for novel molecular assays aiming to predict N. gonorrhoeae susceptibility to cefixime.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Funding was provided by the National Institute of Allergy and Infectious Diseases, grant R21 AI117256, to Jeffrey D. Klausner. Jeffrey D. Klausner has received advisory fees or research support from Cepheid, SpeedX Diagnostics, Shield Diagnostics, Click Diagnostics and Hologic Inc. The results presented are subject of a pending U.S. patent application.
References
[1] Unemo M, Shafer WM. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann N Y Acad Sci 2011; 1230 E19–28.| Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future.Crossref | GoogleScholarGoogle Scholar | 22239555PubMed |
[2] Tapsall JW, Ndowa F, Lewis DA, Unemo M. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther 2009; 7 821–34.
| Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 19735224PubMed |
[3] Tanvir SB, Qasim SSB, Shariq A, Najeeb S, Shah AH. Systematic review and meta-analysis on efficacy of cefixime for treating gonococcal infections. Int J Health Sci (Qassim) 2018; 12 90–100.
[4] Unemo M, Lahra MM, Cole M, Galarza P, Ndowa F, Martin I, Dillon JR, Ramon-Pardo P, Bolan G, Wi T. The WHO Global Gonococcal Antimicrobial Surveillance Programme (67 surveyed countries in 2015–2016) – an observational study emphasising essential global actions. Sex Health 2019; In press.
[5] World Health Organization. WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva: World Health Organization; 2016.
[6] Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64 1–137.
| 26042815PubMed |
[7] Fifer H, Saunders J, Soni S, Sadiq ST, FitzGerald M. British Association for Sexual Health and HIV national guideline for the management of infection with Neisseria gonorrhoeae. 2019; Macclesfield: BASHH; 2019. Available online at: https://pcwhf.co.uk/wp-content/uploads/2019/03/gc-2019.pdf [verified 1 June 2019].
| 31225605PubMed |
[8] Olsen B, Pham TL, Golparian D, Johansson E, Tran HK, Unemo M. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011. BMC Infect Dis 2013; 13 40
| Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011.Crossref | GoogleScholarGoogle Scholar | 23351067PubMed |
[9] Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 2012; 56 1273–80.
| High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure.Crossref | GoogleScholarGoogle Scholar | 22155830PubMed |
[10] Lee H, Unemo M, Kim HJ, Seo Y, Lee K, Chong Y. Emergence of decreased susceptibility and resistance to extended-spectrum cephalosporins in Neisseria gonorrhoeae in Korea. J Antimicrob Chemother 2015; 70 2536–42.
| Emergence of decreased susceptibility and resistance to extended-spectrum cephalosporins in Neisseria gonorrhoeae in Korea.Crossref | GoogleScholarGoogle Scholar | 26084303PubMed |
[11] Ohnishi M, Watanabe Y, Ono E, Takahashi C, Oya H, Kuroki T, Shimuta K, Okazaki N, Nakayama S, Watanabe H. Spread of a chromosomal cefixime-resistant penA gene among different Neisseria gonorrhoeae lineages. Antimicrob Agents Chemother 2010; 54 1060–7.
| Spread of a chromosomal cefixime-resistant penA gene among different Neisseria gonorrhoeae lineages.Crossref | GoogleScholarGoogle Scholar | 20028823PubMed |
[12] Morita-Ishihara T, Unemo M, Furubayashi K, Kawahata T, Shimuta K, Nakayama S, Ohnishi M. Treatment failure with 2 g of azithromycin (extended-release formulation) in gonorrhoea in Japan caused by the international multidrug-resistant ST1407 strain of Neisseria gonorrhoeae. J Antimicrob Chemother 2014; 69 2086–90.
| Treatment failure with 2 g of azithromycin (extended-release formulation) in gonorrhoea in Japan caused by the international multidrug-resistant ST1407 strain of Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 24777907PubMed |
[13] Ryan L, Golparian D, Fennelly N, Rose L, Walsh P, Lawlor B, Mac Aogain M, Unemo M, Crowley B. Antimicrobial resistance and molecular epidemiology using whole-genome sequencing of Neisseria gonorrhoeae in Ireland, 2014–2016: focus on extended-spectrum cephalosporins and azithromycin. Eur J Clin Microbiol Infect Dis 2018; 37 1661–72.
| Antimicrobial resistance and molecular epidemiology using whole-genome sequencing of Neisseria gonorrhoeae in Ireland, 2014–2016: focus on extended-spectrum cephalosporins and azithromycin.Crossref | GoogleScholarGoogle Scholar | 29882175PubMed |
[14] Ito M, Deguchi T, Mizutani KS, Yasuda M, Yokoi S, Ito S, Takahashi Y, Ishihara S, Kawamura Y, Ezaki T. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob Agents Chemother 2005; 49 137–43.
| Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan.Crossref | GoogleScholarGoogle Scholar | 15616287PubMed |
[15] Gianecini R, Romero MLM, Oviedo C, Vacchino M, Galarza P. Emergence and spread of Neisseria gonorrhoeae isolates with decreased susceptibility to extended-spectrum cephalosporins in Argentina, 2009 to 2013. Sex Transm Dis 2017; 44 351–5.
| Emergence and spread of Neisseria gonorrhoeae isolates with decreased susceptibility to extended-spectrum cephalosporins in Argentina, 2009 to 2013.Crossref | GoogleScholarGoogle Scholar | 28499284PubMed |
[16] Martin I, Sawatzky P, Allen V, Hoang L, Lefebvre B, Mina N, Wong T, Gilmour M. Emergence and characterization of Neisseria gonorrhoeae isolates with decreased susceptibilities to ceftriaxone and cefixime in Canada: 2001–2010. Sex Transm Dis 2012; 39 316–23.
| Emergence and characterization of Neisseria gonorrhoeae isolates with decreased susceptibilities to ceftriaxone and cefixime in Canada: 2001–2010.Crossref | GoogleScholarGoogle Scholar | 22421701PubMed |
[17] Serra-Pladevall J, Barbera MJ, Rodriguez S, Bartolome-Comas R, Roig G, Juve R, Andreu A. Neisseria gonorrhoeae antimicrobial susceptibility in Barcelona: penA, ponA, mtrR, and porB mutations and NG-MAST sequence types associated with decreased susceptibility to cephalosporins. Eur J Clin Microbiol Infect Dis 2016; 35 1549–1556.
| Neisseria gonorrhoeae antimicrobial susceptibility in Barcelona: penA, ponA, mtrR, and porB mutations and NG-MAST sequence types associated with decreased susceptibility to cephalosporins.Crossref | GoogleScholarGoogle Scholar | 27255221PubMed |
[18] Jiang FX, Lan Q, Le WJ, Su XH. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates from Hefei (2014–2015): genetic characteristics of antimicrobial resistance. BMC Infect Dis 2017; 17 366
| Antimicrobial susceptibility of Neisseria gonorrhoeae isolates from Hefei (2014–2015): genetic characteristics of antimicrobial resistance.Crossref | GoogleScholarGoogle Scholar | 28545411PubMed |
[19] Takahata S, Senju N, Osaki Y, Yoshida T, Ida T. Amino acid substitutions in mosaic penicillin-binding protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae. Antimicrob Agents Chemother 2006; 50 3638–45.
| Amino acid substitutions in mosaic penicillin-binding protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 16940068PubMed |
[20] Allen VG, Farrell DJ, Rebbapragada A, Tan J, Tijet N, Perusini SJ, Towns L, Lo S, Low DE, Melano RG. Molecular analysis of antimicrobial resistance mechanisms in Neisseria gonorrhoeae isolates from Ontario, Canada. Antimicrob Agents Chemother 2011; 55 703–12.
| Molecular analysis of antimicrobial resistance mechanisms in Neisseria gonorrhoeae isolates from Ontario, Canada.Crossref | GoogleScholarGoogle Scholar | 21098249PubMed |
[21] Carannante A, Vacca P, Ghisetti V, Latino MA, Cusini M, Matteelli A, Vocale C, Prignano G, Leli C, Ober P, Antonetti R, Poletti F, Stefanelli P. Genetic resistance determinants for cefixime and molecular analysis of gonococci isolated in Italy. Microb Drug Resist 2017; 23 247–52.
| Genetic resistance determinants for cefixime and molecular analysis of gonococci isolated in Italy.Crossref | GoogleScholarGoogle Scholar | 27347854PubMed |
[22] Jeverica S, Golparian D, Maticic M, Potocnik M, Mlakar B, Unemo M. Phenotypic and molecular characterization of Neisseria gonorrhoeae isolates from Slovenia, 2006–12: rise and fall of the multidrug-resistant NG-MAST genogroup 1407 clone? J Antimicrob Chemother 2014; 69 1517–25.
| Phenotypic and molecular characterization of Neisseria gonorrhoeae isolates from Slovenia, 2006–12: rise and fall of the multidrug-resistant NG-MAST genogroup 1407 clone?Crossref | GoogleScholarGoogle Scholar | 24535277PubMed |
[23] Endimiani A, Guilarte YN, Tinguely R, Hirzberger L, Selvini S, Lupo A, Hauser C, Furrer H. Characterization of Neisseria gonorrhoeae isolates detected in Switzerland (1998–2012): emergence of multidrug-resistant clones less susceptible to cephalosporins. BMC Infect Dis 2014; 14 106
| Characterization of Neisseria gonorrhoeae isolates detected in Switzerland (1998–2012): emergence of multidrug-resistant clones less susceptible to cephalosporins.Crossref | GoogleScholarGoogle Scholar | 24568221PubMed |
[24] Allen VG, Mitterni L, Seah C, Rebbapragada A, Martin IE, Lee C, Siebert H, Towns L, Melano RG, Low DE. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA 2013; 309 163–70.
| Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada.Crossref | GoogleScholarGoogle Scholar | 23299608PubMed |
[25] Pandori M, Barry PM, Wu A, Ren A, Whittington WL, Liska S, Klausner JD. Mosaic penicillin-binding protein 2 in Neisseria gonorrhoeae isolates collected in 2008 in San Francisco, California. Antimicrob Agents Chemother 2009; 53 4032–4.
| Mosaic penicillin-binding protein 2 in Neisseria gonorrhoeae isolates collected in 2008 in San Francisco, California.Crossref | GoogleScholarGoogle Scholar | 19546370PubMed |
[26] Cámara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, Ardanuy C. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother 2012; 67 1858–60.
| Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain.Crossref | GoogleScholarGoogle Scholar | 22566592PubMed |
[27] Grad YH, Harris SR, Kirkcaldy RD, Green AG, Marks DS, Bentley SD, Tress D, Lipsitch M. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J Infect Dis 2016; 214 1579–87.
| Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013.Crossref | GoogleScholarGoogle Scholar | 27638945PubMed |
[28] Lewis DA, Sriruttan C, Muller EE, Golparian D, Gumede L, Fick D, de Wet J, Maseko V, Coetzee J, Unemo M. Phenotypic and genetic characterization of the first two cases of extended-spectrum-cephalosporin-resistant Neisseria gonorrhoeae infection in South Africa and association with cefixime treatment failure. J Antimicrob Chemother 2013; 68 1267–70.
| Phenotypic and genetic characterization of the first two cases of extended-spectrum-cephalosporin-resistant Neisseria gonorrhoeae infection in South Africa and association with cefixime treatment failure.Crossref | GoogleScholarGoogle Scholar | 23416957PubMed |
[29] Washington MA, Jerse AE, Rahman N, Pilligua-Lucas M, Garges EC, Latif NH, Akhvlediani T. First description of a cefixime- and ciprofloxacin-resistant Neisseria gonorrhoeae isolate with mutations in key antimicrobial susceptibility-determining genes from the country of Georgia. New Microbes New Infect 2018; 24 47–51.
| First description of a cefixime- and ciprofloxacin-resistant Neisseria gonorrhoeae isolate with mutations in key antimicrobial susceptibility-determining genes from the country of Georgia.Crossref | GoogleScholarGoogle Scholar | 29872530PubMed |
[30] Lahra MM, Martin I, Demczuk W, Jennison AV, Lee KI, Nakayama SI, Lefebvre B, Longtin J, Ward A, Mulvey MR, Wi T, Ohnishi M, Whiley D. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis 2018; 24 735–43.
| Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain.Crossref | GoogleScholarGoogle Scholar |
[31] Yokoi S, Deguchi T, Ozawa T, Yasuda M, Ito S, Kubota Y, Tamaki M, Maeda S. Threat to cefixime treatment for gonorrhea. Emerg Infect Dis 2007; 13 1275–7.
| 17953118PubMed |
[32] Unemo M, Golparian D, Syversen G, Vestrheim DF, Moi H. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveill 2010; 15 19721
| 21144442PubMed |
[33] Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill 2011; 16 19833
| 21492528PubMed |
[34] Forsyth S, Penney P, Rooney G. Cefixime-resistant Neisseria gonorrhoeae in the UK: a time to reflect on practice and recommendations. Int J STD AIDS 2011; 22 296–7.
| Cefixime-resistant Neisseria gonorrhoeae in the UK: a time to reflect on practice and recommendations.Crossref | GoogleScholarGoogle Scholar | 21571983PubMed |
[35] Unemo M, Golparian D, Stary A, Eigentler A. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill 2011; 16 19998
| First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011.Crossref | GoogleScholarGoogle Scholar | 22085601PubMed |
[36] Cristillo AD, Bristow CC, Torrone E, Dillon JA, Kirkcaldy RD, Dong H, Grad YH, Nicholas RA, Rice PA, Lawrence K, Oldach D, Shafer WM, Zhou P, Wi TE, Morris SR, Klausner JD. Antimicrobial resistance in Neisseria gonorrhoeae: proceedings of the STAR Sexually Transmitted Infection-Clinical Trial Group Programmatic Meeting. Sex Transm Dis 2019; 46 e18–25.
| Antimicrobial resistance in Neisseria gonorrhoeae: proceedings of the STAR Sexually Transmitted Infection-Clinical Trial Group Programmatic Meeting.Crossref | GoogleScholarGoogle Scholar | 30363025PubMed |
[37] Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: the impending problem of resistance. Expert Opin Pharmacother 2009; 10 555–77.
| The use of cephalosporins for gonorrhea: the impending problem of resistance.Crossref | GoogleScholarGoogle Scholar | 19284360PubMed |
[38] Blank S, Daskalakis DC. Neisseria gonorrhoeae — rising infection rates, dwindling treatment options. N Engl J Med 2018; 379 1795–7.
| Neisseria gonorrhoeae — rising infection rates, dwindling treatment options.Crossref | GoogleScholarGoogle Scholar | 30403946PubMed |
[39] Dougherty TJ, Koller AE, Tomasz A. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother 1980; 18 730–7.
| Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 6778384PubMed |
[40] Ameyama S, Onodera S, Takahata M, Minami S, Maki N, Endo K, Goto H, Suzuki H, Oishi Y. Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother 2002; 46 3744–9.
| Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime.Crossref | GoogleScholarGoogle Scholar | 12435671PubMed |
[41] Gose S, Nguyen D, Lowenberg D, Samuel M, Bauer H, Pandori M. Neisseria gonorrhoeae and extended-spectrum cephalosporins in California: surveillance and molecular detection of mosaic penA. BMC Infect Dis 2013; 13 570
| Neisseria gonorrhoeae and extended-spectrum cephalosporins in California: surveillance and molecular detection of mosaic penA.Crossref | GoogleScholarGoogle Scholar | 24305088PubMed |
[42] Chen CC, Yen MY, Wong WW, Li LH, Huang YL, Chen KW, Li SY. Tracing subsequent dissemination of a cluster of gonococcal infections caused by an ST1407-related clone harbouring mosaic penA alleles in Taiwan. J Antimicrob Chemother 2013; 68 1567–71.
| Tracing subsequent dissemination of a cluster of gonococcal infections caused by an ST1407-related clone harbouring mosaic penA alleles in Taiwan.Crossref | GoogleScholarGoogle Scholar | 23508619PubMed |
[43] Ochiai S, Ishiko H, Yasuda M, Deguchi T. Rapid detection of the mosaic structure of the Neisseria gonorrhoeae penA Gene, which is associated with decreased susceptibilities to oral cephalosporins. J Clin Microbiol 2008; 46 1804–10.
| Rapid detection of the mosaic structure of the Neisseria gonorrhoeae penA Gene, which is associated with decreased susceptibilities to oral cephalosporins.Crossref | GoogleScholarGoogle Scholar | 18367575PubMed |
[44] European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters, version 9.0. Basel: EUCAST; 2019. Available online at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf [verified 1 June 2019].
[45] Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing, 28th edn. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
[46] Shimuta K, Unemo M, Nakayama S, Morita-Ishihara T, Dorin M, Kawahata T, Ohnishi M. Antimicrobial resistance and molecular typing of Neisseria gonorrhoeae isolates in Kyoto and Osaka, Japan, 2010 to 2012: intensified surveillance after identification of the first strain (H041) with high-level ceftriaxone resistance. Antimicrob Agents Chemother 2013; 57 5225–32.
| Antimicrobial resistance and molecular typing of Neisseria gonorrhoeae isolates in Kyoto and Osaka, Japan, 2010 to 2012: intensified surveillance after identification of the first strain (H041) with high-level ceftriaxone resistance.Crossref | GoogleScholarGoogle Scholar | 23939890PubMed |
[47] Tomberg J, Fedarovich A, Vincent LR, Jerse AE, Unemo M, Davies C, Nicholas RA. Alanine 501 mutations in penicillin-binding protein 2 from Neisseria gonorrhoeae: structure, mechanism, and effects on cephalosporin resistance and biological fitness. Biochemistry 2017; 56 1140–50.
| Alanine 501 mutations in penicillin-binding protein 2 from Neisseria gonorrhoeae: structure, mechanism, and effects on cephalosporin resistance and biological fitness.Crossref | GoogleScholarGoogle Scholar | 28145684PubMed |
[48] Tomberg J, Unemo M, Ohnishi M, Davies C, Nicholas RA. Identification of amino acids conferring high-level resistance to expanded-spectrum cephalosporins in the penA gene from Neisseria gonorrhoeae strain H041. Antimicrob Agents Chemother 2013; 57 3029–36.
| Identification of amino acids conferring high-level resistance to expanded-spectrum cephalosporins in the penA gene from Neisseria gonorrhoeae strain H041.Crossref | GoogleScholarGoogle Scholar | 23587946PubMed |
[49] Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 2011; 55 3538–45.
| Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: Detailed characterization of the first strain with high-level resistance to ceftriaxone.Crossref | GoogleScholarGoogle Scholar | 21576437PubMed |
[50] Demczuk W, Sidhu S, Unemo M, Whiley DM, Allen VG, Dillon JR, Cole M, Seah C, Trembizki E, Trees DL, Kersh EN, Abrams AJ, de Vries HJC, van Dam AP, Medina I, Bharat A, Mulvey MR, Van Domselaar G, Martin I. Neisseria gonorrhoeae sequence typing for antimicrobial resistance, a novel antimicrobial resistance multilocus typing scheme for tracking global dissemination of N. gonorrhoeae strains. J Clin Microbiol 2017; 55 1454–68.
| Neisseria gonorrhoeae sequence typing for antimicrobial resistance, a novel antimicrobial resistance multilocus typing scheme for tracking global dissemination of N. gonorrhoeae strains.Crossref | GoogleScholarGoogle Scholar | 28228492PubMed |
[51] Tomberg J, Unemo M, Davies C, Nicholas RA. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry 2010; 49 8062–70.
| Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations.Crossref | GoogleScholarGoogle Scholar | 20704258PubMed |
[52] Pan W, Spratt BG. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol Microbiol 1994; 11 769–75.
| Regulation of the permeability of the gonococcal cell envelope by the mtr system.Crossref | GoogleScholarGoogle Scholar | 8196548PubMed |
[53] Ropp PA, Hu M, Olesky M, Nicholas RA. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 2002; 46 769–77.
| Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 11850260PubMed |
[54] Zhao S, Tobiason DM, Hu M, Seifert HS, Nicholas RA. The penC mutation conferring antibiotic resistance in Neisseria gonorrhoeae arises from a mutation in the PilQ secretin that interferes with multimer stability. Mol Microbiol 2005; 57 1238–51.
| The penC mutation conferring antibiotic resistance in Neisseria gonorrhoeae arises from a mutation in the PilQ secretin that interferes with multimer stability.Crossref | GoogleScholarGoogle Scholar | 16101998PubMed |
[55] Olesky M, Zhao S, Rosenberg RL, Nicholas RA. Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute, and antibiotic permeation through PIB proteins with penB mutations. J Bacteriol 2006; 188 2300–8.
| Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute, and antibiotic permeation through PIB proteins with penB mutations.Crossref | GoogleScholarGoogle Scholar | 16547016PubMed |
[56] Harris SR, Cole MJ, Spiteri G, Sanchez-Buso L, Golparian D, Jacobsson S, Goater R, Abudahab K, Yeats CA, Bercot B, Borrego MJ, Crowley B, Stefanelli P, Tripodo F, Abad R, Aanensen DM, Unemo M, Azevedo J, Balla E, Barbara C. Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: a genomic survey. Lancet Infect Dis 2018; 18 758–68.
| Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: a genomic survey.Crossref | GoogleScholarGoogle Scholar | 29776807PubMed |
[57] Shimuta K, Watanabe Y, Nakayama S, Morita-Ishihara T, Kuroki T, Unemo M, Ohnishi M. Emergence and evolution of internationally disseminated cephalosporin-resistant Neisseria gonorrhoeae clones from 1995 to 2005 in Japan. BMC Infect Dis 2015; 15 378
| Emergence and evolution of internationally disseminated cephalosporin-resistant Neisseria gonorrhoeae clones from 1995 to 2005 in Japan.Crossref | GoogleScholarGoogle Scholar | 26381611PubMed |
[58] Grad YH, Kirkcaldy RD, Trees D, Dordel J, Harris SR, Goldstein E, Weinstock H, Parkhill J, Hanage WP, Bentley S, Lipsitch M. Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study. Lancet Infect Dis 2014; 14 220–6.
| Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study.Crossref | GoogleScholarGoogle Scholar | 24462211PubMed |
[59] Lo JY, Ho KM, Leung AO, Tiu FS, Tsang GK, Lo AC, Tapsail JW. Ceftibuten resistance and treatment failure of Neisseria gonorrhoeae infection. Antimicrob Agents Chemother 2008; 52 3564–7.
| Ceftibuten resistance and treatment failure of Neisseria gonorrhoeae infection.Crossref | GoogleScholarGoogle Scholar | 18663018PubMed |
[60] Unemo M, Nicholas RA, Jerse AE, Davies C, Shafer WM. Molecular mechanisms of antibiotic resistance expressed by the pathogenic Neisseria. In Genco C, Wetzler L, editors. Neisseria: molecular mechanisms of pathogenesis, 2nd edn. Norfolk, UK: Caister Academic Press; 2014. pp. 161–192.
[61] Doná V, Kasraian S, Lupo A, Guilarte YN, Hauser C, Furrer H, Unemo M, Low N, Endimiani A. Multiplex real-time PCR assay with high-resolution melting analysis for characterization of antimicrobial resistance in Neisseria gonorrhoeae. J Clin Microbiol 2016; 54 2074–81.
| Multiplex real-time PCR assay with high-resolution melting analysis for characterization of antimicrobial resistance in Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 27225407PubMed |
[62] Zhao S, Duncan M, Tomberg J, Davies C, Unemo M, Nicholas RA. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob Agents Chemother 2009; 53 3744–51.
| Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 19528266PubMed |
[63] Whiley DM, Jacobsson S, Tapsall JW, Nissen MD, Sloots TP, Unemo M. Alterations of the pilQ gene in Neisseria gonorrhoeae are unlikely contributors to decreased susceptibility to ceftriaxone and cefixime in clinical gonococcal strains. J Antimicrob Chemother 2010; 65 2543–7.
| Alterations of the pilQ gene in Neisseria gonorrhoeae are unlikely contributors to decreased susceptibility to ceftriaxone and cefixime in clinical gonococcal strains.Crossref | GoogleScholarGoogle Scholar | 20940180PubMed |
[64] Lee SG, Lee H, Jeong SH, Yong D, Chung GT, Lee YS, Chong Y, Lee K. Various penA mutations together with mtrR, porB and ponA mutations in Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime or ceftriaxone. J Antimicrob Chemother 2010; 65 669–75.
| Various penA mutations together with mtrR, porB and ponA mutations in Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime or ceftriaxone.Crossref | GoogleScholarGoogle Scholar | 20093260PubMed |
[65] World Health Organization. Global action plan on antimicrobial resistance. Geneva: World Health Organization; 2015.
[66] Buono SA, Watson TD, Borenstein LA, Klausner JD, Pandori MW, Godwin HA. Stemming the tide of drug-resistant Neisseria gonorrhoeae: the need for an individualized approach to treatment. J Antimicrob Chemother 2015; 70 374–81.
| Stemming the tide of drug-resistant Neisseria gonorrhoeae: the need for an individualized approach to treatment.Crossref | GoogleScholarGoogle Scholar | 25331059PubMed |
[67] Klausner JD, Kerndt P. Cephalosporin resistance in Neisseria gonorrhoeae infections. JAMA 2013; 309 1989–91.
| Cephalosporin resistance in Neisseria gonorrhoeae infections.Crossref | GoogleScholarGoogle Scholar | 23677303PubMed |
[68] Wong LKHP, Soge OO, Humphries RM, Klausner JD. Real-time PCR targeting the penA mosaic XXXIV type for prediction of extended-spectrum-cephalosporin susceptibility in clinical Neisseria gonorrhoeae isolates. Antimicrob Agents Chemother 2017; 61 e01339-17
| Real-time PCR targeting the penA mosaic XXXIV type for prediction of extended-spectrum-cephalosporin susceptibility in clinical Neisseria gonorrhoeae isolates.Crossref | GoogleScholarGoogle Scholar |
[69] Lo JY, Ho KM, Lo AC. Surveillance of gonococcal antimicrobial susceptibility resulting in early detection of emerging resistance. J Antimicrob Chemother 2012; 67 1422–6.
| Surveillance of gonococcal antimicrobial susceptibility resulting in early detection of emerging resistance.Crossref | GoogleScholarGoogle Scholar | 22334602PubMed |