The myth of wild dogs in Australia: are there any out there?
Kylie M. Cairns A B E , Mathew S. Crowther
A B E , Mathew S. Crowther  C , Bradley Nesbitt D and Mike Letnic A B
C , Bradley Nesbitt D and Mike Letnic A B
A Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia.
B Evolution and Ecology Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia.
C School of Life and Environmental Sciences, University of Sydney, NSW 2006, Australia.
D School of Environmental and Rural Science, University of New England, Armidale, NSW 2350, Australia.
E Corresponding author. Email: k.cairns@unsw.edu.au
Australian Mammalogy 44(1) 67-75 https://doi.org/10.1071/AM20055
Submitted: 17 August 2020 Accepted: 26 February 2021 Published: 26 March 2021
Journal Compilation © Australian Mammal Society 2022 Open Access CC BY NC ND
Abstract
Hybridisation between wild and domestic canids is a global conservation and management issue. In Australia, dingoes are a distinct lineage of wild-living canid with a controversial domestication status. They are mainland Australia’s apex terrestrial predator. There is ongoing concern that the identity of dingoes has been threatened from breeding with domestic dogs, and that feral dogs have established populations in rural Australia. We collate the results of microsatellite DNA testing from 5039 wild canids to explore patterns of domestic dog ancestry in dingoes and observations of feral domestic dogs across the continent. Only 31 feral dogs were detected, challenging the perception that feral dogs are widespread in Australia. First generation dingo × dog hybrids were similarly rare, with only 27 individuals identified. Spatial patterns of genetic ancestry across Australia identified that dingo populations in northern, western and central Australia were largely free from domestic dog introgression. Our findings challenge the perception that dingoes are virtually extinct in the wild and that feral dogs are common. A shift in terminology from wild dog to dingo would better reflect the identity of these wild canids and allow more nuanced debate about the balance between conservation and management of dingoes in Australia.
Keywords: admixture, Australia, Canis dingo, Canis familiaris, dingo, dog, domestication, feral dog, introgression, wild dog.
Introduction
The occurrence of feral domestic dogs is rare, and distinct from the close to a billion free-breeding or village dogs that exist globally (Gompper 2013; Pilot et al. 2015). Free-breeding or village dogs are those that live in and around human settlements, rely upon anthropogenic food or water sources, breed freely with each other, and are not owned or cared for by people (Hughes and Macdonald 2013). Feral dogs are those that are living in a wild state not in the vicinity of human settlements: they may be escaped pets or self-sustaining populations. Empirical data from remote living free-breeding dog populations suggests these populations rely upon recruitment from stray or owned dogs because their reproductive success is low, i.e. pups rarely survive past 1 year (Boitani et al. 1995, 2006). The only acknowledged example of a true wild-living self-sustaining feral dog population occurred in the Galápagos; it was founded by a variety of breeds in the 1800s and persisted until the 1980s (Barnett 1986; Reponen et al. 2014). Despite there being a robust population of free-breeding or stray dogs associated with towns in the Galápagos, there was limited evidence of mixing between the free-breeding dog and feral dog populations based on genetic analysis (Reponen et al. 2014). The Galápagos feral dog population was eradicated using 1080 poisoning of water and meat baits in the 1980s (Barnett 1986) and the population did not re-establish despite the presence of free-breeding and pet dogs in nearby human settlements.
Dingoes, including New Guinea singing dogs, form a discrete lineage from Eurasian and modern domestic dogs (Bergström et al. 2020; Surbakti et al. 2020; Cairns 2021). Their domestication status and taxonomic nomenclature is disputed, with some considering them Canis familiaris, a feral domestic dog (Jackson et al. 2017, 2019) and others calling them Canis dingo, a wild protodog (Crowther et al. 2014; Smith et al. 2019; Zhang et al. 2020). Globally, most free-breeding, village and breed dogs fall within the modern domestic dog lineage (Bergström et al. 2020; Surbakti et al. 2020; Cairns 2021). A close relationship between dingoes, Asian wolves, and some East Asian dogs has been observed, suggesting the dingoes’ ancestor was of Asian origin (vonHoldt et al. 2010; Oskarsson et al. 2011; Freedman et al. 2014; Surbakti et al. 2020; Zhang et al. 2020; Cairns 2021). Dingoes and New Guinea singing dogs are examples of true wild-living dogs that are not reliant on artificial water or food sources. Dingoes fill the ecological role of terrestrial apex predator on mainland Australia (Newsome et al. 2001; Letnic and Koch 2010; Letnic et al. 2012; Letnic et al. 2013; Morrant et al. 2017). Molecular dating indicates that dingoes and New Guinea singing dogs diverged from their ancestral population approximately 8000–12 000 years ago (Cairns and Wilton 2016; Cairns et al. 2017; Zhang et al. 2020). Dingoes remained reproductively isolated from domestic dogs until 1788.
There is rising global concern about the occurrence of hybridisation between wild and domestic canids or sympatric wild canids (Gopalakrishnan et al. 2018; Salvatori et al. 2020; vonHoldt and Aardema 2020). Hybridisation is the process of interbreeding between two species or varieties, generally F1 offspring would be referred to as hybrids and the offspring of F1 hybrids with animals from a parental species or variety would be called backcrosses. Interbreeding between varieties or species results in the exchange or mixing of genetic material (genetic admixture). The transfer of genetic material from one species into another through hybridisation and backcrossing is called introgression (Harrison and Larson 2014). The occurrence of genetic admixture may be modern or historical, and in some cases is the result of anthropogenic actions. In canids, the phenomenon of interspecific introgression has been observed between species such as grey wolves and dogs (Vilà and Wayne 1999; Anderson et al. 2009; vonHoldt et al. 2011, 2016; Schweizer et al. 2018), coyotes and wolves (Bohling et al. 2016; vonHoldt et al. 2016), red wolves and coyotes (Miller et al. 2003; Adams et al. 2007; Schmutz et al. 2007; Bohling and Waits 2015), jackals and dogs (Galov et al. 2015), dingoes and dogs (Newsome and Corbett 1985; Wilton 2001; Claridge et al. 2014; Stephens et al. 2015; Cairns et al. 2019).
One of the concerns raised by hybridisation is genetic swamping, whereby the genetic identity of a population is threatened by introgression of genes from another population or species. For example, the Scottish wildcat is threatened by hybridisation and subsequent introgression from domestic cats to the extent that contemporary wildcat populations exhibit extensive levels of domestic cat ancestry (Daniels et al. 1998; Kitchener et al. 2005; Macdonald et al. 2010). Indeed, most wildcats in Scotland carry significant domestic cat ancestry, and the occurrence of hybridisation is believed to have accelerated in the last 50–100 years (Mattucci et al. 2019; Senn et al. 2019). Similar concerns have been raised in Australia with many dingoes, particularly in southeastern Australia, exhibiting genetic, morphological or phenotypic evidence of domestic dog ancestry (Newsome and Corbett 1985; Daniels and Corbett 2003; Jones 2009; Stephens et al. 2015). There is also widespread concern that feral domestic dogs have established in the wild across Australia (Fleming et al. 2001; NSW Threatened Species Scientific Committee 2009).
Since the 1980s rising concern about domestic dog ancestry and the occurrence of hybridisation events has led to shifts in the management and conservation status of dingoes but also a duality in their identity. For example, in Victoria dingoes are now listed as a threatened species, but wild dogs are listed as a declared pest, where wild dogs are defined as feral or wild populations of dogs (Canis familiaris) and dingo × dog hybrids (Canis dingo × Canis familiaris) (DEPI 2013). In New South Wales (NSW), the listing of ‘predation and hybridisation by feral dogs (Canis familiaris)’ as a key threatening process implies that dingoes are ‘under serious decline as a consequence of hybridisation’ (NSW Threatened Species Scientific Committee 2009). Indeed, there has been concern in NSW that feral dogs and dingo × dog hybrids with low levels of dingo ancestry have essentially replaced dingoes in the wild (Claridge et al. 2014; Stephens et al. 2015). For example, the NSW key threatening process determination states that ‘due to the constant influx of Domestic Dogs into natural ecosystems, lasting eradication of even local populations of Feral Dogs is difficult’ (NSW Threatened Species Scientific Committee 2009). Accordingly, the term ‘wild dog’ is now used ubiquitously by state government and pest control organisations when communicating about management programs directed at controlling wild canids (Letnic 2012; Kreplins et al. 2019). However, it is clear from social studies of public perception and also expectations about the management of dingoes vs wild dogs, that the general public believe the term wild dog refers only to feral dogs and does not properly understand that the term wild dog is defined as including dingoes, dingo × dog hybrids and feral dogs (van Eeden et al. 2020).
Before the 2000s a majority of our knowledge about dingo × dog hybridisation was based on assessment of skull morphology and physical appearance (Newsome et al. 1980; Newsome and Corbett 1985; Jones 1990; Corbett 2001; Fleming et al. 2001). A microsatellite DNA test for assessing the ancestry of dingoes was developed in 1999 (Wilton et al. 1999; Wilton 2001) and has since become widely used by wildlife managers and conservation groups. Studies assessing the reliability of morphological, physical and genetic methods of ancestry assessment in dingoes have highlighted the importance of using genetic data (Elledge et al. 2008; Parr et al. 2016). Stephens et al. (2015) undertook microsatellite DNA testing of wild canids across Australia and identified regional patterns of domestic dog introgression in dingoes, with dog ancestry particularly prevalent in southeastern Australia. However, a major limitation of Stephens et al. (2015) is the low number of samples (95) from NSW. A more detailed study using the same genetic markers and significantly higher density of sampling across northeastern NSW identified several key hotspots of high dingo ancestry (Cairns et al. 2019). Their finding that a majority of wild dingoes in NSW were pure dingoes or carried more than 75% dingo ancestry is a stark contrast to the common public perception that feral dogs are widespread and established in the wild (NSW Threatened Species Scientific Committee 2009; Claridge et al. 2014; ABC Landline 2019). Here we collate and analyse genetic ancestry data based on microsatellite DNA testing from 5039 samples to examine the occurrence of feral dogs and F1 or F2 dingo × dog hybrids across Australia. Critically, this study includes a broader set of samples from southeastern Australia including from southern NSW filling knowledge gaps about the ancestry and identity of wild canids in southeastern Australia. Spatial patterns of domestic dog introgression across Australia are also examined using the dataset. These data inform ongoing debate about the appropriate terminology and management of wild canids in Australia.
Methods and materials
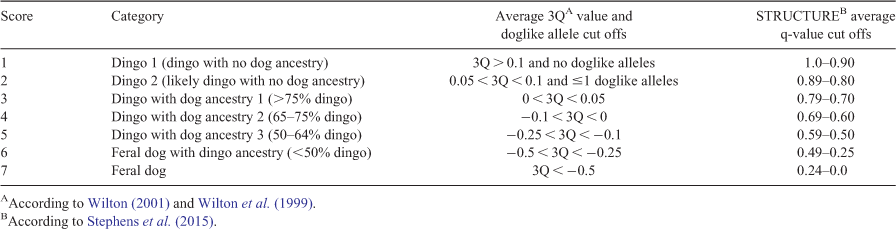
DNA testing based on a widely used 23 microsatellite marker set was used to estimate dingo ancestry in Stephens et al. (2015), Cairns et al. (2019) and a previously unpublished dataset (available in the BioStudies database under accession number S-BSST501 https://www.ebi.ac.uk/biostudies/studies/S-BSST501). Briefly, a panel of 23 microsatellites were amplified and genotyped in the wild canid samples based on the original methodology of Wilton (2001) and Elledge (2008). Ancestry modelling was performed in STRUCTURE with reference populations of known dingoes and dogs using the admixture and correlated allele frequency models. Cairns et al. (2019) and the unpublished dataset used a set of 50 dingoes and 66 mixed breed dogs as reference populations; to account for regional variation these analyses included a set of 13 wild dingoes from northern and western Australia. Stephens et al. (2015) used a set of 322 post-priori identified reference dingoes and 102 domestic dogs. In all three studies simulations were run with 200 000 iterations, a 20 000 iteration burn-in period and 10 replicates of each K = 2 was performed. Previously, modelling demonstrated that K = 2 was the appropriate model for assessing ancestry in Australian wild canids and modelling was run with the USEPOPINFO flag to allow population allele frequencies to be updated only from the defined reference population individuals (Stephens et al. 2015; Cairns et al. 2019). STRUCTURE reports estimated ancestry proportions (q-values) for each genetic cluster for each sample (Stephens et al. 2015; Cairns et al. 2019). In a K = 2 analysis each individual has a q-value for the domestic dog cluster and for the dingo cluster. The dingo cluster q-value is used to define animals as either a pure dingo, probable dingo, dingo with >75% ancestry, dingo with 65–75% ancestry, dingo with 50–65% ancestry, feral dog hybrid or feral domestic dog (Table 1; Stephens et al. 2015; Cairns et al. 2019). We define feral dog hybrids with a q-value between 0.25 and 0.49 to be possible F1 or F2 dingo × dog hybrids (Stephens et al. 2015; Cairns et al. 2019). An F1 hybrid is defined as the offspring of a dingo × a dog and an F2 hybrid is the offspring of two F1 dingo × dog hybrids.
|
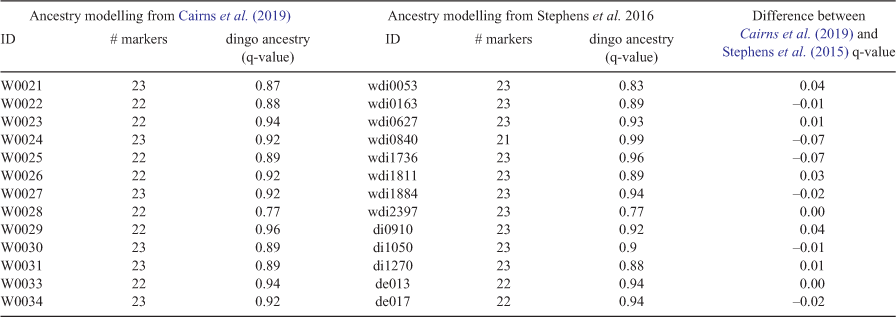
Raw microsatellite data could not be compared because of slightly different microsatellite amplification conditions. To confirm that ancestry estimates were equivalent between Cairns et al. (2019) and Stephens et al. (2015), a set of 13 wild canids were genotyped and ancestry estimates calculated by both laboratories (Table 2).
|
Between the three-studies DNA ancestry estimates from 5039 wild canids collected by trappers, wildlife managers and government agencies across public and private lands in Australia were reported. A majority of the wild canids were trapped/shot as part of broadscale wild canid management to protect livestock from predation. Samples from the three datasets were collected between 1996 and 2014. We collated these DNA ancestry estimates (STRUCTURE q-values) together with location coordinates (Supplementary Material S1 dataset).
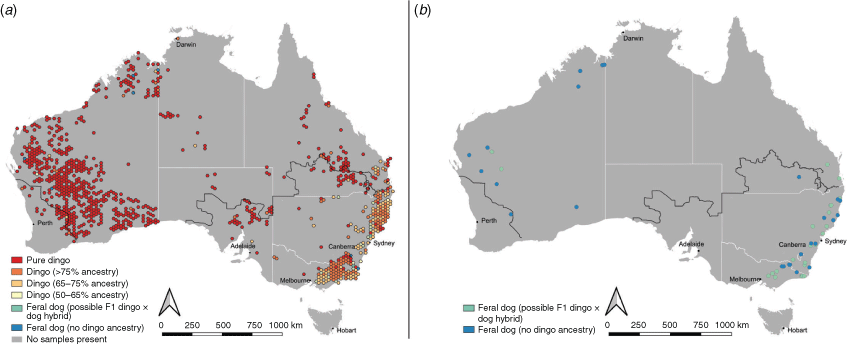
The distribution of feral dogs with no dingo ancestry and possible F1 or F2 dingo × dog hybrids was mapped using QGIS ver. 3.01 (QGIS 2020). We also explore the distribution of dingoes with varying degrees of dog ancestry across Australia as follows: the location of 5039 samples with DNA ancestry results were mapped in QGIS, a 0.3 × 0.3 degree hexagonal grid was drawn and the mean and median ‘q-score’ of the samples within each grid cell was calculated (using the join attributes by location tool). We also mapped the occurrence of feral dogs with no dingo ancestry and possible F1 or F2 dingo × dog hybrids across Australia.
Results
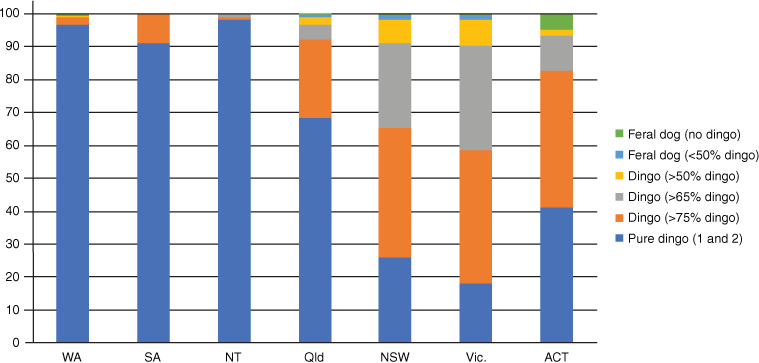
We collated the ancestry results of 3641 samples from Stephens et al. (2015), 753 samples from Cairns et al. (2019) and 611 samples from the unpublished dataset. Ancestry estimates were consistent between Stephens et al. (2015) and Cairns et al. (2019) based on comparison of results for 13 repeated samples (Table 2). Out of 5039 samples that were DNA tested the breakdown of dingo ancestry results are as follows: 33.7% pure dingoes, 30.4% probable dingoes, 19.8% greater than 75% dingo ancestry, 11.7% greater than 65% dingo ancestry and 3.2% greater than 50% dingo ancestry (Table 3). Feral dogs and F1 or F2 dingo-dog hybrids were rarely collected from the wild and made up less than 1.2% of the wild canid population (Table 3). In total, only 31 feral dogs with no evidence of dingo ancestry were observed and 27 probable F1 or F2 dingo × dog hybrids (q-value between 0.25 and 0.49) were identified in the sample. The occurrence of dog introgression differed between states (Fig. 1) and was more prevalent in southeastern Australia.

|
|
Mapping of wild canid ancestry across Australia indicates that domestic dog introgression is largely restricted to southeastern Australia (Fig. 2; Supplementary Material S2). Across northern, central and western Australia, the dingo population is genetically intact, i.e. with limited or no domestic dog introgression. In southeastern Australia there are regions with dingo populations that are genetically intact (Fig. 2) and most populations maintain a dingo-dominant identity (median ancestry is greater than 75% dingo). Feral dogs were restricted mostly to southeastern Australia and were captured in relatively close proximity to human settlements (Fig. 2). Interestingly, no feral dogs were identified in central Australia.
Discussion
Extensive DNA testing across Australia detected very few feral dogs. Out of the 5039 wild canids that were sampled just 31 (0.61%) were inferred to be feral dogs (Table 3). Similarly, there were only 27 animals identified as likely F1 or F2 dingo × dog hybrids. Contrary to widespread understanding, our results show that feral dogs and feral dog × dingo hybrids were very rare across mainland Australia. This suggests that feral dogs have not established a self-sustaining population in the wild and that inter-breeding between dingoes and dogs may occur infrequently.
Our finding that feral dogs were rare and are unlikely to have established a self-sustaining population on mainland Australia is backed up by the rarity of true feral dog populations globally. In Tasmania, there is a similar mix of European derived dog breeds to mainland Australia and a similar environment to southeastern Australia but there is little evidence that a feral dog population has become established (DPIPWE 2013). The rarity of true feral dogs both globally and in Australia suggests that domestic dogs have not retained the ability to persist in the wild in the absence of anthropogenic derived resources. Indeed, the Galápagos remains an isolated exemplar of domestic dogs establishing a feral population (Barnett 1986; Reponen et al. 2014). However, free breeding (‘village or camp dogs’) that rely on anthropogenic food and water sources are of widespread occurrence in many regions of the world (Gompper 2013; Home et al. 2018). In Australia, free-breeding dogs are largely restricted to the fringes of Indigenous communities (Collins and Mills 2013; Newsome et al. 2013, 2014; Hudson et al. 2018; Brookes et al. 2020; Ma et al. 2020).
Our collation of DNA ancestry testing results suggests that most wild canids in Australia are pure dingoes (Fig. 1). Dog introgression is uncommon in Western Australia, the Northern Territory and South Australia, with more than of 90% of dingoes tested in those states being pure dingoes. Of the dingoes which did show domestic dog introgression, most carried more than 75% dingo ancestry. No F1 or F2 dingo × dog hybrids were observed in the Northern Territory or South Australia. In Queensland, 68.5% of wild canids were pure dingoes and a majority of the remaining carried greater than 75% dingo ancestry. Only 7% of wild canids in Queensland were less than 65% dingo, and 0.8% were feral dogs or possible F1 or F2 dingo × domestic dog hybrids.
As highlighted by Stephens et al. (2015), dog introgression is most widespread in southeastern Australia (Fig. 2). Despite this, the occurrence of possible F1 or F2 dingo × dog hybrids was low in southeastern Australia, making up less than 2% of the total population in these regions. It may be that dingo × dog hybridisation events are rare in the wild or that the survival of wild canids with less than 50% dingo ancestry is poor. The widespread introgression of dog genes in southeastern Australia may reflect backcrossing of F1 dingo × dog hybrids, facilitating the spread of dog genes into the wider dingo population over long periods of time rather than a high occurrence of dingo × dog hybridisation in the wild. As emphasised by Stephens et al. (2015), dog introgression in dingoes may be more common in southeastern Australia due to the earlier and more intensive European settlement, resulting in increased contact between domestic dogs and dingoes in these regions. Cairns et al. (2019) added that the widespread occurrence of intensive lethal control, particularly aerial baiting, may increase the likelihood of dingo × dog hybridisation by fracturing dingo social structures. Although this admixture from dogs into the dingo population is a concern, it is important to note that the dingo population still maintains a genetically and morphologically dingo dominant identity (Parr et al. 2016; Cairns et al. 2019; Crowther et al. 2020).
There are several key knowledge gaps about the identity of dingoes in Australia that bear consideration. First, we still lack information about the genetic identity of dingoes across large regions of Australia, particularly central and northern Australia (Fig. 2). Morphological research about the phenotype of dingoes with low levels of dog ancestry may assist on-ground management and conservation efforts, particularly if distinguishing features could be identified. Management plans should be careful not to assume that a given population does or does not carry domestic dog ancestry without the necessary genetic data. Despite this, the broad pattern of dingo ancestry across Australia suggests that in western, central and northern Australia, dog introgression is likely to be limited and feral dogs extremely rare. There is some concern that current microsatellite testing methods may be biased by regional genetic variation within dingoes (Cairns et al. 2017, 2019). It is important to consider that ancestry testing methods rely on the assumption that dingoes form a single homogeneous population (Elledge et al. 2008; Stephens et al. 2015; Cairns et al. 2019), an assumption we now know to be false (Cairns and Wilton 2016; Cairns et al. 2018; Koungoulos 2020). Robust dingo ancestry assessments require broad sampling across Australia to capture regional genetic variation (Cairns et al. 2019). Possibly some dingoes are misclassified as hybrids because of regional variation. As argued by Cairns et al. (2019), the type and number of genetic markers limits accuracy of genetic testing and estimates based on 23 microsatellites may not reflect genome-wide ancestry. Genome-wide SNP genotyping may offer a cost-effective and high-throughput alternative to address the limitations of microsatellite genetic testing in the future. Thus, we caution managers and researchers to evaluate the reliability of ancestry estimates and urge end-users to explore technology improvements for ancestry testing into the future.
There has been ongoing debate about the appropriate terminology for wild canids in Australia, i.e. dingo or wild dog (Letnic 2012). Kreplins et al. (2019) found that the term wild dog was more commonly used in studies funded by livestock industry organisations, compared to conservation-based studies which predominately used the term dingo. van Eeden et al. (2020) studied public understanding of the terms dingo and wild dog. They found that only 19.1% of respondents were aware that wild dog control programs targeted dingoes and furthermore respondents were generally not supportive of lethal dingo management. At the 2019 Royal Zoological Society of NSW symposium titled ‘Dingo Dilemma’ there was strong opposition to the term wild dog, with many participants asserting that the term wild dog disguises lethal management practices on dingoes from the public and hinders debate about dingo management in Australia (Dickman 2019). We add that the term wild dog does not accurately represent the ancestry of wild canids in Australia, particularly as the dominant genetic identity is dingo and feral domestic dogs are virtually absent from the landscape (Fig. 2). Although there are dingoes carrying domestic dog ancestry, particularly in southeastern Australia, there are few F1 or F2 hybrids. The term hybrid generally refers to only F1 crosses, i.e. the offspring of a dingo and domestic dog but F2 animals which are the offspring of two F1 hybrids may also be referred to as hybrids (Hansson et al. 2012). We suggest that dingoes that carry domestic dog ancestry but are not F1/F2 hybrids should be referred to as dingo backcrosses or admixed dingoes.
The finding that feral dogs have not established populations has implications for the management of wild canids in Australia. Dingoes and stray or roaming domestic dogs can cause serious impacts for livestock graziers (Fleming et al. 2001). Management of feral, stray or roaming domestic dogs should focus on responsible pet ownership including spaying and neutering of pet animals, keeping pet and working dogs under control and confined during the night. As feral dogs do not represent a significant portion of the wild canid population, it should be clear in legislation and policy that lethal control programs are targeting dingoes (including admixed dingoes) rather than feral dogs. Although hybridisation is a concern in southeastern Australia (Stephens et al. 2015; Cairns et al. 2019), responsible pet ownership and continued exclusion of domestic dogs from National Parks and conservation areas can reduce the occurrence of future dingo × dog hybridisation events. The low number of F1 or F2 hybrids detected indicates that dingo × dog hybridisation events are uncommon. Despite historical domestic dog introgression, the dingo population maintains a dingo dominant identity, even in southeastern Australia (Fig. 1). It is possible that widespread lethal control programs have increased the likelihood of dingo × dog hybridisation events and facilitating the spread of introgressed dog genes into the wider dingo population. Lethal control has been identified as a factor increasing the likelihood of interspecific hybridisation in other wild canids including coyotes and red wolves by fracturing social structures and altering demographic patterns (Bohling and Waits 2015). Management programs that maintain stable dingo social structures present a better balance to managing the risks to stock predation and dingo conservation (Allen 2014, 2015; Wallach et al. 2017). Additionally, lethal control programs should not occur during the dingo breeding season (winter) as this may facilitate dingo × dog hybridisation events, by fracturing pack structures and reducing the availability of dingo mates. Baiting has also been linked to an increase in the body-size of dingoes, possibly increasing their impact on livestock (Letnic and Crowther 2020).
The lack of public engagement and debate on dingo conservation on private and public lands in Australia has allowed agricultural industry priorities to dominate government policy and decision making on dingo management. Social science studies show the general public are largely unaware of the current threats wild dog control programs have on the remaining dingo populations in Australia (van Eeden et al. 2020). The lack of engagement by the public on dingo conservation can in part be attributed to the renaming of the Australian dingo as a wild dog in government literature and allowing the general misunderstanding that all wild dogs are feral dogs to persist. We propose that a terminology shift is required to reflect the identity of wild canids in Australia: the term dingo needs to be reinstated because genetic testing demonstrates that a majority of animals are of high dingo ancestry and feral dogs are virtually absent. The term wild dog does not reflect the ancestry of wild canids in Australia and is poorly understood by the public, it should be retired from use.
Conflicts of interest
KMC is a scientific advisor to the Australia Dingo Foundation, New Guinea Singing Dog Conservation Society and New Guinea Highland Wild Dog Foundation.
Declaration of funding
KMC is supported by research funding from the Australian Dingo Foundation.
Acknowledgements
The authors thank Barry Traill, Angus Emmott and David Pollock for the impetus to write this paper following discussions at the RZS Dingo Dilemma Symposium in 2019. We acknowledge the extensive work done by the late Associate Professor Alan Wilton on this topic. The authors would also like to thank two anonymous reviewers and the editor for their careful reading of our manuscript and insightful comments that have improved this manuscript.
References
ABC Landline (2019). Wild Dogs. In ‘Meet the ferals’. (Ed. P Adams) Available at https://iview.abc.net.au/show/meet-the-feralsAdams, J. R., Lucash, C., Schutte, L., and Waits, L. P. (2007). Locating hybrid individuals in the red wolf (Canis rufus) experimental population area using a spatially targeted sampling strategy and faecal DNA genotyping. Molecular Ecology 16, 1823–1834.
| Locating hybrid individuals in the red wolf (Canis rufus) experimental population area using a spatially targeted sampling strategy and faecal DNA genotyping.Crossref | GoogleScholarGoogle Scholar | 17444895PubMed |
Allen, L. R. (2014). Wild dog control impacts on calf wastage in extensive beef cattle enterprises. Animal Production Science 54, 214–220.
| Wild dog control impacts on calf wastage in extensive beef cattle enterprises.Crossref | GoogleScholarGoogle Scholar |
Allen, L. R. (2015). Demographic and functional responses of wild dogs to poison baiting. Ecological Management & Restoration 16, 58–66.
| Demographic and functional responses of wild dogs to poison baiting.Crossref | GoogleScholarGoogle Scholar |
Anderson, T. M., vonHoldt, B. M., Candille, S. I., Musiani, M., Greco, C., Stahler, D. R., Smith, D. W., Padhukasahasram, B., Randi, E., Leonard, J. A., Bustamante, C. D., Ostrander, E. A., Tang, H., Wayne, R. K., and Barsh, G. S. (2009). Molecular and evolutionary history of melanism in North American gray wolves. Science 323, 1339–1343.
| Molecular and evolutionary history of melanism in North American gray wolves.Crossref | GoogleScholarGoogle Scholar | 19197024PubMed |
Barnett, B. D. (1986). Eradication and control of feral and free-ranging dogs in the Galapagos Islands. In ‘Proceedings of the Twelfth Vertebrate Pest Conference’. Available at https://digitalcommons.unl.edu/vpc12/8
Bergström, A., Frantz, L., Schmidt, R., Ersmark, E., Lebrasseur, O., Girdland-Flink, L., Lin, A. T., Storå, J., Sjögren, K.-G., Anthony, D., Antipina, E., Amiri, S., Bar-Oz, G., Bazaliiskii, V. I., Bulatović, J., Brown, D., Carmagnini, A., Davy, T., Fedorov, S., Fiore, I., Fulton, D., Germonpré, M., Haile, J., Irving-Pease, E. K., Jamieson, A., Janssens, L., Kirillova, I., Horwitz, L. K., Kuzmanovic-Cvetković, J., Kuzmin, Y., Losey, R. J., Dizdar, D. L., Mashkour, M., Novak, M., Onar, V., Orton, D., Pasarić, M., Radivojević, M., Rajković, D., Roberts, B., Ryan, H., Sablin, M., Shidlovskiy, F., Stojanović, I., Tagliacozzo, A., Trantalidou, K., Ullén, I., Villaluenga, A., Wapnish, P., Dobney, K., Götherström, A., Linderholm, A., Dalén, L., Pinhasi, R., Larson, G., and Skoglund, P. (2020). Origins and genetic legacy of prehistoric dogs. Science 370, 557.
| Origins and genetic legacy of prehistoric dogs.Crossref | GoogleScholarGoogle Scholar | 33122379PubMed |
Bohling, J. H., and Waits, L. P. (2015). Factors influencing red wolf–coyote hybridization in eastern North Carolina, USA. Biological Conservation 184, 108–116.
| Factors influencing red wolf–coyote hybridization in eastern North Carolina, USA.Crossref | GoogleScholarGoogle Scholar |
Bohling, J. H., Dellinger, J., McVey, J. M., Cobb, D. T., Moorman, C. E., and Waits, L. P. (2016). Describing a developing hybrid zone between red wolves and coyotes in eastern North Carolina, USA. Evolutionary Applications 9, 791–804.
| Describing a developing hybrid zone between red wolves and coyotes in eastern North Carolina, USA.Crossref | GoogleScholarGoogle Scholar | 27330555PubMed |
Boitani, L., Francisci, F., Ciucci, P., and Andreoli, G. (1995). Population biology and ecology of feral dogs in central Italy. In ‘The domestic dog: Its evolution, behaviour, and interactions with people’. (Ed. J. Serpell) pp. 217–244. (Cambridge University Press, UK)
Boitani, L., Ciucci, P., and Ortolani, A. (2006). Behaviour and social ecology of free-ranging dogs. In ‘The Behavioural Biology of Dogs’. (Ed. P. Jensen) pp. 147–165. (Cromwell Press: Trowbridge)
Brookes, V. J., Degeling, C., Van Eeden, L. M., and Ward, M. P. (2020). What is a dingo? The phenotypic classification of dingoes by Aboriginal and Torres Strait islander residents in northern Australia. Animals 10, 1230.
| What is a dingo? The phenotypic classification of dingoes by Aboriginal and Torres Strait islander residents in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Cairns, K. M. (2021). What is a dingo – origins, hybridisation and identity. Australian Zoologist , .
| What is a dingo – origins, hybridisation and identity.Crossref | GoogleScholarGoogle Scholar |
Cairns, K. M., and Wilton, A. N. (2016). New insights on the history of canids in Oceania based on mitochondrial and nuclear data. Genetica 144, 553–565.
| New insights on the history of canids in Oceania based on mitochondrial and nuclear data.Crossref | GoogleScholarGoogle Scholar | 27640201PubMed |
Cairns, K. M., Brown, S. K., Sacks, B. N., and Ballard, J. W. O. (2017). Conservation implications for dingoes from the maternal and paternal genome: multiple populations, dog introgression and demography. Ecology and Evolution 7, 9787–9807.
| Conservation implications for dingoes from the maternal and paternal genome: multiple populations, dog introgression and demography.Crossref | GoogleScholarGoogle Scholar | 29188009PubMed |
Cairns, K. M., Shannon, L. M., Koler-Matznick, J., Ballard, J. W. O., and Boyko, A. R. (2018). Elucidating biogeographical patterns in Australian native canids using genome wide SNPs. PLoS ONE 13, e0198754.
| Elucidating biogeographical patterns in Australian native canids using genome wide SNPs.Crossref | GoogleScholarGoogle Scholar | 29889854PubMed |
Cairns, K. M., Nesbitt, B. J., Laffan, S. W., Letnic, M., and Crowther, M. S. (2019). Geographic hot spots of dingo genetic ancestry in southeastern Australia despite hybridisation with domestic dogs. Conservation Genetics 21, 77–90.
| Geographic hot spots of dingo genetic ancestry in southeastern Australia despite hybridisation with domestic dogs.Crossref | GoogleScholarGoogle Scholar |
Claridge, A. W., Spencer, R.-J., Wilton, A. N., Jenkins, D. J., Dall, D., and Lapidge, S. J. (2014). When is a dingo not a dingo? Hybridisation with domestic dogs. In ‘Carnivores of Australia: past, present and future’. (Eds A. Glenn, C. Dickman) pp. 151–172. (CSIRO Publishing: Melbourne)
Collins, B., and Mills, V. (2013). Keeping safe around cheeky camp dogs. In ‘ABC Kimberley’. (Australian Broadcasting Corporation: Australia)
Corbett, L. K. (2001). Conservation status of the dingo. In ‘A symposium on the dingo’. (Eds C. R. Dickman, D. Lunney) pp. 10–19. (Royal Zoological Society of New South Wales: Sydney)
Crowther, M. S., Fillios, M., Colman, N., and Letnic, M. (2014). An updated description of the Australian dingo (Canis dingo Meyer, 1793). Journal of Zoology 293, 192–203.
| An updated description of the Australian dingo (Canis dingo Meyer, 1793).Crossref | GoogleScholarGoogle Scholar |
Crowther, M. S., Cairns, K. M., van Eeden, L. M., and Letnic, M. (2020). Introgression does not influence the positive ecological and functional role of dingo populations. Australian Zoologist , .
| Introgression does not influence the positive ecological and functional role of dingo populations.Crossref | GoogleScholarGoogle Scholar |
Daniels, M. J., and Corbett, L. (2003). Redefining introgressed protected mammals: when is a wildcat a wild cat and a dingo a wild dog? Wildlife Research 30, 213–218.
| Redefining introgressed protected mammals: when is a wildcat a wild cat and a dingo a wild dog?Crossref | GoogleScholarGoogle Scholar |
Daniels, M. J., Balharry, D., Hirst, D., Kitchener, A. C., and Aspinall, R. J. (1998). Morphological and pelage characteristics of wild living cats in Scotland: implications for defining the ‘wildcat’. Journal of Zoology 244, 231–247.
| Morphological and pelage characteristics of wild living cats in Scotland: implications for defining the ‘wildcat’.Crossref | GoogleScholarGoogle Scholar |
DEPI (2013). Action Statement No. 248 Dingo Canis lupus subsp. dingo. (Victorian Government: Melbourne, Victoria)
Dickman, C. R. (2019). The dingo dilemma: a brief history of debate. In ‘The dingo dilemma: cull, contain or conserve’, 2019, Sydney, NSW. (Eds C. R. Dickman, D. Lunney, T. M. Newsome)
DPIPWE (2013). Guideline Managing Wild Dogs. Department of Primary Industries, Water and Environment. (Tasmanian Government: Tasmania, Australia) Available at https://dpipwe.tas.gov.au/Documents/Guideline%20-%20Managing%20Wild%20Dogs%20%28December%202013%20-%20v1.0%29.pdf
Elledge, A. E., Allen, L. R., Carlsson, B.-L., and Leung, L. K.-P. (2008). An evaluation of genetic analyses, skull morphology and visual appearance for assessing dingo purity: implications for dingo conservation. Wildlife Research 35, 812–820.
| An evaluation of genetic analyses, skull morphology and visual appearance for assessing dingo purity: implications for dingo conservation.Crossref | GoogleScholarGoogle Scholar |
Fleming, P., Corbett, L. K., Harden, R., and Thomson, P. (2001). ‘Managing the impacts of dingoes and other wild dogs.’ (Bureau of Rural Sciences: Canberra)
Freedman, A. H., Gronau, I., Schweizer, R. M., Ortega-Del Vecchyo, D., Han, E., Silva, P. M., Galaverni, M., Fan, Z., Marx, P., Lorente-Galdos, B., Beale, H., Ramirez, O., Hormozdiari, F., Alkan, C., Vilà, C., Squire, K., Geffen, E., Kusak, J., Boyko, A. R., Parker, H. G., Lee, C., Tadigotla, V., Siepel, A., Bustamante, C. D., Harkins, T. T., Nelson, S. F., Ostrander, E. A., Marques-Bonet, T., Wayne, R. K., and Novembre, J. (2014). Genome Sequencing Highlights the Dynamic Early History of Dogs. PLoS Genetics 10, e1004016.
| Genome Sequencing Highlights the Dynamic Early History of Dogs.Crossref | GoogleScholarGoogle Scholar | 24453982PubMed |
Galov, A., Fabbri, E., Caniglia, R., Arbanasić, H., Lapalombella, S., Florijančić, T., Bošković, I., Galaverni, M., and Randi, E. (2015). First evidence of hybridization between golden jackal (Canis aureus) and domestic dog (Canis familiaris) as revealed by genetic markers. Royal Society Open Science 2, 150450.
| First evidence of hybridization between golden jackal (Canis aureus) and domestic dog (Canis familiaris) as revealed by genetic markers.Crossref | GoogleScholarGoogle Scholar | 27019731PubMed |
Gompper, M. E. (2013). ‘Free-ranging dogs and wildlife conservation.’ (Oxford University Press)
Gopalakrishnan, S., Sinding, M.-H. S., Ramos-Madrigal, J., Niemann, J., Samaniego Castruita, J. A., Vieira, F. G., Carøe, C., Montero, M. d. M., Kuderna, L., Serres, A., González-Basallote, V. M., Liu, Y.-H., Wang, G.-D., Marques-Bonet, T., Mirarab, S., Fernandes, C., Gaubert, P., Koepfli, K.-P., Budd, J., Rueness, E. K., Sillero, C., Heide-Jørgensen, M. P., Petersen, B., Sicheritz-Ponten, T., Bachmann, L., Wiig, Ø., Hansen, A. J., and Gilbert, M. T. P. (2018). Interspecific Gene Flow Shaped the Evolution of the Genus Canis. Current Biology 28, e5–3449.
| Interspecific Gene Flow Shaped the Evolution of the Genus Canis.Crossref | GoogleScholarGoogle Scholar | 30344120PubMed |
Hansson, B., Tarka, M., Dawson, D. A., and Horsburgh, G. J. (2012). Hybridization but no evidence for backcrossing and introgression in a sympatric population of Great Reed Warblers and Clamorous Reed Warbler PLOS ONE 7, e31667.
| Hybridization but no evidence for backcrossing and introgression in a sympatric population of Great Reed Warblers and Clamorous Reed WarblerCrossref | GoogleScholarGoogle Scholar | 22384052PubMed |
Harrison, R. G., and Larson, E. L. (2014). Hybridization, Introgression, and the Nature of Species Boundaries. Journal of Heredity 105, 795–809.
| Hybridization, Introgression, and the Nature of Species Boundaries.Crossref | GoogleScholarGoogle Scholar |
Home, C., Bhatnagar, Y. V., and Vanak, A. T. (2018). Canine Conundrum: domestic dogs as an invasive species and their impacts on wildlife in India. Animal Conservation 21, 275–282.
| Canine Conundrum: domestic dogs as an invasive species and their impacts on wildlife in India.Crossref | GoogleScholarGoogle Scholar |
Hudson, E., Brookes, V., and Ward, M. (2018). Demographic studies of owned dogs in the Northern Peninsula Area, Australia, to inform population and disease management strategies. Australian Veterinary Journal 96, 487–494.
| Demographic studies of owned dogs in the Northern Peninsula Area, Australia, to inform population and disease management strategies.Crossref | GoogleScholarGoogle Scholar | 30478842PubMed |
Hughes, J., and Macdonald, D. W. (2013). A review of the interactions between free-roaming domestic dogs and wildlife. Biological Conservation 157, 341–351.
| A review of the interactions between free-roaming domestic dogs and wildlife.Crossref | GoogleScholarGoogle Scholar |
Jackson, S. M., Groves, C. P., Fleming, P. J. S., Aplin, K. P., Eldridge, M. D. B., Gonzalez, A., and Helgen, K. M. (2017). The wayward dog: is the Australian native dog or dingo a distinct species? Zootaxa 4317, 201–224.
| The wayward dog: is the Australian native dog or dingo a distinct species?Crossref | GoogleScholarGoogle Scholar |
Jackson, S. M., Fleming, P. J. S., Eldridge, M. D. B., Ingleby, S., Flannery, T., Johnson, R. N., Cooper, S. J. B., Mitchell, K. J., Souilmi, Y., Cooper, A., Wilson, D. E., and Helgen, K. M. (2019). The Dogma of Dingoes-Taxonomic status of the dingo: A reply to Smith et al. Zootaxa 4564, 198–212.
| The Dogma of Dingoes-Taxonomic status of the dingo: A reply to Smith et al.Crossref | GoogleScholarGoogle Scholar |
Jones, E. (1990). Physical characteristics and taxonomic status of wild canids, Canis familiaris, from the eastern highlands of Victoria. Wildlife Research 17, 69–81.
| Physical characteristics and taxonomic status of wild canids, Canis familiaris, from the eastern highlands of Victoria.Crossref | GoogleScholarGoogle Scholar |
Jones, E. (2009). Hybridisation between the dingo, Canis lupus dingo, and the domestic dog, Canis lupus familiaris, in Victoria: a critical review. Australian Mammalogy 31, 1–7.
| Hybridisation between the dingo, Canis lupus dingo, and the domestic dog, Canis lupus familiaris, in Victoria: a critical review.Crossref | GoogleScholarGoogle Scholar |
Kitchener, A. C., Yamaguchi, N., Ward, J. M., and Macdonald, D. W. (2005). A diagnosis for the Scottish wildcat (Felis silvestris): a tool for conservation action for a critically-endangered felid. Animal Conservation 8, 223–237.
| A diagnosis for the Scottish wildcat (Felis silvestris): a tool for conservation action for a critically-endangered felid.Crossref | GoogleScholarGoogle Scholar |
Koungoulos, L. (2020). Old dogs, new tricks: 3D geometric analysis of cranial morphology supports ancient population substructure in the Australian dingo. Zoomorphology 139, 263–275.
| Old dogs, new tricks: 3D geometric analysis of cranial morphology supports ancient population substructure in the Australian dingo.Crossref | GoogleScholarGoogle Scholar |
Kreplins, T. L., Gaynor, A., Kennedy, M. S., Baudains, C. M., Adams, P., Bateman, P. W., and Fleming, P. A. (2019). What to call a dog? A review of the common names for Australian free-ranging dogs. Pacific Conservation Biology 25, 124–134.
| What to call a dog? A review of the common names for Australian free-ranging dogs.Crossref | GoogleScholarGoogle Scholar |
Letnic, M. (2012). Us and them correspondence. In ‘Quarterly Essay - Great Expectations: Government, Entitlement and an Angry Nation’, 46 edn. (Ed. L. Tingle) pp. 79–81. (Black Inc.: Victoria, Australia)
Letnic, M., and Koch, F. (2010). Are dingoes a trophic regulator in arid Australia? A comparison of mammal communities on either side of the dingo fence. Austral Ecology 35, 167–175.
| Are dingoes a trophic regulator in arid Australia? A comparison of mammal communities on either side of the dingo fence.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., and Crowther, M. (2020). Pesticide use is linked to increased body size in a large mammalian carnivore. Biological Journal of the Linnean Society , .
| Pesticide use is linked to increased body size in a large mammalian carnivore.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., Ritchie, E. G., and Dickman, C. R. (2012). Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study. Biological Reviews of the Cambridge Philosophical Society 87, 390–413.
| Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study.Crossref | GoogleScholarGoogle Scholar | 22051057PubMed |
Letnic, M., Baker, L., and Nesbitt, B. (2013). Ecologically functional landscapes and the role of dingoes as trophic regulators in south-eastern Australia and other habitats. Ecological Management & Restoration 14, 101–105.
| Ecologically functional landscapes and the role of dingoes as trophic regulators in south-eastern Australia and other habitats.Crossref | GoogleScholarGoogle Scholar |
Ma, G. C., Ford, J., Lucas, L., Norris, J. M., Spencer, J., Withers, A.-M., and Ward, M. P. (2020). “They reckon they’re man’s best friend and I believe that.” understanding relationships with dogs in Australian Aboriginal communities to inform effective dog population management. Animals 10, 810.
| “They reckon they’re man’s best friend and I believe that.” understanding relationships with dogs in Australian Aboriginal communities to inform effective dog population management.Crossref | GoogleScholarGoogle Scholar |
Macdonald, D. W., Yamaguchi, N., Kitchener, A. C., Daniels, M., Kilshaw, K., and Driscoll, C. (2010). Reversing cryptic extinction: the history, present and future of the Scottish Wildcat. In ‘Biology and conservation of wild felids’. (Eds D. W. Macdonald, A. Loveridge) pp. 471–492. (Oxford University Press: Oxford)
Mattucci, F., Galaverni, M., Lyons, L. A., Alves, P. C., Randi, E., Velli, E., Pagani, L., and Caniglia, R. (2019). Genomic approaches to identify hybrids and estimate admixture times in European wildcat populations. Scientific Reports 9, 11612.
| Genomic approaches to identify hybrids and estimate admixture times in European wildcat populations.Crossref | GoogleScholarGoogle Scholar | 31406125PubMed |
Miller, C. R., Adams, J. R., and Waits, L. P. (2003). Pedigree-based assignment tests for reversing coyote (Canis latrans) introgression into the wild red wolf (Canis rufus) population. Molecular Ecology 12, 3287–3301.
| Pedigree-based assignment tests for reversing coyote (Canis latrans) introgression into the wild red wolf (Canis rufus) population.Crossref | GoogleScholarGoogle Scholar | 14629346PubMed |
Morrant, D. S., Johnson, C. N., Butler, J. R. A., and Congdon, B. C. (2017). Biodiversity friend or foe: land use by a top predator, the dingo in contested landscapes of the Australian Wet Tropics. Austral Ecology 42, 252–264.
| Biodiversity friend or foe: land use by a top predator, the dingo in contested landscapes of the Australian Wet Tropics.Crossref | GoogleScholarGoogle Scholar |
Newsome, A. E., and Corbett, L. K. (1985). The Identity of the Dingo III. The incidence of Dingoes, Dogs and Hybrids and their coat colours in remote and settled regions of Australia. Australian Journal of Zoology 33, 363–373.
| The Identity of the Dingo III. The incidence of Dingoes, Dogs and Hybrids and their coat colours in remote and settled regions of Australia.Crossref | GoogleScholarGoogle Scholar |
Newsome, A. E., Corbett, L. K., and Carpenter, S. M. (1980). The Identity of the Dingo I. Morphological discriminants of Dingo and Dog skulls. Australian Journal of Zoology 28, 615–625.
| The Identity of the Dingo I. Morphological discriminants of Dingo and Dog skulls.Crossref | GoogleScholarGoogle Scholar |
Newsome, A. E., Catling, P. C., Cooke, B. D., and Smyth, R. (2001). Two ecological universes separated by the Dingo Barrier Fence in semi-arid Australia: interactions between landscapes, herbivory and carnivory, with and without dingoes. The Rangeland Journal 23, 71–98.
| Two ecological universes separated by the Dingo Barrier Fence in semi-arid Australia: interactions between landscapes, herbivory and carnivory, with and without dingoes.Crossref | GoogleScholarGoogle Scholar |
Newsome, T. M., Stephens, D., Ballard, G.-A., Dickman, C. R., and Fleming, P. J. S. (2013). Genetic profile of dingoes (Canis lupus dingo) and free-roaming domestic dogs (C. l. familiaris) in the Tanami Desert, Australia. Wildlife Research 40, 196–206.
| Genetic profile of dingoes (Canis lupus dingo) and free-roaming domestic dogs (C. l. familiaris) in the Tanami Desert, Australia.Crossref | GoogleScholarGoogle Scholar |
Newsome, T. M., Ballard, G.-A., Crowther, M. S., Dickman, C. R., and Fleming, P. J. S. (2014). Dietary niche overlap of free-roaming dingoes and domestic dogs: the role of human provided food. Journal of Mammalogy 92, 392–403.
| Dietary niche overlap of free-roaming dingoes and domestic dogs: the role of human provided food.Crossref | GoogleScholarGoogle Scholar |
NSW Threatened Species Scientific Committee (2009). Predation and Hybridisation by Feral Dogs (Canis lupus familiaris) - key threatening process listing. In ‘Schedule 3 of Threatened Species Conservation Act. New South Wales (Australia)’. Available at https://www.environment.nsw.gov.au/topics/animals-and-plants/threatened-species/nsw-threatened-species-scientific-committee/determinations/final-determinations/2008-2010/predation-and-hybridisation-by-feral-dogs-canis-lupus-familiaris-key-threatening-process-listing
Oskarsson, M. C. R., Klütsch, C. F. C., Boonyaprakob, U., Wilton, A., Tanabe, Y., and Savolainen, P. (2011). Mitochondrial DNA data indicate an introduction through Mainland Southeast Asia for Australian dingoes and Polynesian domestic dogs. Proceedings of the Royal Society B: Biological Sciences 279, 967–974.
| Mitochondrial DNA data indicate an introduction through Mainland Southeast Asia for Australian dingoes and Polynesian domestic dogs.Crossref | GoogleScholarGoogle Scholar |
Parr, W. C. H., Wilson, L. A. B., Wroe, S., Colman, N. J., Crowther, M. S., and Letnic, M. (2016). Cranial shape and the modularity of hybridization in dingoes and dogs; hybridization does not spell the end for native morphology. Evolutionary Biology 43, 171–187.
| Cranial shape and the modularity of hybridization in dingoes and dogs; hybridization does not spell the end for native morphology.Crossref | GoogleScholarGoogle Scholar |
Pilot, M., Malewski, T., Moura, A. E., Grzybowski, T., Oleński, K., Ruść, A., Kamiński, S., Fadel, F. R., Mills, D. S., Alagaili, A. N., Mohammed, O. B., Kłys, G., Okhlopkov, I. M., Suchecka, E., and Bogdanowicz, W. (2015). On the origin of mongrels: evolutionary history of free-breeding dogs in Eurasia. Proceedings of the Royal Society B: Biological Sciences 282, 20152189.
| On the origin of mongrels: evolutionary history of free-breeding dogs in Eurasia.Crossref | GoogleScholarGoogle Scholar | 26631564PubMed |
QGIS (2020). QGIS Geographic Information System. 3.12 edn. (Open Source Geospatial Foundation Project: http://qgis.org/)
Reponen, S. E. M., Brown, S. K., Barnett, B. D., and Sacks, B. N. (2014). Genetic and morphometric evidence on a Galápagos Island exposes founder effects and diversification in the first-known (truly) feral western dog population. Molecular Ecology 23, 269–283.
| Genetic and morphometric evidence on a Galápagos Island exposes founder effects and diversification in the first-known (truly) feral western dog population.Crossref | GoogleScholarGoogle Scholar |
Salvatori, V., Donfrancesco, V., Trouwborst, A., Boitani, L., Linnell, J. D. C., Alvares, F., Åkesson, M., Balys, V., Blanco, J. C., Chiriac, S., Cirovic, D., Groff, C., Guinot-Ghestem, M., Huber, D., Kojola, I., Kusak, J., Kutal, M., Iliopulos, Y., Ionescu, O., Majic Skrbinsek, A., Mannil, P., Marucco, F., Melovski, D., Mysłajek, R. W., Nowak, S., Ozolins, J., Rauer, G., Reinhardt, I., Rigg, R., Schley, L., Skrbinsek, T., Svensson, L., Trajce, A., Trbojevic, I., Tzingarska, E., von Arx, M., and Ciucci, P. (2020). European agreements for nature conservation need to explicitly address wolf-dog hybridisation. Biological Conservation 248, 108525.
| European agreements for nature conservation need to explicitly address wolf-dog hybridisation.Crossref | GoogleScholarGoogle Scholar |
Schmutz, S. M., Berryere, T. G., Barta, J. L., Reddick, K. D., and Schmutz, J. K. (2007). Agouti sequence Polymorphisms in coyotes, wolves and dogs suggest hybridization. Journal of Heredity 98, 351–355.
| Agouti sequence Polymorphisms in coyotes, wolves and dogs suggest hybridization.Crossref | GoogleScholarGoogle Scholar |
Schweizer, R. M., Durvasula, A., Smith, J., Vohr, S. H., Stahler, D. R., Galaverni, M., Thalmann, O., Smith, D. W., Randi, E., Ostrander, E. A., Green, R. E., Lohmueller, K. E., Novembre, J., and Wayne, R. K. (2018). Natural selection and origin of a melanistic allele in North American gray wolves. Molecular Biology and Evolution 35, 1190–1209.
| Natural selection and origin of a melanistic allele in North American gray wolves.Crossref | GoogleScholarGoogle Scholar | 29688543PubMed |
Senn, H. V., Ghazali, M., Kaden, J., Barclay, D., Harrower, B., Campbell, R. D., Macdonald, D. W., and Kitchener, A. C. (2019). Distinguishing the victim from the threat: SNP-based methods reveal the extent of introgressive hybridization between wildcats and domestic cats in Scotland and inform future in situ and ex situ management options for species restoration. Evolutionary Applications 12, 399–414.
| Distinguishing the victim from the threat: SNP-based methods reveal the extent of introgressive hybridization between wildcats and domestic cats in Scotland and inform future in situ and ex situ management options for species restoration.Crossref | GoogleScholarGoogle Scholar | 30828363PubMed |
Smith, B. P., Cairns, K. M., Adams, J. W., Newsome, T. M., Fillios, M., Deaux, E. C., Parr, W. C. H., Letnic, M., van Eeden, L. M., Appleby, R. G., Bradshaw, C. J. A., Savolainen, P., Ritchie, E. G., Nimmo, D. G., Archer-Lean, C., Greenville, A. C., Dickman, C. R., Watson, L., Moseby, K. E., Doherty, T. S., Wallach, A. D., Morrant, D. S., and Crowther, M. S. (2019). Taxonomic status of the Australian dingo: the case for Canis dingo Meyer, 1793. Zootaxa 4564, 173–197.
| Taxonomic status of the Australian dingo: the case for Canis dingo Meyer, 1793.Crossref | GoogleScholarGoogle Scholar |
Stephens, D., Wilton, A. N., Fleming, P. J. S., and Berry, O. (2015). Death by sex in an Australian icon: a continent-wide survey reveals extensive hybridization between dingoes and domestic dogs. Molecular Ecology 24, 5643–5656.
| Death by sex in an Australian icon: a continent-wide survey reveals extensive hybridization between dingoes and domestic dogs.Crossref | GoogleScholarGoogle Scholar | 26514639PubMed |
Surbakti, S., Parker, H. G., McIntyre, J. K., Maury, H. K., Cairns, K. M., Selvig, M., Pangau-Adam, M., Safonpo, A., Numberi, L., Runtuboi, D. Y. P., Davis, B. W., and Ostrander, E. A. (2020). New Guinea highland wild dogs are the original New Guinea singing dogs. Proceedings of the National Academy of Sciences 117, 24369.
| New Guinea highland wild dogs are the original New Guinea singing dogs.Crossref | GoogleScholarGoogle Scholar |
van Eeden, L. M., Crowther, M. S., Dickman, C. R., and Newsome, T. M. (2020). Wicked “wild dogs”: Australian public awareness of and attitudes towards dingoes and dingo management. Australian Zoologist , .
| Wicked “wild dogs”: Australian public awareness of and attitudes towards dingoes and dingo management.Crossref | GoogleScholarGoogle Scholar |
Vilà, C., and Wayne, R. K. (1999). Hybridization between wolves and dogs. Conservation Biology 13, 195–198.
| Hybridization between wolves and dogs.Crossref | GoogleScholarGoogle Scholar |
vonHoldt, B. M., and Aardema, M. L. (2020). Updating the Bibliography of Interbreeding among Canis in North America. Journal of Heredity 111, 249–262.
| Updating the Bibliography of Interbreeding among Canis in North America.Crossref | GoogleScholarGoogle Scholar |
vonHoldt, B. M., Pollinger, J. P., Lohmueller, K. E., Han, E., Parker, H. G., Quignon, P., Degenhardt, J. D., Boyko, A. R., Earl, D. A., Auton, A., Reynolds, A., Bryc, K., Brisbin, A., Knowles, J. C., Mosher, D. S., Spady, T. C., Elkahloun, A., Geffen, E., Pilot, M., Jedrzejewski, W., Greco, C., Randi, E., Bannasch, D., Wilton, A., Shearman, J., Musiani, M., Cargill, M., Jones, P. G., Qian, Z., Huang, W., Ding, Z.-L., Zhang, Y.-p., Bustamante, C. D., Ostrander, E. A., Novembre, J., and Wayne, R. K. (2010). Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 464, 898–902.
| Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication.Crossref | GoogleScholarGoogle Scholar | 20237475PubMed |
vonHoldt, B. M., Pollinger, J. P., Earl, D. A., Knowles, J. C., Boyko, A. R., Parker, H., Geffen, E., Pilot, M., Jedrzejewski, W., Jedrzejewska, B., Sidorovich, V., Greco, C., Randi, E., Musiani, M., Kays, R., Bustamante, C. D., Ostrander, E. A., Novembre, J., and Wayne, R. K. (2011). A genome-wide perspective on the evolutionary history of enigmatic wolf-like canids. Genome Research 21, 1294–1305.
| A genome-wide perspective on the evolutionary history of enigmatic wolf-like canids.Crossref | GoogleScholarGoogle Scholar | 21566151PubMed |
vonHoldt, B. M., Cahill, J. A., Fan, Z., Gronau, I., Robinson, J., Pollinger, J. P., Shapiro, B., Wall, J., and Wayne, R. K. (2016). Whole-genome sequence analysis shows that two endemic species of North American wolf are admixtures of the coyote and gray wolf. Science Advances 2, e1501714.
| Whole-genome sequence analysis shows that two endemic species of North American wolf are admixtures of the coyote and gray wolf.Crossref | GoogleScholarGoogle Scholar | 29713682PubMed |
Wallach, A. D., Ramp, D., and O’Neill, A. J. (2017). Cattle mortality on a predator-friendly station in central Australia. Journal of Mammalogy 98, 45–52.
| Cattle mortality on a predator-friendly station in central Australia.Crossref | GoogleScholarGoogle Scholar |
Wilton, A. (2001). DNA methods of assessing Australian dingo purity. In ‘A Symposium on the dingo’. (Eds C. R. Dickman, D. Lunney) pp. 49–55. (Royal Zoological Society of New South Wales: Sydney)
Wilton, A. N., Steward, D. J., and Zafiris, K. (1999). Microsatellite variation in the Australian dingo. Journal of Heredity 90, 108–111.
| Microsatellite variation in the Australian dingo.Crossref | GoogleScholarGoogle Scholar |
Zhang, S.-J., Wang, G.-D., Ma, P., Zhang, L.-l., Yin, T.-T., Liu, Y.-h., Otecko, N. O., Wang, M., Ma, Y.-p., Wang, L., Mao, B., Savolainen, P., and Zhang, Y.-p. (2020). Genomic regions under selection in the feralization of the dingoes. Nature Communications 11, 671.
| Genomic regions under selection in the feralization of the dingoes.Crossref | GoogleScholarGoogle Scholar | 32015346PubMed |