Sexual mixing patterns in men who have sex with men: network approaches for smart resource allocation
M. Kumi Smith A * , Matthew Graham B , Katherine Harripersaud A , Qiuying Zhu C , Guanghua Lan C , Zhiyong Shen C and Shuai Tang C *
A * , Matthew Graham B , Katherine Harripersaud A , Qiuying Zhu C , Guanghua Lan C , Zhiyong Shen C and Shuai Tang C *
A Division of Epidemiology & Community Health, School of Public Health, University of Minnesota Twin Cities, Minneapolis, MN, USA.
B Li Ka Shing Centre for Health Information and Discovery, Oxford, UK.
C Guangxi Center for Disease Control and Prevention, Nanning, China.
Sexual Health 20(2) 126-133 https://doi.org/10.1071/SH22163
Submitted: 29 September 2022 Accepted: 8 February 2023 Published: 27 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Background: Age-based sexual mixing patterns in men who have sex with men (MSM) can greatly inform strategic allocation of intervention resources to subsets of the population for the purpose of preventing the greatest number of new HIV infections.
Methods: Egocentric network data collected from MSM participating in annual HIV sentinel surveillance surveys were used to assess age-dependent mixing and to explore its epidemiological implications on the risk of HIV transmission risk (among those HIV-infected) and HIV acquisition risk (among those not infected).
Results: Mixing in this sample of 1605 Chinese MSM is relatively age assortative (the average of values expressing the degree of preferential mixing were 2.01 in diagonal cells vs 0.87 in off-diagonal cells). Expected numbers of HIV acquisition were highest in the 20–24 years age group; those for HIV transmissions were highest among 25–29 year olds. The risk of both acquisition and transmission was highest in age groups that immediately follow the most commonly reported ages of sexual debut in this population (i.e. age 20).
Conclusions: These findings suggest that combination prevention resources should be targeted at younger MSM who are at higher risk of both transmission and acquisition. Programs may also do well to target even younger age groups who have not yet debuted in order to establish prevention effects before risky sexual behaviours begin. More research on optimal strategies to access these harder-to-reach subsets of the MSM population is needed. Findings also support ongoing efforts for public health practitioners to collect network data in key populations to support more empirically driven strategies to target prevention resources.
Keywords: age mixing, combination prevention, contact matrices, HIV, men who have sex with men (MSM), network analysis, resource allocation, sexual mixing.
Introduction
A decade has passed since the results of two groundbreaking trial demonstrated the efficacy of antiretroviral drugs in reducing the risk of sexual HIV transmission (‘treatment as prevention’ (TasP)1) and acquisition (pre-exposure prophylaxis (PrEP)2). Yet recent epidemiological evidence suggests we have made little progress globally towards the UNAIDS 2021 goal of ‘ending AIDS.’3 Global recommendations now emphasise the delivery of biomedical interventions, such as TasP and PrEP, as part of ‘combination strategies’ designed to address key implementation challenges including long-term patient management, demanding drug adherence regimens, or the need for vast financial resources.4–8 These challenges have raised important questions about the feasibility of timely scale-up for larger populations at risk, particularly in light of added demands that combination interventions be locally tailored, culturally appropriate, and operate within budgetary confines.
These and other debates have sparked calls to target program resources for high-priority groups, including men who have sex with men (MSM). Existing strategies to target interventions, such as TasP and PrEP, include methods such as clinical risk calculators, mathematical models, and transmission networks constructed using phylogenetic data. Although promising, these tools are not without their limitations. Risk score calculators, for example, can produce problematic results when applied to populations different from those to which the original models were calibrated, as when several HIV risk calculators originally developed using data from predominantly white MSM in the northern US9–11 were applied to a cohort of black MSM in the south.12 Similarly, the utility of findings from mathematical models for a particular setting is often constrained by the availability of reliable data to inform model parameterisation. Finally, the use of HIV transmission networks to guide prevention activities requires robust HIV testing programs and laboratory capacity to conduct regular genetic sequencing, a cost prohibitive strategy in many resource constrained settings.
Here, we present an analytical approach to identify subgroups of MSM to guide efficient allocation of prevention resources. Data come from annual HIV sentinel surveillance procedures among MSM in Guangxi Province, a high-transmission region in southern China. We use egocentric network data to characterise sexual mixing patterns, which is then combined with age-specific HIV prevalence to identify subgroups with the greatest risk to transmit HIV (if already infected) or acquire it (if still uninfected). Our analysis is conducted using egocentric network data, a relatively accessible form of data to collect using traditional survey approaches – as compared with full network mapping – which makes this approach replicable across different settings and key populations.
Methods
Data were collected as part of annual HIV sentinel surveillance work conducted by the HIV/AIDS Division of the Guangxi Center for Disease Control and Prevention. Every year, provincial health authorities partner with community-based LGBT organisations local to 10 sites across the province to recruit, survey, and test eligible MSM for HIV and syphilis. Recruitment for the survey data used in this analysis took place in 2019, and was conducted both online (via Wechat and QQ) and through in-person outreach at popular cruising sites including bars, karaoke halls, saunas, massage parlours, parks, and public restrooms. Participants were also invited to personally refer eligible contacts to take part in the study. Eligible participants were born male and aged at least 16 years (the legal age of consent in China) who reported at least one sexual encounter with another male in the last year and who were willing to provide informed consent. Paper surveys were administered through face-to-face interviews by trained staff, data from which were double entered into the DataFax system (Clinical DataFax Systems, Hamilton, Ontario, Canada). Blood samples were collected from all participants for serological HIV and syphilis testing. HIV screening was conducted with enzyme-linked immunoassay (Beijing Wantai Biological, Beijing, China), and positive test results were confirmed by a second enzyme-linked immunoassay test from a different manufacturer (Intec Technology, Xiamen, Fujian, China). Participants testing positive for HIV were referred to their local county disease control centre for counselling, confirmatory testing and treatment eligibility screening (confirmed HIV patients are eligible for free antiretroviral therapy through the Chinese National Free Treatment Program13). Participants were compensated 50 RMB (approximately $US8) for their time.
Egocentric network data were collected using a name-generating method in which each participant is asked to enumerate a list of up to their three most recent male anal sex partners. Follow-up questions are then asked regarding each partner, as informed by the participant’s best guess, including age, education level (did or did not complete high school) and residency status (rural versus urban). Due to challenges with timely staff training and survey implementation, network-related survey questions were not administered in three of the 10 sites, and unevenly in the remaining seven sites (Fig. 1). Because survey eligibility criteria stipulated sex with another man in the past year, participants who did not list a single partner were assumed to have not been administered this set of questions, or to have refused or skipped them. Given the high rates of missingness, we restricted our primary analysis to the subset of respondents who listed at least one partner.
|
|
Network data were used to construct mixing matrices that describe contact patterns between participants and up to three most recent male anal sex partners in 5-year age groupings. This was conducted by estimating a value for each cell of a nine-by-nine mixing matrix (from age >20 years to age ≥54 years) representing the ratio of observed contacts between a particular age group combination and the expected number of contacts between those two age groups under the assumption of proportional (i.e. random) mixing. The observed intensity of contacts was estimated directly from the data by summing the total number of partnerships in each 5-year age group combination. The same value for expected frequencies under the assumption of proportional mixing were calculated as follows:
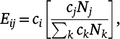
where ci and cj represent the average number of contacts among respondents in age group i and j, respectively, and Nj and Nk are the total sizes of age group j and the entire population, respectively. Confidence intervals (CI) of estimated ratio values were bootstrapped by resampling partnerships over 1000 iterations. The resulting values ranged from 0 to infinity, with values <1.0 indicating less contact between two age groups than would be expected under proportional mixing, and values >1.0 indicating more contact than expected. Assortativity, or the extent to which individuals mix with partners similar to themselves, was assessed for age-based mixing by measuring the diagonality of the mixing matrix; that is, the relative amount of contact occurring among partners of the same or similar age groups as compared with contact among partners of different age groups.
We then combined age-based mixing patterns and age-specific HIV prevalence rates estimated from our data together with literature-derived estimates of per-partner probability of HIV transmission through anal intercourse.14 This information was then used to generate a second matrix in which each cell expressed the expected number of new HIV infections resulting from contact between a given age group combination. These expected values were summed across columns to estimate the expected number of new HIV infections attributed to sexual contact with members of that age group, whereas sums across rows were used to estimate the expected number of HIV infections that were newly acquired in each age group. All statistical analyses were performed using R studio (v1.1.456; www.cran.org).
Study procedures were conducted in accordance with the STROBE guidelines for observational studies. All participants provided written informed consent for analysis of data they provided in the study surveys, and the original study was approved by the institutional review board of the Guangxi Centers for Disease Control; for this analysis, the institutional review board of the University of Minnesota determined that this secondary analysis of deidentified data was not considered human subject research.
Results
Respondent and network characteristics
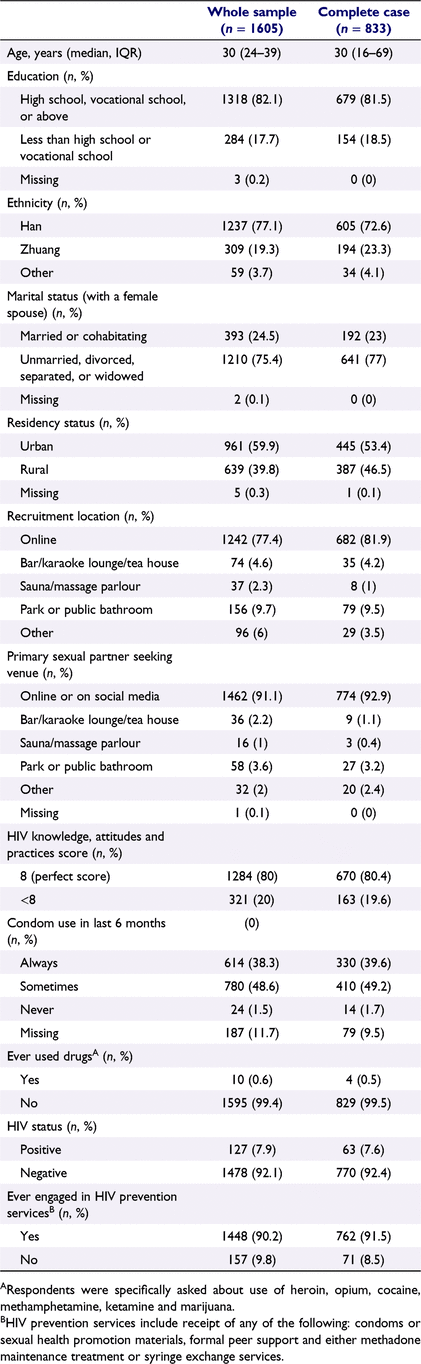
A total of 1605 MSM took part in the survey, which was implemented in 10 sentinel surveillance sites across the province between March and August of 2019 (Table 1). The median age of participants was 30 years (interquartile range 24–39 years), the vast majority of whom had at least a high school or vocational school education (82.1%) and were of the dominant Han ethnicity (77.1%). Only one-quarter were married (24.5%; average age of first marriage for Chinese men was 29 years in 2017), and a majority (59.9%) held an urban (vs rural) residency permit, a classification commonly interpreted in Chinese demography studies as a proxy for the environment in which one was born and raised.15 Most of the participants (77.4%) were recruited into the study online, a pattern reflected in participants’ preferred venue for partner seeking (91.1% online), although in practice most respondents likely find partners through multiple venues. HIV knowledge in this sample was high (80% scored perfectly on a 8-point scale), and drug use was highly uncommon (0.6% reported ever using). Past engagement in HIV-related prevention services, including receipt of condoms or sexual health promotion materials, receiving formal peer support, or taking part in methadone maintenance treatment or syringe exchange services, was very high (90.2% ever engaged). A total of 127 participants (7.9%) tested positive for HIV.
|
Of the 1605 MSM recruited into the main sample, approximately half (51.9%) listed at least one partner and were included in the analysis sample. Among the 833 people in the analysis sample, half listed one partner (50%), 72 (8.6%) listed two and 344 (41.3%) listed three. Demographic and behavioural characteristics were quite similar to those of the full sample (Table 1). The mean difference in age between respondents and their reported partners was 6.9 years (standard deviation 7.1).
Contact patterns
Age-based mixing in 5-year age groups is shown in Fig. 2, and indicates that contact patterns in this population are largely assortative by age, as evidenced by the higher ratio values among those of the same or nearly the same age (i.e. the diagonal and near-diagonal cells of the matrix). Assortativity was also evident in the average of the diagonal cell values (mixing of partners of the same age group) of 2.01, in contrast to the lower average value of 0.87 in the off-diagonal cells (mixing of partners of disparate age groups). When also accounting for mixing between partners of those who differ by one age group, the average fell to 1.69 in contrast to 0.68 in the remaining cells.
Overall, survey respondents tended to report partnerships with partners of the same age group or younger, as indicated by the greater number of cells with ratio values >1.0 (i.e. red coloured) that lie below the diagonal. Contact was particularly frequent in three specific age group combinations: <20 year olds and other <20 year olds (ratio 3.43; 95% CI 2.31–4.99); 50–54 year olds with 45–49 year olds (ratio 3.69; 95% CI 1.55–6.70); and ≥54 yea olds with 45–49 year olds (ratio 4.03; 95% CI 1.73–7.32).
Implications for HIV control
Plots of the expected number of new infections in Fig. 3 show that sex with HIV-infected people in the 25–29 years age group would result in the greatest number of new HIV infections, whereas the greatest number of newly acquired HIV infections would take place in the 20–24 years age group. This suggests that improving coverage of services for prevention of secondary transmission for people living with HIV in the 25–29 years age group – e.g. TasP or ‘prevention for positives’ interventions16 – would result in the greatest reduction in new transmissions. The results similarly suggest that HIV-uninfected members of the 20–24 years age group would be expected to acquire the most new infections, and so in turn primary prevention strategies, such as PrEP, would result in the greatest reduction in new acquisitions.
Discussion
We observed age-assortative sexual mixing in a province-wide sample of MSM in a high HIV transmission region of China. By comparing observed mixing patterns against expected patterns under the assumption of proportional mixing, we were able to discern that similarity in age likely drives partner choice more than relative availability of variously aged partners. Combining information about contact patterns with age-specific HIV prevalence also allowed us to estimate the relative number of HIV transmissions and acquisitions expected to result from sexual contact between each age group. The results provide an empirical basis for targeting of HIV prevention resources to the segments of populations that will benefit from them the most; in this case, PrEP for uninfected 20–24 year olds and TasP for 25–29 year olds living with HIV. The network data on age-based mixing presented here can also empirically inform parameterisation of modelling studies on the impact of combination prevention in Chinese MSM, none of which to date appropriately account for population heterogeneity.17–19
Several studies have reported on MSM sexual mixing patterns based on attributes, such as HIV status, race/ethnicity,20 sexual and drug using behaviours,21 body image type,22 and sexual role (i.e. insertive or receptive anal sex partner).23 Only one of these studies (Weiss et al.) has also examined age as a mixing attribute,20 a surprising fact given the salience of age assortativity in MSM HIV transmission dynamics24,25 and the relative reliability with which researchers can collect partner age data as compared to information on sexual risk or HIV status. Age assortativity was not formally assessed by Weiss et al., but their sample appears to have exhibited less assortativity than our study, given the larger average age difference between partners of 9.5 years (vs 6.9 in this study). Although the two samples had similar age distributions, differences may stem from the fact that the study used by Weiss et al. came from a nationwide sample of American MSM who were recruited and surveyed completely online.
Regarding the implications of matrix findings for HIV prevention resource allocation, we note that the groups with the greatest risk of both transmitting and acquiring HIV lie at the younger end of the age spectrum, with the exception of the youngest age group (>20 years), whose risk levels are comparable with all other age categories (Fig. 3). This pattern may reflect the well documented phenomenon in Chinese MSM of sexual debut with another man around the age of 20 years,26,27 an age that usually coincides with leaving home for the first time for work or schooling. The notable risk increase immediately following the presumed age of sexual debut is consistent with reported patterns of rapid HIV acquisition in younger MSM only shortly after initiating sexual activity.28,29 It also suggests that HIV-infected MSM who transmit to sexual partners are likely themselves only recent acquirers. Together, these findings support the need for interventions that can ideally reach MSM before they begin having sex. Because younger MSM still living at home may have fewer opportunities for in-person engagement with queer-friendly health services, social media or ‘mHealth’ interventions may hold the greatest promise for this population. Promising results of early social media interventions to improve HIV testing uptake30,31 suggest that this is an acceptable platform to not only engage and inform, but to offer services, such as home delivery of HIV self-test kits.32
The results of this study should be interpreted in light of several key limitations. First, due to certain features of the survey, which was designed to facilitate administration in a resource-limited setting, respondents were asked to name only up to three of their most recent sexual partners, regardless of when the partnership took place. Due to right truncation, these data are therefore unable to describe the size of respondents’ sexual networks. However, evidence from past network studies of Chinese MSM show that most reported approximately two or three male sexual partners in the recent 3–6-month period, suggesting that our survey methods may have captured a representative portion of recent relationships.33,34 In addition, because the age of reported partners are provided by the respondent, there is the possibility that differential recall or reporting bias across respondent ages could bias results. For example, respondents may more reliably report the ages of partners closer to them in age as compared with those much older or younger. Without further work to validate reported partner ages, however, it is difficult to assess the possible extent or direction of this bias. Third, the inclusion criteria for this study excluded MSM who did not have at least one male sexual partner in the past year, which inhibited our ability to estimate the proportion of MSM whose reported network size would have been zero. Another notable limitation was in the substantial portion (51.9%) of respondents who were missing network data despite meeting the eligibility criteria of having had at least one partner in the past year (and who did not indicate question refusal). Due to resource limitations, survey staff at each of the 10 sites had received varying levels of training on administration of network-related questions that were newly added in 2019. We have little reason to believe that these drivers of missingness would be associated with behavioural or network factors, although comparisons between the full and analytic sample suggest a few demographic differences (i.e. MSM missing data were more likely rural and from the Zhuang ethnic minority). The fact that Guangxi is a largely rural and that the Zhuang make up over one-third of the population35,36 suggest that this sample may underrepresent those of ethnic minority backgrounds and of lower socioeconomic status. Although less than ideal, the ability of this study to include moderate proportions of these groups represents some improvement on most existing studies of Chinese MSM, which largely focus on urban men of Han ethnicity. Indeed, subgroup analyses comparing mixing patterns by region or socioeconomic status might have revealed further insights to this end, but the sparseness of observations in certain age combinations (particularly for highly age-discordant partnerships) meant that we were underpowered to perform such analyses.
Our findings suggest that mixing in Chinese MSM is relatively age assortative, and that the risk of both HIV transmission and acquisition increases sharply immediately following the age at which most members of this population report sexual debut. Taken together, these findings suggest that targeting combination prevention resources at MSM who are not only younger, but particularly at those who have not yet sexually debuted, may be critical for slowing the spread of HIV in this population. More research on optimal strategies to access these harder-to-reach subsets of the MSM population – that is, those who may not yet self-identify as gay men and may not opt to engage in MSM-facing health services – is needed. This study also supports ongoing efforts for public health practitioners to collect network data in key populations to support more empirically driven strategies to target prevention resources.
Data availability
Data from the Guangxi sentinel surveillance survey are the property of the People’s Republic of China Center for Disease Control and Prevention. They are collected for disease control purposes and can only be used for epidemiological research on a case-by-case basis determined by the China CDC institutional review board. These restrictions are imposed by the National Center for AIDS/STD Control and Prevention, Center for Disease Control and Prevention under the Ministry of Health of the People’s Republic of China. For data requests, please contact Guanghua Lan at lgh605@163.com.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was supported by funds from the Guangxi Bagui Honor Grant from the National Natural Science Foundation of China (12071366).
Acknowledgements
The authors acknowledge the local health officials and MSM community members in the 10 cities who supported and participated in the study.
References
[1] Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365 493–505.| Prevention of HIV-1 infection with early antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar |
[2] Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363 2587–2599.
| Preexposure chemoprophylaxis for HIV prevention in men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[3] Joint United Nations Programme on HIV/AIDS (UNAIDS). End Inequalities. End AIDS. Global AIDS Strategy 2021–2026. Geneva: UNAIDS; 2021. Available at https://www.unaids.org/sites/default/files/media_asset/global-AIDS-strategy-2021-2026_en.pdf
[4] Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep 2011; 8 62–72.
| Combination HIV prevention: significance, challenges, and opportunities.Crossref | GoogleScholarGoogle Scholar |
[5] Vermund SH, Hayes RJ. Combination prevention: New hope for stopping the epidemic. Curr HIV/AIDS Rep 2013; 10 169–186.
| Combination prevention: New hope for stopping the epidemic.Crossref | GoogleScholarGoogle Scholar |
[6] Kippax SC, Holt M, Friedman SR. Bridging the social and the biomedical: engaging the social and political sciences in HIV research. J Int AIDS Soc 2011; 14 S1
| Bridging the social and the biomedical: engaging the social and political sciences in HIV research.Crossref | GoogleScholarGoogle Scholar |
[7] Kessler J, Braithwaite RS. Modeling the cost-effectiveness of HIV treatment: how to buy the most “health” when resources are limited. Curr Opin HIV AIDS 2013; 8 544–549.
| Modeling the cost-effectiveness of HIV treatment: how to buy the most “health” when resources are limited.Crossref | GoogleScholarGoogle Scholar |
[8] McNairy ML, El-Sadr WM. Antiretroviral therapy for the prevention of HIV transmission: What will it take? Clin Infect Dis 2014; 58 1003–1011.
| Antiretroviral therapy for the prevention of HIV transmission: What will it take?Crossref | GoogleScholarGoogle Scholar |
[9] Menza TW, Hughes JP, Celum CL, Golden MR. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis 2009; 36 547–555.
| Prediction of HIV acquisition among men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[10] Hoenigl M, Weibel N, Mehta SR, et al. Development and validation of the San Diego Early Test Score to predict acute and early HIV infection risk in men who have sex with men. Clin Infect Dis 2015; 61 468–475.
| Development and validation of the San Diego Early Test Score to predict acute and early HIV infection risk in men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[11] Smith DK, Pals SL, Herbst JH, Shinde S, Carey JW. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr 2012; 60 421–427.
| Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States.Crossref | GoogleScholarGoogle Scholar |
[12] Jones J, Hoenigl M, Siegler AJ, Sullivan PS, Little S, Rosenberg E. Assessing the performance of three HIV incidence risk scores in a cohort of black and white MSM in the south. Sex Transm Dis 2017; 44 297–302.
| Assessing the performance of three HIV incidence risk scores in a cohort of black and white MSM in the south.Crossref | GoogleScholarGoogle Scholar |
[13] Dou Z, Zhang F, Zhao Y, et al. Progress on China’ s national free antiretroviral therapy strategy in 2002–2014. Zhonghua Liu Xing Bing Xue Za Zhi 2015; 36 1345–1350.
[14] Baggaley RF, White RG, Boily M-C. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol 2010; 39 1048–1063.
| HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention.Crossref | GoogleScholarGoogle Scholar |
[15] Liu C, Fu R, Tang W, et al. Transplantation or rurality? Migration and HIV risk among Chinese men who have sex with men in the urban areas. J Int AIDS Soc 2018; 21 e25039
| Transplantation or rurality? Migration and HIV risk among Chinese men who have sex with men in the urban areas.Crossref | GoogleScholarGoogle Scholar |
[16] Fisher JD, Smith L. Secondary prevention of HIV infection: the current state of prevention for positives. Curr Opin HIV AIDS 2009; 4 279–287.
| Secondary prevention of HIV infection: the current state of prevention for positives.Crossref | GoogleScholarGoogle Scholar |
[17] Lu Z, Wang L, Wang LP, et al. A mathematical model for HIV prevention and control among men who have sex with men in China. Epidemiol Infect 2020; 148 e224
| A mathematical model for HIV prevention and control among men who have sex with men in China.Crossref | GoogleScholarGoogle Scholar |
[18] Li J, Peng L, Gilmour S, et al. A mathematical model of biomedical interventions for HIV prevention among men who have sex with men in China. BMC Infect Dis 2018; 18 600
| A mathematical model of biomedical interventions for HIV prevention among men who have sex with men in China.Crossref | GoogleScholarGoogle Scholar |
[19] Lou J, Blevins M, Ruan Y, et al. Modeling the impact on HIV incidence of combination prevention strategies among men who have sex with men in Beijing, China. PLoS One 2014; 9 e90985
| Modeling the impact on HIV incidence of combination prevention strategies among men who have sex with men in Beijing, China.Crossref | GoogleScholarGoogle Scholar |
[20] Weiss KM, Goodreau SM, Morris M, et al. Egocentric sexual networks of men who have sex with men in the United States: results from the ARTnet study. Epidemics 2020; 30 100386
| Egocentric sexual networks of men who have sex with men in the United States: results from the ARTnet study.Crossref | GoogleScholarGoogle Scholar |
[21] Schneider JA, Cornwell B, Ostrow D, et al. Network mixing and network influences most linked to HIV infection and risk behavior in the HIV epidemic among black men who have sex with men. Am J Public Health 2013; 103 e28–e36.
| Network mixing and network influences most linked to HIV infection and risk behavior in the HIV epidemic among black men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[22] Leung KK, Wong HTH, Naftalin CM, Lee SS. A new perspective on sexual mixing among men who have sex with men by body image. PLoS One 2014; 9 e113791
| A new perspective on sexual mixing among men who have sex with men by body image.Crossref | GoogleScholarGoogle Scholar |
[23] Armbruster B, Roy S, Kapur A, Schneider JA. Sex role segregation and mixing among men who have sex with men: implications for biomedical HIV prevention interventions. PLoS One 2013; 8 e70043
| Sex role segregation and mixing among men who have sex with men: implications for biomedical HIV prevention interventions.Crossref | GoogleScholarGoogle Scholar |
[24] Hurt CB, Matthews DD, Calabria MS, et al. Sex with older partners is associated with primary HIV infection among men who have sex with men in North Carolina. J Acquir Immune Defic Syndr 2010; 54 185–190.
| Sex with older partners is associated with primary HIV infection among men who have sex with men in North Carolina.Crossref | GoogleScholarGoogle Scholar |
[25] Jin F, Grulich AE, Mao L, et al. Sexual partner’s age as a risk factor for HIV seroconversion in a cohort of HIV-negative homosexual men in Sydney. AIDS Behav 2013; 17 2426–2429.
| Sexual partner’s age as a risk factor for HIV seroconversion in a cohort of HIV-negative homosexual men in Sydney.Crossref | GoogleScholarGoogle Scholar |
[26] Liu Y, Qian H-Z, Amico KR, et al. Subsequent sexual risks among men who have sex with men may differ by sex of first partner and age at sexual debut: a cross-sectional study in Beijing, China. AIDS Behav 2017; 21 2913–2923.
| Subsequent sexual risks among men who have sex with men may differ by sex of first partner and age at sexual debut: a cross-sectional study in Beijing, China.Crossref | GoogleScholarGoogle Scholar |
[27] Huai P, Li F, Li Z, et al. Prevalence, risk factors, and medical costs of Chlamydia trachomatis infections in Shandong Province, China: a population-based, cross-sectional study. BMC Infect Dis 2018; 18 534
| Prevalence, risk factors, and medical costs of Chlamydia trachomatis infections in Shandong Province, China: a population-based, cross-sectional study.Crossref | GoogleScholarGoogle Scholar |
[28] Xu R, Dai W, Zhao G, et al. Early sexual debut and HIV infection among men who have sex with men in Shenzhen, China. Biomed Res Int 2016; 2016 2987472
| Early sexual debut and HIV infection among men who have sex with men in Shenzhen, China.Crossref | GoogleScholarGoogle Scholar |
[29] Wang N, Wu G, Lu R, et al. Investigating HIV among Chinese men who have sex with men with recent sexual debut, Chongqing, China, 2011. AIDS Behav 2016; 20 2976–2982.
| Investigating HIV among Chinese men who have sex with men with recent sexual debut, Chongqing, China, 2011.Crossref | GoogleScholarGoogle Scholar |
[30] Huai P, Li F, Chu T, Liu D, Liu J, Zhang F. Prevalence of genital Chlamydia trachomatis infection in the general population: a meta-analysis. BMC Infect Dis 2020; 20 589
| Prevalence of genital Chlamydia trachomatis infection in the general population: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[31] Cao B, Gupta S, Wang J, et al. Social media interventions to promote HIV testing, linkage, adherence, and retention: systematic review and meta-analysis. J Med Internet Res 2017; 19 e394
| Social media interventions to promote HIV testing, linkage, adherence, and retention: systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[32] Jin X, Xu J, Smith MK, et al. An internet-based self-testing model (easy test): Cross-sectional survey targeting men who have sex with men who never tested for HIV in 14 provinces of China. J Med Internet Res 2019; 21 e11854
| An internet-based self-testing model (easy test): Cross-sectional survey targeting men who have sex with men who never tested for HIV in 14 provinces of China.Crossref | GoogleScholarGoogle Scholar |
[33] Choi K-H, Ning Z, Gregorich SE, Pan Q-C. The influence of social and sexual networks in the spread of HIV and syphilis among men who have sex with men in Shanghai, China. J Acquir Immune Defic Syndr 2007; 45 77–84.
| The influence of social and sexual networks in the spread of HIV and syphilis among men who have sex with men in Shanghai, China.Crossref | GoogleScholarGoogle Scholar |
[34] Liu H, Feng T, Liu H, et al. Egocentric networks of chinese men who have sex with men: network components, condom use norms, and safer sex. AIDS Patient Care STDS 2009; 23 885–893.
| Egocentric networks of chinese men who have sex with men: network components, condom use norms, and safer sex.Crossref | GoogleScholarGoogle Scholar |
[35] Zhao YJ, Lu Y. Mapping determinants of rural poverty in Guangxi — a less developed region of China. J Mt Sci 2020; 17 1749–1762.
| Mapping determinants of rural poverty in Guangxi — a less developed region of China.Crossref | GoogleScholarGoogle Scholar |
[36] Guangxi Zhuang Autonomous Region Bureau of Statistics. Guangxi Statistical Yearbook 2019. Nanning: Guangxi Zhuang Autonomous Region Bureau of Statistics; 2020. Available at http://tjj.gxzf.gov.cn/tjsj/tjnj/material/tjnj20200415/2019/zk/indexch.htm


