Would men who have sex with men support less frequent screening for asymptomatic chlamydia and gonorrhoea to improve antibiotic stewardship? A qualitative study
Amelia Margaret Wardley A * , Henrietta Williams B , Jacqueline Coombe
A * , Henrietta Williams B , Jacqueline Coombe  A , Cassandra Caddy A , Christopher Kincaid Fairley
A , Cassandra Caddy A , Christopher Kincaid Fairley  B and Jane Simone Hocking
B and Jane Simone Hocking  A
A
A Melbourne School of Population and Global Health, The University of Melbourne, Melbourne, Vic., Australia.
B Melbourne Sexual Health Centre, Melbourne, Vic., Australia.
Sexual Health 20(2) 148-157 https://doi.org/10.1071/SH22139
Submitted: 25 August 2022 Accepted: 20 February 2023 Published: 16 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Background: Men who have sex with men (MSM) taking pre-exposure prophylaxis (PrEP) are recommended to have screening for asymptomatic chlamydia and gonorrhoea every 3 months with high rates of asymptomatic chlamydia and gonorrhoea detected. However, there is little evidence about the effectiveness of this screening interval and there is increasing concern about antibiotic consumption and its impact on antimicrobial resistance. There have been calls to reconsider this frequent screening for chlamydia and gonorrhoea. We conducted interviews with MSM to assess their attitudes to 3-monthly chlamydia and gonorrhoea screening.
Methods: Individual semi-structured interviews were conducted with MSM living in Victoria, Australia. Participants were aged 20–62 years and had been taking PrEP for at least 6 months. Interviews were audio-recorded and transcribed, and these data were investigated through reflexive thematic analysis.
Results: Thirteen interviews were conducted in August 2021. Participants were hesitant about reducing the screening frequency and reported that testing gave them a sense of security. While MSM recognised antimicrobial resistance was a concern, it did not impact their sexual behaviour, with many participants stating they would rather continue to take antibiotics to treat infections rather than adopt preventative measures such as condom use. Positive attitudes towards screening interval changes are more likely when PrEP patients are informed about the risks and benefits of sexual healthcare recommendations.
Conclusion: While MSM on PrEP were initially hesitant to changes in screening frequency, changes may be acceptable if transparent communication, presenting the benefits and harms of screening and treatment, was delivered by a trusted healthcare professional.
Keywords: AMR, MSM, PrEP, qualitative, sexual behaviour, sexual health, STI, STI screening.
Introduction
Sexually transmitted infection (STI) rates among men who have sex with men (MSM) have increased markedly over the past 20 years, particularly in high income countries at a time when HIV rates have declined considerably.1–3 Several factors are thought to have contributed to the considerable rise in STIs among MSM over this time. For example, the widespread uptake of highly effective anti-retroviral treatments for HIV to reduce the risk of HIV acquisition, have contributed to a phenomenon known as ‘treatment optimism’.3–5 Treatment optimism has prompted changed sexual practices, including reduced condom use, due to the reduced fear towards HIV acquisition or transmission.3,6,7 There are concerns that similar risk compensation behaviours are occurring in pre-exposure prophylaxis (PrEP) users, such as increased partner change and decreased condom use, as recent reviews have established that use of PrEP is associated with increased diagnoses of STIs.8 Additionally, dense sexual networks among MSM on PrEP have facilitated the transmission of STIs and have densified even further in recent years due social factors such as the use of geospatial social networking applications (GSN apps) to find sex partners.9,10 MSM using apps such as ‘Grindr’ have been found to have higher numbers of sex partners and higher rates of gonorrhoea and chlamydia (but not HIV or syphilis).10–12
PrEP has been extraordinarily successful at reducing HIV incidence.3,13 However, it has had an impact on service provision with a dramatic increase in asymptomatic STI testing among those prescribed PrEP. In Australia, the UK and the USA, clinical guidelines require that those prescribed PrEP are screened every 3 months for chlamydia, gonorrhoea, syphilis, and HIV.14–17 For MSM, three infection site testing is conducted (oropharyngeal, urethral, and anorectal) for chlamydia and gonorrhoea.18,19 Despite strong evidence to support this frequency of regular screening for HIV and syphilis,20,21 there is minimal evidence to indicate a noticeable reduction in prevalence of gonorrhoea and chlamydia resulting from 3-monthly asymptomatic screening.15,16 This is attributed to the fact that the STI prevalence in MSM on PrEP is predominantly a result of increased sexual network connectivity and screening frequency does not impact sexual network density.15,16
Furthermore, unlike in women, the adverse health consequences of asymptomatic chlamydia and gonorrhoea infections in men are minimal as these infections naturally clear over time; within approximately 12 months for rectal infections, 3–5 months for urethral infection, and less than 3 months for oropharyngeal infection if left untreated.22,23 This is an important consideration for this research as the negligible health effects of asymptomatic chlamydia and gonorrhoea is only relevant for those with male biological sex characteristics excluding those who have sex with those who are biologically female.22 Further, there is little evidence that chlamydia and gonorrhoea in men on PrEP are associated with incidental HIV infection.19
It is important to note that 3-monthly screening for asymptomatic chlamydia and gonorrhoea may have some unintended negative consequences. The high incidence of asymptomatic gonorrhoea and chlamydia detected during the 3-monthly screens have led to dramatic increases in antibiotic prescribing raising concern about antimicrobial resistance (AMR).15 AMR has been identified as a global health issue.24,25 Macrolide resistance in Mycoplasma genitalium (MG) has occurred in response to the widespread prescription of azithromycin for asymptomatic chlamydia.26 Widespread use of azithromycin for asymptomatic chlamydia is also thought to have contributed to outbreaks of azithromycin-resistant syphilis in Australian communities and globally.26,27 Furthermore, sexual health services and laboratory testing services are strained, and 3-monthly testing increases their costs and already significant workload.15
Concerns about the harms of such regular STI screening have prompted calls by some to reconsider the frequency of asymptomatic screening for chlamydia and gonorrhoea among MSM.15,16,24,27–31 However, sexual public health campaigns in Australia targeted at MSM have predominantly focused on the importance of regular STI screening.32,33 As such, the receptivity of MSM to reduce STI testing frequency as a way of improving public sexual health may be a barrier to implementing changes in guidelines. Therefore, it is critical to investigate the attitudes of MSM and how they feel about the frequency of screening for asymptomatic chlamydia and gonorrhoea and whether they would support reducing the screening frequency. In this qualitative study, we interviewed MSM on PrEP to explore their views about the frequency of asymptomatic screening for chlamydia and gonorrhoea.
Materials and methods
Study design, participants, and recruitment
This was a qualitative study involving semi-structured interviews with MSM. Participants were invited for an interview if they were cis-gender men aged 18 years or older who exclusively had sex with cis-gender men. To be eligible, participants must have been on PrEP for at least 6 months to ensure they had experience with the routine screening process and needed to be proficient in English. Participants were recruited through electronic and paper-based advertising flyers and social media, including Twitter and Instagram. Advertising flyers were placed at sexual health clinics in Victoria. Recruitment was voluntary and self-selective. Potential participants were invited to contact the primary researcher (AW) by email and were sent a plain language statement via email with a description of the study being undertaken. We aimed to recruit 10–15 participants, a sample size consistent with our chosen methodology (semi-structured interviews and reflexive thematic analysis).34,35
Ethics approval was attained from the University of Melbourne Human Research Ethics Committee (Ethics ID: 2021-20798-18258-3.)
Data collection
Participants completed an individual semi-structured interview lasting between 45 min and 1 h, on Zoom or via telephone depending on their preference. All interviews were conducted by the primary researcher (AW). All participants gave informed verbal consent prior to participating in an interview and all interviews were recorded and transcribed.
As standard semi-structured interview methodology,34 interviews involved the same key open-ended questions and probes for every participant, with a slight variance in how and when they were delivered depending on the responses from participants. The interview structure involved asking participants about their perspectives on current screening guidelines and STIs generally, followed by asking their perspective on AMR and changing STI guidelines. Researchers anticipated that not every participant would know what AMR was or about the natural clearance of asymptomatic chlamydia and gonorrhoea. Consequently, the interview schedule provided general information to the participants about these topics, for example:
Asymptomatic chlamydia and gonorrhoea infections in men who have sex with men have no long-term adverse health outcomes and also many of these infections can clear themselves spontaneously without antibiotics – Interviewer
Antibiotic resistance means that bacteria become resistant to the antibiotic and is unable to be treated. – Interviewer
The interview questions explored participant’s lived experiences of attitudes and perceptions towards current STI screening guidelines, how they would feel living with an untreated asymptomatic STI, their attitudes towards antibiotic resistance and probes into their previous experiences of testing. For more information on the interview questions, see the interview schedule available online (Supplementary Material File S1).
Data analysis
Reflexive thematic analysis was used to analyse the data.36,37 Thematic analysis is an iterative process in which themes are generated from the empirical data, and reflexivity ensures the researchers influence on data interpretation is considered in analysis.35,37 As this study aimed to understand the attitudes towards STI screening, reflexive thematic analysis was considered a suitable method for undertaking the analysis. This analysis was facilitated using the qualitative data analysis software NVIVO.38
The reflexive thematic analysis occurred in the following five stages to establish common patterns, themes and sub-themes within the data that was collected.36 Stage one involved familiarisation with the data, including transcribing, reading, and re-reading, and noting down initial ideas. After the completion of each interview, the researcher also kept an analysis log to ensure any initial thoughts about the interview were captured and included. Stage two involved generated codes to systematically organise the data. Stage three involved searching for themes and then sorting all relevant data into each identified theme. Initial themes were then reviewed in consultation with the broader research team (HW, CC), and key themes were generated from the data (stages four and five).
A key conceptual framework to contextualise the attitudes of MSM, is their experience of sexual behaviour stigma particularly when accessing healthcare services.39 Sexual behaviour stigma can be understood as stigma anticipated, perceived, or experienced as a result of one’s sexual experience.40 Such stigma has been well documented to negatively influence sexual health outcomes of MSM. For example, discrimination from healthcare professionals towards MSM has been linked with increased fear and avoidance of health services and treatment.41 This framework is also helpful for understanding the complex experiences of marginalisation of PrEP users among the MSM community. When PrEP was first introduced, there was some stigma held by the broader MSM population that it was only used by those who engage in higher risk sexual behaviours, such as using PrEP as a substitute for condoms.42 However, targeted public health campaigns have fought to shift this stigma, which instead promote PrEP patients as empowered and informed.43,44 As such, in this study, we interpret and contextualise our findings with this stigma in mind, recognising its historical influence on health seeking behaviours among this population, and its potential to impact the current attitudes of MSM towards existing STI screening guidelines.
Results
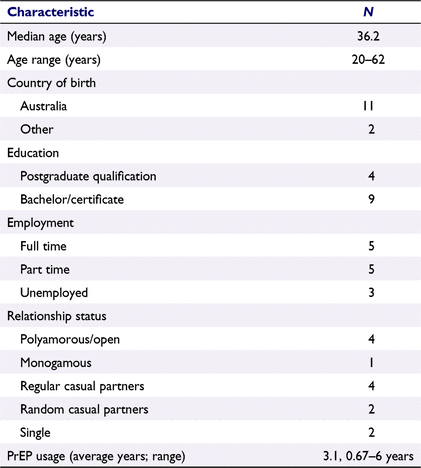
A total of 16 men responded to the advertisement and of these, 13 were eligible, consented to participate and were interviewed between August and September 2021. Table 1 describes the demographics of this sample of men. The average duration of PrEP usage for participants was 3.1 years.
|
We identified three key themes during analysis: (1) resistance to change; (2) perception of risk; and (3) the role of trust in sexual health care
Theme 1: Resistance to change
This theme demonstrates that for many participants, the current 3-monthly screening requirements were reassuring and perceived as necessary to ensure the health of not just themselves but the MSM community broadly. Participants consistently expressed how they would feel worried and concerned if they received less screening, and how being on PrEP has become synonymous with caring about your sexual health because of these screening requirements.
There was an overwhelmingly positive attitude towards the current 3-monthly STI screening requirements for asymptomatic chlamydia and gonorrhoea from participants who felt this requirement created feelings of safety and control. For example, when asked to describe how he feels about STI screening, one participant described his feelings of empowerment after being tested regularly:
Oh, like I said I feel less inhibited to express myself, I feel freer, I feel more confident I just feel much better about what I am doing and enjoying my time with the other guy. Reassuring and confidence are the two things that come to my mind. (Participant B, 56 years of age, regular and casual sexual partners, 1 year on PrEP)
Many participants articulated how the 3-monthly screening was less about their own personal health and more about feeling like they were protecting the sexual health of the wider MSM community. Participants identified how they felt the guidelines were important for ensuring their safety and confidence when engaging in sexual activity.
This was identified as particularly important as many participants thought 3-monthly testing was necessary due to high-risk sexual behaviours of the MSM community. For example, this participant states:
I’m very pro testing I think it is important especially in the gay community. Especially if you go on to sex on site premises, where there is a lot of random casual sex in groups, where the risk of transmission is quite high. Yeah, it is essential that men take responsibility of their sexual health and get regular testing. (Participant B, 56 years of age, regular and casual sexual partners, 1 year on PrEP)
Additionally, a recurring suggestion from participants was that they felt being on PrEP meant other MSM perceived you as responsible for your sexual health. This was articulated clearly by the following participant:
It means if I am on PrEP, I get tested every 3 months…which is a really good shorthand to show you are looking after your sexual health. (Participant L, 34 years of age, open relationship status, 3 years on PrEP)
Concurrently, the idea of a longer screening interval for asymptomatic chlamydia and gonorrhoea was initially received with concern and resistance from almost all participants. This hesitation was attributed to the fear it would increase the prevalence of chlamydia and gonorrhoea in the community and reduce the positive attitudes towards PrEP users and their sexual health. As this participant states:
I would…worry about the community…I would feel more anxious about sex in general…I think PrEP would lose some of its status as sort of a badge of someone who takes care of sexual health. (Participant H, 26 years of age, single, 18 months on PrEP)
Despite being informed that asymptomatic infections would not result in long term health problems for MSM, very few participants would be happy to allow asymptomatic infections to clear on their own. Many participants discussed the moral accountability they would feel passing on any kind of infection to a partner and the stigma around that. As stated by participant D:
I think I am less concerned about my own health in those circumstances I’m more concerned about passing it on and having someone else judge me for that. Or causing any kind of issue in someone else’s life, that’s more where the issue would come in with being asymptomatic. (Participant D, 26 years of age, monogamous, 18 months on PrEP)
Evidently, for this participant, and others in our study, knowingly passing on an infection to a sexual partner was a significant concern.
Theme 2: Perception of risk
This theme explored how participants’ perception of risk for different STIs impacted their understanding of treatment. This theme is critical to understand the attitudes towards STI screening requirements and explains the hesitancy participants feel towards less frequent screening.
An important observation identified was that the level of concern participants felt about certain STIs correlated strongly with the availability of treatment. This was evidenced by the response of participant M when asked if he thought the ease of treatment of gonorrhoea and chlamydia led to his feelings of indifference about contracting these STIs:
That is 100% right. You know that even…before I even had [gonorrhoea] I was sort like oh yeah you got gonorrhoea oh yeah cool. It’s like two or three pills or whatever it is…and a shot in the bum and you are good. You know it’s just a really easy fix! (Participant M, 32 years of age, single, 3 years on PrEP)
Increase in STIs since starting PrEP
A reoccurring sub-theme throughout the data was the significant increase in STIs rates participants reported after starting PrEP. For the following participant, this coincided with a reduction in his use of condoms since starting PrEP:
Before I went on PrEP, I would religiously use condoms, ALL the time EVERY time. So, for the first 20 years of my sexual life before PrEP came along, I would use condoms all the time. Interestingly in the 20 years I never actually got an STI at all. I never got an STI! In 20 years. As soon as I got onto PrEP, and I started not using condoms I found that I was getting STIs left right and centre. (Participant A, 46 years of age, regular and casual partners, 6 years on PrEP)
Many participants mentioned complacency driven by PrEP usage and was exacerbated by the neutrality felt towards STIs such as chlamydia and gonorrhoea:
Now I understand that condoms are much more important for the broader range of STIs. It doesn’t just stop HIV there are a lot of other things you can catch. BUT I would have to say within the community my feeling is that PrEP has reduced the use of condoms. Um… and given people a false sense of security. Yes, it has helped with HIV, but it doesn’t help obviously with the range of other STIs that you can catch. (Participant B, 56 years of age, regular and casual sexual partners, 1 year on PrEP)
The high prevalence of chlamydia and gonorrhoea seemed to reinforce the lack of concern towards contracting these bacterial STIs for our participants. As stated by the following participant when asked about whether he was worried about the spread of chlamydia and gonorrhoea in the MSM community:
In the community at the moment chlamydia and gonorrhoea … it’s really not much of a big deal anymore. I don’t want to say some people there’s a slight degree of complacency…not complacency it’s just not as much of a big deal… so people might not feel as much need to tell everyone because you assume the people you’re having sex with are also on PrEP and also being tested frequently. So, if you don’t tell them that you have something it’s at worst going to be 3 months till they get tested anyway. (Participant G, 28 years of age, regular and casual partners, 5 years on PrEP)
Strong desire to still have asymptomatic infections treated
Despite the recognition that asymptomatic chlamydia and gonorrhoea were not STIs that concerned them greatly and would not endanger their health, participants repeatedly voiced their desire to be treated with antibiotics for asymptomatic infections. This cognitive dissonance was articulated by participant D:
I actually didn’t know that asymptomatic cases can clear on their own. I had no idea, and hearing you say this now I sort of think we are so like scared! I wonder if it is the social stigma that makes us so scared of these things to try and get rid of them with any means necessary. (Participant D, 26 years of age, monogamous relationship, 18 months on PrEP)
Cognitive dissonance in this context refers to how the neutrality towards STIs such as chlamydia and gonorrhoea was dependent on the readily available treatment, yet the threat of AMR was not something that worried participants enough to change their desire for treatment or their sexual behaviour. As discussed by Participant G:
I know some guys who have MG (Mycoplasma genitalium) that is really hard to treat properly. And that’s not nice, I haven’t had any complications for me it’s easy for me to say no I am not worried about it. I am sure if something like that happened to me, I might change my mind. But no… I don’t worry about…there’s no change in my behaviour thinking about whether or not there are resistant STIs. (Participant G, 28 years of age, regular and casual partners, 5 years on PrEP)
Many participants stated their concern about antibiotic resistance, yet not to the point where they would happily forgo treatment of an asymptomatic STI to improve AMR overall. A repeated sentiment among participants was that they would feel obliged to abstain from sex during this recovery period and that was not a realistic option for them:
I think I would rather take the treatments again and again rather than not be treated. Then it’s sort of like well… for how long? I just wait until the infection clears up on its own? What if it doesn’t or lasts for a year. (Participant G, 28 years of age, regular and casual partners, 5 years on PrEP)
Knowing they could currently get treated quickly and efficiently for gonorrhoea and chlamydia, meant participants did not feel these bacterial infections were of great concern.
Theme 3: The role of trust in sexual healthcare
Considering the aforementioned themes and the clear resistance and distress at the thought of extending STI screening time frame for asymptomatic chlamydia and gonorrhoea, it became clear that trusting sexual healthcare providers had a key role to play in making these shifts more acceptable to the MSM population. Many participants expressed their previous experiences of stigma and harmful sexual healthcare experiences. The following participant explained how a negative experience at a specialised sexual healthcare service led him to change healthcare providers multiple times until he found one he trusted:
I didn’t feel like I had a relationship with the doctors there. I felt like I was being talked down to a little bit, you know it felt a little like ‘we the people and you the dirty masses. (Participant D, 26 years of age, monogamous relationship, 18 months on PrEP)
In multiple interviews participants mentioned misdiagnosis and a lack of knowledge around sexual health needs for MSM. For example, Participant L stated:
Yeah, there is a reason I don’t go to my GP! I have been misdiagnosed at least twice for a sexually related issue. By two separate GPs! I absolutely don’t trust non-specialist for that kind of thing anymore. (Participant L, 34 years of age, open relationship status, 3 years on PrEP)
Understanding MSM’s previous experiences of stigma and marginalisation from healthcare professionals is important to contextualise their receptivity towards new STI prevention strategies.
Several participants communicated their concern that reducing screening frequency would just be about health professionals saving money rather than improving health outcomes of MSM, as stated by Participant G:
I think they would have to have a pretty good rationale. If you’ve been telling people to get tested for years and years and that’s the public health messaging... I think you would also need to tell the gay community what the personal benefits are. Because if you are just saying its more cost effective to the government if gay men get 6-monthly tested instead of 3-monthly. I don’t think that would sit well. (Participant G, 28 years of age, regular and casual partners, 5 years on PrEP)
Additionally, some participants stated that they would feel overlooked by the healthcare system if this change in guideline were to occur, suggesting that they would feel as if healthcare professionals were more concerned with cutting costs than their health and wellbeing:
I would feel like they are just trying to save money. I’d feel neglected. (Participant C, 58 years, open relationship, 6 years on PrEP)
However, as the interviews progressed and it was clearly explained to the participants that changing guidelines may be better for AMR and this change would only happen if supported by specialised sexual healthcare professionals, their attitudes shifted. As stated by participant M:
I trust that the health advice is still there to keep us safe and keep us regularly tested and all of that. But there is something happening behind the scenes which I do not know but they are still doing all the great things! (Participant M, 33 years of age, single, 5 years)
Some participants articulated their comfortability with living with an asymptomatic STI without treating it by the end of the interview process:
There are negative consequences to the use of antibiotics. And you know I don’t want to be taking any medication I don’t need to take. I think overtime I would be quite comfortable, um… well, somewhat comfortable with not being treated even though I might have been positive but be asymptomatic, to see if it clears on its own. (Participant B, 56 years of age, regular casual sexual partners, 1 year on PrEP)
Our participants’ comments highlighted that initial hesitation and resistance to changing screening requirements is heavily dependent on how the public health messaging is delivered and the relationship the participants had with their health professional.
Discussion
The findings of this study suggest that while participants were hesitant about increasing time between screens for asymptomatic chlamydia and gonorrhoea, this hesitation could be overcome if recommendations were made by trusted sexual health services and explained properly to patients. MSM on PrEP strongly support 3-monthly screening requirements and consider them to be a crucial part of maintaining the sexual health of not just themselves but the wider MSM community. The observed resistance to changing guidelines was deeply influenced by their perception of bacterial STIs such as chlamydia and gonorrhoea being easily treatable. While MSM acknowledged AMR was a concern, it did not impact their sexual risk-taking behaviour. Ensuring any potential future change in screening intervals is delivered with informed care from trusted sexual healthcare professionals, would be critical to ensuring acceptability and adherence to these changes.
A key concern of participants was that the prevalence of chlamydia and gonorrhoea would increase in MSM communities if the screening frequency was decreased. Participants strongly associated more frequent testing with better sexual health outcomes. This is unsurprising as public health campaigns in Australia have long campaigned for increasing STI testing among those most at risk.42 Therefore, investigating the receptivity of MSM to reducing STI testing frequency as a way of improving public sexual health is an important aspect of our study and reveals how less testing may be met with resistance from MSM. Given the increasing concern about AMR and its implications beyond simply treating STIs, any changes to public health campaigns must be considered in consultation with MSM. Our results add to a growing body of literature that supports antibiotic stewardship and the implementation of strict evidence-based practices that are relevant to specific populations such as MSM. This could indicate a historic shift in sexual health promotion where the impact of AMR is recognised and ‘one size fits all’ approaches to STI treatment are disbanded.
Despite being informed about the negligible health impacts of infection with asymptomatic chlamydia and gonorrhoea for MSM, participants were strongly opposed to letting these infections clear on their own. A unique finding from the study was to investigate how participants would feel living with an asymptomatic infection that was not causing long-term health problems. This has not been explored in previous literature and provides insight into the acceptability of living with an asymptomatic STI. Participants repeatedly discussed the moral concern of passing an asymptomatic infection on to a sexual partner. While this resistance and concern is understandable, particularly when applying the conceptual framework of stigma towards MSM sexual behaviour, it is important that MSM are informed of how the benefits of screening should outweigh the harms and are equipped with accurate information regarding asymptomatic STIs.30 These results suggest that if the screening frequency was to reduce, health professionals would need to clearly explain to MSM that 3-monthly intensive screening for gonorrhoea and chlamydia is not necessarily associated with reduced prevalence of these bacterial infections and asymptomatic infections are not causing any negative impact on their health.16,29 As previous literature suggests, this is likely due to the fact that while 3-monthly screening may reduce prevalence/transmission in the short term, it is not addressing key drivers such as changing sexual practices such as decreased condom usage and dense sexual networks that are maintaining STIs at their endemic state in the MSM community.24,43 Our participants suggested that acceptability of a change in screening frequency is possible with transparent communication delivered by a trusted healthcare professional.
Participants did not perceive asymptomatic chlamydia and gonorrhoea as a major threat to their health and this influenced their sexual behaviour. Results from this study supported findings of earlier research that PrEP can lead to changed sexual behaviour among MSM45 such as increased partner numbers.36,43 Similar to previous findings, participants of this study frequently reported an increase in STI diagnosis since starting PrEP.8 While attitudes to STI prevention vary greatly among the MSM population, the majority participants in this study stated they were very cautious with condom usage prior to starting PrEP and then stopped using condoms altogether once they were taking PrEP. The implications of these findings for health promotion are significant, as they indicate more should be done by health professionals to educate MSM on STI prevention strategies that should be used in conjunction with PrEP.
Furthermore, the observed normalisation of being infected with gonorrhoea and chlamydia was often attributed to being the ‘price of admission’ of being a sexually active gay man and that gonorrhoea and chlamydia are perceived as ‘easier to treat than the common cold’ (Participant H). Participants neutrality towards contracting bacterial STIs such as gonorrhoea and chlamydia, was linked to the availability of treatment; an important consideration due to the impending threat of AMR. Literature has demonstrated comfort with chlamydia and gonorrhoea is attributed to their curability.45,46 Getting tested and treated for curable STIs is of minimal inconvenience to PrEP users.46 The effectiveness of PrEP at preventing HIV, has reduced the apparent need for condoms and other preventative behaviours meaning that incidence rates of gonorrhoea and chlamydia in the MSM population are maintained.14 These attitudes have substantial implications for health promotion in the sexual health field. The fact that participants were not overly concerned about the threat of AMR, revealed a cognitive dissonance as their neutrality towards gonorrhoea and chlamydia was contingent on being able to treat these bacterial STIs easily with available antibiotics. Cognitive dissonance in this context refers to how participants were not worried about chlamydia and gonorrhoea infections because of the effect treatment available, yet would still want to treat asymptomatic infections and not preserve the use of antibiotics for when they were needed to ensure their efficacy was maintained into the future. Exploring alternative initiatives to tackle AMR is critical as the World Health Organization (WHO) has recognised that the threat of AMR is of global concern25 and gonorrhoea is becoming increasingly hard to treat. Research has shown, that intensive screening and treatment is not reducing STI prevalence in this population as intended.25 Instead, the high prevalence of STIs in the MSM community is likely linked to sexual network connectivity.24,25 Intensive screening in MSM on PrEP has resulted in very high consumption levels of azithromycin and ceftriaxone.15 Both are broad-spectrum antibiotics that can also cause resistance in other non-STI organisms and, according to the WHO, should be reserved for infections where there is clear evidence of benefit, and no alternatives exist.44 It is important that healthcare providers understand the importance of AMR and are able inform MSM of the risk of AMR, and how reducing screening frequency could lead to less consumption of antibiotics.
However, addressing risk compensation behaviours of MSM must be balanced with the sexual empowerment that PrEP offers the MSM community. Risk compensation refers to the theory that MSM on PrEP may compensate for the protection afforded against HIV by having more condomless sex or increasing their number of sexual partners.8,45 Previous findings have established that experiences of sexual pleasure and intimacy are enhanced for PrEP users because it reduces the physical and emotional barriers that always using a condom and the risk of HIV poses, a particularly important consideration for a population that has been so stigmatised for their sexual behaviours.47 These results were supported by our findings, which found that participants framed PrEP users as individuals who care about their sexual health. This is particularly significant as it represents a shift from PrEP users being stigmatised for their sexual behaviour as being irresponsible,42 rather they are stepping forward and embracing demanding STI testing guidelines.
STIs are a key public health concern and condoms continue to be the most effective prevention method; however, public health messaging should take into account the reality of sexual behaviours in the MSM community and create health promotion strategies that are likely to be followed.46 Attitudes towards STIs vary significantly in the MSM population,43 and as such adherence to different STI prevention strategies varies greatly as well. For example, condom use for sexual activities such as oral sex, while important to curb transmission of bacterial STIs, is a practice that is rarely enacted.48
Strengths and limitations
Our findings should be considered within their limitations. Recruitment was limited to social media services and sexual health clinics that were accessible to the research team. As such, it may have narrowed the population down to participants who had access to technology, and more affiliated with university networks. The results of this study cannot be generalised to other populations as only MSM who lived in Melbourne were recruited; future research may wish to explore the perspectives of MSM living in different areas of Australia. In addition, further research should be conducted to investigate the attitudes of MSM towards current STI screening guidelines, and why there may be hesitation towards extending screening to longer time frames. The perspectives of health professionals and key informants to provide more robust evidence about the impact of changing guidelines should also be explored.
Our study also has strengths. We were unable to identify any comparable studies that have given insight into the attitudes of MSM on PrEP towards STI screening guidelines and AMR. This study suggests stigma still experienced by this population would impact on the acceptability and feasibility of any future changes to STI screening frequency. Ensuring that medical practices are not just evidence based but are supported by their target population is essential to their success. As such, this study provides nuanced insight into the lived experiences that will help inform future research particularly regarding the acceptability STI screening guidelines and understanding attitudes towards AMR.
Conclusion
While some are calling for reduced asymptomatic chlamydia and gonorrhoea screening among MSM to reduce AMR, any changes should be informed by the beliefs and lived experience of those who will be impacted. Considering the increased recognition that intensive STI screening/treatment is not reducing STI incidence in MSM using PrEP as previously expected, current STI testing recommendations for asymptomatic gonorrhoea and chlamydia should be re-examined. However, increasing the screening frequency from every 3 months to a longer time interval will only be an effective intervention if the MSM population on PrEP adequately understand why this change is occurring and are provided ongoing and supportive sexual health care from a physician they trust. Positive attitudes towards screening changes are more likely when PrEP patients are informed about the risks and benefits of recommendations for sexual health care, and as such are more likely to adhere to them.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study cannot be publicly shared due to ethical or privacy reasons; however, it may be shared upon reasonable request to Ms Amelia Wardley (amelia.wardley@unimelb.edu.au) if appropriate.
Conflicts of interest
Professor Christopher Fairley and Professor Jane Hocking are editors of Sexual Health, but were blinded from the peer review process for this paper. The authors declare no other conflicts of interest.
Declarations of funding
Jane Hocking was supported by a NHMRC Senior Research Fellowship (1136117). No other funds supported this research.
References
[1] Centers for Disease Control and Prevention (CDCP). Sexually transmitted disease surveillance 2019. U.S. Department of Health and Human Services; 2019.[2] Jasek E, Chow EPF, Ong JJ, Bradshaw CS, Chen MY, Hocking JS, et al. Sexually Transmitted infections in Melbourne, Australia from 1918 to 2016: nearly a century of data. Commun Dis Intell Q Rep 2017; 41 E212–E22.
[3] Ramchandani MS, Golden MR. Confronting rising STIs in the era of PrEP and treatment as prevention. Curr HIV/AIDS Rep 2019; 16 244–56.
| Confronting rising STIs in the era of PrEP and treatment as prevention.Crossref | GoogleScholarGoogle Scholar |
[4] Brennan DJ, Welles SL, Miner MH, Ross MW, Rosser BRS, The Positive Connections Team HIV treatment optimism and unsafe anal intercourse among HIV-positive men who have sex with men: findings from the positive connections study. AIDS Educ Prev 2010; 22 126–37.
| HIV treatment optimism and unsafe anal intercourse among HIV-positive men who have sex with men: findings from the positive connections study.Crossref | GoogleScholarGoogle Scholar |
[5] Sullivan PS, Drake AJ, Sanchez TH. Prevalence of treatment optimism-related risk behavior and associated factors among men who have sex with men in 11 states, 2000–2001. AIDS Behav 2007; 11 123–9.
| Prevalence of treatment optimism-related risk behavior and associated factors among men who have sex with men in 11 states, 2000–2001.Crossref | GoogleScholarGoogle Scholar |
[6] Brooks RA, Landovitz RJ, Kaplan RL, Lieber E, Lee S-J, Barkley TW. Sexual risk behaviors and acceptability of HIV pre-exposure prophylaxis among HIV-negative gay and bisexual men in serodiscordant relationships: a mixed methods study. AIDS Patient Care STDs 2012; 26 87–94.
| Sexual risk behaviors and acceptability of HIV pre-exposure prophylaxis among HIV-negative gay and bisexual men in serodiscordant relationships: a mixed methods study.Crossref | GoogleScholarGoogle Scholar |
[7] Golub SA, Kowalczyk W, Weinberger CL, Parsons JT. Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men. J Acquir Immune Defic Syndr 2010; 54 548–55.
| Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[8] Traeger MW, Schroeder SE, Wright EJ, Hellard ME, Cornelisse VJ, Doyle JS, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis 2018; 67 676–86.
| Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[9] Stenger MR, Pathela P, Anschuetz G, Bauer H, Simon J, Kohn R, et al. Increases in the rate of Neisseria gonorrhoeae among gay, bisexual and other men who have sex with men – findings from the sexually transmitted disease surveillance network 2010–2015. Sex Transm Dis 2017; 44 393–7.
| Increases in the rate of Neisseria gonorrhoeae among gay, bisexual and other men who have sex with men – findings from the sexually transmitted disease surveillance network 2010–2015.Crossref | GoogleScholarGoogle Scholar |
[10] Lehmiller JJ, Ioerger M. Social networking smartphone applications and sexual health outcomes among men who have sex with men. PLoS ONE 2014; 9 e86603
| Social networking smartphone applications and sexual health outcomes among men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[11] Phillips G, Magnus M, Kuo I, Rawls A, Peterson J, Jia Y, et al. Use of geosocial networking (GSN) mobile phone applications to find men for sex by men who have sex with men (MSM) in Washington, DC. AIDS Behav 2014; 18 1630–7.
| Use of geosocial networking (GSN) mobile phone applications to find men for sex by men who have sex with men (MSM) in Washington, DC.Crossref | GoogleScholarGoogle Scholar |
[12] Zou H, Fan S. Characteristics of men who have sex with men who use smartphone geosocial networking applications and implications for HIV interventions: a systematic review and meta-analysis. Arch Sex Behav 2017; 46 885–94.
| Characteristics of men who have sex with men who use smartphone geosocial networking applications and implications for HIV interventions: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[13] Schueler K, Ferreira M, Nikolopoulos G, Skaathun B, Paraskevis D, Hatzakis A, et al. Pre-exposure prophylaxis (PrEP) awareness and use within high HIV transmission networks. AIDS Behav 2019; 23 1893–903.
| Pre-exposure prophylaxis (PrEP) awareness and use within high HIV transmission networks.Crossref | GoogleScholarGoogle Scholar |
[14] Brady M, Rodger A, Asboe D, Cambiano V, Clutterbuck D, Desai M, et al. BHIVA/BASHH guidelines on the use of HIV pre-exposure prophylaxis (PrEP) 2018. HIV Med 2019; 20 s2–s80.
| BHIVA/BASHH guidelines on the use of HIV pre-exposure prophylaxis (PrEP) 2018.Crossref | GoogleScholarGoogle Scholar |
[15] Kenyon C. We need to consider collateral damage to resistomes when we decide how frequently to screen for chlamydia/gonorrhoea in preexposure prophylaxis cohorts. AIDS 2019; 33 155–7.
| We need to consider collateral damage to resistomes when we decide how frequently to screen for chlamydia/gonorrhoea in preexposure prophylaxis cohorts.Crossref | GoogleScholarGoogle Scholar |
[16] Kenyon C. Screening is not associated with reduced incidence of gonorrhoea or chlamydia in men who have sex with men (MSM); an ecological study of 23 European countries. F1000Res 2019; 8 160
| Screening is not associated with reduced incidence of gonorrhoea or chlamydia in men who have sex with men (MSM); an ecological study of 23 European countries.Crossref | GoogleScholarGoogle Scholar |
[17] US Preventive Services Task Force Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Preexposure prophylaxis for the prevention of HIV infection: US preventive services task force recommendation statement. JAMA 2019; 321 2203–13.
| Preexposure prophylaxis for the prevention of HIV infection: US preventive services task force recommendation statement.Crossref | GoogleScholarGoogle Scholar |
[18] Tang EC, Vittinghoff E, Philip SS, Doblecki-Lewis S, Bacon O, Chege W, et al. Quarterly screening optimizes detection of sexually transmitted infections when prescribing HIV preexposure prophylaxis. AIDS 2020; 34 1181–6.
| Quarterly screening optimizes detection of sexually transmitted infections when prescribing HIV preexposure prophylaxis.Crossref | GoogleScholarGoogle Scholar |
[19] McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387 53–60.
| Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial.Crossref | GoogleScholarGoogle Scholar |
[20] Gross G, Flaig B, Rode S. Syphilis. Part 2: laboratory diagnostics, therapy and prevention. Hautarzt 2013; 64 851–63.
| Syphilis. Part 2: laboratory diagnostics, therapy and prevention.Crossref | GoogleScholarGoogle Scholar |
[21] Lucas S, Nelson AM. HIV and the spectrum of human disease. J Pathol 2015; 235 229–41.
| HIV and the spectrum of human disease.Crossref | GoogleScholarGoogle Scholar |
[22] Chow EPF, Vodstrcil LA, Williamson DA, Maddaford K, Hocking JS, Ashcroft M, et al. Incidence and duration of incident oropharyngeal gonorrhoea and chlamydia infections among men who have sex with men: prospective cohort study. Sex Transm Infect 2021; 97 452–8.
| Incidence and duration of incident oropharyngeal gonorrhoea and chlamydia infections among men who have sex with men: prospective cohort study.Crossref | GoogleScholarGoogle Scholar |
[23] Chow EPF, Camilleri S, Ward C, Huffam S, Chen MY, Bradshaw CS, et al. Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: a systematic review. Sex Health 2016; 13 199–204.
| Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[24] Kenyon C, Vanbaelen T, Van Dijck C. Recent insights suggest the need for the STI field to embrace a more eco-social conceptual framework: a viewpoint. Int J STD AIDS 2022; 33 404–15.
| Recent insights suggest the need for the STI field to embrace a more eco-social conceptual framework: a viewpoint.Crossref | GoogleScholarGoogle Scholar |
[25] Lahra MM, Dillon J-AR, George CRR, Lewis DA, Wi TE, Whiley DM. From zero to zero in 100 years: gonococcal antimicrobial resistance. Microbiol Aust 2016; 37 173–6.
| From zero to zero in 100 years: gonococcal antimicrobial resistance.Crossref | GoogleScholarGoogle Scholar |
[26] Murray GL, Bradshaw CS, Bissessor M, Danielewski J, Garland SM, Jensen JS, et al. Increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium. Emerg Infect Dis 2017; 23 809–12.
| Increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium.Crossref | GoogleScholarGoogle Scholar |
[27] Kenyon C, Manoharan-Basil SS, Van Dijck C. Is there a resistance threshold for macrolide consumption? positive evidence from an ecological analysis of resistance data from Streptococcus pneumoniae, Treponema pallidum, and Mycoplasma genitalium. Microb Drug Resist 2021; 27 1079–86.
| Is there a resistance threshold for macrolide consumption? positive evidence from an ecological analysis of resistance data from Streptococcus pneumoniae, Treponema pallidum, and Mycoplasma genitalium.Crossref | GoogleScholarGoogle Scholar |
[28] Baker A, Fleury C, Clarke E, Foley E, Samraj S, Rowen D, et al. Increasing screening frequency in men who have sex with men: impact of guidance on risk profiling on workload and earlier diagnosis of sexually transmitted infection and HIV. Int J STD AIDS 2013; 24 613–7.
| Increasing screening frequency in men who have sex with men: impact of guidance on risk profiling on workload and earlier diagnosis of sexually transmitted infection and HIV.Crossref | GoogleScholarGoogle Scholar |
[29] Kenyon C. Risks of antimicrobial resistance in N. gonorrhoeae associated with intensive screening programs in pre-exposure prophylaxis programs. Clin Infect Dis 2018; 67 154–5.
| Risks of antimicrobial resistance in N. gonorrhoeae associated with intensive screening programs in pre-exposure prophylaxis programs.Crossref | GoogleScholarGoogle Scholar |
[30] Tsoumanis A, Hens N, Kenyon CR. Is screening for chlamydia and gonorrhea in men who have sex with men associated with reduction of the prevalence of these infections? A systematic review of observational studies. Sex Transm Dis 2018; 45 615–22.
| Is screening for chlamydia and gonorrhea in men who have sex with men associated with reduction of the prevalence of these infections? A systematic review of observational studies.Crossref | GoogleScholarGoogle Scholar |
[31] Yeung A, Temple-Smith M, Fairley C, Hocking J. Narrative review of the barriers and facilitators to chlamydia testing in general practice. Aust J Prim Health 2015; 21 139–47.
| Narrative review of the barriers and facilitators to chlamydia testing in general practice.Crossref | GoogleScholarGoogle Scholar |
[32] Vella A, Wilkinson A, Pedrana A, Stoové M. Outcome evaluation of HIV prevention initiatives 2012–2013 in men who have sex with men in Victoria. p. 50. Melbourne: Burnet Institute; 2014.
[33] Lee E, Mao L, von Doussa H, Batrouney C, West M, Prestage G, et al. Gay community periodic survey: Melbourne 2014. Sydney: Centre for Social Research in Health, Victorian AIDS Council/Gay Men’s Health Centre, Department of Health Victoira, The Kirby Institute; 2014.
[34] Galletta A, Cross WE Jr. Mastering the semi-structured interview and beyond: from research design to analysis and publication. New York University Press; 2013.
[35] Serry T, Liamputtong P. The in-depth interviewing method in health. In: Liamputtong P, editor. Research methods in health: foundations for evidence-based practice. Oxford University Press; 2013. pp. 39–53.
[36] Braun V, Clarke V, Hayfield N, Terry G. Thematic analysis. In: Liamputtong P, editor. Handbook of research methods in health social sciences. Springer; 2019. pp. 843–860.
[37] Braun V, Clarke V. One size fits all? What counts as quality practice in (reflexive) thematic analysis? Qual Res Psychol 2021; 18 328–52.
| One size fits all? What counts as quality practice in (reflexive) thematic analysis?Crossref | GoogleScholarGoogle Scholar |
[38] QSR International Pty Ltd. NVivo (released in March 2020). 2020. Available at https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home
[39] Brown G, Leonard W, Lyons A, Power J, Sander D, McColl W, et al. Stigma, gay men and biomedical prevention: the challenges and opportunities of a rapidly changing HIV prevention landscape. Sex Health 2017; 14 111–8.
| Stigma, gay men and biomedical prevention: the challenges and opportunities of a rapidly changing HIV prevention landscape.Crossref | GoogleScholarGoogle Scholar |
[40] Brown L, Macintyre K, Trujillo L. Interventions to reduce HIV/AIDS stigma: what have we learned? AIDS Educ Prev 2003; 15 49–69.
| Interventions to reduce HIV/AIDS stigma: what have we learned?Crossref | GoogleScholarGoogle Scholar |
[41] Nyblade LC. Measuring HIV stigma: existing knowledge and gaps. Psychol Health Med 2006; 11 335–45.
| Measuring HIV stigma: existing knowledge and gaps.Crossref | GoogleScholarGoogle Scholar |
[42] Dunn M, Barnett A, McKay FH. Pre-exposure prophylaxis (PrEP) in Australia: are there challenges facing sexual health promotion? Health Promot Int 2022; 37 daab177
| Pre-exposure prophylaxis (PrEP) in Australia: are there challenges facing sexual health promotion?Crossref | GoogleScholarGoogle Scholar |
[43] Holt M, Lea T, Bear B, Halliday D, Ellard J, Murphy D, et al. Trends in attitudes to and the use of HIV pre-exposure prophylaxis by Australian gay and bisexual men, 2011–2017: implications for further implementation from a diffusion of innovations perspective. AIDS Behav 2019; 23 1939–50.
| Trends in attitudes to and the use of HIV pre-exposure prophylaxis by Australian gay and bisexual men, 2011–2017: implications for further implementation from a diffusion of innovations perspective.Crossref | GoogleScholarGoogle Scholar |
[44] Kirby Institute. Monitoring HIV pre exposure prophylaxis uptake in Australia: PBS-subsidised HIV Preexposure Prophylaxis from April 2018 to June 2020. Kirby Institute; 2020.
[45] Kumar S, Haderxhanaj LT, Spicknall IH. Reviewing PrEP’s effect on STI incidence among men who have sex with men – balancing increased STI screening and potential behavioral sexual risk compensation. AIDS Behav 2021; 25 1810–8.
| Reviewing PrEP’s effect on STI incidence among men who have sex with men – balancing increased STI screening and potential behavioral sexual risk compensation.Crossref | GoogleScholarGoogle Scholar |
[46] Sarno EL, Macapagal K, Newcomb ME. “The main concern is HIV, everything else is fixable”: indifference toward sexually transmitted infections in the era of biomedical HIV prevention. AIDS Behav 2021; 25 2657–60.
| “The main concern is HIV, everything else is fixable”: indifference toward sexually transmitted infections in the era of biomedical HIV prevention.Crossref | GoogleScholarGoogle Scholar |
[47] Mabire X, Puppo C, Morel S, Mora M, Rojas Castro D, Chas J, et al. Pleasure and PrEP: pleasure-seeking plays a role in prevention choices and could lead to PrEP initiation. Am J Mens Health 2019; 13 1557988319827396
| Pleasure and PrEP: pleasure-seeking plays a role in prevention choices and could lead to PrEP initiation.Crossref | GoogleScholarGoogle Scholar |
[48] Hui B, Fairley CK, Chen M, Grulich A, Hocking J, Prestage G, et al. Oral and anal sex are key to sustaining gonorrhoea at endemic levels in MSM populations: a mathematical model. Sex Transm Infect 2015; 91 365–9.
| Oral and anal sex are key to sustaining gonorrhoea at endemic levels in MSM populations: a mathematical model.Crossref | GoogleScholarGoogle Scholar |


