Simulation of health care and related costs in people with dementia in Australia
Lachlan Standfield A B D , Tracy Comans A B C and Paul A. Scuffham AA Centre for Applied Health Economics, Menzies Health Institute Queensland, Griffith University, Nathan, Qld 4111, Australia. Email: t.comans@uq.edu.au; p.scuffham@griffith.edu.au
B NHMRC Cognitive Decline Partnership Centre, University of Sydney, Sydney, NSW 2006, Australia.
C Metro North Hospital and Health Service, Herston, Brisbane, Qld 4029, Australia.
D Corresponding author. Email: lachlan.standfield@griffithuni.edu.au
Australian Health Review 43(5) 531-539 https://doi.org/10.1071/AH18022
Submitted: 30 January 2018 Accepted: 24 July 2018 Published: 24 September 2018
Journal Compilation © AHHA 2019 Open Access CC BY-NC-ND
Abstract
Objectives The aim of this study was to develop a validated model to predict current and future Australian costs for people with dementia to help guide decision makers allocate scarce resources in the presence of capacity constraints.
Methods A hybrid discrete event simulation was developed to predict costs borne in Australia for people with dementia from 2015 to 2050. The costs captured included community-based care, permanent and respite residential aged care, hospitalisation, transitional care, pharmaceuticals, aged care assessments, out of hospital medical services and other programs.
Results The costs borne for people with dementia in Australia are predicted to increase from A$11.8 billion in 2015 to A$33.6 billion in 2050 at 2013–14 prices, ceteris paribus. If real per capita health and social expenditure increased by 1.0% annually, these costs are predicted to increase by around A$14.2 billion to a total of around A$47.8 billion by 2050.
Conclusions This simulation provides useful estimates of the potential future costs that will be borne for people with dementia and allows the exploration of the effects of capacity constraints on these costs. The model demonstrates that the level of real annual per capita growth in health and social expenditure has significant implications for the future sustainability of dementia care in Australia.
What is known about the topic? With the aging of the Australian population, the number of people living with dementia is predicted to rise markedly in the next four decades. As the number of people living with dementia increases, so too will the financial burden these debilitating and degenerative diseases place on private and public resources. These increases are likely to challenge the efficiency and sustainability of many health systems in the developed world.
What does this paper add? This research provides a validated model to predict current and future Australian costs for people with dementia to help guide decision makers allocate scarce resources in the presence of capacity constraints (i.e. where the supply of resources does not meet demand). The model predicts an increase in costs for people with dementia from A$11.8 billion in 2015 to A$33.6 billion in 2050 at 2013–14 prices. If real per capita health and social expenditure increased by 1.0% annually, these costs are predicted to increase by around A$14.2 billion to a total of around A$47.8 billion by 2050.
What are the implications for practitioners? This simulation provides useful estimates of the potential future costs that will be borne for people with dementia and allows the exploration of the effects of capacity constraints on these costs. The model demonstrates that the level of real annual per capita growth in health and social expenditure has significant implications for the future sustainability of dementia care in Australia.
Additional keywords: aged care, aging, health economics, health funding and financing, health system.
Introduction
With the aging of the Australian population, the number of people living with dementia is predicted to rise markedly in the next four decades.1 As the number of people living with dementia increases, so too will the financial burden these debilitating and degenerative diseases place on private and public resources. These increases are likely to challenge the efficiency and sustainability of many health systems in the developed world. Therefore, it will be important to develop predictive models to assist decision makers to plan and rationally allocate limited healthcare funds to areas that are likely to maximise benefits most efficiently.
As healthcare systems evolve to meet these increases in dementia, capacity constraints (i.e. where the supply of resources does not meet demand) are likely to emerge. Therefore, it would appear important that any modelling methods used to plan future healthcare resource allocation in this setting have the ability to capture the implications of these effects. The choice of the modelling method in this situation has the potential to result in different resource allocation decisions that may alter the overall efficiency of the healthcare system being studied.2 However, the most commonly used modelling method in health economics to date is the Markov cohort analysis, which does not capture the dynamic effects of resource constraints. In line with this, previous research on the cost of dementia in Australia has focused on standard cohort-based demographic modelling methods in which age–sex dementia prevalence rates are applied to age–sex population projection estimates and costs assigned to these cohorts.3,4 In contrast, the model described herein allows the exploration of the effect of capacity constraints through discrete event simulation methods with dynamic queueing (DES-DQ). This modelling process allows the researcher to investigate the implications on expenditure of altering demand for resources (e.g. people with dementia requiring residential aged care (RAC) beds) and the level of resources available (e.g. the number of RAC beds available). This is important in situations where demand for resources exceeds supply. In these settings, queues may lengthen to such a point that people are redirected to less efficient, less appropriate and more costly healthcare pathways, affecting the efficiency of the healthcare system as a whole.
Methods
This study had two aims. First, the study aimed to develop a validated mathematical model of dementia epidemiology and resource use for Australia. The model was then used to generate projections of potential future costs borne in Australia for people with dementia over the period 2015–50. The research provides a range of different cost projections showing the effects various assumptions regarding future dementia prevalence, capacity constraints and real average growth in per capita health and social care expenditure may have on the future costs borne by the health and welfare sectors in Australia.
The model developed herein is an extension of a previously published model.5 Briefly, the model is a computer-based simulation analysis developed using AnyLogic 7.2.0 multimethod simulation software (AnyLogic North America, Oakbrook Terrace, IL). The simulation comprised a natural history model, which predicts an individual patient’s disease progression over time, and a health system-level model to predict healthcare system resource use (see supplementary fig. 1a, b, respectively in Standfield et al.5). As described previously,5 the simulation was populated with published regression analyses derived from large linked individual patient datasets where possible. These datasets included those from the Pathways in Aged Care project (based on linked data from 32 000 people), the Hospital Dementia Services Project, which included 252 313 patients (20 748 people with dementia, 231 565 people without dementia) and an analysis of linked data from 948 000 hospital discharges used to determine the movement of people between acute hospital care and RAC.6–8 As described previously, it was assumed that the age-standardised incidence of dementia was constant over time and all simulated people with incident dementia were assumed to start in the mild dementia health state.5
The present analysis expands on the previous model by capturing health and welfare system costs (predominantly for RAC) used by people with dementia in Australia. The costs captured in this analysis were sourced from the literature and include the cost of community-based care (e.g. home care packages (HCPs); non-residential respite), permanent and respite RAC (operational, capital and maintenance costs), hospitalisation, transitional care, antidementia pharmaceuticals, aged care assessments, out-of-hospital medical services and other programs. The costs captured in these analyses should be interpreted as the total costs for people with dementia, not the excess health care costs due to dementia per se. Unless otherwise specified, all costs are presented in Australian dollars at 2013–14 price levels.
As described previously,5 a hybrid individual-level patient simulation (IPS) model using state transition (microsimulation) and DES techniques (i.e. hybrid DES model) was used to estimate the potential future prevalence and health care resource use associated with dementia in Australia. This type of model generates simulated individual people who enter the model over time (in line with the incidence and prevalence of dementia in Australia). Each simulated person carries individual attributes (e.g. age, dementia severity, educational attainment, marital status etc.) and these attributes evolve over time (i.e. people age and dementia may progress). Based on progression, patients move through various states within the model (i.e. state transition microsimulation). Some states describe dementia progression and others describe components of the health and social welfare system (e.g. community, hospital, RAC; see supplementary fig. 1a, b in Standfield et al.5). The model also simulates the number of resources that are available at any one time (e.g. RAC beds). Simulated people compete for these resources and, if the demand for these resources exceeds supply, queues form (i.e. DES with DQ). If these queues are protracted, these simulated patients may leave these queues and be redirected to other parts of the healthcare system while waiting for suitable care.1,9–11
The weighted average cost of capital applied in the model was determined using a previously described method.12 Briefly, the cost of equity capital was determined using the capital asset pricing model.13,14 It was assumed that the cost of debt capital for high-care RAC places was derived entirely from the market, whereas debt capital for low-care RAC places was obtained from accommodation bonds. The cost of debt capital derived from the market was determined by the spread of non-financial BBB-rated corporate bonds over 10-year Commonwealth Government Bonds (i.e. acting as a measure of the ‘risk-free’ rate of return).
The model also explores the effects of varying the real growth of per capita health and social welfare expenditure in Australia over time.
Full details of all the input parameters used to derive costs in the model are given in Table 1.
The model’s financial impact estimates were validated against published estimates of the cost of caring for people with dementia in Australia in 2013–14.11 The cost of future healthcare resource use was explored in three scenario analyses in which different levels of dementia incidence were assumed (i.e. low-, mid- and high-incidence scenarios). The low-incidence scenario explored the effect on resource use should the incidence of dementia be assumed to be 20% lower than described by Standfield et al.5 in the mid-incidence scenario. This low estimate is based on the recently published study by Matthews et al.15 that suggested that dementia incidence may have decreased by 20% over the past two decades. The mid-incidence scenario used the incidence estimates described by Standfield et al.5 that were derived from Fratiglioni et al.16 The high-incidence scenario assumes that the incidence of dementia used in the model is around 18% higher than the mid-incidence scenario; this ensures that the model generates similar prevalence estimates to those derived from Anstey et al.10 Each of these scenarios is presented in two ways: the first assumes that there are capacity constraints on the number of RAC beds available for people with dementia, and the second analysis demonstrates the effect of removing all RAC bed capacity constraints from these analyses. The latter analysis was used to determine the percentage increase in RAC beds, and the cost of these additional resources, required to relieve these capacity constraints. In the main analysis, no real growth in per capita expenditure (0%) due to increased use of existing products and services or the shift to newer and more expensive products and services is explicitly applied. The effect of increasing the real growth in per capita expenditure on the cost estimates generated by the model was also explored in detail.
Results
Model validation
To cross-validate the natural history component of the analysis, the model’s predictions of future dementia prevalence were compared with other published projections of future dementia prevalence in Australia.1,9–11 In the mid-incidence scenario, the model predicted that around 298 000 people will have dementia in 2011, increasing to around 387 000 in 2020 and 928 000 by 2050. As described previously,5 these estimates most closely accord with the projections produced by the Australian Institute of Health and Welfare (AIHW)1 and Deloitte Access Economics.9
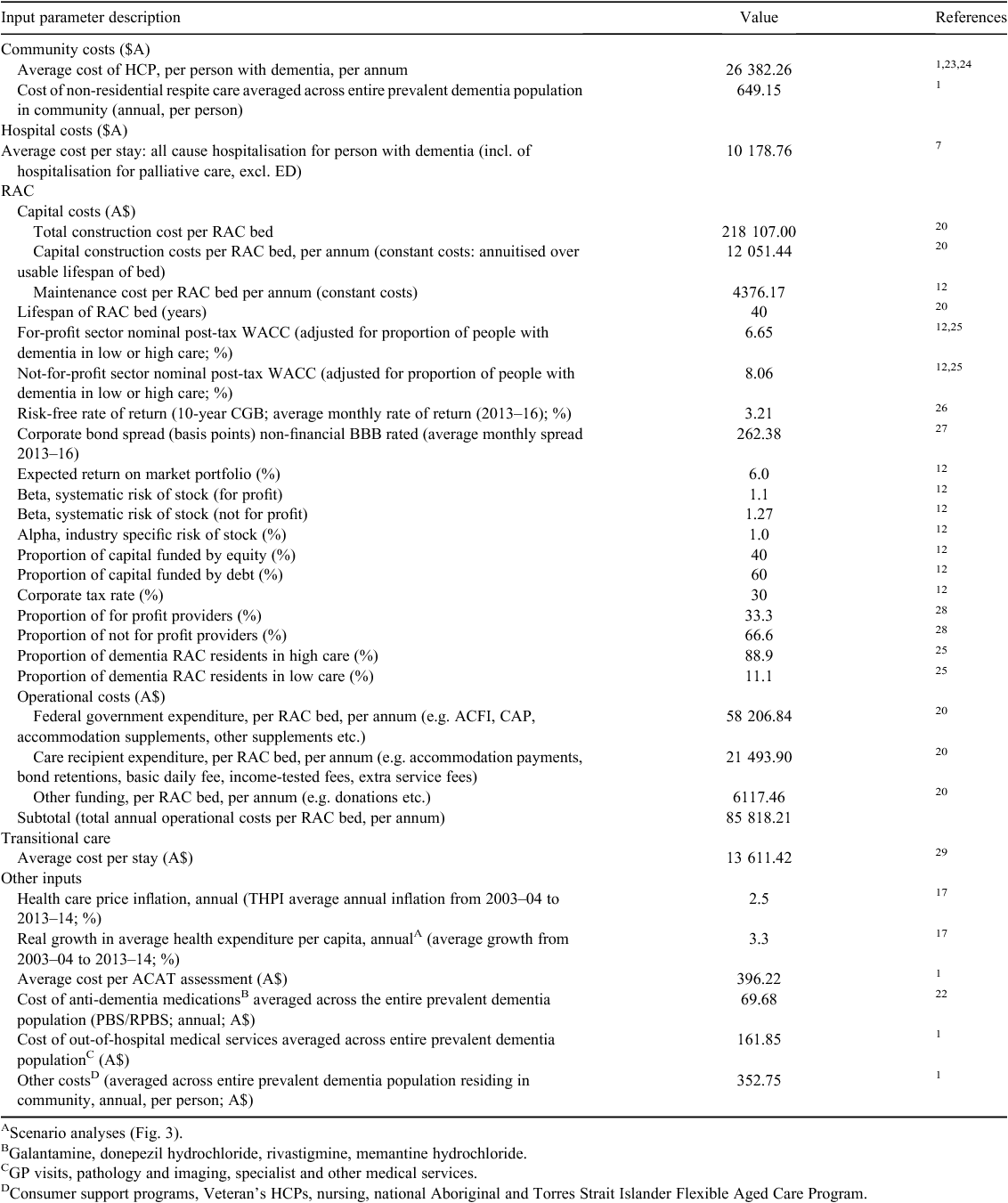
The levels of health service resource use generated by the model were compared and validated against known historical levels of healthcare resource use. Fig. 1 shows the results of this comparison and validation of the disaggregated cost of health service resource use generated by the model compared with known literature-based estimates of the historical costs borne for these services in Australia in 2013–14.11 The model appears to predict similar levels of service expenditure across hospitalisation, permanent RAC (P-RAC) (operational, capital and maintenance), respite RAC (R-RAC), transitional care, HCP, aged care assessment team assessments, antidementia medication use and out-of-hospital, non-residential respite and other costs.
|
Projections of future costs
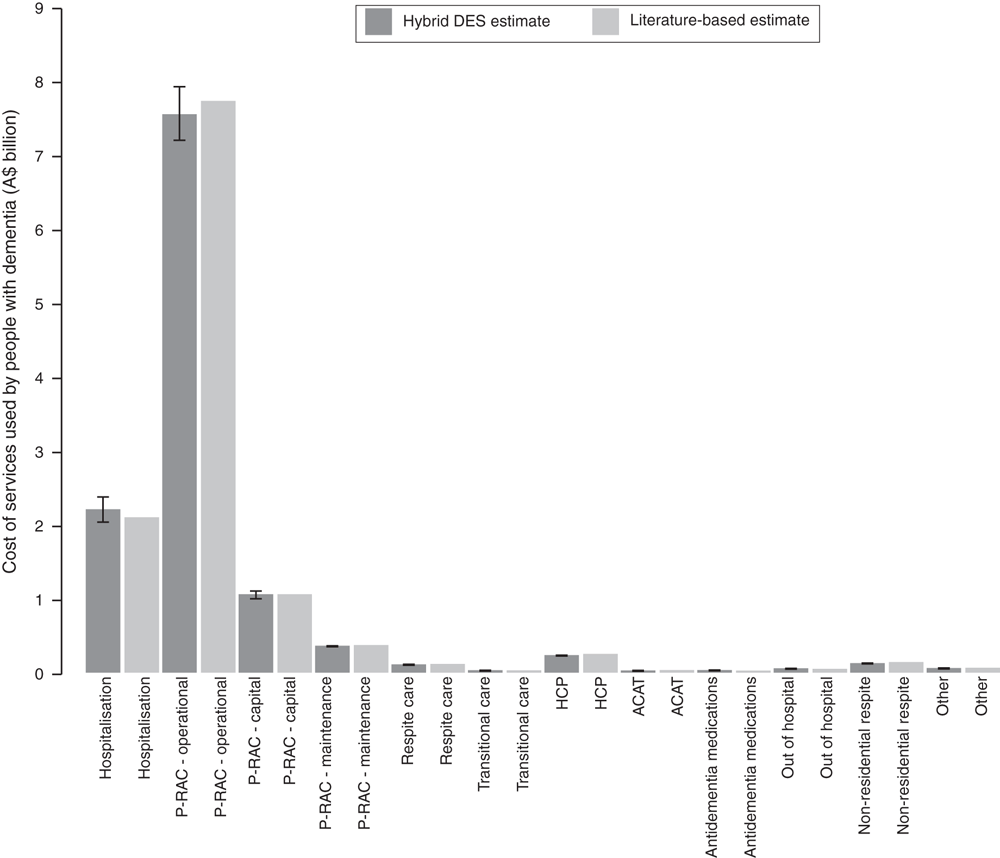
Fig. 2 shows the hybrid DES dementia model’s prediction of costs by health service over time for three scenarios (i.e. low, mid and high incidence; Scenarios 1, 2 and 3 respectively). Each scenario is presented in two ways: the first assumes that there are capacity constraints on the number of RAC beds available for people with dementia and the second analysis demonstrates the effect of removing all RAC bed capacity constraints from the analyses.
|
In all analyses, most costs are borne in the P-RAC sector. Operational costs form the largest cost for P-RAC, followed by capital costs and the cost of ongoing maintenance. The cost of hospitalisation is also a major contributor to the overall costs of services for people with dementia.
In the low-incidence projections of Scenario 1 (Fig. 2a), the constrained and unconstrained analyses produce similar costs across all healthcare resource categories. Both analyses predicted a total of around A$9.8 and A$27.8 billion (constant at 2013–14 dollar values) in costs in 2015 and 2050 respectively. The similarity between these analyses demonstrates that at the lower level of dementia incidence adopted in Scenario 1, the demand for RAC beds does not materially exceed supply in the constrained analysis.
In contrast, in both the mid- and high-incidence scenarios (Scenarios 2 and 3), the constrained and unconstrained analyses differ. The total respective costs of P-RAC (operational, capital and maintenance) in 2015 and 2050 are around A$9.2 and A$25.7 billion (constant) in unconstrained Scenario 2, compared with A$9.0 and A$23.3 billion (constant) in constrained Scenario 2. In contrast, hospital costs are lower by 2050 in unconstrained Scenario 2, with around A$5.9 billion (constant) in costs accrued compared with constrained Scenario 2, in which around A$8.0 billion (constant) in costs are predicted in 2050. These differences reflect the movement of people with dementia away from appropriate care (P-RAC or R-RAC) into hospital as the demand for RAC beds exceeds supply. In Scenario 2, the model predicts that this redirection of people with dementia into hospital care results in excess hospital costs in the final 5 years of the modelled time horizon (i.e. 2045–50), reaching a peak of around A$2.1 billion (constant) in 2050. The unconstrained version of the analysis shows that increases in P-RAC bed places of around 4.4%, 6.6% and 12.8% above those used in the base case projections in 2040, 2045 and 2050 respectively would largely alleviate these excess costs to the hospital sector.
These differences become more apparent in the high-incidence dementia scenarios (Scenario 3). The total cost of P-RAC (operational, capital and maintenance) is predicted to be around A$10.7 and A$30.7 billion (constant) in unconstrained Scenario 3 in 2015 and 2050 respectively, compared with A$9.6 and A$23.4 billion (constant) in constrained Scenario 3. Again, as in Scenario 2, hospital costs in Scenario 3 move in the opposite direction, with around A$2.4 and A$7.0 billion (constant) in costs accrued in unconstrained Scenario 3 and A$3.1 and A$13.8 billion (constant) in constrained Scenario 3 in 2015 and 2050 respectively. In Scenario 3, the model predicts that this redirection of people with dementia into hospital care results in excess hospital costs reaching around A$2.8 billion (constant) in 2040 and peaking at around A$6.8 billion (constant) in 2050. The unconstrained version of the analysis shows that increases in P-RAC bed places of around 8.0%, 9.8%, 27.1% and 32.3% above those applied in the base case projections in 2025, 2035, 2045 and 2050 respectively would largely alleviate these excess costs to the hospital sector. The total cost of the services captured in the capacity constrained version of the model is predicted to increase from A$9.7 to A$27.6, from A$11.8to A$33.6 and from A$13.5 to A$39.7 billion (constant) for the low, mid- and high-incidence scenarios for 2015 and 2050 respectively. In comparison, the unconstrained model predicts that costs will increase from A$9.8 to A$28.0, from A$12.1 to A$34.1 and from A$14.0 to A$40.4 billion (constant) for the low, mid- and high-incidence scenarios for 2015 and 2050 respectively.
If the time to reneging (i.e. leaving from RAC queues due to protracted waiting times and entering hospital) is decreased from 1 month to 2 weeks in Scenario 2 (constrained), the cost of hospitalisations increases to around A$9.4 billion by 2050. Conversely, if this time before reneging is increased to 2 months, the cost of hospitalisation is predicted to decrease to A$7.0 billion in 2050.
Effects of altering the real growth in average health and social care expenditure per capita
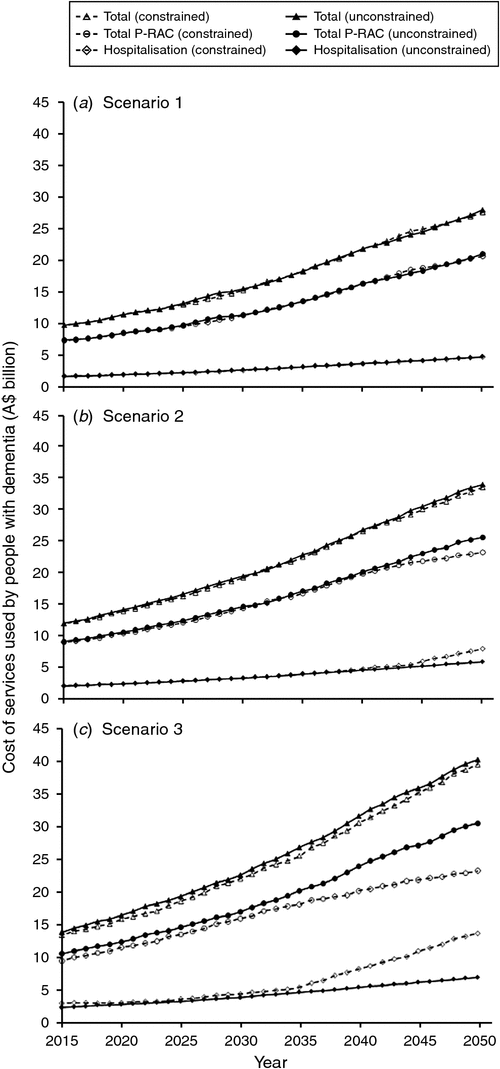
Data collected over the decade from 2003–04 to 2013–14 estimate that per capita expenditure for health has increased by around 3.3% per annum (real).17 This value provides an estimate of the increase in the individual’s consumption of existing healthcare products and services per se (i.e. independent of age) and the shift to newer and more expensive products and services and changing consumption as the population ages. Because there is significant uncertainty surrounding the magnitude of this growth into the future, Fig. 3 shows the effect altering the per capita expenditure has on the total costs generated by the model using the mid-incidence (constrained) scenario assumptions. In the main analysis, no real growth in per capita expenditure is explicitly applied in the model. However, the model captures the changing age distribution of people with dementia over time and will therefore capture the growth in per capita expenditure related to dementia due to aging. If per capita expenditure growth for health and social care consumption (independent of age) were assumed to increase 1.0% per annum (real), the model predicts that the total costs of the services would increase to A$12.2 and A$47.8 billion (constant) in 2015 and 2050 respectively. If this growth were assumed to increase at 3.3% per annum (real), in line with the growth observed in health in the past decade, the model predicts the total costs of the services would increase to A$12.7 and A$114.6 billion (constant) in 2015 and 2050 respectively.
|
Discussion
This study presents a validated hybrid DES model based on a series of large linked individual patient datasets that may be used to generate predictions of the future cost of resources required for people with dementia in Australia. The model has previously been shown5 to generate realistic estimates of dementia prevalence over time when cross-validated with other published projections of dementia prevalence developed for Australia.1,9,11 The model also appears to generate realistic estimates of health service cost when validated against known values. As seen in other jurisdictions, most costs identified were borne in the P-RAC sector for the accommodation of people with dementia.18 This contrasts with other recent Australian research that found that although RAC costs were substantial, hospitalisation costs were the main direct cost associated with dementia.4 The differences between these findings are due primarily to the breadth of costs associated with RAC included in each analysis. The analysis presented herein attempts to capture a broad range of costs to reflect the total expenditure required to provide RAC regardless of the source of this funding. This includes Federal government expenditure (e.g. aged care funding instrument, conditional adjustment payments, accommodation supplements, other supplements etc.), the care recipient’s expenditure (e.g. accommodation payments, bond retentions, basic daily fee, income-tested fees, extra service fees), donations, capital and maintenance costs associated with the provision of RAC beds (see Table 1). In contrast, the other research appears to focus on the expenditure of the care recipient alone (i.e. maximum basic daily fee).4
Modelling allows the researcher to vary important input parameters, such as dementia incidence, to explore their effects on health and welfare costs in people with dementia into the future. Varying dementia incidence in this analysis is particularly important because there is currently conflicting research that has found that dementia incidence has decreased over time,15 whereas other research suggests that dementia incidence may increase beyond what is predicted by demographic aging alone due to increasing mid-life obesity in Australia.19
By incorporating DES dynamic queueing methods, the model also allows exploration of the possible implications capacity constraints on RAC bed numbers may have on the cost of other health services in Australia. Under-resourcing of the number of RAC beds would lead to increased waiting times for these resources and the potential for people with dementia to be redirected away from appropriate care in RAC accommodation and into more expensive and less appropriate care in hospital. These excess costs are evident in the mid- and high-incidence dementia scenarios where demand for RAC beds exceeds supply, leading to increased hospital costs in the constrained analyses, albeit towards the later periods of the modelled time horizon, where uncertainty is greater. Clearly shifts into less appropriate care are likely to have effects beyond the financial, affecting the person with dementia and his/her carers and affecting the capacity of hospitals to manage other cases.
The two most important drivers of increasing future costs identified in the model are the growth in real per capita expenditure per annum and the annual growth in the number of people with dementia, which generates a real increase of around 2.9% in costs per annum. The model predicts that if real growth in per capita expenditure (independent of age) was increased from 0% to 1% per annum over the modelled time horizon, the costs to the health and welfare system would increase in the vicinity of A$1.3, A$3.4, A$8.3 and A$14.2 billion (constant) in 2020, 2030, 2040 and 2050 respectively. If this growth were assumed to increase at 3.3% per annum (real), in line with the growth observed in health in the past decade, the model predicts total costs of the services would increase by A$3.4, A$14.7, A$39.3 and A$81.1 billion (constant) in 2020, 2030, 2040 and 2050 respectively.
To put this into some context, in 2013–14 health expenditure in Australia was estimated at around 9.8% of gross domestic product (GDP), or around A$155 billion.17 The Australian and state governments were estimated to have spent around 1.0% of GDP on RAC and community services for aged care in 2013–14 (around A$16 billion). Other RAC funding (excluding capital costs) received from care recipients and donations, among other things, reached around A$5 billion in 2014, making a total of around A$176 billion in expenditure on health and aged care services (excluding RAC capital costs).20 Based on estimates from the Australian Treasury’s Intergenerational Report 2015,21 under the currently legislated scenario, total healthcare costs and RAC (excluding capital costs) and community services for aged care are predicted to increase to around A$750 billion in today’s dollars by 2054–55 (calculated by upscaling Australian government expenditure to account for state or local government and private expenditure assuming current relative expenditure levels across funding groups are maintained).
This study is subject to the usual limitations of forecasting the future. In addition, the analysis does not model all potential costs, because some costs are omitted. For example, although the majority of out-of-pocket (OOP) copayments are captured in the overall cost of providing the health and welfare services valued in this study, some more minor OOP costs, such as those incurred for over-the-counter medications, alternative therapies and transport, are not captured in the analysis. Further, given the lack of data available to accurately determine the cost of emergency department attendances and non-dementia-specific medications for people with dementia in Australia, these costs have been excluded from the analysis. In addition to this, the model does not attempt to capture productivity losses associated with dementia because the valuation of the extent of these losses can be somewhat contentious. Finally, the model does not explicitly capture the cost of palliative care, although a proportion of the cost of these services will be captured within the costing of other resources (e.g. hospitalisation, P-RAC). Nevertheless, the salient costs likely to drive overall expenditure are well defined and captured in this analysis.
The model presented herein is designed at a reasonably high level of abstraction focusing on Australia as a whole. Implicit within this level of abstraction is the simplifying assumption that RAC beds are equally accessible across the country. This is not necessarily the case and it therefore should be noted that inconsistencies in the supply and demand for RAC beds by region are not captured by the analysis.
One area of particular uncertainty that should be highlighted is the future cost of antidementia medications. Currently, there are four main antidementia medications used in Australia: galantamine, donepezil hydrochloride, rivastigmine and memantine hydrochloride. The cost of these medications to the Pharmaceutical Benefits Scheme in Australia peaked in 2012 (at around A$63.6 million) and has rapidly decreased to a low of A$22.8 million in 2015.22 These decreases are due to patent expiry and government pricing policies. Should newer more effective antidementia medications become available in the future, these are likely to be expensive. The primary analysis does not capture the cost of shifting to newer and more expensive products and services; however, the real growth in per capita expenditure analyses presented (Fig. 3) may provide useful insights into the implications of the growth in these costs over time. Regardless, it is acknowledged that there remains significant uncertainty in these estimates.
It should not be assumed that curing dementia would save all the costs presented. Although a dementia cure would inevitably reduce the costs presented in this analysis, many of these costs would still be borne by the health and welfare systems. For example, the model captures all-cause hospitalisation in dementia patients; it is known from the large Hospital Dementia Services Project that people with dementia have higher rates of hospitalisation and longer stays in hospital than the general population.7 Therefore, curing dementia would reduce the rate and duration of hospitalisation and subsequent costs in the model, but patients would still require hospitalisation for other medical reasons. Similarly, we know that people with dementia are more likely to require P-RAC and, in some instances, curing dementia would obviate the need for P-RAC in these individuals altogether but in others curing dementia may simply delay the need for P-RAC because movement into P-RAC may be required for other reasons (e.g. frailty, falls, incontinence etc.).6,8 To determine the effects of changing the epidemiology and natural history of dementia on costs, we need to undertake incremental analyses that compare the current projections with those that capture the net change in health service use that is directly attributable to dementia per se. This is the focus of future research.
As with any model, in this analysis we rely on historical data and analyses to predict the future. Clearly, over time, the healthcare system, epidemiology, treatment or prevention of dementia may change and this may alter these expenditure predictions. However, the strength of models such as the one presented here is that they can be altered in line with these changes to test the implications of resourcing decisions and the evolution in our understanding of the complex epidemiology and costs associated with dementia. Although it would be comforting to delay decision making until all unknown factors are resolved, this is unlikely to ever occur. Further, decision makers are in the invidious position of having to plan and act today with imperfect information to meet future health service demands. In this way, analyses such as the one presented here, can act as a useful and flexible guide for rational decision making about future health service funding for people with dementia and Australia.
Conclusion
This simulation provides useful estimates of the potential future costs that will be borne for people with dementia in Australia and allows the exploration of the effects of capacity constraints in aged care may have on these costs. Along with the future growth in the number of people with dementia, the model highlights that the level of real annual per capita growth in health and social welfare expenditure may have significant implications for the future sustainability of dementia care in Australia.
Competing interests
None.
Acknowledgements
Financial support for this study was provided to LS by an Australian Postgraduate Award Scholarship grant from the Australian government. The funding agreements ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. This study was also supported by funding provided by the National Health and Medical Research Council (NHMRC) Partnership Centre on Dealing with Cognitive and Related Functional Decline in Older People (Grant no. GNT9100000). The contents of the published materials are solely the responsibility of the individual authors identified, and do not reflect the views of the NHMRC or any other funding bodies or the funding partners.
References
[1] Australian Institute of Health and Welfare (AIHW). Dementia in Australia. Catalogue no. Age 70. Canberra: AIHW; 2012.[2] Standfield LB, Comans TA, Scuffham PA. An empirical comparison of Markov cohort modeling and discrete event simulation in a capacity-constrained health care setting. Eur J Health Econ 2017; 18 33–47.
| An empirical comparison of Markov cohort modeling and discrete event simulation in a capacity-constrained health care setting.Crossref | GoogleScholarGoogle Scholar |
[3] Deloitte Access Economics. Keeping dementia front of mind: incidence and prevalence 2009–2050. Canberra: Alzheimer’s Australia; 2009.
[4] Brown L, Hansnata E, La HA. Economic cost of dementia in Australia 2016–2056. 2017. Available at: https://reports.dementia.org.au/costofdementia [verified 14 August 2018].
[5] Standfield LB, Comans T, Scuffham P. A simulation of dementia epidemiology and resource use in Australia. Aust N Z J Public Health 2018; 42 291–5.
| A simulation of dementia epidemiology and resource use in Australia.Crossref | GoogleScholarGoogle Scholar |
[6] Australian Institute of Health and Welfare (AIHW). Dementia and the take-up of residential respite care: an analysis using the PIAC cohort. Catalogue no. CSI 9. Canberra: AIHW; 2010.
[7] Australian Institute of Health and Welfare (AIHW). Dementia care in hospitals costs and strategies. Catalogue no. Age 72. Canberra: AIHW; 2013.
[8] Australian Institute of Health and Welfare (AIHW). Movement between hospital and residential aged care 2008–09. Data linkage series no. 16. Catalogue no. CSI 16. Canberra: AIHW; 2013.
[9] Deloitte Access Economics. Dementia across Australia: 2011–2050. Canberra: Alzheimer’s Australia; 2011.
[10] Anstey KJ, Burns RA, Birrell CL, Steel D, Kiely KM, Luszcz MA. Estimates of probable dementia prevalence from population-based surveys compared with dementia prevalence estimates based on meta-analyses. BMC Neurol 2010; 10 62
| Estimates of probable dementia prevalence from population-based surveys compared with dementia prevalence estimates based on meta-analyses.Crossref | GoogleScholarGoogle Scholar |
[11] Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making 2012; 32 733–43.
| Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7.Crossref | GoogleScholarGoogle Scholar |
[12] Deloitte Access Economics. The viability of residential aged care providers. Canberra: Deloitte Access Economics; 2011.
[13] Sharpe WF. Capital asset prices: a theory of market equilibrium under conditions of risk. J Finance 1964; 19 425–42.
[14] Lintner J. The valuation of risk assets and the selection of risky investments in stock portfolios and capital budgets. Rev Econ Stat 1965; 47 13–37.
| The valuation of risk assets and the selection of risky investments in stock portfolios and capital budgets.Crossref | GoogleScholarGoogle Scholar |
[15] Matthews FE, Stephan BC, Robinson L, Jagger C, Barnes LE, Arthur A, Brayne C, Cognitive Function and Ageing Studies Collaboration A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun 2016; 7 11398
| A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II.Crossref | GoogleScholarGoogle Scholar |
[16] Fratiglioni L, Launer LJ, Andersen K, Breteler MM, Copeland JR, Dartigues JF, Lobo A, Martinez-Lage J, Soininen H, Hofman A. Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000; 54 S10–5.
[17] Australian Institute of Health and Welfare (AIHW). Health expenditure Australia 2013–14. Canberra: AIHW; 2015.
[18] Åkerborg O, Lang A, Wimo A, Skoldunger A, Fratiglioni L, Gaudig M, Rosenlund M. Cost of dementia and its correlation with dependence. J Aging Health 2016; 28 1448–64.
| Cost of dementia and its correlation with dependence.Crossref | GoogleScholarGoogle Scholar |
[19] Nepal B, Brown L, Anstey K.. Rising midlife obesity will worsen future prevalence of dementia. PLoS One 2014; 9 e99305
| Rising midlife obesity will worsen future prevalence of dementia.Crossref | GoogleScholarGoogle Scholar |
[20] Aged Care Financing Authority (ACFA). Report on the funding and financing of the aged care industry. Canberra: ACFA; 2014.
[21] Commonwealth of Australia. Intergenerational report: Australia in 2055. Canberra: The Commonwealth of Australia, The Treasury; 2015. Available at: https://treasury.gov.au/publication/2015-intergenerational-report/ [verified 14 August 2018].
[22] Department of Human Services. Pharmaceutical Benefits Schedule item reports. 2016. Available at: http://medicarestatistics.humanservices.gov.au/statistics/pbs_item.jsp [verified 8 August 2016].
[23] Department of Social Services. Home care packages programme guidelines. Canberra: Australian Government; 2014.
[24] Australian Institute of Health and Welfare (AIHW). Aged care packages in the community 2010–11: a statistical overview. Canberra: AIHW; 2012.
[25] Australian Institute of Health and Welfare (AIHW). Dementia among aged care residents: first information from the Aged Care Funding Instrument. Aged care statistics series no. 32. Catalogue no. Age 63. Canberra: AIHW; 2011.
[26] Reserve Bank of Australia (RBA). Yields on Commonwealth government bonds, 10 years maturity; Monthly; Series ID: FCMYGBAG10. Available at: http://www.rba.gov.au/statistics/tables/ [verified 12 August 2016]. 2016.
[27] Reserve Bank of Australia (RBA). Non-financial corporate BBB-rated bonds – spread to AGS – 10 year target tenor; Monthly; Series ID FNFCBBB10M; 2016. Available at: http://www.rba.gov.au/statistics/tables/ [verified 12 August 2016].
[28] Australian Institute of Health and Welfare (AIHW). Residential aged care in Australia 2010–11: a statistical overview. Aged care statistics series no. 36. Catalogue no. Age 68. Canberra: AIHW; 2012.
[29] Gray LC, Peel NM, Crotty M, Kurrle SE, Giles LC, Cameron ID. How effective are programs at managing transition from hospital to home? A case study of the Australian Transition Care Program. BMC Geriatr 2012; 12 6
| How effective are programs at managing transition from hospital to home? A case study of the Australian Transition Care Program.Crossref | GoogleScholarGoogle Scholar |