Complementary medicines advertising policy Part II: unethical conduct in the Australian market after July 2018
Ken Harvey A C , Malcolm Vickers
A C , Malcolm Vickers  A and Bruce Baer Arnold
A and Bruce Baer Arnold  B
B
A Public Health and Preventive Medicine, Monash University, 553 St Kilda Road, Melbourne, Vic. 3004, Australia. Email: mvic0003@student.monash.edu
B School of Law and Justice, Faculty of Business, Government and Law, University of Canberra, Bruce, ACT 2617, Australia. Email: bruce.arnold@canberra.edu.au
C Corresponding author. Email: kenneth.harvey@monash.edu
Australian Health Review - https://doi.org/10.1071/AH20047
Submitted: 7 March 2020 Accepted: 20 March 2020 Published online: 19 October 2020
Journal Compilation © AHHA 2020 Open Access CC BY-NC-ND
Abstract
Objective To assess the effects of Australian complementary medicines advertising policy after major changes in 2018. These included a legally enforceable advertising code, stronger investigative and compliance powers for the Therapeutic Goods Administration (TGA) and enhanced educational resources for industry.
Methods Analysis of the TGA complaint outcome database from 1 July 2018 to 30 June 2019 and the new regulatory measures.
Results Of 1821 complaint records analysed, 92% were classified as low priority and closed by sending the advertiser a Regulatory Obligation letter. For low priority complaints, no details of the product, advertiser or alleged Code violation were published, and no follow-up was undertaken. Of 121 higher priority complaints, 79% failed to meet their key performance indicator (KPI) time to closure (60–90 days). These included complaints about dangerous sports supplements and ineffective weight loss and hangover products, some of which had been submitted in July 2018.
Conclusions Complaint classification and actions taken by the TGA were inconsistent. The TGA’s new compliance powers were rarely applied. The new complaint system is less transparent than the one it replaced. There is a high rate of advertising complaints and a low rate of effective regulatory response. Time-based KPIs should be based on outcome measures, not when a case is closed by a process measure. An urgent review of the new system is required. Comment on Australia’s 2018 Royal Commission into Misconduct in Banking is equally applicable to the TGA: ‘Essentially a failure to enforce the law undermines the authority of the regulator whose fundamental responsibility is to do just that.’ It also encourages others to break the law, leading to a race to the bottom and consumer detriment.
What is known about the topic? The previous co-regulatory system for complementary medicines was the subject of long-standing criticism and high levels of regulatory non-compliance. The new system, operated solely by the TGA, was meant to overcome these problems.
What does this paper add? High levels of advertising complaints persist. The TGA was unable to close many higher-priority complaints within the time frame set by its KPIs. These complaints involved serious breaches of the Therapeutic Goods Act 1989 (Cwlth), which can attract both civil and (strict liability) criminal penalties. However, in most cases compliance was achieved by negotiation. The TGA met its KPIs for virtually all complaints it classified as low priority because these were closed by merely sending an obligations letter with no follow-up.
What are the implications for practitioners? The persisting high levels of regulatory violation mean that practitioners cannot trust the claims made for complementary medicines or give good advice. In addition, consumers are wasting their money on useless products and are diverted from seeking more evidence-based remedies.
Introduction
In 2009, Bollen and Whicker highlighted long delays in implementing recommendations for reform that arose from the 2003 recommendations of the Expert Committee on Complementary Medicines.1 This delay had ‘enabled the market to be inundated with a vast range of products with an equally vast range of combinations of active ingredients supported by the limited evidence’.1
In 2011, reform was delayed by an attempt to form a single regulatory agency, the Australia New Zealand Therapeutic Products Agency (ANZTPA). This was abandoned in 2014.2 Subsequently, the Australian government proceeded with an Expert Review of Medicines and Medical Devices Regulation, which included a 2015 report on Regulatory Frameworks for Complementary Medicines and Advertising of Therapeutic Goods.3
In 2018, as recommended by the 2015 report, significant changes were finally made to the regulation of complementary medicines and advertising. These culminated on 1 July 2018, when the Therapeutic Goods Administration (TGA) took over the advertising complaint system and the Complaint Resolution Panel (CRP) was abolished.4 The system that existed before July 2018 is described by Vickers and Harvey in a companion paper.5
The new complaint system triaged complaints based on perceived risk.6 In addition, the TGA was given new investigative and enforcement powers to address advertising non-compliance.7
On 7 March 2018, the Therapeutic Goods (Permissible Indications) Determination No.1 of 2018 (Cwlth) came into effect. This abolished the ‘free-text field’ where sponsors could enter their own (often creative) indications for complementary medicines on the Australian Register of Therapeutic Goods (ARTG) without oversight. Instead, sponsors were required to select from a TGA-approved list of permitted indications. A 3-year transition period was provided for sponsors to relist their products using permitted indications.
On 5 May 2018, the Therapeutic Goods Amendment (2017 Measures No. 1) Bill 2017 (Cwlth) was enacted. This provided an additional pathway, labelled ‘AUST L(A)’, for sponsors of complementary medicines to list their product on the ARTG using intermediate-level indications outside the permitted indications list, if supported by premarket assessment of scientific evidence by the TGA. The aim was to encourage more evidence-based products and help consumers to choose them.8
The original pathway for lower-risk medicines, labelled ‘AUST L’, had no premarket assessment by the TGA; sponsors simply self-certified that all legislative requirements were met. In contrast, higher-risk medicines, labelled ‘AUST R’, are registered in the ARTG after full premarket assessment of quality, safety and efficacy.
On 26 September 2018, the Therapeutic Goods (Excluded Goods) Determination 2018 (Cwlth) removed ear candles from being a therapeutic good. This transferred regulatory responsibility to the Australian Competition and Consumer Commission (ACCC), despite their submission to a prior consultation stating they were overloaded and a specialist regulator was more appropriate. Subsequently, the TGA also referred complaints about misleading therapeutic claims for magnets to the ACCC, despite these products not being listed on the Excluded Goods Determination.
Health Minister Greg Hunt said that the measures proposed ‘will enable potential harms from inappropriate advertising to be comprehensively prevented but at the same time make it clear to industry that they have the responsibility to produce compliant advertisements in the first place’.9 He also promised an independent review of the effect of the new advertising measures within 2 years of implementation.9
This paper reviews the effects of these regulatory reforms, focusing on the period from 1 July 2018 to 30 June 2019.
Methods
On 11 October 2019, the TGA complaint database was searched for complaints received from 1 July 2018 to 30 June 2019 (https://compliance.tga.gov.au/investigations-database/). This delay allowed reasonable time for the TGA to process complaints received during 2018–19. Complaint outcomes were tabulated according to their assigned risk category (priority) and, for each risk category, the action taken by the TGA.
The TGA indications for listed medicines database was searched for scientific indications only, or indications that involved a tradition of use (https://www.ebs.tga.gov.au/). The ARTG was searched (https://tga-search.clients.funnelback.com/s/search.html?query=&collection=tga-artg) for AUST L(A) products and examples of listings that used the new permissible indications. Regulatory decisions and announcements,10 advertising direction notices and the TGA’s inaugural advertising compliance 2018–19 annual report11 were analysed for additional information.
Commencing in July 2018, 37 advertising complaints were submitted to the TGA by one of the authors (KH). These included complaints about ineffective weight loss products, useless hangover products that implicitly encouraged unsafe drinking and dangerous sports supplements containing prohibited ingredients.
Results
The TGA 2018–19 advertising compliance annual report stated that 1468 complaints were received in 2018–19, which generated 2436 ‘cases’ (one complaint may mention multiple advertisers). Of these, 1601 complaints were said to be closed during the reporting period.
At the time of the present analysis (11 October 2019), the outcome of 1821 complaints that were closed in 2018–19 were published on the TGA complaints database. Our count of closed complaints was greater than that of the TGA because we had allowed an additional 3 months for complaints to be processed and closed.
Of the 1821 complaints analysed, the assigned priority classifications were low for 1678 (91.6%), medium for 145 (7.9%), high for two (0.1%) and critical for six (0.3%). These proportions were similar to those in the TGA’s annual report.11
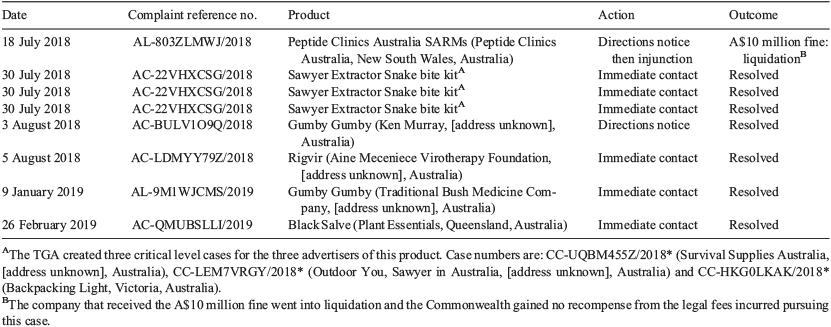
The action taken on the 1678 closed complaints classified as low priority and the 145 closed complaints classified as medium priority is summarised in Table 1. The action taken on the eight closed complaints classified as high or critical priority is summarised in Table 2.
|
Many complaints submitted by KH in July and August 2018 have yet to have outcomes published. These include advertisements for weight loss, homeopathic and hangover products. A complaint submitted in December 2018 for a sports supplement containing illegal selective androgen receptor modulators currently has no published outcome, although we understand the TGA is pursuing legal action. At the time of writing, all these products continue to be advertised.
No AUST L(A) products were found. Of 1021 permissible indications analysed, 86% could be justified by invoking ‘traditional’ rather than scientific evidence. An analysis of ARTG entries using the new permissible indications found that some sponsors had created a huge list of indications for a particular product, many of which appeared to be based on non-existent or dubious scientific evidence, similar to the old ‘free-text’ indications. For example, Thompson’s One-A-Day Echinacea 4000 (Integria Healthcare Australia, Eight Mile Plains, Qld, Australia) had 97 permitted indications, Hyperi-Lift Plus (Bio-Practica, Glen Osmond, SA, Australia) had 85 and Nutrichew Chewable Multivitamin (Nutristar Solutions, Browns Plains, Qld, Australia) had 81.
Many permitted indications invoked the TGA’s controversial ‘traditionally used’ paradigm.12 For example Hyperi-Lift Plus used (among many others) the permitted indications ‘Traditionally used in Chinese medicine to move/promote/increase/augment/generate/promote Qi’.
Discussion
At the October 2019 meeting of the Therapeutic Goods Advertising Consultative Committee, the TGA expressed surprise at the flood of complaints they had received in the first 12 months of the new system.13 These complaints have shown no sign of abating over the past few months. Some of this increase may be due to the promotion of the TGA’s new user-friendly advertising hub, including their use of social media. Some is likely to have resulted from complainants frustrated by the lack of effective sanctions provided to the old CRP who are testing the new system.
The TGA appeared to set the priority level of a complaint based on a perceived risk of direct harm to a consumer. Indirect harm did not appear to concern the TGA. The latter occurs when consumers believe misleading and deceptive claims and, as a result, forgo more evidence-based products, fail to consult a medical professional when they should or waste their scarce financial resources on ineffective products. In addition, the TGA did not appear to consider repeated code violations previously upheld by the CRP as grounds for moving a complaint up the risk-based triage system.
The TGA did not regard ‘low-priority’ complaints as having formal code breaches (‘this is not regulatory action at law’).7 Yet one redacted obligation letter we obtained from the TGA dated 16 November 2018 agreed with the allegation made by the complainant; the advertisement was in breach of s.42DL(12) of the Therapeutic Goods Act 1989 (Cwlth) (hereafter referred to as ‘the Act’). This breach of the Act attracts both civil and criminal penalties, but neither was applied. The letter went on to say, ‘The TGA will not be pursuing this complaint any further at this time’.
It is a contradiction for the TGA to say that low-priority complaints have not broken the law when specific breaches of the Act, Regulations (Therapeutic Goods Regulations 1990 (Cwlth)) and Code (Therapeutic Goods Advertising Code (No.2) 2018 (Cwlth), hereafter referred to as ‘the Code’) were detailed in complaints, and the obligations letter and the action taken (e.g. ‘Requested removal’, ‘Sent for internal review’ and ‘Educational campaign’) are tabulated in the TGA’s annual report.11
The low priority accorded some complaints was also hard to understand. For example, complaint AC-E7JS15BB/2018 was submitted on 3 August 2018 and closed a few days later by a ‘Compliance Notice sent with educational material’. This product won a 2017 Choice ‘Shonky’ award and was the subject of a scathing New Zealand Consumer review.14 An upheld CRP complaint (26 March 2018) was also sent to the TGA by the CRP for non-compliance. The product continues to be promoted at the time of writing.
Neurofolin (Grunbiotics, Melbourne, Vic., Australia) (AC-NLDGK2 LY/2019) was an example of complaint categorised as low priority sent for internal review. This product was promoted as a medical food for the management of depression. It contained l-methylfolate calcium, as did a listed product whose TGA indication requirement was ‘Product presentation must not imply or refer to mental illnesses, disorders or conditions’. The internal review ultimately resulted in a Therapeutic Goods Order that declared certain goods containing folate substances to be therapeutic goods when used, advertised or presented for supply for therapeutic use.15 At the time of writing, neither this outcome nor the product it addressed have been added to the complaint record.
The TGA reported that 97% of 1480 complaints classified as ‘low priority’ met their key performance indicator (KPI) ‘time to close’; that is, closing 90% of complaints within 20 days. Clearly, there is little difficulty in meeting this KPI if complaints classified as ‘low priority’ are closed by merely sending an obligations letter with no follow-up. A more useful KPI would be based on the time taken to achieve compliance with the Code.
For most complaints (those classified as ‘low priority’), the TGA only published the reference number and ‘action taken’ with no information provided about the complaint, product, advertiser or the TGA’s own assessment. This provides much less transparency than the old CRP system. The CRP sent every complaint it judged to have breached the Code to the advertiser, in full, for a considered response. It then published, on its website, details of the advertisement, the offending claims, the name of the product and sponsor and a complete determination of claims alleged to breach the Code, often running to many pages. This was educational for the complainant, the advertiser, consumers and industry.
Research on the old CRP system showed that several large companies consistently broke the law.5 Presumably the profit that accrued from this behaviour outweighed the negligible risk of penalties being applied. These companies, and many others, continue to offend. The main problem with the old complaint system was that the CRP lacked the power to penalise advertisers who breached the Code (and law). This is why the TGA was given enhanced investigative and compliance powers under the new system.
However, the TGA’s new powers were rarely applied. Penalties, not negotiation, are required for sponsors, advertisers and products that repeatedly violate the rules. These penalties must outweigh the profit that comes from breaking the law.
There were also repeated complaints about products where new studies had invalidated older claims that, nevertheless, continued to be made. Examples include, omega-3 for ‘heart health’, glucosamine for osteoarthritis and Ginkgo biloba for mental enhancement. These products should be delisted.
The TGA’s failure to meet its KPIs for higher-level complaints was attributed to the unexpected number of complaints and staffing problems, although Minister Hunt stated on 3 February 2018 that ‘the TGA will be adequately resourced and staffed to manage complaints from July 1, 2018’.9
Several higher-priority complaints that have not yet been dealt with involve products at the food–medicine interface, such as sports supplements. The TGA has invited comment on these matters.16
The reluctance of industry to take up the new AUST L(A) listing pathway suggests that it is aware that few products would qualify and/or a greater return on investment comes from promotional hype and celebrity endorsement rather than research.
When the Therapeutic Goods Amendment (2017 Measures No. 1) Bill 2017 was debated, consumer and health professional organisations called for an educational statement on all traditional medicines,17 similar to that implemented in Canada on the front panel of paediatric homeopathic products: ‘NOTICE – This claim is based on traditional homeopathic references and not modern scientific evidence’.18,19(p.3) This was opposed by the TGA and government, but is still required.
Finally, the data analysed in this study are limited to complaint outcomes published by the TGA at the time of writing. We allowed 3 months to elapse after 30 June 2019 before commencing our analysis, but we are aware that there are still complaints submitted during 2018–19 that have not yet had outcomes published.
Recommendations
-
The TGA must be adequately staffed by people with appropriate expertise to handle the complaint load.
-
The TGA must use its new enforcement powers to reduce the unacceptably high level of advertising non-compliance. More use should be made of advertising direction notices (retractions), infringement notices (financial penalties), enforceable undertakings and civil and criminal penalties.
-
The ‘low priority’ complaint classification must be eliminated. All complaints that the TGA agrees document a Code breach must be sent to the advertiser for a formal response and followed-up to ensure compliance.
-
The status of complaints must be regularly updated in the TGA database.
-
KPIs must be changed to monitor outcomes (when compliance was achieved), not process (when the case was ‘closed’ by a letter sent).
-
Areas of public health concern, such as weight loss and hangover products, that lack evidence of efficacy must be prioritised.
-
The TGA must delist products when new and better research invalidates claims made by older studies.
-
An educational statement must be added to products making ‘traditional’ claims, such as ‘These claims are based on traditional beliefs and practices, not modern scientific evidence’.
-
The TGA must accept responsibility for dealing with all complaints about therapeutic advertising claims. Excluding certain products from the TGA’s remit, such as ear candles and magnets, fails to address the problem.
-
The lack of clarity at the food–medicine interface requires further work by the TGA and Food Standards Australia New Zealand.
Conclusion
Comment on Australia’s 2018 Royal Commission into Misconduct in Banking is equally applicable to the TGA: ‘Essentially a failure to enforce the law undermines the authority of the regulator whose fundamental responsibility is to do just that.’20 It also encourages others to break the law, leading to a race to the bottom and consumer detriment.
Postscript
The independent review of the effect of the new advertising measures, promised by Health Minister Hunt within 2 years of the commencement of the Therapeutic Goods Amendment (2017 Measures No.1) Act 2018 has now reported and the Government has accepted all 22 recommendations made.21
Competing interests
Ken Harvey represented Choice on the old Therapeutic Goods Advertising Code Council and Complaint Resolution Panel and continues to represent Choice on the new Therapeutic Goods Advertising Consultative Committee. He is President of Friends of Science in Medicine (FSM); the other authors are also FSM members.
Acknowledgements
Some of the complaints submitted to the TGA were based on research undertaken by Monash University students. This research did not receive any specific funding.
References
[1] Bollen MD, Whicker SD. Complementary medicines regulatory reform. Aust Health Rev 2009; 33 288–94.| Complementary medicines regulatory reform.Crossref | GoogleScholarGoogle Scholar | 19563317PubMed |
[2] Rollins A. ANZAC spirit fails drug test. Australian Medicine, 20 November 2014. Available at: https://ama.com.au/ausmed/anzac-spirit-fails-drug-test [verified 3 March 2020].
[3] The Department of Health. Expert review of medicines and medical devices regulation. 2015. Available at: https://www1.health.gov.au/internet/main/publishing.nsf/Content/Expert-Review-of-Medicines-and-Medical-Devices-Regulation [verified 3 March 2020].
[4] Therapeutic Goods Administration. Advertising hub. 2019. Available at: https://www.tga.gov.au/advertising-hub [verified 29 November 2019].
[5] Vickers M, Harvey K. Complementary medicines advertising policy Part I: unethical conduct in the Australian market before July 2018. Aust Health Rev 2020;
| Complementary medicines advertising policy Part I: unethical conduct in the Australian market before July 2018.Crossref | GoogleScholarGoogle Scholar |
[6] Therapeutic Goods Administration. Managing advertising complaints by risk category. 2019. Available at: https://www.tga.gov.au/publication/complaints-handling-advertising-therapeutic-goods-australian-public#managing [verified 3 March 2020].
[7] Therapeutic Goods Administration. Complaints handling for the advertising of therapeutic goods to the Australian public. 2020. Available at: https://www.tga.gov.au/publication/complaints-handling-advertising-therapeutic-goods-australian-public [verified 30 August 2020].
[8] Therapeutic Goods Administration. What does ‘TGA assessed’ mean on my medicine label? 2019. Available at: https://www.tga.gov.au/what-does-tga-assessed-mean-my-medicine-label [verified 29 November 2019].
[9] Hunt G. Re: Therapeutic Goods Amendment (2017 Measures No. 1) Bill 2017. [Letter to Catherine King and Tony Zappia] 3 February 2018. Available at: http://www.medreach.com.au/wp-content/uploads/2018/02/Minister-Hunts-Letter-re-TGA-Bill-2018.pdf [verified 29 November 2019].
[10] Therapeutic Goods Administration. Advertising: regulatory decisions and announcements. 2019. Available at: https://www.tga.gov.au/advertising-regulatory-decisions-and-announcements [verified 29 November 2019].
[11] Therapeutic Goods Administration. Therapeutic goods advertising compliance, 2018–19 annual report. 2019. Available at: https://www.tga.gov.au/publication/therapeutic-goods-advertising-compliance-2018-19-annual-report [verified 29 November 2019].
[12] Seidel B. Alarming health claims must not appear on complementary medicine: RACGP President. NewsGP, 9 February 2018. Available at: https://www1.racgp.org.au/newsgp/clinical/alarming-healthcare-claims-must-not-appear-on-comp [verified 29 November 2019].
[13] Therapeutic Goods Advertising Consultative Committee. Therapeutic Goods Advertising Consultative Committee, Communique, 17 October 2019. 2019. Available at: https://www.tga.gov.au/committee-meeting-info/therapeutic-goods-advertising-consultative-committee-17-october-2019 [verified 30 August 2020].
[14] Consumer. Pain Erazor claims. Consumer, 24 February 2017. Available at: https://www.consumer.org.nz/articles/pain-erazor-claims [verified 31 August 2020].
[15] Therapeutic Goods Administration. Therapeutic goods amendment (declared goods) order 2019: folate substances. 2019. Available at: https://www.tga.gov.au/therapeutic-goods-amendment-declared-goods-order-2019-folate-substances [verified 29 November 2019].
[16] Therapeutic Goods Administration. Consultation: proposed clarification that certain sports supplements are therapeutic goods. 2019. Available at: https://www.tga.gov.au/consultation/consultation-proposed-clarification-certain-sports-supplements-are-therapeutic-goods [verified 29 November 2019].
[17] Han E. ‘Softens hardness’: TGA under fire for health claim list that critics say endorses pseudoscience. The Sydney Morning Herald, 8 February 2018. Available at: https://www.smh.com.au/healthcare/softens-hardness-tga-under-fire-for-health-claim-list-that-endorses-pseudoscience-20180207-h0vfst.html [verified 7 November 2019].
[18] Foxcroft T. Unproven homeopathic remedies for kids still promising relief despite new label rules. CBC News, 9 May 2017. Available at: https://www.cbc.ca/news/health/homeopathic-labels-marketplace-1.4104417 [verified 3 September 2020].
[19] Canadian Homeopathic Pharmaceutical Association. Dear Manon, 9 December 2016. [Letter] Available at: https://www.documentcloud.org/documents/3705638-Association-Letter.html [verified 3 September 2020].
[20] Frydenberg J. ASIC’s failure to enforce the law let the sector off much too lightly. The Australian, 29 September 2018. Available at: https://joshfrydenberg.com.au/wp-content/uploads/2018/09/180929-Op-ed-ASICs-failure-to-enforce-the-law-let-the-sector-off-much-too-lightly.pdf [verified 3 September 2020].
[21] The Department of Health. Australian Government response to the review of the reforms to the Therapeutic Goods advertising framework. 2020. Available at: https://www.tga.gov.au/australian-government-response-review-reforms-therapeutic-goods-advertising-framework [verified 3 September 2020].



