Mycoplasma genitalium: enhanced management using expanded resistance-guided treatment strategies
Emma L. Sweeney A * , David M. Whiley A B , Gerald L. Murray C D E and Catriona S. Bradshaw F G
A * , David M. Whiley A B , Gerald L. Murray C D E and Catriona S. Bradshaw F G
A Faculty of Medicine, The University of Queensland Centre for Clinical Research, The University of Queensland, Brisbane, Qld, Australia.
B Pathology Queensland Central Laboratory, Brisbane, Qld, Australia.
C Murdoch Children’s Research Institute, Parkville, Vic., Australia.
D Women’s Centre for Infectious Diseases, The Royal Women’s Hospital, Parkville, Vic., Australia.
E Department of Obstetrics and Gynaecology, The University of Melbourne, Parkville, Vic., Australia.
F Melbourne Sexual Health Centre, Alfred Hospital, Carlton, Vic., Australia.
G Central Clinical School, Monash University, Melbourne, Vic., Australia.
Sexual Health - https://doi.org/10.1071/SH22012
Submitted: 19 January 2022 Accepted: 18 March 2022 Published online: 27 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Mycoplasma genitalium is an emerging sexually transmitted bacterium that is gaining attention because of the impact escalating antimicrobial resistance (AMR) is having on patient management. Of additional concern is that increased availability of testing appears to be resulting in screening practices that are not supported by clinical guidelines. This results in increasing numbers of asymptomatic M. genitalium infections being identified, which when combined with AMR issues, creates significant challenges for patients and clinicians. Rapidly rising levels of AMR, coupled with limited alternative treatment options, means patients can enter cycles of complex antimicrobial regimens that may cause more harm than the infection itself. In this review, we discuss the emergence of AMR and the implication for treatment practices, highlight the recommendations for testing but not screening for M. genitalium, and discuss expansion of individualised treatment strategies, to curb the emergence of resistance and improve outcomes for patients. We also provide suggestions for future research on the transmission and spread of resistance, to enhance global surveillance of this antimicrobial resistant pathogen and inform the revision of local and international treatment strategies.
Keywords: antimicrobials, antimicrobial resistance, enhanced patient management, individualised treatment, mycoplasma genitalium, resistance-guided treatment, STIs, treatment failure.
Introduction
Approximately 4 million Australians (16% of the population) will experience a sexually transmitted infection (STI) during their life.1 Despite ongoing targeted public health strategies designed to reduce the burden of STIs, their prevalence continues to increase. In fact, recent reports have indicated that in Australian states with significant coronavirus disease 2019 (COVID-19)-related lockdowns and enhanced social distancing measures, there were no significant changes in STI diagnoses and genital infections.2 This indicates that STI transmission was still occurring during periods of less contact and diminished ability to travel locally and internationally. Although the bulk of STI infections in Australia are caused by the well-recognised notifiable STIs, chlamydia and gonorrhoea, other less recognised STIs are now causing major concerns due to the treatment challenges they pose to clinicians and patients. One such STI is Mycoplasma genitalium, which commonly causes urethritis, cervicitis and pelvic inflammatory disease3 and is now emerging as an increasingly antimicrobial-resistant STI.
Increasing antimicrobial resistance in M. genitalium
In 2019, M. genitalium was one of only three organisms added to the Centers for Disease Control and Prevention (CDC) ‘antimicrobial resistant threats’ watch list, due to the rapid emergence and spread of antimicrobial resistance (AMR). Notably, M. genitalium has developed high levels of resistance to both macrolides and fluoroquinolones in less than a decade. A recent systematic review and meta-analysis4 of M. genitalium identified that macrolide resistance had increased from 10.4% before 2010 to 51.4% in 2016–17, with the greatest increase in resistance observed in the WHO Western Pacific region. Although there were less data available to assess trends in fluoroquinolone resistance, it was estimated to be around 7.7% globally, and to be highest in the WHO Western Pacific region, compared to European regions. Dual class resistance to both macrolides and fluoroquinolones was estimated to be 2.8% globally and did not vary over time or by geographical region.4 There has been a considerable increase in publications that report increasing resistance to both classes of antimicrobials since 2017, and the meta-analysis is currently being updated, in order to generate contemporary AMR estimates.
In general, the highest number of recent reports of AMR come from the Asia-Pacific region and from men who have sex with men (MSM) populations. A study of samples collected from Australian MSM (n = 1001) between 2016 and 2017 found 9.4% of participants harboured asymptomatic M. genitalium infections, with high levels of both macrolide (84%) and fluoroquinolone resistance mutations (13%).5 In China, a study of 154 M. genitalium-positive males and females attending an STI clinic between 2016 and 2018 found macrolide resistance mutations in 66.4% of M. genitalium samples and parC fluoroquinolone resistance mutations in 77.7%,6 whereas another study indicated levels of fluoroquinolone resistance may be even higher at ∼89%.7 Similarly, high levels of AMR have also recently been reported in a study of 982 asymptomatic MSM from Japan who were tested in 2019–20;8 of the 60 patients with M. genitalium infections, 89.6% were macrolide resistant and 68.3% were fluoroquinolone resistant. These data collectively highlight the need for AMR surveillance and improvements in antimicrobial prescribing practices and stewardship.
Screening and treatment practices for STIs can impact AMR
Although it has been proposed that intensified screening and treatment programs can reduce the prevalence and burden of bacterial STIs, there is also evidence that this approach may, in fact, be doing harm and have little or no sustained impact on STI prevalence. Kenyon et al.9,10 have suggested that the framework to support intense chlamydia and gonorrhoea screening and treatment does not take into account some of the important ecological and social aspects that can impact upon AMR in STIs. For example, the emergence and spread of AMR is multifaceted, and includes interactions at the microbe, host and population level, as well as considerations like the density of sexual networks in which these STIs are identified.9 These recent studies that include data from European countries on Neisseria gonorrhoeae, syphilis and M. genitalium, highlight the unintended harms and collateral damage that can arise from well-intended STI screening and treatment practices. This work also demonstrates that many countries with low rates of STI screening had low levels of antimicrobial consumption and lower rates of AMR in these STIs. In contrast, countries with concentrated STI screening programs had higher rates of antimicrobial consumption, measurable increases in AMR (both minimum inhibitory concentrations [MICs] and resistance-associated mutations) and had minimal or no impact on the prevalence of these STIs.9,11–13 Earlier examples similarly showed no sustained benefit from intensive STI screening campaigns. A mass gonorrhoea treatment campaign in Greenland between 1965 and 1968 yielded a 25–30% decline in the notification rates of gonorrhoea over a 6-month period, after which notification rates quickly returned to pre-campaign levels, accompanied by a sharp rise in the MICs for penicillin in local gonococcal strains.9
Recent studies have shown a positive correlation between the consumption of macrolides in the years preceding the emergence of macrolide resistance in M. genitalium.14,15 Azithromycin, a macrolide with a long half-life, was first discovered in the 1980s and by the 1990s had become a popular choice for the syndromic treatment of STIs, with high efficacy against chlamydia and M. genitalium. The first cases of azithromycin-resistant M. genitalium were reported in 2006.16,17 Resistance is attributed to single nucleotide polymorphisms (SNPs) in the 23S ribosomal RNA gene (A2058T A2058C, A2058G, A2059C and A2059G, Escherichia coli numbering).17 These five SNPs each result in elevated MICs and high-level resistance to azithromycin, and are selected for in vivo in approximately 10–12% of macrolide-susceptible M. genitalium infections following treatment with 1 g of azithromycin.17–19 This high rate of selected resistance, and the widespread use of the 1 g dose in sexual health settings, including in combination with ceftriaxone for N. gonorrhoeae,20 has contributed to the rapid rise in macrolide resistance and a global decline in the efficacy of azithromycin for M. genitalium.21 Following the emergence of macrolide-resistant M. genitalium, the fluoroquinolone, moxifloxacin, was shown to be another highly effective treatment option;16 however, resistance to moxifloxacin was again rapidly reported, with a recent meta-analysis showing significant declines in its efficacy within 10 years.22
Why we don’t screen for M. genitalium
Patient demand, together with increasing availability of diagnostic assays, has resulted in a growing number of molecular assays that can detect M. genitalium, either individually or when multiplexed with other well-known STIs, such as Chlamydia trachomatis and N. gonorrhoeae.23–26 This has apparently led to inadvertent screening (as opposed to only testing individuals with suspected infection) occurring for M. genitalium in patient populations. Despite established screening programs for chlamydia in many countries, there is actually considerable uncertainty surrounding the benefits and impact of chlamydial screening on its prevalence and sequelae.27 For M. genitalium, which is a lower-load and frequently indolent infection, the benefits of screening are even less certain. There is far less knowledge regarding the natural history of M. genitalium, treatment options are increasingly limited due to the rapid rise in AMR, and some treatments are associated with uncommon but serious adverse effects.28–30 There is no evidence that M. genitalium is associated with an increased risk of serious sequelae in men, and concerns are mainly directed towards women. Two large studies on M. genitalium infections in women found a significant association with cervicitis and pelvic inflammatory disease (PID).31,32 Two recent meta-analyses29,33 on M. genitalium in female reproductive tract syndromes, reported associations with PID (pooled odds ratio [pOR] = 2.14), preterm birth (pOR = 1.89), spontaneous abortion (pOR = 1.82), post abortal PID (OR = 6.3), and an elevated, but non-significant risk of tubal factor infertility. However, an analysis of the UK prevention of pelvic inflammation data suggested that significantly fewer M. genitalium infections progress to PID (4.9%), when compared to chlamydial infections (14.4%).34
Putting this information in context, together with increasing awareness of the impact of antimicrobials on the gut microbiota and induction of AMR in the target organism and commensals, current evidence does not favour screening for M. genitalium, and it is not recommended by local or international guidelines.35–37 Although testing for C. trachomatis and N. gonorrhoeae are usually indicated when a patient is being tested for M. genitalium, clinicians should be aware of the rates of coinfection in their clinic populations, which can be as high as 10–14%, so that these infections are appropriately managed when detected. Furthermore, clinicians are increasingly encountering patients requiring repeated courses of antimicrobials, which is associated with prolonged morbidity and increased health anxiety. Although some studies in young women have reported high prevalence estimates for M. genitalium, this combined with tighter estimates around the risk of PID may support the need for targeted screening and treatment strategies in specific sub-populations. However, more research is required, and we would caution against ‘treating a test result’ and, rather, encourage targeted testing and treatment of patients, based on appropriate symptoms and scenarios, as outlined in STI management guidelines.
There is a need to expand resistance testing to optimise treatment success and promote antimicrobial stewardship
The decreasing efficacy of empiric treatments in the face of increasing AMR has highlighted the value of diagnostic assays that incorporate resistance mutations. Resistance-guided treatment approaches for macrolides have been readily adopted for patient management, using molecular tests to detect the 23S rRNA mutations that are associated with high-level resistance to azithromycin.38 Where a mutation is detected, the use of macrolides is not recommended; however, where no resistance mutations are detected, the use of macrolides can be considered. This was shown to be particularly effective when azithromycin was given following syndromic doxycycline in an extended regimen (1 g followed by 500 mg daily for a further 3 days).38 This sequential and individualised treatment approach has been shown to dramatically improve the rate of cure from 50% with syndromic use of 1 g azithromycin to 96% (95% CI = 92–98%) and reduce selection of macrolide-resistance from 12% to <4%. It is now recommended within the Australian, UK and US STI treatment guidelines.35–37 Using this strategy, patients with macrolide-resistant infections are given syndromic doxycycline, followed by moxifloxacin. This approach achieved 92% cure rates in a population where ∼20% of macrolide-resistant infections also had clinically relevant parC mutations that have been associated with moxifloxacin failure.39 As fluoroquinolone resistance rises, the efficacy of this approach will further decline, and with limited alternatives to moxifloxacin, this highlights the need for the next logical step – development of fluoroquinolone-resistance tests.
Fluoroquinolones target the topoisomerase enzymes of bacteria, and resistance can arise from mutations affecting the encoding genes (parC and gyrA in M. genitalium). One issue with more complex individualised treatment approaches is that the relative contribution of specific mutations in parC and gyrA to fluoroquinolone resistance is far less clear than for macrolide resistance.40 This has hampered efforts to design molecular diagnostic tests that have high predictive values for failure of fluoroquinolones. The most common mutation linked with fluoroquinolone resistance and treatment failure is G248T, which changes the serine at position 83 of the ParC protein to an isoleucine (notation of S83I). There are additional mutations affecting S83 and aspartic acid 87 (D87), which are less common and have weaker association with treatment failure.41,42 Our recent data demonstrated that 60–70% of cases carrying the S83I ParC mutation failed treatment with sequential doxycycline + moxifloxacin,39 which in our experience is higher than the cure rate for doxycycline monotherapy. This predictive value is substantially lower than that associated with macrolide-resistance mutations for azithromycin treatment failure.
A recent review of our data indicated there is great value in detecting the absence of mutations in the parC sequence at positions S83 and D87. The presence of a wildtype parC gene in M. genitalium infection is associated with ≥97% cure following extended spectrum fluoroquinolone therapy.5,39,43 Based on this strategy, molecular assays that detect both S83I and ParC wildtype sequences could be used in tandem with macrolide resistance assays, enabling new levels of individualised therapy that would achieve high first-line cure, promote antimicrobial stewardship and reduce the need for tests of cure.
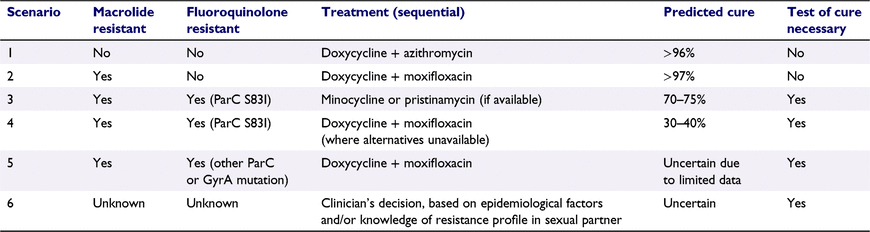
A strategy that incorporates both macrolide and fluoroquinolone resistance/susceptibility profiles could be achieved as follows (summarised in Table 1):
|
-
A patient with an M. genitalium infection that is ‘dual class’ wildtype (no macrolide or fluoroquinolone resistance) could be treated with doxycycline followed by azithromycin first-line, achieving very high (>96%) rates of cure with no requirement for a test of cure.
-
A patient whose infection is macrolide resistant but contains ParC wildtype sequences, could be treated first-line with doxycycline followed by moxifloxacin, and achieve similarly high rates of cure (≥97%) with no need for follow up.39,43
-
Where infections harbour macrolide resistance and the ParC–S83I mutation, ideally clinicians would utilise a non-quinolone antimicrobial, such as minocycline or pristinamycin, which would achieve 70–75% cure.44,45 However, if the only available option was moxifloxacin, rates of cure in the order of 30–40%39 would be expected and a test-of-cure warranted.
-
In cases where M. genitalium infections harbour rare mutations in ParC (e.g. S83R, S83C, D87N, D87Y or D87H), more data are clearly needed to determine their specific contributions to moxifloxacin failure. In these instances, moxifloxacin or a non-quinolone treatment option could be used, and a test-of-cure would be required.
-
Additionally, where the M. genitalium load is not sufficient to determine the susceptibility profile of the infecting strain, clinicians should use the epidemiological profile of the patient to inform their decision-making, or when available, the resistance profile of the sexual partners. For example, an MSM patient with no resistance result would likely be administered moxifloxacin as there is a very high (>85%) probability that the infecting strain is macrolide resistant. In heterosexuals, approximately half of infections are macrolide resistant, and so the clinician’s decision would be based on consultation with the patient and assessment of their specific risk factors.
These enhanced individualised treatment approaches have benefits for antimicrobial stewardship by further individualising care, increasing first line cure, reducing the use/misuse of antibiotics and likely further reducing healthcare costs, with fewer clinic visits and less need for follow up of a significant proportion of patients. With these advantages, we anticipate this strategy would be adopted by sexual health services and recommended in STI management guidelines in the future. However, the majority of assays (commercial or research) are designed for use within pathology laboratories, and as such, have limited utility for the timely treatment of infections at the point-of-care (POC). In order to further reduce time to diagnosis and enhance patient management at presentation, POC tests that incorporate resistance markers have been commercialised and include the ResistancePlus® MG FleXible test on the Cepheid GeneXpert platform. This particular test allows the simultaneous diagnosis of M. genitalium infection and detection of macrolide resistance markers. Evaluation studies have demonstrated that this near patient test offers comparable test performance, when compared to laboratory-based assays, which can expedite the initiation of appropriate sequential treatments with the potential to enhance antimicrobial stewardship.46,47
There are still substantial limitations in our understanding of M. genitalium antimicrobial resistance
Although in recent years AMR in M. genitalium has gained significant attention and we now have improved global estimates,4 our understanding of the dissemination and spread of AMR remains limited. Although clinical studies have demonstrated that 1 g azithromycin treatment strategies were selecting for macrolide resistance in 10–12% of cases,18,19,48 changing to the sequential approach of doxycycline followed by an extended 2.5 g azithromycin regimen was associated with lower levels of selected resistance in two studies.38,49 Importantly, there are no data to show that selection of resistance occurs in patients when treated with moxifloxacin,39 however, fluoroquinolone resistance has clearly risen in countries where fluoroquinolones are more commonly used (particularly in the Asia-Pacific).4,50–53 It is possible that a move towards sequential treatment with doxycycline followed by moxifloxacin may have an impact on resistance; however further studies are required to determine this. Of note, induced resistance during treatment has been reported for sitafloxacin, an alternative fourth generation fluoroquinolone used to treat M. genitalium in some countries.42,50
Studies in Australia and Europe have shown evidence of the de novo acquisition of AMR in M. genitalium, presumably followed by multi-clonal spread;54,55 however, there is still insufficient data to confirm that international transmission of specific M. genitalium clones is occurring, as has been reported for other notifiable STIs like gonorrhea.56–58 Further research is needed to better understand whether fluoroquinolone resistance within Australia is being selected for or imported. It will also be crucial to understand the patterns and dissemination of fluoroquinolone resistance, both locally and globally, in order to reduce the likelihood of further resistance emerging and/or spreading. Finally, we need to focus our attention on the transmission dynamics and relative fitness of global M. genitalium clones that may be of interest, with a view to enhancing surveillance of AMR and potentially inform key areas where public health interventions may be warranted.
Conclusion and final remarks
Mycoplasma genitalium infections are becoming increasingly challenging to treat, due to the continued escalation of AMR and limited available treatment options. Inappropriate and/or inadvertent screening for M. genitalium in asymptomatic individuals, which is not supported by current evidence, leads to increased antimicrobial consumption and further emergence of AMR. Adherence to testing recommendations in STI management guidelines is essential, and the adoption of broader individualised testing and treatment strategies would greatly improve our precision, cure and antimicrobial stewardship. This approach is particularly valuable given the limited treatments currently available and in the pipeline for M. genitalium. Further research into the transmission and spread of AMR in M. genitalium clones/strains is also needed, in order to enhance surveillance of AMR, and to further inform the development and revision of local and international treatment strategies for this challenging pathogen.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors received research funding from SpeeDx Pty Ltd.
Declaration of funding
This work was supported by an Australian Research Council Research hub for antimicrobial resistance, grant number IH190100021.
References
[1] The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia: Annual Surveillance Report 2018. 2018. Available at https://kirby.unsw.edu.au/report/asr2018[2] Chow EPF, Hocking JS, Ong JJ, Phillips TR, Fairley CK. Sexually transmitted infection diagnoses and access to a sexual health service before and after the national lockdown for COVID-19 in Melbourne, Australia. Open Forum Infect Dis 2021; 8 ofaa536
| Sexually transmitted infection diagnoses and access to a sexual health service before and after the national lockdown for COVID-19 in Melbourne, Australia.Crossref | GoogleScholarGoogle Scholar | 33506064PubMed |
[3] Taylor-Robinson D, Jensen JS.. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24 498–514.
| Mycoplasma genitalium: from Chrysalis to multicolored butterfly.Crossref | GoogleScholarGoogle Scholar | 21734246PubMed |
[4] Machalek DA, Tao Y, Shilling H, et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis 2020; 20 1302–14.
| Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 32622378PubMed |
[5] Chua TP, Bodiyabadu K, Machalek DA, et al. Prevalence of Mycoplasma genitalium fluoroquinolone-resistance markers, and dual-class-resistance markers, in asymptomatic men who have sex with men. J Med Microbiol 2021; 70 001429
| Prevalence of Mycoplasma genitalium fluoroquinolone-resistance markers, and dual-class-resistance markers, in asymptomatic men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[6] Ke W, Li D, Tso LS, et al. Macrolide and fluoroquinolone associated mutations in Mycoplasma genitalium in a retrospective study of male and female patients seeking care at a STI Clinic in Guangzhou, China, 2016–2018. BMC Infect Dis 2020; 20 950
| Macrolide and fluoroquinolone associated mutations in Mycoplasma genitalium in a retrospective study of male and female patients seeking care at a STI Clinic in Guangzhou, China, 2016–2018.Crossref | GoogleScholarGoogle Scholar | 33308173PubMed |
[7] Li Y, Su X, Le W, et al. Mycoplasma genitalium in symptomatic male urethritis: macrolide use is associated with increased resistance. Clin Infect Dis 2020; 70 805–10.
| Mycoplasma genitalium in symptomatic male urethritis: macrolide use is associated with increased resistance.Crossref | GoogleScholarGoogle Scholar | 30972419PubMed |
[8] Ando N, Mizushima D, Takano M, et al. High prevalence of circulating dual-class resistant Mycoplasma genitalium in asymptomatic MSM in Tokyo, Japan. JAC Antimicrob Resist 2021; 3 dlab091
| High prevalence of circulating dual-class resistant Mycoplasma genitalium in asymptomatic MSM in Tokyo, Japan.Crossref | GoogleScholarGoogle Scholar | 34223146PubMed |
[9] Kenyon C, Laumen J, Van Dijck C.. Could intensive screening for gonorrhea/chlamydia in preexposure prophylaxis cohorts select for resistance? Historical lessons from a mass treatment campaign in Greenland. Sex Trans Dis 2020; 47 24–7.
| Could intensive screening for gonorrhea/chlamydia in preexposure prophylaxis cohorts select for resistance? Historical lessons from a mass treatment campaign in Greenland.Crossref | GoogleScholarGoogle Scholar |
[10] Kenyon C.. Does intense sexually transmitted infection screening cause or prevent antimicrobial resistance in sexually transmitted infections? It depends on one’s underlying epistemology. A viewpoint. Sex Trans Dis 2020; 47 506–10.
| Does intense sexually transmitted infection screening cause or prevent antimicrobial resistance in sexually transmitted infections? It depends on one’s underlying epistemology. A viewpoint.Crossref | GoogleScholarGoogle Scholar |
[11] Kenyon C.. To what extent should we rely on antibiotics to reduce high gonococcal prevalence? Historical insights from mass-meningococcal campaigns. Pathogens 2020; 9 134
| To what extent should we rely on antibiotics to reduce high gonococcal prevalence? Historical insights from mass-meningococcal campaigns.Crossref | GoogleScholarGoogle Scholar |
[12] Kenyon C.. We need to consider collateral damage to resistomes when we decide how frequently to screen for chlamydia/gonorrhoea in preexposure prophylaxis cohorts. AIDS 2019; 33 155–7.
| We need to consider collateral damage to resistomes when we decide how frequently to screen for chlamydia/gonorrhoea in preexposure prophylaxis cohorts.Crossref | GoogleScholarGoogle Scholar | 30234609PubMed |
[13] Kenyon CR, Schwartz IS.. Effects of sexual network connectivity and antimicrobial drug use on antimicrobial resistance in Neisseria gonorrhoeae. Emerg Infect Dis 2018; 24 1195–203.
| Effects of sexual network connectivity and antimicrobial drug use on antimicrobial resistance in Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 29912682PubMed |
[14] Kenyon C, Manoharan-Basil SS.. Macrolide consumption and resistance in Mycoplasma genitalium. Lancet Infect Dis 2020; 20 1235–6.
| Macrolide consumption and resistance in Mycoplasma genitalium.Crossref | GoogleScholarGoogle Scholar | 33098774PubMed |
[15] Kenyon C, Manoharan-Basil SS, Van Dijck C.. Is there a resistance threshold for macrolide consumption? Positive evidence from an ecological analysis of resistance data from Streptococcus pneumoniae, Treponema pallidum, and Mycoplasma genitalium. Microb Drug Resist (Larchmont, NY) 2021; 27 1079–86.
| Is there a resistance threshold for macrolide consumption? Positive evidence from an ecological analysis of resistance data from Streptococcus pneumoniae, Treponema pallidum, and Mycoplasma genitalium.Crossref | GoogleScholarGoogle Scholar |
[16] Bradshaw CS, Jensen JS, Tabrizi SN, et al. Azithromycin failure in Mycoplasma genitalium urethritis. Emerg Infect Dis 2006; 12 1149–52.
| Azithromycin failure in Mycoplasma genitalium urethritis.Crossref | GoogleScholarGoogle Scholar | 16836839PubMed |
[17] Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R.. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis 2008; 47 1546–53.
| Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance.Crossref | GoogleScholarGoogle Scholar | 18990060PubMed |
[18] Read TRH, Fairley CK, Tabrizi SN, et al. Azithromycin 1.5 g over 5 days compared to 1 g single dose in urethral Mycoplasma genitalium: impact on treatment outcome and resistance. Clin Infect Dis 2017; 64 250–6.
| Azithromycin 1.5 g over 5 days compared to 1 g single dose in urethral Mycoplasma genitalium: impact on treatment outcome and resistance.Crossref | GoogleScholarGoogle Scholar |
[19] Horner P, Ingle SM, Garrett F, et al. Which azithromycin regimen should be used for treating Mycoplasma genitalium? A meta-analysis. Sex Trans Infect 2018; 94 14–20.
| Which azithromycin regimen should be used for treating Mycoplasma genitalium? A meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[20] Workowski KA, Bolan GA. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64 1–137.
| 26042815PubMed |
[21] Lau A, Bradshaw CS, Lewis D, et al. The efficacy of azithromycin for the treatment of genital Mycoplasma genitalium: a systematic review and meta-analysis. Clin Infect Dis 2015; 61 1389–99.
| The efficacy of azithromycin for the treatment of genital Mycoplasma genitalium: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 26240201PubMed |
[22] Li Y, Le W-J, Li S, Cao Y-P, Su X-H.. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS 2017; 28 1106–14.
| Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection.Crossref | GoogleScholarGoogle Scholar | 28118803PubMed |
[23] Tabrizi SN, Su J, Bradshaw CS, et al. Prospective evaluation of ResistancePlus MG, a new multiplex quantitative PCR assay for detection of Mycoplasma genitalium and macrolide resistance. J Clin Microbiol 2017; 55 1915–9.
| Prospective evaluation of ResistancePlus MG, a new multiplex quantitative PCR assay for detection of Mycoplasma genitalium and macrolide resistance.Crossref | GoogleScholarGoogle Scholar | 28381611PubMed |
[24] Tabrizi SN, Tan LY, Walker S, et al. Multiplex assay for simultaneous detection of Mycoplasma genitalium and macrolide resistance using PlexZyme and PlexPrime technology. PLoS ONE 2016; 11 e0156740
| Multiplex assay for simultaneous detection of Mycoplasma genitalium and macrolide resistance using PlexZyme and PlexPrime technology.Crossref | GoogleScholarGoogle Scholar | 27271704PubMed |
[25] Rumyantseva T, Golparian D, Nilsson CS, et al. Evaluation of the new AmpliSens multiplex real-time PCR assay for simultaneous detection of Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis. APMIS 2015; 123 879–86.
| Evaluation of the new AmpliSens multiplex real-time PCR assay for simultaneous detection of Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis.Crossref | GoogleScholarGoogle Scholar | 26299582PubMed |
[26] Goldstein E, Martinez-García L, Obermeier M, et al. Simultaneous identification of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, and Trichomonas vaginalis – multicenter evaluation of the Alinity m STI assay. J Lab Med 2021; 45 213–23.
| Simultaneous identification of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, and Trichomonas vaginalis – multicenter evaluation of the Alinity m STI assay.Crossref | GoogleScholarGoogle Scholar |
[27] Pillay J, Wingert A, MacGregor T, Gates M, Vandermeer B, Hartling L.. Screening for chlamydia and/or gonorrhea in primary health care: systematic reviews on effectiveness and patient preferences. Syst Rev 2021; 10 118
| Screening for chlamydia and/or gonorrhea in primary health care: systematic reviews on effectiveness and patient preferences.Crossref | GoogleScholarGoogle Scholar | 33879251PubMed |
[28] Peel J, Aung E, Bond S, Bradshaw C.. Recent advances in understanding and combatting Mycoplasma genitalium. Fac Rev 2020; 9 3
| Recent advances in understanding and combatting Mycoplasma genitalium.Crossref | GoogleScholarGoogle Scholar | 33659935PubMed |
[29] Cina M, Baumann L, Egli-Gany D, et al. Mycoplasma genitalium incidence, persistence, concordance between partners and progression: systematic review and meta-analysis. Sex Trans Infect 2019; 95 328–35.
| Mycoplasma genitalium incidence, persistence, concordance between partners and progression: systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[30] Baggio D, Ananda-Rajah MR.. Fluoroquinolone antibiotics and adverse events. Aust Prescr 2021; 44 161–4.
| Fluoroquinolone antibiotics and adverse events.Crossref | GoogleScholarGoogle Scholar | 34728881PubMed |
[31] Bjartling C, Osser S, Persson K.. Mycoplasma genitalium in cervicitis and pelvic inflammatory disease among women at a gynecologic outpatient service. Am J Obstet Gynecol 2012; 206 476.e1–8.
| Mycoplasma genitalium in cervicitis and pelvic inflammatory disease among women at a gynecologic outpatient service.Crossref | GoogleScholarGoogle Scholar |
[32] Latimer RL, Vodstrcil LA, Plummer EL, et al. The clinical indications for testing women for Mycoplasma genitalium. Sex Trans Infect 2021;
| The clinical indications for testing women for Mycoplasma genitalium.Crossref | GoogleScholarGoogle Scholar |
[33] Lis R, Rowhani-Rahbar A, Manhart LE.. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015; 61 418–26.
| Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 25900174PubMed |
[34] Lewis J, Horner PJ, White PJ.. Incidence of pelvic inflammatory disease associated with Mycoplasma genitalium infection: evidence synthesis of cohort study data. Clin Infect Dis 2020; 71 2719–22.
| Incidence of pelvic inflammatory disease associated with Mycoplasma genitalium infection: evidence synthesis of cohort study data.Crossref | GoogleScholarGoogle Scholar | 32701123PubMed |
[35] ASHM. Australian STI management guidelines: Mycoplasma genitalium [database on the Internet]. 2019. Available at https://sti.guidelines.org.au/sexually-transmissible-infections/mycoplasma-genitalium/
[36] Soni S, Horner P, Rayment M. British Association for Sexual Health and HIV national guideline for the management of infection with Mycoplasma genitalium (2018). Int J STD AIDS 2019; 30 938–50.
| British Association for Sexual Health and HIV national guideline for the management of infection with Mycoplasma genitalium (2018).Crossref | GoogleScholarGoogle Scholar | 31280688PubMed |
[37] Workowski KA, Bachmann LH, Chan PA. Sexually transmitted infections treatment guidelines, 2021. MMWR 2021; 70 1–187.
| Sexually transmitted infections treatment guidelines, 2021.Crossref | GoogleScholarGoogle Scholar | 34292926PubMed |
[38] Read TRH, Fairley CK, Murray GL, et al. Outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: a prospective evaluation. Clin Infect Dis 2019; 68 554–60.
| Outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: a prospective evaluation.Crossref | GoogleScholarGoogle Scholar | 29873691PubMed |
[39] Vodstrcil L, Doyle M, Plummer EL. A trial of combination therapy for Mycoplasma genitalium, and new insights into the utility of parC mutant detection to improve first-line cure. Clin Infect Dis 2022; –ciab1058.
| A trial of combination therapy for Mycoplasma genitalium, and new insights into the utility of parC mutant detection to improve first-line cure.Crossref | GoogleScholarGoogle Scholar | 34984438PubMed |
[40] Manhart LE, Jensen JS.. Quinolone resistance-associated mutations in Mycoplasma genitalium: not ready for prime time. Sex Trans Dis 2020; 47 199–201.
| Quinolone resistance-associated mutations in Mycoplasma genitalium: not ready for prime time.Crossref | GoogleScholarGoogle Scholar |
[41] Murray GL, Bradshaw CS, Bissessor M, et al. Increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium. Emerg Infect Dis 2017; 23 809–12.
| Increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium.Crossref | GoogleScholarGoogle Scholar | 28418319PubMed |
[42] Murray GL, Bodiyabadu K, Danielewski J, et al. Moxifloxacin and sitafloxacin treatment failure in Mycoplasma genitalium infection: association with parC mutation G248T (S83I) and concurrent gyrA mutations. J Infect Dis 2020; 221 1017–24.
| Moxifloxacin and sitafloxacin treatment failure in Mycoplasma genitalium infection: association with parC mutation G248T (S83I) and concurrent gyrA mutations.Crossref | GoogleScholarGoogle Scholar | 32031634PubMed |
[43] Sweeney EL, Bradshaw CS, Murray GL, Whiley DM.. Individualised treatment of Mycoplasma genitalium infection – incorporation of fluoroquinolone resistance testing into clinical care. Lancet Infect Dis 2022;
| Individualised treatment of Mycoplasma genitalium infection – incorporation of fluoroquinolone resistance testing into clinical care.Crossref | GoogleScholarGoogle Scholar | 35325618PubMed |
[44] Doyle M, Vodstrcil LA, Plummer EL, Aguirre I, Fairley CK, Bradshaw CS.. Nonquinolone options for the treatment of Mycoplasma genitalium in the era of increased resistance. Open Forum Infect Dis 2020; 7 ofaa291
| Nonquinolone options for the treatment of Mycoplasma genitalium in the era of increased resistance.Crossref | GoogleScholarGoogle Scholar | 32782911PubMed |
[45] Read TRH, Jensen JS, Fairley CK, et al. Use of pristinamycin for macrolide-resistant Mycoplasma genitalium infection. Emerg Infect Dis 2018; 24 328–35.
| Use of pristinamycin for macrolide-resistant Mycoplasma genitalium infection.Crossref | GoogleScholarGoogle Scholar | 29350154PubMed |
[46] Murray GL, Doyle M, Bodiyabadu K, et al. Evaluation of ResistancePlus MG FleXible, a ‘near-patient’ test for the detection of Mycoplasma genitalium and macrolide resistance mutations, using freshly collected clinical samples. J Med Microbiol 2021; 70 001271
| Evaluation of ResistancePlus MG FleXible, a ‘near-patient’ test for the detection of Mycoplasma genitalium and macrolide resistance mutations, using freshly collected clinical samples.Crossref | GoogleScholarGoogle Scholar |
[47] Sweeney EL, Mhango LP, Ebeyan S, et al. Evaluation of the ResistancePlus MG FleXible cartridge for near-point-of-care testing of Mycoplasma genitalium and associated macrolide resistance mutations. J Clin Microbiol 2020; 58 e01897-19
| Evaluation of the ResistancePlus MG FleXible cartridge for near-point-of-care testing of Mycoplasma genitalium and associated macrolide resistance mutations.Crossref | GoogleScholarGoogle Scholar | 31896664PubMed |
[48] Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis 2015; 60 1228–36.
| Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens.Crossref | GoogleScholarGoogle Scholar | 25537875PubMed |
[49] Durukan D, Read TRH, Murray G, et al. Resistance-guided antimicrobial therapy using doxycycline-moxifloxacin and doxycycline-2.5 g azithromycin for the treatment of Mycoplasma genitalium infection: efficacy and tolerability. Clin Infect Dis 2020; 71 1461–8.
| Resistance-guided antimicrobial therapy using doxycycline-moxifloxacin and doxycycline-2.5 g azithromycin for the treatment of Mycoplasma genitalium infection: efficacy and tolerability.Crossref | GoogleScholarGoogle Scholar | 31629365PubMed |
[50] Deguchi T, Ito S, Yasuda M. Emergence of Mycoplasma genitalium with clinically significant fluoroquinolone resistance conferred by amino acid changes both in GyrA and ParC in Japan. J Infect Chemother 2017; 23 648–50.
| Emergence of Mycoplasma genitalium with clinically significant fluoroquinolone resistance conferred by amino acid changes both in GyrA and ParC in Japan.Crossref | GoogleScholarGoogle Scholar | 28462860PubMed |
[51] Deguchi T, Ito S, Yasuda M, et al. Surveillance of the prevalence of macrolide and/or fluoroquinolone resistance-associated mutations in Mycoplasma genitalium in Japan. J Infect Chemother 2018; 24 861–7.
| Surveillance of the prevalence of macrolide and/or fluoroquinolone resistance-associated mutations in Mycoplasma genitalium in Japan.Crossref | GoogleScholarGoogle Scholar | 30190106PubMed |
[52] Kikuchi M, Ito S, Yasuda M, et al. Remarkable increase in fluoroquinolone-resistant Mycoplasma genitalium in Japan. J Antimicrob Chemother 2014; 69 2376–82.
| Remarkable increase in fluoroquinolone-resistant Mycoplasma genitalium in Japan.Crossref | GoogleScholarGoogle Scholar | 24894419PubMed |
[53] Murray G, Bodiyabadu K, Vodstrcil L. parC variants in Mycoplasma genitalium: trends over time and association with moxifloxacin failure. Antimicrob Agents Chemother 2022; 66 –e00278-22.
| parC variants in Mycoplasma genitalium: trends over time and association with moxifloxacin failure.Crossref | GoogleScholarGoogle Scholar | 35475636PubMed |
[54] Sweeney EL, Tickner J, Bletchly C, Nimmo GR, Whiley DM.. Genotyping of Mycoplasma genitalium suggests de novo acquisition of antimicrobial resistance in Queensland, Australia. J Clinical Microbiol 2020; 58 e00641–20.
| Genotyping of Mycoplasma genitalium suggests de novo acquisition of antimicrobial resistance in Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
[55] Dumke R, Rust M, Glaunsinger T.. MgpB types among Mycoplasma genitalium strains from men who have sex with men in Berlin, Germany, 2016–2018. Pathogens (Basel, Switzerland) 2019; 9 12
| MgpB types among Mycoplasma genitalium strains from men who have sex with men in Berlin, Germany, 2016–2018.Crossref | GoogleScholarGoogle Scholar |
[56] Chen SC, Han Y, Yuan LF, Zhu XY, Yin YP.. Identification of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, China. Emerg Infect Dis 2019; 25 1427–9.
| Identification of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, China.Crossref | GoogleScholarGoogle Scholar | 30900979PubMed |
[57] Lahra MM, Martin I, Demczuk W, et al. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis 2018; 24 735–40.
| Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain.Crossref | GoogleScholarGoogle Scholar |
[58] Shimuta K, Watanabe Y, Nakayama S, et al. Emergence and evolution of internationally disseminated cephalosporin-resistant Neisseria gonorrhoeae clones from 1995 to 2005 in Japan. BMC Infect Dis 2015; 15 378
| Emergence and evolution of internationally disseminated cephalosporin-resistant Neisseria gonorrhoeae clones from 1995 to 2005 in Japan.Crossref | GoogleScholarGoogle Scholar | 26381611PubMed |


