What is the role of sexual health services in the delivery of primary prevention of sexually transmitted infections? A narrative review
Danielle Jayes A , Rachel Merrick B , Caisey Pulford B , Erna Buitendam B , Hamish Mohammed B C and John Saunders B C *
B C *
A Office for Health Improvement and Disparities, Department for Health and Social Care, London, UK.
B Blood Safety, Hepatitis, STIs and HIV Division, UK Health Security Agency, 61 Colindale Avenue, London NW9 5EQ, UK.
C Institute for Global Health, University College London, London, UK.
Sexual Health - https://doi.org/10.1071/SH22047
Submitted: 10 March 2022 Accepted: 7 July 2022 Published online: 4 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing
Abstract
Sexually transmitted infections (STIs) affect hundreds of millions of people globally. The resulting impact on quality of life and the economy for health systems is huge. Specialist sexual health services (SHS) play a key role in the provision of primary prevention interventions targeted against STIs. We conducted a narrative review to explore the role of SHSs in delivering primary prevention interventions for STIs. Established interventions include education and awareness building, condom promotion, and the provision of vaccines. Nascent interventions such as the use of antibiotics as pre- and post-exposure prophylaxis are not currently recommended, but have already been adopted by some key population groups. The shift to delivering SHS through digital health technologies may help to reduce barriers to access for some individuals, but creates challenges for the delivery of primary prevention and may inadvertently increase health inequities. Intervention development will need to consider carefully these shifting models of service delivery so that existing primary prevention options are not side-lined and that new interventions reach those who can benefit most.
Keywords: condoms, health promotion, health services, hepatitis, HPV, prevention, STIs, vaccines.
Introduction
Sexually transmitted infections (STIs) are a major public health concern. In 2020, the World Health Organization estimated that more than 374 million new infections occurred with either Chlamydia trachomatis, Neisseria gonorrhoeae, Treponema pallidum or Trichomonas vaginalis.1 Many hundreds of millions more individuals are living with genital herpes simplex virus, human papillomavirus (HPV) and chronic hepatitis B infection.1 STIs are also often associated with shame, social stigma and intolerance, all of which can dramatically impact an individual’s wellbeing and quality of life.2
Undiagnosed or untreated STIs can lead to life-changing complications, including pelvic inflammatory disease; tubal factor infertility; ectopic pregnancy; cervical cancer; perinatal, congenital or disseminated infections; cardiovascular and neurological complications; and increased susceptibility to HIV.3 The resulting impacts on morbidity, mortality and costs on the healthcare system are enormous.4–6 Prevention and treatment of STIs can be significantly less costly than treating the sequelae of infections. For example, in the UK, initial treatment of syphilis is estimated to be less than 300 times the management costs for permanent disability caused by neurosyphilis (£77 vs £25 776).7
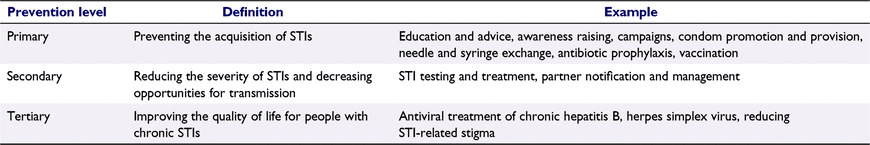
Efforts to prevent the acquisition of infections form a key part of activities undertaken by sexual and public health professionals.8 Broadly, these activities operate at each of the three levels of prevention in public health: primary, secondary and tertiary (Table 1). Primary prevention methods aim to prevent the acquisition of an infection and include activities such as the use of safer sex practices to reduce exposure (e.g. condoms), education on sexual and reproductive health and relationships (e.g. school-based education and public campaigns), the use of chemoprophylaxis (e.g. pre- and post-exposure prophylaxis for HIV, chlamydia and syphilis) and vaccination (e.g. for HPV, hepatitis A and B viruses). Secondary prevention aims to treat the infection to prevent health harms and transmission. Examples include STI testing, treatment, and partner notification and management. Tertiary prevention for STIs aims to reduce any long-term effects of chronic infections; for example, management of genital herpes and HIV.9
|
Although a combination prevention approach should be taken, drawing on interventions across primary, secondary and tertiary prevention options, this narrative review focusses on evidence to support the delivery of primary prevention interventions for STIs, including education, condoms, vaccination and chemoprophylaxis, delivered within specialist sexual health services (SHS), and considers implications for delivery of these within future and emerging models of SHS delivery. A narrative review approach has been taken to allow inclusion of evidence across different populations, interventions, and outcomes relevant to SHS. Although the exact structure of these services will vary between countries and health systems, literature was chosen when the intervention related to primary prevention and it was delivered within a setting where the primary focus is the delivery of sexual health care and have healthcare professionals trained in sexual health. Although primary prevention for STIs can, and should, be delivered in a variety of settings, there are several features that make SHS particularly well suited. Healthcare professionals within these settings specialise in sexual health, a key part of which is having structured, culturally competent discussions about sex, risk and prevention that may be more challenging in other settings or for other healthcare professionals.10 This is important as concerns about being able to take sexual histories and being asked sensitive questions can act as a barrier to the delivery of good care. In many areas, there have been large changes in how these services are delivered over the last decade; for example, with the availability of technologies to allow for remote self-sampling. More recently, the coronavirus disease 2019 (COVID-19) pandemic has led to rapid and sustained changes in service delivery with a shift to more remote and digital delivery models; this includes a range of modalities including via telephone, video, or online.11 In the UK, there has been increased provision of remote consultations and management, and an increase in the number of individuals accessing online self-sampling testing.12,13 This change in delivery is reflective of a trend that was already occurring and has implications for the delivery of primary prevention in SHS. We consider potential consequences of these changes in how services are delivered and accessed on the delivery of primary prevention.
Education, awareness and health promotion
Health promotion encompasses interventions aimed at changing individual behaviours and reducing risk.14 Accessible and high-quality information that empowers people to manage their own sexual health needs is essential to prevent acquisition and transmission of STIs.
Although some health promotion activities are universal in scope, others need to be tailored for key populations and marginalised groups disproportionately affected by STIs. Examples of these key population groups may include gay, bisexual, and other men who have sex with men (MSM), trans and gender-diverse people, and sex workers. Understanding the differences in knowledge and awareness of STIs in these population groups, as well as behaviours, attitudes and other factors that influence their risk of acquiring STIs, are essential for developing effective health promotion interventions.
Among MSM, poorer knowledge of STIs has been shown to be associated with higher risk behaviours. Although some MSM are well informed, there remains widespread lack of knowledge around prevention, modes of STI transmission and treatment options, especially among HIV-negative men or those with unknown HIV status compared with HIV-positive men.15–20 Specialist SHS play an important role in providing health promotion and advice and are widely perceived as acceptable and preferable as a source of information compared to other settings among MSM and young people.21,22 Barriers exist to accessing specialist SHS, including concerns over confidentiality and being identified within their communities among men of Black and Asian ethnicities in the UK, although variation exists with people from a Black Caribbean background more likely than those in other ethnic groups to access UK sexual health clinics.23,24
Community involvement in the design, delivery and evaluation of sexual health promotion and interventions is also recognised as an important factor among both MSM and Black Caribbean groups.25,26 To help stimulate behaviour change, health promotion activities and interventions should therefore be co-developed with key populations to ensure they are culturally relevant, tailored to groups that are at higher risk of acquiring STIs, and reflect different groups’ preferences for their content, format and delivery.
A shift towards remote and digital methods of SHS delivery highlights the need for education, awareness, and promotion activities to be accessible and delivered synergistically. The use of digital interventions have significant potential to provide a cost-effective way to deliver sexual health promotion given the reach and popularity of the internet and mobile phones,27 and could be delivered from SHS given they are broadly viewed as a trusted source of information.21,28,29 Young people consider Reproductive and Sexual Health Education through school as their the main source of information about sexual health, but cite doctors, nurses and clinics as a highly trusted and preferred setting for information about condoms and contraception,28,29 suggesting there is a role for SHS in the delivery of prevention. More targeted in-person or digital brief interventions that address key risk behaviours (e.g. drug and alcohol use) have also been widely used in SHS but, although feasible, have shown mixed results.30–33
Among MSM, the delivery of sexual health information and promotion through social media and dating apps is viewed as acceptable and feasible, but concerns around discrimination and judgement over social media were expressed and to be effective, messaging should be engaging, positive in tone and delivered by trusted healthcare organisations.22
A recent systematic review of digital interventions (i.e. social media, websites, text messages) for sexual health promotion among young people found evidence that these interventions are effective in increasing knowledge around the prevention of STIs and HIV; however, this increase in knowledge does not guarantee improved health outcomes.34,35
Although the provision of factsheets and clear, accurate written information about STIs and their prevention is recommended within STI management guidelines, the evidence to support the effectiveness of these factsheets to prevent reinfection is lacking.36,37
Although the use of digital interventions delivered by SHS is feasible, significant barriers exist to this approach. These include shared computer space (privacy concerns) or filtering software on phones or other devices.27 Moreover, the scale of digital exclusion needs to be considered to ensure the equitable delivery of digital or online health promotion messages.
Condoms
Consistent and correct use of condoms can significantly reduce the risk of acquiring STIs, including HIV.38–41 An international systematic review looking at the effectiveness of global condom promotion programs, including interventions relating to social marketing, promotion of condom brands and HIV/STI prevention programs that specifically promoted condom use, confirms that condoms remain a crucial intervention for the prevention of STI transmission.42 Interventions that teach and encourage correct and consistent condom use, along with communication and negotiation skills, are particularly effective in reducing STI transmission.43 The use of female (internal) and male (external) condoms (when women are given the option to make a choice of one or both at each sexual act) is more effective than use of male condoms only in preventing chlamydia and gonorrhoea, and is potentially more effective in preventing trichomoniasis and other STIs.44 Therefore, access to condoms of all types is an essential component of SHS delivery.
Young people experience the highest diagnosis rates of the most common STIs and studies have shown rising trends in diversity of sexual practices.45–49 Despite changes in behavioural patterns, the use and perception of condoms varies among young people with unintended conception viewed as a greater concern than contracting a STI.29 Young people also report major barriers to accessing SHS, including long waiting times, stigma and embarrassment, lack of clarity on which services are available, and an insufficient number of specialised SHS.29 Many of these access challenges for young people have been compounded by the coronavirus disease 2019 (COVID-19) pandemic and a move to online provision.13 Young people reported that their access to, and use of, free condom schemes was disrupted and that there was uncertainty and confusion about how to access condoms.50,51 These changing patterns of behaviour and barriers to access raise new challenges for health promotion and the provision of condoms through SHS, and there is an increasing need to improve digital sexual health literacy among young people.
Motivational interviewing has been found to be effective at increasing the frequency of condom use during anal or oral sex among MSM living with HIV in the 3 months following the motivational interviewing intervention.50,51 However, this intervention was delivered within an academic centre providing HIV outpatient care and there is a lack of evidence that motivational interviewing reduces the likelihood of unprotected anal intercourse with casual partners among young MSM. There is some evidence to suggest that lay health advisor-delivered interventions could be effective at reducing STI acquisition and improving condom use among young heterosexual African American men.52,53
Biomedical prevention
Vaccination
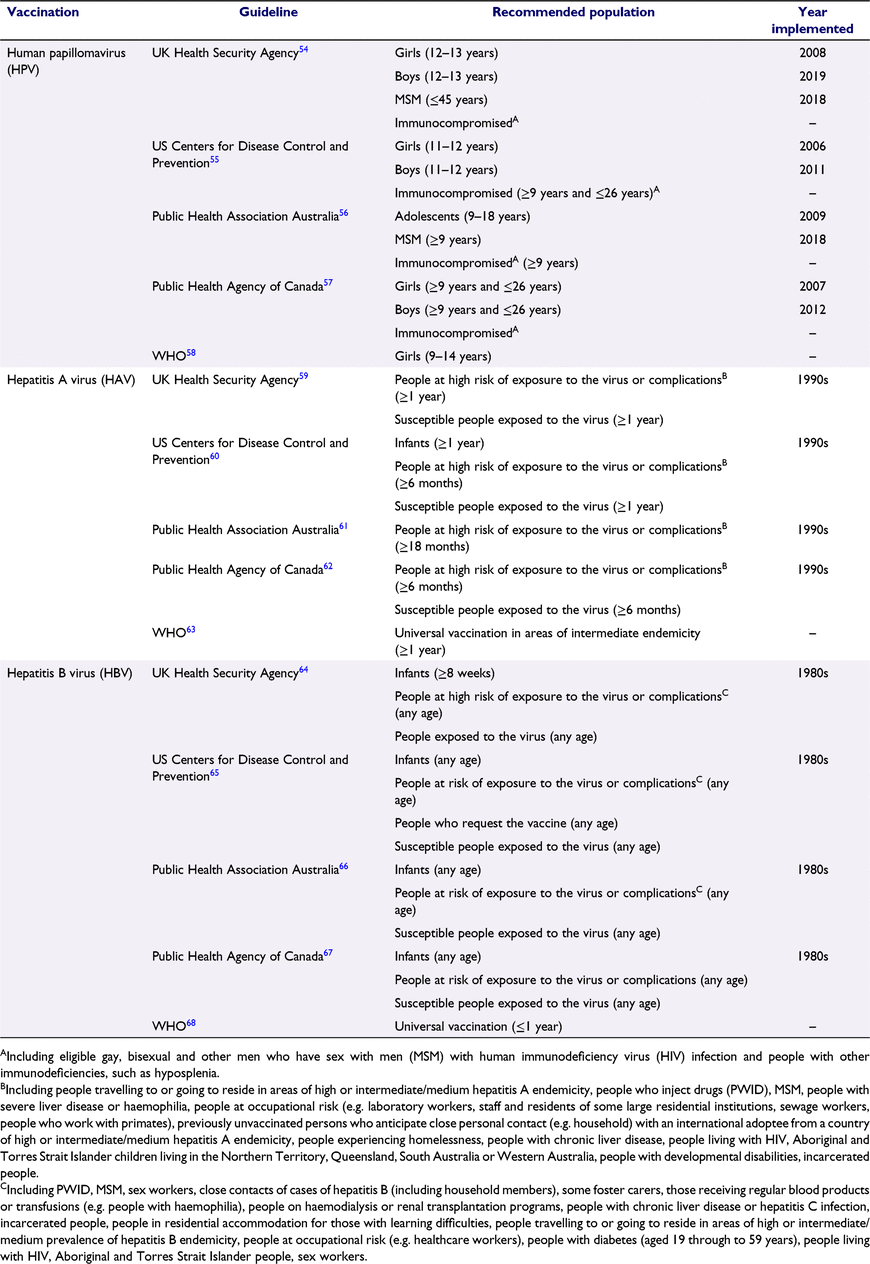
Safe and effective vaccines exist for several STIs and can be delivered to individuals attending SHS, although vaccine coverage varies between and within programs and populations. Within current delivery models, these require administration within the clinic, or by healthcare workers in outreach settings (Table 2); therefore, the shift to remote and digital delivery of SHS may present a challenge to ongoing vaccination efforts.
|
Hepatitis A and B virus
There are 1.4 million new cases of hepatitis A virus (HAV) annually reported globally accounting for approximately 7000 deaths.69 In a number of countries, selective vaccination is recommended for individuals travelling to endemic countries and other key risk groups, including MSM (Table 2). Outbreaks of HAV in MSM do occur, most recently in England in 2017, partly attributable to variable implementation of vaccination in SHS.70
Hepatitis B virus (HBV) also causes an acute or chronic infection of the liver, and is transmitted through sex, blood-to-blood contact and perinatally. If persistent infection develops, it can lead to cirrhosis and liver cancer. There are approximately 250 million people living with chronic HBV infection and almost 900 000 people die from complications of infection annually.71 Vaccination is often available through a variety of healthcare settings, some of which may be more acceptable for some key populations compared to others. For example, infant vaccination programs have been introduced in most countries, but selective immunisation for certain key populations at risk through sexual exposure or blood; for example, MSM, sex workers, prisoners and people who inject drugs, is also recommended in guidelines (Table 2).72
Human papillomavirus
Human papillomavirus (HPV) is one of the most commonly transmitted STIs and worldwide, about 5% of all cancers are linked to the virus, mostly due to subtypes HPV16 and 18, including cervical, penile, anal and genital cancers and some cancers of the head and neck.73 Other subtypes of HPV, such as 6 and 11, cause external genital warts, which can place a significant impact on personal wellbeing, as well as an economic burden on health systems.74
Since the introduction of the HPV vaccine, there have been significant reductions in HPV infections, cervical intraepithelial neoplasia 2+ and anogenital warts diagnoses seen globally and, in England, the HPV immunisation program has almost eliminated cervical cancer in women born since 1 September 1995.75,76 In some countries where universal vaccination of school-aged children was not initially adopted, catch up vaccination of key population groups in SHS has been introduced. For example, in England, the HPV vaccination program has had a phased expansion based on modelling direct and indirect benefits to include MSM up to age 45 years attending SHS (since 2018) and boys via a gender-neutral, school-based adolescent immunisation program in school year 8 (i.e. aged 12 and 13 years (since 2019)).77,78 By the end of December 2019, 93% of specialist SHS in England reported HPV opportunistic vaccination activity for MSM.79
Barriers to HPV vaccination are varied and can range from lack of knowledge of HPV, HPV-related diseases, HPV or vaccine stigma and vaccine beliefs to delivery-specific concerns, such as long intervals between dose, and the inconvenience of multiple doses.80,81 Offering the vaccine when testing for STIs or within SHS, support from the healthcare provider and increasing public awareness of HPV and the health benefits of vaccination to MSM may overcome some of these barriers to facilitate vaccine uptake.80,81
Gonorrhoea and chlamydia
Although there are currently no licensed vaccines for most STIs, such as herpes simplex, chlamydia, gonorrhoea, and syphilis, or sexually transmissible enteric pathogens such as Shigella, several are in development.82–84 Efforts to develop vaccines against Neisseria gonorrhoeae have become increasingly critical given the rising threat of gonococcal antimicrobial resistance (AMR).85 Analyses of surveillance data collected in New Zealand showed that individuals who had received Meningitis B vaccine were at lower risk of subsequently acquiring gonorrhoea, and clinical trials are currently underway to evaluate the efficacy of the four-component meningococcal B vaccine (Bexsero) in preventing gonorrhoea in gay and bisexual men.86,87 An additional anticipated benefit to reducing diagnoses of gonorrhoea would be to slow the development of AMR; however, vaccine hesitancy could be an issue if the stigma associated with STIs extended to STI vaccines.
The complex life cycle of chlamydia has made vaccine development challenging. A recent first-in-human clinical phase 1 trial showed a vaccine candidate and adjuvant were immunogenic, safe and well tolerated with further efficacy studies underway.88
Doxycycline prophylaxis for chlamydia and syphilis
Doxycycline as pre- and post-exposure prophylaxis for syphilis and chlamydia has been shown to reduce the risk of acquiring chlamydia and syphilis in a very small number of published studies.89,90 There are several ongoing international studies to further explore the efficacy and potential harms of doxycycline prophylaxis, particularly in relation to AMR.91,92 Significant uncertainties currently exist about the efficacy, safety, harms and most appropriate target populations for doxycycline prophylaxis. So, although the delivery of antibiotic prophylaxis could feasibly be delivered through SHS, it is not currently recommended in any guidelines internationally. Despite this, there is evidence that some MSM at higher risk of STIs (e.g. MSM on HIV-pre-exposure prophylaxis (PrEP)) are self-sourcing antibiotics to use as STI prophylaxis, and healthcare providers should be aware of the issues.93–98
The future of primary prevention in SHS
Although there are many strengths to the approach of delivering primary prevention in SHS, there are also several potential weaknesses and emerging challenges. SHS are limited in their physical and financial capacity to deliver care to those at risk of STIs. Key populations still experience significant barriers to accessing services and continue to be disproportionately affected by STIs.
The COVID-19 pandemic has accelerated the move to online provision of SHS. This has implications for the delivery of primary prevention in SHS and provides opportunities to enhance the role of digital interventions by providing more targeted, user-friendly, and interactive prevention messaging that is tailored to key populations and groups disproportionately affected by STIs.
Although this may facilitate access for some groups, it may also present a significant barrier to access for others, and it is increasingly important to ensure that key populations remain able to access prevention services, that existing barriers are not exacerbated, and that digital exclusion does not increase inequalities.
Online delivery may provide savings due to factors such as reduced staffing costs, but there is limited existing evidence to support the cost-effectiveness of online service models. Those who are already disproportionately affected by STIs are also those who may struggle most to use digital health care, thereby cementing and worsening health inequalities.99 This highlights the need to ensure that multiple access points are available in addition to the delivery of online services and that both face-to-face and digital prevention is delivered in line with the preferences and needs of key populations. Ongoing evaluation is essential to improve our understanding of the impact that digital interventions could have on sexual health outcomes.
In terms of the delivery of the primary prevention interventions outlined above, there are obvious challenges in delivering many of these through virtual consultations. Online models of PrEP delivery may offer an example of how biomedical STI prevention could be delivered in the future, but vaccination currently requires face-to-face delivery. However, self-administered intradermal and intramuscular influenza vaccination has been shown to be acceptable and feasible among healthcare workers so could be explored for other vaccines and for lay users, although logistical issues particularly around cold-chain management could be an issue.100 Self-administration of heparin and long-acting contraception are other examples that could be drawn upon.
Conclusion
SHS are ideally placed to provide a variety of primary prevention interventions to a range of key population groups. Evidence to support their delivery in these settings predominantly comes from observational data to show it is feasible and acceptable, but quantifying the economic and disease impacts remains challenging. Evidence we have drawn upon in this review are often non-comparative studies, making it challenging to drawn definitive conclusions about the effectiveness of interventions. Interventions to improve uptake and delivery of primary prevention should be co-produced with input from the population groups for whom these interventions are targeted. Although the shift to delivering online services may help to create opportunities for some service users, digital exclusion could contribute to worsening health outcomes for others; therefore, robust evaluation should underpin any intervention development and delivery to improve our understanding of what works for whom, why and in what context. This is important to allow services in other settings to adopt and adapt interventions to best suit their own context.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The authors would like to thank Katy Sinka and Kate Folkard at the UK Health Security Agency; Adam Winter, Sydney Joyce and Andrea Duncan at the UK Department of Health and Social Care for their assistance with this study.
References
[1] World Health Organization. Sexually transmitted infections Factsheet. Geneva: WHO; 2021. Available at https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis)[2] Hood JE, Friedman AL. Unveiling the hidden epidemic: a review of stigma associated with sexually transmissible infections. Sex Health 2011; 8 159–70.
| Unveiling the hidden epidemic: a review of stigma associated with sexually transmissible infections.Crossref | GoogleScholarGoogle Scholar |
[3] World Health Organization. Global health sector strategy on sexually transmitted infections, 2016-2021. Geneva: WHO; 2016.
[4] World Bank. Sexually transmitted infections in developing countries. 2009. Available at https://webworldbankorg/archive/website01213/WEB/0__CO-54HTM
[5] Jackson LJ, Auguste P, Low N, Roberts TE. Valuing the health states associated with Chlamydia trachomatis infections and their sequelae: a systematic review of economic evaluations and primary studies. Value Health 2014; 17 116–30.
| Valuing the health states associated with Chlamydia trachomatis infections and their sequelae: a systematic review of economic evaluations and primary studies.Crossref | GoogleScholarGoogle Scholar |
[6] Centers for Disease Control and Prevention. Sexually transmitted infections prevalence, incidence, and cost estimates in the United States. 2021. Available at https://wwwcdcgov/std/statistics/prevalence-2020-at-a-glancehtm#:∼:text=CDC%20estimates%20indicate%20about%2020%20percent%20of%20the,system%20nearly%20%2416%20billion%20in%20healthcare%20costs%20alone
[7] Public Health England. Sexual and reproductive health return on investment tool. Public Health England; 2020. Available at https://www.gov.uk/government/publications/sexual-and-reproductive-health-return-on-investment-tool
[8] World Health Organization. Defining sexual health. Report of a technical consultation on sexual health, 28–31 January 2002. Geneva: World Health Organization; 2006.
[9] European Centre for Disease Prevention and Control. Developing a national strategy for the prevention and control of sexually transmitted infections. Stockholm: ECDC; 2019.
[10] Yeung A, Temple-Smith M, Fairley C, Hocking J. Narrative review of the barriers and facilitators to chlamydia testing in general practice. Aust J Prim Health 2015; 21 139–47.
| Narrative review of the barriers and facilitators to chlamydia testing in general practice.Crossref | GoogleScholarGoogle Scholar |
[11] Phillips TR, Fairley CK, Donovan B, Ong JJ, McNulty A, Marshall L, et al. Sexual health service adaptations to the coronavirus disease 2019 (COVID-19) pandemic in Australia: a nationwide online survey. Aust N Z J Public Health 2021; 45 622–7.
| Sexual health service adaptations to the coronavirus disease 2019 (COVID-19) pandemic in Australia: a nationwide online survey.Crossref | GoogleScholarGoogle Scholar |
[12] Public Health England. The impact of the COVID-19 pandemic on prevention, testing, diagnosis and care for sexually transmitted infections, HIV and viral hepatitis in England. Provisional data: January to September 2020. Public Health England; 2020.
[13] Dema E, Gibbs J, Clifton S, Copas AJ, Tanton C, Riddell J, et al. Initial impacts of the COVID-19 pandemic on sexual and reproductive health service use and unmet need in Britain: findings from a quasi-representative survey (Natsal-COVID). Lancet Public Health 2022; 7 e36–47.
| Initial impacts of the COVID-19 pandemic on sexual and reproductive health service use and unmet need in Britain: findings from a quasi-representative survey (Natsal-COVID).Crossref | GoogleScholarGoogle Scholar |
[14] World Health Organization. Health promotion. 2022. Available at https://www.who.int/westernpacific/about/how-we-work/programmes/health-promotion
[15] Wayal S, Reid D, Weatherburn P, Blomquist P, Fabiane S, Hughes G, et al. Association between knowledge, risk behaviours, and testing for sexually transmitted infections among men who have sex with men: findings from a large online survey in the United Kingdom. HIV Med 2019; 20 523–33.
| Association between knowledge, risk behaviours, and testing for sexually transmitted infections among men who have sex with men: findings from a large online survey in the United Kingdom.Crossref | GoogleScholarGoogle Scholar |
[16] McDaid LM, Li J, Knussen C, Flowers P. Sexually transmitted infection testing and self-reported diagnoses among a community sample of men who have sex with men, in Scotland. Sex Transm Infect 2013; 89 223–30.
| Sexually transmitted infection testing and self-reported diagnoses among a community sample of men who have sex with men, in Scotland.Crossref | GoogleScholarGoogle Scholar |
[17] Mimiaga MJ, Goldhammer H, Belanoff C, Tetu AM, Mayer KH. Men who have sex with men: perceptions about sexual risk, HIV and sexually transmitted disease testing, and provider communication. Sex Transm Dis 2007; 34 113–9.
| Men who have sex with men: perceptions about sexual risk, HIV and sexually transmitted disease testing, and provider communication.Crossref | GoogleScholarGoogle Scholar |
[18] van der Snoek EM, de Wit JBF, Götz HM, Mulder PGH, Neumann MHA, van der Meijden WI. Incidence of sexually transmitted diseases and HIV infection in men who have sex with men related to knowledge, perceived susceptibility, and perceived severity of sexually transmitted diseases and HIV infection: Dutch MSM–Cohort Study. Sex Transm Dis 2006; 33 193–8.
| Incidence of sexually transmitted diseases and HIV infection in men who have sex with men related to knowledge, perceived susceptibility, and perceived severity of sexually transmitted diseases and HIV infection: Dutch MSM–Cohort Study.Crossref | GoogleScholarGoogle Scholar |
[19] Datta J, Reid D, Hughes G, Mercer CH, Wayal S, Weatherburn P. Awareness of and attitudes to sexually transmissible infections among gay men and other men who have sex with men in England: a qualitative study. Sex Health 2019; 16 18–24.
| Awareness of and attitudes to sexually transmissible infections among gay men and other men who have sex with men in England: a qualitative study.Crossref | GoogleScholarGoogle Scholar |
[20] The EMIS Network. EMIS 2010: The European men-who-have-sex-with-men internet survey. Findings from 38 countries. Stockholm: European Centre for Disease Prevention and Control; 2013.
[21] Gan J, Kularadhan V, Chow EPF, Fairley CK, Hocking JS, Kong FYS, et al. What do young people in high-income countries want from STI testing services? A systematic review. Sex Transm Infect 2021; 97 574
| What do young people in high-income countries want from STI testing services? A systematic review.Crossref | GoogleScholarGoogle Scholar |
[22] Kesten JM, Dias K, Burns F, Crook P, Howarth A, Mercer CH, et al. Acceptability and potential impact of delivering sexual health promotion information through social media and dating apps to MSM in England: a qualitative study. BMC Public Health 2019; 19 1236
| Acceptability and potential impact of delivering sexual health promotion information through social media and dating apps to MSM in England: a qualitative study.Crossref | GoogleScholarGoogle Scholar |
[23] Datta J, Reid D, Hughes G, Mercer CH, Wayal S, Weatherburn P. Places and people: the perceptions of men who have sex with men concerning STI testing: a qualitative study. Sex Transm Infect 2018; 94 46–50.
| Places and people: the perceptions of men who have sex with men concerning STI testing: a qualitative study.Crossref | GoogleScholarGoogle Scholar |
[24] Wayal S, Hughes G, Sonnenberg P, Mohammed H, Copas AJ, Gerressu M, et al. Ethnic variations in sexual behaviours and sexual health markers: findings from the third British National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Lancet Public Health 2017; 2 e458–e72.
| Ethnic variations in sexual behaviours and sexual health markers: findings from the third British National Survey of Sexual Attitudes and Lifestyles (Natsal-3).Crossref | GoogleScholarGoogle Scholar |
[25] Estupinan M, Mercer CH, Hughes G, Winter A, Mohammed H, Jayes D, et al. Sexually transmitted infections: promoting the sexual health and wellbeing of gay, bisexual and other men who have sex with men. From research to public health practice: an evidence-based resource for commissioners, providers and voluntary sector organisations. Public Health England; 2021. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1021121/HPRU1_MSM_PHE_UCL_Report__1_.pdf
[26] Estupinan M, Mercer CH, Woode Owusu M, Gerressu M, Winter A, Hughes G, et al. Sexually transmitted infections: promoting the sexual health and wellbeing of people from a Black Caribbean background. From research to public health practice: an evidence-based resource for commissioners, providers and third sector organisations. Public Health England; 2021. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1021488/HPRU1_BC_PHE_UCL_Report.pdf
[27] Bailey J, Mann S, Wayal S, Hunter R, Free C, Abraham C, et al. Sexual health promotion for young people delivered via digital media: a scoping review. Public Health Research 2015; 3
| Sexual health promotion for young people delivered via digital media: a scoping review.Crossref | GoogleScholarGoogle Scholar |
[28] Tanton C, Jones KG, Macdowall W, Clifton S, Mitchell KR, Datta J, et al. Patterns and trends in sources of information about sex among young people in Britain: evidence from three National Surveys of Sexual Attitudes and Lifestyles. BMJ Open 2015; 5 e007834
| Patterns and trends in sources of information about sex among young people in Britain: evidence from three National Surveys of Sexual Attitudes and Lifestyles.Crossref | GoogleScholarGoogle Scholar |
[29] Lewis R, Blake C, McMellon C, Riddell J, Graham C, Mitchell KR. Understanding young people’s use and non-use of condoms and contraception: a co-developed, mixed-methods study with 16-24 year olds in Scotland. Final report from CONUNDRUM (CONdom and CONtraception UNDerstandings: Researching Uptake and Motivations). MRC/CSO Social and Public Health Sciences Unit: University of Glasgow; 2021.
[30] Carey MP, Rich C, Norris AL, Krieger N, Gavarkovs AG, Kaplan C, et al. A brief clinic-based intervention to reduce alcohol misuse and sexual risk behavior in young women: results from an exploratory clinical trial. Arch Sex Behav 2020; 49 1231–50.
| A brief clinic-based intervention to reduce alcohol misuse and sexual risk behavior in young women: results from an exploratory clinical trial.Crossref | GoogleScholarGoogle Scholar |
[31] Chander G, Hutton HE, Xu X, Canan CE, Gaver J, Finkelstein J, et al. Computer delivered intervention for alcohol and sexual risk reduction among women attending an urban sexually transmitted infection clinic: a randomized controlled trial. Addict Behav Rep 2021; 14 100367
| Computer delivered intervention for alcohol and sexual risk reduction among women attending an urban sexually transmitted infection clinic: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[32] Crawford MJ, Sanatinia R, Barrett B, Byford S, Dean M, Green J, et al. The clinical and cost-effectiveness of brief advice for excessive alcohol consumption among people attending sexual health clinics: a randomised controlled trial. Sex Transm Infect 2015; 91 37–43.
| The clinical and cost-effectiveness of brief advice for excessive alcohol consumption among people attending sexual health clinics: a randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[33] Lane J, Proude EM, Conigrave KM, de Boer JP, Haber PS. Nurse-provided screening and brief intervention for risky alcohol consumption by sexual health clinic patients. Sex Transm Infect 2008; 84 524–7.
| Nurse-provided screening and brief intervention for risky alcohol consumption by sexual health clinic patients.Crossref | GoogleScholarGoogle Scholar |
[34] Chávez NR, Shearer LS, Rosenthal SL. Use of digital media technology for primary prevention of STIs/HIV in youth. J Pediatr Adolesc Gynecol 2014; 27 244–57.
| Use of digital media technology for primary prevention of STIs/HIV in youth.Crossref | GoogleScholarGoogle Scholar |
[35] Wadham E, Green C, Debattista J, Somerset S, Sav A. New digital media interventions for sexual health promotion among young people: a systematic review. Sex Health 2019; 16 101–23.
| New digital media interventions for sexual health promotion among young people: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[36] Clutterbuck DJ, Flowers P, Barber T, Wilson H, Nelson M, Hedge B, et al. UK national guideline on safer sex advice. Int J STD AIDS 2012; 23 381–8.
| UK national guideline on safer sex advice.Crossref | GoogleScholarGoogle Scholar |
[37] Australasian Sexual and Reproductive Health Alliance and Australasian Society for HIV Viral Hepatitis and Sexual Health Medicine. Australian STI management gudelines for use in primary care. ASHM; 2021. Available at https://sti.guidelines.org.au/
[38] Weller SC, Davis-Beaty K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev 2002; 2002 CD003255
| Condom effectiveness in reducing heterosexual HIV transmission.Crossref | GoogleScholarGoogle Scholar |
[39] Crosby RA, Charnigo RA, Weathers C, Caliendo AM, Shrier LA. Condom effectiveness against non-viral sexually transmitted infections: a prospective study using electronic daily diaries. Sex Transm Infect 2012; 88 484–9.
| Condom effectiveness against non-viral sexually transmitted infections: a prospective study using electronic daily diaries.Crossref | GoogleScholarGoogle Scholar |
[40] Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bull World Health Organ 2004; 82 454–61.
[41] Bernabe-Ortiz A, Carcamo CP, Scott JD, Hughes JP, Garcia PJ, Holmes KK. HBV infection in relation to consistent condom use: a population-based study in Peru. PLoS One 2011; 6 e24721
| HBV infection in relation to consistent condom use: a population-based study in Peru.Crossref | GoogleScholarGoogle Scholar |
[42] Evans WD, Ulasevich A, Hatheway M, Deperthes B. Systematic review of peer-reviewed literature on global condom promotion programs. Int J Environ Res Public Health 2020; 17 2262
| Systematic review of peer-reviewed literature on global condom promotion programs.Crossref | GoogleScholarGoogle Scholar |
[43] Petrova D, Garcia-Retamero R. Effective evidence-based programs for preventing sexually-transmitted infections: a meta-analysis. Curr HIV Res 2015; 13 432–8.
| Effective evidence-based programs for preventing sexually-transmitted infections: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[44] Wiyeh AB, Mome RKB, Mahasha PW, Kongnyuy EJ, Wiysonge CS. Effectiveness of the female condom in preventing HIV and sexually transmitted infections: a systematic review and meta-analysis. BMC Public Health 2020; 20 319
| Effectiveness of the female condom in preventing HIV and sexually transmitted infections: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[45] Mercer CH, Tanton C, Prah P, Erens B, Sonnenberg P, Clifton S, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 2013; 382 1781–94.
| Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal).Crossref | GoogleScholarGoogle Scholar |
[46] Lewis R, Tanton C, Mercer CH, Mitchell KR, Palmer M, Macdowall W, et al. Heterosexual practices among young people in Britain: evidence from three national surveys of sexual attitudes and lifestyles. J Adolesc Health 2017; 61 694–702.
| Heterosexual practices among young people in Britain: evidence from three national surveys of sexual attitudes and lifestyles.Crossref | GoogleScholarGoogle Scholar |
[47] Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15-44 years of age, United States, 2002. Adv Data 2005; 1–55.
[48] Chandra A, Mosher WD, Copen C, Sionean C. Sexual behavior, sexual attraction, and sexual identity in the United States: data from the 2006-2008 National Survey of Family Growth. Natl Health Stat Report 2011; Mar 3 1–36.
[49] Centers for Disease Control and Prevention. Sexually transmitted disease surveillance: adolescents and young adults. 2019. Available at https://www.cdc.gov/std/statistics/2019/figures/2019-STD-Surveillance-Adolescents-and-Young-Adults.pptx
[50] Lewis R, Blake C, Shimonovich M, Coia N, Duffy J, Kerr Y, et al. Dirsupted prevention: condom and contraception access and use among young adults during the initial months of the COVID-19 pandemic. An online survey. BMJ Sex Reprod Health 2021; 47 269–76.
| Dirsupted prevention: condom and contraception access and use among young adults during the initial months of the COVID-19 pandemic. An online survey.Crossref | GoogleScholarGoogle Scholar |
[51] Brown JL, Vanable PA, Bostwick RA, Carey MP. A pilot intervention trial to promote sexual health and stress management among HIV-infected men who have sex with men. AIDS Behav 2019; 23 48–59.
| A pilot intervention trial to promote sexual health and stress management among HIV-infected men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[52] Crosby R, DiClemente RJ, Charnigo R, Snow G, Troutman A. A brief, clinic-based, safer sex intervention for heterosexual African American men newly diagnosed with an STD: a randomized controlled trial. Am J Public Health 2009; 99 S96–103.
| A brief, clinic-based, safer sex intervention for heterosexual African American men newly diagnosed with an STD: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[53] Crosby RA, Charnigo RJ, Salazar LF, Pasternak R, Terrell IW, Ricks J, et al. Enhancing condom use among Black male youths: a randomized controlled trial. Am J Public Health 2014; 104 2219–25.
| Enhancing condom use among Black male youths: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[54] UK Health Security Agency. Greenbook Chapter 18a human papillomavirus (HPV). 2022. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/828868/Greenbook_chapter_18a.pdf [accessed 21 February 2022]
[55] Centers for Disease Control and Prevention. HPV vaccination recommendations. 2021. Available at https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html [accessed 23 February 2022]
[56] Australian Government Department of Health. Human papillomavirus (HPV). 2018. Available at https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/human-papillomavirus-hpv [accessed 23 February 2022]
[57] Government of Canada. Human papillomavirus vaccine: Canadian Immunization Guide. 2021. Available at https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-9-human-papillomavirus-vaccine.html [accessed 23 February 2022]
[58] World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017. Available at https://www.who.int/publications/i/item/who-wer9219-241-268. [accessed 22 July 2022]
[59] UK Health Security Agency. Greenbook Chapter 17 Hepatitis A. 2022. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1053507/Greenbook-chapter-17-7Feb22.pdf [accessed 21 February 2022]
[60] Centers for Disease Control and Prevention. Hepatitis A. 2021. Available at https://www.cdc.gov/vaccines/pubs/pinkbook/hepa.html [accessed 23 February 2022]
[61] Australian Government Department of Health. Hepatitis A. 2020. Available at https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/hepatitis-a [accessed 23 February 2022]
[62] Government of Canada. Hepatitis A vaccine: Canadian Immunization Guide. 2021. Available at https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-6-hepatitis-a-vaccine.html [accessed 23 February 2022]
[63] World Health Organization. WHO position papers on Hepatitis A. 2012. Available at https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/position-papers/hepatitis-a [accessed 21 February 2022]
[64] UK Health Security Agency. Greenbook Chapter 18 Hepatitis B. 2022. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1052889/Greenbook-chapter-18-4Feb22.pdf [accessed 21 February 2022]
[65] Centers for Disease Control and Prevention. Hepatitis B. 2021. Available at https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html [accessed 23 February 2022]
[66] Australian Government Department of Health. Hepatitis B. 2021. Available at https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/hepatitis-b [accessed 23 February 2022]
[67] Government of Canada. Hepatitis B vaccine: Canadian Immunization Guide. 2022. Available at https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-7-hepatitis-b-vaccine.html [accessed 23 February 2022]
[68] World Health Organization. Hepatitis B. 2022. Available at https://www.who.int/news-room/fact-sheets/detail/hepatitis-b [accessed 21 February 2022]
[69] World Health Organization. Hepatitis A Factsheet. 2021. Available at https://www.who.int/news-room/fact-sheets/detail/hepatitis-a
[70] Public Health England. Health protection report. Volume 11 Number 17. Public Health England; 2017.
[71] World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis. World Health Organization; 2016.
[72] Nguyen MH, Wong G, Gane E, Kao J-H, Dusheiko G. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin Microbiol Rev 2020; 33 e00046–19
| Hepatitis B virus: advances in prevention, diagnosis, and therapy.Crossref | GoogleScholarGoogle Scholar |
[73] de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017; 141 664–70.
| Worldwide burden of cancer attributable to HPV by site, country and HPV type.Crossref | GoogleScholarGoogle Scholar |
[74] Raymakers AJN, Sadatsafavi M, Marra F, Marra CA. Economic and Humanistic Burden of External Genital Warts. PharmacoEconomics 2012; 30 1–16.
| Economic and Humanistic Burden of External Genital Warts.Crossref | GoogleScholarGoogle Scholar |
[75] Drolet M, Bénard E, Pérez N, Brisson M, Group HPVVIS Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019; 394 497–509.
| Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[76] Falcaro M, Castañon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet 2021; 398 2084–92.
| The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study.Crossref | GoogleScholarGoogle Scholar |
[77] Public Health England. HPV vaccination programme for men who have sex with men (MSM). 2018. Available at https://www.gov.uk/government/collections/hpv-vaccination-for-men-who-have-sex-with-men-msm-programme
[78] Public Health England. HPV vaccine to be given to boys in England. 2018. Available at https://www.gov.uk/government/news/hpv-vaccine-to-be-given-to-boys-in-england
[79] Public Health England. Human papillomavirus (HPV) vaccination uptake in gay, bisexual and other men who have sex with men (MSM). National programme: 2019 annual report. 2019. Available at https://www.gov.uk/government/publications/hpv-vaccination-uptake-in-men-who-have-sex-with-men-msm [accessed 10 February 2022]
[80] Fontenot HB, Fantasia HC, Vetters R, Zimet GD. Increasing HPV vaccination and eliminating barriers: recommendations from young men who have sex with men. Vaccine 2016; 34 6209–16.
| Increasing HPV vaccination and eliminating barriers: recommendations from young men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[81] Gerend MA, Madkins K, Crosby S, Korpak AK, Phillips GL, Bass M, et al. A Qualitative analysis of young sexual minority men’s perspectives on human papillomavirus vaccination. LGBT Health 2019; 6 350–6.
| A Qualitative analysis of young sexual minority men’s perspectives on human papillomavirus vaccination.Crossref | GoogleScholarGoogle Scholar |
[82] Haese EC, Thai VC, Kahler CM. Vaccine candidates for the control and prevention of the sexually transmitted disease gonorrhea. Vaccines 2021; 9 804
| Vaccine candidates for the control and prevention of the sexually transmitted disease gonorrhea.Crossref | GoogleScholarGoogle Scholar |
[83] Gottlieb SL, Deal CD, Giersing B, Rees H, Bolan G, Johnston C, et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: update and next steps. Vaccine 2016; 34 2939–47.
| The global roadmap for advancing development of vaccines against sexually transmitted infections: update and next steps.Crossref | GoogleScholarGoogle Scholar |
[84] Mani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine 2016; 34 2887–94.
| Status of vaccine research and development for Shigella.Crossref | GoogleScholarGoogle Scholar |
[85] Gottlieb SL, Jerse AE, Delany-Moretlwe S, Deal C, Giersing BK. Advancing vaccine development for gonorrhoea and the Global STI Vaccine Roadmap. Sex Health 2019; 16 426–32.
| Advancing vaccine development for gonorrhoea and the Global STI Vaccine Roadmap.Crossref | GoogleScholarGoogle Scholar |
[86] Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 2017; 390 1603–10.
| Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study.Crossref | GoogleScholarGoogle Scholar |
[87] ClinicalTrials.gov. Efficacy Study of 4CMenB (Bexsero®) to Prevent Gonorrhoea Infection in Gay and Bisexual Men (GoGOVax). NCT04415424. Available at https://clinicaltrials.gov/ct2/show/NCT04415424
[88] Abraham S, Juel HB, Bang P, Cheeseman HM, Dohn RB, Cole T, et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect Dis 2019; 19 1091–100.
| Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial.Crossref | GoogleScholarGoogle Scholar |
[89] Bolan RK, Beymer MR, Weiss RE, Flynn RP, Leibowitz AA, Klausner JD. Doxycycline prophylaxis to reduce incident syphilis among HIV-infected men who have sex with men who continue to engage in high-risk sex: a randomized, controlled pilot study. Sex Transm Dis 2015; 42 98–103.
| Doxycycline prophylaxis to reduce incident syphilis among HIV-infected men who have sex with men who continue to engage in high-risk sex: a randomized, controlled pilot study.Crossref | GoogleScholarGoogle Scholar |
[90] Molina J-M, Charreau I, Chidiac C, Pialoux G, Cua E, Delaugerre C, et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis 2018; 18 308–17.
| Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial.Crossref | GoogleScholarGoogle Scholar |
[91] Grant JS, Stafylis C, Celum C, Grennan T, Haire B, Kaldor J, et al. Doxycycline prophylaxis for bacterial sexually transmitted infections. Clin Infect Dis 2020; 70 1247–53.
| Doxycycline prophylaxis for bacterial sexually transmitted infections.Crossref | GoogleScholarGoogle Scholar |
[92] ClinicalTrials.gov. Doxycycline PEP for prevention of sexually transmitted infections among Kenyan women using HIV PrEP (dPEP-KE). Identifier: NCT04050540. 2020. Available at https://clinicaltrials.gov/ct2/show/NCT04050540
[93] O’Halloran C, Croxford S, Mohammed H, Gill ON, Hughes G, Fifer H, et al. Factors associated with reporting antibiotic use as STI prophylaxis among HIV PrEP users: findings from a cross-sectional online community survey, May–July 2019, UK. Sex Transm Infect 2021; 97 429–33.
| Factors associated with reporting antibiotic use as STI prophylaxis among HIV PrEP users: findings from a cross-sectional online community survey, May–July 2019, UK.Crossref | GoogleScholarGoogle Scholar |
[94] Carveth-Johnson T, Stingone C, Nwokolo N, Whitlock G. Doxycycline use in MSM taking PrEP. Lancet HIV 2018; 5 e482
| Doxycycline use in MSM taking PrEP.Crossref | GoogleScholarGoogle Scholar |
[95] Chow EPF, Fairley CK. Use of doxycycline prophylaxis among gay and bisexual men in Melbourne. Lancet HIV 2019; 6 e568–9.
| Use of doxycycline prophylaxis among gay and bisexual men in Melbourne.Crossref | GoogleScholarGoogle Scholar |
[96] Evers YJ, van Liere GAFS, Dukers-Muijrers NHTM, Hoebe CJPA. Use of doxycycline and other antibiotics to prevent STIs among men who have sex with men visiting sexual health clinics in the Netherlands. Sex Transm Infect 2020; 96 550–1.
| Use of doxycycline and other antibiotics to prevent STIs among men who have sex with men visiting sexual health clinics in the Netherlands.Crossref | GoogleScholarGoogle Scholar |
[97] Kohli M, Reid D, Pulford CV, Howarth A, Brown J, Mohammed H, et al. Choice of antibiotics for prophylaxis of bacterial STIs among individuals currently self-sourcing. Sex Transm Infect 2022; 98 158
| Choice of antibiotics for prophylaxis of bacterial STIs among individuals currently self-sourcing.Crossref | GoogleScholarGoogle Scholar |
[98] Saunders J, Kohli M, Medland N, Fifer H. Position statement on doxycycline as prophylaxis for sexually transmitted infections 2021 update. 2021. Available at https://wwwbashhguidelinesorg/media/1296/position_statement_refresh_2021_v11pdf
[99] Sumray K, Lloyd KC, Estcourt C, Burns F, Gibbs J. Scoping review of online postal sexually transmitted infection services: access, usage and clinical outcomes. medRxiv 2021; 2021.12.10.21267608
| Scoping review of online postal sexually transmitted infection services: access, usage and clinical outcomes.Crossref | GoogleScholarGoogle Scholar |
[100] Coleman BL, McNeil SA, Langley JM, Halperin SA, McGeer AJ. Differences in efficiency, satisfaction and adverse events between self-administered intradermal and nurse-administered intramuscular influenza vaccines in hospital workers. Vaccine 2015; 33 6635–40.
| Differences in efficiency, satisfaction and adverse events between self-administered intradermal and nurse-administered intramuscular influenza vaccines in hospital workers.Crossref | GoogleScholarGoogle Scholar |


