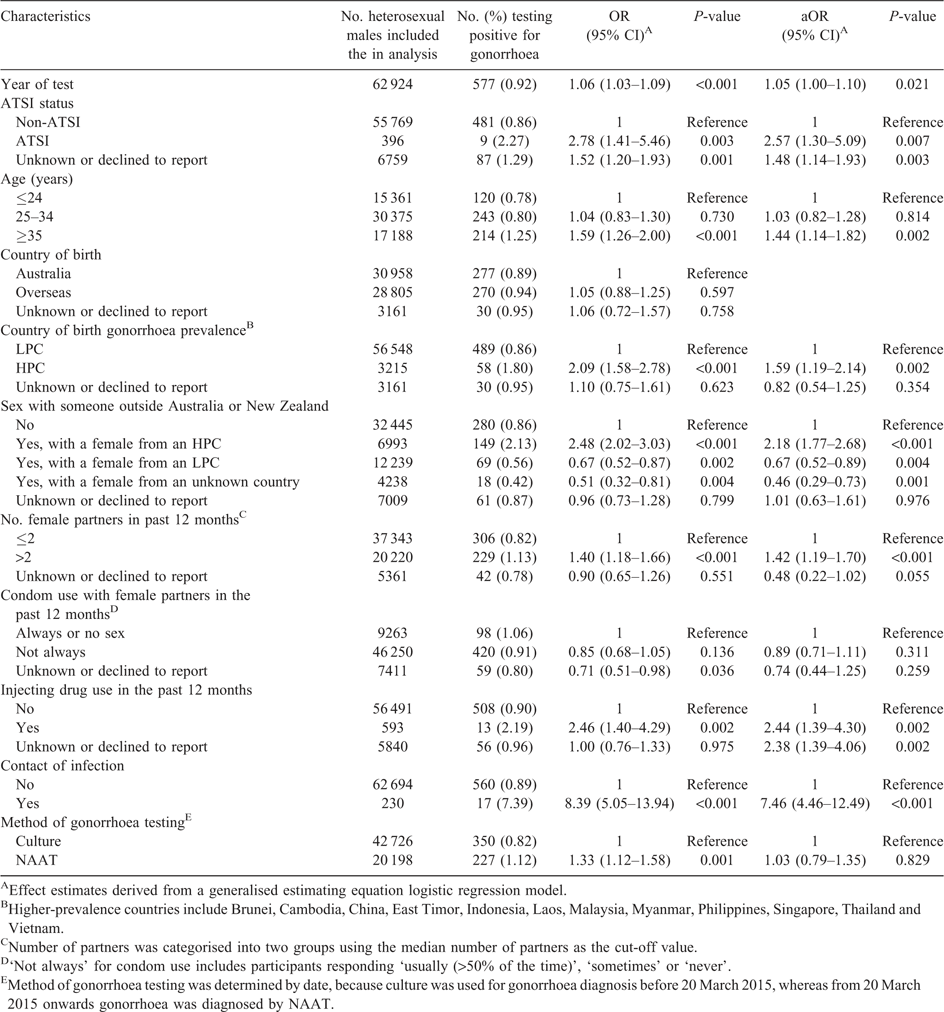
Risk factors for urethral gonorrhoea infection among heterosexual males in Melbourne, Australia: 2007–17
Tiffany R. Phillips A B C , Christopher K. Fairley A B , Marcus Y. Chen A B , Catriona S. Bradshaw A B and Eric P. F. Chow
A B C , Christopher K. Fairley A B , Marcus Y. Chen A B , Catriona S. Bradshaw A B and Eric P. F. Chow  A B
A B
A Melbourne Sexual Health Centre, Alfred Health, 580 Swanston Street, Melbourne, Vic. 3053, Australia.
B Central Clinical School, Faculty of Medicine, Nursing and Health Sciences, Monash University, 99 Commercial Road, Melbourne, Vic. 3004, Australia.
C Corresponding author. Email: TPhillips@mshc.org.au
Sexual Health 16(5) 508-513 https://doi.org/10.1071/SH19027
Submitted: 8 February 2019 Accepted: 1 April 2019 Published: 17 June 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Background: Since 2014 there has been an increase in gonorrhoea among heterosexuals in Australia. Sex with a partner from a country with high gonorrhoea prevalence has been identified as a risk factor for gonorrhoea in heterosexual females, but risk factors for heterosexual males remain unclear. This study determined risk factors for gonorrhoea among heterosexual males. Methods: Retrospective analysis was performed among heterosexual males attending Melbourne Sexual Health Centre (MSHC) between 1 January 2007 and 31 December 2017. Countries for overseas sexual partners were stratified as high-prevalence countries (HPC) or low-prevalence countries (LPC) based on the incidence of gonorrhoea. Results: The annual gonorrhoea positivity increased from 0.72% in 2007 to 1.33% in 2017 (Ptrend <0.001). Males attending MSHC as a contact of gonorrhoea had the highest odds of testing positive (adjusted odds ratio (aOR) 7.46; 95% confidence interval (CI) 4.46–12.49), followed by males identifying as Aboriginal and Torres Strait Islander (aOR 2.57; 95% CI 1.30–5.09), males who had injected drugs in the past 12 months (aOR 2.44; 95% CI 1.39–4.30) and males who had sex with a female from an HPC (aOR 2.18; 95% CI 1.77–2.68). Males aged ≥35 were at higher risk than those aged ≤24 years (aOR 1.44; 95% CI 1.14–1.82). Gonorrhoea positivity increased among males who had sex with females from an LPC (from 0.60% to 1.33%; Ptrend = 0.004) but remained the same over time among males who had sex with females from an HPC (2.14%; Ptrend = 0.143). Conclusions: There was an 80% increase in urethral gonorrhoea among heterosexual males between 2007 and 2017. Having sex with a female from an HPC is a significant risk factor for gonorrhoea. Gonorrhoea positivity among men having sex with a female from an HPC did not change over time, suggesting this risk factor has become less important.
Additional keywords: gonorrhoea prevention, sex overseas, travel.
Introduction
Gonorrhoea notifications have increased markedly among men who have sex with men (MSM) in Australia over the past decade.1 The rates of gonorrhoea among heterosexuals in Australia have been rising steadily over the past decade and accelerated in 2014.2,3 The Annual Surveillance Report for 2018 showed an 80% increase in gonorrhoea notifications between 2013 and 2017 (from 65.5 to 118.0 per 1 00 000), with an increase in gonorrhoea notifications in both males (91%) and females (56%).4 In addition, there was an increase in the ratio of gonorrhoea notifications to Medicare-rebated tests (from 1.4 to 1.9) among both males (38%) and females (31%) between 2013 and 2017, suggesting an increase in transmission through heterosexual sex as opposed to an increase in testing.4
The reasons behind the rise in gonorrhoea among heterosexuals in Australia are unclear. Recently, a study from Melbourne identified that females who had sex with a partner from a country with a high prevalence of gonorrhoea (e.g. South-east or East Asia) had 2.37-fold higher odds of having genital gonorrhoea than females who had no overseas sexual partners.5 However, this association has not been investigated among heterosexual males, so it is not known whether greater overseas travel and sex overseas is a contributing risk factor for the rise in gonorrhoea in this population or whether increasing endemic transmission in Australia is responsible for the increase.
To better understand the causes of rising gonococcal notifications among heterosexual males, this study identified risk factors for urethral gonorrhoea among heterosexual males attending a sexual health clinic in Melbourne over a decade (2007–17).
Methods
Ethics approval for the study was obtained from the Alfred Hospital Ethics Committee, Melbourne, Australia (Project number 56/17).
Study population and setting
Males aged 16–80 years attending the Melbourne Sexual Health Centre (MSHC) between 1 January 2007 and 31 December 2017 who did not have sexual contact with another man were included in this analysis. The MSHC is the largest public sexual health centre located in the State of Victoria, Australia, providing approximately 45 000 consultations a year,6 of which approximately 28% are for heterosexual males.7 Demographic information, including questions on sexual contact with individuals overseas, is recorded for patients at each visit using a computer-assisted self-interview (CASI) or added to a patient’s medical file by the clinician during the consultation.
Study measures
‘Self-reported overseas sexual contact’ refers to sex with someone overseas or sex with someone in Australia who is from an overseas country. Countries were stratified into high-prevalence countries (HPCs) or low-prevalence countries (LPCs) based on the incidence of gonorrhoea among the female population in that country, as described previously.5 Female population data were used because most notification data do not stratify males into heterosexual and MSM, and therefore using male notification data will be skewed due to the high prevalence of gonorrhoea in MSM. The HPCs (i.e. more than 100 cases of gonorrhoea per 100 000 females)8 included China and South-east Asian countries (Brunei, Cambodia, East Timor, Indonesia, Laos, Malaysia, Myanmar, Philippines, Singapore, Thailand and Vietnam).
Self-reported condom use with female partners in the past 12 months refers to condom use with vaginal or anal sex. Clients were asked to select from the following options: always, usually (>50% of the time), sometimes, never, or not applicable.
During the study period, two different laboratory tests were used for gonorrhoea diagnoses. Between 1 January 2007 and 20 March 2015, culture was used for gonorrhoea diagnosis from urethral swabs (chlamydia was tested using a nucleic acid amplification test (NAAT; BD ProbeTec Strand Displacement Amplification Assay; Becton Dickinson, Sparks, MD, USA). However, from 20 March 2015 onwards, gonorrhoea was diagnosed using transcription-mediated amplification (Aptima Combo 2 assay; Hologic Gen-Probe PANTHER system; Hologic, Inc, San Diego, CA, USA) from first void urine. Prior to September 2017, urethral gonorrhoea testing for heterosexual males was only performed at the MSHC for those attending the clinic with urethral symptoms or reported sexual contact with partners with gonorrhoea because gonorrhoea screening is not recommended among heterosexual populations with low prevalence.9 Oropharyngeal gonorrhoea testing in heterosexual males is not currently recommended as per the Australian sexually transmissible infection (STI) screening guidelines.10 Urine testing for chlamydia was part of routine STI screening for heterosexual men during the study period.
Statistical analysis
Descriptive statistics and frequency distributions were calculated. Univariable logistic regression with a generalised estimating equation was performed to examine associations between gonorrhoea positivity from culture or NAAT and potential explanatory risk factors. Included in the analysis were demographic characteristics (age and country of birth), sexual behaviours (number of partners, condom use and sex with someone outside Australia or New Zealand), injecting drug use behaviours and self-reported contact of infection or having symptoms. Multivariable logistic regression was performed on those risk factors with P < 0.10 significance and crude and adjusted (aOR) odds ratios and 95% confidence intervals (CIs) are reported. Overall gonorrhoea positivity was calculated by the number of positives from culture or NAAT divided by the number of urine tests for chlamydia in each calendar year. Chlamydia tests were used as the denominator instead of the number of gonorrhoea tests performed because urethral gonorrhoea in males is almost always symptomatic. Screening for gonorrhoea in asymptomatic heterosexual males were not recommended in this time frame, and thus the positivity would be biased towards symptomatic heterosexual males. This method has been used previously in similar studies.3,11 A χ2 trend test was used to assess the temporal trend of gonorrhoea positivity.
All statistical analyses were performed using Stata version 14 (Stata Corporation, College Station, TX, USA).
Results
There were 1 02 709 clinical consultations for heterosexual males between 1 January 2007 and 31 December 2017. Of these, 50 (0.05%) were excluded for being <16 years of age and 83 (0.08%) were excluded for being >80 years of age. After then excluding consultations with no tests for gonorrhoea (by NAAT or culture) or chlamydia by urine sample (n = 37 500; 36.6%), those who had ever engaged in sex work (n = 583) and duplicate records from the same client (n = 1569), there were 62 924 consultations (41 925 individuals) included in this analysis.
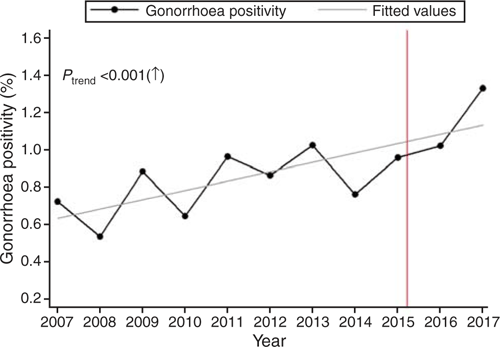
Of the 62 924 consultations included in the analysis, 577 (0.92%) resulted in a positive test for gonorrhoea either by culture or NAAT. Although the gonorrhoea positivity fluctuated during the study period, it increased significantly from 0.72% in 2007 to 1.33% in 2017 (Ptrend < 0.001; Fig. 1).
|
The median age of males was 29 years (interquartile range (IQR) 25–35 years) and the median (IQR) number of female sexual partners in the past 12 months was 2 (1–3). Most men (73.5%) had had condomless sex with their female sexual partners in the 12 months before the consultation.
Multivariable analysis showed that males attending the clinic as a contact of gonorrhoea infection (aOR 7.46; 95% CI 4.46–12.49; Table 1) had the highest odds of testing positive for gonorrhoea. Males identifying as Aboriginal and Torres Strait Islander (ATSI) had the next highest odds of testing positive for gonorrhoea (aOR 2.57; 95% CI 1.30–5.09) compared with non-ATSI males. In multivariable analysis, the aOR of testing positive for gonorrhoea was 2.44 (95% CI 1.39–4.30) among males who had injected drugs in the past 12 months compared with those who had not injected drugs.
Males who reported having sex with a female from an HPC were more likely to test positive for gonorrhoea (aOR 2.18; 95% CI 1.77–2.68) than males who reported no overseas sex (sex only with partners from Australia or New Zealand). However, males who had sex with a female from an LPC were less likely to test positive for gonorrhoea than those with no overseas sex, before and after adjusting for confounding factors (aOR 0.67; 95% CI 0.52–0.89). Being born in an HPC was associated with higher odds of testing positive for gonorrhoea than being born in an LPC (aOR 1.59; 95% CI 1.19–2.14).
Males with more than two female partners in the past 12 months were more likely to test positive for gonorrhoea (aOR 1.42; 95% CI 1.19–1.70) than males with two or fewer female partners. Males aged ≥35 years were more likely to test positive for gonorrhoea than those aged ≤24 years (aOR 1.44; 95% CI 1.14–1.82).
Testing for gonorrhoea by NAAT instead of culture was a risk factor for testing positive for gonorrhoea in the univariable analysis; however, this association disappeared in the multivariable analysis after adjusting for potential confounding factors. There was no association between condomless sex with female partners and urethral gonorrhoea.
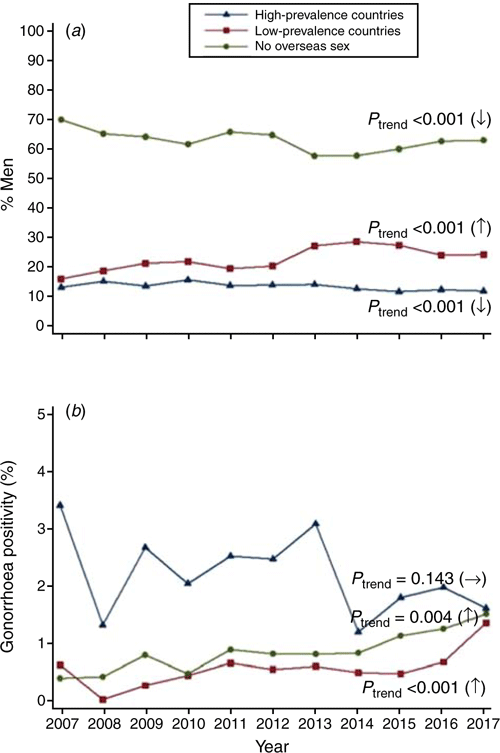
The proportion of males who had no overseas sex decreased significantly over time (from 70.4% in 2007 to 63.3% in 2017; Ptrend < 0.001; Fig. 2a), as did the proportion of males who had sex with a female from an HPC (from 13.4% to 12.1%; Ptrend < 0.001). The proportion of males who had sex with a female from an LPC increased significantly over time (from 16.2% in 2007 to 24.6% in 2017; Ptrend < 0.001). Gonorrhoea positivity among males who had sex with females from an LPC increased twofold over the study period (from 0.60% in 2007 to 1.33% in 2017; Ptrend = 0.004; Fig. 2b). Overall, gonorrhoea positivity among males who had sex with females from HPCs was 2.14%, and this did not change over the period (Ptrend = 0.143). Overall, gonorrhoea positivity among males who did not have any overseas sex was 0.86% and increased over the study period (Ptrend < 0.001).
|
Discussion
This study examined a decade of data to explore the risk factors for urethral gonorrhoea among 41 925 heterosexual males attending a sexual health clinic in Melbourne, Australia, with a particular focus on changes over time. We found an 80% increase in gonorrhoea positivity among heterosexual men between 2007 and 2017, together with some important observations in relation to travel, a risk identified in several countries when there is a relatively low prevalence of gonorrhoea in heterosexuals.12 Sex with a female from an HPC was a significant risk factor for gonorrhoea for heterosexual males, but over time this risk factor became relatively less important, with less sex with females from HPCs and a relatively unchanging positivity of gonorrhoea among those who had sex with females from HPCs. Monitoring changes in risk factors over time can lead to the identification of epidemics: an outbreak of gonorrhoea was associated with changes in sex worker policing in Australia,13 and changes in risks groups (from MSM to young heterosexuals) in a UK gonorrhoea outbreak alerted authorities to its existence.14,15
The findings of the present study suggest that having sex with a female from an HPC, although a statistically significant risk factor, is less important relative to other risk factors that appear to be operating in the heterosexual male population. The key for researchers is to identify what these factors are and how they relate to the usual factors identified, such as partner number and injecting drug use.
The proportion of heterosexual males having sex with females from an HPC fell during the study and the gonorrhoea positivity in this group remained unchanged, suggesting that this risk factor became less important relative to other risk factors during the study period. The absence of any rise in gonorrhoea positivity among these individuals, coupled with the simultaneous rise in gonorrhoea positivity in those with no overseas sex, supports the observation of a true rise in the prevalence of gonorrhoea among the local heterosexual community.
Consistent with previous studies, we found that travel is one of the risk factors for gonorrhoea in heterosexuals.5 A meta-analysis from 2010 concluded that casual sex while traveling is relatively common and that only approximately 50% of people engaging in new sexual relationships while traveling use condoms consistently.12 The fact that gonorrhoea positivity increased in males who had sex with females from LPCs could reflect an overall increase in gonorrhoea globally.
The rise in gonorrhoea among heterosexual males with no overseas sex may be influenced by the rising rates of gonorrhoea among female sex workers, but the present study could not investigate this risk factor because no data were collected on men who have sex with female sex workers during the study period. A retrospective cohort study of female sex workers attending 42 sexual health clinics across Australia showed an overall increase in gonorrhoea from 2009 to 2015, with the highest increase in oropharyngeal cases (increased by 200%; vs 71% and 171% increases in urogenital and anorectal cases respectively).16 Although the condom rate for female sex workers is relatively high in Australia for vaginal and anal sex, around 20–25% of female sex workers reported inconsistent condom use for oral sex (fellatio) in the past 3 months in NSW.17 The asymptomatic nature of oropharyngeal gonorrhoea and inconsistent condom use for oral sex could be contributing to transmission of gonorrhoea. The Second Australian Study of Health and Relationships from 2012 to 2013 reported that 2.2% of Australian males had paid a female for sex in the previous 12 months.18 However, there are no more recent estimates, and hence it is unclear whether this proportion has changed since 2013 along with the increase in gonorrhoea rates. A Sydney-based surveillance study has revealed that of the 69 gonorrhoea cases in heterosexual males during the period 2008–12, 41% of cases were likely to have acquired gonorrhoea from sex workers.19 Future studies should investigate this risk in order to better understand endemic transmission in heterosexuals.
This study has several limitations. First, the study was conducted at one STI clinic that sees a population that is generally at a higher risk of acquiring STIs than the general population, and it may be that the risks of gonorrhoea among clients of the clinic are different to the wider Australian community. Second, two different methods for testing gonorrhoea were used over the study period as the clinic shifted to the use of the duplex NAATs for gonorrhoea and chlamydia20 in March 2015,21 a more-sensitive and less-specific testing method for Neisseria gonorrhoeae. However, our data suggest that the increase in gonorrhoea over time was a true and significant increase, even after adjusting for testing method in the multivariable analysis.
Third, positivity may have changed over time because the clinic only started testing all asymptomatic heterosexual men for urethral gonorrhoea, and not just those presenting as a contact of infection or with symptoms, in September 2017. It is unlikely that this made a measurable difference given that gonorrhoea is rarely asymptomatic, and, indeed, gonorrhoea positivity did not differ in 2017 after the introduction of testing asymptomatic men (data not shown).
Fourth, due to the low rates of testing for gonorrhoea in heterosexual males over the study period, we have had to calculate positivity by using the number of men tested for chlamydia. However, because gonorrhoea is almost always symptomatic in men,22 this should be a reasonably accurate method for calculating positivity because it is unlikely that those not tested for gonorrhoea would be positive, and this method has been previously used in other studies.3,11 Thus, our findings may also be biased towards symptomatic males because they are more likely to present at the clinic and test positive.
Fifth, we are unable to distinguish between males who had sex overseas and those who had sex in Australia with an overseas partner. However, we performed a sensitivity analysis by excluding males who were born overseas in an HPC, and the significant risk factors in the multivariate analysis remained unchanged (data not shown), which indicates the risk is not limited to recent arrivals to Australia from an HPC.
Finally, males were able to report one country for their overseas sexual partner. If males have partners from multiple countries, only one country was captured and it is likely to be the country perceived to be a higher risk, as described previously.5
The rising rates of gonorrhoea in heterosexual males is concerning because they may indicate that the reproductive ratio (R0) has increased and is now greater than 1 in this population. It is also possible that the R0 is <1 and that there is greater spread to heterosexuals from other populations. The most recent estimate of the proportion of bisexual men in Australia is from the Second Australian Study of Health and Relationships (2012–13), which found that 1.3% of men identify as bisexual.23 The gonorrhoea positivity among MSM has risen greatly, so even if the proportion of bisexual men remained stable since 2014, this rising prevalence will have a great effect on heterosexual gonorrhoea. It is very important that the reasons for the rises in gonorrhoea are accurately determined because they will inform public health campaigns aimed at reducing rates.
Conflicts of interest
None declared.
Acknowledgements
Eric P. F. Chow was supported by a National Health and Medical Research Council of Australia Early Career Fellowship (APP1091226). This research did not receive any specific funding.
References
[1] Callander D, Guy R, Fairley CK, McManus H, Prestage G, Chow EPF, et al Gonorrhoea gone wild: rising incidence of gonorrhoea and associated risk factors among gay and bisexual men attending Australian sexual health clinics. Sex Health 2018;| Gonorrhoea gone wild: rising incidence of gonorrhoea and associated risk factors among gay and bisexual men attending Australian sexual health clinics.Crossref | GoogleScholarGoogle Scholar | 30409244PubMed |
[2] Jasek E, Chow EP, Ong JJ, Bradshaw CS, Chen MY, Hocking JS, et al Sexually transmitted infections in Melbourne, Australia from 1918 to 2016: nearly a century of data. Commun Dis Intell Q Rep 2017; 41 E212–22.
| 29720070PubMed |
[3] Mannion PK, Fairley CK, Fehler G, Tabrizi SN, Tan WS, Chen MY, et al Trends in gonorrhoea positivity by nucleic acid amplification test versus culture among Australian heterosexual men with a low prevalence of gonorrhoea, 2007–2014. Sex Transm Infect 2016; 92 625–8.
| Trends in gonorrhoea positivity by nucleic acid amplification test versus culture among Australian heterosexual men with a low prevalence of gonorrhoea, 2007–2014.Crossref | GoogleScholarGoogle Scholar | 26888660PubMed |
[4] Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2018. Sydney: Kirby Institute, UNSW Sydney; 2018.
[5] Misson J, Chow EPF, Chen MY, Read TRH, Bradshaw CS, Fairley CK. Trends in gonorrhoea infection and overseas sexual contacts among females attending a sexual health centre in Melbourne, Australia, 2008–2015. Commun Dis Intell (2018) 2018; 42
| 30626294PubMed |
[6] Needleman R, Chow EPF, Towns JM, Cornelisse VJ, Yang TZT, Chen MY, et al Access to sexual health services after the rapid roll out of the launch of pre-exposure prophylaxis for HIV in Melbourne, Australia: a retrospective cross-sectional analysis. Sex Health 2018;
| Access to sexual health services after the rapid roll out of the launch of pre-exposure prophylaxis for HIV in Melbourne, Australia: a retrospective cross-sectional analysis.Crossref | GoogleScholarGoogle Scholar | 29973331PubMed |
[7] Chow EPF, Carlin JB, Read TRH, Chen MY, Bradshaw CS, Sze JK, et al Factors associated with declining to report the number of sexual partners using computer-assisted self-interviewing: a cross-sectional study among individuals attending a sexual health centre in Melbourne, Australia. Sex Health 2018;
| Factors associated with declining to report the number of sexual partners using computer-assisted self-interviewing: a cross-sectional study among individuals attending a sexual health centre in Melbourne, Australia.Crossref | GoogleScholarGoogle Scholar | 30558710PubMed |
[8] Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10 e0143304
| Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting.Crossref | GoogleScholarGoogle Scholar | 26646541PubMed |
[9] Field N, Clifton S, Alexander S, Ison CA, Hughes G, Beddows S, et al Confirmatory assays are essential when using molecular testing for Neisseria gonorrhoeae in low-prevalence settings: insights from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Sex Transm Infect 2015; 91 338–41.
| Confirmatory assays are essential when using molecular testing for Neisseria gonorrhoeae in low-prevalence settings: insights from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3).Crossref | GoogleScholarGoogle Scholar | 25512673PubMed |
[10] Australasian Sexual Health Alliance. Australian STI management guidelines for use in primary care. 2018. Available online at: http://www.sti.guidelines.org.au [verified 15 March 2019].
[11] Chow EP, Tomnay J, Fehler G, Whiley D, Read TR, Denham I, et al Substantial increases in chlamydia and gonorrhea positivity unexplained by changes in individual-level sexual behaviors among men who have sex with men in an Australian sexual health service from 2007 to 2013. Sex Transm Dis 2015; 42 81–7.
| Substantial increases in chlamydia and gonorrhea positivity unexplained by changes in individual-level sexual behaviors among men who have sex with men in an Australian sexual health service from 2007 to 2013.Crossref | GoogleScholarGoogle Scholar | 25585066PubMed |
[12] Vivancos R, Abubakar I, Hunter PR. Foreign travel, casual sex, and sexually transmitted infections: systematic review and meta-analysis. Int J Infect Dis 2010; 14 e842–51.
| Foreign travel, casual sex, and sexually transmitted infections: systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 20580587PubMed |
[13] Li B, Bi P, Waddell R, Chow EP, Donovan B, McNulty A, et al Was an epidemic of gonorrhoea among heterosexuals attending an Adelaide sexual health services associated with variations in sex work policing policy? Sex Transm Infect 2016; 92 377–9.
| Was an epidemic of gonorrhoea among heterosexuals attending an Adelaide sexual health services associated with variations in sex work policing policy?Crossref | GoogleScholarGoogle Scholar | 26567331PubMed |
[14] Foster K, Cole M, Hotonu O, Stonebridge J, Hughes G, Simms I, et al How to do it: lessons identified from investigating and trying to control an outbreak of gonorrhoea in young heterosexual adults. Sex Transm Infect 2016; 92 396–401.
| How to do it: lessons identified from investigating and trying to control an outbreak of gonorrhoea in young heterosexual adults.Crossref | GoogleScholarGoogle Scholar | 26936653PubMed |
[15] Fairley CK, Ong JJ, Chow EP. Epidemics of STIs; ask why. Sex Transm Infect 2016; 92 329–30. [Editorial]
| Epidemics of STIs; ask why.Crossref | GoogleScholarGoogle Scholar | 27440928PubMed |
[16] Callander D, McManus H, Guy R, Hellard M, O’Connor CC, Fairley CK, et al Rising chlamydia and gonorrhoea incidence and associated risk factors among female sex workers in Australia: a retrospective cohort study. Sex Transm Dis 2018; 45 199–206.
| Rising chlamydia and gonorrhoea incidence and associated risk factors among female sex workers in Australia: a retrospective cohort study.Crossref | GoogleScholarGoogle Scholar | 29420449PubMed |
[17] Read PJ, Wand H, Guy R, Donovan B, McNulty AM. Unprotected fellatio between female sex workers and their clients in Sydney, Australia. Sex Transm Infect 2012; 88 581–4.
| Unprotected fellatio between female sex workers and their clients in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 22875839PubMed |
[18] Rissel C, Donovan B, Yeung A, de Visser RO, Grulich A, Simpson JM, et al Decriminalization of sex work is not associated with more men paying for sex: results from the Second Australian Study of Health and Relationships. Sex Res Soc Policy 2017; 14 81–6.
| Decriminalization of sex work is not associated with more men paying for sex: results from the Second Australian Study of Health and Relationships.Crossref | GoogleScholarGoogle Scholar |
[19] Lusk MJ, Uddin RN, Lahra MM, Garden FL, Kundu RL, Konecny P. Pharyngeal gonorrhoea in women: an important reservoir for increasing Neisseria gonorrhoea prevalence in urban Australian heterosexuals? J Sex Transm Dis 2013; 2013 967471
| Pharyngeal gonorrhoea in women: an important reservoir for increasing Neisseria gonorrhoea prevalence in urban Australian heterosexuals?Crossref | GoogleScholarGoogle Scholar | 26316970PubMed |
[20] Donovan B, Dimech W, Ali H, Guy R, Hellard M. Increased testing for Neisseria gonorrhoeae with duplex nucleic acid amplification tests in Australia: implications for surveillance. Sex Health 2015; 12 48–50.
| Increased testing for Neisseria gonorrhoeae with duplex nucleic acid amplification tests in Australia: implications for surveillance.Crossref | GoogleScholarGoogle Scholar | 25659837PubMed |
[21] Cornelisse VJ, Chow EP, Huffam S, Fairley CK, Bissessor M, De Petra V, et al Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44 114–17.
| Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing.Crossref | GoogleScholarGoogle Scholar | 27984552PubMed |
[22] Ong JJ, Fethers K, Howden BP, Fairley CK, Chow EPF, Williamson DA, et al Asymptomatic and symptomatic urethral gonorrhoea in men who have sex with men attending a sexual health service. Clin Microbiol Infect 2017; 23 555–9.
| Asymptomatic and symptomatic urethral gonorrhoea in men who have sex with men attending a sexual health service.Crossref | GoogleScholarGoogle Scholar | 28257898PubMed |
[23] Richters J, Altman D, Badcock PB, Smith AM, de Visser RO, Grulich AE, et al Sexual identity, sexual attraction and sexual experience: the Second Australian Study of Health and Relationships. Sex Health 2014; 11 451–60.
| Sexual identity, sexual attraction and sexual experience: the Second Australian Study of Health and Relationships.Crossref | GoogleScholarGoogle Scholar | 25376998PubMed |