Breeding ecology of the rainforest dung beetle Cephalodesmius armiger (Scarabaeidae) in Tooloom National Park
Elizabeth A. Dalgleish A B and Mark A. Elgar A CA Department of Zoology, University of Melbourne, Vic. 3010, Australia.
B Present address: Native Title Unit, Department of Justice, 55 St Andrews Place, East Melbourne, Vic. 3001, Australia.
C Corresponding author. Email: m.elgar@unimelb.edu.au
Australian Journal of Zoology 53(2) 95-102 https://doi.org/10.1071/ZO04067
Submitted: 21 September 2004 Accepted: 3 February 2005 Published: 6 April 2005
Abstract
The ecology of the rainforest dung beetle Cephalodesmius armiger was investigated in Tooloom National Park, within the high-altitude rainforest of north-east New South Wales. We observed considerable overlap in the brood-rearing stages of the breeding cycle. The operational sex ratio was female-biased. Biparental care is provided in this species, but it is not obligatory; females reared one brood either in partnership with a male or alone, and males did or did not pair with a female and rear a brood. There was spatial variation in the density of burrows. The availability of brood-burrows for brood rearing appears to be limited and evidence of the recycling of brood-burrows was collected for the first time. We suggest that the temporal variation in the breeding cycle arises from individual variation in the development and degeneration of the reproductive organs, rather than ecological factors favouring burrow and larder preparation. The female-biased operation sex ratio may result in males being more selective in their choice of mates than females and less committed to brood rearing.
Introduction
The explanatory power of studies of the evolution and maintenance of biparental care is often reduced by a lack of knowledge about the ecology of the model species under investigation. In particular, information on the operational sex ratio (the ratio of reproductively active females to males: Emlen and Oring 1977), and the availability of breeding sites (Parker and Simmons 1996; Ahnesjö et al. 2001) is often inadequate despite widespread recognition of their influence on mating systems, care of offspring and reproductive success. When the operational sex ratio is biased, the rarer sex is expected to be choosier and the more abundant sex is expected to compete for mates. Males may reduce or terminate care of offspring when the operational sex ratio is female-biased and other mating opportunities may be available (Keenleyside 1983; Whittingham 1994; Balshine-Earn 1995, 1997; Magrath and Elgar 1997). Similarly, where breeding requires specialised sites or environmental parameters that are limited, competition for such sites between individuals may enhance the attractiveness of individuals retaining such sites. Hence, without an understanding of the ecology of the species under investigation, empirical studies of biparental care may not adequately address theoretical predictions.
Most studies of parental care have focused on vertebrate taxa (Clutton-Brock 1991), largely because of the limited occurrence of uniparental and biparental care among invertebrates. However, the dung beetles (Scarabaeidae) exhibit a range of patterns of care of offspring from absence to uniparental and biparental care. Generally, copulation occurs away from dung sources and, in species with post-oviposition care, males often participate in dung collection and preparation but do not persist in caring beyond oviposition (Matthews 1963; Klemperer and Boulton 1976; Kingston and Coe 1977; Klemperer 1982; Tyndale-Biscoe 1983; Sato and Inamori 1987; Cook 1988; Edwards and Aschenborn 1988; Sowig 1996; Hunt and Simmons 1998; Sato 1998). Among dung beetles, Cephalodesmius armiger (subfamily Scarabaeinae) is unique because it exhibits prolonged biparental care including the ongoing provision of food to larvae throughout their growth (Monteith and Storey 1981). This species provides the opportunity to investigate biparental care in an invertebrate taxon.
Cephalodesmius armiger is a flightless, burrowing dung beetle. It is endemic to Australia and is restricted to higher-altitude subtropical rainforest fragments in south-east Queensland and north-east New South Wales. Some aspects of the natural history of C. armiger are described in Monteith and Storey (1981), who found that individuals forage on the surface primarily for plant material but opportunistically take dung and fruits from which a larder (brood mass) is prepared. Burrows are integral to all stages of the annual breeding cycle; single or paired (female and male) beetles inhabit burrows in the rainforest soil. Burrows have a single entrance in which males usually sit, thus effectively isolating the female in the brood chamber. Copulation occurs on the soil surface following the emergence of the teneral (new season) adults from brood-burrows and just before oviposition in the brood-burrow (Monteith and Storey 1981).
The subterranean structure of inhabited burrows (illustrated in Monteith and Storey 1981) varies seasonally. From late summer to early spring, following the emergence of teneral adults onto the soil surface, burrows may contain a male, a female or a male and female pair. Most burrows during this period consist of a short vertical shaft (5–8 cm) and a small chamber, and are termed feeding burrows. From early winter onwards, most burrows contain a male and female pair and consist of a long entrance that runs vertically (~10 cm) and then horizontally (3–5 cm) before terminating in a large chamber. Females in these brood-burrows oviposit single eggs into small balls of material taken from the larder (brood balls). Larvae hatch inside the brood balls. Brood balls are coated with additional material from the larder throughout growth and development. Females remain in the brood-burrow until death. Males usually remain in the brood-burrow until larvae pupate and sometimes until after the emergence of teneral offspring from brood balls. However, from mid to late summer, the male may leave the brood chamber and reside in a small subchamber above the brood chamber. These burrow complexes are termed double brood-burrows.
While Monteith and Storey (1981) provide valuable information on the natural history of C. armiger, data are lacking in several key areas important for understanding and interpreting the reproductive ecology of this species. For example, Monteith and Storey (1981) could not determine whether biparental care is obligate and their irregular sampling meant that no inferences could be drawn about patterns of pair formation or the operational sex ratio. Finally, the distribution and availablility of burrows for brood-rearing is poorly understood.
The broad aim of this study was to investigate aspects of the ecology of C. armiger that are likely to be important for reproduction. Specifically, we examine the patterns of variation in the breeding cycle, changes in the operational sex ratio in relation to the breeding cycle, variation in brood-rearing associations, the spatial distribution of burrows and the excavation and recycling of brood-burrows. These data are obtained for natural populations by intensive sampling of burrows, using artificial brood-burrows, and marking individuals in artificial brood-burrows and on the soil surface.
Methods
Field site and sampling techniques
Fieldwork was conducted in Tooloom National Park (28°29′S, 152°31′E), north-eastern New South Wales, Australia at an altitude of 700 m. The park contains both subtropical rainforest and wet sclerophyll forest. The study was restricted to subtropical rainforest within the national park and was concentrated along a sidetrack that ran off an old logging road (Tucker Box Road). This area was selected because beetles were common and burrows were easily located. The habitat was bare earth with leaf litter and patchy ground vegetation. Fieldwork was conducted from March 1997 to April 1999. Within this period, monitoring was undertaken in two reproductive seasons: from April 1997 to February 1998, and from April 1998 to March 1999.
Beetles were sexed and individually marked with plastic numbered tags, 2 mm in diameter, that were attached with super glue to either the dorsal or ventral surface. Both abdominal surfaces were utilised because limited combinations of tag numbers and colours were available. There is an overlap of generations during the emergence of teneral adults, which can be identified by a lack of tibial wear (Monteith and Storey 1981).
Separate plots were used for each of four sampling techniques: mapping of burrows, excavated survey of burrows, soil surface activity of individuals and artificial brood-burrows. The soil surface activity of individuals was investigated in 10 × 2 m belt transects. Study plots for all of the other sampling techniques were 4-m2 plots in the track, bounded on two sides by the track boundary, and running along the length of the sidetrack and its branches. All plots were separated by at least 10 m to ensure that only one sampling technique was used at each plot.
Mapping of natural burrows
Burrows were defined as sites of beetle residence and were visible as either a hole in the ground or an area of raised soil. Burrows were marked using bamboo skewers with tags attached, a grid was established on the 2 × 2 m plot, and the x–y coordinates of the burrows on the grid were recorded. Individual burrows were mapped and recorded as active or inactive periodically from November 1997 to April 1999. Thirteen plots were sampled in November 1997 and the original 13 plus another five (n = 18) were sampled from December 1997 until April 1999. Burrows were recorded as active if they satisfied at least one of the four following criteria: (1) leaf material in the entrance, (2) excavated soil around the entrance (push-up), (3) a beetle visible in the entrance, or (4) an open entrance. Burrows were recorded as inactive if the entrance was closed and there was no push-up. Disturbance of plots during the survey meant that it was not possible to determine whether some burrows were active or inactive. These burrows were recorded as undetermined. Active brood-burrows were further classified as newly excavated if there was any fresh dirt in the push-up or recycled if there was brood-rearing debris in the push-up. From these data, the density of active and inactive burrows per plot was calculated for each plot. A box plot of the density of burrows at each plot was used to allocate plots to one of three classes of density. The plots were surveyed and classified at each stage of the breeding cycle. High-burrow-density plots were defined as the higher tail of the box plot, medium-burrow-density plots were defined as the third quartile and low-burrow-density plots were defined as the first quartile and lower tail. These plots were used as control plots for classifying other plots according to burrow density. The plots used for each of the other sampling methods were mapped before field work and classified on the basis of box plots of burrow density at control plots, generated for each stage of the breeding cycle.
Survey of excavated natural burrows
All active and inactive natural burrows were excavated after natural burrows were mapped and burrow-density was calculated. In total, 609 natural burrows in 87 plots were excavated between 26 March 1997 and 16 April 1999. Excavation destroyed burrows. Excavation was undertaken between 1100 and 1500 hours when the surface temperature was highest and beetle activity levels were lowest. Each natural burrow was classified as a feeding, brood or double brood burrow, following Monteith and Storey (1981). The number and sex of beetles, and the presence of larder and brood balls in each natural burrow were recorded.
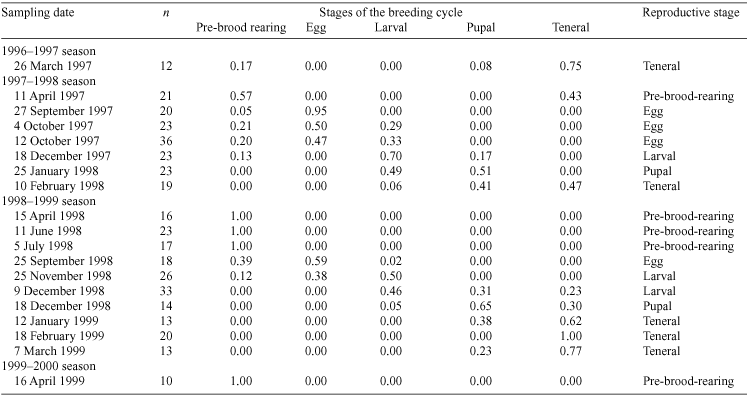
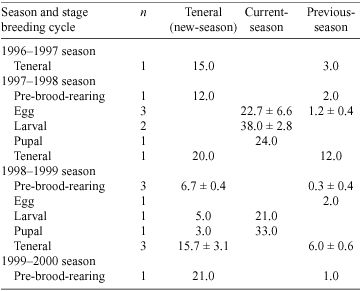
Offspring development was recorded for each brood ball as an egg, larva, pupa or hardening teneral adult. The size of brood balls varied with the stage of offspring development but not enough to differentiate between egg and early larva, late larva and pupa and late pupa and hardening teneral adult. To confirm the stage of offspring development, we scraped the brood ball with a fingernail until the contents were visible. Eggs were immobile within small brood balls. Larvae were identifiable because they defecated into the tear in the outside wall of the brood ball. Pupae were immobile within large brood balls. Hardening teneral adults were maroon in colour and later turned black. For each sampling date, the proportion of brood-burrows in each stage of the breeding cycle was determined. For each sampling date, the stage of the breeding cycle of the population was defined as the stage attained by most of the natural brood-burrows in a sample.
Generally, the operational sex ratio is calculated as the number of sexually receptive females divided by the number of sexually receptive males. In this study, the operational sex ratio was estimated as the proportion of pre-ovipositing and ovipositing females (pre-brood-rearing females and females with eggs) divided by the number of males. Clearly, this metric assumes that these groups of individuals are rarely unable to seek mates and always sexually receptive, and our observations suggest that this is the case (Dalgleish 2002).
Soil surface activity of individuals
To investigate soil surface activity, one transect was surveyed in each of a low-, medium- and high-burrow-density plot on 19 sampling dates between 8 April 1997 and 13 April 1999. The number and sex of beetles observed on the surface, their activity and the x–y coordinates of their position on each transect were recorded. The activity of beetles was recorded as undetermined, copulating or foraging (carrying leaf material). Individuals stand still when disturbed, but become active after a few minutes. To ensure that no animal was sampled twice, all captured individuals were held in containers and were processed after all transects were surveyed. This also standardised sampling time between transects as processing each individual took a few minutes.
Artificial brood-burrows
Artificial brood-burrows were based on the dimensions of real brood-burrows and were constructed from clear plastic containers (5 × 5 × 4 cm) with lids. Grey plastic conduit pipe of internal diameter 1.5 cm and length 10 cm was attached to the base of the container to provide an entrance tunnel to the brood chamber. Containers were buried so that the opening of the entrance was flush with the ground surface. Artificial brood-burrows were reburied in the low-, medium- or high-burrow-density plots from which they were initially excavated. In each season, low-burrow-density plots were 8 m2 in area to ensure a sufficient sample size of artificial brood-burrows. In each season, one plot was selected for use as each of a medium- and high-burrow-density plot. After mapping, active natural brood-burrows were excavated and their residents (single females, single males and pairs) and any larder and leaf material were transferred into artificial brood-burrows in either April 1997 (1997–1998 season) or April 1998 (1998–1999 season). In total, 56 artificial brood-burrows were established in six plots. Individual artificial brood-burrows were re-excavated and the number and sex of beetles, presence of any larder and brood balls, and the offspring stage of development were recorded before the artificial brood-burrow was replaced.
Results
Breeding cycle
The proportion of excavated natural brood-burrows at each stage of the breeding cycle is given in Table 1. In both seasons, there was considerable overlap between stages of the breeding cycle: some natural brood-burrows were still pre-brood-rearing when most contained larvae. In the 1997–1998 season, the egg stage of the breeding cycle was the most synchronised, while the pre-brood-rearing and teneral stages of the breeding cycle were the most synchronised in the 1998–1999 season. Teneral adults independent of their parents were observed earlier in the 1997–1998 than in the 1998–1999 season.
Associations among beetles and the operational sex ratio
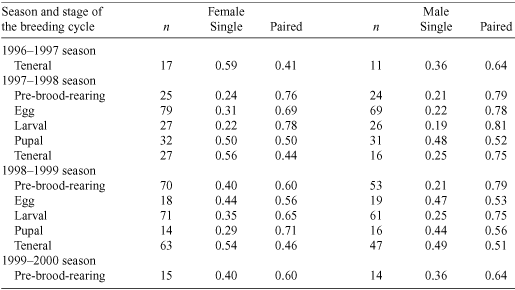
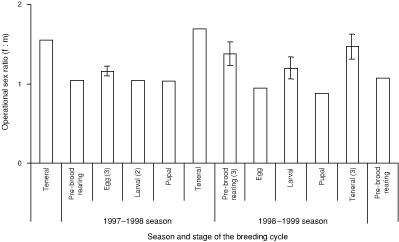
The proportions of single and paired individuals in excavated natural burrows varied according to the sex of the individual and the stage of the breeding cycle (Table 2). In the pre-brood-rearing and egg, larval and pupal stages of the breeding cycle there were nearly always more paired than single individuals (Table 2). The operational sex ratio was female-biased (χ2(1) = 5.97, P = 0.05) (Fig. 1) and did not vary significantly across stages of the breeding cycle in either season (1997–98: χ2(1) = 0.07, P = 0.79; 1998–99: χ2(1) = 0.89, P = 0.34) (Fig. 1).
|
|
Brood-rearing associations
Slightly more females reared broods in a pair than alone. In artificial brood-burrows, 61% of females reared their broods in a pair (n = 51 females). In excavated natural burrows, at least 65% of all females were in pairs in the larval stage of the breeding cycle in both seasons (Table 2). In contrast, males were nearly always in a pair. In artificial brood-burrows, 90% of males reared brood in a pair. In excavated natural burrows, ~75% of males were in pairs in the larval stage of the life cycle in both seasons (Table 2). In artificial brood-burrows, two unusual brood-rearing associations were noted: one male reared a brood alone after the female died during the larval stage, and one burrow contained two males and a female. One male remained in the burrow until the larval stage of offspring development and the other until the pupal stage of offspring development.
Soil surface activity
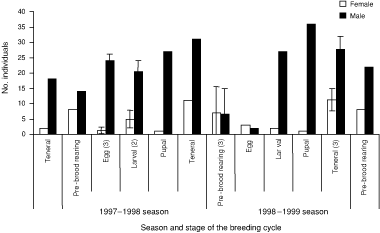
Males were always more active on the surface than females throughout different stages of the breeding cycle (Fig. 2). Surface activity was greatest in the teneral and pupal stages of the breeding cycle. Females from the previous season were never observed on the surface during the pupal, teneral and pre-brood-rearing stages of the breeding cycle. However, previous-season males were observed on the surface in the teneral and pre-brood-rearing stages of the breeding cycle (Table 3). The surface activity of teneral males was greater than that of previous-season males in the teneral stage of the breeding cycle in each season (Table 3).
|
|
Burrows
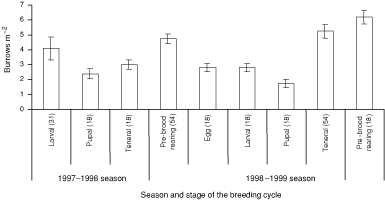
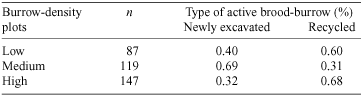
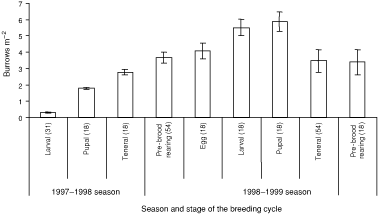
Natural brood-burrows were recycled between seasons or were newly excavated. Newly excavated burrows on the main track were washed away as a result of severe erosion associated with rain in both seasons. Burrows on sidetracks with lower gradients and those in the rainforest were not adversely affected by rain. The density of active natural burrows within plots varied significantly with stages of the breeding cycle (repeated-measures ANOVA: F9,33 = 14.82, P < 0.01). The density of active natural burrows declined in the pupal stage of the breeding cycle and peaked in the pre-brood-rearing stage of the breeding cycle in both seasons (Fig. 3). There were significantly more recycled brood-burrows in low- and high- than medium-burrow-density sites (χ2(2) = 39.08, P < 0.01) (Table 4). Pairs always occupied natural double brood-burrows. The density of inactive natural burrows within plots varied significantly with stages of the breeding cycle (repeated-measures ANOVA: F9,33 = 65.56, P < 0.01). Inactive natural burrows declined in density in the teneral stage during the 1998–1999 breeding season (Fig. 4).
|
|
|
Discussion
The decrease in the proportion of solitary individuals from the teneral to the brood-rearing stages of the breeding cycle suggests that pair formation typically occurs at this time. Interestingly, some males remain single throughout the brood-rearing stages of the breeding cycle, suggesting that single males are either non-breeding or that single males secure paternity in the broods of single and/or paired females without contributing to the care of offspring.
There was considerable variation in the timing of the stages of the breeding cycle. For example, some females were ovipositing when other brood-burrows were in the pupal stage of the breeding cycle, although the proportion of females ovipositing is lowest in the larval stage of the breeding cycle. This asynchrony implies that, for males, mating opportunities with females in their fertile period were available throughout the breeding cycle. This may influence whether or not paired males remain and care for offspring or desert and seek mating opportunities elsewhere. It is unclear whether temporal variation in the breeding cycle is a function of temporal variation in burrow preparation, larder preparation and/or maturation of the reproductive organs of females and males. It seems unlikely that burrow or larder preparation inhibit oviposition as pairs often form several months before oviposition, suggesting that there is ample time for both burrow and larder preparation. Thus, intraspecific variation in the maturation of the reproductive organs is a more probable explanation of temporal variation in the breeding cycle.
There is an association between the breeding cycle of C. armiger and ovarian and testicular development and degeneration. Ovarian degeneration occurs towards the end of the female lifespan, usually in the pupal and teneral stages of the breeding cycle (Monteith and Storey 1981; Lopez and Guerrero 1995) and is apparently associated with reduced food availability during brood-rearing (Lopez-Guerrero 1996). In the laboratory, testicular development occurred in January, February and March (equivalent to the teneral stage of the breeding cycle in the current study), mature sperm were present from the end of March to October (equivalent to the pre-brood-rearing, egg and larval stages of the breeding cycle in the current study) and testicular degeneration was observed from October to December (equivalent to the pupal and teneral stages of the breeding cycle in the current study) (Lopez-Guerrero and de Halffter 1991). These studies do not contradict our results, but rather the variation in the breeding cycle suggests that there is considerable intraspecific variation in the maturation and degeneration of reproductive organs.
The female bias in the operational sex ratio may be a product of sex differences in survivorship (Emlen and Oring 1977; Partridge and Endler 1987) and/or a bias to female offspring in broods (Clutton-Brock 1991). This bias is unlikely to be a product of sex differences in survivorship given that the female bias was most pronounced in the teneral stage of the breeding cycle when females on the soil surface and those in burrows were teneral, while males were either from the previous season or teneral. A female sex-ratio bias in broods is the most likely explanation for the female bias in the operational sex ratio observed in the field and such a bias was observed in both the field and laboratory (Dalgleish 2002). The female bias in the operational sex ratio implies that males may initially be more selective in their choice of mates than females. It also suggests that males may be less committed to brood-rearing than females given that other mating and pairing opportunities exist.
Both single and paired females were observed brood-rearing. In all, 39% of females were single when excavated and transferred to artificial brood-burrows, and all of these females reared broods alone. Monteith and Storey (1981) did not report single females rearing broods. The single females observed in the field were not a consequence of male desertion because male desertion was restricted exclusively to post-oviposition stages of the breeding cycle, suggesting that in this study single females were the result of females initiating brood-rearing alone (Dalgleish 2002). Instead, brood-rearing by single females is most likely a consequence of the lack of available males during pair formation. This suggests that biparental care is not obligatory and raises questions about what factors influence variation in the observed patterns of brood-rearing associations and why some males provide care.
Natural brood-burrows are often recycled, suggesting that the cost of excavating is substantial. There are several plausible explanations for the greater proportion of recycled natural brood-burrows in both low- and high-burrow-density plots compared with medium-burrow-density plots. At low burrow-density the soil root mass was greater and there were frequent gaps in the canopy than at other burrow-density plots. These factors may have resulted in decreased burrow humidity and suitable habitat for burrow construction may have been limited in these plots. Conversely, high-burrow-density plots were usually associated with a complete canopy and the absence of large trees adjacent to the track. High-burrow-density plots may reflect optimal habitat and habitat saturation may lead to greater pressure to recycle brood-burrows. This explanation suggests that brood-burrows are a limiting resource for reproduction. Accordingly, individuals that secure brood-burrows are expected to show enhanced attractiveness over individuals that do not and this attractiveness may be independent of the operational sex ratio. It is unclear how brood-burrows were recycled. Brood-burrows were recycled only between generations, suggesting that recycling may occur as either a result of the inheritance of brood-burrows from parents by natal offspring or by the occupation of brood-burrows by unrelated teneral individuals.
Acknowledgments
We thank John Alcock, Cybele Heddle, Mariella Herberstein, Peter Dwyer, Therésa Jones, Mick Keough, Michael Magrath, Angus Martin, Deidre Mattiske, Natasha McLean, Monica Minnegal, Geoff Monteith, Chris Nave, Jutta Schneider, Melissa Thomas and especially John Wright for their thoughtful insights and comments. We also appreciate the help provided in the field by Michael and Grant Wolstenholme, Beverley and Russell Robertson, Ron Dalgleish, Yasmine and Kai Jade, Ian and Mary Dalgleish, Jean and Les Bennett, Bob Tenner and the residents of Urbenville. The University of Melbourne and the Australian Research Council (grant A19802502) provided financial support.
Ahnesjö, I. , Kvarnemo, C. , and Mevilaita, S. (2001). Using potential reproductive rates to predict mating competition among individuals qualified to mate. Behavioral Ecology 12, 397–401.
| Crossref | GoogleScholarGoogle Scholar |
Cook, D. (1988). Sexual selection in dung beetles. II. Female fecundity as an estimate of male reproductive success in relation to horn size, and alternative behavioural strategies in Onthophagus binodis Thunberg (Scarabaeidae: Onthophagini). Australian Journal of Zoology 36, 521–532.
Edwards, P. B. , and Aschenborn, H. H. (1988). Male reproductive behaviour of the African ball-rolling dung beetle, Kheper nigroaeneus (Coleoptera; Scarabaeidae). Coleopterists Bulletin 42, 17–27.
Sato, H. (1998). Male participation in nest building in the dung beetle Scarabaeus catenatus (Coleoptera: Scarabaeidae): mating effort versus parental effort. Journal of Insect Behavior 11, 833–843.
| Crossref | GoogleScholarGoogle Scholar |

Sato, H. , and Inamori, M. (1987). Nesting behaviour of a sub-social African ball-roller Kheper platynotus (Coleoptera, Scarabaeidae). Ecological Entomology 12, 415–425.

Sowig, P. (1996). Duration and benefits of biparental brood care in the dung beetle Onthophagus vacca (Coleoptera: Scarbaeidae). Ecological Entomology 21, 81–86.

Tyndale-Biscoe, M. (1983). Effects of ovarian condition on nesting behaviour in a brood-caring dung beetle, Copris diversus Waterhouse (Coleoptera: Scarabaeidae). Bulletin of Entomological Research 73, 45–52.

Whittingham, L. A. (1994). Additional mating opportunities and male parental care in red-winged blackbirds. Animal Behaviour 48, 875–883.
| Crossref | GoogleScholarGoogle Scholar |