Incident haemodialysis and outcomes in the Top End of Australia
Jaquelyne T. Hughes A B G , Sandawana W. Majoni A B C , Federica Barzi A , Tegan M. Harris A , Selina Signal B , Gwendoline Lowah B , Jola Kapojos B , Asanga Abeyaratne B , Madhivanan Sundaram B , Paul Goldrick D , Sarah L. Jones D , Robert McFarlane E , Lewis T. Campbell A C D , Dianne Stephens C D F and Alan Cass AA Menzies School of Health Research, Charles Darwin University, PO Box 41096, Casuarina, NT 0811, Australia. Email: william.majoni@nt.gov.au; federica.barzi@nt.gov.au; Tegan.Harris@menzies.edu.au; Lewis.Campbell@nt.gov.au; alan.cass@menzies.edu.au
B Department of Nephrology, Division of Medicine, Royal Darwin Hospital, PO Box 41326, Casuarina, NT 0811, Australia. Email: Selina.Signal@nt.gov.au; Gwendoline.Lowah@nt.gov.au; Jola.Kapojos@sa.gov.au; Asanga.Abeyaratne@nt.gov.au; Madhivanan.Sundaram@nt.gov.au
C Northern Territory Clinical School, Flinders University, Darwin, NT 0811, Australia. Email: Dianne.Stephens@nt.gov.au
D Intensive Care Unit, Royal Darwin Hospital, PO Box 40596, Casuarina, NT 0811, Australia. Email: Paul.Goldrick@nt.gov.au; SarahL.Jones@nt.gov.au
E Chemical Pathology, Territory Pathology, Department of Health, PO Box 41326, Casuarina, NT 0811, Australia. Email: Robert.McFarlane@nt.gov.au
F National Critical Care and Trauma Response Centre, Royal Darwin Hospital, Darwin, NT 0810, Australia.
G Corresponding author. Email: Jaqui.hughes@menzies.edu.au
Australian Health Review 44(2) 234-240 https://doi.org/10.1071/AH18230
Submitted: 2 November 2018 Accepted: 6 January 2019 Published: 18 April 2019
Journal Compilation © AHHA 2020 Open Access CC BY-NC-ND
Abstract
Objective The Northern Territory has the highest incidence of haemodialysis care for end-stage kidney disease in Australia. Although acute kidney injury (AKI) is a recognised risk for chronic kidney disease (CKD), the effect of AKI causing incident haemodialysis (iHD) is unknown. Audits identifying antecedents of iHD may inform health service planning. Thus, the aims of this study were to describe: (1) the development of an iHD recording system involving patients with AKI and CKD; and (2) the incidence, patient characteristics and mortality for patients with dialysis-requiring AKI.
Methods A retrospective data linkage study was conducted using eight clinical and administrative datasets of adults receiving iHD during the period from July 2011 to December 2012 within a major northern Australian hospital for AKI without CKD (AKI), AKI in people with pre-existing CKD (AKI/CKD) and CKD (without AKI). The time to death was identified by the Northern Territory Register of deaths.
Results In all, 121 iHD treatments were provided for the cohort, whose mean age was 51.5 years with 53.7% female, 68.6% Aboriginal ethnicity and 46.3% with diabetes. iHD was provided for AKI (23.1%), AKI/CKD (47.1%) and CKD (29.8%). The 90-day mortality rate was 25.6% (AKI 39.3%, AKI/CKD 22.8%, CKD 19.4%). The 3-year mortality rate was 45.5% (AKI 53.6%, AKI/CKD 22.8%, CKD 19.4%). The time between requesting data from custodians and receipt of data ranged from 15 to 1046 days.
Conclusion AKI in people with pre-existing CKD was a common cause of iHD. Health service planning and community health may benefit from AKI prevention strategies and the implementation of sustainable and permanent linkages with the datasets used to monitor prospective incident haemodialysis.
What is known about the topic? AKI is a risk factor for CKD. The Northern Territory has the highest national incidence rates of dialysis-dependent end-stage kidney disease, but has no audit tool describing outcomes of dialysis-requiring AKI.
What does this paper add? We audited all iHD and showed 25.6% mortality within the first 90 days of iHD and 45.5% overall mortality at 3 years. AKI in people with pre-existing CKD caused 47.1% of iHD.
What are the implications for practitioners? Health service planning and community health may benefit from AKI prevention strategies and the implementation of sustainable and permanent linkages with the datasets used to monitor prospective incident haemodialysis.
Additional keywords: acute kidney injury, end-stage kidney disease, health services, Indigenous Australian.
Introduction
Acute kidney injury (AKI) is a clinical syndrome marked by an abrupt decline in kidney function due to altered pathophysiological pathways.1 Severe AKI that requires dialysis (Stage III AKI) is an internationally recognised marker of increased mortality, with some long-term survivors requiring maintenance dialysis.2,3 Chronic kidney disease (CKD) is defined by structural kidney damage or sustained loss of kidney function (at least 3 months) and marked by reduced glomerular filtration rate and/or albuminuria.4 Renal replacement therapy (RRT), using maintenance dialysis or kidney transplantation, is required to support metabolic functions for patients with Stage 5 CKD and uraemic symptoms.
Risk markers for CKD include older age, hypertension, diabetes, disadvantage and being part of an Indigenous population. In people with acute illness, the following factors increase the risk of developing AKI: age >65 years, CKD, past history of AKI, coexisting illness, hypovolaemia, sepsis, use of iodinated contrast agents within the previous week, current or recent nephrotoxic agents and being in the perioperative period. Although susceptibility factors for AKI have also included ethnicity (Black race), a multiethnic Canadian study of AKI and critical illness found that First Nation race was not predictive of higher mortality.5 Auditing the frequency and cause of Stage III AKI events in populations with endemic CKD may generate hypotheses regarding factors leading to rapid kidney injury progression. However, there are few studies that describe the incidence of dialysis requiring AKI or patient outcomes in Indigenous populations living in CKD endemic areas, which could inform the development of interventions to maintain health and preserve kidney function.
Australia has one of the highest prevalence rates of CKD internationally.6 The Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) is the main resource used nationally for renal service and dialysis planning. ANZDATA documents patients receiving chronic RRT who survive at least 90 days after incident haemodialysis (iHD). ANZDATA does not require information recording the period between incident dialysis and the first 90 days, and even then only records maintenance dialysis.7 During 2011, ANZDATA reported an Australian incidence rate for maintenance haemodialysis of 83 per million population,8 where Indigenous Australians had a 4.5-fold higher burden than non-Indigenous Australians (337 vs 75 per million population).8 Although ANZDATA recorded an incidence rate for chronic maintenance haemodialysis in the Northern Territory (NT) of 523 per million population,8 there was no auditable recording system of CKD trajectory before a client’s first record within ANZDATA.9 Furthermore, there was no recording system for antecedents of and outcomes following dialysis-requiring AKI. Therefore, we sought to define the feasibility of creating a recording system to define the characteristics and outcomes of patients admitted for dialysis-requiring AKI. We hypothesised that dialysis-requiring AKI resulted in a permanent loss of kidney function, which contributed to the high burden of end-stage kidney disease (ESKD) in the NT. The aims of this study were to describe the development of an iHD recording system (iHD-RS), and to describe the incidence, patient characteristics and mortality (at 90 days and 3 years) of patients with severe dialysis-requiring AKI. This research question aligns with Indigenous patient priorities for clear and useful information about kidney health and the regionally specific expected health journey for people with CKD.10 The audit was supported at the outset by the Top-End Renal Patient Advisory and Advocacy Committee, and supports Indigenous data custodian principles relating to Aboriginal kidney health data.11,12
Methods
We designed a data linkage study to enable an audit of health service use for iHD. The research proposal was supported by the Top-End Renal Patient Advisory and Advocacy Committee, and was approved with waiver of individual consent by the NT Department of Health and Menzies School of Health Research Human Research Ethics Committee, including the Aboriginal Ethics Subcommittee.
Inclusion criteria
Adults (age >18 years) who received an iHD treatment at the only hospital providing iHD in the region, in either the dialysis unit or intensive care unit (ICU), were included in the study. Kidney impairment for admissions involving haemodialysis was recorded as AKI, AKI with pre-existing CKD (AKI/CKD) or CKD (without AKI). We included the earliest recording period of iHD within both units (July 2011). Patients with prevalent ESKD care or previously registered in ANZDATA were excluded.13
Data linkage
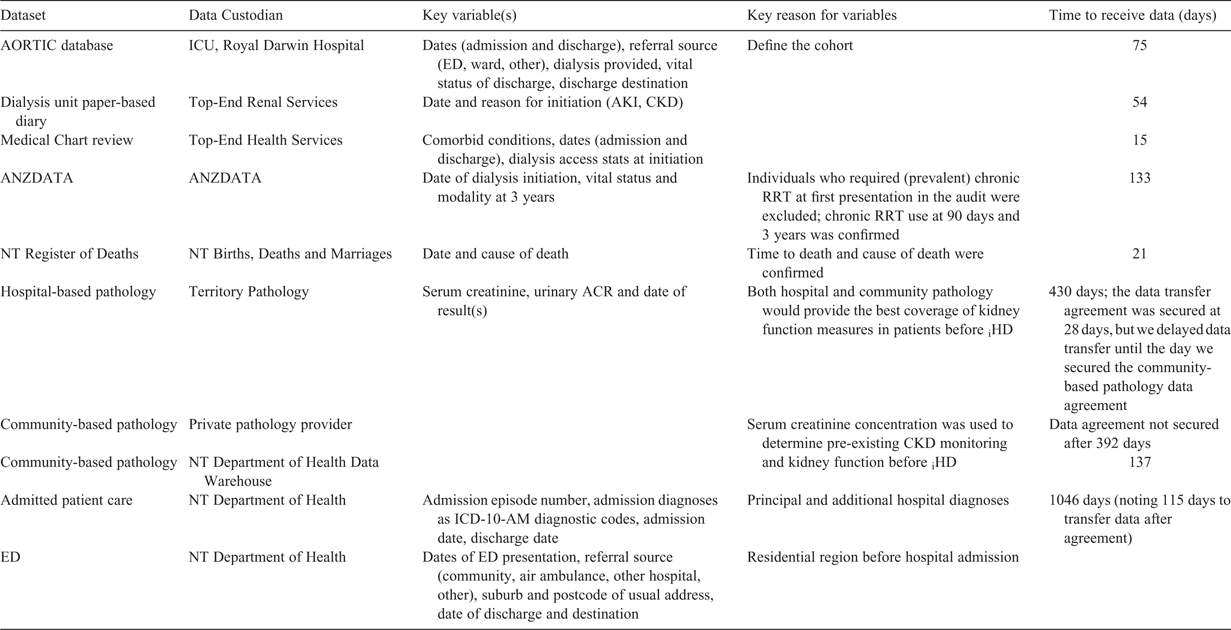
We developed a ground-up recording system, starting with the ICU dataset (Australian Outcomes Research Tool for Intensive Care (AORTIC)), the dialysis unit diary and the clinical record. In total, we secured access to eight key datasets to define the cohort, describe the availability and results of kidney function testing and serum creatinine trajectory leading up to iHD treatment and describe the cause of admission. We recorded the duration (in days) between the data access request and receipt of data for each dataset. Linkage of datasets was managed independently of the research team using a unique identifying code and secure data portal.
The dialysis unit diary, AORTIC database and ANZDATA defined the cohort of interest and location at iHD, community- and hospital-based pathology confirmed pre-existing kidney function, the NT Department of Health Emergency Department dataset defined the location of residence (postcode or locality) preceding hospital presentation and the admitted patient care, medical chart review and AORTIC datasets defined the circumstances of the hospital admission.
Measures
Kidney impairment categories were defined from the admitted patient cohort admission diagnoses, coded into International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification (ICD-10-AM) diagnostic codes. AKI was defined by ICD-10-AM code as either acute nephritic syndrome (N00), acute tubulointerstitial nephritis (N10) or acute kidney failure (N17).14 All AKI was classified as Stage III15 Since all patients required intermittent or continuous haemodialysis therapy. CKD was defined by ICD-10-AM codes E10.2, E11.2, E13.2, E14.2, I12, I13, I15.0, I15.1, N00–N09, N11, N12, N14, N15, N16, N18, N19, N25–N28, N39.1, N39.2, Q60–Q63, T82.4, T86.1, Z49.0, Z94.0 and Z99.2.14 Diabetes was defined by ICD-10-AM codes E10–E14 and 04*.
Principal causes of admission, including infection and cardiovascular diseases, were identified.2 Suburb or postcode provided before admission was converted to an Accessibility/Remoteness Index of Australia (ARIA) 2011+ score.16 In this analysis, ‘remote’ and ‘very-remote’ were reported as a single category.
Baseline kidney function was reported using the estimated glomerular filtration rate (eGFR) reported by the Chronic Kidney Disease Epidemiology Collaborative (CKD-EPI) formula without correction for African American peoples,17 using serum creatinine within 90 days and not less than 8 days before admission.15 Patients with iHD in the ICU were also scored using an Acute Physiology and Chronic Health Evaluation (APACHE) III score, where higher values predict hospital mortality.18
Outcomes included survival at 90 days after the date of hospital admission with and without maintenance RRT (confirmed by ANZDATA), and survival at a minimum of 3 years of follow-up2 (or censored to 31 December 2015). The date and causes of mortality were provided by the NT Register of Deaths, encoded to ICD-10-AM and subclassified into five categories (infection, cardiovascular, malignancy, metabolic and other).2
Statistical analyses
Data are reported as the mean ± s.d. for continuous, normally distributed variables and as the median and interquartile range (IQR) for non-normally distributed variables. Categorical variables are described as a percentage. Differences between kidney impairment groups were described by analysis of variance (ANOVA) for continuous variables and the Chi-squared statistic for categorical variables. Differences in survival were described by Kaplan–Meier survival estimates. Analyses were performed using Stata 14 (Stata Corp., College Station, TX, USA).
Results
Developing the iHD-RS
The iHD-RS was created through linkage of data from eight datasets (Table 1). The time between the request for data and receipt of data ranged from 15 to 1046 days. Confirmation of data sharing agreements was a requirement before agreement to link to the admitted patient cohort and emergency presentation datasets (which was received on Day 1046). The data access request for community pathology (for serum creatinine) was abandoned after 392 days, and was subsequently accessed from an alternative source (NT Department of Health Data Warehouse).
Cohort characteristics
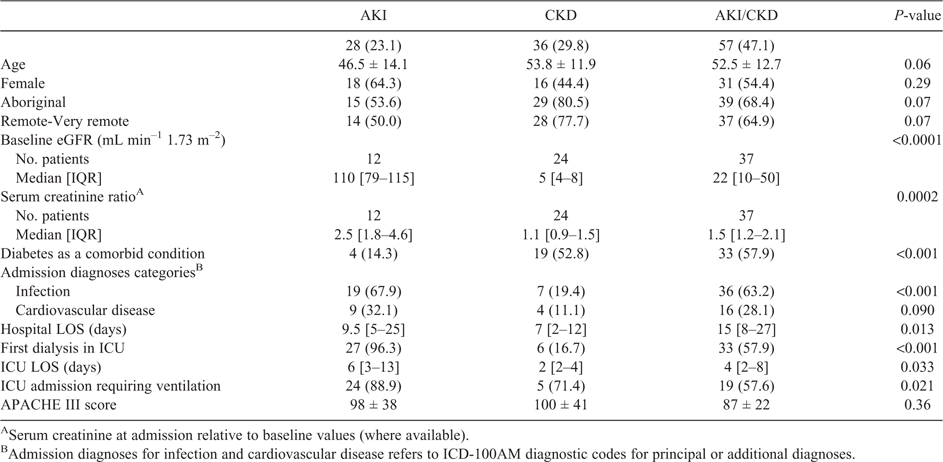
During the 18-month observation period, 121 adults received an iHD treatment (6.7 patients per month; Table 2). The mean age of patients was 51.5 years (range 18.5–84.3 years), 53.7% were female, 68.6% were Aboriginal, 46.3% of patients had diabetes and 65.3% were living in remote or very remote localities relative to the admitting hospital before admission. In this cohort, 59.5% had an eGFR test recorded at all three time points in the previous 2 years (admission, 90 days prior and 2 years prior), including 35.7% in the AKI group, 63.2% in the AKI/CKD group and 72.2% in the CKD group.
Admissions
The median hospital length of stay for all patients was 11 days (IQR 5–23 days). AKI/CKD was the most common kidney impairment category (57/121; 47.1%; Table 2), followed by CKD (36/121; 29.8%) and AKI (28/121; 23.1%). Thirty-three of 36 patients with CKD were known to the renal service before iHD.
The median length of stay in hospital was 15 days for AKI/CKD, 9.5 days for AKI and 7 days for CKD (P = 0.001). iHD was provided in the ICU setting for 54.5% of patients. Six patients with CKD had iHD provided in the ICU (Table 2). Diagnoses of infection (either principal or additional diagnoses) were observed in 67.9%, 63.2% and 19.4% of AKI, AKI/CKD and CKD patients respectively (P < 0.001). In patients with AKI or AKI/CKD who had baseline serum creatinine information, there was an at least 1.5-fold increase in serum creatinine from baseline.
Overall mortality by kidney impairment category and dialysis requirement
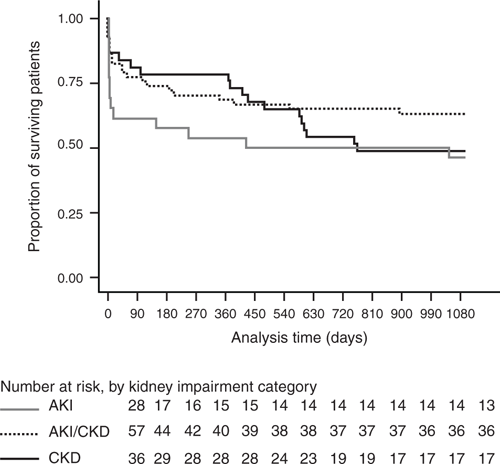
Patients were followed up for a median of 1107 days (~3.0 years) and maximum of 1621 days (4.44 years). Fifty-five deaths were recorded (45.5% overall mortality; Fig. 1), including 25.6% who died within 90 days. Over the entire follow-up period, infection and cardiovascular diseases were the principal cause of death in 25.5% and 47.3% of cases respectfully.
|
Survival at 90 days (with regard to chronic maintenance dialysis)
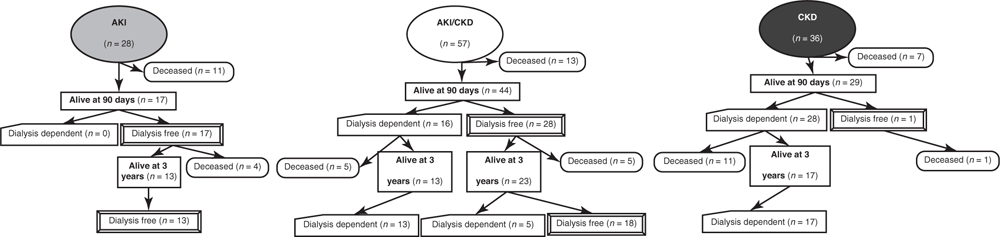
All patients with AKI who survived 90 days (60.7%) were surviving dialysis free (Fig. 2). At 90 days, 16 of 44 (36.4%) patients with AKI/CKD and 80.5% of patients with CKD were receiving chronic maintenance RRT.
|
Discussion
This is the first detailed study describing the outcomes of all patients requiring iHD support in a major northern Australian hospital in a region with endemic CKD. One in four patients died within the first 90 days, with almost half who survived this initial period already undergoing chronic maintenance RRT. At 3 years, approximately half the cohort had died. The cohort was relatively young, with two-thirds of Aboriginal background and the majority who were accessing care from remote or very remote regions.
Australian clinical quality registries record health services information to document health service utilisation and to benchmark between service providers.19 Patients, families and clinicians supported this study and the development of the iHD-RS. First, we responded to a priority request of the Indigenous patient community for clinicians to clearly communicate region-specific information about the causes of dialysis-dependent kidney disease.10 Second, the health service had dialysis unit capacity issues with high and unpredictable acute iHD needs, but there was no recording system to reflect the range of service activity. This study helps explain challenges in matching resources with service demand for acute care dialysis. ANZDATA, which is the main resource used nationally for renal service and dialysis planning, documents patients receiving chronic RRT who survive at least 90 days after iHD. In this study, we report that only one-third of patients who required iHD were recorded in ANZDATA; two-thirds of the patients had either died before 90 days or had recovered renal function.
Critical illness at dialysis initiation
The ICU supported 57% of the cohort at iHD, and included patients with all three kidney impairment conditions (AKI, CKD and AKI/CKD). As expected, high APACHE III scores and ventilator requirements were observed in critical illness, and the AKI group recorded the highest mortality at 90 days. Sepsis is a recognised comorbidity risk for people who have concurrent CKD,20,21 and this audit confirmed infection as a potentially modifiable precipitant of dialysis initiation in Aboriginal people, which was not previously reported from studies involving Australian ICUs.2
The inception of the study was derived from clinicians and supported by patients who perceived, but needed confirmation, that AKI/CKD, particularly in the setting of sepsis, precipitated unplanned chronic maintenance RRT. The study has quantified the prognosis after iHD, and improved the quality of local healthcare information for patients, families and health services.
Relevance to public and strategic health needs
Stage III AKI is internationally recognised as an indicator of increased mortality.2,3 We recommend individual and health system preventative approaches for AKI, which may reduce the need for critical illness care at iHD and delay the onset of chronic maintenance RRT. These approaches include community-wide annual kidney health screening in high-risk populations, targeted health promotion material for Indigenous communities, information and resource support for Indigenous Australian knowledge exchange health teams about the relationship between AKI and CKD, individual- and system-level approaches to improve and respond to early infections and cardiovascular health and collaborative management approaches between primary healthcare, nephrology and ICU healthcare teams.
An auditable kidney health clinical register is needed in northern Australia, and the iHD-RS may improve the identification of high-risk patients, support clinician feedback and community-level knowledge of CKD. We show a feasible methodological approach to link data silos at the time of iHD, which has potential benefit across multiple northern Australian sites. The observation period of 18 months, although brief, highlighted the clinical journey of patients at the time of iHD and reported a high mortality. However, we recognise the small sample size limits the ability to ascertain the relationship of outcomes (such as explained by remote locality or Indigenous ethnicity). We always intended this study to inform the implementation of a prospective iHD-RS, but we first needed to identify the key region-specific variables. This data linkage method was designed from the ground up given there was no auditable process available.9,22
One major delay to the development of the iHD-RS was privacy concerns from the government-contracted community pathology provider. Furthermore, there was no agent who could mediate between the research team and pathology provider, which may expedite future safety and quality research projects. We have since led consultation meetings with patients living with ESKD in northern Australia to guide data use and inform clinical care recommendations that enhance and advance health for this population.23,24
This study was retrospective in nature, which has inherent limitations. The audit focused on iHD, and excluded peritoneal dialysis initiation, because, like other Australian hospitals, there was no acute peritoneal dialysis program locally during the time of the study. The major strength of the research was confirming the feasibility of this approach, which can now support a prospective study of outcomes following incident dialysis for this high-risk population.
Conclusions
In this northern Australian study of characteristics and outcomes following iHD, we report three times as many patients requiring iHD care as were eligible for recording in the ANZDATA clinical registry. Within the overall cohort, one in four had died at 90 days, and one in two had died at 3 years. Region-specific AKI prevention strategies are recommended to preserve kidney function in northern Australia. Defining the iHD-RS and strengthening relationships with data custodians will support a prospective reporting tool for AKI in this region, which has a high concurrent CKD burden.
Competing interests
All authors declare they have no competing interests.
Acknowledgements
The authors gratefully acknowledge the support of Top End NT Renal Patient Advisory and Advocacy Committee who supported the project proposal, all data sponsors and Robyn Liddle for assistance with database management. ANZDATA collates and securely archives patient RRT care data. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of ANZDATA. The AKI study was funded by Belberry Limited and the Menzies Renal Research program. J. T. Hughes was supported by a Jacquot Research Establishment Award from the Royal Australasian College of Physicians and an Australia National Health and Medical Research Council Fellowship (1092576).
References
[1] Ostermann M, Liu K. Pathophysiology of AKI. Best Pract Res Clin Anaesthesiol 2017; 31 305–14.| Pathophysiology of AKI.Crossref | GoogleScholarGoogle Scholar | 29248138PubMed |
[2] Gallagher M, Cass A, Bellomo R, Finfer S, Gattas D, Lee J, Lo S, McGuinness S, Myburgh J, Parke R, Rajbhandari D, for the POST-RENAL Study Investigators and the ANZICS Clinical Trials Group Long-term survival and dialysis dependency following acute kidney injury in intensive care: extended follow-up of a randomized controlled trial. PLoS Med 2014; 11 e1001601
| Long-term survival and dialysis dependency following acute kidney injury in intensive care: extended follow-up of a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar | 24523666PubMed |
[3] Lafrance J-P, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 2010; 21 345–52.
| Acute kidney injury associates with increased long-term mortality.Crossref | GoogleScholarGoogle Scholar | 20019168PubMed |
[4] Levey AS, de Jong PE, Coresh J, Nahas ME, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies conference report. Kidney Int 2011; 80 17–28.
| The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies conference report.Crossref | GoogleScholarGoogle Scholar | 21150873PubMed |
[5] Bagshaw SM, Sood MM, Long J, Fowler RA, Adhikari NK. Acute kidney injury among critically ill patients with pandemic H1N1 influenza A in Canada: cohort study. BMC Nephrol 2013; 14 123
| Acute kidney injury among critically ill patients with pandemic H1N1 influenza A in Canada: cohort study.Crossref | GoogleScholarGoogle Scholar | 23763900PubMed |
[6] Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FDR. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One 2016; 11 e0158765
| Global prevalence of chronic kidney disease – a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 27383068PubMed |
[7] Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). New patient registration form. Adelaide: ANZDATA; 2017. Available at: http://www.anzdata.org.au/forms/ANZDATA-New-Patient-Form-20170307.pdf [verified 22 August 2018].
[8] Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). Chapter 12: End stage kidney disease among Indigenous peoples of Australia and New Zealand. In: Clayton P, editor. Australia and New Zealand Dialysis and Transplant Registry 39th Annual Report. Adelaide: ANZDATA; 2017. Available at: http://www.anzdata.org.au/anzdata/AnzdataReport/39thReport/c12_indigenous_v5.0_20170821 [verified 5 March 2019].
[9] Holwell A, Cherian S, Barzi F, Brady S, Hughes J. Rapid progression of chronic kidney disease in five years prior to haemodialysis initiation in Central Australia. Renal Soc Australas J 2017; 13 5–8.
[10] Hughes JT, Dembski L, Kerrigan V, Majoni SW, Lawton PD, Cass A. Gathering perspectives – finding solutions for chronic and end stage kidney disease. Nephrology (Carlton) 2018; 23 5–13.
| Gathering perspectives – finding solutions for chronic and end stage kidney disease.Crossref | GoogleScholarGoogle Scholar |
[11] Hughes JT, Walker CJ, Kara T, McDonald S, Jose M, Rosman J, Lawton P, Kim S, Palmer S. Exploring data custodianship, ownership, and governance within the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry. Nephrology (Carlton) 2016; 21 204
[12] Hughes JT, Palmer SC. Strengthening ANZDATA structure and processes to improve Aboriginal and Torres Strait Islander and Mãori Health. Presented at ANZSN Clinical Policy Advisory Committee Workshop; 28 February 2017; Adelaide, SA, Australia. Available at: http://www.anzdata.org.au/documents/presentations/DNT_2017_ANZDATA%20Session%208_JH01032017.pdf [verified 5 March 2019].
[13] Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) 38th Annual Report. Adelaide: ANZDATA; 2016.
[14] Australian Institute of Health and Welfare (AIHW). Acute kidney injury in Australia: a first national snapshot. Catalogue no. PHE 190. Canberra: AIHW; 2015.
[15] Kellum JA, editor. KDIGO clinical practice guideline for acute kidney injury. Section 2: AKI definition. Kidney Int Suppl 2012; 2: 19–36.
[16] The Hugo Centre. Accessibility/Remoteness Index of Australia. ARIA 2011+. 2017. Available at: https://www.adelaide.edu.au/hugo-centre/services/aria#example-aria-calculation [verified 5 March 2019].
[17] Maple-Brown LJ, Hughes JT, Lawton PD, Jones GRD, Ellis AG, Drabsch K, Brown ADH, Cass A, Hoy WE, MacIsaac RJ, O’Dea K, Jerums G. Accurate assessment of kidney function in Indigenous Australians: the estimated gfr study. Am J Kidney Dis 2012; 60 680–2.
| Accurate assessment of kidney function in Indigenous Australians: the estimated gfr study.Crossref | GoogleScholarGoogle Scholar | 22884671PubMed |
[18] Zimmerman JE, Wagner DP, Draper EA, Wright L, Alzola C, Knaus WA. Evaluation of Acute Physiology And Chronic Health Evaluation III predictions of hospital mortality in an independent database. Crit Care Med 1998; 26 1317–26.
| Evaluation of Acute Physiology And Chronic Health Evaluation III predictions of hospital mortality in an independent database.Crossref | GoogleScholarGoogle Scholar | 9710088PubMed |
[19] McDonald SP, Russ GR. Australian registries – ANZDATA and ANZOD. Transplant Rev 2013; 27 46–9.
| Australian registries – ANZDATA and ANZOD.Crossref | GoogleScholarGoogle Scholar |
[20] Davis JS, He V, Anstey NM, Condon JR. Long term outcomes following hospital admission for sepsis using relative survival analysis: a prospective cohort study of 1,092 patients with 5 year follow up. PLoS One 2014; 9 e112224
| 25486241PubMed |
[21] Chalmers RM, Majoni SW, Ward L, Perry GJ, Jabbar Z, Currie BJ. Melioidosis and end-stage renal disease in tropical northern Australia. Kidney Int 2014; 86 867–70.
| Melioidosis and end-stage renal disease in tropical northern Australia.Crossref | GoogleScholarGoogle Scholar | 25360487PubMed |
[22] Lawton PD, Cunningham J, Hadlow N, Zhao Y, Jose MD. Chronic kidney disease in the Top End of the Northern Territory of Australia, 2002–2011: a retrospective cohort study using existing laboratory data. BMC Nephrol 2015; 16 168
| Chronic kidney disease in the Top End of the Northern Territory of Australia, 2002–2011: a retrospective cohort study using existing laboratory data.Crossref | GoogleScholarGoogle Scholar | 26494472PubMed |
[23] Hughes J, Mick-Ramsamy L, Mills P, Ross L, Kelly J. Summary report, Darwin, catching some air – asserting Aboriginal and Torres Strait Islander information rights in renal disease. Darwin: Menzies School of Health Research; 2018.
[24] Duff D, Jesudason S, Howell M, Hughes JT. A partnership approach to engage Aboriginal and Torres Strait Islander peoples with clinical guideline development for chronic kidney disease. Renal Soc Australas J 2018; 14 84–8.