Effects of temperature and exercise on metabolism of three species of Australian freshwater turtles: implications for responses to climate change
Bruce C. Chessman
Centre for Ecosystem Science, University of New South Wales, Sydney, NSW 2052, Australia. Email: brucechessman@gmail.com
Australian Journal of Zoology 66(6) 317-325 https://doi.org/10.1071/ZO18062
Submitted: 17 September 2018 Accepted: 6 May 2019 Published: 21 May 2019
Abstract
Oxygen consumption (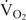 ) of Chelodina expansa, C. longicollis and Emydura macquarii (Pleurodira: Chelidae) was measured at rest and during induced exercise at 8, 13, 18, 22, 26, 30 and 34°C. Resting
) of Chelodina expansa, C. longicollis and Emydura macquarii (Pleurodira: Chelidae) was measured at rest and during induced exercise at 8, 13, 18, 22, 26, 30 and 34°C. Resting 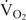 varied significantly among species, being lowest in C. expansa, which is the most sedentary of the three species in nature, and highest in E. macquarii, which is the most energetic, but active
varied significantly among species, being lowest in C. expansa, which is the most sedentary of the three species in nature, and highest in E. macquarii, which is the most energetic, but active 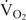 did not differ significantly among the three species overall. For both Chelodina species, resting
did not differ significantly among the three species overall. For both Chelodina species, resting 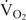 was appreciably lower than expected from regression of
was appreciably lower than expected from regression of 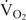 on body mass for non-marine turtles globally, a result that reinforces previous evidence of low resting metabolism in Australian chelid turtles. Active
on body mass for non-marine turtles globally, a result that reinforces previous evidence of low resting metabolism in Australian chelid turtles. Active 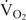 of all three species at higher temperatures was similar to
of all three species at higher temperatures was similar to 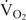 reported for active freshwater cryptodires. Resting
reported for active freshwater cryptodires. Resting 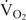 of all three species increased similarly with temperature, but active
of all three species increased similarly with temperature, but active 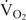 and aerobic scope did not. In C. expansa and E. macquarii, active
and aerobic scope did not. In C. expansa and E. macquarii, active 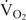 and aerobic scope increased over the full temperature range assessed but in C. longicollis these variables reached a plateau above 22°C. Projected increases in freshwater temperatures in south-eastern Australia as a result of global warming are likely to enhance activity, feeding and growth of the three species (subject to food availability), especially in cooler seasons for C. longicollis and warmer seasons for C. expansa and E. macquarii. However, other aspects of predicted climate change, especially increased drought, are likely to be detrimental.
and aerobic scope increased over the full temperature range assessed but in C. longicollis these variables reached a plateau above 22°C. Projected increases in freshwater temperatures in south-eastern Australia as a result of global warming are likely to enhance activity, feeding and growth of the three species (subject to food availability), especially in cooler seasons for C. longicollis and warmer seasons for C. expansa and E. macquarii. However, other aspects of predicted climate change, especially increased drought, are likely to be detrimental.
Additional keywords: aerobic scope, Chelodina expansa, Chelodina longicollis, Emydura macquarii, oxygen consumption.
References
Addinsoft (2019). XLSTAT statistical and data analysis solution. Addinsoft, Long Island, NY. https://www.xlstat.comArnall, S. G., Kuchling, G., and Mitchell, N. J. (2014). A thermal profile of metabolic performance in the rare Australian chelid, Pseudemydura umbrina. Australian Journal of Zoology 62, 448–453.
| A thermal profile of metabolic performance in the rare Australian chelid, Pseudemydura umbrina.Crossref | GoogleScholarGoogle Scholar |
Ashton, D.T., Bettaso, J.B., and Welsh, H.H. (2015). Changes across a decade in size, growth, and body condition of western pond turtle (Actinemys marmorata) populations on free-flowing and regulated forks of the Trinity River in northwest California. Copeia 103, 621–633.
| Changes across a decade in size, growth, and body condition of western pond turtle (Actinemys marmorata) populations on free-flowing and regulated forks of the Trinity River in northwest California.Crossref | GoogleScholarGoogle Scholar |
Avery, H. W., Spotila, J. R., Congdon, J. D., Fischer, R. U., Standora, E. A., and Avery, S. B. (1993). Roles of diet protein and temperature in the growth and nutritional energetics of juvenile slider turtles, Trachemys scripta. Physiological Zoology 66, 902–925.
| Roles of diet protein and temperature in the growth and nutritional energetics of juvenile slider turtles, Trachemys scripta.Crossref | GoogleScholarGoogle Scholar |
Bagatto, B., and Henry, R. P. (1999). Exercise and forced submergence in the pond slider (Trachemys scripta) and softshell turtle (Apalone ferox): influence on bimodal gas exchange, diving behaviour and blood acid–base status. The Journal of Experimental Biology 202, 267–278.
| 9882639PubMed |
Baudinette, R. V., Miller, A. M., and Sarre, M. P. (2000). Aquatic and terrestrial locomotory energetics in a toad and a turtle: a search for generalisations among ectotherms. Physiological and Biochemical Zoology 73, 672–682.
| Aquatic and terrestrial locomotory energetics in a toad and a turtle: a search for generalisations among ectotherms.Crossref | GoogleScholarGoogle Scholar | 11121342PubMed |
Belkin, D. A. (1965). Reduction of metabolic rate in response to starvation in the turtle Sternothaerus minor. Copeia 1965, 367–368.
| Reduction of metabolic rate in response to starvation in the turtle Sternothaerus minor.Crossref | GoogleScholarGoogle Scholar |
Bennett, A. F. (1982). The energetics of reptilian activity. In ‘Biology of the Reptilia. Volume 13. Physiology D, Physiological Ecology’. (Eds C. Gans and F. H. Pough.) pp. 155–199. (Academic Press: London.)
Bennett, A. F. (1990). Thermal dependence of locomotor capacity. The American Journal of Physiology 259, R253–R258.
| 2201218PubMed |
Bennett, A. F., and Dawson, W. R. (1976). Metabolism. In ‘Biology of the Reptilia. Volume 5. Physiology A’. (Eds C. Gans and W. R. Dawson.) pp. 127–223. (Academic Press: London.)
Booth, D. T. (1998). Effects of incubation temperature on the energetics of embryonic development and hatchling morphology in the Brisbane river turtle Emydura signata. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 168, 399–404.
| Effects of incubation temperature on the energetics of embryonic development and hatchling morphology in the Brisbane river turtle Emydura signata.Crossref | GoogleScholarGoogle Scholar | 9706707PubMed |
Booth, D. T. (2000). Incubation of eggs of the Australian broad-shelled turtle, Chelodina expansa (Testudinata: Chelidae), at different temperatures: effects on pattern of oxygen consumption and hatchling morphology. Australian Journal of Zoology 48, 369–378.
| Incubation of eggs of the Australian broad-shelled turtle, Chelodina expansa (Testudinata: Chelidae), at different temperatures: effects on pattern of oxygen consumption and hatchling morphology.Crossref | GoogleScholarGoogle Scholar |
Booth, D. T. (2010). The natural history of nesting in two Australian freshwater turtles. Australian Zoologist 35, 198–203.
| The natural history of nesting in two Australian freshwater turtles.Crossref | GoogleScholarGoogle Scholar |
Bowen, K. D., Spencer, R.-J., and Janzen, F. J. (2005). A comparative study of environmental factors that affect nesting in Australian and North American freshwater turtles. Journal of Zoology 267, 397–404.
| A comparative study of environmental factors that affect nesting in Australian and North American freshwater turtles.Crossref | GoogleScholarGoogle Scholar |
Bower, D. S., and Hodges, K. M. (2014). Chelodina expansa Gray 1857 – broad-shelled turtle, giant snake-necked turtle. In ‘Conservation Biology of Freshwater Turtles and Tortoises: a Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group’. (Eds A. G. J. Rhodin, P. C. H. Pritchard, P. P. van Dijk, R. A. Saumure, K. A. Buhlmann, J. B. Iverson, and R. A. Mittermeier.) Chelonian Research Monographs 5, 071.1–071.5.
Brown, G. P., Bishop, C. A., and Brooks, R. J. (1994). Growth rate, reproductive output, and temperature selection of snapping turtles in habitats of different productivities. Journal of Herpetology 28, 405–410.
| Growth rate, reproductive output, and temperature selection of snapping turtles in habitats of different productivities.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A. (1967). The biology of south-western Australian tortoises. Ph.D. Thesis, University of Western Australia, Perth.
Chessman, B. C. (1978). Ecological studies of freshwater turtles in south-eastern Australia. Ph.D. Thesis, Monash University, Melbourne.
Chessman, B. C. (1983). Observations on the diet of the broad-shelled turtle, Chelodina expansa Gray (Testudines: Chelidae). Australian Wildlife Research 10, 169–172.
| Observations on the diet of the broad-shelled turtle, Chelodina expansa Gray (Testudines: Chelidae).Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1984a). Evaporative water loss from three south-eastern Australian species of freshwater turtle. Australian Journal of Zoology 32, 649–655.
| Evaporative water loss from three south-eastern Australian species of freshwater turtle.Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1984b). Food of the snake-necked turtle, Chelodina longicollis (Shaw) (Testudines: Chelidae) in the Murray Valley, Victoria and New South Wales. Australian Wildlife Research 11, 573–578.
| Food of the snake-necked turtle, Chelodina longicollis (Shaw) (Testudines: Chelidae) in the Murray Valley, Victoria and New South Wales.Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1986). Diet of the Murray turtle, Emydura macquarii (Gray) (Testudines: Chelidae). Australian Wildlife Research 13, 65–69.
| Diet of the Murray turtle, Emydura macquarii (Gray) (Testudines: Chelidae).Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1987). Atmospheric and aquatic basking of the Australian freshwater turtle Emydura macquarii (Gray) (Testudines: Chelidae). Herpetologica 43, 301–306.
Chessman, B. C. (1988a). Habitat preferences of freshwater turtles in the Murray Valley, Victoria and New South Wales. Australian Wildlife Research 15, 485–491.
| Habitat preferences of freshwater turtles in the Murray Valley, Victoria and New South Wales.Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1988b). Seasonal and diel activity of freshwater turtles in the Murray Valley, Victoria and New South Wales. Australian Wildlife Research 15, 267–276.
| Seasonal and diel activity of freshwater turtles in the Murray Valley, Victoria and New South Wales.Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (2011). Declines of freshwater turtles associated with climatic drying in Australia’s Murray–Darling Basin. Wildlife Research 38, 664–671.
| Declines of freshwater turtles associated with climatic drying in Australia’s Murray–Darling Basin.Crossref | GoogleScholarGoogle Scholar |
Clark, N. J., Gordos, M. A., and Franklin, C. E. (2008a). Diving behaviour, aquatic respiration and blood respiratory properties: a comparison of hatchling and juvenile Australian turtles. Journal of Zoology 275, 399–406.
| Diving behaviour, aquatic respiration and blood respiratory properties: a comparison of hatchling and juvenile Australian turtles.Crossref | GoogleScholarGoogle Scholar |
Clark, N. J., Gordos, M. A., and Franklin, C. E. (2008b). Thermal plasticity of diving behavior, aquatic respiration, and locomotor performance in the Mary River turtle Elusor macrurus. Physiological and Biochemical Zoology 81, 301–309.
| Thermal plasticity of diving behavior, aquatic respiration, and locomotor performance in the Mary River turtle Elusor macrurus.Crossref | GoogleScholarGoogle Scholar | 18419556PubMed |
Clark, N. J., Gordos, M. A., and Franklin, C. E. (2009). Implications of river damming: the influence of aquatic hypoxia on the diving physiology and behaviour of the endangered Mary River turtle. Animal Conservation 12, 147–154.
| Implications of river damming: the influence of aquatic hypoxia on the diving physiology and behaviour of the endangered Mary River turtle.Crossref | GoogleScholarGoogle Scholar |
Dillon, M. E., Wang, G., and Huey, R. B. (2010). Global metabolic impacts of recent climate warming. Nature 467, 704–706.
| Global metabolic impacts of recent climate warming.Crossref | GoogleScholarGoogle Scholar | 20930843PubMed |
Eiby, Y. A., and Booth, D. T. (2011). Determining optimal incubation temperature for a head-start program: the effect of incubation temperature on hatchling Burnett River snapping turtles (Elseya albagula). Australian Journal of Zoology 59, 18–25.
| Determining optimal incubation temperature for a head-start program: the effect of incubation temperature on hatchling Burnett River snapping turtles (Elseya albagula).Crossref | GoogleScholarGoogle Scholar |
FitzGibbon, S. I., and Franklin, C. E. (2010). The importance of the cloacal bursae as the primary site of aquatic respiration in the freshwater turtle, Elseya albagula. Australian Zoologist 35, 276–282.
| The importance of the cloacal bursae as the primary site of aquatic respiration in the freshwater turtle, Elseya albagula.Crossref | GoogleScholarGoogle Scholar |
Frazer, N. B., Greene, J. L., and Gibbons, J. W. (1993). Temporal variation in growth rate and age at maturity of male painted turtles, Chrysemys picta. American Midland Naturalist 130, 314–324.
| Temporal variation in growth rate and age at maturity of male painted turtles, Chrysemys picta.Crossref | GoogleScholarGoogle Scholar |
Gatten, R. E. (1974). Effects of temperature and activity on aerobic and anaerobic metabolism and heart rate in the turtles Pseudemys scripta and Terrapene ornata. Comparative Biochemistry and Physiology Part A: Physiology 48, 619–648.
| Effects of temperature and activity on aerobic and anaerobic metabolism and heart rate in the turtles Pseudemys scripta and Terrapene ornata.Crossref | GoogleScholarGoogle Scholar |
Gatten, R. E. (1978). Aerobic metabolism in snapping turtles, Chelydra serpentina, after thermal acclimation. Comparative Biochemistry and Physiology Part A: Physiology 61, 325–337.
| Aerobic metabolism in snapping turtles, Chelydra serpentina, after thermal acclimation.Crossref | GoogleScholarGoogle Scholar |
Gatten, R. E. (1980). Aerial and aquatic oxygen uptake by freely-diving snapping turtles (Chelydra serpentina). Oecologia 46, 266–271.
| Aerial and aquatic oxygen uptake by freely-diving snapping turtles (Chelydra serpentina).Crossref | GoogleScholarGoogle Scholar | 28309683PubMed |
Georges, A. (1988). Sex determination is independent of incubation temperature in another chelid turtle, Chelodina longicollis. Copeia 1988, 248–254.
| Sex determination is independent of incubation temperature in another chelid turtle, Chelodina longicollis.Crossref | GoogleScholarGoogle Scholar |
Georges, A., and McInnes, S. (1998). Temperature fails to influence hatchling sex in another genus and species of chelid turtle, Elusor macrurus. Journal of Herpetology 32, 596–598.
| Temperature fails to influence hatchling sex in another genus and species of chelid turtle, Elusor macrurus.Crossref | GoogleScholarGoogle Scholar |
Georges, A., Norris, R. H., and Wensing, L. (1986). Diet of the freshwater turtle Chelodina longicollis (Testudines: Chelidae) from the coastal dune lakes of the Jervis Bay Territory. Australian Wildlife Research 13, 301–308.
| Diet of the freshwater turtle Chelodina longicollis (Testudines: Chelidae) from the coastal dune lakes of the Jervis Bay Territory.Crossref | GoogleScholarGoogle Scholar |
Georges, A., Guarino, F., and White, M. (2006). Sex-ratio bias across populations of a freshwater turtle (Testudines: Chelidae) with genotypic sex determination. Wildlife Research 33, 475–480.
| Sex-ratio bias across populations of a freshwater turtle (Testudines: Chelidae) with genotypic sex determination.Crossref | GoogleScholarGoogle Scholar |
Georges, A., Gruber, B., Pauly, G. B., White, D., Adams, M., Young, M. J., Kilian, A., Zhang, X., Shaffer, H. B., and Unmack, P. J. (2018). Genomewide SNP markers breathe new life into phylogeography and species delimitation for the problematic short-necked turtles (Chelidae: Emydura) of eastern Australia. Molecular Ecology 27, 5195–5213.
| 30403418PubMed |
Gibbons, J. W. (1970). Reproductive dynamics of a turtle (Pseudemys scripta) population in a reservoir receiving heated effluent from a nuclear reactor. Canadian Journal of Zoology 48, 881–885.
| Reproductive dynamics of a turtle (Pseudemys scripta) population in a reservoir receiving heated effluent from a nuclear reactor.Crossref | GoogleScholarGoogle Scholar |
Goode, J., and Russell, J. (1968). Incubation of eggs of three species of chelid tortoises, and notes on their embryological development. Australian Journal of Zoology 16, 749–761.
| Incubation of eggs of three species of chelid tortoises, and notes on their embryological development.Crossref | GoogleScholarGoogle Scholar |
Howard, K., Beesley, L., Ward, K., and Stokeld, D. (2016). Preliminary evidence suggests freshwater turtles respond positively to an environmental water delivery during drought. Australian Journal of Zoology 64, 370–373.
| Preliminary evidence suggests freshwater turtles respond positively to an environmental water delivery during drought.Crossref | GoogleScholarGoogle Scholar |
Hulin, V., Delmas, V., Girondot, M., Godfrey, M. H., and Guillon, J.-M. (2009). Temperature-dependent sex determination and global change: are some species at greater risk? Oecologia 160, 493–506.
| Temperature-dependent sex determination and global change: are some species at greater risk?Crossref | GoogleScholarGoogle Scholar | 19277719PubMed |
Hutchison, V. H., and Kosh, R. J. (1964). The effect of photoperiod on the critical thermal maxima of painted turtles (Chrysemys picta). Herpetologica 20, 233–238.
Kennett, R., and Christian, K. (1994). Metabolic depression in estivating long-neck turtles (Chelodina rugosa). Physiological Zoology 67, 1087–1102.
| Metabolic depression in estivating long-neck turtles (Chelodina rugosa).Crossref | GoogleScholarGoogle Scholar |
Kennett, R. M., and Georges, A. (1990). Habitat utilization and its relationship to growth and reproduction of the eastern long-necked turtle, Chelodina longicollis (Testudinata: Chelidae), from Australia. Herpetologica 46, 22–33.
Kennett, R., Roe, J., Hodges, K., and Georges, A. (2009). Chelodina longicollis (Shaw 1794) – eastern long-necked turtle, common long-necked turtle, common snake-necked turtle. In ‘Conservation Biology of Freshwater Turtles and Tortoises: a Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group’. (Eds A. G. J. Rhodin, P. C. H. Pritchard, P. P. van Dijk, R. A. Saumure, K. A. Buhlmann, J. B. Iverson, and R. A. Mittermeier.) Chelonian Research Monographs 5, 031.1–031.8.
Kepenis, V., and McManus, J. J. (1974). Bioenergetics of young painted turtles, Chrysemys picta. Comparative Biochemistry and Physiology Part A: Physiology 48, 309–317.
| Bioenergetics of young painted turtles, Chrysemys picta.Crossref | GoogleScholarGoogle Scholar |
King, P., and Heatwole, H. (1994). Partitioning of aquatic oxygen uptake among different respiratory surfaces in a freely diving pleurodiran turtle, Elseya latisternum. Copeia 1994, 802–806.
| Partitioning of aquatic oxygen uptake among different respiratory surfaces in a freely diving pleurodiran turtle, Elseya latisternum.Crossref | GoogleScholarGoogle Scholar |
Kirsch, R., and Vivien-Roels, B. (1984). Oxygen consumption in the tortoise, Testudo hermanni G., subjected to temperature changes in summer and autumn. Comparative Biochemistry and Physiology Part A: Physiology 79, 513–517.
| Oxygen consumption in the tortoise, Testudo hermanni G., subjected to temperature changes in summer and autumn.Crossref | GoogleScholarGoogle Scholar |
Legler, J. M. (1978). Observations on behavior and ecology in an Australian turtle, Chelodina expansa (Testudines: Chelidae). Canadian Journal of Zoology 56, 2449–2453.
| Observations on behavior and ecology in an Australian turtle, Chelodina expansa (Testudines: Chelidae).Crossref | GoogleScholarGoogle Scholar |
Ligon, D. B., and Lovern, M. B. (2009). Temperature effects during early life stages of the alligator snapping turtle (Macrochelys temminckii). Chelonian Conservation and Biology 8, 74–83.
| Temperature effects during early life stages of the alligator snapping turtle (Macrochelys temminckii).Crossref | GoogleScholarGoogle Scholar |
Lowell, W. R. (1990). Aerobic metabolism and swimming energetics of the turtle, Chrysemys picta. Experimental Biology 48, 349–355.
| 2365028PubMed |
Mathie, N. J., and Franklin, C. E. (2006). The influence of body size on the diving behaviour and physiology of the bimodally respiring turtle, Elseya albagula. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology 176, 739–747.
| The influence of body size on the diving behaviour and physiology of the bimodally respiring turtle, Elseya albagula.Crossref | GoogleScholarGoogle Scholar | 16791587PubMed |
Meathrel, C. E., Suter, P. J., and Radford, N. M. (2002). Niche segregation between three species of freshwater turtle in a large billabong during flood. Victorian Naturalist 119, 160–173.
Meathrel, C. E., Suter, P. J., and Reid, S. (2004). Habitat and dietary preferences of freshwater turtles in ephemeral billabongs on the Ovens River, north-east Victoria. Victorian Naturalist 121, 4–14.
Micheli-Campbell, M. A., Campbell, H. A., Cramp, R. L., Booth, D. T., and Franklin, C. E. (2011). Staying cool, keeping strong: incubation temperature affects performance in a freshwater turtle. Journal of Zoology 285, 266–273.
| Staying cool, keeping strong: incubation temperature affects performance in a freshwater turtle.Crossref | GoogleScholarGoogle Scholar |
Mitchell, N. J., and Janzen, F. J. (2010). Temperature-dependent sex determination and contemporary climate change. Sexual Development: Genetics, Molecular Biology, Evolution, Endocrinology, Embryology, and Pathology of Sex Determination and Differentiation 4, 129–140.
| Temperature-dependent sex determination and contemporary climate change.Crossref | GoogleScholarGoogle Scholar |
Mitchell, N. J., Jones, T. V., and Kuchling, G. (2012). Simulated climate change increases juvenile growth in a critically endangered tortoise. Endangered Species Research 17, 73–82.
| Simulated climate change increases juvenile growth in a critically endangered tortoise.Crossref | GoogleScholarGoogle Scholar |
Mitchell, N., Hipsey, M. R., Arnall, S., McGrath, G., Bin Tareque, H., Kuchling, G., Vogwill, R., Sivapalan, M., Porter, W. P., and Kearney, M. R. (2013). Linking eco-energetics and eco-hydrology to select sites for the assisted colonization of Australia’s rarest reptile. Biology 2, 1–25.
| Linking eco-energetics and eco-hydrology to select sites for the assisted colonization of Australia’s rarest reptile.Crossref | GoogleScholarGoogle Scholar |
Mitchell, N. J., Rodriguez, N., Kuchling, G., Arnall, S. G., and Kearney, M. R. (2016). Reptile embryos and climate change: modelling limits of viability to inform translocation decisions. Biological Conservation 204, 134–147.
| Reptile embryos and climate change: modelling limits of viability to inform translocation decisions.Crossref | GoogleScholarGoogle Scholar |
Ocock, J. F., Bino, G., Wassens, S., Spencer, J., Thomas, R. F., and Kingsford, R. T. (2018). Identifying critical habitat for Australian freshwater turtles in a large regulated floodplain: implications for environmental water management. Environmental Management 61, 375–389.
| Identifying critical habitat for Australian freshwater turtles in a large regulated floodplain: implications for environmental water management.Crossref | GoogleScholarGoogle Scholar | 28280912PubMed |
Parmenter, R. R. (1981). Digestive turnover rates in freshwater turtles: the influence of temperature and body size. Comparative Biochemistry and Physiology Part A: Physiology 70, 235–238.
| Digestive turnover rates in freshwater turtles: the influence of temperature and body size.Crossref | GoogleScholarGoogle Scholar |
Parmenter, C. J. (1985). Reproduction and survivorship of Chelodina longicollis (Testudinata: Chelidae). In ‘Biology of Australasian Frogs and Reptiles’. (Eds G. Grigg, R. Shine, and H. Ehmann.) pp. 53–61. (Royal Zoological Society of New South Wales: Sydney.)
Petrov, K., Lewis, J., Malkiewicz, N., Van Dyke, J. U., and Spencer, R.-J. (2018a). Food abundance and diet variation in freshwater turtles from the mid-Murray River, Australia. Australian Journal of Zoology 66, 67–76.
| Food abundance and diet variation in freshwater turtles from the mid-Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
Petrov, K., Stricker, H., Van Dyke, J. U., Stockfeld, G., West, P., and Spencer, R.-J. (2018b). Nesting habitat of the broad-shelled turtle (Chelodina expansa). Australian Journal of Zoology 66, 4–14.
| Nesting habitat of the broad-shelled turtle (Chelodina expansa).Crossref | GoogleScholarGoogle Scholar |
Pörtner, H. O., and Farrell, A. P. (2008). Physiology and climate change. Science 322, 690–692.
| Physiology and climate change.Crossref | GoogleScholarGoogle Scholar | 18974339PubMed |
Rapatz, G. L., and Musacchia, X. J. (1957). Metabolism of Chrysemys picta during fasting and during cold torpor. The American Journal of Physiology 188, 456–460.
| Metabolism of Chrysemys picta during fasting and during cold torpor.Crossref | GoogleScholarGoogle Scholar | 13411235PubMed |
Refsnider, J., and Janzen, F. J. (2012). Behavioural plasticity may compensate for climate change in a long-lived reptile with temperature-dependent sex determination. Biological Conservation 152, 90–95.
| Behavioural plasticity may compensate for climate change in a long-lived reptile with temperature-dependent sex determination.Crossref | GoogleScholarGoogle Scholar |
Refsnider, J. M., Bodensteiner, B. L., Reneker, J. L., and Janzen, F. J. (2013). Nest depth may not compensate for sex ratio skews caused by climate change in turtles. Animal Conservation 16, 481–490.
| Nest depth may not compensate for sex ratio skews caused by climate change in turtles.Crossref | GoogleScholarGoogle Scholar |
Roe, J. H., and Georges, A. (2007). Heterogeneous wetland complexes, buffer zones, and travel corridors: landscape management for freshwater reptiles. Biological Conservation 135, 67–76.
| Heterogeneous wetland complexes, buffer zones, and travel corridors: landscape management for freshwater reptiles.Crossref | GoogleScholarGoogle Scholar |
Roe, J. H., and Georges, A. (2008a). Maintenance of variable responses for coping with wetland drying in freshwater turtles. Ecology 89, 485–494.
| Maintenance of variable responses for coping with wetland drying in freshwater turtles.Crossref | GoogleScholarGoogle Scholar | 18409437PubMed |
Roe, J. H., and Georges, A. (2008b). Terrestrial activity, movements and spatial ecology of an Australian freshwater turtle, Chelodina longicollis, in a temporally dynamic wetland system. Austral Ecology 33, 1045–1056.
| Terrestrial activity, movements and spatial ecology of an Australian freshwater turtle, Chelodina longicollis, in a temporally dynamic wetland system.Crossref | GoogleScholarGoogle Scholar |
Roe, J. H., and Georges, A. (2010). Responses of freshwater turtles to drought: the past, present and implications for future climate change in Australia. In ‘Meltdown: Climate Change, Natural Disasters and Other Catastrophes – Fears and Concerns for the Future’ (Ed. K. Gow.) pp. 175–190. (Nova Science Publishers: New York.)
Santori, C., Spencer, R. J., Van Dyke, J. U., and Thompson, M. B. (2018). Road mortality of the eastern long-necked turtle (Chelodina longicollis) along the Murray River, Australia: an assessment using citizen science. Australian Journal of Zoology 66, 41–49.
| Road mortality of the eastern long-necked turtle (Chelodina longicollis) along the Murray River, Australia: an assessment using citizen science.Crossref | GoogleScholarGoogle Scholar |
Schwanz, L. E., and Janzen, F. J. (2008). Climate change and temperature-dependent sex determination: can individual plasticity in nesting phenology prevent extreme sex ratios? Physiological and Biochemical Zoology 81, 826–834.
| Climate change and temperature-dependent sex determination: can individual plasticity in nesting phenology prevent extreme sex ratios?Crossref | GoogleScholarGoogle Scholar | 18831689PubMed |
Seebacher, F., Sparrow, J., and Thompson, M. B. (2004). Turtles (Chelodina longicollis) regulate muscle metabolic enzyme activity in response to seasonal variation in body temperature. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology 174, 205–210.
| Turtles (Chelodina longicollis) regulate muscle metabolic enzyme activity in response to seasonal variation in body temperature.Crossref | GoogleScholarGoogle Scholar | 14722721PubMed |
Setlalekgomo, M. R., and Winter, P. E. D. (2016). Evidence for a circadian rhythm in the oxygen consumption of resting angulate tortoise (Chersina angulata). Journal of Entomology and Zoology Studies 4, 945–949.
Seymour, R. S. (1973). Physiological correlates of forced activity and burrowing in the spadefoot toad, Scaphiopus hammondii. Copeia 1973, 103–115.
| Physiological correlates of forced activity and burrowing in the spadefoot toad, Scaphiopus hammondii.Crossref | GoogleScholarGoogle Scholar |
Sievert, L. M., Sievert, G. A., and Cupp, P. V. (1988). Metabolic rate of feeding and fasting juvenile midland painted turtles, Chrysemys picta marginata. Comparative Biochemistry and Physiology Part A: Physiology 90, 157–159.
| Metabolic rate of feeding and fasting juvenile midland painted turtles, Chrysemys picta marginata.Crossref | GoogleScholarGoogle Scholar |
Snover, M. L., Adams, M. J., Ashton, D. T., Bettaso, J. B., and Welsh, H. H. (2015). Evidence of counter-gradient growth in western pond turtles (Actinemys marmorata) across thermal gradients. Freshwater Biology 60, 1944–1963.
| Evidence of counter-gradient growth in western pond turtles (Actinemys marmorata) across thermal gradients.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J., Thompson, M. B., and Hume, I. D. (1998). The diet and digestive energetics of an Australian short-necked turtle, Emydura macquarii. Comparative Biochemistry and Physiology Part A: Physiology 121, 341–349.
| The diet and digestive energetics of an Australian short-necked turtle, Emydura macquarii.Crossref | GoogleScholarGoogle Scholar |
Stockard, M. E., and Gatten, R. E. (1983). Activity metabolism of painted turtles (Chrysemys picta). Copeia 1983, 214–221.
| Activity metabolism of painted turtles (Chrysemys picta).Crossref | GoogleScholarGoogle Scholar |
Telemeco, R. S., Abbott, K. C., and Janzen, F. J. (2013). Modeling the effects of climate change-induced shifts in reproductive phenology on temperature-dependent traits. American Naturalist 181, 637–648.
| Modeling the effects of climate change-induced shifts in reproductive phenology on temperature-dependent traits.Crossref | GoogleScholarGoogle Scholar | 23594547PubMed |
Thompson, M. B. (1988). Influence of incubation temperature and water potential on sex determination in Emydura macquarii (Testudines: Pleurodira). Herpetologica 44, 86–90.
Thornhill, G. M. (1982). Comparative reproduction of the turtle, Chrysemys scripta elegans, in heated and natural lakes. Journal of Herpetology 16, 347–353.
| Comparative reproduction of the turtle, Chrysemys scripta elegans, in heated and natural lakes.Crossref | GoogleScholarGoogle Scholar |
Ultsch, G. R. (2013). Metabolic scaling in turtles. Comparative Biochemistry and Physiology Part A: Physiology 164, 590–597.
| Metabolic scaling in turtles.Crossref | GoogleScholarGoogle Scholar |
van Vliet, M. T. H., Franssen, W. H. P., Yearsley, J. R., Ludwig, F., Haddeland, I., Lettenmaier, D. P., and Kabat, P. (2013). Global river discharge and water temperature under climate change. Global Environmental Change 23, 450–464.
| Global river discharge and water temperature under climate change.Crossref | GoogleScholarGoogle Scholar |
Vestjens, W. J. M. (1969). Nesting, egg-laying and hatching of the snake-necked tortoise at Canberra, A.C.T. Australian Zoologist 15, 141–149.
Vleck, D. (1987). Measurement of O2 consumption, CO2, production, and water vapor production in a closed system. Journal of Applied Physiology 62, 2103–2106.
| Measurement of O2 consumption, CO2, production, and water vapor production in a closed system.Crossref | GoogleScholarGoogle Scholar | 3110127PubMed |
Webb, G. J. W., and Witten, G. J. (1973). Critical thermal maxima of turtles: validity of body temperature. Comparative Biochemistry and Physiology Part A: Physiology 45, 829–832.
| Critical thermal maxima of turtles: validity of body temperature.Crossref | GoogleScholarGoogle Scholar |
Williamson, L. U., Spotila, J. R., and Standora, E. A. (1989). Growth, selected temperature and CTM of young snapping turtles, Chelydra serpentina. Journal of Thermal Biology 14, 33–39.
| Growth, selected temperature and CTM of young snapping turtles, Chelydra serpentina.Crossref | GoogleScholarGoogle Scholar |
Wood, S. C., Lykkeboe, G., Johansen, K., Weber, R. E., and Maloiy, G. M. O. (1978). Temperature acclimation in the pancake tortoise, Malacochersus tornieri: metabolic rate, blood pH, oxygen affinity and red cell organic phosphates. Comparative Biochemistry and Physiology Part A: Physiology 59, 155–160.
| Temperature acclimation in the pancake tortoise, Malacochersus tornieri: metabolic rate, blood pH, oxygen affinity and red cell organic phosphates.Crossref | GoogleScholarGoogle Scholar |
Xu, W., Dang, W., Geng, J., and Lu, H.-L. (2015). Thermal preference, thermal resistance, and metabolic rate of juvenile Chinese pond turtles Mauremys reevesii acclimated to different temperatures. Journal of Thermal Biology 53, 119–124.
| Thermal preference, thermal resistance, and metabolic rate of juvenile Chinese pond turtles Mauremys reevesii acclimated to different temperatures.Crossref | GoogleScholarGoogle Scholar | 26590464PubMed |
Zhao, T., and Dai, A. (2015). The magnitude and causes of global drought changes in the twenty-first century under a low–moderate emissions scenario. Journal of Climate 28, 4490–4512.
| The magnitude and causes of global drought changes in the twenty-first century under a low–moderate emissions scenario.Crossref | GoogleScholarGoogle Scholar |


