Seasonal productivity drives aggregations of killer whales and other cetaceans over submarine canyons of the Bremer Sub-Basin, south-western Australia
Chandra Salgado Kent A B C H , Phil Bouchet
A B C H , Phil Bouchet  D E , Rebecca Wellard
D E , Rebecca Wellard  A F , Iain Parnum
A F , Iain Parnum  A , Leila Fouda
A , Leila Fouda  A G and Christine Erbe
A G and Christine Erbe  A
A
A Centre for Marine Science and Technology, Curtin University, Kent Street, Bentley, WA 6102, Australia.
B Oceans Blueprint, Coogee, WA 6166, Australia.
C Centre for Marine Ecosystems Research, Edith Cowan University, Joondalup Drive, Joondalup, WA 6027, Australia.
D School of Biological Sciences, University of Western Australia, Crawley, WA 6009, Australia.
E Centre for Research into Ecological & Environmental Modelling, The Observatory, Buchanan Gardens, University of St Andrews, Fife KY16 9LZ, Scotland, UK.
F Project ORCA, Perth, WA 6026, Australia.
G School of Biological and Chemical Sciences, Queen Mary University of London, Mile End Road, London E1 4NS, UK.
H Corresponding author. Email: c.salgado@oceansblueprint.com
Australian Mammalogy 43(2) 168-178 https://doi.org/10.1071/AM19058
Submitted: 2 October 2019 Accepted: 3 May 2020 Published: 25 June 2020
Journal Compilation © Australian Mammal Society 2021 Open Access CC BY-NC-ND
Abstract
Cetaceans are iconic predators that serve as important indicators of marine ecosystem health. The Bremer Sub-Basin, south-western Australia, supports a diverse cetacean community including the largest documented aggregation of killer whales (Orcinus orca) in Australian waters. Knowledge of cetacean distributions is critical for managing the area’s thriving ecotourism industry, yet is largely sporadic. Here we combined aerial with opportunistic ship-borne surveys during 2015–2017 to describe the occurrence of multiple cetacean species on a regional scale. We used generalised estimating equations to model variation in killer whale relative density as a function of both static and dynamic covariates, including seabed depth, slope, and chlorophyll a concentration, while accounting for autocorrelation. Encountered cetacean groups included: killer (n = 177), sperm (n = 69), long-finned pilot (n = 29), false killer (n = 2), and strap-toothed beaked (n = 1) whales, as well as bottlenose (n = 12) and common (n = 5) dolphins. Killer whale numbers peaked in areas of low temperatures and high primary productivity, likely due to seasonal upwelling of nutrient-rich waters supporting high prey biomass. The best predictive model highlighted potential killer whale ‘hotspots’ in the Henry, Hood, Pallinup and Bremer Canyons. This study demonstrates the value of abundance data from platforms of opportunity for marine planning and wildlife management in the open ocean.
Additional keywords: generalised estimating equations, habitat modelling, submarine canyons, temporal autocorrelation, whale watching.
Introduction
From swarms of microscopic plankton to seabird mega-colonies, animal aggregations are commonplace in the marine environment and represent some of the most striking biological patterns observed in nature (Parrish and Edelstein-Keshet 1999). In pelagic systems, reports of ‘super-groups’ sometimes exceeding hundreds of baleen and toothed whales are not uncommon (e.g. Butler et al. 2017; Findlay et al. 2017), and may act as useful indicators of oceanographically productive habitats where dense and predictable patches of prey likely occur. As such, identifying and understanding cetacean aggregations is critical to supporting ecosystem-based management and spatial conservation planning in the open ocean (McClellan et al. 2014).
Mapping wildlife at-sea distributions is a challenge, not least because most air-breathing marine vertebrates are elusive, highly mobile, and occur in remote habitats that prove difficult to access or demand complex logistics for dedicated biological data collection (Williams et al. 2006a). For instance, numerous whale species concentrate in deep submarine canyons far away from coasts, where complex seafloor topography and ocean currents interact to promote suitable foraging conditions (Rennie et al. 2009; Moors-Murphy 2014; Santora et al. 2018). In this context, aerial surveys (e.g. visual counts conducted by observers onboard an aircraft or helicopter) have traditionally been a prominent tool in studies of offshore megafauna (Laran et al. 2017), mostly due to their ability to reach distant areas and cover broad stretches of ocean space within short timeframes. However, manned aerial methods present non-negligible risks to personnel safety and usually incur significant costs associated with platform hire and staffing, limiting their implementation.
With the growth of wilderness-based tourism globally, new opportunities have opened to gather vast amounts of ecological information at minimal costs by capitalising on existing marine infrastructure such as passenger ferries, cruise ships or whale-watching vessels (Kiszka et al. 2007b). Such ‘platforms of opportunity’ (PoPs) can provide valuable means of monitoring cetaceans in data-deficient regions, and have been successfully used to assess the abundance and occurrence of common dolphins (Delphinus delphis) and killer (Orcinus orca), humpback (Megaptera novaeangliae), Bryde’s (Balaenoptera edeni) and minke (Balaenoptera acutorostrata) whales, among many other species (see Appendix S1, available as Supplementary Material to this paper for examples). In order to maximise profits, commercial operators tend to go out to sea on a regular basis (e.g. daily) throughout the tourism season and target areas where wildlife sightings are expected to be made reliably, giving PoPs an unprecedented level of temporal replication and resolution. Although this behaviour is advantageous, it can also introduce problems associated with patterns of spatial bias and temporal autocorrelation that must be accounted for in statistical inference. To our knowledge, limited efforts have been made to address these issues in analyses of PoP data.
We combined sightings from both aerial and ship-borne PoP surveys (Fig. 1) conducted over 3 years (2015–2017) to provide one of the first descriptions of the cetacean assemblage of the Bremer Sub-Basin, off the southern coast of Western Australia (Fig. 2). The region encompasses an array of deep and biologically active submarine canyons (Huang et al. 2018) that attract a diversity of cetaceans, including sperm whales (Physeter macrocephalus) (Johnson et al. 2016), beaked whales (Mesoplodon spp.) (Wellard et al. 2016; Pitman et al. 2019) and killer whales (Wellard et al. 2015; Wellard and Erbe 2017). It is also the focus of multiple human uses, including seismic exploration, commercial fishing, and vessel traffic, with rates of increase in the cumulative impacts of anthropogenic activities among the highest on the globe (Halpern et al. 2019). There is limited information on beaked whales in the Bremer Sub-Basin. In contrast, sperm whales were a target for commercial whaling during the last century, with long-lasting effort in offshore waters near Albany providing support for the historic regional significance for the species. Despite recent habitat suitability modelling pointing to south-west canyons off the continental shelf as suitable for sperm whales occupancy (Johnson et al. 2016), aerial surveys in 2009 did not provide evidence for sperm whale population recovery since the ban on whaling (Carroll et al. 2014). In recent years, however, the region has been recognised as having the largest seasonal aggregation of killer whales so far recorded in Australian waters (Wellard et al. 2015; Wellard and Erbe 2017). Although killer whales now support a thriving local ecotourism industry, they have been the focus of surprisingly little dedicated research (Waples and Raudino 2018), and the ecological determinants of their abundance patterns have yet to be explored. This is important as continued interactions with human activities are likely to make killer whales a species of rapidly increasing management interest in the future (Waples and Raudino 2018).
|
Recently, Jones et al. (2019) mapped the expected distribution of suitable habitats for killer whales within the Bremer Sub-Basin using the software package MaxEnt (Elith et al. 2011). MaxEnt has been extensively applied in terrestrial systems and is now gaining traction in marine studies both in Australia and elsewhere (e.g. Smith et al. 2012; Prieto et al. 2017). However, a growing body of literature indicates that MaxEnt can be sensitive to input parameters and subject to potentially substantial bias, especially when model calibration is performed under default settings (e.g. Merow et al. 2013; Yackulic et al. 2013; Fiedler et al. 2018). Further, MaxEnt provides insights only into relative probabilities of presence (Pearce and Ferrier 2001). In contrast, count data and estimates of population trends over space and time relate more directly to habitat quality, species threat status, and extinction risk, leading to models with improved predictive performance that can better support management actions (Howard et al. 2014; Johnston et al. 2015). As abundance data are often prohibitively expensive and laborious to collect, particularly for highly mobile animals like cetaceans (Evans and Hammond 2004), there has been rising interest in comparing environmental suitability and abundance models to determine whether their outputs correlate and whether the former can be estimated from, or act as a surrogate for, the latter (Yin and He 2014). Although several studies found reasonable agreement between the two approaches (Weber et al. 2017), evidence suggests that this is highly species-specific, and varies with sampling conditions, species prevalence, breeding strategies, and behaviour (e.g. Nielsen et al. 2005).
With this in mind, the aims of our study were three-fold: (i) provide a baseline assessment of the regional distribution and diversity of cetaceans in the Bremer Sub-Basin; (ii) model the influence of physical habitat features and ocean productivity on the occurrence of the most sighted species, the killer whale; and (iii) explore the suitability–abundance relationship for killer whales by comparing resulting model predictions to those of Jones et al. (2019). Importantly, we build upon the work of Jones et al. (2019) by analysing a larger dataset, exploring a wider range of relevant ecological predictors, and demonstrating how statistical techniques can be used to successfully deal with the correlation patterns present in data obtained incidentally aboard whale-watching ships. Our approach allows us to map expected patterns in killer whale density across adjacent canyon systems. This knowledge can help identify similar environments around Australia that are likely to be occupied by killer whales, as a basis for improving management outcomes for the species.
Materials and methods
The Supplementary Material accompanying this paper provides additional literature relevant to various aspects of the study.
Study region
The Bremer Sub-Basin (Fig. 2) lies within Australia’s South-west Marine Bioregion between the cities of Albany and Esperance. It extends over an area of ~15 000 km2 under the outer continental slope, and encompasses an extensive network of steep, shelf-incising submarine canyons that plunge to depths in excess of 4500 m across the nearby abyssal plain (Huang et al. 2014). Due to their topographic complexity and proximity to the shelf break, most of these canyons are relatively productive features that sustain periodic upwellings, an abundant oxygen supply, and elevated particulate organic carbon concentrations (Huang et al. 2018). Such conditions favour biological activity, with both historical records and anecdotal evidence suggesting that a range of pelagic species including marine mammals, sharks, seabirds and fishes occur throughout the region (Johnson et al. 2016; Bouchet et al. 2018). The centre of the Sub-Basin is straddled by the Bremer Marine Park, a large regulated area that covers 4472 km2 and combines an offshore no-take zone (IUCN class II) and an inshore Special Purpose zone (IUCN class IV, https://parksaustralia.gov.au/marine/parks/south-west/bremer/, accessed 28 May 2020).
Ship-borne surveys
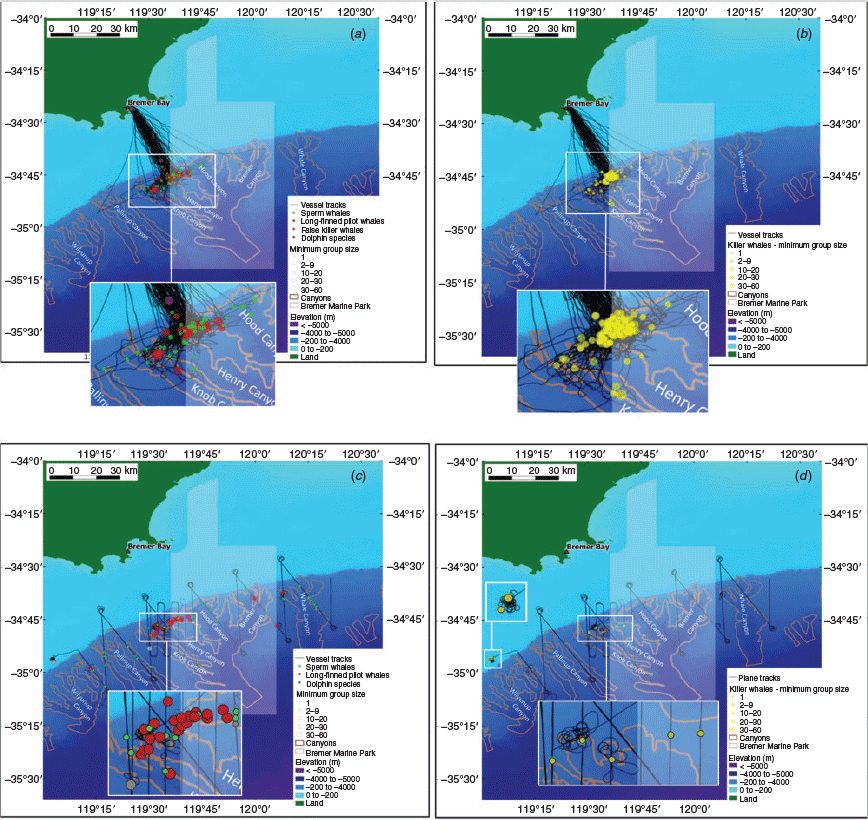
Data were collected aboard two ships of opportunity (i.e. commercial ecotourism vessels Cetacean Explorer and chartered fishing vessel Dhu Force) throughout an area of ~4000 km2 within the canyon system (centred at 34°44.30′S, 119°35.55′E) between February and April 2015 and 2016 (Fig. 2). The research vessel RV Big Dreams was chartered to carry out opportunistic surveys from 25 March 2017 to 4 April 2017 (see Supplementary Material, Appendix S2 for vessel details). Each trip departed from Bremer Bay, and followed a non-systematic design along pre-determined routes converging on the heads of the Hood and Henry canyons, where groups of killer whales are regularly sighted. Vessel speeds did not exceed 15 kn, and surveys were conducted during daylight hours in Beaufort Sea States of 5 or less. Two experienced observers were employed, with the exception of research voyages in 2017 that employed two to four observers (two of whom were dedicated to scanning for cetaceans). Observers continuously scanned the surface of the ocean with the naked eye and binoculars (7 × 50) in search of cetaceans (during passing mode) from the time of departure of the vessel. When animals were encountered, relevant sighting details (i.e. date, time, global positioning system (GPS) position, group size, group composition and behavioural state) were immediately logged. Behavioural state was assigned to one of four categories, which mirrored previous killer whale studies: (1) travelling, (2) foraging, (3) socialising, (4) milling (Baird and Dill 1995). ‘Closing mode’ was subsequently engaged, with the vessel approaching each individual to within 50 m in order to confirm group size estimations and take identification photographs (Wellard and Erbe 2017). If there were no occurrences of killer whales within 30 min or more, the following sighting was deemed a new sighting.
Aerial surveys
Aerial surveys were undertaken in March 2017 over a period of 6 days. Surveys were flown with a twin-engine high-wing Cessna 337 aircraft fitted with bubble windows, at an altitude of 300 m and a speed of 120 kn, as per standard protocols (Salgado Kent et al. 2012). Survey routes were generated in software Distance v6.0 (Thomas et al. 2010) using two different designs. The first (Route A) consisted of wide, equally-spaced saw-toothed transects (20-km apart) designed to enable adequate coverage of waters inside and adjacent to the Bremer Marine Park on a regional scale. The second (Route B) comprised a series of denser parallel transects (3-km apart) restricted to a small site where killer whales are known to aggregate (Fig. 2). Both routes were travelled in passing mode, although deviations from the flight path were allowed to gather close-up information following detections of killer whales. Search effort resumed when the aircraft re-joined the initial transect line. Onboard personnel included one pilot and two trained observers, who sat on either side of the aircraft and logged wildlife sightings concurrently and independently. Observers measured vertical and horizontal angles to each sighting using Suunto PM-5/360PC clinometers and a compass board, while the pilot recorded the angle of drift of the aircraft from the flight path, as per Salgado Kent et al. (2012). All relevant sighting information was recorded via headset onto an Olympus digital voice recorder, including the time of encounter, species, confidence in species identification (definite, probable, likely), group size and composition (number of adults and calves), swim direction (relative to the plane) and sighting cue.
Data processing
Several environmental variables were collated based on their putative influence on killer whale distribution and mapped onto a 1.1-km2 grid overlaid on the study region. High-quality, 9 arc-second (0.0025° or ~250 m at the equator) depth data were obtained from a digital bathymetric raster of Australian waters curated by Geoscience Australia (http://www.ausseabed.gov.au/, accessed 8 January 2019). Seabed slope (in degrees) was derived for each cell from the depth raster by taking the arctangent of the two-dimensional gradient calculated from the nearest neighbours, one either side of the cell being calculated. Distance to shore (in km) was computed as the distance to the nearest 0 m contour. Sea surface temperature (SST) and chlorophyll a concentration (Chl a) datasets were created by the Integrated Marine Observing System (IMOS) Satellite Remote Sensing Facility and obtained from the Australian Ocean Data Network portal (http://imos.aodn.org.au/, accessed 8 January 2019). The SST dataset consisted of daily (night-time) measurements and was derived from the Advanced Very High Resolution Radiometer (AVHRR) with corrections made for sensor-specific error statistics (SSES) bias; and only data of quality level 5 were retained. The Chl a dataset was daily and derived from Moderate Resolution Imaging Spectroradiometer (MODIS) data using the OC3 model (Hu et al. 2012), and only data of level 3 quality was used. Summary statistics were calculated for both SST and Chl a, and included: minimum, maximum, mean, standard deviation, median, 25th quantile and 75th quantile. Potential issues of multicollinearity among explanatory variables were investigated using pairwise correlation plots and variance inflation factors (VIF) (Pirotta et al. 2018). Variables associated with VIFs > 3 were taken to indicate collinearity (Zuur et al. 2009) and were omitted from further analysis. The centroid location of each grid cell was used to extract values of all environmental layers. Grid cells associated with little survey effort (<10 km) or extreme environmental conditions relative to the rest of the region were discarded.
Statistical analysis
The relative density of killer whale groups was modelled as a function of environmental conditions using generalised estimating equations (GEEs), a technique that permits correlated residuals and therefore can account for the spatio-temporal bias present in many forms of PoP data (Zorn 2001; Zuur et al. 2009; Scott-Hayward et al. 2014). Data only from ship-borne platforms were used, as survey effort and sightings from the 2017 aerial surveys were too limited to warrant inclusion. Due to zero-inflation on both daily and seasonal scales, the total number of groups sighted over the study period (all days and years surveyed) was computed for each grid cell and used as the response variable. Models were built with a log-link function (for Poisson distributed counts) using the MRSea (Scott-Hayward et al. 2013) and geepack (Yan and Fine 2004; Johnson et al. 2016) packages in R ver. 3.5.1 (R Core Team 2013), with effort (in km) coded as an offset. Smoothing was achieved using a complex region spatial smoother (CReSS) framework (Scott-Hayward et al. 2013, 2014) to allow for robust smoothing in areas of convoluted topography, following Scott-Hayward et al. (2015). GEEs require the specification of ‘panels’ (or ‘blocks’) between which model residuals are assumed to be independent (Scott-Hayward et al. 2015). Here, a spatial blocking structure was applied as a vector of integers numbered as sequential ~3 km2 blocks, using grid cell as the identification (ID). An AR-1 correlation structure was initially selected (Zuur et al. 2009), although independence, unstructured and exchangeable correlation structures were also tested in models, and the best fit was assessed using the quasi-likelihood information criterion (QIC; Pan 2001).
Model selection began with a full model that contained the maximum possible number of uncorrelated variables, as well as their pairwise interactions. Several sets of uncorrelated variables were tested for inclusion in turn. A backward stepwise selection procedure was then applied, whereby non-significant explanatory terms were eliminated one by one, and the resulting submodel refitted until all terms were significant. Residual checks were performed for each model, and the one with the fewest terms that reduced the penalised Quasi-likelihood information criterion (QICu) by more than two units was selected as the best, most parsimonious candidate (Cui 2007). Model validation was undertaken using 10-fold cross-validation, and predictions of killer whale group density from the final model were generated across a 130-km longitudinal range along the shelf edge and canyon systems of the region. Estimated relationships were visualised with partial residuals plots, and uncertainty in both model coefficients and predictions quantified via bootstrapping, as per Scott-Hayward et al. (2013) (using n = 1000 iterations).
Results
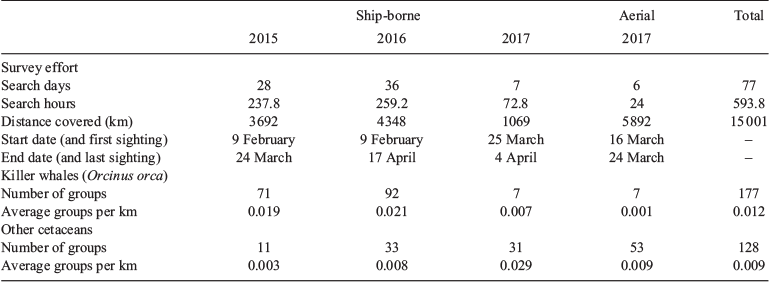
A total of 2470 individuals in 305 cetacean groups was sighted during 594 search hours across 77 days within a 3-year period (Table 1). Species sighted included the killer whale, sperm whale, long-finned pilot whale (Globicephala melas), bottlenose dolphin (Tursiops spp.), common dolphin (Delphinus delphis), false killer whale (Pseudorca crassidens) and strap-toothed beaked whale (Mesoplodon layardii); (see Supplementary Material, Appendix S3). Long-finned pilot, killer, and sperm whales and bottlenose dolphins were sighted during all three years of the study. Common dolphins were only seen during 2 of the years (2015 and 2016), and false killer and strap-toothed beaked whales were only sighted during 2016. Species sighted during both aerial and ship-borne surveys include long-finned pilot, killer and sperm whales and bottlenose dolphins. Common dolphins and strap-toothed beaked and false killer whales were seen only during ship-borne surveys.
|
Ship-borne surveys
Twenty-eight surveys were conducted aboard whale watching and opportunistically on research vessels between 2015 and 2017, totalling 570 h of visual search effort over 71 days. During these surveys, 1099 individuals in 170 groups of killer whales, 81 individuals in 37 groups of sperm whales (Physeter macrocephalus), 601 individuals in 21 groups of long-finned pilot whales (Globicephala melas), 55 individuals in 9 groups of bottlenose dolphins (Tursiops spp.), 33 individuals in 5 groups of common dolphins (Delphinus delphis), 40 individuals in 2 groups of false killer whales (Pseudorca crassidens) and 1 strap-toothed beaked whales (Mesoplodon layardii) were observed (Table 1; Supplementary Material, Appendix S3). Of the 170 killer whale groups observed, 158 were travelling and 12 were foraging or socialising. All long-finned pilot whales were observed travelling and sperm whales were either travelling or milling. All species were observed in offshore waters, except for Indo-Pacific bottlenose dolphins which were seen in inshore waters within Bremer Bay (Fig. 2). Vessel tracks crossed a total of 1130 grid cells over which effort varied. Most grid cells had <1 h and 10 km of effort (Supplementary Material, Appendix S4). The mean number of killer whale groups per grid cell observed was 0.15 and ranged between 0 and 17.
Aerial surveys
Six surveys were flown in March 2017 (Table 1), representing 25 h of visual search effort. In addition to killer whales (n = 7 groups, N = 57 individuals), three other cetacean species could be reliably identified from the air, namely long-finned pilot whales (n = 8, N = 306), sperm whales (n = 32, N = 34) and bottlenose dolphins (n = 3, N = 61) (Fig. 2). More than two-thirds of sightings (68%, n = 42) consisted of single animals or pairs, although several larger groups of pilot whales and bottlenose dolphins comprising between 10 and 60 individuals were also detected (Fig. 2). The distribution of both pilot and sperm whales was widespread throughout the region, with the latter recorded on virtually every transect. In contrast, killer whales were only encountered west of 119.75°E in pod sizes up to 30 animals, and with most sightings (71%, n = 5) being made outside the boundaries of the Bremer Marine Park. Young calves were present in 11% (n = 7) of groups, and rarely exceeded two.
Killer whale density
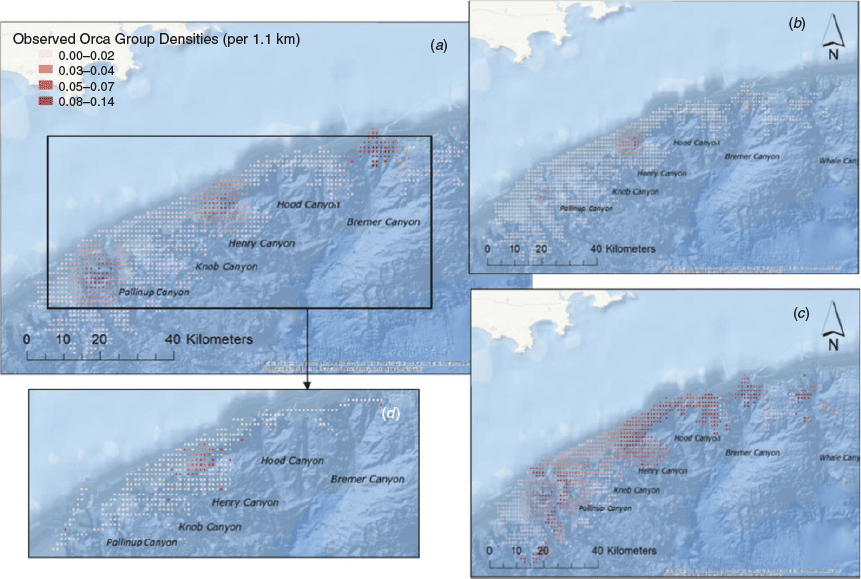
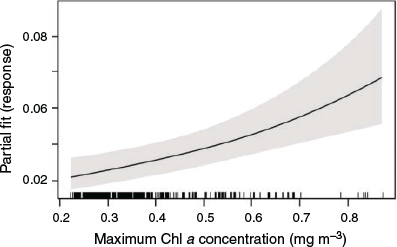
The observed density of killer whale groups was greatest ca. 38 km from the coast, in depths ranging from 800 to 1000 m and moderate to steep seabed slopes (5–12°). Peak numbers of killer whale groups occurred in grid cells showing average temperature conditions (across years) of 20.1°C (ranging from 19.4 to 22.1°C), and average Chl a concentrations of 0.15 mg m−3 (0.05–0.5 mg m−3). The final subset of data used for modelling included 360 h of visual effort over 4284 km of tracklines during both 2015 and 2016. The final best-fit model only included a single term, maximum Chl a (Table 2), and indicated a preference for areas of higher maximum primary productivity (Fig. 3). The mean number of killer whale groups observed per grid cell (1.1 km−2) in the survey area was 0.015 (s.e. = 0.004), and is comparable to predictions made across the broader Sub-Basin (0.012; s.e. = 0.0006). Areas of highest predicted killer whale density (‘hotspots’) were identified over four canyon systems, including the Bremer, Henry, Hood and Pallinup Canyons (Fig. 4). Model prediction mean squared error was 0.728, and remained consistent across multiple cross-validation runs. Spatial bootstrap 95% confidence intervals were wide (Fig. 4).
|
Discussion
This study provides the first baseline assessment of regional patterns in cetacean abundance and occurrence throughout the Bremer Sub-Basin during the austral summer–autumn. As such, it symbolises an important initial step towards the establishment of an ecosystem monitoring framework in the pelagic waters of Australia’s South-west Marine Bioregion (Fletcher et al. 2010). Our observations indicate that a minimum of seven odontocetes (i.e. bottlenose and common dolphins and killer, false killer, strap-toothed beaked, long-finned pilot and sperm whales) frequent the Bremer and adjacent submarine canyons, at least seasonally, and that the assemblage appears to be dominated by sperm, long-finned pilot, and killer whales, as well as bottlenose dolphins. These findings corroborate the possible occupancy of these species that past reported sightings, stranding records and habitat suitability modelling have alluded to (see Supplementary Material, Appendix S5), all of which depend upon opportunistic reports biased towards locations of prevalent human activity.
Although taxonomic diversity is lower here than in comparable systems around the Australian continent (Gill et al. 2015), and elsewhere (Kiszka et al. 2007a; Carrillo et al. 2010; Laran et al. 2017), the species diversity recorded in this study can only be taken as a minimum. This study has shown that the Bremer Sub-Basin remains an important offshore habitat that supports high biological activity and attracts a variety of pelagic organisms, including sharks, fishes, cephalopods, and seabirds (Bouchet et al. 2018). In particular, killer whales aggregate at the heads of the Hood and Henry canyons in unprecedented numbers. With over 140 individuals recognised to date (Wellard and Erbe 2017), this constitutes the largest reported seasonal aggregation of the species in Australian waters. Despite this, current understanding of the spatial ecology of Australian killer whales remains limited, both within the Bremer Sub-Basin and throughout other parts of the country’s waters (Morrice 2004; Pitman et al. 2015). For instance, only sporadic matches of photo-identified individuals are available to describe the animals’ broad-scale movements and speculate about their habitat usage and preferences in the Southern Ocean (de Bruyn et al. 2013), with no known killer whales catalogued in the Bremer Sub-Basin matched across Australian regions to date (Totterdell 2015; Donnelly et al. 2016; Wellard and Erbe 2017). Previous research shows that oceanic circulation (including tidal flows) around complex canyon floor topographies forces critical benthic–pelagic coupling by promoting the re-suspension of particulate organic matter, aiding carbon transport offshore and funnelling deep nutrients to surface layers (Ryan et al. 2005). This is particularly true of shelf-incising canyons, which provide consistently higher ‘habitat potential’ for pelagic species than slope-confined canyons (Huang et al. 2018). Because of this, submarine canyons often act as predator foraging grounds (Rennie et al. 2009; Moors-Murphy 2014; Fernandez-Arcaya et al. 2017). Little is known of the bio-physical mechanisms underlying productivity and prey biomass in the region, but the presence of killer whales in the Bremer Sub-Basin is likely attributable to foraging opportunities afforded by intermittent upwelling of cold Antarctic water up the canyons’ edges (Akhir 2010). Indeed, the first published account of active killer whale predation on beaked whales is from the Bremer Sub-Basin (Wellard et al. 2016), with anecdotal reports suggesting that the animals target a wide range of other prey (e.g. fish, squid; R. Wellard, pers. obs.).
Our modelling of killer whale relative density indicated a positive relationship with maximum Chl a concentration, consistent with the known physiology of other members of the Delphinidae family, which exhibit elevated energetic demands and are thus dependent on productivity (Mannocci et al. 2014). By contrast, seabed depth, slope, and SST were not significant predictors in the final model, although sightings were largely made in intermediate depths (600–1200 m with a peak around 800 m), over steep bathymetric slopes (5–8°), and in low regional temperatures (between 19–22°C) (Supplementary Material, Appendix S6), in alignment with similar observations made in the Pacific and Atlantic Oceans, and the Mediterranean Sea (Ferguson et al. 2006; Esteban et al. 2014; Passadore et al. 2014). Contrary to this study, the presence-only models developed by Jones et al. (2019) identified depth as the most important predictor of habitat suitability, resulting in model predictions that largely (>70%) favoured flatter, more homogeneous areas between canyon heads, against the authors’ own expectations. Such disparities between our results and those of Jones et al. (2019) add to an ongoing debate around the nature and consistency of abundance–suitability relationships for many taxa (e.g. contrast Weber et al. 2017 with; Dallas and Hastings 2018), and offer a first line of evidence that occurrence probability may not correlate well with density in killer whales. In general, density models ought to be more informative than their presence-only counterparts (Howard et al. 2014), and best suited for setting priorities in conservation planning (Johnston et al. 2015; Veloz et al. 2015). We therefore modelled counts, out of the desire to provide outputs of maximum utility for decision-making. Additionally, remotely-sensed Chl a is a more proximal indicator of biological productivity than seabed depth, and is thus expected to be a more ecologically meaningful predictor of killer whale distribution within an area subject to seasonally variable conditions, like the Bremer Sub-Basin. Significant improvements in predictive capacity may be gained from models that incorporate knowledge of prey patches (although see Torres et al. 2008), but these will not be possible until detailed information on the diet and feeding ecology of Australian killer whales becomes available.
This study was limited to surveys conducted during the summer–autumn season over a period of 3 years. Because of this, repeated visual surveys over multiple months and years will be required to fully elucidate the fine scale environmental associations of cetaceans across the Bremer Sub-Basin, and their variability over time. In this context, the use of aircraft as sampling platforms would be advantageous to cover more ground in shorter timeframes, and would provide ground truthing of model predictions regionally. Indeed, few sightings of killer whales were made outside the Hood and Henry canyons, despite several hotspots of high density (e.g. within the Bremer Marine Park) being identified through modelling. While robust abundance estimates can often be obtained from aerial survey data (Salgado Kent et al. 2012), ship-borne methods can usually accommodate more observers, enable more sophisticated search protocols, yield higher detection rates (provided good weather), and ensure longer sighting times with better species identification potential due to the moderate speed of the vessel relative to the animals’ own movements (Laran et al. 2017). Importantly, ship-borne data collection can be a cost-effective alternative to dedicated effort when considered in tandem with wildlife tourism operations. There is now considerable interest in, and rising acceptance of, the value of PoPs as tools in studies of cetacean distribution, abundance, and behaviour (Higby et al. 2012). In some regions, PoP programmes have been in place for decades (e.g. ORCA, https://www.orcaweb.org.uk/, accessed 28 May 2020), having been launched by scientists and implemented in the field by trained personnel. As a result, many operators are eager to accommodate scientific activities, as participation in science is perceived to enhance company reputation, increase attendance and revenues, promote public engagement, and foster wildlife stewardship (Lück 2015). Robust inference from PoP data is challenging, as limited temporal and geographic coverage and non-randomised, unequal sampling effort generally complicate analyses (Isojunno et al. 2012). However, when these biases are accounted for using appropriate statistical modelling machinery (as was done here), PoPs may provide meaningful insights into target species’ ecology that could not be otherwise obtained with other approaches (Isojunno et al. 2012; Scott-Hayward et al. 2015). That said, model validation on independent data remains critical to verifying the reliability of model predictions and identifying conditions limiting model applications. Collecting the data necessary to perform such assessments should be seen as an important avenue for future research on killer whales in Australia.
Fisheries by-catch, pollution, and a variety of other anthropogenic threats to marine megafauna are becoming increasingly prominent in the Indian and Southern Oceans, with high proportions of marine mammals currently at risk around Australia (Davidson et al. 2012). The Bremer Sub-Basin is no exception, with vessel activity present, numerous oil and gas exploration campaigns, and rates of warming surpassing those observed in many other marine habitats. Quantifying the population-level consequences of lethal and non-lethal human impacts is difficult yet necessary to implement optimal management strategies that can successfully buffer against species declines. In this context, an important next step in research on Bremer killer whales will be the application of quantitative modelling approaches such as the Population Consequences of Disturbance (PCoD) framework (Pirotta et al. 2018), which has been developed specifically to assess the effects of disturbance on marine mammal populations. Implementing this framework will allow novel insights into the fitness costs associated with repeated exposure to tourism vessels within the Bremer Sub-Basin, but will require additional data on demographic rates and physiological status (Pirotta et al. 2018), which are currently lacking. We recommend that photo-identification surveys be continued in the future as a means of informing exposure risk, residency patterns (including seasonal), and individual health. These may be usefully complemented by biotelemetry studies.
Contrary to other studies (e.g. Reisinger et al. 2015), model results indicated that seasonal upwelling of nutrient-rich waters, as captured in remote-sensed Chl a imagery, may be a strong driver of killer whale presence within the Bremer Sub-Basin during the austral summer–autumn. There have indeed been multiple accounts of killer whale predation on squid, fish, and cetaceans in the Bremer Sub-Basin (Wellard et al. 2016), confirming the idea that the submarine canyons within the Albany complex exhibit high habitat potential for pelagic species (Huang et al. 2018). Killer whales have been shown to respond to vessel-induced disturbance, and to be particularly vulnerable to exposure when feeding, consistent with observed risk avoidance strategies in long-lived mammals (Lusseau et al. 2009). In some cases, lost foraging opportunities have been estimated to result in potentially substantial decreases in energy intake for some individuals (Williams et al. 2006b), such that spatial planning strategies targeting critical foraging grounds are expected to confer greater conservation benefits for this species than those designed to protect habitats more generically (Ashe et al. 2010). This underscores the importance of both (i) granting the Albany canyon complex strong and immediate protection, and (ii) having in place and enforcing an effective code of conduct for mitigating disturbance caused by whale-watching activities throughout the Bremer Sub-Basin (e.g. surrounding vessel behaviour and proximity to animals).
Faced with limited budgets and lingering uncertainty about which pressures to address as a priority, conservation agencies often focus interventions on single (indicator) species, whose status is deemed an ultimate measure of management efficacy (Laran et al. 2017). Although this rationale has some merits, broader assessments of communities as a whole also bear relevance for management, especially when considered over larger geographic areas. By combining multiple sampling platforms, each operating on different scales, our work offers both novel taxon-oriented (i.e. killer whale) and community-wide knowledge about the cetaceans occupying the Bremer Sub-Basin. Both approaches emphasise the importance of the region’s outer continental shelf and slope to a diversity of cetaceans, which should be seen as a strong candidate for conservation attention.
Data availability
The aerial survey data are publicly available on the Research Data Australia portal (https://researchdata.ands.org.au/aerial-visual-survey-project-ep2/1098271) and can be visualised in an R Shiny app (https://pjbouchet.shinyapps.io/bremer_ep2/). The SST and Chl a data were sourced from Australia’s Integrated Marine Observing System (IMOS; http://imos.org.au/facilities/aodn/), and the bathymetry was downloaded from Geoscience Australia (http://www.ausseabed.gov.au/). The ship-borne sightings data are supporting ongoing projects. Please contact Rebecca Wellard (becwellard@gmail.com) regarding the ship-borne data.
Author contributions
CSK led the data analysis and writing of the manuscript. PB participated greatly in data analysis and writing. RW designed and led the fieldwork, collected field data in all years, pre-processed all sightings data, and secured funds. IP sourced and processed the environmental data. LF co-led the fieldwork in 2015, collected sightings data in 2015, and secured funds for the 2015 season. CE conceived and initiated the study and secured funds. All authors contributed to the writing of this manuscript and approved its final version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Naturaliste Charters for operating vessels Cetacean Explorer and Dhu Force in 2015–2016, and to John Totterdell, CETREC WA, for chartering Big Dreams during the 2017 field season. We thank Norwest Air Work pilot Conor O’Reilly and marine wildlife consultant Verity Steptoe for their assistance with the 2017 aerial surveys. The 2015–2017 visual data were collected under the Curtin University Animal Ethics Committee Approval No. AEC_2013_27 and under the Australian Government Department of the Environment Research Permits Nos. 2014/0008 and 2017/0001. The 2015 data were obtained under a contract between the SeaWorld & Busch Gardens Conservation Fund and Curtin University (recipients CE and LF). The 2016 and 2017 ship-borne data were collected with support from The Holsworth Wildlife Research Endowment of The Ecological Society of Australia, The Australian Geographic Society, and the Australian Acoustical Society Education Grant (for 2017) (recipient RW). The 2017 aerial surveys were made possible by the Australian Government’s National Environmental Science Programme (NESP) Marine Biodiversity Hub under the Emerging Priorities research scheme.
References
Akhir, M. F. M. (2010). Physical processes along the southern continental shelf and slope of Western Australia. PhD thesis, School of Environmental Systems Engineering, University of Western Australia, Perth, WA, Australia.Ashe, E., Noren, D. P., and Williams, R. (2010). Animal behaviour and marine protected areas: incorporating behavioural data into the selection of marine protected areas for an endangered killer whale population. Animal Conservation 13, 196–203.
| Animal behaviour and marine protected areas: incorporating behavioural data into the selection of marine protected areas for an endangered killer whale population.Crossref | GoogleScholarGoogle Scholar |
Baird, R. W., and Dill, L. M. (1995). Occurrence and behaviour of transient killer whales: seasonal and pod-specific variability, foraging behaviour, and prey handling. Canadian Journal of Zoology 73, 1300–1311.
| Occurrence and behaviour of transient killer whales: seasonal and pod-specific variability, foraging behaviour, and prey handling.Crossref | GoogleScholarGoogle Scholar |
Bouchet, P., Meeuwig, J., Erbe, C., Salgado Kent, C., Wellard, R., and Pattiaratchi, C. (2018). Bremer Canyon Emerging Priorities Project EP2: Final report for the National Environmental Science Programme, Marine Biodiversity Hub. University of Western Australia, Perth, WA, Australia.
Butler, R. W., MacVicar, R. S., and Hollick-Kenyon, S. (2017). Observation of a super pod of Pacific Harbor porpoises (Phocoena phocoena vomerina) in the Salish Sea. Northwestern Naturalist 98, 137–138.
| Observation of a super pod of Pacific Harbor porpoises (Phocoena phocoena vomerina) in the Salish Sea.Crossref | GoogleScholarGoogle Scholar |
Carrillo, M., Pérez-Vallazza, C., and Álvarez-Vázquez, R. (2010). Cetacean diversity and distribution off Tenerife (Canary Islands). Marine Biodiversity Records 3, e97.
| Cetacean diversity and distribution off Tenerife (Canary Islands).Crossref | GoogleScholarGoogle Scholar |
Carroll, G., Hedley, S., Bannister, J., Ensor, P., and Harcourt, R. (2014). No evidence for recovery in the population of sperm whale bulls off Western Australia, 30 years post-whaling. Endangered Species Research 24, 33–43.
| No evidence for recovery in the population of sperm whale bulls off Western Australia, 30 years post-whaling.Crossref | GoogleScholarGoogle Scholar |
Cui, J. (2007). QIC program and model selection in GEE analyses. The Stata Journal 7, 209–220.
| QIC program and model selection in GEE analyses.Crossref | GoogleScholarGoogle Scholar |
Dallas, T. A., and Hastings, A. (2018). Habitat suitability estimated by niche models is largely unrelated to species abundance. Global Ecology and Biogeography 27, 1448–1456.
| Habitat suitability estimated by niche models is largely unrelated to species abundance.Crossref | GoogleScholarGoogle Scholar |
Davidson, A. D., Boyer, A. G., Kim, H., Pompa-Mansilla, S., Hamilton, M. J., Costa, D. P., Ceballos, G., and Brown, J. H. (2012). Drivers and hotspots of extinction risk in marine mammals. Proceedings of the National Academy of Sciences of the United States of America 109, 3395–3400.
| Drivers and hotspots of extinction risk in marine mammals.Crossref | GoogleScholarGoogle Scholar | 22308490PubMed |
de Bruyn, P. N., Tosh, C. A., and Terauds, A. (2013). Killer whale ecotypes: Is there a global model? Biological Reviews of the Cambridge Philosophical Society 88, 62–80.
| Killer whale ecotypes: Is there a global model?Crossref | GoogleScholarGoogle Scholar |
Donnelly, D., McInnes, J., Morrice, M., and Andrews, C. (2016). ‘Killer whales of eastern Australia: photo-identification catalogue.’ (Killer Whales Australia: Box Hill South, Vic., Australia)
Elith, J., Phillips, S. J., Hastie, T., Dudík, M., Chee, Y. E., and Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists. Diversity & Distributions 17, 43–57.
| A statistical explanation of MaxEnt for ecologists.Crossref | GoogleScholarGoogle Scholar |
Esteban, R., Verborgh, P., Gauffier, P., Giménez, J., Afán, I., Cañadas, A., García, P., Murcia, J. L., Magalhães, S., and Andreu, E. (2014). Identifying key habitat and seasonal patterns of a critically endangered population of killer whales. Journal of the Marine Biological Association of the United Kingdom 94, 1317–1325.
| Identifying key habitat and seasonal patterns of a critically endangered population of killer whales.Crossref | GoogleScholarGoogle Scholar |
Evans, P. G. H., and Hammond, P. S. (2004). Monitoring cetaceans in European waters. Mammal Review 34, 131–156.
| Monitoring cetaceans in European waters.Crossref | GoogleScholarGoogle Scholar |
Ferguson, M. C., Barlow, J., Fiedler, P., Reilly, S. B., and Gerrodette, T. (2006). Spatial models of delphinid (family Delphinidae) encounter rate and group size in the eastern tropical Pacific Ocean. Ecological Modelling 193, 645–662.
| Spatial models of delphinid (family Delphinidae) encounter rate and group size in the eastern tropical Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Fernandez-Arcaya, U., Ramirez-Llodra, E., Aguzzi, J., Allcock, A. L., Davies, J. S., Dissanayake, A., Harris, P., Howell, K., Huvenne, V. A. I., Macmillan-Lawler, M., Martín, J., Menot, L., Nizinski, M., Puig, P., Rowden, A. A., Sanchez, F., and Van den Beld, I. M. J. (2017). Ecological role of submarine canyons and need for canyon conservation: a review. Frontiers in Marine Science 4, 5.
| Ecological role of submarine canyons and need for canyon conservation: a review.Crossref | GoogleScholarGoogle Scholar |
Fiedler, P. C., Redfern, J. V., Forney, K. A., Palacios, D. M., Sheredy, C., Rasmussen, K., García-Godos, I., Santillán, L., Tetley, M. J., Félix, F., and Ballance, L. T. (2018). Prediction of large whale distributions: a comparison of presence–absence and presence-only modeling techniques. Frontiers in Marine Science 5, 419.
| Prediction of large whale distributions: a comparison of presence–absence and presence-only modeling techniques.Crossref | GoogleScholarGoogle Scholar |
Findlay, K. P., Seakamela, S. M., Meÿer, M. A., Kirkman, S. P., Barendse, J., Cade, D. E., Hurwitz, D., Kennedy, A. S., Kotze, P. G., and McCue, S. A. (2017). Humpback whale ‘super-groups’ – a novel low-latitude feeding behaviour of Southern Hemisphere humpback whales (Megaptera novaeangliae) in the Benguela Upwelling System. PLoS One 12, e0172002.
| Humpback whale ‘super-groups’ – a novel low-latitude feeding behaviour of Southern Hemisphere humpback whales (Megaptera novaeangliae) in the Benguela Upwelling System.Crossref | GoogleScholarGoogle Scholar | 28249036PubMed |
Fletcher, W. J., Shaw, J., Metcalf, S. J., and Gaughan, D. J. (2010). An ecosystem based fisheries management framework: the efficient, regional-level planning tool for management agencies. Marine Policy 34, 1226–1238.
| An ecosystem based fisheries management framework: the efficient, regional-level planning tool for management agencies.Crossref | GoogleScholarGoogle Scholar |
Gill, P. C., Pirzl, R., Morrice, M. G., and Lawton, K. (2015). Cetacean diversity of the continental shelf and slope off southern Australia. The Journal of Wildlife Management 79, 672–681.
| Cetacean diversity of the continental shelf and slope off southern Australia.Crossref | GoogleScholarGoogle Scholar |
Halpern, B. S., Frazier, M., Afflerbach, J., Lowndes, J. S., Micheli, F., O’Hara, C., Scarborough, C., and Selkoe, K. A. (2019). Recent pace of change in human impact on the world’s ocean. Scientific Reports 9, 11609.
| Recent pace of change in human impact on the world’s ocean.Crossref | GoogleScholarGoogle Scholar | 31406130PubMed |
Higby, L. K., Stafford, R., and Bertulli, C. G. (2012). An evaluation of ad hoc presence-only data in explaining patterns of distribution: cetacean sightings from whale-watching vessels. International Journal of Zoology 2012, art428752.
| An evaluation of ad hoc presence-only data in explaining patterns of distribution: cetacean sightings from whale-watching vessels.Crossref | GoogleScholarGoogle Scholar |
Howard, C., Stephens, P. A., Pearce-Higgins, J. W., Gregory, R. D., and Willis, S. G. (2014). Improving species distribution models: the value of data on abundance. Methods in Ecology and Evolution 5, 506–513.
| Improving species distribution models: the value of data on abundance.Crossref | GoogleScholarGoogle Scholar |
Hu, C., Lee, Z., and Franz, B. (2012). Chlorophyll a algorithms for oligotrophic oceans: a novel approach based on three-band reflectance difference. Journal of Geophysical Research 117, C01011.
| Chlorophyll a algorithms for oligotrophic oceans: a novel approach based on three-band reflectance difference.Crossref | GoogleScholarGoogle Scholar |
Huang, Z., Nichol, S., Harris, P., and Caley, J. (2014). Classification of submarine canyons of the Australian continental margin. Marine Geology 357, 362–383.
| Classification of submarine canyons of the Australian continental margin.Crossref | GoogleScholarGoogle Scholar |
Huang, Z., Schlacher, T. A., Nichol, S., Williams, A., Althaus, F., and Kloser, R. (2018). A conceptual surrogacy framework to evaluate the habitat potential of submarine canyons. Progress in Oceanography 169, 199–213.
| A conceptual surrogacy framework to evaluate the habitat potential of submarine canyons.Crossref | GoogleScholarGoogle Scholar |
Isojunno, S., Matthiopoulos, J., and Evans, P. G. (2012). Harbour porpoise habitat preferences: robust spatio-temporal inferences from opportunistic data. Marine Ecology Progress Series 448, 155–170.
| Harbour porpoise habitat preferences: robust spatio-temporal inferences from opportunistic data.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. M., Beckley, L. E., Kobryn, H., Johnson, G. E., Kerr, I., and Payne, R. (2016). Crowdsourcing modern and historical data identifies sperm whale (Physeter macrocephalus) habitat offshore of South-Western Australia. Frontiers in Marine Science 3, 167.
| Crowdsourcing modern and historical data identifies sperm whale (Physeter macrocephalus) habitat offshore of South-Western Australia.Crossref | GoogleScholarGoogle Scholar |
Johnston, A., Fink, D., Reynolds, M. D., Hochachka, W. M., Sullivan, B. L., Bruns, N. E., Hallstein, E., Merrifield, M. S., Matsumoto, S., and Kelling, S. (2015). Abundance models improve spatial and temporal prioritization of conservation resources. Ecological Applications 25, 1749–1756.
| Abundance models improve spatial and temporal prioritization of conservation resources.Crossref | GoogleScholarGoogle Scholar | 26591443PubMed |
Jones, A., Bruce, E., Davies, K. P., Blewitt, M., and Sheehan, S. (2019). Assessing potential environmental influences on killer whale (Orcinus orca) distribution patterns in the Bremer Canyon, south-west Australia. The Australian Geographer 50, 381–405.
| Assessing potential environmental influences on killer whale (Orcinus orca) distribution patterns in the Bremer Canyon, south-west Australia.Crossref | GoogleScholarGoogle Scholar |
Kiszka, J., Ersts, P., and Ridoux, V. (2007a). Cetacean diversity around the Mozambique Channel Island of Mayotte (Comoros archipelago). The Journal of Cetacean Research and Management 9, 105.
Kiszka, J., Macleod, K., Van Canneyt, O., Walker, D., and Ridoux, V. (2007b). Distribution, encounter rates, and habitat characteristics of toothed cetaceans in the Bay of Biscay and adjacent waters from platform-of-opportunity data. ICES Journal of Marine Science 64, 1033–1043.
| Distribution, encounter rates, and habitat characteristics of toothed cetaceans in the Bay of Biscay and adjacent waters from platform-of-opportunity data.Crossref | GoogleScholarGoogle Scholar |
Laran, S., Authier, M., Van Canneyt, O., Dorémus, G., Watremez, P., and Ridoux, V. (2017). A comprehensive survey of pelagic megafauna: their distribution, densities, and taxonomic richness in the tropical Southwest Indian Ocean. Frontiers in Marine Science 4, 139.
| A comprehensive survey of pelagic megafauna: their distribution, densities, and taxonomic richness in the tropical Southwest Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Lück, M. (2015). Education on marine mammal tours – but what do tourists want to learn? Ocean and Coastal Management 103, 25–33.
| Education on marine mammal tours – but what do tourists want to learn?Crossref | GoogleScholarGoogle Scholar |
Lusseau, D., Bain, D. E., Williams, R., and Smith, J. C. (2009). Vessel traffic disrupts the foraging behavior of southern resident killer whales Orcinus orca. Endangered Species Research 6, 211–221.
| Vessel traffic disrupts the foraging behavior of southern resident killer whales Orcinus orca.Crossref | GoogleScholarGoogle Scholar |
Mannocci, L., Catalogna, M., Dorémus, G., Laran, S., Lehodey, P., Massart, W., Monestiez, P., Van Canneyt, O., Watremez, P., and Ridoux, V. (2014). Predicting cetacean and seabird habitats across a productivity gradient in the South Pacific gyre. Progress in Oceanography 120, 383–398.
| Predicting cetacean and seabird habitats across a productivity gradient in the South Pacific gyre.Crossref | GoogleScholarGoogle Scholar |
McClellan, C. M., Brereton, T., Dell’Amico, F., Johns, D. G., Cucknell, A.-C., Patrick, S. C., Penrose, R., Ridoux, V., Solandt, J.-L., Stephan, E., Votier, S. C., Williams, R., and Godley, B. J. (2014). Understanding the distribution of marine megafauna in the English Channel region: identifying key habitats for conservation within the busiest seaway on Earth. PLoS One 9, e89720.
| Understanding the distribution of marine megafauna in the English Channel region: identifying key habitats for conservation within the busiest seaway on Earth.Crossref | GoogleScholarGoogle Scholar | 24586985PubMed |
Merow, C., Smith, M. J., and Silander, J. A. (2013). A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography 36, 1058–1069.
| A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter.Crossref | GoogleScholarGoogle Scholar |
Moors-Murphy, H. B. (2014). Submarine canyons as important habitat for cetaceans, with special reference to the Gully: a review. Deep-sea Research. Part II, Topical Studies in Oceanography 104, 6–19.
| Submarine canyons as important habitat for cetaceans, with special reference to the Gully: a review.Crossref | GoogleScholarGoogle Scholar |
Morrice, M. G. (2004). Killer whales (Orcinus orca) in Australian territorial waters. Report for the Whale and Dolphin Conservation Society. Deakin University, Warrnambool, Vic., Australia.
Nielsen, S. E., Johnson, C. J., Heard, D. C., and Boyce, M. S. (2005). Can models of presence-absence be used to scale abundance? Two case studies considering extremes in life history. Ecography 28, 197–208.
| Can models of presence-absence be used to scale abundance? Two case studies considering extremes in life history.Crossref | GoogleScholarGoogle Scholar |
Pan, W. (2001). Akaike’s information criterion in generalized estimating equations. Biometrics 57, 120–125.
| Akaike’s information criterion in generalized estimating equations.Crossref | GoogleScholarGoogle Scholar | 11252586PubMed |
Parrish, J. K., and Edelstein-Keshet, L. (1999). Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284, 99–101.
| Complexity, pattern, and evolutionary trade-offs in animal aggregation.Crossref | GoogleScholarGoogle Scholar | 10102827PubMed |
Passadore, C., Domingo, A., Szephegyi, M., and Secchi, E. R. (2014). Influence of environmental and longline fishing operational variables on the presence of killer whales (Orcinus orca) in south-western Atlantic. Journal of the Marine Biological Association of the United Kingdom 94, 1267–1276.
| Influence of environmental and longline fishing operational variables on the presence of killer whales (Orcinus orca) in south-western Atlantic.Crossref | GoogleScholarGoogle Scholar |
Pearce, J., and Ferrier, S. (2001). The practical value of modelling relative abundance of species for regional conservation planning: a case study. Biological Conservation 98, 33–43.
| The practical value of modelling relative abundance of species for regional conservation planning: a case study.Crossref | GoogleScholarGoogle Scholar |
Pirotta, E., Booth, C. G., Costa, D. P., Fleishman, E., Kraus, S. D., Lusseau, D., Moretti, D., New, L. F., Schick, R. S., and Schwarz, L. K. (2018). Understanding the population consequences of disturbance. Ecology and Evolution 8, 9934–9946.
| Understanding the population consequences of disturbance.Crossref | GoogleScholarGoogle Scholar | 30386587PubMed |
Pitman, R. L., Totterdell, J. A., Fearnbach, H., Ballance, L. T., Durban, J. W., and Kemps, H. (2015). Whale killers: prevalence and ecological implications of killer whale predation on humpback whale calves off Western Australia. Marine Mammal Science 31, 629–657.
| Whale killers: prevalence and ecological implications of killer whale predation on humpback whale calves off Western Australia.Crossref | GoogleScholarGoogle Scholar |
Pitman, R. L., Totterdell, J., Wellard, R., Cullen, P., and de Boer, M. (2019). Long in the tooth: biological observations from at-sea sightings of strap-toothed beaked whales (Mesoplodon layardii). Marine Mammal Science 35, 1141–1161.
| Long in the tooth: biological observations from at-sea sightings of strap-toothed beaked whales (Mesoplodon layardii).Crossref | GoogleScholarGoogle Scholar |
Prieto, R., Tobeña, M., and Silva, M. A. (2017). Habitat preferences of baleen whales in a mid-latitude habitat. Deep-sea Research. Part II, Topical Studies in Oceanography 141, 155–167.
| Habitat preferences of baleen whales in a mid-latitude habitat.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2013). R: a language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria) Available at http://www.R-project.org/ [Verified 15 May 2020]
Reisinger, R. R., Keith, M., Andrews, R. D., and de Bruyn, P. J. N. (2015). Movement and diving of killer whales (Orcinus orca) at a Southern Ocean archipelago. Journal of Experimental Marine Biology and Ecology 473, 90–102.
| Movement and diving of killer whales (Orcinus orca) at a Southern Ocean archipelago.Crossref | GoogleScholarGoogle Scholar |
Rennie, S., Hanson, C. E., McCauley, R. D., Pattiaratchi, C., Burton, C., Bannister, J., Jenner, C., and Jenner, M. N. (2009). Physical properties and processes in the Perth Canyon, Western Australia: links to water column production and seasonal pygmy blue whale abundance. Journal of Marine Systems 77, 21–44.
| Physical properties and processes in the Perth Canyon, Western Australia: links to water column production and seasonal pygmy blue whale abundance.Crossref | GoogleScholarGoogle Scholar |
Ryan, J. P., Chavez, F. P., and Bellingham, J. G. (2005). Physical–biological coupling in Monterey Bay, California: topographic influences on phytoplankton ecology. Marine Ecology Progress Series 287, 23–32.
| Physical–biological coupling in Monterey Bay, California: topographic influences on phytoplankton ecology.Crossref | GoogleScholarGoogle Scholar |
Salgado Kent, C., Jenner, K. C. S., Jenner, M.-N., Bouchet, P., and Rexstad, E. (2012). Southern Hemisphere breeding stock ‘D’ humpback whale population estimates from north west cape, Western Australia. The Journal of Cetacean Research and Management 12, 29–38.
Santora, J. A., Zeno, R., Dorman, J. G., and Sydeman, W. J. (2018). Submarine canyons represent an essential habitat network for krill hotspots in a large marine ecosystem. Scientific Reports 8, 7579.
| Submarine canyons represent an essential habitat network for krill hotspots in a large marine ecosystem.Crossref | GoogleScholarGoogle Scholar | 29765085PubMed |
Scott-Hayward, L., Oedekoven, C. S., Mackenzie, M. L., Walker, C. G., and Rexstad, E. (2013). User guide for the MRSea package: statistical modelling of bird and cetacean distributions in offshore renewables development areas. University of St Andrews, Report for Marine Scotland No. CR/2012/05, St Andrews, Fife, UK.
Scott-Hayward, L. A. S., MacKenzie, M. L., Donovan, C. R., Walker, C., and Ashe, E. (2014). Complex region spatial smoother (CReSS). Journal of Computational and Graphical Statistics 23, 340–360.
| Complex region spatial smoother (CReSS).Crossref | GoogleScholarGoogle Scholar |
Scott-Hayward, L. A., Mackenzie, M. L., Ashe, E., and Williams, R. (2015). Modelling killer whale feeding behaviour using a spatially adaptive complex region spatial smoother (CReSS) and generalised estimating equations (GEEs). Journal of Agricultural Biological & Environmental Statistics 20, 305–322.
| Modelling killer whale feeding behaviour using a spatially adaptive complex region spatial smoother (CReSS) and generalised estimating equations (GEEs).Crossref | GoogleScholarGoogle Scholar |
Smith, J. N., Grantham, H. S., Gales, N., Double, M. C., Noad, M. J., and Paton, D. (2012). Identification of humpback whale breeding and calving habitat in the Great Barrier Reef. Marine Ecology Progress Series 447, 259–272.
| Identification of humpback whale breeding and calving habitat in the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Thomas, L., Buckland, S. T., Rexstad, E. A., Laake, J. L., Strindberg, S., Hedley, S. L., Bishop, J. R. B., Marques, T. A., and Burnham, K. P. (2010). Distance software: design and analysis of distance sampling surveys for estimating population size. Journal of Applied Ecology 47, 5–14.
| Distance software: design and analysis of distance sampling surveys for estimating population size.Crossref | GoogleScholarGoogle Scholar | 20383262PubMed |
Torres, L. G., Read, A. J., and Halpin, P. (2008). Fine-scale habitat modeling of a top marine predator: do prey data improve predictive capacity. Ecological Applications 18, 1702–1717.
| Fine-scale habitat modeling of a top marine predator: do prey data improve predictive capacity.Crossref | GoogleScholarGoogle Scholar | 18839765PubMed |
Totterdell, J. A. (2015). WA Orca Research Report - Ningaloo ID Catalogue. Marine Information and Research Group (MIRG).
Veloz, S., Salas, L., Altman, B., Alexander, J., Jongsomjit, D., Elliott, N., and Ballard, G. (2015). Improving effectiveness of systematic conservation planning with density data. Conservation Biology 29, 1217–1227.
| Improving effectiveness of systematic conservation planning with density data.Crossref | GoogleScholarGoogle Scholar | 25873240PubMed |
Waples, K., and Raudino, H. (2018). Setting a course for marine mammal research in Western Australia. Pacific Conservation Biology 24, 289–303.
| Setting a course for marine mammal research in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Weber, M. M., Stevens, R. D., Diniz-Filho, J. A. F., and Grelle, C. E. V. (2017). Is there a correlation between abundance and environmental suitability derived from ecological niche modelling? A meta-analysis. Ecography 40, 817–828.
| Is there a correlation between abundance and environmental suitability derived from ecological niche modelling? A meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Wellard, R., and Erbe, C. (2017). ‘Killer whales of the Bremer Sub-Basin: a photo-ID catalogue’, 2nd edn. (Project ORCA and Centre for Marine Science and Technology, Curtin University: Perth, WA, Australia)
Wellard, R., Erbe, C., Fouda, L., and Blewitt, M. (2015). Vocalisations of killer whales (Orcinus orca) in the Bremer Canyon, Western Australia. PLoS One 10, e0136535.
| Vocalisations of killer whales (Orcinus orca) in the Bremer Canyon, Western Australia.Crossref | GoogleScholarGoogle Scholar | 26462069PubMed |
Wellard, R., Lightbody, K., Fouda, L., Blewitt, M., Riggs, D., and Erbe, C. (2016). Killer whale (Orcinus orca) predation on beaked whales (Mesoplodon spp.) in the Bremer Sub-Basin, Western Australia. PLoS One 11, e0166670.
| Killer whale (Orcinus orca) predation on beaked whales (Mesoplodon spp.) in the Bremer Sub-Basin, Western Australia.Crossref | GoogleScholarGoogle Scholar | 27923044PubMed |
Williams, R., Hedley, S. L., and Hammond, P. S. (2006a). Modeling distribution and abundance of Antarctic baleen whales using ships of opportunity. Ecology and Society 11, 1.
| Modeling distribution and abundance of Antarctic baleen whales using ships of opportunity.Crossref | GoogleScholarGoogle Scholar |
Williams, R., Lusseau, D., and Hammond, P. S. (2006b). Estimating relative energetic costs of human disturbance to killer whales (Orcinus orca). Biological Conservation 133, 301–311.
| Estimating relative energetic costs of human disturbance to killer whales (Orcinus orca).Crossref | GoogleScholarGoogle Scholar |
Yackulic, C. B., Chandler, R., Zipkin, E. F., Royle, J. A., Nichols, J. D., Campbell Grant, E. H., and Veran, S. (2013). Presence-only modelling using MAXENT: when can we trust the inferences? Methods in Ecology and Evolution 4, 236–243.
| Presence-only modelling using MAXENT: when can we trust the inferences?Crossref | GoogleScholarGoogle Scholar |
Yan, J., and Fine, J. (2004). Estimating equations for association structures. Statistics in Medicine 23, 859–874.
| Estimating equations for association structures.Crossref | GoogleScholarGoogle Scholar | 15027075PubMed |
Yin, D., and He, F. (2014). A simple method for estimating species abundance from occurrence maps. Methods in Ecology and Evolution 5, 336–343.
| A simple method for estimating species abundance from occurrence maps.Crossref | GoogleScholarGoogle Scholar |
Zorn, C. J. (2001). Generalized estimating equation models for correlated data: a review with applications. American Journal of Political Science 45, 470–490.
| Generalized estimating equation models for correlated data: a review with applications.Crossref | GoogleScholarGoogle Scholar |
Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A., and Smith, G. M. (2009). ‘Mixed effects models and extensions in ecology using R.’ (Springer: New York, NY, USA)