Canids potentially threaten bilbies at Astrebla Downs National Park
John Augusteyn A E , Maree Rich B , Georgeanna Story C and Barry Nolan DA Queensland Parks and Wildlife Service and Partnerships, PO Box 3130, Red Hill, Qld 4701, Australia.
B Queensland Parks and Wildlife Service and Partnerships, PO Box 202, Longreach, Qld 4730, Australia.
C Scat About Ecological, PO Box 45, Majors Creek, NSW 2622, Australia.
D Queensland Parks and Wildlife Service and Partnerships, PO Box 5332, Airlie Beach, Qld 4802, Australia.
E Corresponding author. Email: john.augusteyn@des.qld.gov.au
Australian Mammalogy 43(3) 300-310 https://doi.org/10.1071/AM20034
Submitted: 21 April 2020 Accepted: 14 August 2020 Published: 10 September 2020
Journal Compilation © Australian Mammal Society 2021 Open Access CC BY NC ND
Abstract
The ecological role of canids in arid Australia is unresolved. Some argue they play a role regulating populations of herbivores and introduced mesopredators such as feral cats (Felis catus) and foxes (Vulpes vulpes). However, evidence also suggests they pose a threat to native species populations. The aims of this study were to determine the extent of canid predation on the bilby population at Astrebla Downs National Park, Queensland, to improve our understanding of the ecological role that canids serve in the park and to determine whether seasonal changes in the canid diet can be used to predict if and when management should intervene. Canid scats (n = 723) were collected over seven years and their content examined. The percentage of bilby remains in the canid scats varied from 13 to 85% (mean = 43%) and was 20–100% by volume. In total, 23 vertebrate species were identified in canid scats. The percentage of cat remains was 0–44% (mean = 11%), peaking in 2013 during a cat plague and coinciding with canids actively hunting cats. Fox remains were not detected in dog scats. These results indicate that canids had a varied diet and at times threatened the bilby population at Astrebla.
Additional keywords: bilby, canids, dietary analysis, Macrotis lagotis, predation, scat analysis, threatened species, wild dogs.
Introduction
The role that wild canids (dingoes/wild dogs (Canis familiaris) and their hybrids) (hereafter dogs) serve is the subject of debate (Allen et al. 2011; Glen 2012; Fancourt et al. 2019). Some argue that dogs help conserve native species by preying on or displacing more effective predators, i.e. mesopredator control (Johnson et al. 2007; Brook et al. 2012; Newsome et al. 2015), and controlling herbivores (Letnic et al. 2012). Others consider dogs a threat to native species (Oakwood 2000; Fisher et al. 2001; Banks et al. 2003) and/or believe the evidence relating to mesopredator regulation is inconclusive (Allen et al. 2011; Glen 2012; Fleming et al. 2013; Fancourt et al. 2019). The species they prey upon most likely vary both spatially and temporally (Corbett and Newsome 1987; Corbett 2001; Doherty 2015), which changes in response to prey availability or activity (e.g. reptiles in summer (Read et al. 2012) and when pups are being reared (Allen et al. 2012)). Dogs also have distinct prey preferences that can sometimes be disproportionate to prey availability (Robertshaw and Harden 1986). The impact that dogs have on a species may depend on how other threats interact. For example, a drought-affected common brushtail possum (Trichosurus vulpecula) population went extinct after being preyed upon by dogs and other predators, even though they have coexisted for thousands of years (3500–4000 years: Corbett 2008) (Kerle et al. 1992). The combined effect of predation and drought caused what is known as a ‘predator pit’ – and the population was not able to recover, even when conditions improved. The ability of dogs to be both specialist hunters (Robertshaw and Harden 1986) and generalist predators means they are well suited to arid environments that oscillate between periods of abundance, the boom periods, and periods when resources are scarce, the busts (Tatler et al. 2019). It can mean that favoured prey species are targeted even when their numbers become scarce, particularly in areas where alternative prey subsidise their food requirements (Allen and Leung 2012).
Although dogs have been living with native species long enough that some have evolved an innate antipredator response (Steindler et al. 2018), introduced predators and increased food and breeding opportunities due to modified land management practices may be affecting the way dogs now interact with their environment. In an Australia-wide study of dog diets, Doherty et al. (2019) found that rabbits (Oryctolagus cuniculus), reptiles and arthropods were encountered more frequently in the arid and semiarid zones compared with other areas. They also found that, where rabbits were rare, the percentage of other medium-sized mammals increased, suggesting that prior to rabbits, dogs predominantly preyed on medium-sized mammals (Doherty et al. 2019; Tatler et al. 2019), many of which are now either extinct or threatened (McKenzie et al. 2007; Woinarski et al. 2014). Thirty-nine native terrestrial species that are Threatened or Near Threatened have been found in dog diets (Doherty et al. 2019).
At Astrebla Downs National Park (hereafter Astrebla), an area that contains Queensland’s largest bilby population, dogs are known to prey on bilbies (8–25% frequency of occurrence (% FO) is the proportion of scats containing a prey item) and kowaris (Dasyuroides byrnei) (4% FO) (n =105) (Palmer 1999). Paltridge (2002) found 1.3% FO (n = 77) of dogs scats collected contained bilby remains and considered dog predation combined with cat and fox predation to be a serious threat to the future of the species. Tatler et al. (2019) found the % FO for bilbies to be less than 10%. To reduce the risk of predation to threatened species many organisations have started installing predator proof fencing, but this option is expensive (Hayward and Kerley 2009; Hayward et al. 2014).
Although resource shortages have been considered the primary driving factor controlling the dynamics of arid fauna communities, there is increasing evidence that predation may play an overriding role in shaping species abundance and composition at times (Pavey et al. 2008; Letnic et al. 2011; Greenville et al. 2014). Understanding factors that have recently changed an ecosystem and shifted the balance in favour of predators is important to be able to conserve threatened species, particularly in unfenced reserves like Astrebla. The challenges for wildlife managers are: predicting when predation events are likely to occur; understanding how dogs and mesopredators interact; and knowing whether and when to intervene, i.e. before, during or immediately after a boom period and/or during the bust (Pavey et al. 2014; Yip et al. 2015). The aims of this study were to determine the extent of dog predation on the bilby population at Astrebla, improve our understanding of the ecological role that dogs serve on the park and determine whether seasonal changes in the dog’s diet can be used to predict if and when management should intervene.
Material and methods
Study area
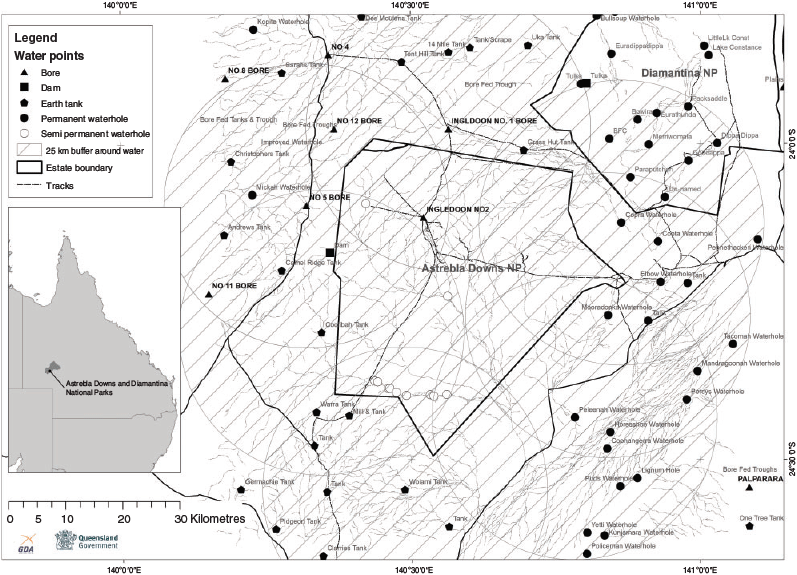
Astrebla is surrounded by stock fences and is located 100 km east of Bedourie in the Mitchell Grass Downs and Channel Country Bioregions and covers an area of approximately 176 000 ha. Cattle grazing enterprises surround the park, all of which contain artificial water points. The main bore on Astrebla, Ingledoon No. 2 bore (No2), was turned off completely in 2013 but several permanent water points remain, either on-park (Mooradonka Waterhole) or immediately off-park. The greatest distance anywhere on park to the nearest permanent water point is approximately 25 km (Fig. 1).
|
Scat collection
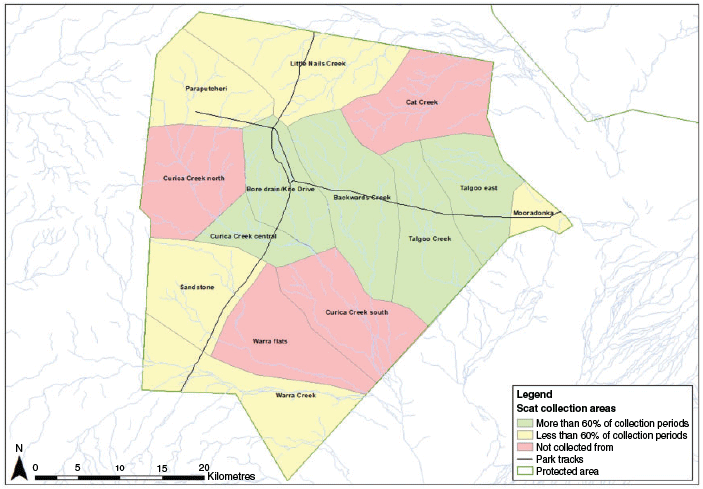
The park was divided into 14 areas, 10 of which were searched for dog scats on up to 17 occasions during 2012–19 (Fig. 2). Bilbies were known to be present in each of these 10 areas. Five of these areas were checked 11–13 times and the other five were checked 7–10 times. Dog scats were distinguished from other scats by their shape and size (Triggs 1996). Confirmation that scats were from dogs was obtained from an experienced scat analyst using the aforementioned characters as well as the presence of grooming hairs and the pattern of prey contents, i.e. fragment size and hair damage. The dog scats (n = 723) were mainly collected along roads and creek lines. Search effort was not standardised, and the number of scats collected for each period varied according to the number of scats that were present and, to a lesser extent, the time spent collecting scats. Each scat was placed in a separate paper bag and the collection site was recorded using a GPS. Three categories of scats were analysed – old scats were odourless, dry and white all over; medium-aged scats contained some odour with mixed colouration; and fresh scats had consistent colouration, and were usually darker with a mucus coating and strong smell. Old scats were considered to have been deposited up to two months prior to collection and were added to the previous survey period/season if the collection date was less than two months from the last survey period. Really old scats (white in colour and disintegrating and most likely more than three months old) were not collected.
Scat analysis
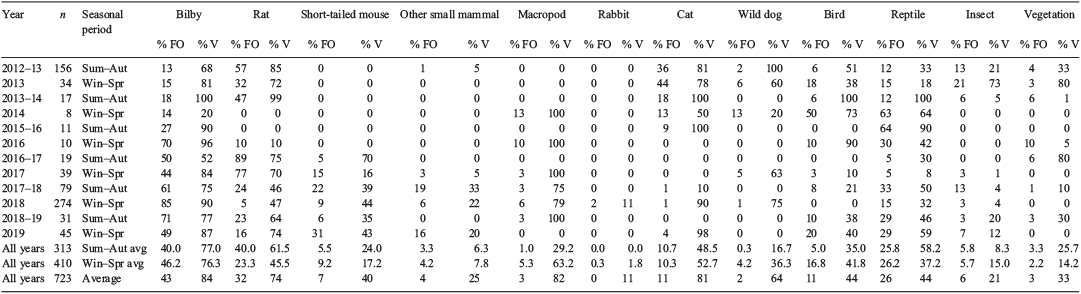
Scats were oven-dried at 100°C for 6 h, then washed in nylon bags to remove all but the identifiable fragments. Prey components were identified to the lowest possible taxonomic level through comparison of remains, such as teeth and claws, with known reference material or the literature (Watts and Aslin 1981; Triggs and Brunner 2002) and hair identified using the technique described by Brunner and Coman (1974). Bilby hair has unique characteristics amongst arid-zone species and is readily distinguished from all other species. The use of an experienced analyst also ensured correct identification of bilby hair. For comparison, samples were pooled into yearly summer–autumn and winter–spring categories. Scats were not collected during winter–spring 2015. Prey data were also pooled into broad taxonomic groups for species that were infrequently detected or unable to be identified to species level. Many studies have used % FO to report on predator diets (Croft and Hone 1978; Catling 1988; Paltridge 2002; Allen and Leung 2012; Doherty et al. 2019) even though this method tends to over-represent the importance of small prey items (Corbett 1989). Percentage volume estimates (% V) (that is, the proportion of the volume of prey matter represented by a species/taxonomic group), were also included to help address this limitation. The % V was visually estimated using a grid within a sorting tray. The average volume for each prey group was calculated for all scats within each collection period where the prey group was present. The average % V for scats within a collection period was also calculated for bilbies. This calculation was to test the assumption that one scat containing bilby remains was equal to one dead bilby, by recording a high percentage volume of bilby within the scat. It was also assumed that if a bilby was eaten, its remains would show in the dog scat. Dogs are known to consume approximately 1.4 kg of food per day (Green and Catling 1977 in Newsome et al. 1983). Male and female bilbies weigh, on average, 1–2.5 kg and 0.8–1.1 kg, respectively (Johnson 2013), meaning that a dog could consume one bilby per day.
Seasonal differences in the amount of bilby, long-haired rat (Rattus villosissimus) (hereafter rat), reptile and bird were tested separately using a paired t-test in QED Statistics (Pisces Conservation Ltd 2007). We also used a combination of species and broad taxonomic groups (10 taxa listed in Table 1) to perform a simple regression analysis in Excel to test whether the % FO for rats, which was thought to be a measure of resource availability, correlated with the diversity of species consumed.
Rainfall
Rainfall and average rainfall data for No2 were obtained from the Scientific Information for Land Owners database (DES 2019) drill system (Silo) (http://www.longpaddock.qld.gov.au/silo). Monthly rainfall totals were converted to residual rainfall by deducting the average monthly rainfall from the actual monthly rainfall.
Results
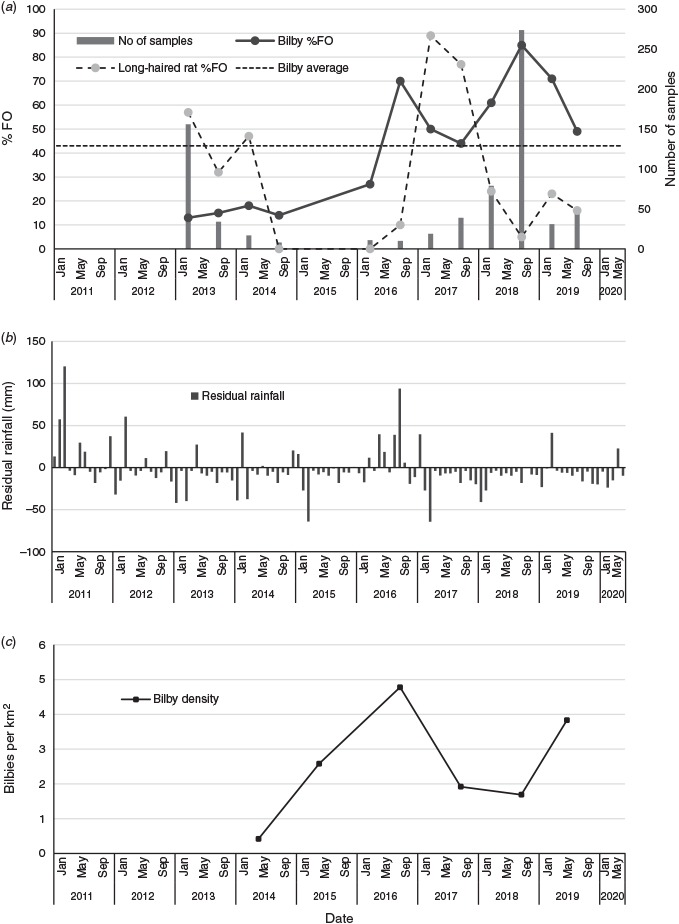
The % FO and % V of prey species and other broad taxonomic groupings are presented in Table 1. Sixteen mammal species and several other taxa that could not be identified to species level were encountered. No foxes were detected but cats were consumed, particularly prior to winter 2016. The proportion of dog scats containing bilby peaked in winter–spring 2018, when 233 of the 274 scats (85% FO) collected contained bilby remains. The average % FO for bilby was consistently lower between 2012 and 2015 (13–24%) than after 2015 (44–85%). Bilbies were consumed at a higher rate than most other species or broad taxonomic groups, regardless of the period, and contributed to an average of 84% of the prey volume within scats and 46% volume within a sampling period. No significant seasonal differences were found in the portion of scats containing bilby (t = −0.957, d.f. = 5, P > 0.05), rat (t = 0.960566, d.f. = 5, P > 0.05), reptile (t = −0.16507, d.f. = 5, P > 0.05) and bird (t = −0.27735, d.f. = 5, P > 0.05) remains. Rats were consumed in all periods except winter–spring 2014 and summer–autumn 2015–16 and were the dominant prey (% V) item when consumed except in winter–spring 2016 when they contributed to only 1% of prey scat volume. The % FO for rats peaked in summer–autumn 2016–17 and this coincided with a slight decrease in the % FO for bilbies (Fig. 3a). A regression analysis comparing the % FO for rats with the diversity of other taxa consumed found that dog prey did not vary during resource pulses (y = 0.017x + 5.5305, R2 = 0.0007), when rats were relatively more abundant than other groups. Short-tailed mice (Leggadina forresti) were not detected until the summer of 2016, after which time they were consistently detected, although at a lower frequency and volume than rats. Reptiles were detected in every sampling season, occasionally in high frequencies and volumes. For example, in summer–autumn 2015–16 reptiles were in 64% of scats and contributed 90% of the prey volume. Pogona sp. and Varanus sp. were commonly encountered. The remaining prey, including large macropods, were consistently found at lower frequencies and volumes across the entire sampling period. Rabbits were detected only in winter–spring 2018, when five scats contained a low volume of rabbit (<20%).
|
Between 2012 and 2020, monthly rainfall was above average on 18 occasions. Rainfall was often well below average outside of these periods (Fig. 3b). The 2016–17 period was one of potentially higher resources.
Discussion
Impact to bilbies
Australia once supported a diverse assemblage of medium‐sized marsupials that would have fed dingoes (Caughley et al. 1980; Johnson 2006; Tatler et al. 2019) and thylacines (Thylacinus cynocephalus) (Figueirido and Janis 2011). Several dog dietary studies (Allen and Leung 2012; Doherty et al. 2019; Tatler et al. 2019) found that rabbits have effectively replaced the medium-sized marsupials that are part of the group known as the ‘critical weight range’ species (35–5500 g) (Johnson and Isaac 2009). However, at Astrebla rabbits are rare and the original medium-sized marsupial, the bilby, is still extant.
This study revealed that the extent of dog predation on Astrebla’s bilby population varied. At times predation was low but occasionally the term ‘hyperpredation’ (Smith and Quin 1996) adequately described the extent. Resource availability is thought to be one of the key drivers of this variation in predation. In the arid zone, populations are known to fluctuate between boom and bust periods (Predavec and Dickman 1994; Greenville et al. 2013). The booms usually follow periods of above-average rainfall and the busts occur during droughts when predation and/or starvation cause both prey and predator populations to decline (Carstairs 1974; Newsome and Corbett 1975; Predavec and Dickman 1994, Pavey et al. 2008; Pavey and Nano 2013; Greenville et al. 2014). Some studies of these boom–bust events have suggested that both bottom-up (food limited) and top-down (predation) pressures interact to regulate native mammals (Greenville et al. 2014). The scale and the spatial extent of these boom events depend on the amount, location and timing of the rainfall (authors’ obs.). Sometimes patchy rainfall can result in isolated resource pulses or ‘mini-booms’. At Astrebla, the rats and the vegetation that the rats feed on are some of the primary drivers of ecosystem productivity (Rich et al. 2014). Tatler et al. (2019) found that rats were encountered 4.5 times more often in dog diets sampled during boom periods than during non-boom periods. The results from this study also suggest that, in addition to rats, bilbies may help increase predator populations. Bilbies were consumed throughout the study period but peaked, albeit at slightly lower % FO than rodents, during the resource booms, which is similar to the results of Tatler et al. (2019), who found that the occurrence of medium-sized mammals remained high during resource booms and sometimes was disproportionate to their abundance.
When this study commenced (2012), the bilby population was high, but this changed rapidly and by 2013, the population had declined considerably (authors’ obs.), most likely due to predation by both feral cats and dogs (Rich et al. 2014). High rainfall in 2010–11 led to an irruption of rodents on Astrebla and cat and dog numbers increased (Rich et al. 2014). As rat numbers dwindled in 2013, both feral cats and, to lesser extent, dogs rapidly switched to bilbies and other small mammals on the park (Rich et al. 2014), a phenomenon that was also described by Letnic and Dickman (2010) and Letnic et al. (2011). Cat stomachs sampled in the first week of May 2013 contained almost all rat. A third of the stomachs sampled a few weeks later contained bilby remains (authors’ obs.). The sheer number of cats at the time, which was considerably higher than the density of dogs, suggests that feral cats posed the biggest threat to bilbies. But this was compounded by the enlarged dog population and the cats themselves may have contributed a partial food source for dogs, extending the time that dogs remained in the area. Stokeld et al. (2018) found that dingoes are likely to limit the recovery of threatened species populations and management programs that focus solely on mitigating cat predation may be inadequate in landscapes where other factors such as the provision of water are at play and where dingoes can be another predator.
Despite over 2500 cats and more than 32 dogs being removed from the park between April 2012 and December 2013 (Rich et al. 2014), bilby and kowari numbers dwindled to the point where they were undetectable, by the end of 2013 (authors’ obs.). Although the efforts to control predators did not prevent the bilby population crashing, they probably enabled the population to recover more quickly. Further, the rapid decline in the bilby population due to predation meant that they probably had not depleted all the available resources left from the boom and some food (bulbs, seeds, termites) persisted in the soil. Patchy rainfall midway through 2016 also helped to boost both rodent and bilby populations and record numbers of bilbies were recorded in 2015–16 (Mitchell 2015; Augusteyn et al. 2020a). But this boom was short-lived and between 2016 and 2018 bilby density declined by up to 96% (Augusteyn et al. 2020a, 2020b, unpublished report) (Fig. 3c) and dog predation was implicated as being the most likely cause. The percentage of dog scats containing bilby increased in winter 2016 (70% FO) and peaked in winter–spring 2018 (85% FO). Cats were actively managed throughout the entire period by frequent spotlight shoots.
Unlike cats, dogs were not controlled between 2014 and 2018. The large bilby population and the presence of rats in 2016–17 are thought to have led to an increase in the dog population. We speculate that the cause of the 2018 winter and spring hyperpredation event was due to there being more dogs and bilbies and a higher encounter rate between the two species, particularly after the rat numbers dwindled towards the end of 2017. The % FO for rats peaked in summer–autumn 2016–17 and then declined, suggesting that the rodent population had dropped and/or the dogs shifted their prey preference to other species. The lack of rats and other prey meant that bilbies became the main target and because there was no other readily available food source for dogs to shift to, bilbies were selectively hunted disproportionately to their population size and bilbies became scarce. A limitation of our study is that we do not have information on either predator numbers or prey availability, other than bilby density and general observations made during line-transect surveys. Therefore, we cannot be confident that prey groups were selected relative to their population size. The events described above follow a similar pattern to that observed in the early to mid-1990s (McRae 2004). Palmer (1999) and McRae (2004) both considered dogs to have an impact on bilbies during the ‘bust’ and during ‘mini-boom’ periods and the results from this study agree with these findings.
Newsome et al. (1983) and Paltridge (2002) described four major prey categories for vertebrate predators including staple, supplementary, opportunistic and seasonal staples. We suggest that bilbies at Astrebla fall into the staple (a food source that predators rely upon through time) category because they were encountered in every season sampled. They may also occasionally represent an opportunistic (during boom periods) food source when high numbers of bilbies provide an extra resource that enables predator populations to increase.
Ecological role
Our results are consistent with several studies (Smith and Quin 1996; Doherty et al. 2019; Tatler et al. 2019) that found that dogs have a flexible diet and perform a mixed ecological role. However, unlike other studies (Pavey et al. 2008; Tatler et al. 2019), the diversity of dog prey did not seem to vary significantly according to resource pulses. This may be because the current study did not start until the end of the 2011–13 rat plague and dogs had already shifted to other prey groups as rat numbers dwindled.
McRae (2004) argued that dogs could be beneficial to bilbies due to their interaction with other feral predators (principally foxes). Foxes have not been detected on Astrebla and the lack of rabbits, which are often linked to their spread (Saunders and McLeod 2007), may limit their extent. However, Moseby et al. (2012) showed that dingoes can kill foxes but do not always eat them and therefore the analysis of scats is not a reliable indicator of fox and dog interactions. Southgate et al. (2007) found that foxes prefer habitats that contain rock features and calcareous substrates, which are largely absent from Astrebla. Foxes are able to survive without free water, but their daily movement is restricted to only a few kilometres per day (Coman et al. 1991; Southgate et al. 2007) which might be problematic at Astrebla. It may mean that the park is unsuitable for foxes except possibly after flood events. Brawata and Neeman (2011) found that at sites where dogs were uncontrolled, foxes were less likely to be found within 5 km of water, suggesting that dogs limit fox populations. However, this hypothesis does not account for the few periods when flooding rain creates multiple semipermanent waterpoints across the landscape and when foxes are still not detected on the park. We suggest that more work needs to be conducted to determine whether dogs and/or the lack of suitable habitat/water are the reasons that foxes are absent from the park.
Dogs were observed hunting cats and scavenging from piles of cat carcasses (authors’ obs.) and this may have contributed to the high % FO for cats found in dog scats collected in 2013. Palmer (1999) found 1% FO of dog scats contained cat remains. Tatler et al. (2019) found that dogs preyed on feral cats and suggested that they form an important part of their prey. While it is possible that dogs may regulate cat numbers or interfere with their habitat use, the evidence from the 2013 boom suggests that dogs have very little influence on cat numbers or behaviour when resources are plentiful. Cats were able to avoid or escape dog predation even on the open, treeless plains by living in bilby burrows and in the tree-lined creeks (Rich et al. 2014).
Dogs on Astrebla were not controlled between 1997 and 2012 and between 2014 and 2018 and were most likely in stable pack structures. Pettigrew (1993) attributed the invasion of cats in the early 1990s to a reduction in dog numbers, even though McRae (2004) disputed this argument, stating that only four dogs were removed and not 50 as suggested. The lack of any dog control in the lead up to the more recent 2013 cat/dog plague, suggests that cat populations can reach plague proportions in response to an abundance of rodents and not because their populations are released from dog predation.
The arid-zone fauna, prior to the introduction of cats and foxes, would have naturally existed in a constant fluctuation between population booms followed by population crashes. The change from having one major predator (dingo or thylacine) to two or three with the introduction of cats and foxes may have been enough to disrupt this cycle to the point where some local extirpations have occurred or may occur in the future. Even though predation has been considered a major cause of species decline in general (Finlayson 1961; Woinarski et al. 2014), dogs and, to a lesser extent, cats (1824–86: Abbott 2002), have coexisted with native species (Gibson et al. 1994; Fisher et al. 2001; Fisher et al. 2014; Abbott 2002; Fancourt et al. 2015) long before some of the more serious declines were observed in the mid-1900s (Burbidge and McKenzie 1989). The reason this balance has suddenly changed, particularly in areas where foxes are absent, is largely unknown but Aboriginal peoples may have persecuted non-commensal dogs (Short et al. 2002). The provision of free water, which has improved the extent and reliability of palatable grasses (Newsome 1975) and favoured large macropods, introduced stock and rabbits (James et al. 1999; Corbett 2001; Fensham and Fairfax 2008; Davies et al. 2010; Allen 2011) and provided breeding opportunities for dogs and other species (Thomson 1992; Short et al. 2002) may have shifted the balance in favour of dogs. Large macropods and rabbits are, however, normally rare at Astrebla and movement data from a study conducted in the Strzelecki Desert found that dogs regularly went 3–5 days without water and were capable of walking approximately 150 km without visiting any water for 22 days (Allen 2012). This suggests that most parts of Astrebla would be well within reach of the natural waterholes that existed prior to European settlement and that neither prey increases nor water for drinking are likely to be important on the park. However, Thomson (1992) found that nearly half of dog dens were located within 500 m of water and only 19% were more than 2.5 km from a known water source, suggesting that increasing the number of artificial water sources on neighbouring properties may have increased the amount of dog breeding and predation on Astrebla.
Changes to the livestock industry, with properties grazing cattle instead of sheep, going organic and/or being used to fatten cattle instead of breeding (Phelps 2007), are changing the way dogs are being managed (van Eeden et al. 2019). The introduction of organic farming, particularly since the mid-1990s (Wynen 2006; Phelps 2014), may limit the capacity of landholders to manage dogs using sodium monofluoroacetate (1080). The increased weight and size of the herd potentially reduces the impact that dogs can have and consequently the need for graziers to manage dogs. This shift potentially means that dog numbers are increasing over large areas of land surrounding Astrebla. The lack of grazing on both Astrebla and the nearby Diamantina National Park also means that there are more resources on the parks for native mammals (Palmer 1999) and food for dogs. Wallach et al. (2009) found that dog control led to their populations being socially fractured and fluctuating between below and above carrying capacity. Without control, dogs existed at carrying capacity and were socially stable. The change of management from dog control to no control on neighbouring lands at Astrebla may mean that dogs are transitioning from a socially fractured to a recovered state. However, the rapid fluctuation in the carrying capacity of the land, as conditions oscillate between boom and bust, may mimic the impact of control and hinder the recovery process, thereby causing the dog population to remain above carrying capacity and socially fractured. The combination of higher dog populations, other feral predators and changes to dog management practices are potentially what is causing the decline and/or extinction of some threatened species populations (Allen and Fleming 2012).
Seasonality
Being able to predict when predators are likely to target a threatened species enables managers to be more strategic and more efficient, and potentially reduce the extent of some of the large fluctuations that have occurred in the bilby population at Astrebla in the past. The type and amount of prey consumed is generally thought to be dependent on the abundance of the prey relative to other prey items (Paltridge 2002; Pavey et al. 2008). However, for favoured prey this may continue to the point where it is no longer in proportion with prey abundance (Robertshaw and Harden 1986). Understanding different predator–prey cycles and how abiotic factors drive these cycles is critical to enable targeted intervention without the need for large-scale, onsite population studies each time. In the arid zone, rainfall is considered to be the main driver of ecosystem productivity (Dickman et al. 1999; Morton et al. 2011; Read et al. 2012; Greenville et al. 2016) but evaporation rates and temperature may also be important. The lack of a significant seasonal difference in the % FO for bilby, rat, reptile and bird was surprising. Predation was expected to be greater for mammals in winter than for reptiles, which are usually less active when conditions are cool (Greer 1989; Paltridge 2002). The lack of seasonal difference could be due to the broad seasonal categories used in the analysis (summer–autumn and winter–spring), both of which include warm periods. These categories were chosen to increase the number of the samples and to pick up the influence of rain, which usually falls during summer. However, the study was punctuated by above-average rainfall in winter 2016. Grass seed production and germination (temperatures must exceed 15°C: Orr 1975) and the rats may not have been able to respond to the rain until the following summer, which caused a lag in the results. We suggest that more work is required to model seed production of Mitchell grass and the mechanism by which rats reach both plague and subplague proportions to better predict when predator control is likely to be required.
Management lessons
In 2018, dog control commenced because of concerns about the high bilby % FO, and the reduced bilby counts. Four dogs were shot and an additional 16 dogs were baited with 1080 (Rich 2018). Cats were also controlled during spotlight shoots. In 2019, rainfall combined with the predator control possibly enabled the bilby population to recover by 78% compared with 2018 (Augusteyn et al. 2020b). These results suggest that controlling predators before their numbers build up is an effective strategy in years following average to slightly above average rainfall. The timing of predator control depends on the size of the boom and the extent of the bust. During boom years Pavey et al. (2014) and Yip et al. (2015) suggested waiting until the bust before attempting control. However, the evidence from the 2011–13 boom suggests that the transition from boom to bust and the subsequent shift in prey can occur rapidly, leaving little time to effectively reduce predator numbers. Letnic and Dickman (2006) also found that predators had their strongest impacts on native mammals in the 1–2 years following very high rainfall and considered this time to be the most critical for wildlife managers. Palmer (1999) considered dogs to have the greatest impact on bilbies during droughts and data from this study suggest that controlling dogs during the bust period has merit. We therefore recommend that for major booms control commences before predator numbers build and continues until predator populations are reduced to the point where they are not impacting threatened species. For ‘mini-booms’ predator control could occur when predators increase their preferences for bilbies. Predator control is also recommended during droughts and periods of low resource availability, particularly if they immediately follow a resource pulse.
Regardless of whether dogs do or do not regulate mesopredators at Astrebla, the extremely high % FO for bilby observed during this study suggests that dogs are major predators of bilbies such that the impact of predation outweighs any potential benefit afforded through mesopredator regulation. We therefore consider that it is appropriate at times to reduce both cat and dog populations, such as during seasonal boom–bust resource cycles, when dog populations are high, or they are likely to be targeting bilbies. The capacity of the Astrebla population to recover from a major predation event also highlights the resilience of the population and that it is possible to maintain a healthy population without having to resort to expensive predator-proof fences.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Thanks to all the Queensland Parks and Wildlife Service staff and volunteers, including the Conservation and Wildlife Management personnel from Sporting Shooters Association of Australia for helping to collect scats. Thanks go to Rhonda Melzer, Marnie Augusteyn, Ross Goldingay and the two anonymous reviewers for their comments on earlier drafts and to Anthony Pople, Alistair Melzer and Graeme Armstrong for their assistance with the data analysis. This research did not receive any specific funding.
References
Abbott, I. (2002). Origin and spread of the cat, Felis catus, on mainland Australia, with a discussion of the magnitude of its early impact on native fauna. Wildlife Research 29, 51–74.| Origin and spread of the cat, Felis catus, on mainland Australia, with a discussion of the magnitude of its early impact on native fauna.Crossref | GoogleScholarGoogle Scholar |
Allen, B. L. (2011). A comment on the distribution of historical and contemporary livestock grazing across Australia: implications for using dingoes for biodiversity conservation. Ecological Management and Restoration 12, 26–30.
| A comment on the distribution of historical and contemporary livestock grazing across Australia: implications for using dingoes for biodiversity conservation.Crossref | GoogleScholarGoogle Scholar |
Allen, B. L. (2012). Do desert dingoes drink daily? Visitation rates at remote waterpoints in the Strzelecki Desert. Australian Mammalogy 34, 251–256.
| Do desert dingoes drink daily? Visitation rates at remote waterpoints in the Strzelecki Desert.Crossref | GoogleScholarGoogle Scholar |
Allen, B. L., and Fleming, P. J. S. (2012). Reintroducing the dingo: the risk of dingo predation to threatened vertebrates of western New South Wales. Wildlife Research 39, 35–50.
| Reintroducing the dingo: the risk of dingo predation to threatened vertebrates of western New South Wales.Crossref | GoogleScholarGoogle Scholar |
Allen, B. L., and Leung, L. K. P. (2012). Assessing predation risk to threatened fauna from their prevalence in predator scats: dingoes and rodents in arid Australia. PLOS ONE 7, e36426.
| Assessing predation risk to threatened fauna from their prevalence in predator scats: dingoes and rodents in arid Australia.Crossref | GoogleScholarGoogle Scholar | 22563498PubMed |
Allen, B. L., Engeman, R. M., and Allen, L. R. (2011). Wild dogma II: the role and implications of wild dogma for wild dog management in Australia. Current Zoology 57, 737–740.
| Wild dogma II: the role and implications of wild dogma for wild dog management in Australia.Crossref | GoogleScholarGoogle Scholar |
Allen, L. R., Goullet, M., and Palmer, R. (2012). The diet of the dingo (Canis lupus dingo and hybrids) in north-eastern Australia: a supplement to the paper of Brook and Kutt (2011). The Rangeland Journal 34, 211–217.
| The diet of the dingo (Canis lupus dingo and hybrids) in north-eastern Australia: a supplement to the paper of Brook and Kutt (2011).Crossref | GoogleScholarGoogle Scholar |
Augusteyn, J., Pople, A., and Rich, M. (2020a). Evaluating the use of thermal image cameras to monitor the endangered greater bilby at Astrebla Downs National Park. Australian Mammalogy 42, 329–340.
| Evaluating the use of thermal image cameras to monitor the endangered greater bilby at Astrebla Downs National Park.Crossref | GoogleScholarGoogle Scholar |
Augusteyn, J., Pople, A., and Rich, M. (2020b). Astrebla bilby density estimates (2017–2019). Unpublished report. Queensland Parks and Wildlife Service, Rockhampton.
Banks, S. C., Horsup, A., Wilton, A. N., and Taylor, A. C. (2003). Genetic marker investigation of the source and impact of predation on a highly endangered species. Molecular Ecology 12, 1663–1667.
| Genetic marker investigation of the source and impact of predation on a highly endangered species.Crossref | GoogleScholarGoogle Scholar | 12755893PubMed |
Brawata, R. L., and Neeman, T. (2011). Is water the key? Dingo management, intraguild interactions and predator distribution around water points in arid Australia. Wildlife Research 38, 426–436.
| Is water the key? Dingo management, intraguild interactions and predator distribution around water points in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Brook, L. A., Johnson, C. N., and Ritchie, E. G. (2012). Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression. Journal of Applied Ecology 49, 1278–1286.
| Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression.Crossref | GoogleScholarGoogle Scholar |
Brunner, H., and Coman, B. J. (1974). ‘The Identification of Mammalian Hair.’ (Inkata Press.)
Burbidge, A. A., and McKenzie, N. L. (1989). Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications. Biological conservation 50, 143–198.
| Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications.Crossref | GoogleScholarGoogle Scholar |
Carstairs, J. L. (1974). The distribution of Rattus villosissimus (Waite) during plague and non-plague years. Wildlife Research 1, 95–106.
| The distribution of Rattus villosissimus (Waite) during plague and non-plague years.Crossref | GoogleScholarGoogle Scholar |
Catling, P. C. (1988). Similarities and contrasts in the diets of foxes, Vulpes vulpes, and cats, Felis catus, relative to fluctuating prey populations and drought. Australian Wildlife Research 15, 307–317.
| Similarities and contrasts in the diets of foxes, Vulpes vulpes, and cats, Felis catus, relative to fluctuating prey populations and drought.Crossref | GoogleScholarGoogle Scholar |
Caughley, G., Grigg, G. C., Caughley, J., and Hill, G. J. E. (1980). Does dingo predation control the densities of kangaroos and emus? Wildlife Research 7, 1–12.
| Does dingo predation control the densities of kangaroos and emus?Crossref | GoogleScholarGoogle Scholar |
Coman, B. J., Robinson, J., and Beaumont, C. (1991). Home range, dispersal and density of red foxes (Vulpes vulpes L.) in central Victoria. Wildlife Research 18, 215–223.
| Home range, dispersal and density of red foxes (Vulpes vulpes L.) in central Victoria.Crossref | GoogleScholarGoogle Scholar |
Corbett, K. (2001). ‘The Dingo in Australia and Asia.’ (JB Books: Marleston, South Australia.)
Corbett, L. K. (1989). Assessing the diet of dingoes from feces: a comparison of 3 methods. Journal of Wildlife Management 53, 343–346.
| Assessing the diet of dingoes from feces: a comparison of 3 methods.Crossref | GoogleScholarGoogle Scholar |
Corbett, L. (2008). Dingo Canis lupus. In ‘The Mammals of Australia’. (Eds S. Van Dyck, and R. Strahan.) pp. 737–739. (New Holland: Sydney.)
Corbett, L., and Newsome, A. E. (1987). The feeding ecology of the dingo – III. Dietary relationships with widely fluctuating prey populations in arid Australia: an hypothesis of alternation of predation. Oecologia 74, 215–227.
| The feeding ecology of the dingo – III. Dietary relationships with widely fluctuating prey populations in arid Australia: an hypothesis of alternation of predation.Crossref | GoogleScholarGoogle Scholar | 28311993PubMed |
Croft, J. D., and Hone, L. D. (1978). The stomach contents of foxes, Vulpes vulpes, collected in New South Wales. Australian Wildlife Research 5, 85–92.
| The stomach contents of foxes, Vulpes vulpes, collected in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Davies, K. F., Melbourne, B. A., James, C. D., and Cuningham, R. B. (2010). Using traits of species to understand responses to land use change: birds and livestock grazing in the Australian arid zone. Biological Conservation 143, 78–85.
| Using traits of species to understand responses to land use change: birds and livestock grazing in the Australian arid zone.Crossref | GoogleScholarGoogle Scholar |
Dickman, C. R., Letnic, M., and Mahon, P. S. (1999). Population dynamics of two species of dragon lizards in arid Australia: the effects of rainfall. Oecologia 119, 357–366.
| Population dynamics of two species of dragon lizards in arid Australia: the effects of rainfall.Crossref | GoogleScholarGoogle Scholar | 28307758PubMed |
Doherty, T. S. (2015). Dietary overlap between sympatric dingoes and feral cats at a semiarid rangeland site in Western Australia. Australian Mammalogy 37, 219–224.
| Dietary overlap between sympatric dingoes and feral cats at a semiarid rangeland site in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Doherty, T. S., Davis, N. E., Dickman, C. R., Forsyth, D. M., Letnic, M., Nimmo, D. G., Palmer, R., Ritchie, E. G., Benshemesh, J., Edwards, G., and Lawrence, J. (2019). Continental patterns in the diet of a top predator: Australia’s dingo. Mammal Review 49, 31–44.
| Continental patterns in the diet of a top predator: Australia’s dingo.Crossref | GoogleScholarGoogle Scholar |
Department of Environment and Science (DES) (2019). SILO (Scientific Information for Land Owners) database. Available at http://www.longpaddock.qld.gov.au/silo [accessed 30 May 2019].
Fancourt, B. A., Hawkins, C. E., Cameron, E. Z., Jones, M. E., and Nicol, S. C. (2015). Devil declines and catastrophic cascades: is mesopredator release of feral cats inhibiting recovery of the eastern quoll? PLoS One 10, e0119303.
| Devil declines and catastrophic cascades: is mesopredator release of feral cats inhibiting recovery of the eastern quoll?Crossref | GoogleScholarGoogle Scholar | 25760348PubMed |
Fancourt, B. A., Cremasco, P., Wilson, C., and Gentle, M. N. (2019). Do introduced apex predators suppress introduced mesopredators? A multiscale spatiotemporal study of dingoes and feral cats in Australia suggests not. Journal of Applied Ecology , 1–12.
| Do introduced apex predators suppress introduced mesopredators? A multiscale spatiotemporal study of dingoes and feral cats in Australia suggests not.Crossref | GoogleScholarGoogle Scholar |
Fensham, R., and Fairfax, R. (2008). Water-remoteness for grazing relief in Australian arid-lands. Biological Conservation 141, 1447–1460.
| Water-remoteness for grazing relief in Australian arid-lands.Crossref | GoogleScholarGoogle Scholar |
Figueirido, B., and Janis, C. M. (2011). The predatory behaviour of the thylacine: Tasmanian tiger or marsupial wolf? Biology Letters 7, 937–940.
| The predatory behaviour of the thylacine: Tasmanian tiger or marsupial wolf?Crossref | GoogleScholarGoogle Scholar | 21543392PubMed |
Finlayson, H. H. (1961). ‘On Central Australian Mammals: The Distribution and Status of Central Australian Species.’ (South Australian Museum: Adelaide.)
Fisher, D. O., Blomberg, S. P., and Hoyle, S. D. (2001). Mechanisms of drought-induced population decline in an endangered wallaby. Biological Conservation 102, 107–115.
| Mechanisms of drought-induced population decline in an endangered wallaby.Crossref | GoogleScholarGoogle Scholar |
Fisher, D. O., Johnson, C. N., Lawes, M. J., Fritz, S. A., McCallum, H., Blomberg, S. P., VanDerWal, J., Abbott, B., Frank, A., Legge, S., and Letnic, M. (2014). The current decline of tropical marsupials in Australia: is history repeating? Global Ecology and Biogeography 23, 181–190.
| The current decline of tropical marsupials in Australia: is history repeating?Crossref | GoogleScholarGoogle Scholar |
Fleming, P. J., Allen, B. L., and Ballard, G. A. (2013). Cautionary considerations for positive dingo management: a response to the Johnson and Ritchie critique of Fleming et al. (2012). Australian Mammalogy 35, 15–22.
| Cautionary considerations for positive dingo management: a response to the Johnson and Ritchie critique of Fleming et al. (2012).Crossref | GoogleScholarGoogle Scholar |
Gibson, D. F., Lundie-Jenkins, G., Langford, D. G., Cole, J. R., Clarke, D. E., and Johnson, K. A. (1994). Predation by feral cats, Felis catus, on the rufous hare-wallaby, Lagorchestes hirsutus, in the Tanami Desert. Australian Mammalogy 17, 103–107.
Glen, A. S. (2012). Enough dogma: seeking the middle ground on the role of dingoes. Current Zoology 58, 856–858.
| Enough dogma: seeking the middle ground on the role of dingoes.Crossref | GoogleScholarGoogle Scholar |
Greenville, A. C., Wardle, G. M., and Dickman, C. R. (2013). Extreme rainfall events predict irruptions of rat plagues in central Australia. Austral Ecology 38, 754–764.
| Extreme rainfall events predict irruptions of rat plagues in central Australia.Crossref | GoogleScholarGoogle Scholar |
Greenville, A. C., Wardle, G. M., Tamayo, B., and Dickman, C. R. (2014). Bottom-up and top-down processes interact to modify intraguild interactions in resource-pulse environments. Oecologia 175, 1349–1358.
| Bottom-up and top-down processes interact to modify intraguild interactions in resource-pulse environments.Crossref | GoogleScholarGoogle Scholar | 24908053PubMed |
Greenville, A. C., Wardle, G. M., and Nguyen, V. (2016). Spatial and temporal synchrony in reptile population dynamics in variable environments. Oecologia 182, 475–485.
| Spatial and temporal synchrony in reptile population dynamics in variable environments.Crossref | GoogleScholarGoogle Scholar | 27337964PubMed |
Greer, A. E. (1989). ‘The Biology and Evolution of Australian Lizards.’ (Surrey Beatty and Sons: Sydney.)
Hayward, M. W., and Kerley, G. I. (2009). Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes? Biological Conservation 142, 1–13.
| Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes?Crossref | GoogleScholarGoogle Scholar |
Hayward, M. W., Moseby, K., and Read, J. L. (2014). The role of predator exclosures in the conservation of Australian fauna. In ‘Carnivores of Australia: Past, Present and Future’. (Eds A. Glen, and C. Dickman.) pp. 355–371. (CSIRO Publishing: Melbourne.)
James, C. D., Landsberg, J., and Morton, S. R. (1999). Provision of watering points in the Australian arid zone: a review of effects on biota. Journal of Arid Environments 41, 87–121.
| Provision of watering points in the Australian arid zone: a review of effects on biota.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. N. (2006). ‘Australia’s Mammal Extinctions: a 50,000 Year History.’ (Cambridge University Press: Melbourne, Australia.)
Johnson, C. N., and Isaac, J. L. (2009). Body mass and extinction risk in Australian marsupials: the ‘Critical Weight Range’ revisited. Austral Ecology 34, 35–40.
| Body mass and extinction risk in Australian marsupials: the ‘Critical Weight Range’ revisited.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. N., Isaac, J. L., and Fisher, D. O. (2007). Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proceedings of the Royal Society, Biological Sciences Series B 274, 341–346.
| Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia.Crossref | GoogleScholarGoogle Scholar |
Johnson, K. A. (2013). Bilby, Macrotis lagotis. In ‘Field Companion to the Mammals of Australia’. (Eds S. Van Dyck, I. Gynther, and A. Baker.) p. 73. (New Holland: Chatswood, New South Wales.)
Kerle, J. A., Foulkes, J. N., Kimber, R. G., and Papenfus, D. (1992). The decline of the brushtail possum, Trichosurus vulpecula (Kerr 1798), in arid Australia. The Rangeland Journal 14, 107–127.
| The decline of the brushtail possum, Trichosurus vulpecula (Kerr 1798), in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., and Dickman, C. R. (2006). Boom means bust: interactions between the El Niño/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia. Biodiversity and Conservation 15, 3847–3880.
| Boom means bust: interactions between the El Niño/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., and Dickman, C. R. (2010). Resource pulses and mammalian dynamics: conceptual models for hummock grasslands and other Australian desert habitats. Biological Reviews 85, 501–521.
| 20015313PubMed |
Letnic, M., Ritchie, E. G., and Dickman, C. R. (2012). Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study. Biological Reviews 87, 390–413.
| Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study.Crossref | GoogleScholarGoogle Scholar | 22051057PubMed |
Letnic, M., Story, P., Story, G., Field, J., Brown, O., and Dickman, C. R. (2011). Resource pulses, switching trophic control, and the dynamics of small mammal assemblages in arid Australia. Journal of Mammalogy 92, 1210–1222.
| Resource pulses, switching trophic control, and the dynamics of small mammal assemblages in arid Australia.Crossref | GoogleScholarGoogle Scholar |
McKenzie, N. L., Burbidge, A. A., Baynes, A., Brereton, R. N., Dickman, C. R., and Gordon, G. (2007). Analysis of factors implicated in the recent decline of Australia’s mammal fauna. Journal of Biogeography 34, 597–611.
| Analysis of factors implicated in the recent decline of Australia’s mammal fauna.Crossref | GoogleScholarGoogle Scholar |
McRae, P. (2004). Aspects of the ecology of the greater bilby, Macrotis lagotis, in Queensland. M.Sc. Thesis, University of Sydney.
Mitchell, C. (2015). An aerial survey of burrows of the greater bilby (Macrotis lagotis) in Astrebla – a preliminary report of the survey conducted during September 2015. Unpublished report. Queensland Parks and Wildlife Service, Diamantina National Park.
Morton, S. R., Smith, D. S., Dickman, C. R., Dunkerley, D. L., Friedel, M. H., McAllister, R. R. J., Reid, J. R. W., Roshier, D. A., Smith, M. A., Walsh, F. J., and Wardle, G. M. (2011). A fresh framework for the ecology of arid Australia. Journal of Arid Environments 75, 313–329.
| A fresh framework for the ecology of arid Australia.Crossref | GoogleScholarGoogle Scholar |
Moseby, K. E., Neilly, H., Read, J. L., and Crisp, H. A. (2012). Interactions between a top order predator and exotic mesopredators in the Australian rangelands. International Journal of Ecology , .
| Interactions between a top order predator and exotic mesopredators in the Australian rangelands.Crossref | GoogleScholarGoogle Scholar |
Newsome, A. (1975). An ecological comparison of the two arid-zone kangaroos of Australia, and their anomalous prosperity since the introduction of ruminant stock to their environment. The Quarterly Review of Biology 50, 389–424.
| An ecological comparison of the two arid-zone kangaroos of Australia, and their anomalous prosperity since the introduction of ruminant stock to their environment.Crossref | GoogleScholarGoogle Scholar | 1221459PubMed |
Newsome, A. E., Catling, P. C., and Corbett, L. K. (1983). The feeding ecology of the dingo. II. Dietary and numerical relationships with fluctuating prey populations in south-eastern Australia. Austral Ecology 8, 345–366.
| The feeding ecology of the dingo. II. Dietary and numerical relationships with fluctuating prey populations in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Newsome, A. E., and Corbett, L. K. (1975). Outbreaks of rodents in semi-arid and arid Australia: causes, preventions, and evolutionary considerations. In ‘Rodents in Desert Environments. Monographiae Biologicae, vol 28.’ (Eds I. Prakash and P. K. Ghosh) pp. 117–153. (Springer: Dordrecht.)
Newsome, T. M., Ballard, G. A., Crowther, M. S., Dellinger, J. A., Fleming, P. J. S., and Glen, A. S. (2015). Resolving the value of the dingo in ecological restoration. Restoration Ecology 23, 201–208.
| Resolving the value of the dingo in ecological restoration.Crossref | GoogleScholarGoogle Scholar |
Oakwood, M. (2000). Reproduction and demography of the northern quoll, Dasyurus hallucatus, in the lowland savanna of northern Australia. Australian Journal of Zoology 48, 519–539.
| Reproduction and demography of the northern quoll, Dasyurus hallucatus, in the lowland savanna of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Orr, D. M. (1975). A review of Astrebla (Mitchell grass) pastures in Australia. Tropical Grasslands 9, 21–36.
Palmer, R. (1999). The ecology of feral cats in the mid-reaches of the Diamantina River in far western Queensland. Report, University of Queensland.
Paltridge, R. (2002). The diets of cats, foxes and dingoes in relation to prey availability in the Tanami Desert, Northern Territory. Wildlife Research 29, 389–403.
| The diets of cats, foxes and dingoes in relation to prey availability in the Tanami Desert, Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Pavey, C. R., Eldridge, S. R., and Heywood, M. (2008). Population dynamics and prey selection of native and introduced predators during a rodent outbreak in arid Australia. Journal of Mammalogy 89, 674–683.
| Population dynamics and prey selection of native and introduced predators during a rodent outbreak in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Pavey, C. R., and Nano, C. E. (2013). Changes in richness and abundance of rodents and native predators in response to extreme rainfall in arid Australia. Austral Ecology 38, 777–785.
| Changes in richness and abundance of rodents and native predators in response to extreme rainfall in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Pavey, C. R., Cole, J. R., McDonald, P. J., and Nano, C. E. (2014). Population dynamics and spatial ecology of a declining desert rodent, Pseudomys australis: the importance of refuges for persistence. Journal of Mammalogy 95, 615–625.
| Population dynamics and spatial ecology of a declining desert rodent, Pseudomys australis: the importance of refuges for persistence.Crossref | GoogleScholarGoogle Scholar |
Pettigrew, J. D. (1993). A burst of feral cats in the Diamantina: a lesson for the management of pest species? In ‘Cat Management Workshop Proceedings’. (Eds G. Siepen, and C. Owens.) pp. 25–32. (Queensland Department of Environment and Heritage: Brisbane.)
Phelps, D. (2007). Sustainable grazing in the Channel Country floodplains (Phase 2): a technical report on findings between March 2003 and June 2006.
Phelps, D. G. (2014). How unique is the channel country? In ‘A Climate of Change in the Rangelands. Proceedings of the 15th Australian Rangeland Society Biennial Conference’. (Ed. D. Orr). pp. 421–422. (Australian Rangeland Society: Australia.)
Predavec, M., and Dickman, C. R. (1994). Population dynamics and habitat use of the rat (Rattus villosissimus) in south-western Queensland. Wildlife Research 21, 1–9.
| Population dynamics and habitat use of the rat (Rattus villosissimus) in south-western Queensland.Crossref | GoogleScholarGoogle Scholar |
Read, J. L., Kovac, K. J., Brook, B. W., and Fordham, D. A. (2012). Booming during a bust: asynchronous population responses of arid zone lizards to climatic variables. Acta Oecologica 40, 51–61.
| Booming during a bust: asynchronous population responses of arid zone lizards to climatic variables.Crossref | GoogleScholarGoogle Scholar |
Rich, M. (2018). Astrebla Downs National Park: Preliminary 1080 baiting report, October 2018. Department of Environment and Science, Queensland Government.
Rich, M., Nolan, B., Speed, J., and Gentle, M. (2014). Lessons in feral cat control – can adaptive management provide the solution? In ‘16th Australasian Pest Conference, Brisbane, Qld’. (Ed. M. Gentle). p. 43. [Abstract.] (Biosecurity Queensland: Brisbane.)
Robertshaw, J. D., and Harden, R. H. (1986). The ecology of the dingo in northeastern New South Wales. 4. Prey selection by dingoes, and its effect on the major prey species, the swamp wallaby, Wallabia bicolor (Desmarest). Wildlife Research 13, 141–163.
| The ecology of the dingo in northeastern New South Wales. 4. Prey selection by dingoes, and its effect on the major prey species, the swamp wallaby, Wallabia bicolor (Desmarest).Crossref | GoogleScholarGoogle Scholar |
Saunders, G., and McLeod, L. (2007). ‘Improving Fox Management Strategies in Australia.’ (Bureau of Rural Sciences: Canberra.)
Short, J., Kinnear, J. E., and Robley, A. (2002). Surplus killing by introduced predators in Australia – evidence for ineffective anti-predator adaptations in native prey species? Biological Conservation 103, 283–301.
| Surplus killing by introduced predators in Australia – evidence for ineffective anti-predator adaptations in native prey species?Crossref | GoogleScholarGoogle Scholar |
Smith, A. P., and Quin, D. G. (1996). Patterns and causes of extinction and decline in Australian conilurine rodents. Biological Conservation 77, 243–267.
| Patterns and causes of extinction and decline in Australian conilurine rodents.Crossref | GoogleScholarGoogle Scholar |
Southgate, R., Paltridge, R., Masters, P., and Ostendorf, B. (2007). Modelling introduced predator and herbivore distribution in the Tanami Desert, Australia. Journal of Arid Environments 68, 438–464.
| Modelling introduced predator and herbivore distribution in the Tanami Desert, Australia.Crossref | GoogleScholarGoogle Scholar |
Steindler, L., Blumstein, D., West, R., Moseby, K., and Letnic, M. (2018). Discrimination of introduced predators by ontogenetically naïve prey scales with duration of shared evolutionary history. Animal Behaviour 137, 133–139.
| Discrimination of introduced predators by ontogenetically naïve prey scales with duration of shared evolutionary history.Crossref | GoogleScholarGoogle Scholar |
Stokeld, D., Fisher, A., Gentles, T., Hill, B., Triggs, B., Woinarski, J. C., and Gillespie, G. R. (2018). What do predator diets tell us about mammal declines in Kakadu National Park? Wildlife Research 45, 92–101.
| What do predator diets tell us about mammal declines in Kakadu National Park?Crossref | GoogleScholarGoogle Scholar |
Tatler, J., Prowse, T. A., Roshier, D. A., Allen, B. L., and Cassey, P. (2019). Resource pulses affect prey selection and reduce dietary diversity of dingoes in arid Australia. Mammal Review 49, 263–275.
| Resource pulses affect prey selection and reduce dietary diversity of dingoes in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Thomson, P. C. (1992). The behavioural ecology of dingoes in north-western Australia. III. Hunting and feeding behaviour, and diet. Wildlife Research 19, 531–541.
| The behavioural ecology of dingoes in north-western Australia. III. Hunting and feeding behaviour, and diet.Crossref | GoogleScholarGoogle Scholar |
Triggs, B. E. (1996). ‘Tracks, Scats and Other Traces.’ (Oxford University Press: Melbourne.)
Triggs, B., and Brunner, H. (2002). ‘Hair ID. An Interactive Tool for Identifying Australian Mammal Hair.’ CD-ROM. (CSIRO Publishing: Melbourne.)
van Eeden, L. M., Dickman, C. R., Crowther, M. S., and Newsome, T. M. (2019). A snapshot of changes in graziers’ management and attitudes towards dingoes over 60 years. Pacific Conservation Biology 25, 413–420.
| A snapshot of changes in graziers’ management and attitudes towards dingoes over 60 years.Crossref | GoogleScholarGoogle Scholar |
Wallach, A. D., Ritchie, E. G., Read, J., and O’Neill, A. J. (2009). More than mere numbers: the impact of lethal control on the social stability of a top-order predator. PLoS One 4, .
| More than mere numbers: the impact of lethal control on the social stability of a top-order predator.Crossref | GoogleScholarGoogle Scholar | 19724642PubMed |
Watts, C. H., and Aslin, H. J. (1981). ‘The Rodents of Australia.’ (Angus and Robertson.)
Woinarski, J. C. Z., Burbidge, A. A., and Harrison, P. L. (2014). ‘The Action Plan for Australian Mammals 2012.’ (CSIRO Publishing: Melbourne.)
Wynen, E. (2006). Organic beef production and marketing in Australia. Journal of Organic Systems 1, .
Yip, S. J., Rich, M. A., and Dickman, C. R. (2015). Diet of the feral cat, Felis catus, in central Australian grassland habitats during population cycles of its principal prey. Mammal Research 60, 39–50.
| Diet of the feral cat, Felis catus, in central Australian grassland habitats during population cycles of its principal prey.Crossref | GoogleScholarGoogle Scholar |