Potential for suppression of Rhizoctonia root rot is influenced by nutrient (N and P) and carbon inputs in a highly calcareous coarse-textured topsoil
Rowena S. Davey A B , Ann M. McNeill A D , Stephen J. Barnett B and Vadakattu V. S. R. Gupta C D
A D , Stephen J. Barnett B and Vadakattu V. S. R. Gupta C D
A School of Agriculture, Food and Wine and The Waite Research Institute, University of Adelaide, PMB1, Urrbrae, SA, Australia.
B Soil Biology and Diagnostics, South Australian Research and Development Institute (SARDI), GPO Box 397, Adelaide, SA, Australia.
C CSIRO Agriculture and Food, Locked Bag No. 2, Glen Osmond, SA 5064, Australia.
D Corresponding authors. Email: ann.mcneill@adelaide.edu.au; gupta.vadakattu@csiro.au
Soil Research 59(4) 329-345 https://doi.org/10.1071/SR20247
Submitted: 25 August 2020 Accepted: 20 November 2020 Published: 27 January 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Bioassays were undertaken in a controlled environment to assess whether the potential for suppression of Rhizoctonia root rot of wheat, in a highly calcareous topsoil, was positively influenced by nutrient (nitrogen (N) or phosphorus (P)) addition and whether any disease suppression response to augmented nutrition was affected by the addition of carbon (C), either as a readily available C source (sucrose) or as wheat stubble. The soil was P deficient, which limited plant growth, populations of putatively beneficial soil microorganisms, and microbial activity and diversity. This ultimately reduced potential for suppression of Rhizoctonia solani AG8. Addition of fertiliser P to the soil increased R. solani AG8 DNA and percent root infection but not the effectiveness of the pathogen. A positive effect of P fertiliser on plant growth partially compensated for the negative effect of increased root infection. Addition of P increased DNA for Microbacterium spp. where labile C had been added and in the presence of plant roots. Stubble addition alone, after 6 weeks of incubation, increased DNA for Pantoea agglomerans, Trichoderma A and Microbacterium spp. although differences in microbial activity and diversity between stubble treatments were only detected after the bioassay had commenced and P was added. Fertiliser P addition to stubble-amended soil resulted in less Rhizoctonia infection compared with that in soil without P or stubble addition. Effectiveness of R. solani AG8 was decreased by 50% with stubble amendment. The application of N alone did not have a marked effect on plant growth or potential for suppression of Rhizoctonia root disease. Agronomic management practices that affect quantity and lability of C input to soil, when combined with strategic P fertiliser decisions, are likely to improve the potential for development of suppression of Rhizoctonia root rot disease in cereal crops on alkaline and highly calcareous soils.
Keywords: calcareous, microbial diversity, nitrogen, phosphorus, soil-borne diseases, Rhizoctonia, stubble management, suppression.
Introduction
A large proportion of cereal production in Australia occurs in semiarid regions on coarse-textured soils that have many edaphic limitations to agricultural productivity (McKenzie et al. 2004). These limitations include not only inherent low water holding capacity (WHC) but also poor fertility exacerbated by low amounts of organic matter (OM) and hence limited cycling of essential nutrients such as nitrogen (N). Additionally, in alkaline coarse-textured topsoils that comprise a high proportion of carbonate, the ‘tie-up’ of nutrients such as phosphorus (P) and zinc, osmotic pressure of soil solution and toxicity from high concentrations of bicarbonate, are all factors likely to constrain plant growth and production (Rengasamy 2010). This complex of edaphic limitations to crop production is characteristic of alkaline calcareous soils (>2 million ha) that support many low-input rain-fed farming systems in southern Australia, which are important contributors to regional and national grain production. Furthermore, such edaphic constraints not only directly affect crop growth and productivity but will affect soil microorganisms (Lauber et al. 2008; Kamble et al. 2014) and indirectly influence plant–soil microorganism interactions (Hartman and Tringe 2019). Low carbon (C) content and poor fertility in particular are likely to limit microbial activity and growth in sandy soils and may support less diverse microbial communities. Indeed, these factors may contribute to the dominance of fungal soil-borne root pathogens in continuous cereal crops on coarse-textured soils (Gupta et al. 2012), especially Rhizoctonia solani AG8 which appears to thrive at low soil moistures typical of coarse-textured soils in semiarid environments (MacNish and Neate 1996; Gill et al. 2001a, 2001b).
Rhizoctonia bare patch resulting from root disease caused by the soil-borne fungus R. solani AG8 is a major biological constraint to cereal crop production in the semiarid cropping regions of southern and Western Australia, and in Pacific North-West regions of the USA (MacNish and Neate 1996; Paulitz 2006; Murray and Brennan 2009, 2010). Roots of plants infected with R. solani show characteristic brown and rotten root tips (spear-tips) that restrict the growth of the root system and result in reduced ability of plants to access water and nutrients. Additionally, growth response to applied fertiliser has been shown to be greater for plants with healthy roots than those with root disease (Wall et al. 1994). Despite several significant advances in the control of Rhizoctonia bare patch, viz. reduced tillage and crop rotation based management practices and some chemical and biocontrol options, complete and reliable control of the disease has not been achieved. Biological disease suppression mediated by a diverse community of soil bacteria and fungi has been suggested to reduce or remove root disease naturally, even in the presence of pathogen, and suppression of cereal root diseases has been identified in agricultural fields in southern and Western Australia (Roget 1995; Penton et al. 2014; Gupta et al. 2019), as well as in soils of the Pacific North-West cropping region in the United States (Lucas et al. 1993; Yin et al. 2013; Schillinger and Paulitz 2014).
It has been widely reported that addition of OM to soil can increase suppression of soil-borne root diseases (van Overbeek et al. 2012; Bonanomi et al. 2018), and this is principally attributed to the influence of C in the OM, especially readily available or labile C. Inputs of C can improve root disease suppression by (i) a general increase in total microbial activity, and thus competitive exclusion of pathogen; (ii) increased abundances of specific microbial communities that reduce pathogens and (iii) induced disease tolerance capacity in plants (Gupta et al. 2011; Schlatter et al. 2017; Vida et al. 2020). Long-term studies of the effects of conservation agriculture practices, including crop residue (cereal stubble) retention for increasing C inputs and microbial activity, have been shown to alter microbial community composition resulting in suppression of soil-borne diseases such as Rhizoctonia bare patch and take-all (Cook 2007; Gupta et al. 2019). There are a range of soil organisms identified as being involved in the development of root-disease suppression (Weller et al. 2002). Soil organisms isolated from agricultural soils in southern Australia that have been shown to act individually or in concert to decrease root infection by Rhizoctonia include bacteria (Exiguobacterium acetylicum, Pantoea agglomerans, Microbacterium spp., Pseudomonas spp., Streptomyces sp. and Bacillus spp.), fungi (Penicillium griseofulvum and Trichoderma spp.) and mycophagous amoebae (Gupta et al. 1999; Barnett 2005; Yang et al. 2005a, 2005b; Barnett et al. 2006).
The development of disease suppression can take 7–10 years, often with a disease peak in the intervening 3–5 years (Roget 1995; Cook 2007). However, in highly alkaline soils of the upper Eyre Peninsula region of South Australia, practices including crop stubble retention, reduced or no-till, crop rotation and enhanced nutrient supply changed microbial population activity and diversity after 8 years but disease suppression of Rhizoctonia and take-all did not develop (Cook et al. 2012). Furthermore, although a controlled environment study of three soils from the same region indicated that addition of OM increased the potential for soil-borne root disease suppression by reducing root infection and increasing DNA of potentially suppressive organisms (E. acetylicum, P. agglomerans and Microbacterium and Trichoderma spp.), the effect was least in the soil that was the most calcareous (Davey et al. 2019). This limited disease suppression potential was postulated to be the result of edaphic limitations, in particular low P availability, that reduced both plant and soil organism growth and function despite C inputs. Although varied effects of N nutrition in agriculture on crop root disease expression have been described (Srihuttagum and Sivasithamparam 1991; Lucas et al. 1993; Smiley et al. 1996), and the application of P fertiliser has been observed to reduce incidence of cereal root diseases (Brennan 1989, 1992), the causes of these effects are poorly understood in terms of the interactions among nutrients, plants, pathogens and potential biocontrol biota (Dordas 2008).
Current understanding of the driving forces behind interactions among soil properties, OM and fertiliser additions to soil and communities of soil microorganisms is limited (Johnson et al. 2003), and hinders the development of management options for suppression of soil-borne diseases. Thus, the aim of the work reported here was to assess how the potential for disease suppression to Rhizoctonia root rot, in a highly calcareous soil from a semiarid agricultural region in southern Australia, was influenced by N or P addition with or without C amendment.
Materials and methods
Three experiments were undertaken using a highly calcareous coarse-textured soil collected by randomly sampling the top 100 mm depth across an agricultural field located near Streaky Bay on the Eyre Peninsula (32′48.956°S, 134′10.175°E) in South Australia. The climate of this cereal production region is classified as semiarid, characterised by hot dry summers and cool wet winters (Nix 1975; Cawood and McDonald 1996), with long-term annual median rainfall (370 mm) being less than half of the annual potential evapotranspiration. The majority of the soil profiles in the upper Eyre Peninsula around Streaky Bay are classified as Calcarasols according to the Australian classification system and tend to have a highly calcareous alkaline topsoil (Isbell 2002; Hall et al. 2009). The farming systems are mainly low-input based cereal–annual legume pasture rotations (often utilising no N fertiliser and low rates of P) and are highly susceptible to cereal root-disease caused by R. solani AG8. The soil was air dry when collected over the summer fallow period, following a wheat (Triticum aestivum) crop in the previous growing season, and was coarsely sieved through a 2-mm mesh to remove stones and other large material. Commercial laboratory analysis of a subsample reported a pH(H2O) of 8.4, electrical conductivity (EC1:5) of 0.208 dS m–1, extractable nitrate-N and ammonium-N (Searle 1984) of 49 and 4 mg kg–1 respectively, extractable potassium and P (Colwell 1963) of 403 and 48 mg kg–1 respectively, a calcium carbonate content (Martin and Reeve 1955) of 73%, an organic C content (Walkley and Black 1934) of 2.25%, and extractable (DTPA) micronutrients (Rayment and Lyons 2011) of 0.20 mg kg–1 copper, 1.32 mg kg–1 zinc, 5.6 mg kg–1 manganese and 4.28 mg kg–1 iron. Further subsamples of soil were analysed for P using resin extraction and diffusive gradients in thin films (DGT) methodologies that are considered highly appropriate for assessing plant-available P, particularly in calcareous soils (Mason et al. 2010). Resin extractable P was 2 mg kg–1 (<6 is considered P deficient) and DGT CE was 298 ug L–1 (<800 is considered P deficient). Field capacity water content of the soil was determined as 24% w/w using a 1-m column technique (Marshall and Holmes 1979).
For each experiment, bulk amounts of dry soil were moistened with nutrient solutions (made using sterilised reverse osmosis (RO) water) and thoroughly mixed. Soil for all experiments received trace element solution (H3BO3 and MnSO4·4H2O at 0.31 mg kg–1 soil; ZnCl2, CuCl2·H2O and MoO3 at 0.01 mg kg–1 soil) plus another nutrient solution containing MgSO4·7H2O (0.06 g kg–1 soil) and ferric citrate (0.01 g kg–1 soil). After the specific treatments for each experiment were imposed (as described in the following sections), the soil was again mixed, divided and allocated into small pots (0.3 L; 0.1 m height) to be used for growing plants in a bioassay.
Bioassay to assess potential for expression of soil-borne disease suppression
A bioassay was used in each experiment to assess potential for expression of soil-borne disease suppression (Wiseman et al. 1996; Roget et al. 1999). Following set up of the treatments for an experiment, the soil in each bioassay pot was inoculated with two 10-mm agar plugs of R. solani AG8 strain W19 (isolated from diseased wheat roots) placed at approximately half pot-height equidistant from each other and incubated for 2 weeks in a controlled environment room at 15°C with a 12-h day/night regime. Along with the inoculated bioassay pots, a second set of uninoculated pots were set up and designated healthy controls. The soil utilised in this study had previously been assessed as causing minimal or no R. solani root infection in wheat seedlings (Davey 2013). Wheat (T. aestivum cv. Yitpi) plants were then grown in all the pots of soil from surface-sterilised seeds planted at seven per pot and thinned to five plants after emergence. Plants were grown for 4 weeks and soil maintained at 75% WHC (18% w/w) by watering to weight every 2–3 days. After 4 weeks, shoots were cut off at the base of the stem, dried in an oven for 4 days at 60°C and weighed to enable an average dry weight per plant to be calculated. Roots were extracted from soil, washed and stored frozen before being rated for disease. Following disease rating roots were dried in an oven for 4 days and dry weight recorded.
Root disease assessment
Roots were visually scored for severity of Rhizoctonia root rot (McDonald and Rovira 1983) on a scale of 0 (no infection) to 5 (severe infection), and also assessed for percent root infection (PRI) using the method described by Barnett et al. (2006). Briefly this involved counting the total number (No.) of seminal roots, the number of seminal roots infected by Rhizoctonia root rot and truncated to less than the depth of the pot, and the number infected with Rhizoctonia but not truncated. Greater weighting was given to truncated roots than those infected but not truncated. These counts were then used to calculate PRI as follows:

Experiment 1: Effects of added N or P, with and without sucrose, on plant growth and Rhizoctonia infection of roots
Four treatments were set up for this experiment using a fully randomised complete block design (RCBD) with replicates as the blocks: (i) nil addition of N and P, (ii) 100 mg ammonium-N kg–1 soil added as a solution of (NH4)2SO4 (0.12 mg kg–1 soil) plus CaCl2 (0.07 mg kg–1 soil) and K2SO4 (0.06 mg kg–1 soil) in order to balance cation additions with other treatments, (iii) 100 mg nitrate-N kg–1 soil added as a solution of Ca (NO3)2 (0.15 mg kg–1 soil) and KNO3 (0.06 mg kg–1 soil) and (iv) 50 mg P per kg soil added as a solution of KH2PO4 (0.02 mg kg–1 soil). All solutions were made up using sterilised RO water. Four replicates of each treatment were set up for use in the bioassay with or without the addition of sucrose at 1 g per 100 g dry soil (1% soil dry weight).
Experiment 2: Effect of added P on plant growth and PRI, DNA of Rhizoctonia and suppressive organisms, microbial activity and diversity (Microresp®), and pathogen virulence
Four P treatments were set up in this fully RCBD experiment: nil applied P and three rates of applied P (5, 25 and 50 mg P kg–1 soil) as Na2HPO4 in solution. Soil from each treatment was divided into three sets of six replicate pots (300 mL) and adjusted to 75% WHC using RO water before use in the previously described bioassay.
In addition to shoot and root dry weight determination and root disease assessment as described for Experiment 1, the negative effect of the disease on plant growth in the bioassay in Experiment 2 was assessed as:
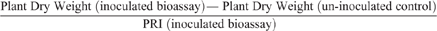
Assessment of soil microorganism DNA and microbial activity and diversity
Sets of replicates of soil from the treatments were destructively sampled at three stages during Experiment 2. The inoculated zero P treatment only was sampled immediately before inoculation and incubation (i.e. at the start of the bioassay) to assess the baseline amount of DNA present for the soil microorganisms of interest. Inoculated and uninoculated P treatments were sampled during the bioassay (i) immediately before sowing but after the 2-week incubation period and (ii) at the termination of the bioassay after 4 weeks of plant growth.
Soil samples were sent to the SARDI Molecular Diagnostic laboratory (https://pir.sa.gov.au/research/services/molecular_diagnostics) for quantification of DNA of selected organisms using real time PCR (Ophel-Keller et al. 2008). The pathogen assayed was R. solani AG8 and the putative beneficial organisms P. agglomerans, Microbacterium spp., Trichoderma Group A (sect. Pachybasium) and Trichoderma Group B (sect. Trichoderma) (Kullnig-Gradinger et al. 2002; Barnett et al. 2006; Ophel-Keller and Barnett 2006; Vinale et al. 2008b). These specific organisms were investigated because they had been detected in other agricultural soils from the same region where the soil in this study was collected (Davey 2013) and have been shown to be associated with decreased root infection in wheat in a bioassay (Davey et al. 2019).
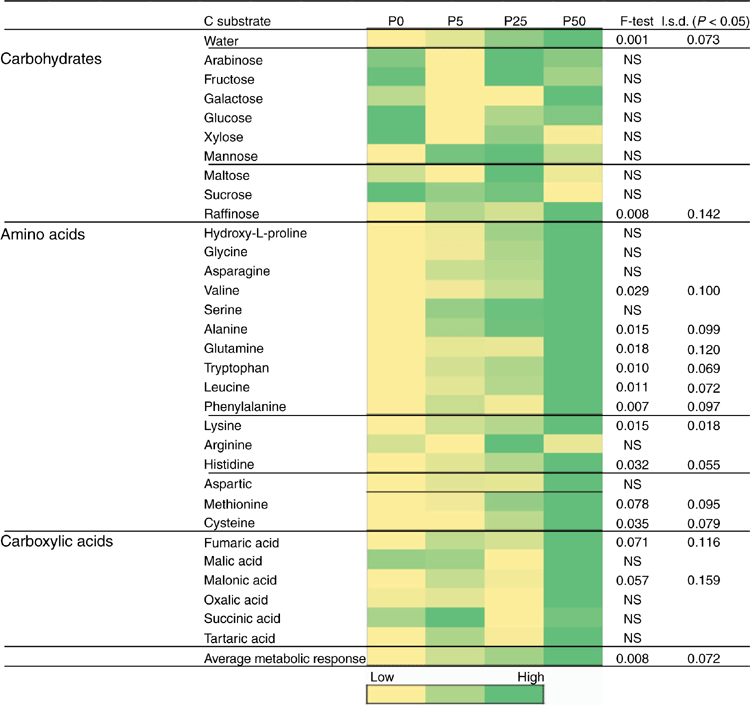
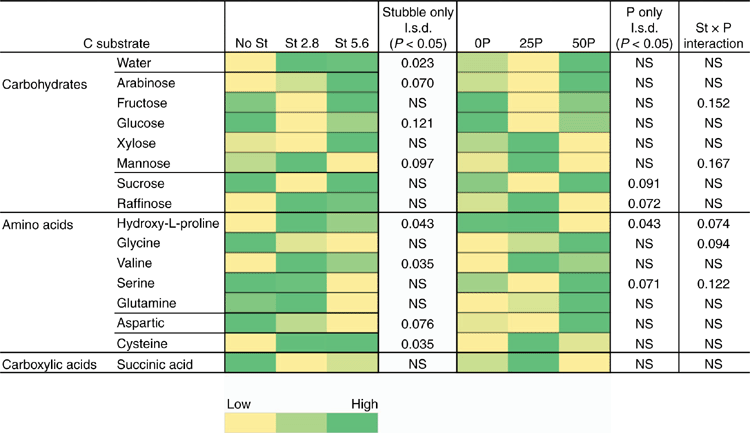
Further subsamples of soils from after the 2-week incubation of the bioassay (before sowing) were analysed to assess microbial activity and diversity using a modified Microresp® technique (Campbell et al. 2003; Knox et al. 2009). Microbial catabolic activity was measured as CO2 production in response to the addition of specific C-containing substrates. Average well colour development (AWCD; Spectromax M4, Moleculardevices.com) from Microresp®, and the respiration values for different substrates were normalised against average CO2 response for all 15 C substrates for each sample (Garland 1997). This average metabolic response represented the overall functional capability of soil heterotrophic microbial communities. Microbial diversity was assessed using the C substrate utilisation data from which ‘community-level physiological profiles’ (CLPP) were constructed (Campbell et al. 2003) and heatmaps to illustrate the relative responses to various C substrates as influenced by the treatments were generated (Jones et al. 2019).
Pathogen effectiveness
Pathogen effectiveness in this study was specified as a measure of ability to cause disease on plant roots per unit of pathogen, and was estimated as:
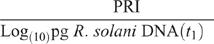
where t1 is the soil sampling immediately before sowing following the initial 2-week incubation period for the bioassay and PRI is measured at the conclusion of the bioassay after 4 weeks of growth.
Experiment 3: Effect of added P, with and without wheat stubble amendment, on plant growth and PRI, DNA for Rhizoctonia and suppressive organisms, and microbial activity and diversity
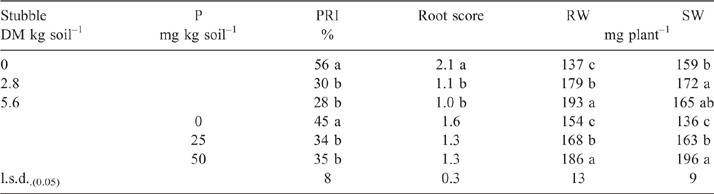
Bulk amounts of the highly calcareous soil amended with finely chopped wheat stubble at 2.8 or 5.6 mg dry matter (DM) kg–1 dry soil and unamended (control) soils were incubated moist (75% WHC) at 15°C for 6 weeks before P was applied as Na2HPO4 in solution at either 25 or 50 mg P kg–1 soil, or no P was applied. Soil from each treatment was then put into pots for a bioassay as previously described with six replicates per treatment and additional sets of replicates for soil sampling and set out in a fully RCBD.
Sets of replicates from each of the nine treatments were destructively sampled at three stages during Experiment 3: (i) when the bioassay experiment was initially set up at the end of the 6-week incubation period following stubble addition, (ii) immediately before sowing in the bioassay which was after a further 2 weeks of incubation with or without pathogen inoculation and (iii) at the end of the bioassay when plants that had been grown for 4 weeks were sampled and processed for measurement of dry weights and assessment of root infection as described for the previous experiments. Soil subsamples from the first two sampling times were assessed for quantitative analysis of soil microorganism DNA and for microbial activity and catabolic diversity as previously described.
Statistical analysis for the three experiments
Data were analysed as an RCBD using ANOVA in Genstat Tenth Edition (VSN International Ltd). Normality and distribution of data were assessed before analysis. Least significant difference (l.s.d.) at P = 0.05 was used for comparison of treatment means and interaction effects. Multivariate analysis on the CO2 evolution and AWCD Microresp® data was undertaken using PRIMER-E software (https://www.primer-e.com/; Auckland, New Zealand) (Clarke and Gorley 2006). The patterns of CLPP derived from the Microresp® data were analysed using multivariate statistical techniques such as nonmetric multidimensional scaling analysis (NMDA) to evaluate the relative degree of similarity among environmental samples (Knox et al. 2009). Significant differences in patterns of CLPP were tested for treatments with permutational multivariate analysis of variance (PERMANOVA) (Anderson 2001) and analysis of similarity (ANOSIM) (Clarke and Ainsworth 1993).
Results
Experiment 1: Effects of added N or P, with and without sucrose addition, on plant growth and Rhizoctonia infection of roots
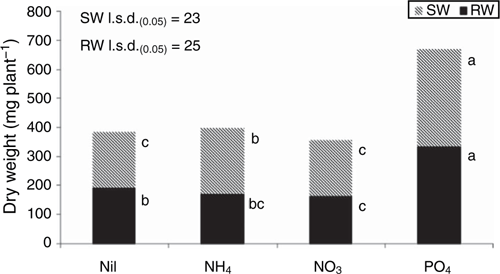
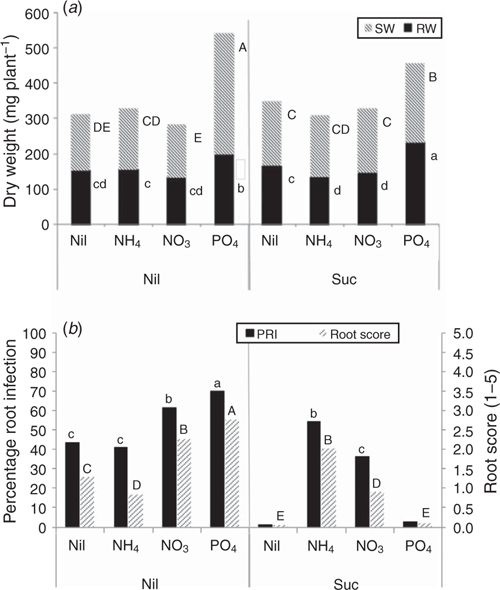
There was no significant plant growth response to addition of N as nitrate in either the uninoculated (Fig. 1) or inoculated bioassays (Fig. 2a), and there was a slight but significant increase in plant dry weight for plants given ammonium-N in the uninoculated bioassay (Fig. 1). Addition of nitrate-N in the absence of sucrose (labile C) increased root infection caused by R. solani AG8 (Fig. 2b) whereas ammonium-N did not, and this possibly contributed to the dry weight of plants in this treatment with addition of ammonium-N being greater than those with nitrate-N (Fig. 2a). Sucrose addition markedly reduced root infection for the nitrate-N treatment but slightly increased it in the ammonium-N treatment.
|
|
Addition of P in the absence of sucrose caused a marked increase in shoot and root growth in the uninoculated (Fig. 1) and inoculated bioassays (Fig. 2a), despite also increasing PRI in the inoculated bioassay (Fig. 2b). Addition of sucrose with or without P addition markedly suppressed root infection to negligible levels (Fig. 2b).
The qualitative visual scoring of root disease symptoms generally resulted in comparable rankings of the treatment effects on root disease expression as those obtained using the quantitative technique of measuring PRI (Fig. 2b), suggesting that this could prove a useful method for disease assessment where rapid screening of large numbers of plants is needed.
Experiment 2: Effect of added P on DNA of Rhizoctonia and suppressive organisms, microbial activity and diversity (Microresp®), and pathogen effectiveness
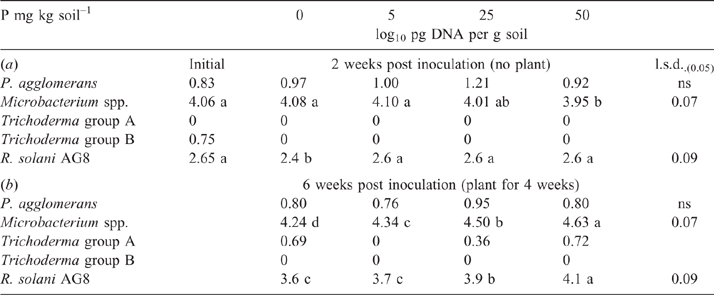
After the initial 2-week incubation period of the bioassay (before sowing), the amount of R. solani AG8 DNA for the P addition treatments was unchanged from that measured initially, although it had significantly decreased in the treatment where no P had been added (Table 1a). During this same period, DNA for Microbacterium spp. was maintained at the quantity measured initially, irrespective of P application, with the exception of a significant decrease where 50 units of P had been applied (Table 1a). The DNA for P. agglomerans at this sampling time was not affected by P addition (Table 1a). The DNA for Trichoderma Group B was below detection in all of the treatments although it was initially present in small amounts (Table 1a), and DNA for Trichoderma Group A was below detection in all soils sampled at this time (Table 1a). Microbial activity (Microresp® CO2 data) and catabolic diversity (CLPP) of soils at this sampling time demonstrated some significant increases with P application, and this was particularly evident for the highest P rate with several amino acid C sources and some carboxylic acid C sources, but not for carbohydrate C sources except raffinose (Fig. 3).
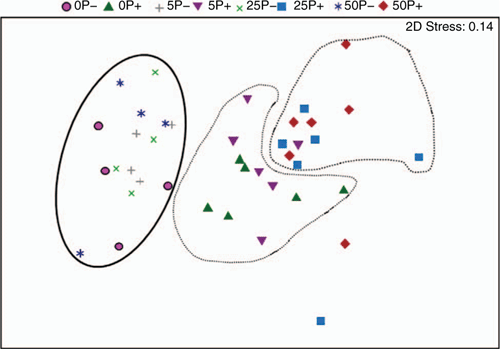
Inoculation of the soil with R. solani AG8 changed the microbial profile of the soil. Soil microbial catabolic diversity (CLPP) significantly differed between soils incubated for 2 weeks with and without R. solani AG8 addition. The NMDA analysis showed distinct grouping between soils with and without inoculation (Fig. 4). Inoculation with R. solani AG8 resulted in significant increases in CO2 evolution (Microresp®) from a range of amino acid C sources compared with uninoculated soils (Table 2). Within the grouping of communities from the inoculated soils there was a tendency for those at zero P and the lowest P rate to cluster closer to the inoculated grouping and away from those at the higher P rates (Fig. 4). NDMA analysis across all soils (inoculated and uninoculated) sampled at this time indicated no significant effect of P on microbial community diversity (Fig. 4).
|
By the end of the bioassay, after 4 weeks of growth of plants, the amount of DNA for R. solani AG8 and the putative suppressive organisms Microbacterium spp. had increased in all treatments compared with the previous sampling, although it was still significantly lower in the soil without added P than where P had been applied (Table 1b). The amount of R. solani AG8 DNA increased as the rate of P applied increased, as did the DNA of Microbacterium spp. (Table 1b). The DNA for P. agglomerans remained similar across P treatments (Table 1b) and amounts did not differ from those measured at the first sampling (Table 1a). The DNA for Trichoderma Group A was detected after the bioassay, but the amount was not affected by P treatment, and Trichoderma Group B was below detection (Table 1b).
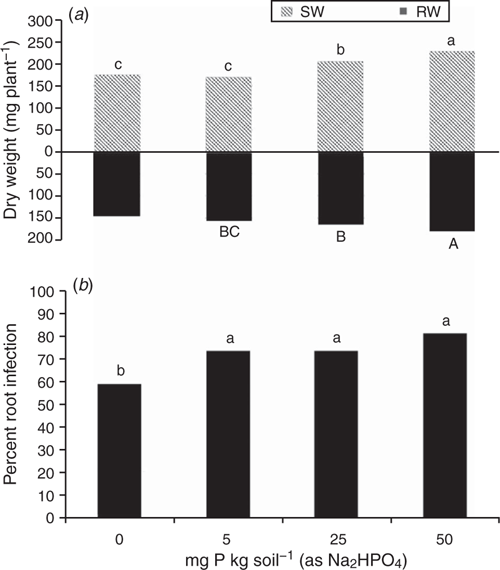
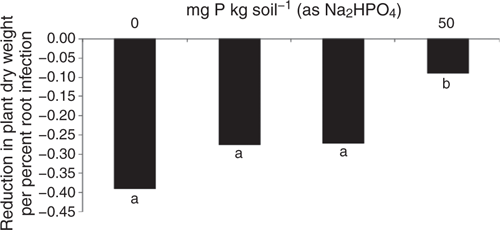
The positive effect of P fertiliser on plant growth in this highly calcareous soil seen in Experiment 1 was confirmed in Experiment 2 (Fig. 5a), and this effect appeared to partially compensate for the fact that P addition increased root infection in this experiment (Fig. 5b) as in the previous one. Consequently, the negative effect of Rhizoctonia root disease on relative reduction in plant dry weight was less at the higher rate of applied P (Fig. 6). Furthermore, the effectiveness of the pathogen did not significantly increase when P was added, with a mean value across all treatments of 28 and a range of 25–30 units of root infection per unit of pathogen DNA.
|
|
Experiment 3: Effect of added P, with and without wheat stubble amendment, on DNA for Rhizoctonia and suppressive organisms, and microbial activity and diversity (Microresp®)
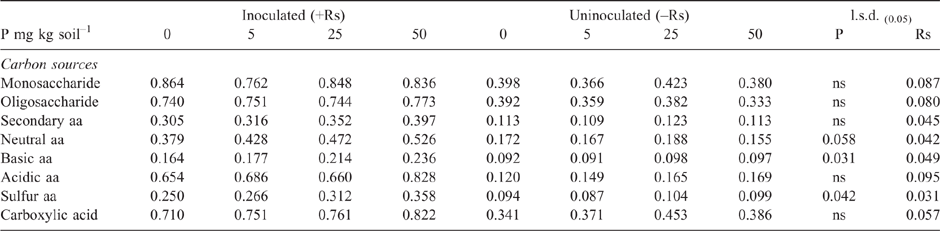
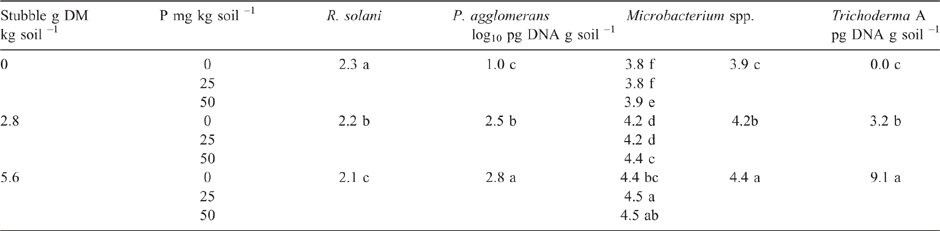
As in Experiment 2, the addition of P to the highly calcareous soil in Experiment 3 significantly increased DNA of Microbacterium spp. but not P. agglomerans or Trichoderma Group A, as measured during the bioassay after 2 weeks incubation with R. solani AG8 (Table 3). Furthermore, at this sampling time, where the soil had been amended with stubble there was a significant increase in the DNA of both Microbacterium spp. and P. agglomerans, with greatest amounts observed at the higher stubble addition (Table 3). Addition of P did not significantly affect the amount of R. solani AG8 DNA detected after 2 weeks of incubation (data not shown), whereas it decreased in soils that had been amended with stubble (Table 3).
Stubble amendment per se followed by incubation for 6 weeks had no effect on the amount of DNA for the native population of R. solani AG8 (Table 4) but increased the DNA for P. agglomerans, Trichoderma Group A and Microbacterium spp., with the latter increasing concurrently with increasing rate of stubble amendment (Table 4). Trichoderma Group B was largely absent from these soils. Microbial activity (Microresp® CO2 data) and diversity (CLPP) for stubble-amended soils at this sampling time (6 weeks after incubation with stubble) showed no systematic variation between the treatments (PERMANOVA data not shown).
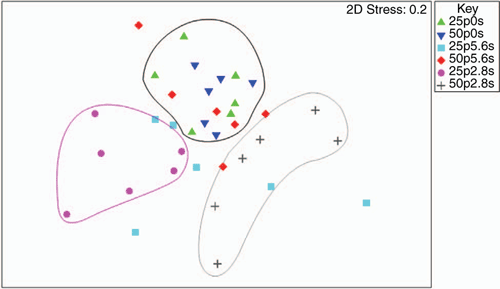
However, CLPP data did indicate significant changes in the microbial community structure during the bioassay after inoculation with R. solani AG8 and a further 2 weeks of incubation with added P fertiliser Fig. 7), in particular where stubble had been previously added (Fig. 7) and there were some significant interaction in combination with P fertiliser (Fig. 7). The main effect of stubble addition varied depending on the different types of C substrates with some significant positive and negative shifts in the use of specific amino acids and carbohydrates (Fig. 7). There was a strong similarity in the community profiles (CLPP) for soils that received P but were not amended with stubble (see within the black ring in Fig. 8). In soil amended with stubble at 2.8 g kg–1, community profiles were different to those for soil without added stubble; in addition the profiles in the 25P and 50P treatment differed (indicated by the pink and grey rings in Fig. 8; PERMANOVA – for P treatments coefficient of variation (CV) = 1.27, P = 0.002, for C treatments CV = 1.28, P = 0.001; for P × C interaction CV = 1.91, P = 0.003). Similar to the previous experiment, a significant effect of addition of P on CLPP was observed with some C sources (Fig. 7).
|
The overall effect of P addition to stubble-amended soil on plants in the bioassay was less Rhizoctonia infection on wheat seedling roots compared with that in soil without P or stubble addition (Table 5). Effectiveness of R. solani AG8 (PRI per unit of pathogen DNA) was not influenced by P addition but was decreased by 50% with stubble amendment: it was 26 units of root infection per unit of pathogen DNA in the nil stubble treatment and reduced to 14 and 13 where soils had been amended with 2.8 or 5.6 g stubble DM respectively.
Discussion
Abiotic stress conditions, including edaphic constraints such as availability of C, nutrient deficiencies and other chemical toxicities, can influence the composition and functioning of the soil microbiome and plant–microbe interactions (van Elsas et al. 2002). It is clear from the results of this study that the investigated highly calcareous topsoil presents both abiotic and biotic constraints to the potential for development of suppression for root rot disease, which are linked to limited bioavailability of C and P. Whereas N application alone did not appear to have a major effect on plant growth or potential for disease suppression, indicating the system was not N limited. The study highlighted that labile C input (sucrose addition), P fertiliser addition and the synergy between C added in wheat stubble and P fertiliser were important factors in determining the responses of plants, pathogen and putative disease-suppressing microorganisms that ultimately lead to root infection by R. solani AG8. These factors, as well as the agronomic implications of the results, are discussed further in the following sections.
Availability of labile C is a major biotic constraint for disease suppression potential in this highly calcareous soil
The positive effects on plants (seen in Experiment 1) of slightly increased plant growth and significantly reduced root infection following addition of a labile C source (sucrose), even in the absence of added nutrients (N or P), highlight that availability of C is a key biotic constraint to the potential for root disease suppression in this soil. This is despite it being a coarse-textured soil that should afford a low level of protection to OM decomposition (Baldock and Skjemstad 2000). Since the soil is from a low-rainfall environment, where annual inputs from crop residues are relatively small in amount and of low quality in terms of C to nutrient ratio, it may be that the predominant forms of organic C in this soil are largely recalcitrant (Baldock et al. 1992). Furthermore, it has been suggested that some calcareous soils occlude soluble organic C (Schmidt et al. 2012), hence, although the organic C content of the soil in this study was reasonably high for a semiarid environment (~2.5%) it is likely that much of that C is not readily available (Demoling et al. 2007). Available C is a key driver for biological activity in soil, and more specifically the effect of OM addition for increased root disease suppression and enhanced beneficial biota is well documented (Alabouvette 1999; Hoitink and Boehm 1999; Bonanomi et al. 2018). Indeed, an effect of C inputs on potential for root-disease suppression in the soil used for this study has previously been reported, although other edaphic constraints were still considered to be limiting (Davey et al. 2019). Rhizoctonia solani AG8 is known to be highly and competitively saprophytic (Sneh et al. 2013), and possibly could access more recalcitrant C sources in the soil OM which might give it an advantage over other microorganisms in a soil where available C is limited.
Although the addition of labile C markedly suppressed root infection in Experiment 1, results from Experiment 2 for putative beneficial organisms suggest that the soil may be inherently limited in terms of a biotic component. Trichoderma species are considered important antagonists for R. solani AG8 (Vinale and Sivasithamparam 2020) but DNA for the two Trichoderma groups (Trichoderma A and B) was either below detection or detected intermittently in small amounts, even in the presence of plants. Similarly, in the presence of plants, the DNA for Microbacterium spp. also increased, but not P. agglomerans for which DNA amounts were constant. The rhizosphere is well recognised as an environment to support growth and function of microorganisms via labile C and other nutrients in root exudates (Bais et al. 2006; de Boer et al. 2006). As mentioned before there are many other putative beneficial organisms that were not assessed in this study but have been isolated under bioassay conditions from other suppressive soils in southern Australia, and if these were assessed it would more fully characterise the suppressive potential of this soil. Indeed, research using metagenomic and transcriptomics tools suggests the involvement of a wide community of bacteria (Yin et al. 2013; Hayden et al. 2018) and fungi (Penton et al. 2014) in the suppression of Rhizoctonia root rot; including several fungi belonging to the Xylariaceae, Bionectriaceae and Hypocreaceae families that have known antifungal capability and were found to be the dominant communities in suppressive compared with nonsuppressive field soils.
The different dynamics of soil organisms measured in this study during plant growth highlights the complex of plant–pathogen–beneficial interactions involved in the expression of disease suppression. In fact, inoculation with pathogen per se for the bioassay also caused a change in catabolic diversity from the indigenous microbial population, as evident by the altered CLPP at 2 weeks after inoculation in Experiment 2, which emphasises the pathogen–soil microbiome interaction. Recent research using ‘omics’ tools have improved our understanding of the general response of microbiomes to multiple influences in natural and agricultural ecosystems (Delgado-Baquerizo et al. 2018); however, the specific drivers for microbial community change in relation to pathogen dynamics, plant–microbe interactions and consequences to disease suppression are yet to be fully resolved (Penton et al. 2014; Delgado-Baquerizo et al. 2020). It is highly likely in this soil also that edaphic factors such as the high pH and salt and carbonate toxicity will influence diversity and function of organisms (Wakelin et al. 2008; Rath et al. 2019), but currently there appears to be limited evidence as to the effects of these specific edaphic factors on potential for suppression of soil-borne root disease (Höper et al. 1995; Senechkin et al. 2014).
Overall, addition of labile C per se did appear to improve potential for disease suppression in this soil, resulting in substantial reduction in root infection and associated small increases in plant growth, although the addition of P had an even greater influence on plant growth and root infection, as discussed in the next section.
Availability of P constrains plants, pathogen and select putative beneficial microorganisms and limits potential for soil-borne disease suppression in this highly calcareous soil
The plants were responsive to P application as predicted from the DGT and resin-extraction based assessments of soil P status which indicated likely P deficiency for crop production (Mason et al. 2010) and confirmed that the Colwell P test (which indicated P sufficiency for this soil) may not be suitable for measuring P status in certain soils due to extraction of P from nonlabile sources (Mason et al. 2008). It is well known that soluble P rapidly forms insoluble complexes in these highly calcareous soils of southern Australia (Bertrand et al. 2003; Lombi et al. 2005). Furthermore, the finding that availability of P is a major constraint for plant growth in this highly calcareous soil was not unexpected given reports from other glasshouse and field studies demonstrating that calcareous soils from the region where the soil in this study was collected are highly responsive to fertiliser P, particularly in fluid form (Holloway et al. 2001).
It is evident that P availability in this highly calcareous soil directly constrains the Rhizoctonia pathogen since pathogen DNA decreased 2 weeks after inoculation in the absence of P application, suggesting that the R. solani AG8 population was constrained by P deficiency. However, although P addition maintained pathogen DNA at a constant amount for 2 weeks after inoculation, it was only after the introduction of plants in the bioassay that DNA of R. solani AG8 increased and was responsive to P, as was the DNA for Microbacterium spp. which are commonly associated with plant rhizospheres (Barnett et al. 2006). Also, DNA for Trichoderma Group A was below detection until after plants were grown. Trichoderma species are well documented as antagonistic fungi to a variety of pathogens including R. solani (Grosch et al. 2006; Verma et al. 2007; Vinale et al. 2008a), as well as being plant growth promoting microorganisms (Avis et al. 2008; Vinale et al. 2008b; Vinale and Sivasithamparam 2020). The results from Experiment 2 therefore suggest a role for the plant rhizosphere in both stimulating putative suppressive organisms, potentially via provision of C-rich substrates and other signal molecules in root exudates (Jones et al. 2009; Carvalhais et al. 2015) and increasing P availability in this highly calcareous soil via solubilisation in the rhizosphere (Richardson et al. 2009). Along with the measured increases in DNA of specific soil organisms after the bioassay in Experiment 2 there were some observed changes to the community physiological profiles, indicative of root induced effects on catabolic diversity (Grayston et al. 1998), specifically where P was in sufficient supply.
Despite no increase in DNA for measured organisms before plants in the bioassay, added P was observed to rapidly increase overall microbial activity (Microresp®) 2 weeks after addition. In P-limited soils from moist tropical forests in Costa Rica, addition of P increased microbial activity over a 30-day period (Cleveland et al. 2002), and in tropical pasture soils in Mexico addition of P increased both microbial activity (CO2 evolution) and C-immobilisation in the microbial biomass after 20 days (Galicia and Garcia-Olivia 2004). The increased microbial activity observed with added P in this current study coincided with a shift in the CLPP from chiefly carbohydrates with no P addition to include a range of amino acids and some carboxylic acids with P addition, especially at the higher rates of P; and this supported an observed trend for the communities of organisms at higher rates of P (25 and 50) to group together and shift slightly away from those receiving low (5) or no P. Given the limited suite of putative organisms measured it is difficult to speculate as to whether the observed trend for community to shift in response to high P is likely to increase or decrease potential for suppression, but it is clear that the application of P, in the absence of sufficient labile C (as seems to be the inherent state for this soil as discussed earlier), increases the abundance of Rhizoctonia pathogen as well as root infection and general microbial activity, which should potentially lead to increased pathogen effectiveness. However, the effectiveness of the pathogen (PRI per unit of pathogen DNA) did not increase, which was possibly attributable to greater root growth in response to applied P, but also the community changes induced by P could have favoured suppressive organisms that were not assessed in this study. Indeed, addition of nutrients to soil can cause changes in microbial biomass C and N which are linked to microbial community structure (Wardle 1992) and addition of P has been reported to shift the microbial community structure in some soils (Liu et al. 2012). Furthermore, the results for this study indicate that if the applied P rate is high enough (greater than ~25 kg P ha–1) any increased root infection is unlikely to compromise shoot growth. The increase in plant growth despite the presence of the pathogen and root infection is partly a synergistic effect in which a nutrient-sufficient plant tends to be healthier and more robust. Plants that experience greater stress including nutrient deficiencies (Dubuis et al. 2005) or lack of moisture or suboptimal soil temperature (Duveiller et al. 2007) are reported to be more susceptible to disease; supporting the observation in this study that the plants receiving no or low P had a greater reduction in plant dry weight per unit of root infection than plants receiving higher rates of P.
In summary, the observations of (i) apparent stable pathogen effectiveness and no response in P. agglomerans populations regardless of P application, (ii) an increase in overall soil microbial activity and plant root infection with increasing addition of P alone, (iii) a lack of detectable Trichoderma populations and (iv) increased populations of soil organisms in the presence of plant roots, indicate that the indigenous biota in this highly calcareous soil may be constrained in the development of suppression to Rhizoctonia root rot, not only by the availability of P but also C. Other studies (Roget et al. 1999; Gupta et al. 2009b) have reported that addition of labile C as sucrose without any additional nutrition in calcareous soils had limited beneficial effect in suppressing Rhizoctonia disease, which supports the suggestion that any effect of added C could potentially be improved by the addition of limiting nutrients such as P.
Wheat stubble addition in this highly calcareous soil has positive effects for disease suppression potential and synergies with P addition
Given observations of the effect of labile C addition to the soil on potential for disease suppression in Experiment 1 of this study, it was not surprising that the addition of stubble per se decreased disease severity on plant roots in the bioassay and the amount of pathogen inoculum (DNA for R. solani AG8). The link between OM and labile C input to soil and an increased potential for root disease suppression is well documented as mentioned earlier, and has specifically been demonstrated for cereal stubble inputs in some Australian soils and farming systems (Roget 1995; Pankhurst et al. 2002a, 2002b; Davey et al. 2019). Further evidence for increased suppression potential from addition of stubble per se is seen in this study since abundance of the putatively suppressive organisms, P. agglomerans and Microbacterium spp., increased after 6 weeks of incubation with stubble, and Trichoderma A was detected after it was initially below detection. The increase in some organisms indicates likely increased population size, although Microresp® data at this time were too variable to confirm any increased microbial activity or diversity. It is possible that any labile C input from stubble addition to a C-limited soil may have been depleted after 6 weeks, and therefore sampling earlier after stubble addition in Experiment 3 could have improved the likelihood of measuring any significant differences associated with stubble-derived labile C effects on microbial community composition (Gupta and Reddy 2010). However, the significant responses in the CLPP data reflecting the changes in microbial community structure after a further 2 weeks of incubation following addition of P fertiliser suggests a combined influence of C and P fertiliser addition in modifying the microbial functional capacity.
The effect of P addition alone on soil organisms in the bioassay in Experiment 3 was similar to Experiment 2; that is, population size (DNA) for beneficial organisms increased, with less of an effect than stubble alone, whereas the pathogen R. solani AG8 did not increase. Furthermore, the CLPP data indicated that microbial communities significantly changed in response to P, confirming the trend observed in Experiment 2. Whereas with stubble amendment plus P, the increase in the beneficial species combined with the reduction in R. solani AG8 confirms the earlier suggestion that both P and C are limiting the biota, although C appears a key input. Stubble plus P addition also reduced root infection reflecting a potential increase in disease suppression. These results indicate that general suppression (Baker 1968), via antagonistic actions including antibiosis (Fravel 1988) and competition for resources and space (Paulitz 2000), and specific suppression (Mazzola 2002) are likely to both be acting to reduce the effect of P on increasing infection that was seen under low C availability in Experiment 2.
In summary, stubble amendment plus P fertiliser acted in synergy to increase the disease-suppressive potential of this highly calcareous soil by addressing abiotic and biotic edaphic constraints. Addition of stubble without fertiliser P in this highly calcareous soil had no effect on the native population of R. solani AG8 but it did result in an increase in the populations of specific potentially suppressive organisms. Effectiveness of R. solani AG8 (PRI per unit of pathogen DNA) was not influenced by P addition alone but was decreased by 50% when P was added to a stubble-amended soil, as was severity of Rhizoctonia root rot (i.e. PRI). Shifts in microbial community structure that are likely to contribute to general disease suppression appeared to occur when both C and P limitations were addressed. These findings suggest that future research, investigating changes in microbial community composition following C and nutrient addition with functional characterisation using metagenomic techniques, would help identify specific members of the community and/or functional characteristics that contribute to the suppression of soil-borne root diseases.
N application alone did not increase potential for disease suppression
Application of N alone in this soil did not apparently improve the potential for suppression of disease as evident by the absence of any reduction in root infection relative to unfertilised plants in the inoculated bioassay. However, there were some differences between the two fertiliser N-sources in terms of effects on plant growth and root infection in this bioassay in relation to labile C, whereby plants given nitrate-N were smaller and had higher root infection than those given ammonium-N in the absence of labile C addition (sucrose), but the reverse was the case in the presence of sucrose. There is conflicting evidence regarding the effects of N fertilisers on Rhizoctonia disease–plant growth interactions (MacNish and Neate 1996). A greater increase in mycelial growth of R. solani given nitrate-N relative to ammonium-N has been reported (Ghini et al. 2007), so that higher infection could potentially occur where nitrate is applied, as was the case in the present study in the absence of added C. There are field studies in Australia that also suggest accumulation of mineral N in the surface soil during the autumn period increases disease severity (Gupta and Roget 2011) although others have reported the opposite effect (MacNish 1985). Studies on the severity of take-all under different N forms found that ammonium increased the populations of Pseudomonads, which were antagonistic to take-all, while nitrate increased populations of those that were deleterious to plant growth (Sarniguet et al. 1992).
Overall, N, at the rate applied in Experiment 1 of this study and in either nitrate or ammonium form, did not appear to greatly limit plant growth per se in this highly alkaline soil. However, this study only investigated the individual effects of added P or N and not the effect in combination which has been shown to be important for wheat production on grey calcareous soils (Hancock et al. 2003). Further work to determine the effects of N fertiliser form on potential for root disease suppression in these alkaline soils is merited once P limitations have been addressed using fertiliser application.
Agronomic implications of the results from this study
The results from this study suggest that increasing stubble and fertiliser P inputs in semiarid farming systems on highly calcareous topsoils should improve the potential for development of root disease suppression where the pathogen R. solani AG8 is present. Indeed, it has been proposed that the long-term adoption of crop management practices that supply greater biologically-available C inputs, either through crop residues or addition of composts and organic manures, can support higher levels of suppression in low OM soils in semiarid southern and Western Australia (Gupta et al. 2011). However, at present there is limited evidence from agronomic field trials in semiarid southern Australia regarding the effectiveness of such management strategies for developing strong disease-suppressive capacity in all soil types (Gupta et al. 2009a, 2012), and some research suggesting that management practices that included stubble retention over the medium term (8 years) did not develop suppression in calcareous soils in the Eyre Peninsula region of South Australia (Cook et al. 2012). It has been said that agronomic trials present the outcome of complex synergistic interactions among many interventions working together (Watt et al. 2006) and this may partly explain why disease suppression is not necessarily improved in some trials, in particular in soils with abiotic or chemical constraints. For example, the pot studies reported here were undertaken under optimal well-watered conditions and did not mimic the seasonal fluctuations in surface soil moisture and temperature in low-rainfall environments typical of southern Australia that are known to affect the incidence and severity of cereal root-rot diseases (Gill et al. 2001a, 2001b, 2002). The rates of P used in this study were somewhat higher than those widely applied in the field by most farmers in the region. Addition of C as stubble was based on average input of 5.6 mg C kg–1 soil given an average yield of 1.6 t ha–1 on the Eyre Peninsula and a harvest index of 0.38. Recent yields in this region on the highly calcareous soils are often much lower than the average and when several poor seasons occur in succession, inputs from stubbles will be very low. The soil–plant–microorganism relationships that underpin development of disease suppression and how these are affected by seasonal and annual supply of water in semiarid farming systems remains to be unravelled. Research presented here highlights the need for future targeted and temporally-based approaches preferably in a field environment to further investigate the role of specific differentially expressed microbial taxa (and functional genes) that contribute to the suppression of Rhizoctonia root rot and to ultimately develop suitable management options.
Conclusion
Addition of P fertiliser with labile C input in a highly calcareous topsoil can alleviate edaphic constraints to the growth of plants, as well as increase the activity and diversity of soil microorganisms including the abundance of soil-borne pathogens and some putative beneficial organisms; all of which can enhance the potential for suppression of Rhizoctonia root rot. However, the application of P in the absence of sufficient labile C is likely to increase root infection and the amount of DNA for R. solani AG8, although this may not necessarily increase the effectiveness of the pathogen. Furthermore, if the applied P rate is high enough (greater than ~25 kg P ha–1) any increased root infection is unlikely to compromise shoot growth, suggesting that improved plant nutrition could mitigate effects of root disease. Overall, results from these studies suggest that management practices which increase the quantity of C in topsoil and biological availability of that C, such as stubble retention but also possibly crop rotation choice or green manure strategies, when combined with strategic P fertiliser decisions can improve the potential for development of suppression to Rhizoctonia root rot in highly calcareous topsoils by addressing biotic and abiotic edaphic constraints. It is recognised that outcomes from agronomic interventions are the result of multiple plant–soil synergistic and antagonistic interactions, and it is therefore difficult to definitively predict the extent of, and time frame for, development of disease-suppressive soil in any farming system. In semiarid systems, the influence of dynamic seasonal and annual water deficits on plant growth, soil community function and amount of residues, may be particularly important in relation to the potential development of disease suppression in the field, but the interactions are not well understood.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Financial support for this work was provided by the Australian Grains Research and Development Corporation in the form of a postgraduate scholarship to Rowena Davey as well as project funding for Ann McNeill (EPFS II UA00087) and Vadakattu Gupta (CSP000135). Thanks to Phil Wheaton on the Eyre Peninsula for allowing access to land for soil sampling, the staff at Minnipa Agricultural Centre (especially Mandy Cooke, Wade Shepherd and Nigel Wilhelm) for field assistance, scientific input and general support, Simon Anstis (SARDI) for providing R. solani AG8 inoculum and Stasia Kroker (CSIRO) for technical assistance.
References
Alabouvette C (1999) Fusarium wilt suppressive soils: an example of disease suppressive soils. Australasian Plant Pathology 28, 57–64.| Fusarium wilt suppressive soils: an example of disease suppressive soils.Crossref | GoogleScholarGoogle Scholar |
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecology 26, 32–46.
Avis T, Gravel V, Antoun H, Tweddell R (2008) Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biology & Biochemistry 40, 1733–1740.
| Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity.Crossref | GoogleScholarGoogle Scholar |
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms Annual Review of Plant Biology 57, 233–266.
| The role of root exudates in rhizosphere interactions with plants and other organismsCrossref | GoogleScholarGoogle Scholar | 16669762PubMed |
Baker R (1968) Mechanisms of biological control of soil-borne pathogens. Annual Review of Phytopathology 6, 263–294.
| Mechanisms of biological control of soil-borne pathogens.Crossref | GoogleScholarGoogle Scholar |
Baldock JA, Skjemstad JO (2000) Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Organic Geochemistry 31, 697–710.
| Role of the soil matrix and minerals in protecting natural organic materials against biological attack.Crossref | GoogleScholarGoogle Scholar |
Baldock JA, Oades JM, Waters AG, Peng X, Vassallo AM, Wilson MA (1992) Aspects of the chemical structure of soil organic matter as revealed by solid-state 13C NMR spectroscopy. Biogeochemistry 16, 1–42.
| Aspects of the chemical structure of soil organic matter as revealed by solid-state 13C NMR spectroscopy.Crossref | GoogleScholarGoogle Scholar |
Barnett SJ (2005) Microorganisms and mechanisms that contribute to Rhizoctonia disease suppression on wheat. Shandong Science 18, 16–21.
Barnett SJ, Roget DK, Ryder MH (2006) Suppression of Rhizoctonia solani AG8 induced disease on wheat by the interaction between Pantoea, Exiguobacterium, and Microbacteria. Soil Research 44, 331–342.
| Suppression of Rhizoctonia solani AG8 induced disease on wheat by the interaction between Pantoea, Exiguobacterium, and Microbacteria.Crossref | GoogleScholarGoogle Scholar |
Bertrand I, Holloway RE, Armstrong RD, McLaughlin MJ (2003) Chemical characteristics of phosphorus in alkaline soils from southern Australia. Australian Journal of Soil Research 41, 61–76.
| Chemical characteristics of phosphorus in alkaline soils from southern Australia.Crossref | GoogleScholarGoogle Scholar |
Bonanomi G, Lorito M, Vinale F, Woo SL (2018) Organic Amendments, Beneficial Microbes, and Soil Microbiota: Toward a Unified Framework for Disease Suppression. Annual Review of Phytopathology 56, 1–20.
| Organic Amendments, Beneficial Microbes, and Soil Microbiota: Toward a Unified Framework for Disease Suppression.Crossref | GoogleScholarGoogle Scholar | 29768137PubMed |
Brennan RF (1989) Effect of superphosphate and superphosphate plus flutriafol on yield and take-all of wheat. Australian Journal of Experimental Agriculture 29, 247–252.
| Effect of superphosphate and superphosphate plus flutriafol on yield and take-all of wheat.Crossref | GoogleScholarGoogle Scholar |
Brennan RF (1992) Effect of superphosphate and nitrogen on yield and take-all of wheat. Fertilizer Research 31, 43–49.
| Effect of superphosphate and nitrogen on yield and take-all of wheat.Crossref | GoogleScholarGoogle Scholar |
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Applied and Environmental Microbiology 69, 3593–3599.
| A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil.Crossref | GoogleScholarGoogle Scholar | 12788767PubMed |
Carvalhais LC, Dennis PG, Badri DV, Kidd BN, Vivanco JM, Schenk PM (2015) Linking Jasmonic Acid Signaling, Root Exudates, and Rhizosphere Microbiomes. Molecular Plant-Microbe Interactions 28, 1049–1058.
| Linking Jasmonic Acid Signaling, Root Exudates, and Rhizosphere Microbiomes.Crossref | GoogleScholarGoogle Scholar | 26035128PubMed |
Cawood R, McDonald G (1996) Climate of south-eastern Australia. In ‘Climate, temperature and crop production in south eastern Australia.’ (Ed. R Cawood.) pp. 21–33. (Agriculture Victoria: Melbourne)
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables Marine Ecology Progress Series 92, 205–219.
| A method of linking multivariate community structure to environmental variablesCrossref | GoogleScholarGoogle Scholar |
Clarke KR, Gorley RN (2006) PRIMER v6: User Manual/Tutorial. (PRIMER-E Ltd: Plymouth)
Cleveland CC, Townsend AR, Schmidt SK (2002) Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5, 680–691.
| Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies.Crossref | GoogleScholarGoogle Scholar |
Colwell J (1963) The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Australian Journal of Experimental Agriculture 3, 190–197.
| The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis.Crossref | GoogleScholarGoogle Scholar |
Cook R (2007) Take-all decline model system in the science of biological control and clue to the success of intensive cropping In ‘Biological control: a global perspective’ (Eds C Vincent, M Goettel, G Lazarovits) pp. 399–414. (CAB International: Wallingford UK)
Cook A, Wilhelm N, Gupta VVSR, Frischke A (2012) The impact of crop rotation and nutrition on Rhizoctonia disease incidence in cereals on grey calcareous soils of upper Eyre Peninsula. In ‘Proceedings of 16th Australian Agronomy Conference, Armidale’. Available at http://www.regional.org.au/au/asa/2012/disease/8041_cooka.htm
Davey RS (2013) Soil-borne disease suppression to Rhizoctonia solani AG8 in agricultural soils from a semi-arid region of South Australia. PhD thesis, University of Adelaide.
Davey RS, McNeill AM, Barnett SJ, Gupta VVSR (2019) Organic matter input influences incidence of root rot caused by Rhizoctonia solani AG8 and microorganisms associated with plant root disease suppression in three Australian agricultural soils. Soil Research 57, 321–332.
| Organic matter input influences incidence of root rot caused by Rhizoctonia solani AG8 and microorganisms associated with plant root disease suppression in three Australian agricultural soils.Crossref | GoogleScholarGoogle Scholar |
de Boer W, Kowalchuk GA, Veen JA (2006) ‘Root-food’ and the rhizosphere microbial community composition. New Phytologist 170, 3–6.
| ‘Root-food’ and the rhizosphere microbial community composition.Crossref | GoogleScholarGoogle Scholar |
Delgado-Baquerizo M, Reith F, Dennis PG, Hamonts K, Powell JR, Young A, Singh BK, Bissett A (2018) Ecological drivers of soil microbial diversity and soil biological networks in the Southern Hemisphere. Ecology 99, 583–596.
| Ecological drivers of soil microbial diversity and soil biological networks in the Southern Hemisphere.Crossref | GoogleScholarGoogle Scholar | 29315530PubMed |
Delgado-Baquerizo M, Reich PB, Trivedi C, Eldridge DJ, Abades S, Alfaro FD, Bastida F, Berhe AA, Cutler NA, Gallardo A, García-Velázquez L, Hart SC, Hayes PE, He J-Z, Hseu Z-Y, Hu H-W, Kirchmair M, Neuhauser S, Pérez CA, Reed SC, Santos F, Sullivan BW, Trivedi P, Wang J-T, Weber-Grullon L, Williams MA, Singh BK (2020) Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nature Ecology & Evolution 4, 210–220.
| Multiple elements of soil biodiversity drive ecosystem functions across biomes.Crossref | GoogleScholarGoogle Scholar |
Demoling F, Figueroa D, Bååth E (2007) Comparison of factors limiting bacterial growth in different soils. Soil Biology & Biochemistry 39, 2485–2495.
| Comparison of factors limiting bacterial growth in different soils.Crossref | GoogleScholarGoogle Scholar |
Dordas C (2008) Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agronomy for Sustainable Development 28, 33–46.
| Role of nutrients in controlling plant diseases in sustainable agriculture. A review.Crossref | GoogleScholarGoogle Scholar |
Dubuis P-H, Marazzi C, Stadler E, Mauch F (2005) Sulphur deficiency causes a reduction in antimicrobial potential and leads to increased disease susceptibility of oilseed rape. Phytopathology 153, 27–36.
| Sulphur deficiency causes a reduction in antimicrobial potential and leads to increased disease susceptibility of oilseed rape.Crossref | GoogleScholarGoogle Scholar |
Duveiller E, Singh R, Nicol J (2007) The challenges of maintaining wheat productivity: pests, diseases, and potential epidemics. Euphytica 157, 417–430.
| The challenges of maintaining wheat productivity: pests, diseases, and potential epidemics.Crossref | GoogleScholarGoogle Scholar |
Fravel DR (1988) Role of antibiosis in the biocontrol of plant diseases. Annual Review of Phytopathology 26, 75–91.
| Role of antibiosis in the biocontrol of plant diseases.Crossref | GoogleScholarGoogle Scholar |
Galicia L, Garcia-Olivia F (2004) The effects of C, N and P additions on soil microbial activity under two remnant tree species in a tropical seasonal pasture. Applied Soil Ecology 26, 31–39.
| The effects of C, N and P additions on soil microbial activity under two remnant tree species in a tropical seasonal pasture.Crossref | GoogleScholarGoogle Scholar |
Garland JL (1997) Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiology Ecology 24, 289–300.
| Analysis and interpretation of community-level physiological profiles in microbial ecology.Crossref | GoogleScholarGoogle Scholar |
Ghini R, Patrício FRA, Bettiol W, de Almeida IMG, Maia AdHN (2007) Effect of sewage sludge on suppressiveness to soil-borne plant pathogens. Soil Biology & Biochemistry 39, 2797–2805.
| Effect of sewage sludge on suppressiveness to soil-borne plant pathogens.Crossref | GoogleScholarGoogle Scholar |
Gill JS, Sivasithamparam K, Smettem KRJ (2001a) Effect of soil moisture at different temperatures on Rhizoctonia root rot of wheat seedlings. Plant and Soil 231, 91–96.
| Effect of soil moisture at different temperatures on Rhizoctonia root rot of wheat seedlings.Crossref | GoogleScholarGoogle Scholar |
Gill JS, Sivasithamparam K, Smettem KRJ (2001b) Soil moisture affects disease severity and colonisation of wheat roots by Rhizoctonia solani AG-8. Soil Biology & Biochemistry 33, 1363–1370.
| Soil moisture affects disease severity and colonisation of wheat roots by Rhizoctonia solani AG-8.Crossref | GoogleScholarGoogle Scholar |
Gill JS, Sivasithamparam K, Smettem KRJ (2002) Size of bare-patches in wheat caused by Rhizoctonia solani AG-8 is determined by the established mycelial network at sowing. Soil Biology & Biochemistry 34, 889–893.
| Size of bare-patches in wheat caused by Rhizoctonia solani AG-8 is determined by the established mycelial network at sowing.Crossref | GoogleScholarGoogle Scholar |
Grayston S, Wang S, Campbell C, Edwards A (1998) Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biology & Biochemistry 30, 369–378.
| Selective influence of plant species on microbial diversity in the rhizosphere.Crossref | GoogleScholarGoogle Scholar |
Grosch R, Scherwinski K, Lottmann J, Berg G (2006) Fungal antagonists of the plant pathogen Rhizoctonia solani: selection, control efficacy and influence on the indigenous microbial community. Mycological Research 110, 1464–1474.
| Fungal antagonists of the plant pathogen Rhizoctonia solani: selection, control efficacy and influence on the indigenous microbial community.Crossref | GoogleScholarGoogle Scholar | 17127047PubMed |
Gupta VVSR, Reddy NPE (2010) Response of soil microbial communities to stubble addition differs between disease suppressive and non-suppressive soils. In ‘Proceedings of the Sixth Australasian Soilborne Disease Symposium. Queensland Australia’. (Ed. GR Stirling) pp. 50.
Gupta VVSR, Roget DK (2011) Biological suppression of Rhizoctonia disease in wheat – effect of carbon and available nitrogen in soil. In 'New frontiers in plant pathology for Asia and Oceania. Darwin Northern Territory Australia', 26–29 April 2011. pp. 33. (Asian Conference on Plant Pathology and Australasian Plant Pathology Society).
Gupta VVSR, Neate SM, Dumitrescu I (1999) Effects of microfauna and mesofauna on Rhizoctonia solani in a south Australian soil. In ‘Proceedings of the First Australasian Soilborne Disease Symposium.’ Gold Coast, Queensland, Australia. (Ed. R Magarey) pp. 134–136. (Watson Ferguson Company Ltd: Brisbane)
Gupta VVSR, McDonough C, Davoren B, Roget DK (2009a) Effect of intensive no-till cropping systems on Rhizoctonia disease incidence at the Waikerie site. Mallee Sustainable Farming 2009 Research Compendium. pp. 32–37. (Mallee Sustainable Farming Inc.: Mildura, Victoria, Australia)
Gupta VVSR, Roget DK, Coppi J, Kroker S (2009b) Soil type and rotation effects on the suppression of Rhizoctonia bare patch disease in Wheat. In ‘Proceedings of the 5th Australasian Soilborne Disease Symposium’ Thredbo NSW. Volume Extended Abstracts. pp. 85–87
Gupta VVSR, Rovira AD, Roget DK (2011) Principles and management of soil biological factors for sustainable rainfed farming systems. In ‘Rainfed farming systems’. (Eds P Tow, I Cooper, N Partridge, C Birch.) pp. 149–184. (Springer: Berlin Heidelberg)
Gupta VVSR, Llewellyn R, McBeath TM, Kroker S, Davoren C, McKay A, Ophel-Keller K, Whitbread A (2012) Break crops for disease and nutrient management in intensive cereal cropping Capturing opportunities and overcoming obstacles in Australian agronomy. In ‘Proceedings of 16th Australian Agronomy Conference’ Armidale, NSW, 14–18 October 2012. (Ed I Yunusa) (Australian Society of Agronomy) Available at www.regional.org.au/au/asa/2012/nutrition/7961_vadakattugupta.htm
Gupta VVSR, Roper MM, Thompson J (2019) Harnessing the benefits of soil biology in conservation agriculture In ‘Australian Agriculture in 2020: From Conservation to Automation’. (Eds J Pratley, JA Kirkegaard) pp. 237–253. (Agronomy Australia and Charles Sturt University: Wagga Wagga)
Hall JAS, Maschmedt DJ, Billing NB (2009) The soils of Southern South Australia, The South Australian Land and Soil Book Series, Volume 1: Geological Survey of South Australia. Bulletin 56, Volume 1. Department of Water, Land and Biodiversity Conservation, Government of South Australia.
Hancock J, Holloway R, McNeill A, McDonald G (2003) Yield and protein benefits from application of nitrogen fertiliser to wheat on upper Eyre Peninsula Solutions for a better environment. In ‘Proceedings of the 11th Australian Agronomy Conference’ Geelong, Victoria, 2–6 February 2003. (Eds M Unkovich, G O’Leary). Available at http://www.regional.org.au/au/asa/2003/c/17/hancock.htm
Hartman K, Tringe SG (2019) Interactions between plants and soil shaping the root microbiome under abiotic stress. The Biochemical Journal 476, 2705–2724.
| Interactions between plants and soil shaping the root microbiome under abiotic stress.Crossref | GoogleScholarGoogle Scholar | 31654057PubMed |
Hayden HL, Savin KW, Wadeson J, Gupta VVSR, Mele PM (2018) Comparative Metatranscriptomics of Wheat Rhizosphere Microbiomes in Disease Suppressive and Non-suppressive Soils for Rhizoctonia solani AG8. Frontiers in microbiology 9, 859
| Comparative Metatranscriptomics of Wheat Rhizosphere Microbiomes in Disease Suppressive and Non-suppressive Soils for Rhizoctonia solani AG8.Crossref | GoogleScholarGoogle Scholar | 29780371PubMed |
Hoitink HAJ, Boehm MJ (1999) Biocontrol within the context of soil microbial communities: A substrate-dependent phenomenon. Annual Review of Phytopathology 37, 427–446.
| Biocontrol within the context of soil microbial communities: A substrate-dependent phenomenon.Crossref | GoogleScholarGoogle Scholar | 11701830PubMed |
Holloway RE, Bertrand I, Frischke AJ, Brace DM, McLaughlin MJ, Shepperd W (2001) Improving fertiliser efficiency on calcareous and alkaline soils with fluid sources of P, N and Zn. Plant and Soil 236, 209–219.
| Improving fertiliser efficiency on calcareous and alkaline soils with fluid sources of P, N and Zn.Crossref | GoogleScholarGoogle Scholar |
Höper H, Steinberg C, Alabouvette C (1995) Involvement of clay type and pH in the mechanisms of soil suppressiveness to fusarium wilt of flax. Soil Biology & Biochemistry 27, 955–967.
| Involvement of clay type and pH in the mechanisms of soil suppressiveness to fusarium wilt of flax.Crossref | GoogleScholarGoogle Scholar |
Isbell RF (2002) ‘The Australian Soil Classification.’ (CSIRO Publishing: Melbourne)
Johnson M, Lee K, Scow K (2003) DNA fingerprinting reveals links among agricultural crops, soil properties, and the composition of soil microbial communities. Geoderma 114, 279–303.
| DNA fingerprinting reveals links among agricultural crops, soil properties, and the composition of soil microbial communities.Crossref | GoogleScholarGoogle Scholar |
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface Plant and Soil 321, 5–33.
| Carbon flow in the rhizosphere: carbon trading at the soil-root interfaceCrossref | GoogleScholarGoogle Scholar |
Jones AR, Gupta VVSR, Buckley S, Brackin R, Schmidt S, Dalal RC (2019) Drying and rewetting effects on organic matter mineralisation of contrasting soils after 36 years of storage. Geoderma 342, 12–19.
| Drying and rewetting effects on organic matter mineralisation of contrasting soils after 36 years of storage.Crossref | GoogleScholarGoogle Scholar |
Kamble P, Kuchekar S, Baath V (2014) Microbial growth, biomass, community structure and nutrient limitation in high pH and salinity soils from Pravaranagar (India). European Journal of Soil Biology 65, 87–95.
| Microbial growth, biomass, community structure and nutrient limitation in high pH and salinity soils from Pravaranagar (India).Crossref | GoogleScholarGoogle Scholar |
Knox OGG, Gupta VVSR, Lardner R (2009) Cotton cultivar selection impacts on microbial diversity and function. Aspects of Applied Biology 98, 129–136.
Kullnig-Gradinger CM, Szakacs G, Kubicek CP (2002) Phylogeny and evolution of the genus Trichoderma: a multigene approach. Mycological Research 106, 757–767.
| Phylogeny and evolution of the genus Trichoderma: a multigene approach.Crossref | GoogleScholarGoogle Scholar |
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biology & Biochemistry 40, 2407–2415.
| The influence of soil properties on the structure of bacterial and fungal communities across land-use types.Crossref | GoogleScholarGoogle Scholar |
Liu L, Gundersen P, Zhang T, Mo J (2012) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biology & Biochemistry 44, 31–38.
| Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China.Crossref | GoogleScholarGoogle Scholar |
Lombi E, McLaughlin MJ, Johnston C, Armstrong RD, Holloway RE (2005) Mobility, solubility and lability of fluid and granular forms of P fertiliser in calcareous and non-calcareous soils under laboratory conditions. Plant and Soil 269, 25–34.
| Mobility, solubility and lability of fluid and granular forms of P fertiliser in calcareous and non-calcareous soils under laboratory conditions.Crossref | GoogleScholarGoogle Scholar |
Lucas P, Smiley RW, Collins HP (1993) Decline of Rhizoctonia root rot on wheat in soils infested with Rhizoctonia solani AG-8. Phytopathology 83, 260–265.
| Decline of Rhizoctonia root rot on wheat in soils infested with Rhizoctonia solani AG-8.Crossref | GoogleScholarGoogle Scholar |
MacNish G (1985) Mapping rhizoctonia patch in consecutive cereal crops in Western Australia. Plant Pathology 34, 165–174.
| Mapping rhizoctonia patch in consecutive cereal crops in Western Australia.Crossref | GoogleScholarGoogle Scholar |
MacNish GC, Neate SM (1996) Rhizoctonia bare patch of cereals: An Australian perspective. Plant Disease 80, 965–971.
| Rhizoctonia bare patch of cereals: An Australian perspective.Crossref | GoogleScholarGoogle Scholar |
Marshall TJ, Holmes JW (1979) ‘Soil Physics.’ (Cambridge University Press: London UK)
Martin A, Reeve R (1955) A rapid manometric method for determination of soil carbonate Soil Science 79, 187–198.
| A rapid manometric method for determination of soil carbonateCrossref | GoogleScholarGoogle Scholar |
Mason SD, Hamon RE, Zhang H, Anderson J (2008) Investigating chemical constraints to the measurement of phosphorus in soils using diffusive gradients in thin films (DGT) and resin methods. Talanta 74, 779–787.
| Investigating chemical constraints to the measurement of phosphorus in soils using diffusive gradients in thin films (DGT) and resin methods.Crossref | GoogleScholarGoogle Scholar |
Mason S, McNeill A, McLaughlin MJ, Zhang H (2010) Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods. Plant and Soil 337, 243–258.
| Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods.Crossref | GoogleScholarGoogle Scholar |
Mazzola M (2002) Mechanisms of natural soil suppressiveness to soilborne diseases. Antonie van Leeuwenhoek 81, 557–564.
| Mechanisms of natural soil suppressiveness to soilborne diseases.Crossref | GoogleScholarGoogle Scholar | 12448751PubMed |
McDonald H, Rovira A (1983) Development of an inoculation technique for Rhizoctonia solani and its application to screening cereal cultivars for resistance. In ‘4th International Congress of Plant Pathology’ Melbourne Australia.
McKenzie N, Jacquier D, Isbell R, Brown KM (2004) ‘Australian soils and landscapes.’ (CSIRO: Melbourne)
Murray GM, Brennan JP (2009) Estimating disease losses to the Australian wheat industry. Australasian Plant Pathology 38, 558–570.
| Estimating disease losses to the Australian wheat industry.Crossref | GoogleScholarGoogle Scholar |
Murray G, Brennan J (2010) Estimating disease losses to the Australian barley industry. Australasian Plant Pathology 39, 85–96.
| Estimating disease losses to the Australian barley industry.Crossref | GoogleScholarGoogle Scholar |
Nix HA (1975) The Australian climate and its effect on grain yield and quality. In ‘Australian Field Crops Vol 1: Wheat and Other Temperate Cereals.’ (Eds A Lazenby, EM Matheson.) pp. 183–225. (Angus and Robertson: Sydney)
Ophel-Keller K, Barnett SJ (2006) Beneficial wheat rhizobiota. Final report for project DAS00027. Grains Research and Development Corporation Canberra. Available at https://grdc.com.au/research/reports/report?id=270.
Ophel-Keller K, McKay A, Hartley D, Herdina , Curran J (2008) Development of a routine DNA-based testing service for soilborne diseases in Australia. Australasian Plant Pathology 37, 243–253.
| Development of a routine DNA-based testing service for soilborne diseases in Australia.Crossref | GoogleScholarGoogle Scholar |
Pankhurst C, Kirkby C, Hawke B, Harch B (2002a) Impact of a change in tillage and crop residue management practice on soil chemical and microbiological properties in a cereal-producing red duplex soil in NSW, Australia. Biology and Fertility of Soils 35, 189–196.
| Impact of a change in tillage and crop residue management practice on soil chemical and microbiological properties in a cereal-producing red duplex soil in NSW, Australia.Crossref | GoogleScholarGoogle Scholar |
Pankhurst C, McDonald H, Hawke B, Kirkby C (2002b) Effect of tillage and stubble management on chemical and microbiological properties and the development of suppression towards cereal root disease in soils from two sites in NSW, Australia. Soil Biology & Biochemistry 34, 833–840.
| Effect of tillage and stubble management on chemical and microbiological properties and the development of suppression towards cereal root disease in soils from two sites in NSW, Australia.Crossref | GoogleScholarGoogle Scholar |
Paulitz TC (2000) Population dynamics of biocontrol agents and pathogens in soils and rhizospheres. European Journal of Plant Pathology 106, 401–413.
| Population dynamics of biocontrol agents and pathogens in soils and rhizospheres.Crossref | GoogleScholarGoogle Scholar |
Paulitz TC (2006) Low input no-till cereal production in the Pacific Northwest of the US: The challenges of root diseases. European Journal of Plant Pathology 115, 271–281.
| Low input no-till cereal production in the Pacific Northwest of the US: The challenges of root diseases.Crossref | GoogleScholarGoogle Scholar |
Penton CR, Gupta VVSR, Tiedje JM, Neate SM, Ophel-Keller K, Gillings M, Harvey P, Pham A, Roget DK (2014) Fungal Community Structure in Disease Suppressive Soils Assessed by 28S LSU Gene Sequencing. PLoS One 9, e93893
| Fungal Community Structure in Disease Suppressive Soils Assessed by 28S LSU Gene Sequencing.Crossref | GoogleScholarGoogle Scholar | 24699870PubMed |
Rath KM, Murphy DN, Rousk J (2019) The microbial community size, structure, and process rates along natural gradients of soil salinity. Soil Biology & Biochemistry 138, 107607
| The microbial community size, structure, and process rates along natural gradients of soil salinity.Crossref | GoogleScholarGoogle Scholar |
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods – Australasia.’ (CSIRO publishing: Melbourne)
Rengasamy P (2010) Soil processes affecting crop production in salt-affected soils. Functional Plant Biology 37, 613–620.
Richardson A, Barea J-M, McNeill A, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant and Soil 321, 305–339.
| Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms.Crossref | GoogleScholarGoogle Scholar |
Roget DK (1995) Decline in root rot (Rhizoctonia solani AG8) in wheat in a tillage and rotation experiment at Avon, South Australia. Australian Journal of Experimental Agriculture 35, 1009–1013.
| Decline in root rot (Rhizoctonia solani AG8) in wheat in a tillage and rotation experiment at Avon, South Australia.Crossref | GoogleScholarGoogle Scholar |
Roget D, Coppi J, Herdina GV, Gupta VVSR (1999) Assessment of suppression to Rhizoctonia solani in a range of soils across SE Australia. In ‘Proceedings, First Australian Soilborne Disease Symposium’ Gold Coast Queensland Australia (Ed. RC Magarey) pp. 129–130. (Watson Ferguson Company Ltd: Brisbane, Qld).
Sarniguet A, Lucas P, Lucas M, Samson R (1992) Soil conduciveness to take-all of wheat: Influence of the nitrogen fertilizers on the structure of populations of fluorescent pseudomonads. Plant and Soil 145, 29–36.
| Soil conduciveness to take-all of wheat: Influence of the nitrogen fertilizers on the structure of populations of fluorescent pseudomonads.Crossref | GoogleScholarGoogle Scholar |
Schillinger WF, Paulitz TC (2014) Natural suppression of Rhizoctonia bare patch in a long-term no-till cropping systems experiment. Plant Disease 98, 389–394.
| Natural suppression of Rhizoctonia bare patch in a long-term no-till cropping systems experiment.Crossref | GoogleScholarGoogle Scholar | 30708450PubMed |
Schlatter DC, Kinkel L, Thomashow LS, Weller DM, Paulitz TC (2017) Disease suppressive soils: New Insights from the soil microbiome. Phytopathology 107, 1284–1297.
| Disease suppressive soils: New Insights from the soil microbiome.Crossref | GoogleScholarGoogle Scholar |
Schmidt A, Smernik RJ, McBeath TM (2012) Measuring organic carbon in Calcarosols: understanding the pitfalls and complications. Soil Research 50, 397–405.
| Measuring organic carbon in Calcarosols: understanding the pitfalls and complications.Crossref | GoogleScholarGoogle Scholar |
Searle PL (1984) The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen - A review. Analyst (London) 109, 549–568.
| The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen - A review.Crossref | GoogleScholarGoogle Scholar |
Senechkin IV, van Overbeek LS, van Bruggen AHC (2014) Greater Fusarium wilt suppression after complex than after simple organic amendments as affected by soil pH, total carbon and ammonia-oxidizing bacteria. Applied Soil Ecology 73, 148–155.
| Greater Fusarium wilt suppression after complex than after simple organic amendments as affected by soil pH, total carbon and ammonia-oxidizing bacteria.Crossref | GoogleScholarGoogle Scholar |
Smiley RW, Collins HP, Rasmussen PE (1996) Diseases of wheat in long-term agronomic experiments at Pendleton, Oregon. Plant Disease 80, 813–820.
| Diseases of wheat in long-term agronomic experiments at Pendleton, Oregon.Crossref | GoogleScholarGoogle Scholar |
Sneh B, Jabaji-Hare S, Neate SM, Dijst G (2013) ‘Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control.’ (Springer: Netherlands)
Srihuttagum M, Sivasithamparam K (1991) The influence of fertilizers on root rot of field peas caused by Fusarium oxysporum, Pythium vexans and Rhizoctonia solani inoculated singly or in combination. Plant and Soil 132, 21–27.
| The influence of fertilizers on root rot of field peas caused by Fusarium oxysporum, Pythium vexans and Rhizoctonia solani inoculated singly or in combination.Crossref | GoogleScholarGoogle Scholar |
van Elsas JD, Garbeva P, Salles J (2002) Effects of agronomical measures on the microbial diversity of soils as related to the suppression of soil-borne plant pathogens. Biodegradation 13, 29–40.
| Effects of agronomical measures on the microbial diversity of soils as related to the suppression of soil-borne plant pathogens.Crossref | GoogleScholarGoogle Scholar | 12222952PubMed |
van Overbeek L, Senechkin I, van Bruggen A (2012) Variation in microbial responses and Rhizoctonia solani AG2–2IIIB growth in soil under different organic amendment regimes Canadian Journal of Plant Pathology 34, 268–276.
| Variation in microbial responses and Rhizoctonia solani AG2–2IIIB growth in soil under different organic amendment regimesCrossref | GoogleScholarGoogle Scholar |
Verma M, Brar S, Tyagi R, Surampalli R, Valero J (2007) Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochemical Engineering Journal 37, 1–20.
| Antagonistic fungi, Trichoderma spp.: Panoply of biological control.Crossref | GoogleScholarGoogle Scholar |
Vida C, de Vicente A, Cazorla FM (2020) The role of organic amendments to soil for crop protection: Induction of suppression of soilborne pathogens. Annals of Applied Biology 176, 1–15.
| The role of organic amendments to soil for crop protection: Induction of suppression of soilborne pathogens.Crossref | GoogleScholarGoogle Scholar |
Vinale F, Sivasithamparam K (2020) Beneficial effects of Trichoderma secondary metabolites on crops. Phytotherapy Research 34, 2835–2842.
| Beneficial effects of Trichoderma secondary metabolites on crops.Crossref | GoogleScholarGoogle Scholar | 32578292PubMed |
Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Barbetti MJ, Li H, Woo SL, Lorito M (2008a) A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiological and Molecular Plant Pathology 72, 80–86.
| A novel role for Trichoderma secondary metabolites in the interactions with plants.Crossref | GoogleScholarGoogle Scholar |
Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M (2008b) Trichoderma-plant-pathogen interactions. Soil Biology & Biochemistry 40, 1–10.
| Trichoderma-plant-pathogen interactions.Crossref | GoogleScholarGoogle Scholar |
Wakelin SA, Macdonald LM, Rogers SL, Gregg AL, Bolger TP, Baldock JA (2008) Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils. Soil Biology & Biochemistry 40, 803–813.
| Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils.Crossref | GoogleScholarGoogle Scholar |
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science 37, 29–38.
| An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method.Crossref | GoogleScholarGoogle Scholar |
Wall PC, Neate SM, Graham RD, Reuter DJ, Rovira AD (1994) The effect of Rhizoctonia root disease and applied nitrogen on growth, nitrogen uptake and nutrient concentrations in spring wheat. Plant and Soil 163, 111–120.
| The effect of Rhizoctonia root disease and applied nitrogen on growth, nitrogen uptake and nutrient concentrations in spring wheat.Crossref | GoogleScholarGoogle Scholar |
Wardle DA (1992) A comparative assessment or factors which influence microbial biomass carbon and nitrogen levels in soil. Biological Reviews of the Cambridge Philosophical Society 67, 321–358.
| A comparative assessment or factors which influence microbial biomass carbon and nitrogen levels in soil.Crossref | GoogleScholarGoogle Scholar |
Watt M, Kirkegaard JA, Passioura JB (2006) Rhizosphere biology and crop productivity - a review. Australian Journal of Soil Research 44, 299–317.
| Rhizosphere biology and crop productivity - a review.Crossref | GoogleScholarGoogle Scholar |
Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annual Review of Phytopathology 40, 309–348.
| Microbial populations responsible for specific soil suppressiveness to plant pathogens.Crossref | GoogleScholarGoogle Scholar | 12147763PubMed |
Wiseman BM, Neate SM, Keller KO, Smith SE (1996) Suppression of Rhizoctonia solani anastomosis group 8 in Australia and its biological nature. Soil Biology & Biochemistry 28, 727–732.
| Suppression of Rhizoctonia solani anastomosis group 8 in Australia and its biological nature.Crossref | GoogleScholarGoogle Scholar |
Yang H, Ryder M, Tang W (2005a) Characterisation and identification of Trichoderma isolates from a South Australian soil suppressive to Rhizoctonia solani on wheat. Shandong Science 18, 36–49.
Yang H, Ryder M, Tang W (2005b) Isolation and biocontrol potential of bacteria and actinomycetes from soils suppressive to Rhizoctonia bare-patch disease in South Australia. Shandong Science 18, 68–77.
Yin C, Hulbert SH, Schroeder KL, Mavrodi O, Mavrodi D, Dhingra A, Schillinger WF, Paulitz TC (2013) Role of Bacterial Communities in the Natural Suppression of Rhizoctonia solani Bare Patch Disease of Wheat (Triticum aestivum L.). Applied and Environmental Microbiology 79, 7428–7438.
| Role of Bacterial Communities in the Natural Suppression of Rhizoctonia solani Bare Patch Disease of Wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 24056471PubMed |