Optimising the hatching success of artificially incubated eggs for use in a conservation program for the western saw-shelled turtle (Myuchelys bellii)
Louise M. Streeting A * , Deborah S. Bower A , Martin L. Dillon A B , Phil Spark C , Michael Gough D , Adam Skidmore E , Paul G. McDonald A , Hannah Delaney A , Adrienne Burns A , Sandy Watson A , Duminda S. B. Dissanayake F , Arthur Georges
A * , Deborah S. Bower A , Martin L. Dillon A B , Phil Spark C , Michael Gough D , Adam Skidmore E , Paul G. McDonald A , Hannah Delaney A , Adrienne Burns A , Sandy Watson A , Duminda S. B. Dissanayake F , Arthur Georges  F and Donald T. McKnight A G
F and Donald T. McKnight A G
A School of Environmental and Rural Science, University of New England, Armidale, NSW 2350, Australia.
B Northern Tablelands Local Land Services, Armidale, NSW 2350, Australia.
C North West Ecological Services, Tamworth, NSW 2340, Australia.
D Latisternum, Dubbo, NSW 2830, Australia.
E Taronga Conservation Society Australia, Mosman, NSW 2088, Australia.
F Institute for Applied Ecology, University of Canberra, Canberra, ACT 2601, Australia.
G Department of Environment and Genetics, School of Agriculture, Biomedicine and Environment, La Trobe University, Wodonga, Vic. 3690, Australia.
Australian Journal of Zoology 70(2) 74-82 https://doi.org/10.1071/ZO22014
Submitted: 30 March 2022 Accepted: 24 October 2022 Published: 9 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Artificial incubation of eggs and the release of hatchlings into the wild is a common conservation intervention designed to augment threatened turtle populations. We investigate a range of incubation temperatures to establish an optimal temperature for maximum hatching success of western saw-shelled turtle (Myuchelys bellii) eggs. We report on the influence of incubation temperature on incubation duration and hatching success and describe two experimental incubation methods which, for the same incubation temperature (27°C), resulted in 77% and 97% hatching success, respectively. Eggs were incubated at constant temperatures (27°C, 28°C and 29°C) to determine the influence of temperature on incubation period, hatchling morphology and external residual yolk. Incubation duration was negatively correlated with incubation temperature. We report on the morphology of eggs and hatchlings and show that their dimensions are positively correlated with maternal adult size and mass. A constant incubation temperature of 27°C produced the highest hatching success and smallest external residual yolk on hatching and is therefore recommended for incubation of eggs for population reinforcement programs. Our study is the first to optimise artificial incubation procedures for M. bellii and will be a valuable resource for M. bellii and other threatened freshwater turtle conservation initiatives.
Keywords: artificial incubation, Bell’s turtle, conservation, endangered species, freshwater turtle, head-starting, population augmentation, population reinforcement, wildlife management.
Introduction
Almost half of Australian freshwater turtle species are under threat, with 11 of the 27 species listed as vulnerable, endangered, or critically endangered (Van Dyke et al. 2018; Turtle Taxonomy Working Group 2021). Predation of nests by the introduced European red fox (Vulpes vulpes) decreases juvenile recruitment and has been suggested as a predominant driver of decline in many Australian freshwater turtle populations (Thompson 1983; Spencer et al. 2017; Van Dyke et al. 2019). The artificial incubation of turtle eggs and release of neonate hatchlings into the wild is an important conservation strategy designed to avoid threats to the most vulnerable stage in turtle life history, and to increase recruitment of juveniles in threatened turtle populations. This strategy is particularly beneficial where the direct management of threats to turtle populations is difficult or unachievable.
Incubation temperature does not influence the sex of Australian chelid turtles (Mazzoleni et al. 2020). Nevertheless, temperature has a profound influence on incubation duration, hatching success, hatchling condition, phenotype, and posthatching growth, fitness and survival (Micheli-Campbell et al. 2011; Noble et al. 2018). Turtle eggs have a narrow range of incubation temperatures conducive to a high proportion of viable hatchlings, and temperatures outside of this viable incubation range reduce embryo survival (Birchard 2004). It is therefore imperative that artificial incubation methods use temperature regimes that are appropriate to each species.
The western saw-shelled turtle (Myuchelys bellii) is an endangered Australian freshwater turtle (IUCN 2021). Also known as Bell’s turtle, M. bellii is endemic to the high-elevation rivers and streams of the Namoi River, Gwydir River and Border Rivers basins (Chessman 2015). Populations predominantly comprise mature individuals, and juvenile recruitment is low, possibly due to high levels of nest predation by the introduced European red fox (Fielder et al. 2014). Hence, artificial egg incubation may be a valuable conservation strategy for M. bellii. The optimal techniques for incubating M. bellii eggs are unknown so a better understanding of the factors affecting hatching success will improve conservation prospects and outcomes for future population reinforcement programs. Our study aimed to investigate the influence of incubation temperature on incubation duration, identify an optimal constant incubation temperature and hydric conditions, and establish an incubation method to maximise hatching success for head-starting M. bellii. This method should help secure viable populations of the species as part of a New South Wales Government conservation program entitled ‘Turtles Forever: Securing Australia’s Wild Populations of Bell’s Turtle’.
Methods
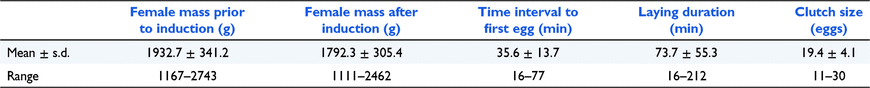
Our study was conducted over two breeding seasons (breeding season 1: November 2017 to March 2018; and breeding season 2: November 2018 to March 2019). Turtles were captured in the Macdonald River within 20 km of Bendemeer, New South Wales (30.870°S, 151.151°E) Australia. Field capture methods were identical to those reported by Fielder et al. (2014). Adult females were palpated in the rear leg inguinal pockets to detect hard-shelled eggs, and gravid females were transported to the University of New England. Gravid female turtles were weighed (±0.01 g), and egg-laying was induced by intramuscular injection of 2.2 mL kg−1 of Ilium Syntocin (Troy Animal Healthcare, Sydney), active constituent 10 IU mL−1 oxytocin, into the rear thigh (Fielder et al. 2014). Following injection, females were placed unrestrained into individual opaque plastic tubs (650 × 400 × 300 mm) containing 10 L of aged water. The tubs were covered with a dark cloth and the females were monitored throughout oviposition. Over the two breeding seasons, a total of 27 separate turtles were induced and each laid a single clutch of 11–30 eggs. In breeding season 1, 13 turtles were induced and in breeding season 2, 14 turtles were induced. Following egg-laying, female turtles were weighed and later released at their point of capture.
As each egg was deposited, the time was recorded, and eggs were immediately weighed (±0.001 g) and measured using Vernier callipers (±0.1 mm). Eggs were handled gently, and care was taken to maintain egg orientation (Limpus et al. 1979). Eggs were placed into clear plastic containers, directly onto a bed of moist vermiculite (1.1:1 parts water to vermiculite by weight) (Fielder et al. 2014) such that approximately one-third of the shell was in contact with the vermiculite. The eggs were placed in two rows and spaced at least 20 mm apart. To account for clutch effects, in breeding season 1, a split clutch design was used in which eggs from each female were systematically divided into three groups that were each assigned to one of three constant incubation temperatures: 27°C (n = 83 eggs), 28°C (n = 82 eggs) or 29°C (n = 80 eggs). Eggs were incubated in Panasonic MIR-554 406L incubators (±0.5°C). In breeding season 2, all eggs (n = 279) were incubated in a single incubator at a constant 27°C.
In breeding season 1, eggs were placed into plastic incubation containers (110 mm × 170 mm × 70 mm) and covered with loose-fitting plastic lids to allow airflow, and vermiculite moisture was replenished every 2–3 days. In breeding season 2, the plastic incubation containers were larger (150 mm × 220 mm × 70 mm) and were sealed with plastic film (Glad® Wrap, Clorox Australia Pty Ltd) held in place with a rubber band and a tight-fitting clip-on lid. The lid had a 146 mm × 216 mm area cut out of the centre leaving a 20 mm border. The containers were weighed fortnightly to track moisture loss. As necessary, a syringe was used to replenish water directly into the vermiculite, and care was taken to avoid water directly contacting the eggs. Eggs were checked daily and the date of hatching for each egg was recorded as the day on which the hatchling had completely emerged from the egg.
Upon hatching, the length and width of the external portion of yolk sac was measured using a ruler (±0.5 mm) to approximate the two-dimensional area (mm2) of yolk that had not been internalised. The total amount of residual yolk, including the proportion of yolk that had been internalised, was not recorded. In both breeding seasons, hatchlings were placed back into their incubator until external yolk was internalised, usually within 24–48 h, although some hatchlings took up to 72 h for complete internalisation of yolk. We used containers with 3–5 mm depth of aged water to individually house hatchlings in the incubator. Once yolk sacs had been internalised, hatchlings were weighed (±0.001 g), and Vernier callipers (±0.1 mm) were used to measure straight carapace length, carapace width, midline plastron length and plastron width (just posterior to the bridge). To derive a proxy for body condition index, the area of the plastron was calculated using the formula for finding the area of an ellipse. Body condition index = body mass/((plastron length/2) × (plastron width/2) × π). After being weighed and measured the hatchlings were placed into plastic holding tubs (320 mm × 570 mm × 380 mm) with an overhead UV-B light source, a basking platform, and 50 mm depth of aged water maintained at 22°C using a submersible aquarium heater. Hatchlings were released at their mother’s point of capture 3–4 weeks after hatching.
To identify the genotypic sex of a sample of hatchlings from breeding season 2, blood samples were collected from 73 hatchlings using QIAcard FTA Elute Micro cards (QIAGEN, Melbourne) and analysed using a PCR sex test (Dissanayake et al. 2022).
Statistical analyses
We conducted tests in R (ver. 3.5.0; R Core Team 2018) to examine the effects of female body size on clutch size and mass. Parametric statistics were used for analyses when assumptions of the tests were met (Pearson’s), otherwise non-parametric Spearman rank correlations were used. We tested the effect of female mass on clutch size (number of eggs), clutch mass, time lag from injection with oxytocin to commencement of oviposition, and duration of oviposition. We also tested the effect of female carapace length on clutch size and clutch mass. Finally, we tested the influence of clutch size on duration of oviposition after injection.
To examine factors influencing individual eggs (rather than entire clutches), we constructed mixed-effects models in R (ver. 3.5.0; R Core Team 2018) using the package lme4 (ver. 1.1-15; Bates et al. 2015). To control for female (and clutch) effects and avoid pseudoreplication, we included female ID as a random effect for all mixed-effects models. We assessed the significance of comparisons using the Anova function in the car package (ver. 2.1-6; Fox and Weisberg 2011) with Type II sums of squares and the Chi-square test statistic. We used quantile–quantile plots (qqp) and residual plots to check model fit. When necessary, the qqp function in the car package and the fitdistr function in the MASS package (ver. 7.3-51.4; Venables and Ripley 2002) were used to determine the appropriate model distribution. All models were linear models without data transformations unless otherwise specified.
We used these models to conduct the following tests: the effect of female mass on egg mass, hatchling mass, and hatchling carapace length; the effect of female carapace length on egg mass and hatchling mass; the effect of egg mass on hatchling mass; the effect of incubation temperature on incubation duration (after accounting for egg mass), hatching success (glmer) (Binomial distribution), residual yolk sac area (linear model on log10-transformed data), hatchling mass, hatchling straight carapace length, and hatchling body condition index; the effect of egg mass on incubation duration; and the effect of the incubation method applied during each breeding season on hatching success of eggs incubated at 27°C. For tests using incubation temperature, only data from breeding season 1 (during which eggs were randomised among three temperature treatments) were used. The influence of different incubation temperatures on hatching success, external yolk sac area, and on body condition index was tested with post hoc Tukey tests using the glht function in the multcomp package (Hothorn et al. 2008). We tested whether the sex ratio of a sample of hatchlings differed from parity using an exact binomial test.
Results
In this study we developed an egg incubation protocol that resulted in a high hatching success rate for M. bellii. Eggs incubated at 27°C in breeding season 2 had a significantly higher hatching success rate (97%) (261 hatched/269 fertile eggs) than eggs incubated at 27°C in breeding season 1 (77%) (64 hatched/83 fertile eggs) (χ2 = 17.59, P < 0.0001, d.f. = 1).
For the 27 female M. bellii induced in breeding seasons 1 and 2, the time interval between injection of synthetic oxytocin and the commencement of oviposition, the duration of oviposition, and clutch size varied between individual turtles (Table 1). Adult female mass was not correlated with either the duration of oviposition (rs = −0.30, P = 0.123) or the interval between the injection of oxytocin and the onset of oviposition (rs = 0.01, P = 0.955). Clutch size was not correlated with the interval between the injection of oxytocin and the completion of oviposition (rs = 0.05, P = 0.816).
|
Over breeding seasons 1 and 2, a total of 27 separate turtles were induced and each laid a single clutch of eggs. In breeding season 1, 13 turtles were induced and in breeding season 2, 14 turtles were induced. A total of 25 eggs failed to develop a white patch during incubation and were considered infertile (Thompson 1985; Fielder et al. 2014).
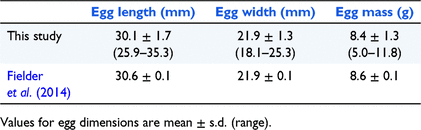
Egg dimensions were similar between breeding seasons 1 and 2 and were similar to those reported by Fielder et al. (2014) (Table 2). Mass of adult females was positively correlated with clutch size (rs = 0.50, P = 0.008, d.f. = 25) (Fig. 1), clutch mass (rs = 0.72, P < 0.0001, d.f. = 25), egg mass (χ2 = 24.43, P < 0.0001, d.f. = 1), hatchling mass (χ2 = 24.86, P < 0.0001, d.f. = 1), and hatchling carapace length (χ2 = 21.19, P < 0.0001, d.f. = 1). Straight carapace length of adult females was positively correlated with clutch size (rs = 0.45, P = 0.020, d.f. = 25), clutch mass (rs = 0.56, P = 0.002, d.f. = 25), egg mass (χ2 = 10.46, P = 0.001, d.f. = 1), and hatchling mass (χ2 = 8.54, P = 0.004, d.f. = 1). There was a strong positive correlation between egg mass and hatchling mass (χ2 = 249.72, P < 0.0001, d.f. = 1).
|
Incubation temperature was negatively correlated with incubation duration (χ2 = 496.00, P < 0.0001, d.f. = 2) (Fig. 2). In breeding season 1, eggs incubated at 27°C had an incubation duration of 59.5 ± 1.3 days (range 57–64 days), eggs incubated at 28°C had an incubation duration of 54.1 ± 1.8 days (range 48–60 days), and eggs incubated at 29°C had an incubation duration of 50.3 ± 2.6 days (range 49–58 days). In breeding season 2, eggs incubated at 27°C had an incubation duration of 60.3 ± 0.9 days (range 58–64 days). The incubation duration for eggs incubated at 27°C was significantly different between breeding seasons 1 and 2 (χ2 = 0.011, P < 0.011, d.f. = 1); however, incubation duration was recorded in whole days and the difference in mean incubation duration of less than one day may be an artefact of variability in the time of day at which daily checks for hatched eggs were conducted. Egg mass did not influence incubation duration (χ2 = 0.65, P = 0.420, d.f. = 1). Incubation temperature strongly influenced hatching success (χ2 = 36.59, P < 0.0001, d.f. = 2). In breeding season 1, eggs incubated at 27°C had a higher hatching success rate (77.1%) than eggs incubated at 28°C (40.2%) or 29°C (29.6%) (Tukey’s post hoc tests: P < 0.0001 for both comparisons); however, hatching successes of eggs incubated at 28°C or 29°C were not significantly different from each other (Tukey’s post hoc test, P = 0.135).
In breeding season 1, hatchlings from eggs incubated at 27°C had a significantly smaller external residual yolk sac area than hatchlings from eggs incubated at 28°C and 29°C (Tukey’s post hoc tests: P < 0.05 and P < 0.001 respectively); however, the external residual yolk sac areas of hatchlings from eggs incubated at 28°C or 29°C were not significantly different from each other (Tukey’s post hoc test, P = 0.162) (Fig. 3).
Incubation temperature within the range 27–29°C did not influence hatchling mass (χ2 = 0.07, P = 0.967, d.f. = 2) or carapace length (χ2 = 3.07, P = 0.216, d.f. = 2) but there was mixed evidence of its influencing the body condition index of hatchlings. Eggs incubated at 27°C had significantly higher body condition index than eggs incubated at 28°C (Tukey’s post hoc test, P = 0.035) but not eggs incubated at 29°C (Tukey’s post hoc test, P = 0.866), and eggs incubated at 28°C and 29°C were not significantly different (Tukey’s post hoc test, P = 0.261). Average hatchling dimensions, mass and body condition were similar between breeding seasons (Table 3). Two hatchlings (siblings incubated at 27°C in breeding season 2) had developmental anomalies: both were missing a right eye and both had a lower mandible protruding to the left side.

|
The sex ratio of 73 hatchlings from eggs incubated at constant 27°C in breeding season 2 did not significantly differ from parity (29 males, 44 females, exact binomial test, 95% CI = 0.481–0.715, P = 0.101).
Discussion
Incubation temperature influenced hatching success, with the lowest temperature (27°C) maximising hatching success rate. Hatching success at 27°C was 77% in breeding season 1 and 97% in breeding season 2. In breeding season 2 we modified our incubation method with the aim of improving hatching success. The key improvements employed were the use of plastic film to seal egg containers, weighing of egg containers at fortnightly intervals to quantify water loss, replenishing lost water using a syringe to place water directly into the vermiculite bedding and avoid any direct wetting of eggs. We hypothesise that this method reduced the occurrence of fungal or bacterial infections and improved control of hydric conditions. Although beyond the scope of this study, it is worth mentioning that this improved method was applied in two subsequent breeding seasons, resulting in 100% (141 hatched/141 fertile eggs) and 99% (294 hatched/295 fertile eggs) hatching success rates. The mean hatching success rate over breeding seasons 2, 3 and 4 was 99%. The consistent 97–100% hatching success rates suggest that 27°C may be close to the optimal incubation temperature for M. bellii, and that our incubation method used in season 2 is highly effective. We recommend the incubation method we describe for breeding season 2 for future M. bellii egg incubation. Future refinements to our method could include testing fluctuating incubation temperature regimes, cooler temperatures, and measuring posthatching fitness. There is a body of evidence that fluctuating incubation temperature regimes more closely resembling natural nest conditions are beneficial for both hatching success and posthatching fitness (Niehaus et al. 2012; Noble et al. 2018).
Six studies that report hatching success rates greater than 90% for Australian freshwater turtles applied constant incubation temperatures between 26°C and 32°C and provide data on hatching success for Chelodina expansa (Booth 2002), Chelodina longicollis (Georges 1988; Booth 2014), Emydura macquarii (Thompson 1988; Booth and Yu 2009) and Elusor macrurus (Georges and McInnes 1998). Most of these studies achieved hatching success rates greater than 90% by incubating eggs at a constant temperature of 28°C or higher. In contrast, our highest hatching success rate for M. bellii was achieved at 27°C. The distribution of M. bellii is restricted to high altitudes (600–1100 m) with a temperate climate (Fielder et al. 2015). Our comparatively low optimal incubation temperature may reflect the likely cooler natural incubation conditions within this turtle’s geographic distribution. However, egg incubation studies for Australian freshwater turtles have employed a range of incubation methods with various hydric and temperature regimes and varying substrate media. This variation in methods means that reported egg hatching success rates may not be directly comparable between studies.
Our results for incubation duration align with other published data for turtles, in which incubation duration is shorter at higher temperatures (Lawson and Rollinson 2021). Our results for eggs incubated at 29°C align with the results of Fielder et al. (2014) in which M. bellii eggs from two clutches incubated at a constant 29°C had durations of 49 and 51 days. However, our result of 60 days for eggs (n = 325) incubated at 27°C differs from the 80-day duration reported by Cann (1998) for M. bellii eggs incubated at approximately 27°C. The reason for this difference is unknown, but we suggest it is likely that the approximate incubation temperature reported by Cann (1998) was either lower or more variable than our incubation regime.
In many turtle species, lower incubation temperatures can result in more yolk being converted into hatchling body tissue (Booth 2006, 2018). In our study, hatchlings from eggs incubated at lower temperatures tended to internalise a greater proportion of yolk prior to hatching and so hatchlings incubated at 27°C had comparatively smaller external residual yolk sac areas upon hatching. Shorter incubation duration resulting in a lesser amount of yolk converted to body tissues has been reported for other freshwater turtles including Chelydra serpentina (Packard et al. 1987) and C. expansa (Booth 2000). An exception is reported by Booth (1998) for E. macquarii where residual yolk mass was not affected by incubation temperature in eggs incubated at a range of constant temperatures between 24°C and 31°C. Comparatively larger amounts of residual yolk have also been reported for hatchling turtles incubated under drier conditions (Packard et al. 1987; Booth 2002) and under more saline conditions (Bower et al. 2013). It should be noted that these studies euthanised the hatchlings and dissected out total residual yolk for measurement, whereas our study measured only the external portion of the residual yolk of live hatchlings. In our study, incubation temperature had no significant effect on hatchling carapace length, which may suggest that thermal effects on the conversion of yolk to tissue may not be readily differentiated within the thermal range of 27–29°C. In breeding season 1 of our study, hydric conditions were not precisely maintained, so it is unknown whether incubation temperature and/or hydric condition influenced external residual yolk sac area.
Moisture interacts with temperature to influence hatching success, incubation duration, and yolk absorption (e.g. Packard et al. 1987). This interaction is less pronounced in turtle species with rigid-shelled eggs compared with those with flexible-shelled eggs that exchange water more readily with the environment (Packard et al. 1981). M. bellii has rigid-shelled eggs. Booth (2002) and Booth and Yu (2009) found that relative to flexible-shelled species, hydric incubation conditions had less of an influence on the embryonic development and hatchling fitness in two other chelid species with rigid-shelled eggs (C. expansa and E. macquarii). In breeding season 2 we controlled the hydric environment within the egg incubation containers. We sealed the egg containers with plastic film, monitored egg container weight fortnightly to track moisture loss, we used a syringe to add measured quantities of water to the vermiculite (as required), and took care to ensure that added water did not come into direct contact with eggs. This process entailed less handling, less variation in moisture levels, reduced risk of exposure of eggs to pathogens, and, at the incubation temperature of 27°C, resulted in a higher hatching success rate than in breeding season 1.
Maternal influences on neonate size have been reported across all groups of reptiles (e.g. Packard et al. 1987; Janzen 1993; Congdon et al. 1995; Reichling and Gutzke 1996; Nelson et al. 2004). Our study found a positive correlation between adult female size and clutch size, egg mass, hatchling mass and hatchling size. This aligns with other studies (Congdon and Gibbons 1987; McKnight et al. 2018; Iverson et al. 2019). Furthermore, we found hatchling mass was positively correlated with egg mass but was not influenced by incubation temperature. This finding is consistent with the reviews of Booth (2006, 2018) that found overall hatchling mass is not influenced by incubation temperature, but the fractions of mass contained as hatchling tissue and as residual yolk is influenced by incubation temperature – hatchlings incubated at cooler temperatures have proportionally more tissue and less residual yolk.
Genetic sex determination is believed to be ubiquitous in the family Chelidae (Martinez et al. 2008; Warner 2011; Mazzoleni et al. 2020) and therefore we hypothesised that incubation temperature would not influence the sex of M. bellii hatchlings. Incubation of eggs at a constant 27°C produced both male and female hatchlings and the sex ratio did not significantly differ from 1:1. Although these results are likely indicative of genetic sex determination in M. bellii, they do not definitively rule out temperature-dependent sex determination, as 27°C could be the pivotal incubation temperature that produces a 1:1 sex ratio in M. bellii.
Our study draws on a robust sample size of 27 clutches and 524 eggs to report on M. bellii egg dimensions and mass, clutch size, and hatchling dimensions and mass. Information on Australian freshwater turtle egg dimensions, clutch size and female size is not often reported, yet this information may be valuable for distinguishing between eggs of species and subspecies (McKnight et al. 2018) and may also be useful for identifying the nests (intact or predated) of sympatric species. However, the sympatric eastern long-necked turtle (C. longicollis) has eggs that are about 20 mm wide and 30 mm long with a mass of 6–7 g (Kennett et al. 2009) and therefore the eggs or eggshells of M. bellii and C. longicollis cannot be reliably distinguished based on length or width.
Although the average clutch size of 19.4 eggs that we report here for M. bellii is larger than the average clutch size of 14 eggs reported for C. longicollis by Kennett et al. (2009), the range of clutch sizes has considerable overlap between the two species and therefore clutch size cannot be reliably used to distinguish between the two species. The clutch size, egg dimensions and egg mass we report for M. bellii are similar to data presented for two M. bellii clutches reported by Fielder et al. (2014) (Table 2), and hatchling dimensions are also similar.
Fielder et al. (2014) report that M. bellii has one of the lowest reproductive outputs of any Australian chelid, with annual fecundity estimated at 14.3 eggs per adult female. This assertion was based on their reported average clutch size of 18.3 eggs (derived from a sample of three clutches with a total of 55 eggs), their reported proportion of 78% of mature females that are productive in any one season (based on a sample of 84 mature females), and their finding that individual females lay only one clutch per breeding season (based on data from seven consecutive breeding seasons). Our study reports an average clutch size of 19.4 eggs based on 27 clutches (524 eggs), and therefore our data suggest that annual fecundity (calculated on the basis of clutch size × clutches per season × average proportion of mature females breeding annually) is 15.1 eggs per female. This value is only slightly higher than the annual fecundity of 14.3 eggs per female reported by Fielder et al. (2014) and therefore we support their conclusion that M. bellii has low annual fecundity relative to values reported for many other Australian freshwater turtles.
Head-starting is conducted as part of many turtle conservation programs (Burke 2015) and aims to increase juvenile recruitment by artificially improving hatching success (Eiby and Booth 2011). The release of head-started hatchlings produced by the artificial incubation of eggs aims to bypass threats to embryo survival in the wild. Our study suggests that artificial incubation is a strategy that could successfully be employed to boost hatchling numbers in wild M. bellii populations. Neonate turtles tend to have low survival rates (Heppell et al. 1996) and therefore large quantities of hatchlings need to be produced (whether naturally or through artificial incubation) to ensure that some reach maturity and are recruited into the adult breeding population. In the case of M. bellii, artificial incubation is an efficient and productive augmentation strategy, as adult turtles are locally abundant, gravid females can be readily captured for induction, and our described incubation method results in high hatching success rates. The occurrence of nesting behaviour several weeks after the harvesting of all eggs via hormonal induction has been reported in E. macquarii and C. expansa (McCosker 2002). It is unknown whether M. bellii carry out nesting behaviour once released into the wild following the harvesting of eggs via hormonal induction but, if so, hormonal induction would not reduce the risk of predation of nesting turtles by terrestrial predators. Releasing artificially incubated neonates soon after hatching, rather than raising them in captivity, has the advantage of avoiding any risk of hatchlings becoming habituated to captivity and failing to develop appropriate behaviours for predator avoidance, foraging and habitat selection (Bennett et al. 2017; Santori et al. 2021).
Some authors warn that artificial incubation and/or head-starting programs may be ineffective relative to the benefit of conserving adult turtles (e.g. Heppell et al. 1996; Seigel and Dodd 2000; Páez et al. 2015). However, in the case of M. bellii, nest depredation by a novel invasive predator, the European red fox, is potentially so high (>95%) that the breeding effort of wild adult turtles is effectively negated, and juvenile recruitment may be insufficient to replenish wild populations (Streeting et al. 2021). Other benefits associated with head-starting programs include opportunities to engage the community by using hatchling turtles as ambassadors to generate public interest in conservation (Burke 2015). The survival, growth, and movement of released hatchlings from this study are the subject of ongoing research, as are other conservation strategies for M. bellii including the in situ protection of wild nests (Streeting et al 2021). Population reinforcement programs require long-term monitoring to assess their effectiveness (Carstairs et al. 2019). The hormonal induction of wild-caught gravid females and the incubation of eggs at 27°C proved to be an efficient and successful way of harvesting M. bellii eggs and producing hatchlings for release into the wild. We recommend these methods to support population augmentation programs for the western saw-shelled turtle.
Data availability
The data that support this study are available in the Dryad repository (Streeting et al. 2022).
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study has been supported by the New South Wales Government through its Environmental Trust (Saving Our Species Partnership Grant 2015/SS/0017) and through additional funding from the Biodiversity and Conservation Division of New South Wales Department of Planning and Environment.
Acknowledgements
We acknowledge the Anaiwan and Kamilaroi people, Traditional Custodians of the lands on which this study was conducted. We thank Jesse, Kael and Charlotte Streeting, Lachlan and Ruby Spark, Kelly Twigge, Geoff Hughes, Megan, Abigail and Emily McDonald, Ashley Saint, Todd Soderquist and Sam Doak for their assistance with the induction of turtles and fieldwork. We thank Craig and Sarah Bailey for veterinary advice. Thank you to Cathy Dillon, David Booth and an anonymous reviewer for comments that improved the manuscript. We also thank the landowners who allowed us access to their properties and have taken an active interest in supporting this conservation research. This research was authorised by the New South Wales Department of Primary Industry Scientific Collection Permit No. P17_0062, New South Wales Department of Planning, Industry and Environment Scientific Licence No. SL101876, and University of New England Animal Ethics Committee Approval No. AEC17-110. This study has been assisted by the New South Wales Government through its Environmental Trust.
References
Bates, D, Mächler, M, Bolker, B, and Walker, S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bennett, AM, Steiner, J, Carstairs, S, Gielens, A, and Davy, CM (2017). A question of scale: replication and the effective evaluation of conservation interventions. Facets 2, 892–909.
| A question of scale: replication and the effective evaluation of conservation interventions.Crossref | GoogleScholarGoogle Scholar |
Birchard GF (2004) Effects of incubation temperature. In ‘Reptilian incubation: environment, evolution and behaviour’. (Ed. DC Deeming) pp. 103–123. (Nottingham University Press: Nottingham)
Booth, DT (1998). Effects of incubation temperature on the energetics of embryonic development and hatchling morphology in the Brisbane river turtle Emydura signata. Journal of Comparative Physiology B 168, 399–404.
| Effects of incubation temperature on the energetics of embryonic development and hatchling morphology in the Brisbane river turtle Emydura signata.Crossref | GoogleScholarGoogle Scholar |
Booth, DT (2000). Incubation of eggs of the Australian broad-shelled turtle, Chelodina expansa (Testudinata: Chelidae), at different temperatures: effects on pattern of oxygen consumption and hatchling morphology. Australian Journal of Zoology 48, 369–378.
| Incubation of eggs of the Australian broad-shelled turtle, Chelodina expansa (Testudinata: Chelidae), at different temperatures: effects on pattern of oxygen consumption and hatchling morphology.Crossref | GoogleScholarGoogle Scholar |
Booth, DT (2002). Incubation of rigid-shelled turtle eggs: do hydric conditions matter? Journal of Comparative Physiology B 172, 627–633.
| Incubation of rigid-shelled turtle eggs: do hydric conditions matter?Crossref | GoogleScholarGoogle Scholar |
Booth, DT (2006). Influence of incubation temperature on hatchling phenotype in reptiles. Physiological and Biochemical Zoology 79, 274–281.
| Influence of incubation temperature on hatchling phenotype in reptiles.Crossref | GoogleScholarGoogle Scholar |
Booth, DT (2014). Longer incubation periods are energetically costly for turtle embryos. Annual Research & Review in Biology 4, 2931–2937.
| Longer incubation periods are energetically costly for turtle embryos.Crossref | GoogleScholarGoogle Scholar |
Booth, DT (2018). Incubation temperature induced phenotypic plasticity in oviparous reptiles: Where to next? Journal of Experimental Zoology Part A: Ecological and Integrative Physiology 329, 343–350.
| Incubation temperature induced phenotypic plasticity in oviparous reptiles: Where to next?Crossref | GoogleScholarGoogle Scholar |
Booth, DT, and Yu, CY (2009). Influence of the hydric environment on water exchange and hatchlings of rigid-shelled turtle eggs. Physiological and Biochemical Zoology 82, 382–387.
| Influence of the hydric environment on water exchange and hatchlings of rigid-shelled turtle eggs.Crossref | GoogleScholarGoogle Scholar |
Bower, DS, Hodges, KM, and Georges, A (2013). Salinity of incubation media influences embryonic development of a freshwater turtle. Journal of Comparative Physiology B 183, 235–241.
| Salinity of incubation media influences embryonic development of a freshwater turtle.Crossref | GoogleScholarGoogle Scholar |
Burke, RL (2015). Head-starting turtles: learning from experience. Herpetological Conservation and Biology 10, 299–308.
Cann J (1998) ‘Freshwater turtles of Australia.’ (Beaumont Publishing: Singapore)
Carstairs, S, Paterson, JE, Jager, KL, Gasbarrini, D, Mui, AB, and Davy, CM (2019). Population reinforcement accelerates subadult recruitment rates in an endangered freshwater turtle. Animal Conservation 22, 589–599.
| Population reinforcement accelerates subadult recruitment rates in an endangered freshwater turtle.Crossref | GoogleScholarGoogle Scholar |
Chessman, BC (2015). Distribution, abundance and population structure of the threatened western saw-shelled turtle, Myuchelys bellii, in New South Wales, Australia. Australian Journal of Zoology 63, 245–252.
| Distribution, abundance and population structure of the threatened western saw-shelled turtle, Myuchelys bellii, in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Congdon, JD, and Gibbons, JW (1987). Morphological constraint on egg size: a challenge to optimal egg size theory? Proceedings of the National Academy of Sciences of the United States of America 84, 4145–4147.
| Morphological constraint on egg size: a challenge to optimal egg size theory?Crossref | GoogleScholarGoogle Scholar |
Congdon, JD, Fischer, RU, and Gatten, RE (1995). Effects of incubation temperatures on characteristics of hatchling American alligators. Herpetologica 51, 497–504.
Dissanayake, DSB, Streeting, LM, Georges, A, and Bower, DS (2022). A male-specific sex marker for the endangered western sawshelled turtle (Myuchelys bellii) using in silico whole-genome subtraction. Conservation Genetics Resources 14, 231–236.
| A male-specific sex marker for the endangered western sawshelled turtle (Myuchelys bellii) using in silico whole-genome subtraction.Crossref | GoogleScholarGoogle Scholar |
Eiby, YA, and Booth, DT (2011). Determining optimal incubation temperature for a head-start program: the effect of incubation temperature on hatchling Burnett River snapping turtles (Elseya albagula). Australian Journal of Zoology 59, 18–25.
| Determining optimal incubation temperature for a head-start program: the effect of incubation temperature on hatchling Burnett River snapping turtles (Elseya albagula).Crossref | GoogleScholarGoogle Scholar |
Fielder, DP, Limpus, DJ, and Limpus, CJ (2014). Reproduction and population ecology of the vulnerable western sawshelled turtle, Myuchelys bellii, in the Murray–Darling Basin, Australia. Australian Journal of Zoology 62, 463–476.
| Reproduction and population ecology of the vulnerable western sawshelled turtle, Myuchelys bellii, in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Fielder D, Chessman B, Georges A (2015) Myuchelys bellii (Gray 1844) – Western Saw-shelled Turtle, Bell’s Turtle. In ‘Conservation biology of freshwater turtles and tortoises: a compilation project of the IUCN/SSC tortoise and freshwater turtle specialist group’. Chelonian research monographs, vol. 5. (Eds AGJ Rhodin, PCH Pritchard, PP van Dijk, RA Saumure, KA Buhlmann, JB Iverson, RA Mittermeier) pp. 088.1–088.7. (IUCN SSC) https://doi.org/10.3854/crm.5.088.bellii.v1.2015
Fox J, Weisberg S (2011) ‘An R companion to applied regression.’ 2nd edn (Sage: Thousand Oaks, California)
Georges, A (1988). Sex determination is independent of incubation temperature in another chelid turtle, Chelodina longicollis. Copeia 1988, 248–254.
| Sex determination is independent of incubation temperature in another chelid turtle, Chelodina longicollis.Crossref | GoogleScholarGoogle Scholar |
Georges, A, and McInnes, S (1998). Temperature fails to influence hatchling sex in another genus and species of chelid turtle, Elusor macrurus. Journal of Herpetology 32, 596–598.
| Temperature fails to influence hatchling sex in another genus and species of chelid turtle, Elusor macrurus.Crossref | GoogleScholarGoogle Scholar |
Heppell, SS, Crowder, LB, and Crouse, DT (1996). Models to evaluate headstarting as a management tool for long-lived turtles. Ecological Applications 6, 556–565.
| Models to evaluate headstarting as a management tool for long-lived turtles.Crossref | GoogleScholarGoogle Scholar |
Hothorn, T, Bretz, F, and Westfall, P (2008). Simultaneous inference in general parametric models. Biometrical Journal 50, 346–363.
| Simultaneous inference in general parametric models.Crossref | GoogleScholarGoogle Scholar |
IUCN (2021) The IUCN Red List of Threatened Species. Version 2021-3. Available at https://www.iucnredlist.org [Accessed 13 March 2022]
Iverson, JB, Lindeman, PV, and Lovich, JE (2019). Understanding reproductive allometry in turtles: a slippery “slope”. Ecology and Evolution 9, 11891–11903.
| Understanding reproductive allometry in turtles: a slippery “slope”.Crossref | GoogleScholarGoogle Scholar |
Janzen, FJ (1993). An experimental analysis of natural selection on body size of hatchling turtles. Ecology 74, 332–341.
| An experimental analysis of natural selection on body size of hatchling turtles.Crossref | GoogleScholarGoogle Scholar |
Kennett R, Roe J, Hodges K, Georges A (2009) Chelodina longicollis (Shaw 1794) – eastern long-necked turtle, common longnecked turtle, common snake-necked turtle. In ‘Conservation biology of freshwater turtles and tortoises: a compilation project of the IUCN/SSC tortoise and freshwater turtle specialist group’. Chelonian research monographs, vol. 5 (Eds AGJ Rhodin, PCH Pritchard, PP van Dijk, RA Saumure, KA Buhlmann, JB Iverson, RA Mittermeier) pp. 031.1–031.8. (IUCN SSC)
Lawson, L, and Rollinson, N (2021). A simple model for the evolution of temperature-dependent sex determination explains the temperature sensitivity of embryonic mortality in imperiled reptiles. Conservation Physiology 9, coab020.
| A simple model for the evolution of temperature-dependent sex determination explains the temperature sensitivity of embryonic mortality in imperiled reptiles.Crossref | GoogleScholarGoogle Scholar |
Limpus, CJ, Baker, V, and Miller, JD (1979). Movement induced mortality of loggerhead eggs. Herpetologica 35, 335–338.
Martinez, PA, Ezaz, T, Valenzuela, N, Georges, A, and Marshall Graves, JA (2008). An XX/XY heteromorphic sex chromosome system in the Australian chelid turtle Emydura macquarii: a new piece in the puzzle of sex chromosome evolution in turtles. Chromosome Research 16, 815–825.
| An XX/XY heteromorphic sex chromosome system in the Australian chelid turtle Emydura macquarii: a new piece in the puzzle of sex chromosome evolution in turtles.Crossref | GoogleScholarGoogle Scholar |
Mazzoleni, S, Augstenová, B, Clemente, L, Auer, M, Fritz, U, Praschag, P, Protiva, T, Velenský, P, Kratochvíl, L, and Rovatsos, M (2020). Sex is determined by XX/XY sex chromosomes in Australasian side-necked turtles (Testudines: Chelidae). Scientific Reports 10, 4276.
| Sex is determined by XX/XY sex chromosomes in Australasian side-necked turtles (Testudines: Chelidae).Crossref | GoogleScholarGoogle Scholar |
McCosker, JR (2002). Chelodina expansa (broad shell river turtle) and Emydura signata (Brisbane shortneck turtle) reproduction. Herpetological Review 33, 189–199.
McKnight, DT, Hollender, EC, Howell, HJ, Carr, JL, Buhlmann, KA, and Ligon, DB (2018). Egg and clutch sizes of western chicken turtles (Deirochelys reticularia miaria). Acta Herpetologica 13, 191–194.
| Egg and clutch sizes of western chicken turtles (Deirochelys reticularia miaria).Crossref | GoogleScholarGoogle Scholar |
Micheli-Campbell, MA, Campbell, HA, Cramp, RL, Booth, DT, and Franklin, CE (2011). Staying cool, keeping strong: incubation temperature affects performance in a freshwater turtle. Journal of Zoology 285, 266–273.
| Staying cool, keeping strong: incubation temperature affects performance in a freshwater turtle.Crossref | GoogleScholarGoogle Scholar |
Nelson, NJ, Thompson, MB, Pledger, S, Keall, SN, and Daugherty, CH (2004). Egg mass determines hatchling size, and incubation temperature influences post-hatching growth, of tuatara Sphenodon punctatus. Journal of Zoology 263, 77–87.
| Egg mass determines hatchling size, and incubation temperature influences post-hatching growth, of tuatara Sphenodon punctatus.Crossref | GoogleScholarGoogle Scholar |
Niehaus, AC, Angilletta, MJ, Sears, MW, Franklin, CE, and Wilson, RS (2012). Predicting the physiological performance of ectotherms in fluctuating thermal environments. Journal of Experimental Biology 215, 694–701.
| Predicting the physiological performance of ectotherms in fluctuating thermal environments.Crossref | GoogleScholarGoogle Scholar |
Noble, DWA, Stenhouse, V, and Schwanz, LE (2018). Developmental temperatures and phenotypic plasticity in reptiles: a systematic review and meta-analysis. Biological Reviews 93, 72–97.
| Developmental temperatures and phenotypic plasticity in reptiles: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Packard, GC, Taigen, TL, Packard, MJ, and Boardman, TJ (1981). Changes in mass of eggs of softshell turtles (Trionyx spiniferus) incubated under hydric conditions simulating those of natural nests. Journal of Zoology 193, 81–90.
| Changes in mass of eggs of softshell turtles (Trionyx spiniferus) incubated under hydric conditions simulating those of natural nests.Crossref | GoogleScholarGoogle Scholar |
Packard, GC, Packard, MJ, Miller, K, and Boardman, TJ (1987). Influence of moisture, temperature, and substrate on snapping turtle eggs and embryos. Ecology 68, 983–993.
| Influence of moisture, temperature, and substrate on snapping turtle eggs and embryos.Crossref | GoogleScholarGoogle Scholar |
Páez, VP, Lipman, A, Bock, BC, and Heppell, SS (2015). A plea to redirect and evaluate conservation programs for South America’s podocnemidid river turtles. Chelonian Conservation and Biology 14, 205–216.
| A plea to redirect and evaluate conservation programs for South America’s podocnemidid river turtles.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2018) ‘R: A language and environment for statistical computing (Version 3.5.0).’ R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/ [Accessed 5 March 2021]
Reichling, SB, and Gutzke, WHN (1996). Phenotypic consequences of incubation environment in the African elapid genus Aspidelaps. Zoo Biology 15, 301–308.
| Phenotypic consequences of incubation environment in the African elapid genus Aspidelaps.Crossref | GoogleScholarGoogle Scholar |
Santori, C, Spencer, R-J, Thompson, MB, Whittington, CM, and Van Dyke, JU (2021). Hatchling short-necked turtles (Emydura macquarii) select aquatic vegetation habitats, but not after one month in captivity. Aquatic Ecology 55, 85–96.
| Hatchling short-necked turtles (Emydura macquarii) select aquatic vegetation habitats, but not after one month in captivity.Crossref | GoogleScholarGoogle Scholar |
Seigel RA, Dodd CK Jr (2000) Manipulation of turtle populations for conservation: halfway technologies or viable options? In ‘Turtle conservation’. (Ed. MW Klemmens) pp. 218–238 (Smithsonian Institution Press: Washington, DC)
Spencer, R-J, Van Dyke, JU, and Thompson, MB (2017). Critically evaluating best management practices for preventing freshwater turtle extinctions. Conservation Biology 31, 1340–1349.
| Critically evaluating best management practices for preventing freshwater turtle extinctions.Crossref | GoogleScholarGoogle Scholar |
Streeting LM, Spark PD, Nesbitt B, Nesbitt J, Baker L, Dillon ML (2021) ‘Bell’s turtle nest protection guidelines.’ (Local Land Services: New South Wales)
Streeting LM, Bower DS, Dillon ML, Spark P, Gough M, Skidmore A, McDonald PG, Delaney H, Burns A, Watson S, Dissanayake DSB, Georges A, McKnight DT (2022) Data for: Optimising the hatching success of artificially incubated eggs for use in a conservation program for the western saw-shelled turtle (Myuchelys bellii). Dryad, Dataset. https://doi.org/10.5061/dryad.0cfxpnw69
Thompson, MB (1983). Populations of the Murray River tortoise, Emydura (Chelodina): the effect of egg predation by the red fox, Vulpes vulpes. Wildlife Research 10, 363–371.
| Populations of the Murray River tortoise, Emydura (Chelodina): the effect of egg predation by the red fox, Vulpes vulpes.Crossref | GoogleScholarGoogle Scholar |
Thompson MB (1985) Functional significance of the opaque white patch in eggs of Emydura macquarii. In ‘Biology of Australasian frogs and reptiles’. (Eds G Grigg, R Shine, HE Ehmann) pp. 387–395. (Australian Zoological Society of New South Wales: Sydney)
Thompson, MB (1988). Influence of incubation temperature and water potential on sex determination in Emydura macquarii (Testudines: Pleurodira). Herpetologica 44, 86–90.
Turtle Taxonomy Working Group [Rhodin AGJ, Iverson JB, Bour R, Fritz U, Georges A, Shaffer HB, van Dijk PP] (2021) Turtles of the world: annotated checklist and atlas of taxonomy, synonymy, distribution, and conservation status. In ‘Conservation biology of freshwater turtles and tortoises: a compilation project of the IUCN/SSC tortoise and freshwater turtle specialist group. 9th edn. Chelonian research monographs, vol. 8. (Eds AGJ Rhodin, JB Iverson, PP van Dijk, CB Stanford, EV Goode, KA Buhlmann, RA Mittermeier) pp. 1–472. (IUCN SSC) Available at https://doi.org/10.3854/crm.8.checklist.atlas.v9.2021
Van Dyke, JU, Ferronato, BDO, and Spencer, R-J (2018). Current conservation status of Australian freshwater turtles. Australian Journal of Zoology 66, 1–3.
| Current conservation status of Australian freshwater turtles.Crossref | GoogleScholarGoogle Scholar |
Van Dyke, JU, Spencer, R-J, Thompson, MB, Chessman, B, Howard, K, and Georges, A (2019). Conservation implications of turtle declines in Australia’s Murray River system. Scientific Reports 9, 1998.
| Conservation implications of turtle declines in Australia’s Murray River system.Crossref | GoogleScholarGoogle Scholar |
Venables WN, Ripley BD (2002) ‘Modern applied statistics with S.’ 4th edn. (Springer: New York)
Warner DA (2011) Chapter 1 – Sex determination in reptiles. In ‘Hormones and reproduction of vertebrates: reptiles’. vol. 3 (Eds DO Norris, KH Lopez) pp. 1–38. (Academic Press: Amsterdam)


