Investigating the decline in Lymphogranuloma venereum diagnoses in men who have sex with men in the United Kingdom since 2016: an analysis of surveillance data
Hester Allen A F , Rachel Pitt A , Megan Bardsley
A F , Rachel Pitt A , Megan Bardsley  A , Christa Smolarchuk
A , Christa Smolarchuk  A , Ann Sullivan
A , Ann Sullivan  A , Hamish Mohammed
A , Hamish Mohammed  A , Michelle Cole
A , Michelle Cole  A , Helen Fifer A , Lesley Wallace B , Daniel Thomas C , Neil Irvine D , Kate Templeton
A , Helen Fifer A , Lesley Wallace B , Daniel Thomas C , Neil Irvine D , Kate Templeton  E , Gwenda Hughes
E , Gwenda Hughes  A and Ian Simms
A and Ian Simms  A
A
A National Infection Service, Public Health England, 61 Colindale Avenue, London, NW9 5EQ, UK.
B Health Protection Scotland, 5 Cadogan Street, Glasgow, G2 6QE, UK.
C Communicable Disease Surveillance Centre, Public Health Wales, 2 Capital Quarter, Tyndall Street, Cardiff, CF10 4B, UK.
D Public Health Agency, Health and Social Care Northern Ireland, 12–22 Linenhall Street, Belfast, BT2 8BS, UK.
E Scottish Bacterial Sexually Transmitted Infections Reference Laboratory, Royal Infirmary of Edinburgh, 51 Little France Crescent, Edinburgh, EH16 4SA, UK.
F Corresponding author. Email: hester.allen@phe.gov.uk
Sexual Health 17(4) 344-351 https://doi.org/10.1071/SH20001
Submitted: 3 January 2020 Accepted: 26 May 2020 Published: 7 August 2020
Journal compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Background: Following an upward trajectory in Lymphogranuloma venereum (LGV) diagnoses in the UK from 2004 to 2016, with annual diagnoses increasing from 28 to 904, diagnoses fell to 641 in 2017; this was inconsistent with the upward trend in other bacterial sexually transmissible infections (STIs) between 2016 and 2017. An analysis of surveillance data from multiple sources to investigate the possible factors contributing to this decline in LGV was performed. Methods: LGV tests and diagnoses in the UK from 2004 to 2018 were captured through laboratory data from the LGV Reference Laboratories and laboratories conducting in-house LGV testing. These data and clinical diagnoses data from England were analysed alongside the national management guidelines issued over the course of the epidemic. Results: LGV diagnoses increased between 2004 and 2015 and then decreased between 2016 and 2018. LGV testing increased from 2010 to 2018 (2690–10 850). Test positivity halved between 2015 (14.8%, 929–6272) and 2018 (7.3%, 791–10 850). Peaks in LGV testing and diagnoses appeared to coincide with the publication of national LGV management guidelines and changes to clinical practice. The proportion of LGV diagnoses among HIV-positive men who have sex with men (MSM) fell between 2013 and 2018 (74–48%). Conclusions: The fall in diagnoses and positivity were likely due to increasing earlier clinical diagnosis and treatment. Changes to the national management guidelines, the clinical policy and practice of some larger clinics and potentially changes to the guidelines for the treatment of chlamydia broadened the scope of testing and increased testing in asymptomatic patients which, in combination, likely had a positive effect on the control of LGV infection.
Additional keywords: asymptomatic, Chlamydia trachomatis, LGV, MSM, sexually transmitted infections.
Introduction
Lymphogranuloma venereum (LGV) is a sexually transmissible infection (STI) caused by the invasive L1, L2 and L3 serovars of Chlamydia trachomatis.1 Increases in LGV were first seen among gay, bisexual and other men who have sex with men (MSM) in Western Europe in 2003, and outbreaks were subsequently reported in other high-income countries, including Australia.2 Diagnoses in the UK and across Europe rose between 2004 and 2016, with the highest European rates seen in the UK.3 Surveillance of LGV in the UK showed a consistent ongoing pattern of the infection being concentrated in MSM living in London, many of whom were co-infected HIV and other STIs. Acquisition was associated with reported behaviours such as condomless anal intercourse; fisting and the use of sex toys; and the increased use of chemsex drugs and group sex facilitated by geospatial networking applications.4–9
The increase in LGV diagnoses seen between 2009 and 2016 was consistent with trends of other STIs in England. Diagnoses of chlamydia, syphilis and gonorrhoea seen in those attending sexual health services increased by 26%, 178% and 280% respectively and, among MSM, by 338%, 234% and 642% respectively.10
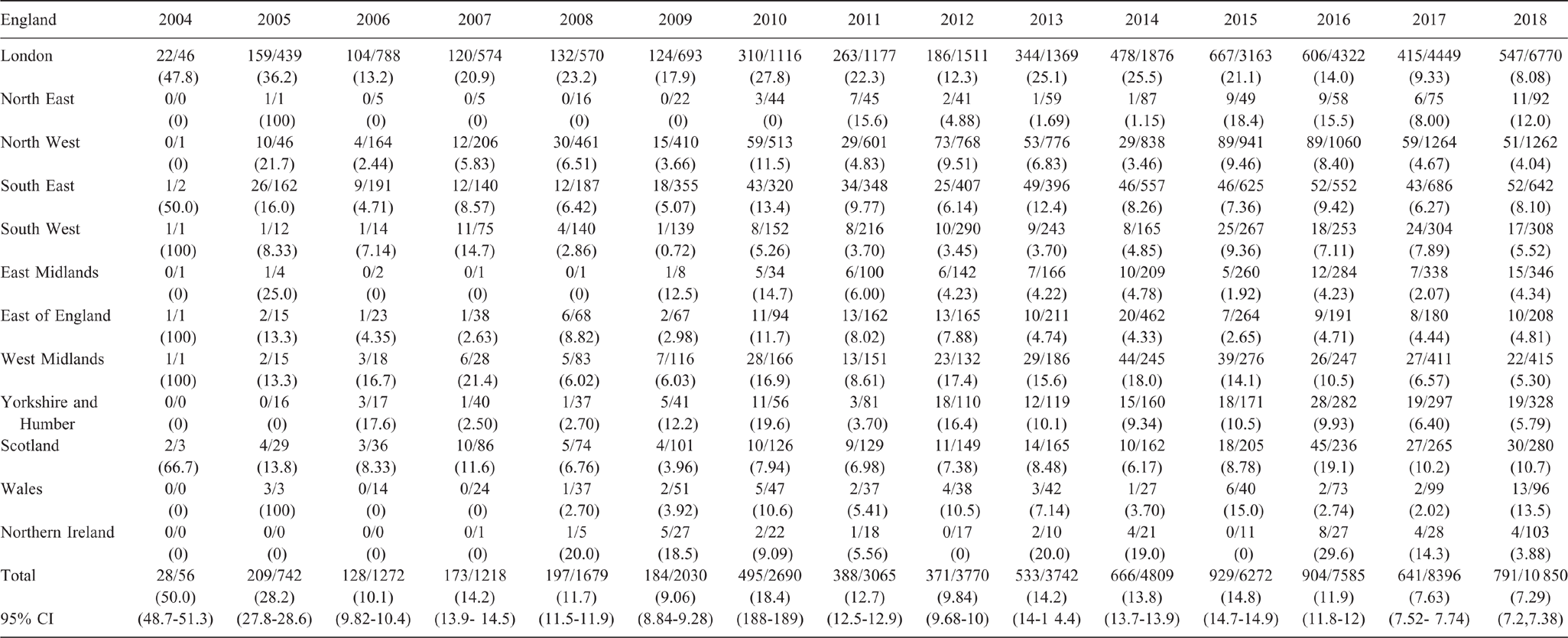
National UK LGV management guidelines have evolved over the course of the epidemic in response to increased insight into the burden of asymptomatic infection, clinical presentation and groups at risk of infection. The initial test for C. trachomatis offered at sexual health services does not distinguish serovars D–K from LGV serovars (L1, L2 and L3). The 2006 LGV guidelines recommended that testing for LGV serovars should be performed on clinical suspicion based on the presence of rectal symptoms and after the exclusion of other aetiologies of rectal bleeding.11 The 2010 UK national chlamydia management guidelines recommended that LGV testing should only be offered to MSM who testing positive for C. trachomatis at the rectal site and those presenting with any rectal symptoms, together with the contacts of confirmed LGV cases (Fig. 1).12 In 2013, the UK national LGV management guidelines extended the testing of LGV to include testing all MSM who test a positive for rectal or pharyngeal C. trachomatis and those who present with rectal symptoms consistent with LGV.13 Results of a 2012 case-finding study showed that 27% of LGV infections were seen in patients without rectal symptoms, indicating that the burden of infection and the level of ongoing transmission may have been higher than previously recognised.14 Consequently, scope for LGV testing was extended in the 2015 chlamydia guidelines. These guidelines recommended that, alongside testing patients with signs and symptoms consistent with LGV infection, LGV testing was conducted on samples from all MSM living with HIV who test positive for C. trachomatis at any site (rectal, genital or pharyngeal), regardless of the presence of symptoms consistent with LGV.15
|
After a consistent upward trajectory, a reduction in LGV diagnoses in the UK was seen from the end of 2015 to 2018. This divergence from the continued, sustained increases in non-LGV chlamydia, syphilis and gonorrhoea in England was assessed by Public Health England (PHE). Here, we present the findings of this investigation, which considered the influence of variations in testing guidelines and practices; clinical and prescribing practices; and the test performance of the diagnostic assay on the course of the epidemic and the control of LGV in the UK.
Methods
Data sources
In the UK, LGV surveillance is coordinated by PHE using four laboratory data sources that conduct LGC testing using the same assay: (1) the PHE National Reference Laboratory, which has performed LGV testing for all sexual health clinics in England, Wales and Northern Ireland since 2004; (2) three London hospital laboratories, which began providing in-house LGV testing to some London sexual health clinics from 2015 to 2018; (3) the Scottish Bacterial Sexually Transmitted Infections Reference Laboratory, which performs LGV testing for all sexual health clinics in Scotland; and (4) laboratory data from two of the health and social care trusts in Northern Ireland, which began providing in-house LGV testing to some sexual health clinics in 2018. Laboratory data, which are collected quarterly from primary diagnostic laboratories through the Chlamydia Testing Activity Dataset surveillance system,16 provided both denominator (number of LGV tests done) and numerator (number of positive tests) data.
Since 2009, all English-commissioned sexual health services had a mandatory responsibility to return data to PHE through the clinical coding-based GUMCAD STI Surveillance System.17 A code for LGV diagnosis was introduced in 2011. Due to underreporting of clinical diagnoses as a result of the sequence of testing for LGV following a positive non-LGV chlamydia result, ~70% of LGV diagnoses captured in the laboratory data are reported through GUMCAD. This dataset includes information on patient characteristics, diagnoses of other STIs and services provided, but does not capture data on LGV tests conducted.
Data analysis
To monitor the trends in both LGV tests performed and diagnoses made, the UK laboratory data were de-duplicated and combined to provide an accurate measure of the number of individuals tested and diagnoses made by excluding instances where individuals were tested for LGV twice within a 6-week window. This was in line with the data cleaning performed on clinical data.18
Laboratory testing data from 2004 to 2018 were descriptively analysed to assess the long-term trends in LGV diagnoses. Annual test positivity (number of diagnoses/total tests, expressed as a percentage) was calculated for each country in the UK and for each PHE Centre in England, and was descriptively analysed alongside the number of annual tests performed and number of tests that were positive for LGV.
The number of LGV tests performed and number of positive tests were also calculated for each quarter and stratified by gender. These data were descriptively analysed alongside the relevant management guidelines that were introduced between 2004 and 2018. LGV test positivity per quarter was also calculated and descriptively analysed alongside relevant guideline changes and changes in prescribing and testing practices within large London-based clinics with a high throughput of MSM. Clinical diagnoses data from the GUMCAD STI Surveillance Systems were used to inform the changing trends in diagnoses by sexual orientation and HIV status; information that is not available via laboratory data.
All data cleaning, collating and statistical analyses were carried out used STATA 15.1 (College Station, TX, USA).
Investigating the performance of the diagnostic assay
The real-time–polymerase chain reaction (RT–PCR) assay used for LGV diagnoses at the PHE National Reference Laboratory was investigated to determine whether the decrease in the number of LGV-positive specimens was due to a reduction in assay sensitivity.19–21 ompA genotyping19 was performed on 40 clinical specimens, accounting for approximately one-third of samples sent to the reference laboratory during the investigation period. Twenty clinical specimens that were chlamydia positive/LGV negative were selected at random from a routine PCR run and 20 clinical specimens were from individuals who had clinical signs of LGV infection and were positive for chlamydia, but were PCR negative for LGV.
Ethical approval
Ethical approval was not required as collection and analysis of LGV laboratory testing data were conducted as part of routine national surveillance by PHE.22 Clinical data were obtained from the GUMCAD STI Surveillance System, the mandatory surveillance system for STIs in England. Public Health England collects pseudo-anonymised, electronic data on all STI tests and diagnoses from all commissioned sexual health services in England.17,23
Results
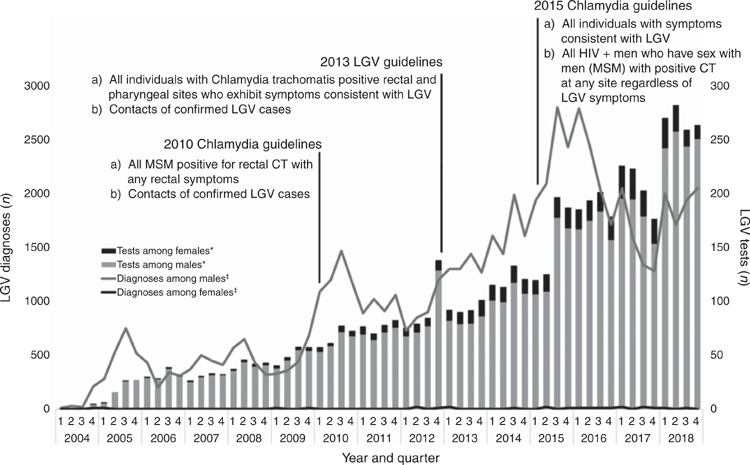
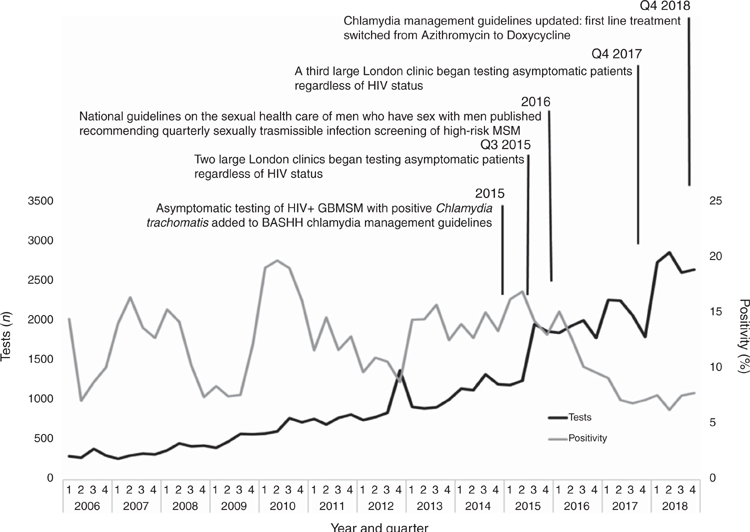
National trends in LGV testing and diagnoses are presented within the context of changes to national management guidelines (Fig. 1).12,13,15 A further detailed view of trends in LGV testing and positivity from 2006 to 2018 is presented within the context of local clinical practice and national management guidelines (Fig. 2).15,24,25 The geographic distribution of LGV diagnoses, tests and positivity from 2004 to 2018 across the UK is shown in Table 1.
|
Trends in diagnoses
Between 2004 and 2016, annual diagnoses of LGV in the UK rose from 28 to 904, with 99% of this 32-fold increase seen among males (Fig. 1). Diagnoses in males peaked at 280 in Q1 2016 before decreasing by 53% to 129 in Q4 2017. Despite a slight upturn in diagnoses since the start of 2018, overall diagnoses were 26% lower in Q4 2018 (206) than at the peak in 2016. The distribution of diagnoses by gender changed little over this period, with a total of 24 LGV diagnoses made among females between 2004 and 2018, 20 of which, were made after 2011.
Trends in testing
Across all UK countries, the number of LGV tests began to increase gradually from 2010. Peaks in testing were seen at the end of both 2012 and 2015, and testing was at its highest level throughout 2017 and 2018 (Fig. 2). Testing was consistently much higher among men (Fig. 1), but LGV testing among females increased rapidly from 159 in 2010 to 1042 in 2017 and then fell to 792 in 2018. A 21% decline in overall testing was seen in 2017 (2266 in Q1, 1800 in Q4).
The annual positivity of LGV tests in the UK fluctuated between 2006 and 2018, peaking at 18% in 2010 and 14% in 2014 (Fig. 2). Alongside an increase in testing, the proportion of total tests that were positive halved between 2015 (14%) and 2018 (7%).
Changes in guidelines and clinical practice
Peaks in LGV testing and diagnoses appear to coincide with the publication of revised national management guidelines concerning LGV testing and changes to clinical practice (Figs 1, 2). From 2010 to 2011, the annual number LGV tests increased by 14% (2690 to 3065), after modifications to the chlamydia guidelines in 2010.12 Following the publication of LGV guidelines in 2013, testing increased 28% (3742 to 4809) between 2013 and 2014; a 16% increase (6772 to 7885) in testing was seen between 2015 and 2016 after the 2015 chlamydia guidelines were published and testing practices were changed at two large London clinics; and a 29% increase (8396 to 10 850) was seen between 2017 and 2018 after a third large London clinic changed its testing practice.13,15 Additionally, the publication of the 2016 MSM guidelines, which recommend quarterly STI screening of high-risk MSM, are likely to have contributed to the national increase in LGV tests performed.25
Geographic variation
Between 2004 and 2018, the majority of LGV diagnoses were made in London (4477/6637, 67%), followed by the North West (602 diagnoses, 9.4%) and the South East (468 diagnoses, 7%) (Table 1).
Lower numbers of diagnoses were reported in Wales (44 diagnoses), Scotland (202) and Northern Ireland (31). Both Scotland and Northern Ireland had a similar trend to England (Table 1). Diagnoses increased in Scotland from 10 to 45 between 2010 and 2015 and then fell to 30 in 2018. In Northern Ireland, diagnoses increased from two to eight from 2010 to 2016 and fell to four annual diagnoses in 2017 (Table 1). Reductions in positivity from 2015 that reflect the national trend were seen in Wales and most PHE centres within England (excluding the East Midlands and East of England) (Table 1).
Patient characteristics
Analysis of clinical data submitted via the GUMCAD STI Surveillance System shows that between 2013 and 2018, the proportion of annual LGV diagnoses in England made among MSM increased from 90% to 96%. Over the same period, the proportion of MSM diagnosed with LGV who were living with HIV fell from 74% to 48%, with the biggest year-to-year decrease seen between 2016 and 2017 (67–59%).
Performance of the diagnostic assay
An investigation at PHE of the RT–PCR assay used for LGV diagnoses showed that all ompA genotyping results were concordant with the results of the RT–PCR assay used for routine testing.
Discussion
After a sustained increase in diagnoses, the LGV epidemic changed trajectory between 2015 and 2017. This fall, which was concurrent with a reduction in positivity, is unlikely to be explained in terms of a decline in testing or under-reporting of LGV diagnoses. In addition, it is unlikely the diagnostic assay missed the LGV target due to an escape mutant. Rather, the decreases in LGV diagnoses are likely related to changes in the LGV and chlamydia national guidelines, suggested through peaks in tests and diagnoses occurring within a year of the publication of new guidelines. The observed increase in testing may have led to the earlier clinical diagnosis, resulting in a shorter infectious period and therefore reduced transmission of LGV and the subsequent reduction in both diagnoses and positivity since 2016.
The lack of available information concerning the extent to which clinics adhered to national management guidelines, specifically, the absence of data on clinical presentation, LGV treatment regimens and testing coverage within specific risk groups and data on the performance of partner notification and management of contacts, has limited the understanding of the course of the epidemic. Despite this, both clinical and laboratory diagnostic and testing data allowed a comprehensive assessment of LGV trends together with sexual risk and demographic characteristics. Additionally, an investigation of the RT–PCR assay showed that the performance of the diagnostic test was as expected. It should be noted that the assay was only investigated at PHE, so some other escape mutants may have been missed in other centres. This is, however, unlikely as all centres used the same LGV targets and any circulating escape mutants should have been detected at PHE where the majority of specimens were tested.
Similar to the trend seen in the UK, between 2010 and 2016 increased LGV diagnoses were also reported in France, the Netherlands, Belgium and the Republic of Ireland,6,26,27 and both France and the Republic of Ireland saw a decrease in diagnoses from 2016 to 201727 (C Bebear, pers. comm.), whereas in the Netherlands, diagnoses increased by 11% between 2016 and 2017.26 Despite a reduction in LGV diagnoses, there is likely to be substantial underdiagnoses of LGV across much of Europe, and testing coverage and management need to be improved.28
In the UK, revisions to national management guidelines that detail testing guidelines for LGV were made in response to developments in our knowledge of the epidemic, including increased insight into the burden of asymptomatic infection, clinical presentation and groups at risk of infection. The scope of LGV testing has been extended from performing LGV testing only among patients with rectal symptoms to include testing of those who were asymptomatic, recommending LGV testing of samples from all MSM living with HIV who test positive for C. trachomatis at any site12 and to focus testing on those at highest risk of infection.
Changes in sexual health guidelines for MSM (2016), which recommend quarterly STI screening of high-risk MSM and the extension of LGV testing to include MSM with positive rectal chlamydia, regardless of the presence of LGV symptoms (2015), are likely to have contributed to the national increase in LGV tests performed.25 From 2015, some large London sexual health clinics that see a high throughput of MSM and higher risk MSM initiated in-house testing for LGV within their local laboratories, rather than referring samples to the PHE National Reference Laboratory for LGV testing. In some large London clinics, reflex testing, that is, testing all samples positive for C. trachomatis for LGV regardless of site of infection or risk group, is likely to have had a substantial effect on the number of LGV tests performed, especially where the patient was asymptomatic.
Despite higher annual diagnoses of LGV among HIV-positive MSM compared with HIV-negative/untested MSM up to 2015, the subsequent fall in LGV positivity was greater in HIV-positive MSM, with almost equal proportions of diagnoses among both groups seen in 2018. This fall may be explained by increased disassortative sexual mixing by HIV status and increased LGV testing within both MSM groups, which may have extended the sexual network considered at highest risk of LGV acquisition beyond that detailed within the testing guidelines. This could be associated with the increased, widespread availability of treatment for prevention of HIV and pre-exposure prophylaxis..29,30 Additionally, reflex LGV testing in some London-based clinics, which have developed in-house testing for LGV and see a high throughput of MSM, may have increased testing of MSM who do not fall within the testing criteria detailed in the 2015 guidance. This may have further improved LGV case ascertainment.
Trends seen for LGV in the UK have not mirrored those seen for gonorrhoea, syphilis and chlamydia, with the number of individuals diagnosed with these STIs continuing to increase.10 LGV is, however, less common and more localised within specific risk groups and may therefore be easier to control through targeted testing strategies.
A drop in LGV diagnoses may also have been influenced by the switch in first-line treatment for chlamydia from 1 g single-dose azithromycin to 7-days of 100 mg doxycycline. Single-dose azithromycin was suggested to be less effective than doxycycline for treating rectal C. trachomatis in MSM.31 Based on this evidence published in 2015, a change in treatment regimen is likely to have been implemented across several clinics in the UK before the update of the national guidelines in 2018.22 As the revised treatment regimen may be sufficient to treat very early asymptomatic and undiagnosed LGV infection, by further reducing the pool of asymptomatic LGV, this change may have acted as an additional driver in the reduction in LGV diagnoses. Additionally, the European Chlamydia management guidelines were also updated in 2015 to recommend the use of doxycycline as first-line treatment for chlamydia32 and the fall in diagnoses seen in some European countries from 2015 may have been related to this change.
This investigation suggests that the changes in national LGV and chlamydia management guidelines have had a positive effect on the control of LGV infection, especially among individuals considered at high risk of acquiring the infection. As PHE seeks to reduce the burden of STIs in England, it is encouraging to see the apparent public health benefit of combined interventions. While the declining UK trend is encouraging, there was some local variation, and continued vigilance is important to identify any local transmission clusters in areas where awareness of LGV may be lower. Furthermore, it is unclear whether the recent drop in LGV diagnoses seen in the UK will be sustained over the coming years or will follow a trajectory more in line with other bacterial STIs. Further evaluation of the intervention strategies and revisions to the management guidelines may be warranted in the near future.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We would like to thank all clinicians and data providers who participated in LGV surveillance. We also thank all past and present members of the PHE National Reference Unit, the PHE Clinical Service Unit, in particular Simon Carne, LGV reporters and the CTAD and GUMCAD teams (PHE, HIV and STI Department): Graham Hogan (The Doctors Laboratories pathologies) and Bruce Macrae (University College London Hospitals NHS foundation trust); Manfred Almida, Paula Nacmanson and Monica Rebec (Imperial College Healthcare NHS Trust); Stephen Duffell, Aarti Khanna, Dolores Mullen, Natasha Ratna, Ammi Shah, Alireza Talebi (GUMCAD and CTAD teams). We thank Laia Fina (Public Health Wales, Cardiff) for providing LGV data for Wales, Birgit van Benthem (National Institute for Public Health and the Environment, Netherlands) and Cecile Bebear (National Reference Centre for Bacterial Sexually Transmitted Infection, France) for providing insight into the recent trends in the Netherlands and France. We also thank Hannah Charles (PHE) for producing the data table and Dr John Saunders (PHE) for clinical input.
References
[1] De Vries HJC. Lymphogranuloma venereum in men who have sex with men. An ongoing epidemic since 10 years, but still not tackled. Sex Transm Infect 2013; 89 A24–24.| Lymphogranuloma venereum in men who have sex with men. An ongoing epidemic since 10 years, but still not tackled.Crossref | GoogleScholarGoogle Scholar |
[2] Kotevski DP, Lam M, Selvey CE, Templeton DJ, Donovan LG, Sheppeard V. Epidemiology of lymphogranuloma venereum in New South Wales, 2006–2015. Commun Dis Intell 2018; 43
[3] Nieuwenhuis RF, Ossewaarde JM, Götz HM, Dees J, Bing Thio H, Thomeer MGJ, et al Resurgence of lymphogranuloma venereum in Western Europe: an outbreak of Chlamydia trachomatis serovar l2 proctitis in The Netherlands among men who have sex with men. Clin Infect Dis 2004; 39 996–1003.
| Resurgence of lymphogranuloma venereum in Western Europe: an outbreak of Chlamydia trachomatis serovar l2 proctitis in The Netherlands among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 15472852PubMed |
[4] Hughes G, Alexander S, Simms I, Conti S, Ward H, Powers C, et al Lymphogranuloma venereum diagnoses among men who have sex with men in the U.K.: interpreting a cross-sectional study using an epidemic phase-specific framework. Sex Transm Infect 2013; 89 542–7.
| Lymphogranuloma venereum diagnoses among men who have sex with men in the U.K.: interpreting a cross-sectional study using an epidemic phase-specific framework.Crossref | GoogleScholarGoogle Scholar | 23851189PubMed |
[5] Childs T, Simms I, Alexander S, Eastick K, Hughes G, Field N. Rapid increase in lymphogranuloma venereum in men who have sex with men, United Kingdom, 2003 to September 2015. Euro Surveill 2015; 20 30076
| Rapid increase in lymphogranuloma venereum in men who have sex with men, United Kingdom, 2003 to September 2015.Crossref | GoogleScholarGoogle Scholar | 26675210PubMed |
[6] Boutin CA, Venne S, Fiset M, Fortin C, Murphy D, Severini A, et al Lymphogranuloma venereum in Quebec: re-emergence among men who have sex with men. Can Commun Dis Rep 2018; 44 55–61.
| Lymphogranuloma venereum in Quebec: re-emergence among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 29770100PubMed |
[7] Foschi C, Gaspari V, Sgubbi P, Salvo M, D'Antuono A, Marangoni A. Sexually transmitted rectal infections in a cohort of ‘men having sex with men. J Med Microbiol 2018; 67 1050–7.
| Sexually transmitted rectal infections in a cohort of ‘men having sex with men.Crossref | GoogleScholarGoogle Scholar | 29927376PubMed |
[8] Aghaizu A, Wayal S, Nardone A, Parsons V, Copas A, Mercey D, et al Sexual behaviours, HIV testing, and the proportion of men at risk of transmitting and acquiring HIV in London, UK, 2000–13: a serial cross-sectional study. Lancet HIV 2016; 3 e431–40.
| Sexual behaviours, HIV testing, and the proportion of men at risk of transmitting and acquiring HIV in London, UK, 2000–13: a serial cross-sectional study.Crossref | GoogleScholarGoogle Scholar | 27562744PubMed |
[9] Daskalopoulou M, Rodger AJ, Phillips AN, Sherr L, Elford J, McDonnell J, et al Condomless sex in HIV-diagnosed men who have sex with men in the UK: prevalence, correlates, and implications for HIV transmission. Sex Transm Infect 2017; 93 590–8.
| Condomless sex in HIV-diagnosed men who have sex with men in the UK: prevalence, correlates, and implications for HIV transmission.Crossref | GoogleScholarGoogle Scholar | 28679630PubMed |
[10] Public Health England. Sexually transmitted infections and screening for chlamydia in England, 2018: Health Protection Report. 2019.
[11] Clinical Effectiveness Group of the British Association for Sexual Health and HIV (CEG/BASHH). 2006 National Guideline for the Management of Lymphogranuloma venereum (LGV). 2006.
[12] British Association for Sexual Health and HIV. Chlamydia trachomatis UK testing guidelines. 2010.
[13] White J, O’Farrell N, Daniels D. 2013 UK National Guideline for the management of lymphogranuloma venereum: Clinical Effectiveness Group of the British Association for Sexual Health and HIV (CEG/BASHH) Guideline development group. Int J STD AIDS 2013; 24 593–601.
| 2013 UK National Guideline for the management of lymphogranuloma venereum: Clinical Effectiveness Group of the British Association for Sexual Health and HIV (CEG/BASHH) Guideline development group.Crossref | GoogleScholarGoogle Scholar | 23970591PubMed |
[14] Saxon C, Hughes G, Ison C. Asymptomatic lymphogranuloma venereum in men who have sex with men, United Kingdom. Emerg Infect Dis 2016; 22 112–6.
| Asymptomatic lymphogranuloma venereum in men who have sex with men, United Kingdom.Crossref | GoogleScholarGoogle Scholar | 26691688PubMed |
[15] Nwokolo NC, Dragovic B, Patel S, Tong CYW, Barker G, Radcliffe K. 2015 UK national guideline for the management of infection with Chlamydia trachomatis. Int J STD AIDS 2016; 27 251–67.
| 2015 UK national guideline for the management of infection with Chlamydia trachomatis.Crossref | GoogleScholarGoogle Scholar | 26538553PubMed |
[16] Public Health England. CTAD Chlamydia Surveillance System. 2012. Available online at: https://www.gov.uk/guidance/ctad-chlamydia-surveillance-system [verified 01 Dec 2019].
[17] Public Health England. GUMCAD STI Surveillance System. 2019. Available online at: https://www.gov.uk/guidance/gumcad-sti-surveillance-system [verified 03 Dec 2019].
[18] Public Health England. GUMCAD STI Surveillance System Data Specification and Technical Guidance. 2019.
[19] Morré SA, Spaargaren J, Fennema JS, de Vries HJ, Coutinho RA, Peña AS. Real-time polymerase chain reaction to diagnose lymphogranuloma venereum. Emerg Infect Dis 2005; 11 1311–2.
| Real-time polymerase chain reaction to diagnose lymphogranuloma venereum.Crossref | GoogleScholarGoogle Scholar | 16110579PubMed |
[20] Alexander S, Martin I, Ison C. Confirming the Chlamydia trachomatis status of referred rectal specimens. Sex Transm Infect 2007; 83 327–9.
| Confirming the Chlamydia trachomatis status of referred rectal specimens.Crossref | GoogleScholarGoogle Scholar | 17475685PubMed |
[21] Chen CY, Chi K, Alexander S, Martin IMC, Liu H, Ison CA, Ballard RC. The molecular diagnosis of lymphogranuloma venereum: evaluation of a real-time multiplex polymerase chain reaction test using rectal and urethral specimens. Sex Transm Dis 2007; 34 451–5.
| The molecular diagnosis of lymphogranuloma venereum: evaluation of a real-time multiplex polymerase chain reaction test using rectal and urethral specimens.Crossref | GoogleScholarGoogle Scholar | 17075436PubMed |
[22] Public Health England. Lymphogranuloma venereum (LGV): guidance, data and analysis. 2009. Available online at: https://www.gov.uk/government/collections/lymphogranuloma-venereum-lgv-guidance-data-and-analysis [verified 03 Dec 2019].
[23] Public Health England. PHE HIV and STI data sharing policy; 2015.
[24] Dragovic B, Nwokolo NC. Update on the treatment of Chlamydia trachomatis (CT) infection. 2018.
[25] Clutterbuck D, Asboe D, Barber T, Emerson C, Field N, Gibson S, et al United Kingdom national guideline on the sexual health care of men who have sex with men. Int J STD AIDS 2016; 2018 956462417746897
[26] Slurink IAL, van Aar F, Op de Cou ELM, Heijne1 JCM, van Wees DA, Hoenderboom BM, et al. Sexually transmitted infections in the Netherlands in 2017. Bilthoven, The Netherlands: National Institute for Public Health and the Environment; 2018.
[27] European Centre for Disease Prevention and Control. ECDC surveillance atlas of infectious diseases. 2018. Available online at: https://atlas.ecdc.europa.eu/public/index.aspx [verified 03 Dec 2019].
[28] Cole MJ, Field N, Pitt R, Amato-Gauci AJ, Begovac J, French PD, et al Substantial underdiagnosis of lymphogranuloma venereum in men who have sex with men in Europe: preliminary findings from a multicentre surveillance pilot. Sex Transm Infect 2020; 96 137–42.
| Substantial underdiagnosis of lymphogranuloma venereum in men who have sex with men in Europe: preliminary findings from a multicentre surveillance pilot.Crossref | GoogleScholarGoogle Scholar | 31235527PubMed |
[29] PrEP Impact Trial. PrEP Impact Trial. City: Publisher; 2018. Available online at: https://www.prepimpacttrial.org.uk/ [verified 03 Dec 2019].
[30] Ong JJ, Baggaley RC, Wi TE, Tucker JD, Fu H, Smith MK, et al Global epidemiologic characteristics of sexually transmitted infections among individuals using preexposure prophylaxis for the prevention of HIV infection: a systematic review and meta-analysis. JAMA Netw Open 2019; 2 e1917134
| Global epidemiologic characteristics of sexually transmitted infections among individuals using preexposure prophylaxis for the prevention of HIV infection: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 31825501PubMed |
[31] Kong FY, Tabrizi SN, Fairley CK, Vodstrcil LA, Huston WM, Chen M, et al The efficacy of azithromycin and doxycycline for the treatment of rectal chlamydia infection: a systematic review and meta-analysis. J Antimicrob Chemother 2015; 70 1290–7.
| The efficacy of azithromycin and doxycycline for the treatment of rectal chlamydia infection: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 25637520PubMed |
[32] Lanjouw E, Ouburg S, de Vries HJ, Stary A, Radcliffe K, Unemo M. 2015 European guideline on the management of Chlamydia trachomatis infections. Int J STD AIDS 2016; 27 333–48.
| 2015 European guideline on the management of Chlamydia trachomatis infections.Crossref | GoogleScholarGoogle Scholar | 26608577PubMed |