Horizontal or vertical? Camera trap orientations and recording modes for detecting potoroos, bandicoots and pademelons
Brendan D. Taylor A C , Ross L. Goldingay A and John M. Lindsay BA School of Environment, Science and Engineering, Southern Cross University, Lismore, NSW 2480, Australia.
B PO Box 544, Mullumbimby, NSW 2482, Australia.
C Corresponding author. Email: brendan.taylor@scu.edu.au
Australian Mammalogy 36(1) 60-66 https://doi.org/10.1071/AM13012
Submitted: 26 April 2013 Accepted: 23 September 2013 Published: 6 December 2013
Abstract
Camera traps can detect rare and cryptic species, and may enable description of the stability of populations of threatened species. We investigated the relative performance of cameras oriented horizontally or vertically, and recording mode (still and video) to detect the vulnerable long-nosed potoroo (Potorous tridactylus) as a precursor to population monitoring. We established camera traps for periods of 13–21 days across 21 sites in Richmond Range National Park in north-east New South Wales. Each camera trap set consisted of three KeepGuard KG680V cameras directed at a bait container – one horizontal and one vertical camera in still mode and one horizontal camera in video mode. Potoroos and bandicoots (Perameles nasuta and Isoodon macrourus) were detected at 14 sites and pademelons (Thylogale stigmatica and T. thetis) were detected at 19 sites. We used program Presence to compare detection probabilities for each camera category. The detection probability for all three taxa groups was lowest for the vertical still and similar for the horizontal cameras. The detection probability (horizontal still) was highest for the potoroos (0.43) compared with the bandicoots (0.16) and pademelons (0.25). We estimate that the horizontal stills camera could achieve a 95% probability of detection of a potoroo within 6 days compared with 8 days using a vertical stills camera. This suggests that horizontal cameras in still mode have great potential for monitoring the dynamics of this potoroo population.
Additional keywords: camera orientation, Richmond Range National Park.
Introduction
Camera traps have become integral to the study of terrestrial wildlife in Australia and overseas (Rowcliffe and Carbone 2008; Wang and Macdonald 2009; O’Connell and Bailey 2011; Meek and Pittet 2012; Taylor and Goldingay 2012). They enable remote, non-invasive and covert monitoring over long deployment times, making them highly versatile as a survey tool (Cutler and Swann 1999). In particular, they provide an effective means of detecting rare and trap-shy species (e.g. Carbone et al. 2001; Claridge et al. 2004; Jackson et al. 2006). Camera traps are being used increasingly to monitor populations of such species (e.g. Karanth 1995; Jackson et al. 2006; McCain and Childs 2008; Rowcliffe et al. 2008; Wang and Macdonald 2009). This leads to the prospect of using this method to evaluate management actions that aim to conserve elements of biodiversity (e.g. Li et al. 2012).
The conservation of threatened species hinges on an understanding of population stability over time. Such information may be very costly to collect, which may lead to studies not being conducted and management being based on incomplete knowledge. The advent of camera-trapping provides a possible way forward for species that are difficult to survey. This point is evident for the long-nosed potoroo (Potorous tridactylus), a Federally listed vulnerable species. Although some populations have been studied in detail over several years (e.g. Frankham et al. 2011), others have been studied for relatively short periods (e.g. Mason 1997; Norton et al. 2010). Securing the long-term conservation of this species will depend on an understanding of its population dynamics at multiple locations across its geographic range, which extends from eastern South Australia to southern Queensland, and includes Tasmania (Johnston 2008). The sensitivity of this species to habitat alteration (Claridge and Barry 2000) and fox predation (Dexter and Murray 2009) is well documented and attests to a need for population monitoring. Camera traps now provide the most cost-effective and least intrusive method for doing this (e.g. Claridge et al. 2010).
A significant population of long-nosed potoroos occurs in Richmond Range National Park in north-east New South Wales and requires monitoring to investigate its long-term persistence. However, a key issue with using camera traps to do this is that their performance may vary across camera models (see Swann et al. 2004) and also vary according to their placement (see De Bondi et al. 2010). Smith and Coulson (2012) compared detection of long-nosed potoroos and southern brown bandicoots (Isoodon obesulus) by cameras directed in a horizontal or vertical plane. They found that vertically oriented cameras had detection probabilities for these species 2–5 times higher than horizontally oriented cameras. Thus, as a precursor to initiating population monitoring we investigated the influence of camera position and recording mode (still/video) on the detection of potoroos and other medium-sized mammals. We included video because this may facilitate species identification.
Methods
Study area
The study was conducted within the Richmond Range National Park (RRNP), ~31 km west of Casino in north-east New South Wales (28°47′08″S, 152°44′21″E). RRNP covers an area of 15 420 ha and features wet and dry sclerophyll forest and large areas of subtropical rainforest (Edwards 2005). Flooded gum (Eucalyptus grandis) dominates the wet sclerophyll forests and Richmond Range spotted gum (Corymbia variegata) and tallowwood (E. microcorys) dominate the dry forest areas. Sclerophyll forests feature shrubby and grassy understoreys dominated by forest oak (Allocasuarina torulosa), red ash (Alphitonia excelsa) and blady grass (Imperata cylindrica). The exotic weed lantana (Lantana camara) dominates some understorey areas, particularly where logging has previously occurred (Edwards 2005). RRNP is located within one of the most bio-diverse regions in Australia and includes 50 mammal species, 17 of which are listed as threatened (BioNet 2012).
Survey methods
We established 21 sites dispersed along 20.5 km of Cambridge Plateau Forest Road, which extends along the north–south oriented ridgeline of RRNP. Sites were located within areas of dry sclerophyll forest at least 500 m apart to maintain independence and positioned 50–1100 m from the road. Owing to lengthy set up times, monitoring of all 21 sites occurred across seven consecutive sessions (three sites per session). Due to time and logistical constraints, session duration ranged between 13 and 21 days. Monitoring sessions began at the southern end of RRNP on 22 August 2012, progressed northwards and was completed on 11 December 2012.
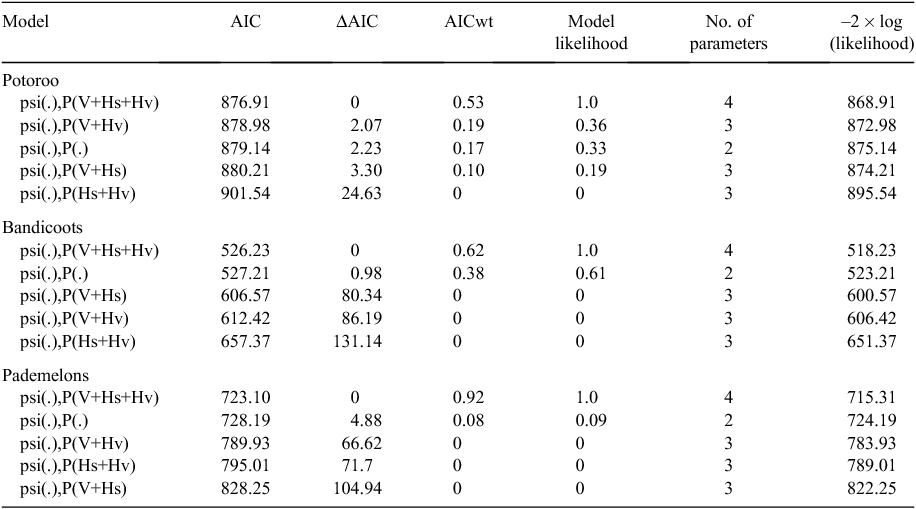
Camera traps were set up similarly to that described by Smith and Coulson (2012). At each site we used a scent lure consisting of a perforated PVC pipe (50 × 100 mm) filled with a mixture of peanut butter and oats to attract animals to the detection zone. Lure chambers were attached to a star picket ~0.3 m above ground level (Fig. 1). At each site we installed three KeepGuard KG680V cameras (ScoutGuard, USA), one in a vertical orientation (hereafter referred to as vertical) and two in a horizontal orientation (hereafter referred to as horizontal). All three cameras were aimed at the lure. The vertical camera was attached to the star picket housing the lure at a height of ~1.3 m above ground level. The horizontal cameras were each attached to star pickets at a height of 0.4 m above ground level, positioned ~0.3 m apart and ~2.0 m from the lure. One of the horizontal cameras (chosen at random) and the vertical camera were set to take three successive still photographs when activated. The second horizontal camera was set to take 20 s of video upon activation. All cameras were set to ‘normal’ sensitivity level and 20-min forced-delay post-photograph activation. To reduce the likelihood of false triggers, areas of reduced vegetation cover were selected so minimal vegetation around the cameras was removed.
|
At the completion of each session, images stored on SD memory cards were uploaded onto a laptop computer and stored in individual folders according to site number and camera orientation/mode. Images and videos were viewed using Windows Photo Viewer. Medium-sized mammals were mostly identified to species. Pademelon species were often difficult to distinguish in the black and white images so were recorded as pademelon. There were instances for all cameras where only a portion of an animal could be seen and identification not possible. These were recorded as ‘identification unknown’ and not used further. For each medium-sized mammal image we recorded data on species or species-group, date, time, site number, camera orientation and recording mode in an Excel spreadsheet (Microsoft Office 2010, Microsoft Corporation, Redmond, WA, USA). A detection history (presence/absence for each 24-h period) for the most frequently detected medium-sized mammals (potoroo, bandicoots, pademelons) was then constructed for each camera. Each series of three photographs or a 20-s video was defined as an ‘ event’ and any not containing an animal were recorded as false triggers.
To enable an unbiased comparison between recording modes and camera orientations, only data from cameras that were functional for the full duration of each survey period (i.e. 13–21 days) were included in analyses. Cameras that malfunctioned part way through (e.g. continuous triggering resulting in rapid filling of memory card, infrared flash not functioning, camera inactive during period(s) of the survey) were not included. Camera malfunction occurred on seven occasions (two Vertical; three Horizontal Video; two Horizontal Stills), leaving 56 functioning cameras for analyses. For Sites 1–6, the vertical camera was mistakenly set to video mode instead of still. To use these data as equivalent to a still camera we viewed only the first 3 s of each video to equate with a three-photograph burst.
Statistical methods
We followed a similar procedure to that of Smith and Coulson (2012). We used program Presence (Version 5.7; USGS Patuxent Wildlife Research Center, Laurel, MD 20708, USA) to compare detection probabilities for each camera type. We used the single-season occupancy model (MacKenzie et al. 2006) to determine the effect of camera orientation and recording mode on detection probability based on detection history. The model thereby controls for variations in survey period. Concurrent deployment of the three cameras and the relatively short periods of survey at each site ensured that populations could be considered closed during our sampling. This also meant that site and weather parameters were equivalent across our camera comparisons. Presence estimates occupancy (psi) and detection probability (P) simultaneously using maximum likelihood. We compared a null model where psi and P are constant across cameras and sites (defined as: psi(.),P(.)) with models that included an influence of camera position/mode as covariates on P but in which psi was constant.
Models were ranked by Presence using Akaike’s Information Criterion (AIC) to identify the most parsimonious model (Burnham and Anderson 2002). Models are ranked from the lowest to the highest AIC value and the difference in AIC (ΔAIC) calculated between each model and the top model. Where ΔAIC is <2 then there is little difference between the models. For ΔAIC values of 2–7 there is support that the models differ and for values >7 there is strong support that they differ (see Burnham and Anderson 2002).
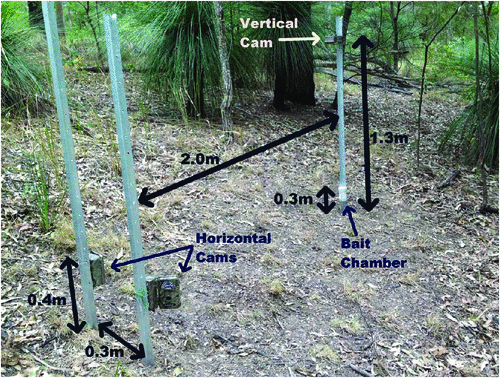
Again following Smith and Coulson (2012), we used the estimated detection probability from the best model for each camera type to calculate the cumulative detection probability for each day of the survey (Pn). We used the formula:
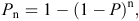
where P = detection probability and n = number of survey days.
Results
Our cameras recorded 3764 events and detected animals (any species) in 3030 of these events. The mean percentage (±s.e.) of false triggers was highest for vertical camera orientation (35.2 ± 5.6%), followed by horizontal still (24.7 ± 5.7%) and horizontal video (21.1 ± 5.1%). There was no significant difference (F2,53 = 1.55, P = 0.22) in mean false triggers for camera orientation (data were arcsine transformed).
The most frequently detected species were the long-nosed potoroo (14 sites), northern brown bandicoot (Isoodon macrourus) (12 sites), long-nosed bandicoot (Perameles nasuta) (6 sites), and two pademelon species that could not be readily distinguished in a black and white image, the red-legged pademelon (Thylogale stigmatica) and the red-necked pademelon (T. thetis) (19 sites) (Fig. 2). To facilitate analysis we pooled images for the two bandicoot species.

|
For all species-groups, horizontal cameras recorded more events than vertical cameras. Potoroos were captured in 440 events (166 horizontal still events, 164 horizontal videos and 110 vertical events). Bandicoots were captured in 101 events (39 horizontal videos, 38 horizontal stills, 24 vertical stills) and pademelons in 209 events (92 horizontal stills, 94 horizontal videos, 23 vertical stills). At sites where all cameras functioned for the entire survey period and target taxa were detected, potoroos were detected by the three cameras, bandicoots evaded detection by vertical stills and horizontal video cameras on one plot each and pademelons evaded detection by vertical cameras on five plots.
All three camera types also detected echidna (Tachyglossus aculeatus) (14 occasions), koala (Phascolarctos cinereus) (6 occasions), mountain brushtail possum (Trichosurus caninus) (17 occasions), black-striped wallaby (Macropus dorsalis) (28 occasions), red-necked wallaby (M. rufogriseus) (10 occasions), swamp wallaby (Wallabia bicolor) (16 occasions) and wild dog (Canis lupus) (6 occasions). Only the horizontal still and video cameras detected the rufous bettong (Aepyprymnus rufescens) (5 occasions). A variety of small mammals, reptiles and birds were also detected.
We found that the statistical model that incorporates a difference in P based on camera type was ranked the top for all three species-groups comparisons (Table 1). For the potoroo data the ΔAIC between this model and the next best model was >2, suggesting that they differ. Comparison of the AICwt values suggests that the top model has twice as much support as the next. In particular, the top model had much more support than the null model. For the bandicoot data the ΔAIC between the top model and the next best (the null model) was ~1, suggesting little difference (Table 1). For the pademelon data the ΔAIC between the top model and the next best (the null model) was >4, suggesting that they differ. Comparison of the AICwt values suggests that the top model has 11.5 times as much support as the null model (Table 1).
The probability of detecting potoroos with the horizontal still camera was 0.43, which was ~0.1 higher than for the vertical camera (Fig. 3). For bandicoots, the detection probability for the horizontal still camera was 0.16, which was slightly higher than for the vertical camera (Fig. 3). For pademelons, the detection probability for the horizontal still camera was 0.25, which was higher than for either the vertical camera or the horizontal video camera (Fig. 3).
|
The number of nights required to achieve a 95% probability of detection if present varied across the three species groups for the three camera types (Fig. 4). This could be achieved in 6 nights for potoroos, 11 nights for pademelons and 17 nights for bandicoots using a horizontal still camera. In comparison, achieving a 95% detection probability using a vertical camera at our sites would require 8, 23 and 31 nights for these species, respectively.
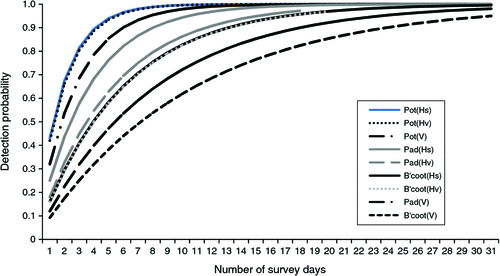
|
Discussion
Effective deployment of camera traps is fundamental to using them to evaluate the status of species’ populations (e.g. Hohnen et al. 2013). In our case we needed to test camera set-up before we embarked on a more detailed survey of a larger segment of the local population of long-nosed potoroos. Our investigation followed a similar one by Smith and Coulson (2012). Although the findings of both studies have particular relevance to the detection of potoroos and other medium-sized ground-dwelling mammals they offer some other important insights to using camera traps for wildlife monitoring in general.
Smith and Coulson (2012) found that a vertical camera was much more likely to detect potoroos and bandicoots than a horizontal camera and postulated that this difference could have been caused by the positioning of the horizontal camera (30 cm above ground) creating gaps in the detection area of their PixController camera. We used a KeepGuard camera with the horizontal camera installed 40 cm above ground and found that the horizontal camera performed better than did the vertical camera in detecting potoroos and bandicoots but was most pronounced for pademelons. This difference may have been mediated by the wider detection zone of the horizontal camera compared with the vertical camera. Our horizontal camera was placed 2 m from the lure compared with 3 m by Smith and Coulson (2012). Their greater distance may have reduced the effectiveness of their horizontal camera due to the detection zone of their camera. We have subsequently compared pairs of horizontal cameras (one positioned 1.3 m and the other 2 m from the lure) at 10 sites in our study area for periods of 13–19 nights. We found no significant difference in the number of detections for potoroos and pademelons (Taylor, Goldingay and Lindsay, unpubl. data). Bandicoots were detected too infrequently to compare. Differences between studies may also be due to differences in the performance of different camera brands (Swann et al. 2004; Kelly and Holub 2008).
We recorded false triggers in a mean of 21–35% of activation events compared with 73–85% by Smith and Coulson (2012). We set our cameras to minimise false-triggering with three photographs or one video taken followed by a 20-min forced delay. Smith and Coulson (2012) allowed continuous triggering for 30 s and no forced delay. There is a trade-off here where additional images facilitate identification but uninterrupted triggering will produce a plethora of images to view. We recommend a period of forced delay between events to minimise image accumulation from false-triggering or excessive numbers of photographs of a single individual remaining at a bait lure for some time. Hamel et al. (2013) examined the influence of time delays and motion-activated versus time-activated cameras and although time delays may increase the rate of daily false-negatives, the influence on detection and occupancy was negligible compared with the influence of sampling period. Similarly, Tobler et al. (2008) reported that the number of species recorded was highly correlated with survey effort and not camera spacing or survey area.
Differences in detectability of potoroos between our study (0.43 horizontal-still) and that of Smith and Coulson (2012) (0.17 vertical) may partly reflect differences in local population size. They detected potoroos at 50% of 16 sites whereas we detected potoroos at 67% of 21 sites. They detected bandicoots at 50% of sites with a detectability of 0.18 (vertical) whereas we detected bandicoots (two species combined) at 67% of sites with a detectability of 0.16 (horizontal-still). Our minimum sampling period (13 nights) was longer (by two nights) than that of Smith and Coulson (2012), which may have also influenced detection. Hamel et al. (2013) found for several species that sampling periods of 20–30 days were needed to stabilise detection probability and occupancy whereas Claridge et al. (2010) reported that periods of 14 days or longer were required to detect bandicoots and potoroos.
Smith and Coulson (2012) observed that the vertical camera greatly facilitated distinguishing potoroos and bandicoots. In contrast, we found that images/videos from horizontally oriented cameras made identification easier because the very different head profiles are more pronounced when viewed side-on compared with top-down (Fig. 2). Depending on the animal’s position in the frame, potoroos and bandicoots could appear similar when viewed from above. This difference in opinion may reflect that we had only three photographs in a sequence whereas their cameras were on rapid fire for up to 30 s. Another consideration is that we are also interested in documenting the frequency of reproductive events among individuals in our population. We found we could identify females with distended pouches with the horizontal camera (Fig. 2) and often identify males from their prominent testes. Therefore, horizontal cameras allow us to satisfy several study objectives more effectively than will vertical cameras.
One important lesson from our study is the importance of conducting presurvey trials with camera traps. Cameras will differ in their ability to detect species (Swann et al. 2004) and the way they are positioned may influence detection (Smith and Coulson 2012). Furthermore, because of differences in detectability, surveys need to be of sufficient duration to achieve high confidence that target species are detected when present in a survey area. Therefore, presurvey testing will be critical to ensure optimal camera positioning and setting, and to determine sufficient survey effort to detect target species and to allow study objectives to be met. Such trials need not be as extensive or take as long as in our investigation but they should enable many aspects of camera set-up to be fine-tuned and minimum periods of deployment determined.
Acknowledgements
We thank the local National Parks and Wildlife Service office for allowing access to the study area. David Rohweder provided helpful comments on an earlier draft. This project was conducted under approval 12/27 from the SCU Animal Care & Ethics Committee and under scientific licence SL100330 from the New South Wales Office of Environment & Heritage.
References
BioNet (2012). Atlas Search. New South Wales Government, Sydney. http://www.environment.nsw.gov.au/atlaspublicapp/UI_Modules/ATLAS_/AtlasSearch.aspx [Verified 1 November 2012.]Burnham, K. P., and Anderson, D. R. (2002). ‘Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach.’ (Springer: New York.)
Carbone, C., Christie, S., Coulson, T., Franklin, N., Ginsberg, J. R., Griffiths, M., Holden, J., Kawanishi, K., Kinnaird, M. F., Laidlaw, R., Lynam, A., Macdonald, D. W., Martyr, D., McDougal, C., Nath, L., Obrien, T., Seidensticker, J., Smith, D. J. L., Sunquist, M., Tilson, R., and Wan Shahruddin, W. N. (2001). The use of photographic rates to estimate densities of tigers and other cryptic mammals. Animal Conservation 4, 75–79.
| The use of photographic rates to estimate densities of tigers and other cryptic mammals.Crossref | GoogleScholarGoogle Scholar |
Claridge, A. W., and Barry, S. C. (2000). Factors influencing the distribution of medium-sized ground-dwelling mammals in southeastern mainland Australia. Austral Ecology 25, 676–688.
| Factors influencing the distribution of medium-sized ground-dwelling mammals in southeastern mainland Australia.Crossref | GoogleScholarGoogle Scholar |
Claridge, A. W., Mifsud, G., Dawson, J., and Saxon, M. J. (2004). Use of infrared digital cameras to investigate the behaviour of cryptic species. Wildlife Research 31, 645–650.
| Use of infrared digital cameras to investigate the behaviour of cryptic species.Crossref | GoogleScholarGoogle Scholar |
Claridge, A. W., Paull, D. J., and Barry, S. C. (2010). Detection of medium-sized ground-dwelling mammals using infrared digital cameras: an alternative way forward? Australian Mammalogy 32, 165–171.
| Detection of medium-sized ground-dwelling mammals using infrared digital cameras: an alternative way forward?Crossref | GoogleScholarGoogle Scholar |
Cutler, T. L., and Swann, D. E. (1999). Using remote photography in wildlife ecology: a review. Wildlife Society Bulletin 27, 571–581.
De Bondi, N., White, J. G., Stevens, M., and Cooke, R. (2010). A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities. Wildlife Research 37, 456–465.
| A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities.Crossref | GoogleScholarGoogle Scholar |
Dexter, N., and Murray, A. J. (2009). The impact of fox control on the relative abundance of forest mammals in east Gippsland, Victoria. Wildlife Research 36, 252–261.
| The impact of fox control on the relative abundance of forest mammals in east Gippsland, Victoria.Crossref | GoogleScholarGoogle Scholar |
Edwards, D. (2005). The parks and reserves of the northern Richmond Range (including Richmond Range, Toonumbar and Mallanganee National Parks and Hogarth Range Nature Reserve). Plan of Management. National Parks and Wildlife Service, NSW.
Frankham, G. J., Reed, R. L., Fletcher, T. P., and Handasyde, K. A. (2011). Population ecology of the long-nosed potoroo (Potorous tridactylus) on French Island, Victoria. Australian Mammalogy 33, 73–81.
| Population ecology of the long-nosed potoroo (Potorous tridactylus) on French Island, Victoria.Crossref | GoogleScholarGoogle Scholar |
Hamel, S., Killengreen, S. T., Henden, J.-A., Eide, N. E., Roed-Eriksen, L., Ims, R. A., and Yoccoz, N. G. (2013). Towards good practice guidance in using camera-traps in ecology: influence of sampling design on validity of ecological inferences. Methods in Ecology and Evolution 4, 105–113.
| Towards good practice guidance in using camera-traps in ecology: influence of sampling design on validity of ecological inferences.Crossref | GoogleScholarGoogle Scholar |
Hohnen, R., Ashby, J., Tuft, K., and McGregor, H. (2013). Individual identification of northern quolls (Dasyurus hallucatus) using remote cameras. Australian Mammalogy 35, 131–135.
| Individual identification of northern quolls (Dasyurus hallucatus) using remote cameras.Crossref | GoogleScholarGoogle Scholar |
Jackson, R. M., Roe, J. D., Wangchuk, R., and Hunter, D. O. (2006). Estimating snow leopard population abundance using photography and capture–recapture techniques. Wildlife Society Bulletin 34, 772–781.
| Estimating snow leopard population abundance using photography and capture–recapture techniques.Crossref | GoogleScholarGoogle Scholar |
Johnston, P. G. (2008). Long-nosed potoroo. In ‘The Mammals of Australia’. (Eds S. Van Dyck and R. Strahan.) pp. 302–304. (Reed New Holland: Sydney.)
Karanth, K. U. (1995). Estimating tiger Panthera tigris populations from camera-trap data using capture–recapture models. Biological Conservation 71, 333–338.
| Estimating tiger Panthera tigris populations from camera-trap data using capture–recapture models.Crossref | GoogleScholarGoogle Scholar |
Kelly, M. J., and Holub, E. L. (2008). Camera trapping of carnivores: trap success among camera types and across species, and habitat selection by species, on Salt Pond Mountain, Giles County, Virginia. Northeastern Naturalist 15, 249–262.
| Camera trapping of carnivores: trap success among camera types and across species, and habitat selection by species, on Salt Pond Mountain, Giles County, Virginia.Crossref | GoogleScholarGoogle Scholar |
Li, S., McShea, W. J., Wang, D., Lu, Z., and Gu, X. (2012). Gauging the impact of management expertise on the distribution of large mammals across protected areas. Diversity & Distributions 18, 1166–1176.
| Gauging the impact of management expertise on the distribution of large mammals across protected areas.Crossref | GoogleScholarGoogle Scholar |
MacKenzie, D. I., Nichols, J. D., Royle, J. A., Pollock, K. H., Bailey, L. L., and Hines, J. E. (2006). ‘Occupancy Estimation and Modelling: Inferring Patterns and Dynamics of Species Occurrence.’ (Academic Press: Burlington.)
Mason, R. J. (1997). Habitat use and population size of the long-nosed potoroo, Potorous tridactylus (Marsupialia: Potoroidae) in a coastal reserve, north-eastern New South Wales. Australian Mammalogy 20, 35–42.
McCain, E. B., and Childs, J. L. (2008). Evidence of resident jaguars (Panthera onca) in the southwestern United States and the implications for conservation. Journal of Mammalogy 89, 1–10.
| Evidence of resident jaguars (Panthera onca) in the southwestern United States and the implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Meek, P. D., and Pittet, A. (2012). User-based design specifications for the ultimate camera trap for wildlife research. Wildlife Research 39, 649–660.
| User-based design specifications for the ultimate camera trap for wildlife research.Crossref | GoogleScholarGoogle Scholar |
Norton, M. A., Claridge, A. W., French, K., and Prentice, A. (2010). Population biology of the long-nosed potoroo (Potorous tridactylus) in the southern highlands of New South Wales. Australian Journal of Zoology 58, 362–368.
| Population biology of the long-nosed potoroo (Potorous tridactylus) in the southern highlands of New South Wales.Crossref | GoogleScholarGoogle Scholar |
O’Connell, A. F., and Bailey, L. L. (2011). Inference for occupancy and occupancy dynamics. In ‘Camera Traps in Animal Ecology Methods and Analyses’. (Eds A. F. O’Connell, J. D. Nichols and K. U. Karanth.) pp. 191–206. (Springer: New York.)
Rowcliffe, J. M., and Carbone, C. (2008). Surveys using camera traps: are we looking to a brighter future? Animal Conservation 11, 185–186.
| Surveys using camera traps: are we looking to a brighter future?Crossref | GoogleScholarGoogle Scholar |
Rowcliffe, J. M., Field, J., Turvey, S. T., and Carbone, C. (2008). Estimating animal density using camera traps without the need for individual recognition. Journal of Applied Ecology 45, 1228–1236.
| Estimating animal density using camera traps without the need for individual recognition.Crossref | GoogleScholarGoogle Scholar |
Smith, J. K., and Coulson, G. (2012). A comparison of vertical and horizontal camera trap orientations for detection of potoroos and bandicoots. Australian Mammalogy 34, 196–201.
| A comparison of vertical and horizontal camera trap orientations for detection of potoroos and bandicoots.Crossref | GoogleScholarGoogle Scholar |
Swann, D.E., Hass, C.C., Dalton, D.C., and Wolf, S.A. (2004). Infrared-triggered cameras for detecting wildlife: an evaluation and review. Wildlife Society Bulletin 32, 357–365.
Taylor, B. D., and Goldingay, R. L. (2012). Restoring connectivity in landscapes fragmented by major roads: a case study using wooden poles as ‘stepping stones’. Restoration Ecology 20, 671–678.
| Restoring connectivity in landscapes fragmented by major roads: a case study using wooden poles as ‘stepping stones’.Crossref | GoogleScholarGoogle Scholar |
Tobler, M. W., Carrillo-Percastegui, S. E., Pitman, R. L., Mares, R., and Powell, G. (2008). An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals. Animal Conservation 11, 169–178.
| An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals.Crossref | GoogleScholarGoogle Scholar |
Wang, S. W., and Macdonald, D. W. (2009). The use of camera traps for estimating tiger and leopard populations in the high altitude mountains of Bhutan. Biological Conservation 142, 606–613.
| The use of camera traps for estimating tiger and leopard populations in the high altitude mountains of Bhutan.Crossref | GoogleScholarGoogle Scholar |