A pragmatic workflow towards the generation of pXRF datasets for large-scale soil monitoring programs
Xueyu Zhao A * , Uta Stockmann A , Mark Farrell
A * , Uta Stockmann A , Mark Farrell  B and Senani Karunaratne
B and Senani Karunaratne  A
A
A
B
Abstract
Compared to conventional laboratory methods, portable X-ray fluorescence (pXRF) can rapidly estimate total elemental concentrations of soil samples. Developing an optimised and efficient data collection protocol is crucial for processing large sample numbers. Factors such as sample preparation methods, manufacturer calibration modes, and associated analysis times can influence pXRF performance. We evaluated the performance of different containers and detection modes (Soil vs Geochem) to determine the most time-efficient scanning protocol under standard instrument operation while maintaining acceptable accuracy for large-scale total elemental concentration analysis. Using 130 representative soil samples from the CSIRO National Soil Archive, results showed strong correlations for most elements (K, Ca, Ti, Fe, Cu, and Zr) across different containers. However, Mg, which has a low atomic number element, showed poor correlations (R2 = 0.05), likely due to the limits of detection (LOD) of the Geochem mode. Al and Si exhibited better R2 values but showed a low Lin’s Concordance Correlation Coefficient (LCCC) value of less than 0.1. Among different modes, elements including K, Ca, Ti, Fe, Cu and Zr maintained strong correlations (R2 > 0.65 and LCCC > 0.7). Scanning soil samples through plastic bags in Geochem mode is recommended due to its shorter measurement and sample preparation time and ability to detect lighter elements (Mg, Al, and Si). This optimised protocol will support national scale soil monitoring programs with large sample sizes (e.g. n > 3000 soil samples). For future work, elemental data acquired can support for example investigations of how soil mineralogy influences carbon storage capacity and provide insights to the biological-chemical stabilisation of soil organic carbon.
Keywords: containers, detection modes, large-scale soil data collection, portable X-ray fluorescence, proximal sensing, soil monitoring, time-efficient scanning protocol, total elemental concentrations.
As an environmentally friendly and non-destructive tool, portable X-ray fluorescence (pXRF) has been increasingly utilised for the rapid estimation of total elemental concentrations in soil samples in the laboratory environment (Ravansari et al. 2020). With different manufacturer calibration modes (e.g. Soil and Geochem detection mode of the Olympus Vanta M Series) operating at varying energy levels, pXRF is widely applied for detection of macro- and trace elements (e.g. Stockmann et al. 2016), as well as soil contaminants (e.g. Weindorf et al. 2013). Beyond elemental analysis, pXRF has also been applied to predict soil properties through the development of empirical models related to mineral composition, such as cation exchange capacity (CEC), and soil organic matter (SOM), demonstrating adequate analytical accuracy across diverse studies (e.g. O’Rourke et al. 2016; Andrade et al. 2020). Additionally, pXRF-generated datasets also provided valuable proxies for the stability of SOM (Ravansari et al. 2020). As a result, pXRF presents a promising alternative or complementary tool alongside conventional laboratory methods for soil analysis.
For large-scale soil analysis, such as Australian national-scale soil organic carbon monitoring initiatives (Karunaratne et al. 2023) or European continental Land Use/Cover Area frame statistical Survey Soil program (Orgiazzi et al. 2018), optimising data collection protocols is essential to balance accuracy, efficiency and cost. Most studies and pXRF manufacturers recommend using thin film-packed sample cups to generate consistent pXRF elemental datasets, as supported by Towett et al. (2016). However, the time and cost associated with this method becomes significant when processing large sample numbers. Plastic bags, such as standard household sealable polypropylene bags, offer a more convenient and readily available alternative (Adams et al. 2017). Nonetheless, further evaluation of the accuracy of using a plastic bag compared to conventional pXRF cups is necessary to assess suitability of use for large scale soil analysis applications.
To identify the most time-efficient scanning protocol that achieves acceptable accuracy for rapid elemental concentration measurements across large sample numbers, a total of 130 national-scale soil samples were selected from the CSIRO National Soil Archive. Originally collected through the Soil Carbon Research Program (SCaRP; Baldock et al. 2013), these samples represent 4180 farmer paddock sites across Australian cropping and pasture regions, covering a wide range of soil types and landscapes. All samples were air-dried at 40°C and sieved to <2 mm to obtain the fine earth fraction. For more details, see the Supplementary materials.
Samples were prepared for pXRF scanning using two types of soil sample containers: (1) sample cups with 4 μm polypropylene thin film; and (2) standard polypropylene plastic bags with a thickness of 50 μm (50 mm × 75 mm). Sample cups were packed with fine earth soil (<2 mm) (at least 1 cm thick), filled with cling wrap, and sealed tightly with thin film (Fig. 1a). Each cup cost approximately AUD2 and required about 2 min of preparation, including assembly. For plastic bag containers, sample bags were filled with consistent quantities of 25–30 g of fine earth soil (Fig. 1a), folded and compacted for scanning (Fig. 1b). At approximately AUD0.02 and 1 min of preparation per bag, this method significantly reduced costs and packing time. As pXRF manufacturers (e.g. Olympus) generally recommend sample cups (e.g. as provided by Choice Analytical Ltd, Thornleigh, Australia) for optimal results across a wide range of elements, the sample cup dataset was used as the standard for comparison in this study.
(a) Soil packing using plastic cups within thin film (top) and bags (below). (b) Compacted soil in plastic bags for pXRF measurement operated in the field stand kit.
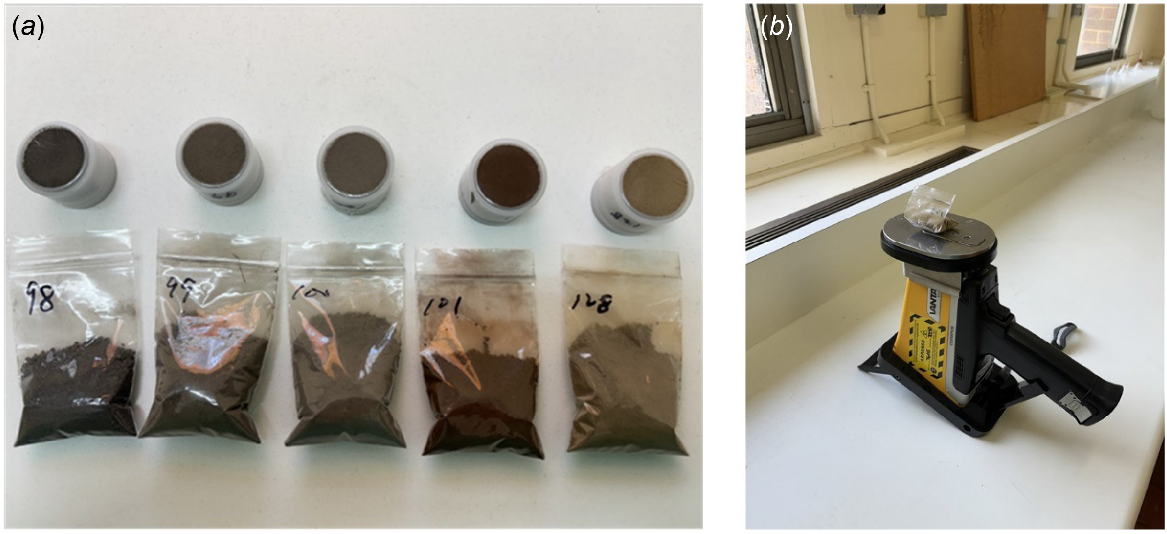
Data acquisition was carried out using an Olympus Vanta pXRF analyser (Vanta M Series, Olympus Scientific Solutions, Waltham, USA), with two manufacturer calibration modes that are commonly used in soil science: (1) Soil, which operates with three energy levels (i.e. beams) (50, 40 and 15 kV) with a total of 90 s standard analysis time (e.g. Ross et al. 2024); and (2) Geochem, which uses two beams (40 and 10 kV) with 60 s standard analysis time (e.g. Zhang and Hartemink 2019). These two calibration modes are designed to optimise elemental detection in soil-like materials. Their primary difference is their limit of detection (LOD) and elemental range that they capture (see Supplementary Table S1). In this study, we compared Mg, Al, and Si, which were only detectable in Geochem mode, other macro-nutrients (K and Ca), micro-nutrients (Fe and Cu), and elements important for pedological studies (Ti and Zr) (Solleiro-Rebolledo et al. 2019). The accuracy and agreement between datasets were evaluated using the coefficient of determination (R2), Lin’s concordance correlation coefficient (LCCC), and the root mean square error (RMSE).
Low atomic number elements of Mg, Al, and Si, which are only available in Geochem detection mode, showed the largest discrepancies in concentrations (%) between sample cups vs sample bags. Fig. 2a shows no significant correlation for Mg, with a very weak R2 (0.05), an LCCC close to 0, and an RMSE of 0.38%. This is likely because most samples fell below the LOD (0.25%). In contrast, for Al (Fig. 2b) and Si (Fig. 2c), there was a tendency towards a linear relationship, with R2 values of 0.65 and 0.75, respectively. However, the agreements along the 1:1 line for both elements were poor with low LCCC (<0.1), indicating an underestimation of Al and Si concentrations when using sample bags. For macro-nutrients, K and Ca (Fig. 2d, e), displayed high R2 values (>0.95), and similarly high LCCC values close to 1 for both modes. However, only when using bags, K and Ca were not detected in 24 and 35 samples, respectively. Thus, the plastic film thickness of the containers appears to influence the detection of low atomic number elements. This can be explained by the Beer-Lambert law (Evans 1955), which suggests that signal attenuation occurs due to material thickness. The thickness of the plastic bags caused significant attenuation of low-energy X-rays compared to the thinner film of the sample cups. Nevertheless, the linear relationship for Al and Si suggests that varying concentrations of these elements in soil samples remain detectable in the sample bag scanning mode.
Measured element concentrations (%) packed in bags versus cups for (a) Mg, (b) Al, and (c) Si in Geochem mode, and (d) K, (e) Ca, (f) Ti, (g) Fe, (h) Cu, and (i) Zr in Geochem and Soil modes. LOD, limit of detection; N, number of R2: coefficient of determination; LCCC, Lin’s concordance correlation coefficient; RMSE, root mean squared error.

Higher atomic number elements showed strong linear relationships for both Geochem and Soil modes. For micronutrients Ti, Fe, Cu, and Zr (Fig. 2f–i), also showed high R2 values over 0.9 and LCCC over 0.85. Notably, Soil mode generally resulted in lower RMSE than Geochem mode for these elements, except for K, Ca, and Fe. This is likely because Soil mode with 50 kV energy level provides a broader detection range for these elements compared to Geochem mode.
To better illustrate the correlation between Geochem and Soil modes, Fig. 3a–f shows the measured element concentrations (%) for K, Ca, Ti, Fe, Cu, and Zr in samples packed in cups. Most R2 values were above 0.9, indicating a strong correlation, except for Ti. In addition, most of LCCC were also high as over 0.85, except for Fe of 0.74. The RMSE were also low for most elements.
Measured element concentrations (%) packed in cups for (a) K, (b) Ca, (c) Ti, (d) Fe, (e) Cu, and (f) Zr in Soil vs Geochem modes. R2, coefficient of determination; LCCC, Lin’s concordance correlation coefficient; RMSE, root mean squared error.

Based on our findings, we recommend using the Geochem mode due to its consistent ability to measure a broad range of macro- and microelements, including Mg, Al, and Si, which are not detectable in Soil mode (Table S1). In addition to this, the Geochem mode provides a quicker 60-s standard analysis, compared to the 90-s standard analysis duration required for Soil mode. Fedeli et al. (2024) evaluated the performance of pXRF in both Soil and Geochem mode across various certified reference materials of soil and plant origin and recommended Geochem mode because it provided more reliable results for a broader range of elements.
In this study, we used pXRF and the Olympus pXRF range manufacturer calibration modes and their standard measurement time of 30 s per energy beam to measure elemental concentrations of soil samples. The impact of differing energy beam scanning time settings was not investigated here but could be explored in further research. Recent studies using the raw pXRF spectral responses of each energy beam and a scanning time of only 2 s found that this fast pXRF measurement can be used effectively to predict a range of soil properties (Tavares et al. 2023).
Furthermore, we only investigated the use of a standard single plastic bag thickness (50 μm). Future research should investigate also the impact of differing plastic bag thickness (25 and 75 μm, for example) on measurement accuracy and assess the associated costs in order to refine and possibly optimise the protocol further. Also, regarding those elements under LOD, it is also necessary to explore and the results from complementary chemical analytical approaches (e.g. Microwave assisted digestion with HF).
Current pXRF applications in soil research have been limited to relatively small sample sizes at field or regional scales, such as 180 samples on the campus of the Federal University of Lavras, Brazil (Bócoli et al. 2025), and 300 samples in the Afzar region, Iran (Naimi et al. 2022). This study provides an optimised protocol for ongoing large soil monitoring projects at national and global scales (e.g. >3000 samples). Using the Geochem mode, combined with standard 50-μm plastic bags has the potential to significantly reduce both time and costs. Rapid and cost-effective acquisition of XRF measurements for large-scale studies is desired, as soil elemental data can provide valuable insights into the role of soil mineralogy in determining carbon storage capacity (Georgiou et al. 2022) and the biological-chemical stabilisation of SOM (McNally et al. 2017) across different environments.
Data availability
The data supporting this study can be made available upon request to the corresponding author.
Conflicts of interest
Mark Farrell and Senani Karunaratne are Associate Editors of Soil Research but were not involved in the peer review or any decision-making process for this paper. The authors have no further conflicts of interest to declare.
Declaration of funding
The Australian Government Department of Climate Change, Energy, Environment, and Water funded this work under the Soil Organic Carbon Monitoring project awarded to Senani Karunaratne and Mark Farrell.
Acknowledgements
The project team acknowledges Georgia Reed and Seija Tuomi from CSIRO support in accessing the archived SCaRP soil samples.
References
Andrade R, Silva SHG, Weindorf DC, Chakraborty S, Faria WM, Mesquita LF, Guilherme LRG, Curi N (2020) Assessing models for prediction of some soil chemical properties from portable X-ray fluorescence (pXRF) spectrometry data in Brazilian Coastal Plains. Geoderma 357, 113957.
| Crossref | Google Scholar |
Baldock J, Macdonald L, Sanderman J (2013) Foreword to ‘soil carbon in Australia’s agricultural lands’. Soil Research 51(8), 1-2.
| Crossref | Google Scholar |
Bócoli FA, Ribeiro D, Mancini M, De Sousa LA, Barbosa SM, Serafim ME, Silva BM, Avanzi JC, Guilherme LRG, Curi N, Silva SHG (2025) Can environmental variables, high sampling density and machine learning deliver detailed maps of soil organic carbon and carbon stock in tropical regions? CATENA 249, 108718.
| Crossref | Google Scholar |
Fedeli R, Di Lella LA, Loppi S (2024) Suitability of XRF for routine analysis of multi-elemental composition: a multi-standard verification. Methods and Protocols 7(4), 53.
| Crossref | Google Scholar | PubMed |
Georgiou K, Jackson RB, Vindušková O, Abramoff RZ, Ahlström A, Feng W, Harden JW, Pellegrini AFA, Polley HW, Soong JL, Riley WJ, Torn MS (2022) Global stocks and capacity of mineral-associated soil organic carbon. Nature Communications 13(1), 3797.
| Crossref | Google Scholar | PubMed |
McNally SR, Beare MH, Curtin D, Meenken ED, Kelliher FM, Calvelo Pereira R, Shen Q, Baldock J (2017) Soil carbon sequestration potential of permanent pasture and continuous cropping soils in New Zealand. Global Change Biology 23(11), 4544-4555.
| Crossref | Google Scholar | PubMed |
Naimi S, Ayoubi S, Di Raimo LADL, Dematte JAM (2022) Quantification of some intrinsic soil properties using proximal sensing in arid lands: application of Vis-NIR, MIR, and pXRF spectroscopy. Geoderma Regional 28, e00484.
| Crossref | Google Scholar |
O’Rourke SM, Stockmann U, Holden NM, McBratney AB, Minasny B (2016) An assessment of model averaging to improve predictive power of portable vis-NIR and XRF for the determination of agronomic soil properties. Geoderma 279, 31-44.
| Crossref | Google Scholar |
Orgiazzi A, Ballabio C, Panagos P, Jones A, Fernández-Ugalde O (2018) LUCAS soil, the largest expandable soil dataset for Europe: a review. European Journal of Soil Science 69(1), 140-153.
| Crossref | Google Scholar |
Ravansari R, Wilson SC, Tighe M (2020) Portable X-ray fluorescence for environmental assessment of soils: not just a point and shoot method. Environment International 134, 105250.
| Crossref | Google Scholar | PubMed |
Ross P-S, Beaudette M, Daoudene Y (2024) Portable XRF applied to regional bedrock mapping in Quebec, Canada. Journal of Geochemical Exploration 258, 107397.
| Crossref | Google Scholar |
Solleiro-Rebolledo E, Rivera-Uria Y, Chávez-Vergara B, Díaz-Ortega J, Sedov S, Alcalá-Martínez JR, Beltrán-Paz OI, Jardines-Martínez LG (2019) Evolution of the landscape and pedodiversity on volcanic deposits in the south of the Basin of Mexico and its relationship with agricultural activities. Terra Latinoamericana 37(4), 501-518.
| Crossref | Google Scholar |
Stockmann U, Cattle SR, Minasny B, McBratney AB (2016) Utilizing portable X-ray fluorescence spectrometry for in-field investigation of pedogenesis. CATENA 139, 220-231.
| Crossref | Google Scholar |
Tavares TR, Molin JP, Alves EEN, Melquiades FL, De Carvalho HWP, Mouazen AM (2023) Towards rapid analysis with XRF sensor for assessing soil fertility attributes: effects of dwell time reduction. Soil and Tillage Research 232, 105768.
| Crossref | Google Scholar |
Towett EK, Shepherd KD, Lee Drake B (2016) Plant elemental composition and portable X-ray fluorescence (pXRF) spectroscopy: quantification under different analytical parameters. X-Ray Spectrometry 45(2), 117-124.
| Google Scholar |
Weindorf DC, Paulette L, Man T (2013) In-situ assessment of metal contamination via portable X-ray fluorescence spectroscopy: Zlatna, Romania. Environmental pollution 182, 92-100.
| Crossref | Google Scholar | PubMed |
Zhang Y, Hartemink AE (2019) Soil horizon delineation using vis-NIR and pXRF data. CATENA 180, 298-308.
| Crossref | Google Scholar |


