Understanding consumer preference for vascular access safety and quality measurement: an international survey
Jessica Schults A B C K , Rebecca Paterson A , Tricia Kleidon A B C , Marie Cooke A B , Amanda Ullman A B , Keith McNeil D , Vineet Chopra F , Karina Charles A C , Gillian Ray-Barruel A B E , Nicole Marsh A B G , Clair Sullivan H I , David J. Sturgess J and Claire Rickard A BA Alliance for Vascular Access Teaching and Research Group, Menzies Health Institute Queensland, Griffith University, Nathan, Qld, Australia. Email: r.paterson@griffith.edu.au; m.cooke@griffith.edu.au; a.ullman@griffith.edu.au; g.ray-barruel@griffith.edu.au; c.rickard@griffith.edu.au
B School of Nursing and Midwifery, Griffith University, Nathan, Qld, Australia.
C Department of Anaesthesia and Pain Management, Queensland Children’s Hospital, Brisbane, Qld, Australia. Email: tricia.kleidon@health.qld.gov.au; karina.charles@health.qld.gov.au
D Prevention Division, Queensland Health, Brisbane, Qld, Australia. Email: Keith.McNeil@health.qld.gov.au
E Queen Elizabeth II Jubilee Hospital, Coopers Plains, Qld, Australia.
F The Patient Safety Enhancement Program, Division of Hospital Medicine, Department of Medicine, University of Michigan Health System and VA Ann Arbor Healthcare System. Email: vineetc@med.umich.edu
G Nursing and Midwifery Research Centre, Royal Brisbane and Women’s Hospital, Herston, Qld, Australia. Email: nicole.marsh@health.qld.gov.au
H Digital Metro North, Metro North Hospital and Health Service, Herston, Qld, Australia. Email: clair.sullivan@health.qld.gov.au
I Centre for Health Services Research, Faculty of Medicine, University of Queensland, Herston, Qld, Australia.
J Department of Anaesthesia, Surgical, Treatment and Rehabilitation Service (STARS), The University of Queensland, Herston, Qld, Australia. Email: david.sturgess@health.qld.gov.au
K Corresponding author. Email: j.schults@griffith.edu.au
Australian Health Review 46(1) 12-20 https://doi.org/10.1071/AH21053
Submitted: 22 February 2021 Accepted: 6 June 2021 Published: 15 December 2021
Journal Compilation © AHHA 2022 Open Access CC BY-NC-ND
Abstract
Objectives The aim of this study was to examine patient perceptions regarding vascular access quality measurement.
Methods A web-based, cross-sectional survey was performed using a convenience sample of healthcare consumers with vascular access experience, recruited from September 2019 to June 2020. Survey respondents were asked to rate the perceived importance of 50 vascular access data items, including patient demographics, clinical and device characteristics, and insertion, management and complication data. Data were ranked using a five-point Likert scale (1, least important; 5, most important), and are reported as median values. Respondents proposed additional items and explored broader perspectives using free-text responses, which were analysed using inductive thematic analysis.
Results In all, 68 consumers completed the survey. Participants were primarily female (82%), aged 40–49 years (29%) and living in Australia or New Zealand (84%). All respondents indicated that measuring the quality of vascular access care was important. Of the 50 items, 37 (74%) were perceived as ‘most important’ (median score 5), with measures of quality (i.e. outcomes and complications) rated highly (e.g. thrombosis and primary blood stream infection). Participants proposed 16 additional items. ‘Gender’ received the lowest perceived importance score (median score 3). Two themes emerged from the qualitative analysis of broader perspectives: (1) measurement of vascular access device complication severity and associated factors; and (2) patient experience.
Conclusion Measuring vascular access quality and safety is important to consumers. Outcome and complication measures were rated ‘most important’, with respondents identifying a need for increased monitoring of their overall vascular access journey through the health system.
What is known about the topic? The use of vascular access devices is common among hospitalised patients. Quality surveillance is not standardised, with no incorporation of patient preference.
What does this paper add? We identify the data items consumers perceive as valuable to measure related to their vascular access journey; most importantly, consumers perceived the collecting of vascular access data as important.
What are the implications for practitioners? Health services can use these data to develop platforms to monitor the quality and safety of vascular access care.
Keywords: adults, co-design, consumer engagement, consumer priorities, patient safety measurement, pediatrics, quality and safety, vascular access.
Introduction
It has become increasingly recognised that patients’ subjective experiences related to disease, treatment or both can uniquely inform targets for disease intervention to ensure that results directly translate to benefits for the patient.1 This is reflected in a shift in patient-centred outcomes research, with the US National Institutes of Health (NIH) highlighting the need for measures of clinical outcomes that are important to patients in their Roadmap for Medical Research,2 and the US Food and Drug Administration (FDA) identifying patient-reported outcomes as a regulatory standard for drug approval and labelling.3 As such, patient input has become a cornerstone for global safety and quality health service standards, leading to patient input informing clinical decision making, contributions to clinical practice guidelines, health policy, drug approval, pricing and reimbursement decisions and shared decision-making and consent for treatment.4
Vascular access devices (VADs) are essential to deliver medical treatment during hospitalisation. For this reason, millions of VADs are inserted annually,5 facilitating the delivery of infusates such as chemotherapeutics, antimicrobials, analgesic agents and contrast media for diagnostic studies. Despite their necessity, device complications are common: one in two peripheral intravenous catheters and one in four central venous catheters fail before treatment completion.6–9 This high failure rate is unacceptable and results in poor-quality care, placing significant burden on patients and health service resources.6,10 In addition, approximately 50% of adults report significant pain and/or anxiety during VAD insertion,11 and studies exploring patient experiences of VADs report varying satisfaction with devices.12–16 Patient input into the decision-making processes and care of VADs can reduce the risk of complications and failure,17 and prevent unnecessary device insertion.18 Patient perspectives are therefore important for ensuring active participation in care and are integral to ensuring value-based care. However, to date, patient involvement in the management of vascular access care quality has been largely overlooked.
The patient is a fundamental part of the vascular access care journey, yet consumers have had little involvement in determining what is quality vascular access care. Historically, the measurement of vascular access safety and quality has relied on local audits, incident reporting or siloed, simple electronic databases.19–21 This ad hoc non-standardised approach limits benchmarking and is associated with a range of errors, including missing data. Further, current practice neglects more intelligent systems of monitoring and feedback, such as integrated electronic platforms that can facilitate external benchmarking of key outcome metrics for patients with VADs worldwide. Current methods rely on resource-intensive clinical audits and non-standardised outcome measurement. Therefore, most healthcare organisations have limited capacity at present to analyse, monitor or learn from safety and quality information related to vascular access care. One exception is central venous catheters and blood stream infection surveillance; however, given peripheral catheters are used at much higher rates than central catheters, increased efforts need to focus on measuring quality across all catheters and outcomes. With the global adoption of electronic medical records and increasing application of clinical quality registries, the quality and value of vascular access care can be monitored and improved for every patient. In order for these advances to contribute to improved clinical outcomes, the patient perspective and care experience must be considered.
As efficient capabilities for electronic medical record data extraction are refined, the promise of data-driven decision making is becoming a reality for many health disciplines.22,23 However, in vascular access, medical record ontologies are fragmented,19,24 making data extraction challenging and unreliable. To date, limited enquiry has been undertaken with consumers regarding what aspects of care are valuable and important to measure for them. With efficient vascular access care based on the provision of easily accessible and reliable data, current methods for quality monitoring are suboptimal. The provision of these data could augment clinical decision making and reduce low-value healthcare practices8,25 (e.g. routine peripheral intravenous catheter replacement); failure to consider the patient perspective in this process could hinder progress, and so the patient perspective is of paramount importance. Implementing clinical decision support systems and streaming analytics in vascular access has immense potential when coupled with consumer engagement, with such functionality promising wide impact and significant clinical and research implications that may contribute to improved patient outcomes and quality of life.
The first step in creating patient-centred, data-driven support systems for vascular access care is the standardisation of data items, including item definitions and associated elements. Given previous research has shown that patient and clinician preferences may differ with respect to vascular access decision making,26 the next step is to incorporate patient perspectives to ensure patient-centred outcomes are included in quality measurement. The aim of the present consumer-driven quality improvement project was to understand which vascular access quality and safety measures consumers consider should be measured and collected.
Methods
Study design
This study was a cross-sectional opt-in Internet survey conducted from September 2019 to June 2020 using non-probability convenience sampling. We followed the American Association for Public Opinion Research (AAPOR) reporting guideline.27 Ethics exemption was granted by the Children’s Health Queensland ethics committee before study commencement. Implied informed consent was provided by all survey participants and based upon completion of survey items. Participants were able to terminate the survey at any time. Partial results were included with missing data described. The survey was anonymous, and confidentiality of information was assured.
Participants and sampling strategy
To include international perspectives and representation, sampling was not limited to geographical location (i.e. regions outside of the host country could participate). Consumers who had access to an electronic device with Internet connectivity, could read the English language and had experience with VADs, either as a patient or parent representative, were eligible to participate. We invited consumer participation via advertisement with consumer groups (e.g. Parenteral Nutrition Down Under), professional organisations (Australian Vascular Access Society), investigator networks and a general invitation on social media (Facebook and Twitter). We identified consumer groups through a web-based search using the key terms ‘vascular access’, ‘patient’ and ‘healthcare consumer’. We contacted 24 consumer groups, and six agreed to disseminate the survey information and link. The sampling strategy was limited to healthcare consumers because we have previously reported healthcare providers’ experiences and perceptions with vascular access data collection.24 Due to the broad dissemination strategy (used to minimise coverage and sampling error28), we were not able to calculate a denominator and subsequent response rate. To encourage a greater sample size, the survey link remained active until no new responses had been received for 7 days. Two reminder notices were published during this time, along with three retweets/shares of social media posts.
Outcomes and tool development
Questions were based on prior work to develop international recommendations for a vascular access minimum dataset29 and included: a scoping review of vascular access outcome measures and quality indicators;19 international30 and local31,32 quality databases; interviews with healthcare professionals;32 and international peripheral intravenous catheter5 and central venous catheter research.33 Consumers rated 50 items across the domains of patient demographics, device characteristics, insertion items, management items and complication and removal items using a five-point Likert scale, rated from 1, ‘least important’, to 5, ‘most important’. Demographic questions (five items) were included to capture respondent characteristics. Four open-ended response questions were included at the end of the survey to capture broader perceptions: (1) what additional variables do you believe are important to collect; (2) what information related to VADs do you believe is a priority for the hospital to collect; (3) what has been your overall experience with VADs; and (4) what was your biggest worry related to your VAD? The final survey included 59 items.
Prior to survey distribution, the tool was piloted with four consumers who provided feedback on the clarity and feasibility (ability to answer) of survey questions using a four-point level of agreement (1, not; 2, somewhat; 3, quite; 4, highly).34,35 All items scored >3 for clarity and feasibility, except for catheter material and catheter-to-vein ratio, which scored 2 for both clarity and feasibility. Consumers were then asked to recommend major revision, minor revision or to keep the item as it was. Overall, consumers recommended minor revisions to eight items, suggesting additional detail to explain complex medical terminology (e.g. ‘catheter material’ and ‘catheter-to-vein ratio’). The feasibility of the tool was established, with the consumer panel reporting the survey took 15 min to complete and that the questions were presented in a logical sequence. No technical difficulties were reported.
Data analysis
Data were analysed using IBM SPSS Version 25. Descriptive statistics were used to summarise respondents’ characteristics and demographic details including counts and percentages. The median and interquartile range (IQR) were calculated for each item. All responses were included in the analysis, with missing data described in tables.
Qualitative data were analysed using iterative and inductive thematic analysis as per Braun and Clarke’s six phases of thematic analysis36 and in line with similar studies.37,38 Initially, two researchers (JS, KC) read the transcribed interviews and independently generated initial codes. An audit trail was used to enhance dependability.39 Following this, the codes were collated into potential themes. The themes were reviewed by both researchers in relation to coded extracts and a thematic map was generated. To ensure authenticity, the resulting themes were reviewed by a third team member (RP). A selection of extract examples is provided in the text to support the final themes. Several strategies were used to enhance data quality, credibility and increase rigour, including data immersion, using a critical approach to analysis and confirmation of emerging findings between the two researchers.40 Further, a wide inclusive sampling technique was adopted to enhance the transferability of findings.41
Patient and public involvement
Patients were involved in the pilot testing of the tool and helped develop the initial minimum dataset as members of a hospital advisory group. The results of the study, in the form of a short summary of study findings, will be disseminated to study participants who provided an email on the first page of the survey.
Results
Demographic characteristics
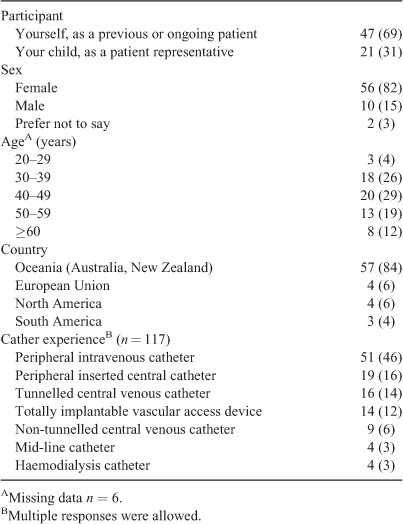
Overall, 68 surveys were returned, with 60 respondents (88%) completing the full survey and 8 (12%) surveys partially completed due to breakoffs or missing data. Of the responding participants, 69% (n = 47) identified as a previous or ongoing patient and 31% (n = 21) were patient representatives. The demographic and geographical data of the respondents are presented in Table 1. Most participants (84%) were from the Oceania region, with female respondents comprising 82% of the cohort. Respondents reported most experience with peripheral intravenous catheters.
|
Importance of vascular access data items
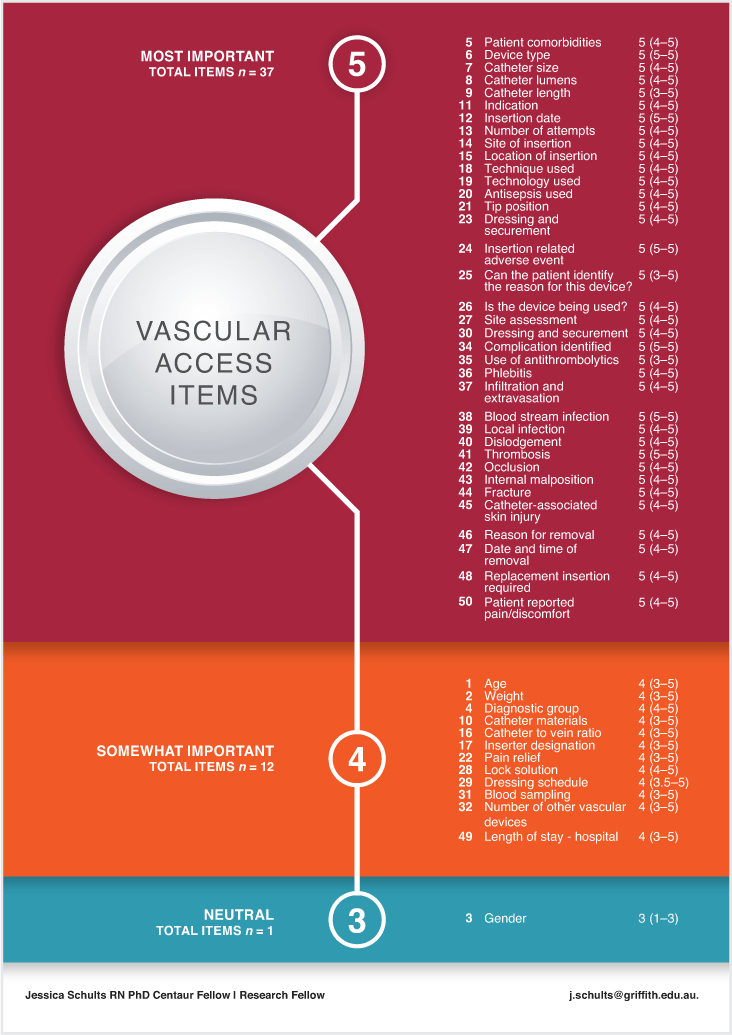
All participants perceived the collection of vascular access data for quality and safety measurement as ‘most important’ (median score 5; IQR 5–5). When evaluating responses to questions of items’ perceived importance, 37 (74%) were rated as ‘most important’ and 12 (24%) were rated as ‘somewhat important’. Gender was the lowest scoring item (median importance rating 3; IQR 1–3). Across domains and items measured, all device complications received a median score of 5, as did the item ‘complication identified’ (median 5; IQR 5–5). Pain relief for device insertion received a median score of 4 (IQR 3–5), along with the demographic items ‘age’, ‘weight’ and ‘diagnostic group’. Perceived importance scores are outlined in Fig. 1 and Supplementary material Table S1.
|
Additional measures of care quality
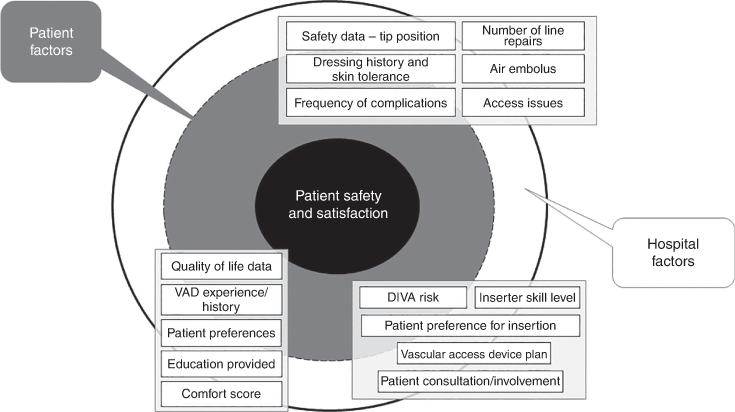
Respondents proposed an additional 16 items to integrate into a vascular access dataset. Items were focused on device complications (n = 6; e.g. frequency of complications and safety data), insertion difficultly (n = 5; e.g. difficult intravenous access risk), patient experience (n = 5; e.g. quality of life, previous vascular access experience). Fig. 2 outlines the additional factors proposed by respondents with integration of the qualitative feedback.
Broader perceptions of vascular access device data collection
Two priority themes emerged from the respondents’ broader perceptions of VAD data collection: (1) measurement of VAD complication severity and associated factors and (2) patient experience, influenced by patient or personal factors such as disease chronicity and hospital factors such as staff experience.
Measurement of vascular access device complication severity and associated factors
Participants identified a perceived ‘deficit’ in how the ‘degree’ or ‘severity’ of vascular access complications was tracked:
I think the hospital needs to survey patients to determine how much difficultly patients have.
One participant suggested there needs to be improved measurement of ‘ALL complications – currently this is poorly done’. Participants perceived a focus on the measurement of infection, with one participant identifying this as a concern:
Throughout my cancer treatment the clinicians seemed most concerned with infection; however, when my catheter tip moved out of the correct place, I felt terrible, irregular heartbeats, and the risk of the catheter accidentally falling out always worried me. I think there are far more painful complications to measure than infection, so it surprised me this was the focus. Although I recognise infection may lead to death.
Many respondents shared the perception that healthcare facilities need improved mechanisms to track patient preferences related to their ‘vascular access history’. Respondents highlighted a need to collect data around effects on activities of daily living or ‘restriction(s) to lifestyle due to line’, which should be used to inform future device decisions. Approximately half the respondents reported feeling uncertain regarding long-term treatment and the lack of a documented long-term vascular access plan, and they stressed the importance of collecting data that informs future vascular access decisions to prevent ‘damage to veins’ and facilitate ‘long-term venous access’ and therapy. Respondents believed collecting data around previous experiences and ‘what works’ could ‘prevent multiple and traumatic tries’ and affect patient satisfaction with care.
Patient experience
Measures of the difficultly of insertion, including ‘number of attempts’ and ‘difficult intravenous risk’, were discussed as important inclusions in data collection tools. Respondents described their experience with establishing reliable vascular access as ‘stressful and overwhelming’, with many participants relating multiple needle sticks and parents reporting traumatic insertions:
My son got held down by two people and it was very traumatic for him.
The insertion procedure was long, traumatic and painful for my daughter.
Measurement of pain and discomfort were highlighted by participants, with pain mentioned in 90% (45/50) of comments related to ‘What was your biggest worry related to your VAD?’ A considerable proportion of respondents expressed frustration at the inadequate or poor measurement of ‘pain and comfort’. Participants noted there was ‘not enough…[information collected] about pain issues with vascular [access] devices’, describing their experience as ‘…quite confronting’ and ‘it was always a source of low-level pain’. Parents discussed a need for change in relation to procedural pain measurement, commenting:
…maybe this study will prompt a change in the way these things are done and as a result our children are less traumatised.
Some respondents did not perceive themselves to be active participants in their vascular access care decisions, which they deemed important and believed could be improved with the documentation of patient preferences (e.g. ‘site of insertion or pain relief’).
Discussion
Vascular access is a critical issue for the healthcare system. Approximately 70% of hospitalised patients require a VAD; poor clinical practices and VAD-associated morbidity entail significant additional healthcare costs per annum.5,42,43 To the best of our knowledge, this study is the first to evaluate consumer perceptions regarding vascular access quality measurement using health data. Not only do we report consumer preferences for quality measurement, but the data we have presented also demonstrate agreement between clinicians (previously established consensus24,29) and consumers regarding what data are ‘important’ to measure. These data are primarily concerned with outcomes and complications, which are measures of the quality of care and the patient’s vascular access journey. The consumer engagement in this study is vital to the development, implementation and sustainability of a vascular access dataset to measure safety and quality. Without patient input into the design of vascular access datasets, outcomes important to patients may not be captured, data capture during hospitalisation may become overly burdensome and patient safety and overall satisfaction could be negatively affected.
Overall, respondents indicated that the measurement of device complications was important to them. Previous studies have shown that patients report frequent and burdensome complications associated with their VADs.11,44 Therefore, although unsurprising, the present study highlights that, for consumers, the measurement and collection of vascular access complication data across the patient’s lifespan and healthcare journey is important and should be a priority for healthcare institutions. This is even more relevant given that current deficits in standardised vascular access complication measurement prohibit international benchmarking.19 In order to reduce the VAD-related burden on the healthcare system, we need to monitor VAD outcomes from the point of insertion to removal and beyond, for every patient. This requires a defined dataset collected as part of routine care for every patient, which can be extracted and monitored. Any adverse safety signals must then be addressed.45
Measurement of the patient experience associated with VADs is understandably challenging. One device-related factor noted to contribute to a negative patient experience was pain and discomfort. Respondents rated ongoing ‘pain and discomfort’ as most important to measure in clinical practice, a finding that aligns with an international survey of consumer experiences.11 Interestingly, the importance ratings for ‘pain relief for insertion’ received a median importance rating of 4 (IQR 3–5). Although it was beyond the scope of this study to estimate the proportion of respondents who received sedation or analgesia for device insertion, it is possible that participants perceived the recording of analgesics on insertion as less important for the healthcare institution or less valuable to prevent complications during device dwell time. It is also likely that pain during catheter dwell is more ‘bothersome’ than pain on insertion, as reflected in previously published consumer experience work.11 Standardised and routine collection of pain measures in practice may be challenging, but these difficulties may be overcome using an integrated electronic medical record and validated numerical rating scale. The present study demonstrates that consumers believe the consistent and reliable measurement of this variable is important.
Some ways that researchers, clinician informaticians and healthcare providers can use the information generated in this study to strengthen their vascular access performance measurement include collaborating on more user-friendly, data-driven support systems and incorporating the measures outlined in this study in addition to hospital (context)-specific variables.46 For national policy makers and local safety and quality managers, our findings have important implications for vascular access quality measurement. Using patient-reported outcomes is an essential aspect for improving clinical care and should be included in the design and implementation of hospital-reported outcome frameworks. The inclusion of such measures supports the creation of a learning healthcare system, a system of continuous knowledge development, improvement and application. Ultimately, this will require systematic problem solving and the development of computing capabilities and analytics that offer real-time information on patient care to support continuous improvement in health care through outcomes.
This work has demonstrated consumers perceive the collection of vascular access data as an important aspect of their healthcare journey. To advance outcome and quality management in vascular access care, it would be useful for future research to explore improved shared decision making in patients’ vascular access journeys to further understand the effects of vascular access care on outcomes and how to improve patient quality of life and experience. Although the automated, integrated and standardised measurement of vascular access care quality and safety has received little attention to date, this work shows that more research and quality initiatives are needed to develop platforms to support measurement of care quality in this space.
Limitations
There are several limitations to our work. The nature of sampling, small sample size and large representation from female consumers from the Oceania region limit the generalisability of findings. We also engaged individuals with a history of vascular access interventions, potentially biasing our findings, with qualitative data limited to four questions. Further, due to the diverse and broad survey dissemination strategy, we were unable to calculate a survey response rate. However, the broad dissemination of the survey using online social media and consumer groups is a strength of the sampling framework. Finally, we used a purpose-built survey that had not been previously validated, and we did not estimate survey internal consistency. However, the tool was piloted and the questions were based on extensive prior work.
Conclusion
The results of this study, in combination with existing work, suggest agreement between clinicians, researchers and patients about which vascular access data are important to measure. Future quality improvement projects should focus on how the collection and reporting of vascular access data can contribute to a patient’s overall health care and vascular access experience, along with producing tangible benefits to the healthcare system and patient safety.
Author’s contributions
Jessica Schults, Rebecca Paterson, Tricia Kleidon, Marie Cooke and Claire Rickard conceived the study, drafted the protocol, collected and analysed data, drafted the manuscript and approved the final manuscript. Amanda Ullman, Gillian Ray-Barruel and Nicole Marsh reviewed the protocol and data analysis, and reviewed and approved the final manuscript. Karina Charles analysed the data and reviewed and approved the final manuscript. Keith McNeil, Vineet Chopra, Claire Sullivan and David Sturgess reviewed the methods and data analysis and reviewed and approved the final manuscript.
Data availability
Data supporting the findings of this study are not available due to the qualitative and confidential nature of interview data.
Competing interests
Jessica Schults has received grant funding from Griffith University and the Children’s Hospital Foundation, as well as investigator-initiated research and educational grants provided to Griffith University by Becton Dickinson, unrelated to this project. Tricia Kleidon’s employer has received the following on her behalf: grant funding from the Children’s Hospital Foundation, Griffith University, National Health and Medical Research Council (NHMRC) and Emergency Medicine Foundation; and investigator-initiated research grants and speaker fees provided to Griffith University from 3M Medical, Access Scientific, BD-Bard, Cardinal Health, Medical Specialties Australia, Smiths Medical and Vygon. Marie Cooke has received grant funding from Griffith University, the Children’s Hospital Foundation, the Royal Brisbane and Women’s Hospital Foundation, Cancer Council Queensland and the Australasian College for Infection Prevention and Control, as well as investigator-initiated research and educational grants and speaker fees provided to Griffith University by Baxter, Becton Dickinson and Entrotech Life Sciences, unrelated to this project. Amanda Ullman reports fellowships and grants from the NHMRC, grants from the Children’s Hospital Foundation, the Royal Brisbane and Women’s Hospital Foundation, Emergency Medicine Foundation and the Australian College of Critical Care Nursing and investigator-initiated research grants and speaker fees provided to Griffith University from 3M, Cardinal Health and Becton Dickinson. Vineet Chopra has received grants from the National Institutes of Health, Agency for Healthcare Research Quality, Centers for Disease Control, American Heart Association and the Blue Cross/Blue Shield Foundation of Michigan, as well as royalties from Oxford University Press and Wolters Kluwers related to chapters and book titles. Gillian Ray-Barruel has received research grant funding from Griffith University and the Australian College of Infection Prevention and Control; Griffith University has received, on her behalf, unrestricted research grants from 3M, BBraun and Becton Dickinson, as well as consultancy payments from Ausmed, 3M, Becton Dickinson, ResQDevices, Medline and Wolters Kluwer unrelated to this project. Nicole Marsh reports investigator-initiated research grants and speaker fees provided to Griffith University from Becton Dickinson, 3M and Cardinal Health and a consultancy payment for expert advice from Becton Dickinson. Claire Rickard reports investigator-initiated research grants and speaker fees provided to Griffith University from 3M, Angiodynamics, Baxter, BBraun, BD-Bard, Medtronic, ResQDevices and Smiths Medical unrelated to this project.
The remaining authors report no conflicts of interest.
Declaration of funding
This work was supported by an unrestricted education grant from Becton Dickinson (Franklin Lakes, NJ, USA) and the Menzies Health Institute (Griffith University). The funders had no role in study design; the collection, analysis and interpretation of the data; the writing of the report; or the decision to submit the article for publication.
Acknowledgements
We gratefully acknowledge the clinicians and consumers who gave freely of their time to complete the survey.
References
[1] Gliklich RE, Dreyer NA, Leavy MB. Use of Patient-Reported Outcomes in Registries. In: Gliklich RE, Dreyer NA, Leavy MB, editors. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality. 3rd edn; 2014. Available at: https://www.ncbi.nlm.nih.gov/books/NBK208628/.[2] Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007; 45 S3–11.
| The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years.Crossref | GoogleScholarGoogle Scholar | 17443116PubMed |
[3] US Department of Health and Human Services FDA Center for Drug Evaluation and Research US Department of Health and Human Services FDA Center for Biologics Evaluation Research US Department of Health and Human Services FDA Center for Devices and Radiological Health Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006; 4 79
| Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance.Crossref | GoogleScholarGoogle Scholar | 17034633PubMed |
[4] Rivera SC, Kyte DG, Aiyegbusi OL, Slade AL, McMullan C, Calvert MJ. The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis. Health Qual Life Outcomes 2019; 17 156
| The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis.Crossref | GoogleScholarGoogle Scholar | 31619266PubMed |
[5] Alexandrou E, Ray-Barruel G, Carr PJ, Frost SA, Inwood S, Higgins N, et al Use of Short Peripheral Intravenous Catheters: Characteristics, Management, and Outcomes Worldwide. J Hosp Med 2018; 13
| Use of Short Peripheral Intravenous Catheters: Characteristics, Management, and Outcomes Worldwide.Crossref | GoogleScholarGoogle Scholar | 29813140PubMed |
[6] Ullman AJ, Cooke M, Kleidon T, Rickard CM. Road map for improvement: Point prevalence audit and survey of central venous access devices in paediatric acute care. J Paediatr Child Health 2017; 53 123–30.
| Road map for improvement: Point prevalence audit and survey of central venous access devices in paediatric acute care.Crossref | GoogleScholarGoogle Scholar | 27709723PubMed |
[7] Malyon L, Ullman AJ, Phillips N, Young J, Kleidon T, Murfield J, Rickard CM. Peripheral intravenous catheter duration and failure in paediatric acute care: A prospective cohort study. Emerg Med Australas 2014; 26 602–8.
| Peripheral intravenous catheter duration and failure in paediatric acute care: A prospective cohort study.Crossref | GoogleScholarGoogle Scholar | 25346034PubMed |
[8] Rickard CM, Webster J, Wallis MC, Marsh N, McGrail MR, French V, et al Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet 2012; 380 1066–74.
| Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial.Crossref | GoogleScholarGoogle Scholar | 22998716PubMed |
[9] Marsh N, Webster J, Larson E, Cooke M, Mihala G, Rickard CM. Observational Study of Peripheral Intravenous Catheter Outcomes in Adult Hospitalized Patients: A Multivariable Analysis of Peripheral Intravenous Catheter Failure. J Hosp Med 2018; 13 83–9.
| 29073316PubMed |
[10] Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med 2012; 125 733–41.
| Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence.Crossref | GoogleScholarGoogle Scholar | 22840660PubMed |
[11] Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: Consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One 2018; 13 e0193436
| Not “just” an intravenous line: Consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries.Crossref | GoogleScholarGoogle Scholar | 29489908PubMed |
[12] Nicholson J, Davies L. Patients’ experiences of the PICC insertion procedure. Br J Nurs 2013; 22 S16–23.
| Patients’ experiences of the PICC insertion procedure.Crossref | GoogleScholarGoogle Scholar | 24261003PubMed |
[13] Sharp R, Grech C, Fielder A, Mikocka-Walus A, Cummings M, Esterman A. The patient experience of a peripherally inserted central catheter (PICC): A qualitative descriptive study. Contemp Nurse 2014; 48 26–35.
| The patient experience of a peripherally inserted central catheter (PICC): A qualitative descriptive study.Crossref | GoogleScholarGoogle Scholar | 25410192PubMed |
[14] Gabriel J. What Patients Think of a PICC. J Vasc Access Devices 2000; 5 26–9.
| What Patients Think of a PICC.Crossref | GoogleScholarGoogle Scholar |
[15] Mutti C, Fumagalli A, Monni P, Rancati S, Rosi IM. Ti racconto della mia scatoletta”: studio fenomenologico sull’esperienza di vivere con un catetere venoso centrale totalmente impiantato [‘Let me tell you about my little box’: phenomenological study on the experience of living with a totally implantable central venous catheter.] Assist Inferm Ric 2016; 35 180–6.
| 28151510PubMed |
[16] Chernecky C. Satisfaction versus dissatisfaction with venous access devices in outpatient oncology: a pilot study. Oncol Nurs Forum 2001; 28 1613–6.
| 11759308PubMed |
[17] Weingart SN, Zhu J, Chiappetta L, Stuver SO, Schneider EC, Epstein AM, et al Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care 2011; 23 269–77.
| Hospitalized patients’ participation and its impact on quality of care and patient safety.Crossref | GoogleScholarGoogle Scholar | 21307118PubMed |
[18] McHugh SM, Corrigan MA, Dimitrov BD, Morris-Downes M, Fitzpatrick F, Cowman S, et al Role of patient awareness in prevention of peripheral vascular catheter-related bloodstream infection. Infect Control Hosp Epidemiol 2011; 32 95–6.
| Role of patient awareness in prevention of peripheral vascular catheter-related bloodstream infection.Crossref | GoogleScholarGoogle Scholar | 21087126PubMed |
[19] Schults JA, Rickard CM, Kleidon T, Hughes R, Macfarlane F, Hung J, Ullman AJ. Building a Global, Pediatric Vascular Access Registry: A Scoping Review of Trial Outcomes and Quality Indicators to Inform Evidence-Based Practice. Worldviews Evid Based Nurs 2019; 16 51–9.
| Building a Global, Pediatric Vascular Access Registry: A Scoping Review of Trial Outcomes and Quality Indicators to Inform Evidence-Based Practice.Crossref | GoogleScholarGoogle Scholar | 30604496PubMed |
[20] Vincent C, Burnett S, Carthey J. Safety measurement and monitoring in healthcare: a framework to guide clinical teams and healthcare organisations in maintaining safety. BMJ Qual Saf 2014; 23 670–7.
| Safety measurement and monitoring in healthcare: a framework to guide clinical teams and healthcare organisations in maintaining safety.Crossref | GoogleScholarGoogle Scholar | 24764136PubMed |
[21] Baskin KM, Durack JC, Abu-Elmagd K, Doellman D, Drews BB, Journeycake JM, et al Chronic Central Venous Access: From Research Consensus Panel to National Multistakeholder Initiative. J Vasc Interv Radiol 2018; 29 461–9.
| Chronic Central Venous Access: From Research Consensus Panel to National Multistakeholder Initiative.Crossref | GoogleScholarGoogle Scholar | 29456059PubMed |
[22] Durieux P, Nizard R, Ravaud P, Mounier N, Lepage E. A Clinical Decision Support System for Prevention of Venous ThromboembolismEffect on Physician Behavior. JAMA 2000; 283 2816–21.
| A Clinical Decision Support System for Prevention of Venous ThromboembolismEffect on Physician Behavior.Crossref | GoogleScholarGoogle Scholar | 10838650PubMed |
[23] Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. with the HI. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak 2017; 17 36
| with the HI. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system.Crossref | GoogleScholarGoogle Scholar | 28395667PubMed |
[24] Schults JA, Woods C, Cooke M, Kleidon T, Marsh N, Ray-Barruel G, Rickard CM. Healthcare practitioner perspectives and experiences regarding vascular access device data: An exploratory study. Int J Healthc Manag 2020;
| Healthcare practitioner perspectives and experiences regarding vascular access device data: An exploratory study.Crossref | GoogleScholarGoogle Scholar |
[25] Sypes EE, de Grood C, Clement FM, Parsons Leigh J, Whalen-Browne L, Stelfox HT, Niven DJ. Understanding the public’s role in reducing low-value care: a scoping review. Implement Sci 2020; 15 20
| Understanding the public’s role in reducing low-value care: a scoping review.Crossref | GoogleScholarGoogle Scholar | 32264926PubMed |
[26] van der Veer SN, Haller MC, Pittens CA, Broerse J, Castledine C, Gallieni M, et al Setting Priorities for Optimizing Vascular Access Decision Making–An International Survey of Patients and Clinicians. PLoS One 2015; 10 e0128228
| Setting Priorities for Optimizing Vascular Access Decision Making–An International Survey of Patients and Clinicians.Crossref | GoogleScholarGoogle Scholar | 26151822PubMed |
[27] American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 9th edn. AAPOR; 2016. Available at https://www.aapor.org/AAPOR_Main/media/publications/Standard-Definitions20169theditionfinal.pdf
[28] Fincham JE. Response rates and responsiveness for surveys, standards, and the Journal. Am J Pharm Educ 2008; 72 43
| Response rates and responsiveness for surveys, standards, and the Journal.Crossref | GoogleScholarGoogle Scholar | 18483608PubMed |
[29] Schults JAKleidon TChopra CCooke MPaterson RSUllman AJet al International recommendations for a vascular access minimum data set: a Delphi consensus-building study. BMJ Safety & Quality 2021 ; 30 : 722–30
[30] Michigan Hospital Medicine Safety Consortium. Peripherally Inserted Central Catheter (PICC) Use Initiative: HMS; 2020. Available at https://www.mi-hms.org/quality-initiatives/peripherally-inserted-central-catheter-picc-use-initiative
[31] Kleidon TM, Horowitz J, Rickard CM, Ullman AJ, Marsh N, Schults J, Ratz D, Chopra V. Peripherally inserted central catheter thrombosis after placement via electrocardiography vs traditional methods. Am J Med 2021; 134 e79–88.
| Peripherally inserted central catheter thrombosis after placement via electrocardiography vs traditional methods.Crossref | GoogleScholarGoogle Scholar | 32673624PubMed |
[32] Kleidon TM, Rickard CM, Schults JA, Mihala G, McBride CA, Rudkin J, Chaseling B, Ullman AJ. Development of a paediatric central venous access device database: A retrospective cohort study of practice evolution and risk factors for device failure. J Paediatr Child Health 2020; 56 289–97.
| Development of a paediatric central venous access device database: A retrospective cohort study of practice evolution and risk factors for device failure.Crossref | GoogleScholarGoogle Scholar | 31436918PubMed |
[33] Takashima M, Ray-Barruel G, Ullman A, Keogh S, Rickard CM. Randomized controlled trials in central vascular access devices: A scoping review. PLoS One 2017; 12 e0174164
| Randomized controlled trials in central vascular access devices: A scoping review.Crossref | GoogleScholarGoogle Scholar | 28323880PubMed |
[34] Polit DF, Beck CT. The content validity index: are you sure you know what’s being reported? Critique and recommendations. Res Nurs Health 2006; 29 489–97.
| The content validity index: are you sure you know what’s being reported? Critique and recommendations.Crossref | GoogleScholarGoogle Scholar | 16977646PubMed |
[35] Polit DF, Beck CT, Owen SV. Is the CVI an acceptable indicator of content validity? Appraisal and recommendations. Res Nurs Health 2007; 30 459–67.
| Is the CVI an acceptable indicator of content validity? Appraisal and recommendations.Crossref | GoogleScholarGoogle Scholar | 17654487PubMed |
[36] Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006; 3 77–101.
| Using thematic analysis in psychology.Crossref | GoogleScholarGoogle Scholar |
[37] Casey JR, Hanson CS, Winkelmayer WC, Craig JC, Palmer S, Strippoli GF, Tong A. Patients’ perspectives on hemodialysis vascular access: a systematic review of qualitative studies. Am J Kidney Dis 2014; 64 937–53.
| Patients’ perspectives on hemodialysis vascular access: a systematic review of qualitative studies.Crossref | GoogleScholarGoogle Scholar | 25115617PubMed |
[38] Ritchie M, Kelly LJ, Moss J, Paul J, Shaw R. Exploring Attitudes towards a Randomised Controlled Trial of Venous access Devices – a Nested Pre-trial Qualitative Study. J Vasc Access 2015; 16 407–12.
| Exploring Attitudes towards a Randomised Controlled Trial of Venous access Devices – a Nested Pre-trial Qualitative Study.Crossref | GoogleScholarGoogle Scholar | 26349872PubMed |
[39] Koch T. Establishing rigour in qualitative research: the decision trail. J Adv Nurs 2006; 53 91–100.
| Establishing rigour in qualitative research: the decision trail.Crossref | GoogleScholarGoogle Scholar | 16422698PubMed |
[40] Lincoln YS, Guba EG. But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation. New Dir Program Eval 1986; 1986 73–84.
| But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation.Crossref | GoogleScholarGoogle Scholar |
[41] Creswell JW, Plano Clark VL. Designing and conducting mixed methods research. Thousand Oaks, CA: SAGE; 2018.
[42] Tuffaha HW, Marsh N, Byrnes J, Gavin N, Webster J, Cooke M, Rickard CM. Cost of vascular access devices in public hospitals in Queensland. Aust Health Rev 2019; 43 511–5.
| Cost of vascular access devices in public hospitals in Queensland.Crossref | GoogleScholarGoogle Scholar | 30176985PubMed |
[43] Tuffaha HW, Rickard CM, Inwood S, Gordon L, Scuffham P. The epic3 recommendation that clinically indicated replacement of peripheral venous catheters is safe and cost-saving: how much would the NHS save? J Hosp Infect 2014; 87 183–4.
| The epic3 recommendation that clinically indicated replacement of peripheral venous catheters is safe and cost-saving: how much would the NHS save?Crossref | GoogleScholarGoogle Scholar | 24928785PubMed |
[44] Sharp R, Grech C, Fielder A, Mikocka-Walus A, Cummings M, Esterman A. The patient experience of a peripherally inserted central catheter (PICC): A qualitative descriptive study. Contemp Nurse 2014; 48 26–35.
| The patient experience of a peripherally inserted central catheter (PICC): A qualitative descriptive study.Crossref | GoogleScholarGoogle Scholar | 25410192PubMed |
[45] Barnett A, Winning M, Canaris S, Cleary M, Staib A, Sullivan C. Digital transformation of hospital quality and safety: real-time data for real-time action. Aust Health Rev 2019; 43 656–61.
| Digital transformation of hospital quality and safety: real-time data for real-time action.Crossref | GoogleScholarGoogle Scholar | 30384880PubMed |
[46] National Health and Hospitals Reform Commission. A healthier future for all Australians: final report June 2009. Canberra: Department of Health and Ageing; 2009.