Weathering Kangaroo Island’s extremes: insights into captures, health, and diet of introduced platypuses in the Rocky River
Tahneal Hawke A B , Gilad Bino A * , Paris Hughes C , Alice Hunter C , Guido Parra Vergara C , Jessica Clayton C , Robert Ellis A and Ryan Baring CA
B
C
Abstract
The platypus (Ornithorhynchus anatinus) is increasingly threatened by habitat loss, climatic extremes, and genetic isolation. Kangaroo Island hosts the only introduced population of the species outside its natural range, offering a rare opportunity to examine population resilience under environmental stress. We conducted live-trapping surveys in the Rocky River catchment in 2021 and 2022, following severe drought, bushfires, and flooding, and compared results with historical data from 1998 to 2000. Capture rates in 2021–2022 were approximately half those recorded two decades earlier, with declining catch-per-unit-effort (CPUE) observed further upstream, suggesting altered spatial dynamics or reduced abundance. Juveniles were captured in both years, indicating continued reproduction despite recent disturbances. Dietary analysis showed a shift in prey composition, with a higher prevalence of Decapoda in 2022, and blood analyses identified year-to-year differences in red cell counts, haemoglobin, and glucose concentrations. These findings point to a population that remains reproductively active and behaviourally flexible, but potentially vulnerable to ongoing environmental change. Given the catchment’s limited extent, low genetic diversity, and projections of increasing drought frequency and reduced rainfall, this population may face heightened extinction risks in the coming decades. Continued monitoring of abundance, health, and habitat use is essential for assessing long-term viability and informing conservation strategies, both for this population and as a model for managing isolated platypus populations under climate stress.
Keywords: drought, environmental disturbance, fire, Kangaroo Island, metabarcoding, monotreme, platypus, population.
Introduction
Natural disturbances such as bushfires, droughts, and floods are ecological drivers that influence species coexistence and community structure (Gill et al. 2002; Falk et al. 2007; Bond et al. 2008; Brehme et al. 2011; Arthur et al. 2012). Bushfires and water availability fluctuations affect ecosystem processes, including vegetation patterns, biodiversity, and species fitness (Jayson et al. 2018). The impact of bushfires on ecosystems has shifted in recent times because of anthropogenic activity, with such events increasing in both frequency and intensity (Mitchell et al. 2022). Many Australian species have adapted to cope with fire; however, over the past few decades detrimental impacts of fires on biodiversity have increased as a result of the increased frequency and intensity of bushfires (van Eeden et al. 2020).
Fire can have significant impacts to freshwater ecosystems. Loss of the riparian vegetation canopy cover can affect nutrient availability and water temperatures in rivers (Royer and Minshall 1997; Bixby et al. 2015). Post-fire rainfall and flooding poses the biggest risk to freshwater ecosystems following fire, with run-off containing increased sediment loads, increasing siltation and driving changes in water quality (Dahm et al. 2015). These changes in water quality, including reductions in dissolved oxygen concentrations, can affect the survival of fish and macroinvertebrate species (Minshall 2003; Lyon and O’connor 2008).
Between June 2019 and February 2020, fires burned 19 million hectares in Australia, particularly affecting eastern and south-eastern regions (Filkov et al. 2020; van Eeden et al. 2020; Collins et al. 2021). Kangaroo Island, situated off the coast of South Australia, was among the areas affected during the hottest, driest year on record for the country (Bonney et al. 2020). The fires burned approximately half of the 4400 km2 land mass and 96% of the Flinders Chase National Park on the western side of the island, resulting in the worst bushfire the island has experienced (Bonney et al. 2020).
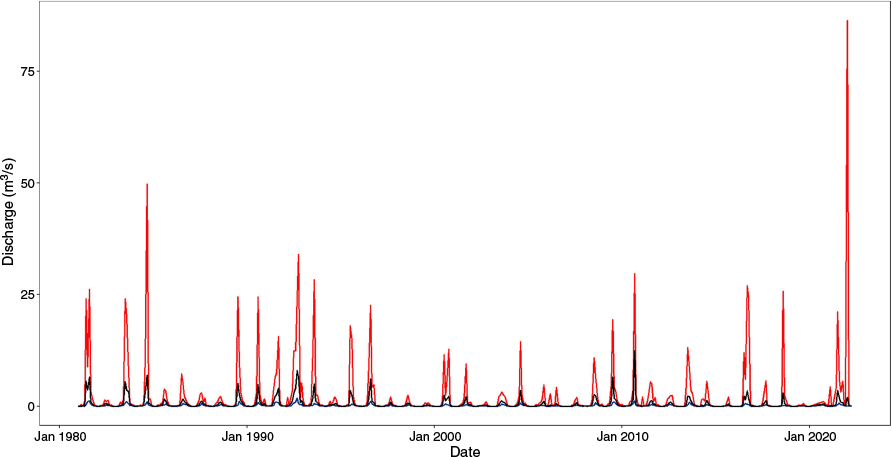
The 2019 bushfire significantly affected rivers and creeks within the Flinders Chase National Park, including the Rocky River. Riparian vegetation in the park was destroyed, and substantial sedimentation was still present 18 months following the fires (Marsh and Glatz 2022). In early 2022, the Rocky River catchment also experienced its largest recorded flood (https://water.data.sa.gov.au/Data/Location/Summary/Location/A5130501; Appendix 1), likely intensified by runoff from burnt vegetation (R. Ellis, pers. comm.). Additionally, over the past 20 years, there has been a marked reduction in streamflow within the Rocky River catchment, with the annual average streamflow from 1997 to 2009 being less than half of that for 1974 and 1996 (Meridian Urban 2024).
The platypus (Ornithorhynchus anatinus), a semi-aquatic mammal endemic to Australia, is classified as Endangered in South Australia (National Parks and Wildlife Act 1972) and Vulnerable in Victoria (Flora and Fauna Guarantee Act 1998). Platypuses face risks from habitat destruction, invasive species, and intensified extreme weather (Bino et al.2019, 2021). Between 1928 and 1946, 19 platypuses were introduced to Kangaroo Island (Serena and Williams 1997). In 1928, two males and one female were translocated from Wynyard, Tasmania, and released into the Rocky River. An additional eight females and eight males were translocated from Healesville, Victoria, with five pairs released in Rocky River in 1941 and three pairs released in Breakneck River in 1946 (Serena and Williams 1997; Furlan et al. 2012). All platypuses were released into Flinders Chase National Park, where there is a restricted area of suitable aquatic habitat for the species.
Kangaroo Island now represents the only known introduced population of platypuses outside the species’ natural extent. Live-trapping surveys between 1996 and 1997 estimated platypus densities at 1.3–5.2 platypuses/km (Serena and Williams 1997). Surveys between 1996 and 2000 captured 70 individuals (Ellis 2000). Most recently, the population size on the island was estimated to be ~110 individuals (Furlan et al. 2012). Given the small founding population, genetic diversity of the population is far lower than that of mainland populations, raising concerns for a continued reduction in genetic diversity because of the small number of founder individuals, geographic isolation of the island population, and the limited availability of suitable habitat (Furlan et al. 2012).
Fires that coincided with a significant drought have been shown to affect platypus densities in the Mid Coast region of New South Wales (Bino et al. 2021). In Victoria, no detrimental impacts of post-fire sediment slugs have been reported, with platypuses successfully reproducing in the first post-fire breeding season (Serena et al. 2022). One hypothesis is that platypuses may respond to post-fire sedimentation by moving their foraging activity to outside the affected area (Serena et al. 2022), a strategy platypuses on Kangaroo Island would have been unable to implement because of the widespread impacts of the fire within their restricted suitable habitat. Additionally, flooding occurring when juveniles are still confined to their nesting burrows (November–January) has been shown to compromise reproductive success (Serena and Grant 2017), suggesting flooding in early 2022 may have affected juvenile recruitment in the catchment.
In this study, we aimed to assess the capture rates, distribution, diet, and health of platypuses in the Rocky River system on Kangaroo Island, following the recent extreme weather events that affected the island. Surveys were conducted in 2021 and 2022, at 15 and 25 months respectively following the severe drought and fires of 2019–2020. The 2022 survey was also conducted 2 months after a significant flood event. Capture rates and distribution patterns were also compared with those recorded between 1998 and 2000, to evaluate any differences between the two capture periods. Additionally, for recent surveys we analysed cheek pouch samples, blood haematology, chemistry, and trace metals, so as to gain baseline insights into the diet and health of the population. These data provide critical information on the status of this vulnerable, isolated population and offers valuable insights into post-disturbance recovery.
Methods
Study area
Kangaroo Island is situated 112 km south-west of Adelaide, South Australia. The island covers 4405 km2, featuring several nature reserves and national parks. Kangaroo Island has a Mediterranean climate with hot days during summer (December–February; mean maximum in January 26.8°C) and cool days during winter (mean minimum in August 5.8°C) (Bureau of Meteorology 2025). Unreliable rainfall during warmer months creates consecutive hot days, with the number of consecutive days with temperatures >30°C becoming more frequent (Bureau of Meteorology 2019).
Rocky River is located within the protected area of Flinders Chase National Park (326 km2) on the western end of Kangaroo Island in South Australia (Fig. 1). The river flows in a south-westerly direction and is approximately 40 km long. The Rocky River catchment is the only catchment in South Australia that has been minimally affected by anthropogenic modification, such as the development of minor access tracks and built assets (Stewart 2005). The river has variable flows, recording no flows on 20.2% of days over a 40 year period (Whiting and Green 2015) and frequently becoming remnant pools during dry months (Ellis 2000).
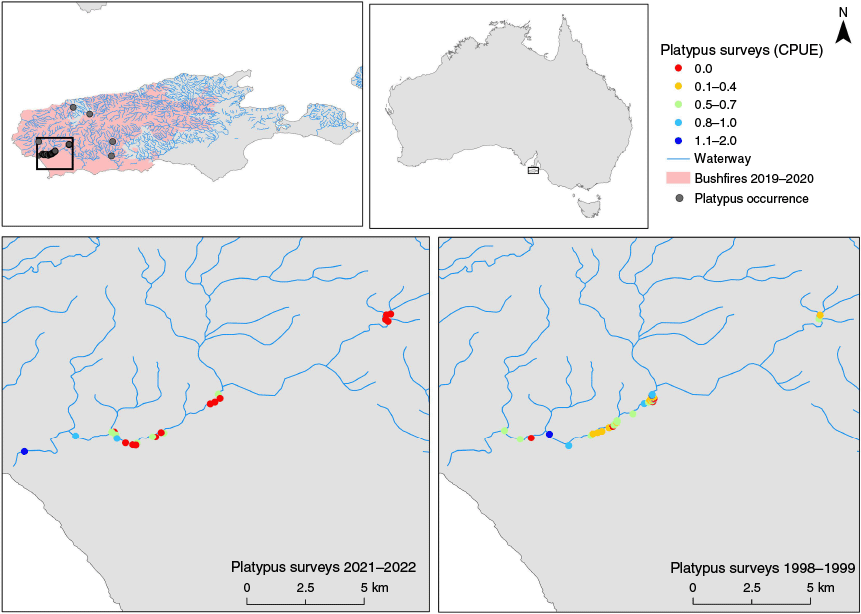
Comparison of platypus catch-per-unit-effort (CPUE) from surveys conducted in the Rocky River catchment on Kangaroo Island, South Australia. Top panels show the location of the study area within Australia (right) and the broader catchment context, including waterway networks, 2019–2020 bushfire extent, and historical platypus occurrence records (left). Bottom panels display CPUE values at survey sites during two periods: 2021–2022 (left) and 1998–1999 (right). CPUE is categorised and colour-coded from 0.0 (red) to 1.1–2.0 (dark blue).
Platypus surveys
This study draws on data from two live-trapping surveys of platypuses conducted in the Rocky River catchment on Kangaroo Island. The first survey (1998–2000) involved monthly trapping across 33 sites from February 1998 to January 2000; further methodological details are provided in Ellis (2000). The second survey (2021–2022) was conducted at 22 sites during two periods, namely May 2021 and March 2022, with each survey comprising seven trap-nights. Both were timed to occur outside the breeding season and after the expected emergence of juveniles (Bino et al. 2019).
In shallow sections (<1.5 m), double-winged fyke nets (30 mm, 1 m and 0.8 m wings) were placed in pairs, facing upstream and downstream, with four pairs set across each site over 500 m. Fyke nets were deployed from 1700 hours and checked every 3 h until sunrise. All surveys undertaken between 1998 and 2000 utilised fyke nets exclusively. For deeper pools (>1.5 m), unweighted mesh nets (80 mm, 100 m max) were set parallel to stream banks from 1700 hours to 0100 hours, checked every 2–3 min by spotlight and hourly to prevent snags or by-catch. Mesh nets were set at two of the surveys sites in 2021–2022, with the remaining sites being surveyed using fyke nets.
Captured platypuses were processed differently between the surveys. In 1998–2000, animals were placed in calico bags, scanned for microchips, transported to a laboratory for processing, and released by morning (Ellis 2000). In 2021–2022, platypuses were held in pillowcases, anaesthetised in the field (Bino et al. 2018) and monitored for oxygen saturation, temperature, and heart rate (Bino and Hawke 2025). Standard measurements were taken (weight, size, sex, age), with age and sex determined by spur morphology (Williams et al. 2013), and fat storage assessed using tail volume index (TVI) (Grant and Carrick 1978). Newly tagged individuals received subcutaneous passive integrated transponder (PIT) tags, and cheek pouch and blood samples were collected (Faragher et al. 1979; Whittington and Grant 1984).
We compared differences in capture rates between the two survey periods by using a standardised metric of catch-per-unit-effort (CPUE). CPUE was calculated as the total number of platypuses captured divided by the total number of trap-net nights, where one trap-net night was defined as either one pair of fyke nets deployed overnight (1998–2000 and 2021–2022) or, in the 2021–2022 surveys, 50 m of mesh net deployed for approximately 6 h. Mesh nets were used only at two sites in 2021–2022, in deeper sections of river unsuitable for fyke nets. For the 1998–2000 surveys, CPUE was calculated per site as the number of individuals captured divided by the number of trap-nights at that site, allowing for both site-level and overall estimates of trapping success. For the 2021–2022 surveys, CPUE was calculated separately for each year and site, and also pooled across both years by using the same standardised approach. Although the combined metric ensures comparability of effort within each period, we acknowledge that differences in net type and site conditions may have influenced capture probability.
To compare differences across survey periods and to account for spatial variation in sampling effort, CPUE values were aggregated into 1 km × 1 km grid cells, a common approach in spatial ecology to address heterogeneous sampling density and autocorrelation (Dormann et al. 2007; Elith et al. 2010). This spatial framework standardised the data structure and facilitated comparisons despite uneven site distribution. Aggregating to grid cells also mitigated spatial autocorrelation and heterogeneity in sampling density, providing a clearer basis for examining platypus distribution and activity patterns. We used a generalised linear mixed model (GLMM) implemented in the ‘glmmTMB’ package (version 1.1.11, Brooks 2017) in R, which accommodates complex variance structures and flexible distribution families. Given that CPUE values included zeros and positive continuous values, we specified a Tweedie distribution, which is well suited to compound data comprising both discrete and continuous components (Dunn and Smyth 2005). In the model, survey period was included as a fixed effect, along with the distance of each grid cell from the river mouth (continuous), a proxy for longitudinal environmental gradients (Sheldon et al. 2002). Grid cell ID was included as a random effect to account for unmeasured spatial heterogeneity and local environmental variation not explicitly modelled.
Dietary analysis
We analysed the diet of platypuses captured from surveys in 2021 and 2022 by using DNA metabarcoding (Hawke et al. 2022). We extracted DNA from cheek pouch samples of platypus by using the DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany), following instructions from the manufacturer. All polymerase chain reactions (PCR) and sequencing were completed by Mr DNA (Molecular Research, LP, Shallowater, TX, USA, http://www.mrdnalab.com). A 313 base pair (bp) region of the COI gene was amplified using the primers mlCOIintF (GGWACWGGWTGAACWGTWTAYCCYCC) and jgHCO2198 (TAIACYTCIGGRTGICCRAARAAYCA) (Leray et al. 2013). The 16S rRNA gene V4 variable region PCR primers 515/806 with barcode on the forward primer were used in a 30 cycle PCR using the HotStarTaq Plus Master Mix Kit (Qiagen, USA). Thermocycling protocols consisted of 94°C for 3 min, followed by 30–35 cycles of 94°C for 30 s, 53°C for 40 s and 72°C for 1 min, and a final elongation step at 72°C for 5 min. Amplification success was determined by running each sample product in a 2% agarose gel and looking at the relative intensity of bands (Hawke et al. 2022)
Sequence data from each sample were demultiplexed b using custom-built FASTq Processor script (http://www.mrdnafreesoftware.com/). The Greenfield Hybrid Analysis Pipeline (GHAP, version 2.2 Commonwealth Scientific and Industrial Research Organisation, Australia) was used for processing data and creating operational taxonomic unit (OTU) tables (Hawke et al. 2022). Sequences that had an overlap of over 25 bp and a homology of 80% minimum were merged, and sequences between 288 and 342 bp long were preserved for further analyses (Shackleton et al. 2021). Sequences were grouped into OTUs on the basis of the 97% threshold that corresponds reasonably well with COI delimitation between invertebrate species. OTUs that made up less than 0.01% of the total reads in a sample were removed to eliminate possible sequencing artifacts. Final OTUs were taxonomically classified using nucleotide basic local alignment search tool (BLASTn) against a curated database derived from National Centre for Biotechnology Information (NCBI) (www.ncbi.nlm.nih.gov). Taxonomic assignment of prey was refined using the assigned homology percentage thresholds (Shackleton et al. 2021) assigned as part of the GHAP pipeline, as follows: 97% or greater for species, 95–<97% for genus, 90–<95% for family, 85–<90% for order, and those with <85% were not assigned because of taxonomic uncertainty (Hawke et al. 2022).
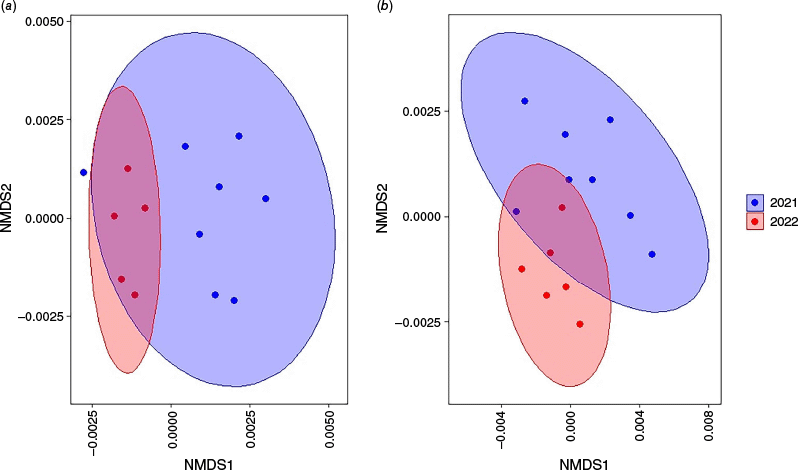
We compared differences in the number of orders and families in each sample between samples collected in 2021 and 2022 by using non-parametric Kruskal–Wallis tests. To assess differences in the composition of diet between years, we first transformed the dataset into a presence/absence matrix at the order level. A Jaccard distance matrix was then calculated from these binary data by using the ‘vegdist’ function to measure dissimilarities between samples. Non-metric multidimensional scaling (nMDS) was performed on the Jaccard distance matrix with the ‘metaMDS’ function, specifying two dimensions and a maximum of 100 iterations. nMDS scores were associated with sample years and visualised using ‘ggplot2’ (version 3.5.1, Gómez-Rubio 2017), with points coloured by year and 95% confidence ellipses for each year group. Following the nMDS ordination, we applied the ‘envfit’ function to identify which individual orders contributed most to the variation in dietary composition across samples. To statistically test for significant differences in dietary composition between 2021 and 2022, we conducted permutational analysis of variance (PERMANOVA) by using the ‘adonis2’ function. nMDS and PERMANOVA analyses were replicated at the family level.
Blood analysis
To analyse blood haematology, whole blood samples were stored in K2EDTA and analysed using an Abaxis VetScan HM5 machine to determine white blood cell concentration, lymphocyte concentration, lymphocyte percentage, red blood cell concentration, and haemoglobin concentration. In total, 11 samples were used to assess blood haematology, including seven from 2021 surveys and four from 2022 surveys. One platypus was captured twice in 2022, so values from both samples were averaged, resulting in one value for each variable for this animal.
To analyse blood chemistry, whole blood samples were stored in lithium heparin vials and centrifuged at 2000 rpm for 10 min at room temperature (Sigma 1-15, equivilant to ~366.8 g) to separate the protein plasma layer. A 100 µL sample of the plasma was added to an Abaxis Comprehensive Diagnostic Profile Router. The plasma was run through the router, while ensuring that no air bubbles were present. The router was then placed into an Abaxis VetScan VS2, which used spectroscopy to determine blood chemistry concentrations within the sample. After running for 20 min, the Abaxis VetScan VS2 calculated the concentrations of alanine aminotransferase, albumin, alkaline phosphate, amylase, total bilirubin, blood urea nitrogen, calcium, phosphorus, glucose, sodium, potassium, total protein, and globulin in each sample. Amylase, total bilirubin and potassium concentrations could not be calculated for two samples from 2021 and potassium concentrations could not be calculated for one sample from 2022. Creatinine concentrations were removed from the analysis because of the inability to determine exact concentrations. Thirteen samples were analysed for blood chemistry, with eight being from 2021 surveys and five from 2022 surveys.
In total, 11 samples were analysed for trace metal toxicology, seven from 2021 and four from 2022. Samples were digested prior to analysis by pipetting individual samples into 15 mL digestion tubes and each sample was then weighed (mg). In the next step, 0.5 mL of concentrated nitric (HNO3) and 0.5 mL of hydrogen peroxide (H2O2) were added to each tube, before they were left to pre-digest overnight. Samples were then digested in a block digestor by increasing the temperature to 80°C for 20 min and then left at 80°C for a further 30 min. The temperature was then increased to 120°C over 15 min and maintained at 120°C for a further 120 min, before being removed and allowed to cool at room temperature. Samples were then diluted up to 10 mL, resulting in a HNO3 concentration of 5%.
A calibration was completed for selected elements on a Perkin Elmer Nexion 350D inductively coupled plasma–mass spectrometry (ICP-MS), by using 5% HNO3 to matrix match the samples. The selected elements for the analysis were iron (Fe), lead (Pb), zinc (Zn), manganese (Mn), chromium (Cr), copper (Cu), barium (Ba), cadmium (Cd), and arsenic (As). The calibration started at 0.05 ppb and went to 100 ppb; however, this was altered for some elements because of high concentrations in the sample (i.e. such as Fe), or to narrow down readings.
All results were converted back to the undiluted concentrations. Internal standards for the ICP-MS were used to correct for drift and were analysed using normal standard mode with no gas and KED (helium) mode. Small volumes of blood (<0.5 mL), owing to the small maximum volume collected from each platypus (2 mL), resulted in difficulties in obtaining results for some trace metals, meaning that some of those candidates were on the limit of detectability. All toxicology analysis was completed by the laboratory technical team at Flinders Analytical at Flinders University. Given the small sample sizes for blood haematology, chemistry, and trace metals, they were compared between the sampling years by using non-parametric Kruskal–Wallis tests. All statistical tests were completed in R (ver. 4.0.2) (R Development Core Team 2024).
Results
Platypus captures
During the 1998–2000 surveys, 149 captures (representing 51 unique individuals) were recorded over 67 trapping nights at 33 sites, amounting to a total of 263 trap-net nights (Tables A1 and A2). The mean CPUE was 0.54 ± 0.08 s.e. platypuses. Of the captured individuals, 22 were adult females, 26 adult males, three juvenile females, and two juvenile males.
In May 2021, eight platypuses were captured across seven trapping nights at 22 sites, yielding a mean CPUE of 0.36 ± 0.20 s.e. platypuses. The captured animals included two adult females, two juvenile females, one adult male, one subadult male, and two juvenile males (Tables A1 and A2). Tail volume index (TVI) values ranged between 2 and 3, indicating moderate to good body condition. Six of the eight individuals were caught in deep waterholes (>2 m depth) located in the lower reaches of Rocky River (Fig. 1).
In March 2022, six captures of five individuals were recorded over seven trapping nights at the same 22 sites, with a mean CPUE of 0.27 ± 0.15 s.e. platypuses. One individual was a recapture from 2021, who was captured twice in 2022, resulting in a total of 12 unique individuals from 14 captures across both years. In 2022, two adult females, two adult males, and one juvenile male were captured. Most animals had a TVI of 2–3, again indicating moderate to good condition, and the juvenile male had a TVI of 4. Unlike the previous year, no individuals were captured in deep pools. Across both 2021–2022 survey periods, the mean CPUE was 0.32 ± 0.12 s.e. platypuses.
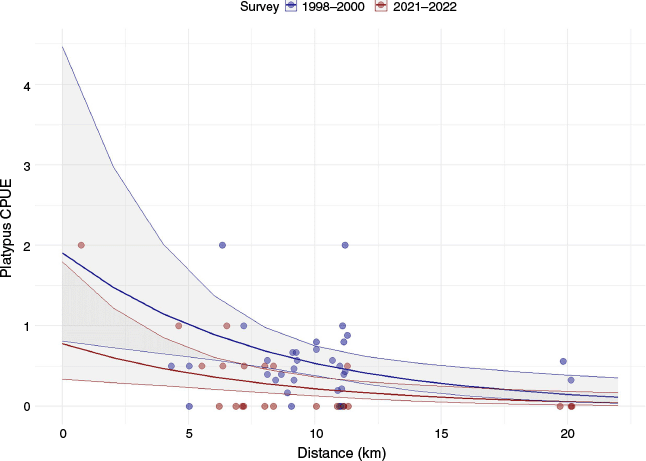
Significantly fewer platypuses were captured during the 2021–2022 surveys than in the 1998 and 2000 periods (estimate = −1.09 ± 0.28 s.e., P < 0.001), (Table 1). CPUE also declined significantly with an increasing distance from the river mouth, indicating a spatial gradient in platypus activity (estimate = −0.14 ± 0.04 s.e., P = 0.001; Fig. 2). Overall, the model explained a substantial portion of the variability in CPUE, with a residual deviance of 54.2 and dispersion parameter for the Tweedie family was estimated at 0.67, indicating a reasonable fit of the model to the data.
Predicted relationship between platypus catch-per-unit-effort (CPUE) and river distance from the mouth (in km), based on Tweedie regression model estimates. The model compares survey data from two periods: 1998–2000 and 2021–2022. The solid lines represent predicted CPUE values across distances for each survey period, with shaded ribbons showing the 95% confidence intervals. Points indicate observed CPUE values from the combined dataset, with colours corresponding to the respective survey period (dark blue for 1998–2000 and dark red for 2021–2022).
Diet
In total, 10 macroinvertebrate orders and 16 families were detected from platypus cheek pouch samples from surveys undertaken in 2021 and 2022, with an average of 2.85 ± 0.41 s.e. orders per sample and 2.62 ± 0.35 s.e. families. Diptera (flies) was the most prominent order, detected in 64.3% of samples, followed by Hemiptera (true bugs; 57.1%) and Decapoda (crustaceans; 42.9%; all Parastacidae (crayfishes)).
In total, 10 orders and 14 families were detected in 2021, compared with six orders and seven families in 2022. There was no significant difference in the number of orders detected in each sample between 2021 (3.00 ± 0.44 s.e.) and 2022 (2.67 ± 0.76 s.e.; χ2 = 1.20, P = 0.27). The number of families detected in samples was significantly higher in 2021 (3.00 ± 0.49 s.e.) than in 2022 (2.17 ± 0.48 s.e.; χ2 = 4.16, P = 0.04). Orders Decapoda and Diptera were detected in more samples in 2022 (83.3% of samples), than in 2021 (12.5%, 50% respectively; Fig. 3).
The percentage of samples that (a) orders and (b) families were detected from dietary samples for platypuses on Kangaroo Island from surveys undertaken in 2021 and 2022.
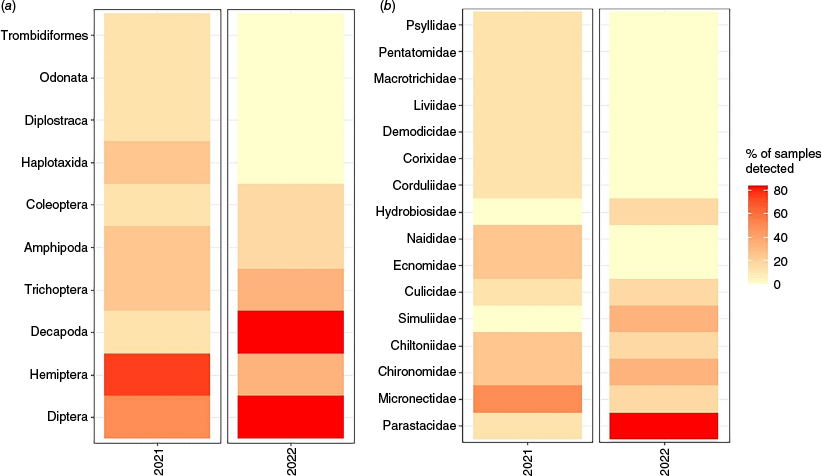
There was a significant difference in overall dietary composition at the order level between 2021 and 2022 (F = 8.81, P = 0.003, Fig. 4a). Individual orders driving differences in the diet between years included Decapoda (P = 0.001), Diptera and Hemiptera (P = 0.002), and Haplotaxida (P = 0.012). Similarly, a significant difference in dietary composition was observed at the family level (P = 0.003, Fig. 4b), with the families Chironomidae (P = 0.007) and Parastacidae (P = 0.002) contributing most to the variation between years.
Blood haematology and chemistry
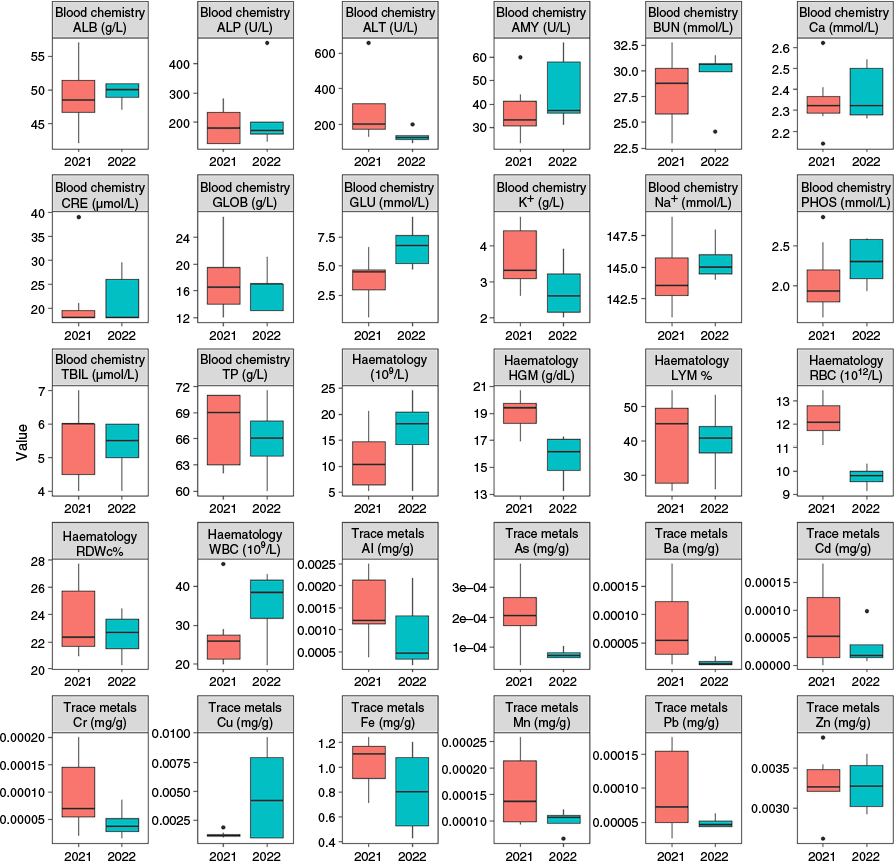
Blood haematology analyses indicated that red blood cell counts were significantly higher in 2021 (average 12.24 ± 0.31 s.e.) than in 2022 (9.74 ± 0.25 s.e.; χ2 = 7.00, P = 0.01), (Table 2). This was also the case for haemoglobin concentrations, which were higher in 2021 (19.00 g/dl ± 0.48 s.e.) than in 2022 (15.69 ± 0.94 s.e.; χ2 = 5.14, P = 0.02). There were no significant differences in white blood cell counts, lymphocyte concentration, or lymphocyte percentage between the two sampling years (Fig. A2).
| Group | Variable | Mean | s.d. | Range | RI | |
|---|---|---|---|---|---|---|
| Adults | TP | 67.25 | 3.77 | 63–72 | 46.5–78 | |
| ALB (g/L) | 48.88 | 2.03 | 46–51 | 14.7–42.4 | ||
| UREA (mmol/L) | 29.66 | 2.458 | 24.1–32.7 | 19.62–36.73 | ||
| CRE (µmol/L) | 21.88 | 5.62 | 18–32 | 15–75 | ||
| GLOB (g/L) | 18.25 | 3.49 | 13–24 | 23–57.5 | ||
| Juveniles | TP | 66.20 | 4.87 | 60–71 | 33–74.9 | |
| ALB (g/L) | 49.00 | 5.10 | 42–56 | 18.3–40.9 | ||
| UREA (mmol/L) | 27.80 | 3.83 | 22.9–31.5 | 21.74–34.94 | ||
| CRE (µmol/L) | 18.75 | 1.50 | 18–21 | 15.4–62 | ||
| GLOB (g/L) | 16.80 | 6.14 | 12–27 | |||
| Males | TP | 65.86 | 4.38 | 60–71 | 53.1–76.6 | |
| ALB (g/L) | 50.86 | 4.22 | 46–57 | 15.3–44 | ||
| UREA (mmol/L) | 27.13 | 3.40 | 22.9–31.5 | 17.46–37.07 | ||
| CRE (µmol/L) | 22.57 | 7.83 | 18–39 | 15–75 | ||
| GLOB (g/L) | 14.86 | 2.12 | 12–17 | 20.8–57.5 | ||
| Females | TP | 68.43 | 3.55 | 63–72 | 44.2–81.4 | |
| ALB (g/L) | 48.14 | 3.18 | 42–51 | 13.4–41.3 | ||
| UREA (mmol/L) | 30.36 | 1.48 | 28.0–32.7 | 18.9–36.19 | ||
| CRE (µmol/L) | 21.83 | 6.15 | 18–32 | 15–74.5 | ||
| GLOB (g/L) | 20.00 | 4.76 | 13–27 | 22.7–51.5 |
Blood means indicate value is outside the RI for that variable.
Glucose concentrations were significantly higher in 2022 (6.68 mmol/L ± 0.82 s.e.) than in 2021 (3.78 ± 0.73 s.e.; χ2 = 6.2, P = 0.01), whereas the opposite was true for alanine aminotransferase, being higher in 2021 (269.87 U/L ± 60.19 s.e.) than in 2022 (133.40 ± 18.86 s.e.; χ2 = 4.8, P = 0.03). There were no significant differences in the concentrations of albumin, blood urea nitrogen, creatinine, total protein, alkaline phosphate, amylase, total bilirubin, calcium, phosphorous, sodium, potassium, or globulin between the two sampling years (Fig. A2).
When comparing blood chemistry results to available references intervals, calculated from studies of mainland platypus populations, across all age and sex categories albumin concentrations were higher than reference intervals, whereas globulin concentrations were lower (Fig. A2).
Trace metals
For trace metals, barium concentrations were significantly higher in 2021 (0.08 µg/g ± 0.03 s.e.) than in 2022 (0.02 µg/g ± 0.003 s.e.; χ2 = 3.57, P = 0.06), as was the case for arsenic (0.21 µg/g ± 0.04 s.e. in 2021 and 0.08 µg/g ± 0.009 s.e. in 2022; χ2 = 3.57, P = 0.06). Although the concentrations of cadmium, chromium, iron, manganese, lead, and zinc were higher in 2021 than in 2022, these differences were not statistically significant (Appendix 3). Copper was the only trace metal with higher concentrations in 2022, although this was also not significant.
Discussion
Our study has provided the first integrative assessment of platypus captures, diet, and physiological health on Kangaroo Island following a sequence of extreme environmental disturbances, namely, drought, bushfire, and flooding, affecting the Rocky River catchment. Despite these pressures, our results indicated that the introduced platypus population has persisted and continues to reproduce, with juveniles comprising a notable proportion of captures. Nevertheless, catch-per-unit-effort (CPUE) was approximately half of that observed two decades earlier, with significant declines in captures with an increasing distance upstream, suggesting a spatial contraction in activity or abundance. Dietary analysis showed marked interannual shifts, including a striking increase in the prevalence of Decapoda in 2022, whereas blood parameters showed modest but significant differences between years. This study also provided the first insights on the diet and blood haematology and chemistry of animals on the island, suggesting differences from mainland populations as well as reporting slight differences between the 2021 and 2022 survey years. Together, these findings suggest a resilient but potentially vulnerable population exhibiting behavioural and physiological responses to environmental stressors. They provide an essential baseline for ongoing monitoring and a rare insight into the ecology of a genetically depauperate, geographically isolated population facing an uncertain climatic future.
Changes in trapping rates between the 1998–2000 surveys and 2021–2022 surveys may reflect increasing pressures from limited habitat availability and extreme weather events, including fires, droughts, and flooding. Over the past 20 years, average stream flows in the Rocky River catchment have declined by more than 50% (1997–2009 compared with 1974–1996) (Meridian Urban 2024). In addition to hydrological changes, Kangaroo Island experienced two of its most widespread fires in recent history, namely, the 2007–2008 fires, which burned 19.5% of the island, and the 2019–2020 fires, which affected nearly half the island (Bonney et al. 2020). Further compounding these challenges, the most severe flooding ever recorded in the Rocky River catchment occurred in 2022. These environmental changes have affected the primary habitat of platypuses on Kangaroo Island and may have been a contributing factor to the reported differences in CPUEs.
During the 2021 surveys, six of the eight platypuses were captured in deeper water holes towards the mouth of Rocky River, compared with only two individuals being captured further upstream in shallower sections. Capture rates at sites nearby these pools were reported to be lower in previous studies, with the highest density of platypuses being recorded further upstream (Serena and Williams 1997; Ellis 2000). While the surveys in 2021 took place more than a year after the severe drought and fires on the island, the higher capture rates in these deeper pools may suggest that platypuses utilised these areas as refugia during dry conditions, or that these areas were less subject to post-fire impacts. However, these conclusions warrant ongoing research, given the limited sample size in this study. While the deeper pools in this section of Rocky River may offer vital refugia for platypuses, the river geomorphology introduces potential risks. These pools are located near the mouth of the river where it cascades into the Southern Ocean via a series of rocky waterfalls. Anecdotal records of platypuses found on the adjacent beach and in the ocean suggest that although platypuses are capable climbers, some individuals may have difficulty navigating back up the waterfalls to return to the river, particularly during period of high flow (R. Ellis, pers. comm., May 2021). In contrast to 2021, when several platypuses were captured in these deeper pools, no individuals were found in 2022, 2 months after severe flooding. This could suggest that the platypuses either dispersed upstream under more favourable conditions or were displaced by flash floods in the rocky section. Nevertheless, because of the limited scope of the current study, no definitive conclusions can be drawn. Further research is essential to understand how environmental factors such as floods and geomorphological features influence platypus habitat use and movement patterns in this river system.
In 2021, four of the captures (50%) were juveniles and, in 2022, one juvenile was captured (16.6% of captures). Breeding in 2020, which resulted in the juveniles being captured in 2021, would have occurred prior to the fires, but still during a period of drought. Despite these juveniles emerging from their burrows soon after the fires, TVIs indicated that they had healthy fat storages, indicative of successful foraging in their first few months post-emergence. The capture of one juvenile in 2022 suggests successful breeding in the first breeding season following the fires, which indicates a capacity of platypuses to reproduce even after fires in certain conditions (Serena et al. 2022). However, the proportion of juvenile captures in 2022 was much lower than that in 2021, which could suggest some impact of the floods on recruitment, but because of the small sample size and lack of available pre-fire breeding data, further monitoring is required to validate these impacts.
Surveys from 1998 to 2000 indicated that <5% of captures were juveniles in the Kangaroo Island population, which is extremely low compared with estimates of 33% from the mainland (Furlan et al. 2012). This was suggested by Furlan et al. (2012) to indicate that the population on Kangaroo Island had reached carrying capacity within its distribution on the island. A high proportion of juvenile captures in the more recent 2021/2022 surveys may suggest that the platypus population no longer exists at carrying capacity, resulting in high reproductive rates.
The use of DNA metabarcoding of cheek pouch samples is a relatively new tool for assessing platypus diet (Hawke et al. 2022). Overall, Diptera was found to be the most frequent order in the diet of platypuses on the island, supporting recent evidence that this important dietary component may be underestimated using other methods of dietary assessments (Hawke et al. 2022). Hemiptera was the most common order in platypus cheek pouch samples in 2021, whereas in 2022 it was the order Decapoda. In comparison, no studies of the diet of wild platypuses on the Australian mainland have reported Hemiptera or Decapoda being the prevalent orders in the species diet (Hawke et al. 2022). Decapoda is rarely reported in platypus dietary analyses and, when present, appears in only a small percentage of samples (Grant 1982; Hawke et al. 2022). Burrowing crayfish (Parastacoides tasmanicus) have been reported in the diet of platypuses in Lake Lea in Tasmania (Bethge 2002) and freshwater crayfish (Cherax destructor and Cherax albidus) are an important food item for captive platypuses (Thomas et al. 2017).
The high proportion of Decapoda (specifically Paratacidae) in the diet is likely to reflect the abundance of the introduced marron (Cherax cainii), established in island waterways since the 1980s (Department of Primary Industries and Regions, SA). Parastacidae may have been detected less in the diet in 2021, owing to differences in capture locations of the platypuses between the years, or because of the impacts of the bushfires on the marron. There is limited research specifically on the impacts of fires on yabby species, but there are known thermal tolerance and oxygen reduction impacts to aquatic invertebrates resulting from post-fire changes in the water quality (Silva et al. 2020; Cramp et al. 2021). In 2021, when platypuses were consuming less Paratacidae, the number of families detected in their diet was significantly higher, suggesting that they were foraging on a greater variety of other species when not consuming marron.
Platypuses on Kangaroo Island had higher red blood cells counts and haemoglobin in 2021 than in 2022. Juvenile platypuses have been shown to have higher red blood cell counts than do adults (Whittington and Grant 1983), which may explain differences between the two sampling years, given the higher proportion of juveniles captured in 2021. Seasonality and latitude have also been shown to influence blood haematology and chemistry in platypuses (Stewart et al. 2021). Changes in glucose and alanine aminotransferase in blood samples between sampling years may reflect differences in age of platypus captures, changes in the diet, or changes in environmental conditions, but no conclusions about this can be drawn from the current study. Albumin concentrations in platypus blood samples were higher than mainland reference intervals, but this may be associated with geographical differences, given albumin has been shown to decrease with latitude in this species (Stewart et al. 2021). Arsenic and barium concentrations in platypus blood samples were also higher in 2021, which may be a result of the bushfires (Rust et al. 2018) or environmental pollutants, and this also warrants further research.
There were several limitations to this study, which hinder conclusions regarding platypus abundances, recruitment, diet, and health. The smaller number of trapping nights and the difference in trapping method between the 1998 and 2000 surveys and the 2021–2022 surveys might have influenced comparisons of CPUE between the two periods. Increasing the number of trapping nights is shown to increase the probability of capture, and capture rates can vary between net types (Bino et al. 2015), and in 2021–2022, mesh nets were used at a subset of sites where fyke nets could not be deployed, unlike in the earlier survey period. This inconsistency in net type may have influenced capture probability and should be considered when interpreting CPUE comparisons. Nevertheless, CPUE remains a widely used and practical tool in long-term wildlife monitoring, providing a standardised index of relative abundance where direct density estimates are unfeasible (Maunder and Punt 2004). These limitations, along with the lack of pre-fire trapping data, mean that conclusions regarding the impacts of the extreme weather on abundances and reproductive success requires ongoing research. Additionally, inconsistency in the survey month between 2021 and 2022 might have contributed to some differences in capture rates (Serena and Williams 2012) and results from the blood and dietary analyses (McLachlan‐Troup et al. 2010). Despite these limitations, this study has still provided valuable information on the distribution and capture rates of platypuses in the Rocky River and provides baseline insights on the diet and health of this population.
Evidence of lower capture rates of platypuses on Kangaroo Island from 2000 to 2022 is of concern for the future viability of this small and isolated, but valuable population. By 2030, the island is projected to face drought conditions 65% of the time, a 13% reduction in spring rainfall, and a 4% increase in evapotranspiration by 2050 (Meridian Urban 2024). These changes are likely to exacerbate habitat loss, particularly for the platypus, a species highly dependent on aquatic ecosystems. Moreover, the population’s genetic health is a critical concern. Isolated from mainland Australia and descended from a limited number of founding individuals, the population shows signs of a genetic bottleneck. Previous studies have estimated an effective population size of only 10, and key indicators of genetic diversity, namely heterozygosity and allelic richness, are significantly lower (30% and 50% respectively) than in mainland Victoria populations (Furlan et al. 2012). Such reduced genetic diversity may impair the population’s adaptability to environmental stressors.
Genetic reinforcement through addition of new individuals should be considered to bolster the resilience of this isolated population. Sustained and ongoing monitoring of abundances, habitat use, diet, movements, and distribution of the platypus population on the island remains paramount to understanding population dynamics. Live-trapping surveys, supplemented by eDNA monitoring, in-stream chip readers, and camera traps should be considered to improve understanding of platypus movements and distribution within the catchment. Implementing these strategies will be crucial for securing the long-term viability of the Kangaroo Island platypus population.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
Surveys in 2021 were funded by UNSW and WWF – Australia, and surveys in 2022 were funded by the Department of Industry, Science and Resources (MSSPI000013). Additional funding was received from the Department for Environment and Water, and Donors to the GoFundMe Happy Platy Program.
Acknowledgements
Our thanks go to Heinrich Klein the Conservation Ecologist at National Parks and Wildlife Service South Australia for his support and assistance. We also thank Dr Lewis Vaughan and Mr Max Boulton at Flinders University for their help during surveys.
References
Arthur, A. D., Catling, P. C., and Reid, A. (2012). Relative influence of habitat structure, species interactions and rainfall on the post-fire population dynamics of ground-dwelling vertebrates. Austral Ecology 37(8), 958-970.
| Crossref | Google Scholar |
Bino, G., Grant, T. R., and Kingsford, R. T. (2015). Life history and dynamics of a platypus (Ornithorhynchus anatinus) population: four decades of mark-recapture surveys. Scientific Reports 5, 16073.
| Crossref | Google Scholar | PubMed |
Bino, G., and Hawke, T. (2025). Field Anaesthesia for Platypuses: a proven method and the case for non-veterinarian accreditation pathways. Australian Mammalogy 47(1), AM24029.
| Crossref | Google Scholar |
Bino, G., Kingsford, R. T., Grant, T., Taylor, M. D., and Vogelnest, L. (2018). Use of implanted acoustic tags to assess platypus movement behaviour across spatial and temporal scales. Scientific Reports 8(1), 5117-5112.
| Crossref | Google Scholar | PubMed |
Bino, G., Kingsford, R. T., Archer, M., Connolly, J. H., Day, J., Dias, K., Goldney, D., Gongora, J., Grant, T., Griffiths, J., Hawke, T., Klamt, M., Lunney, D., Mijangos, L., Munks, S., Sherwin, W., Serena, M., Temple-Smith, P., Thomas, J., et al. (2019). The platypus: evolutionary history, biology, and an uncertain future. Journal of Mammalogy 100(2), 308-327.
| Crossref | Google Scholar | PubMed |
Bino, G., Hawke, T., and Kingsford, R. T. (2021). Synergistic effects of a severe drought and fire on platypuses. Science of The Total Environment 777, 146137.
| Crossref | Google Scholar | PubMed |
Bixby, R. J., Cooper, S. D., Gresswell, R. E., Brown, L. E., Dahm, C. N., and Dwire, K. A. (2015). Fire effects on aquatic ecosystems: an assessment of the current state of the science. Freshwater Science 34(4), 1340-1350.
| Crossref | Google Scholar |
Bond, N. R., Lake, P. S., and Arthington, A. H. (2008). The impacts of drought on freshwater ecosystems: an Australian perspective. Hydrobiologia 600(1), 3-16.
| Crossref | Google Scholar |
Bonney, M. T., He, Y., and Myint, S. W. (2020). Contextualizing the 2019–2020 Kangaroo Island bushfires: quantifying landscape-level influences on past severity and recovery with Landsat and Google earth engine. Remote Sensing 12(23), 3942.
| Crossref | Google Scholar |
Brehme, C. S., Clark, D. R., Rochester, C. J., and Fisher, R. N. (2011). Wildfires alter rodent community structure across four vegetation types in southern California, USA. Fire Ecology 7(2), 81-98.
| Crossref | Google Scholar |
Brooks, M., Kristensen, K., Benthem, K., Magnusson, A., Berg, C., Nielsen, A., Skaug, H., Mächler, M., and Bolker, B. (2017). glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. The R Journal 9(2), 378.
| Crossref | Google Scholar |
Bureau of Meteorology (2019) Kangaroo Island, SA climate guide. (Australian Government) Available at http://www.bom.gov.au/climate/climate-guides/guides/049-Kangaroo-Island-SA-Climate-Guide.pdf [retrieved 12 March 2025].
Bureau of Meteorology (2025) Climate statistics for Australian locations: Kingscote (Kangaroo Island Airport) Available at http://www.bom.gov.au/climate/data/ [retrieved 12 March 2025].
Collins, L., Bradstock, R. A., Clarke, H., Clarke, M. F., Nolan, R. H., and Penman, T. D. (2021). The 2019/2020 mega-fires exposed Australian ecosystems to an unprecedented extent of high-severity fire. Environmental Research Letters 16(4), 044029.
| Crossref | Google Scholar |
Cramp, R., Mulvey, C., Cameron, J., Wintour, M., Gomez Isaza, D., and Franklin, C. (2021) Impacts of post-fire ash and runoff sediment on the physiological tolerances of Australian freshwater aquatic fauna. Available at https://www.nespthreatenedspecies.edu.au/media/re2dcnx5/8-3-7-impacts-of-post-fire-ash-and-runoff-freshwater-aquatic-fauna-report_v6.pdf
Dahm, C. N., Candelaria‐Ley, R. I., Reale, C. S., Reale, J. K., and Van Horn, D. J. (2015). Extreme water quality degradation following a catastrophic forest fire. Freshwater Biology 60(12), 2584-2599.
| Crossref | Google Scholar |
Dormann, C. F., McPherson, J. M, Araújo, M. B., Bivand, R., Bolliger, J., Carl, G., Davies, R. G., Hirzel, A., Jetz, W., and Daniel Kissling, W. (2007). Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30(5), 609-628.
| Google Scholar |
Dunn, P. K., and Smyth, G. K. (2005). Series evaluation of Tweedie exponential dispersion model densities. Statistics and Computing 15, 267-280.
| Crossref | Google Scholar |
Elith, J., Kearney, M., and Phillips, S. (2010). The art of modelling range‐shifting species. Methods in Ecology and Evolution 1(4), 330-342.
| Crossref | Google Scholar |
Falk, D. A., Miller, C., McKenzie, D., and Black, A. E. (2007). Cross-scale analysis of fire regimes. Ecosystems 10(5), 809-823.
| Crossref | Google Scholar |
Faragher, R., Grant, T., and Carrick, F. (1979). Food of the platypus (Ornithorhynchus anatinus) with notes on the food of brown trout (Salmo trutta) in the Shoalhaven River, NSW. Australian Journal of Ecology 4(2), 171-179.
| Crossref | Google Scholar |
Filkov, A. I., Ngo, T., Matthews, S., Telfer, S., and Penman, T. D. (2020). Impact of Australia’s catastrophic 2019/20 bushfire season on communities and environment. Retrospective analysis and current trends. Journal of Safety Science and Resilience 1(1), 44-56.
| Crossref | Google Scholar |
Furlan, E., Stoklosa, J., Griffiths, J., Gust, N., Ellis, R., Huggins, R. M., and Weeks, A. R. (2012). Small population size and extremely low levels of genetic diversity in island populations of the platypus, Ornithorhynchus anatinus. Ecology and Evolution 2(4), 844-857.
| Crossref | Google Scholar | PubMed |
Gómez-Rubio, V. (2017). ggplot2 - Elegant Graphics for Data Analysis (2nd Edition). Journal of Statistical Software 77(Book Review 2),.
| Crossref | Google Scholar |
Grant, T. R. (1982). Food of the platypus, Ornithorhynchus anatinus (Monotremata: Ornithorhynchidae), from various water bodies in New South Wales. Australian Mammalogy 5, 235-236.
| Crossref | Google Scholar |
Grant, T. R., and Carrick, F. N. (1978 Available at). Some aspects of the ecology of the platypus, Ornithorhynchus anatinus, in the upper Shoalhaven River, New South Wales. Australian Zoologist 20, 181-199 https://www.scopus.com/inward/record.uri?eid=2-s2.0-0000010268&partnerID=40&md5=990249892ad88ca98e17ca6b2d40521c.
| Google Scholar |
Hawke, T., Bino, G., Shackleton, M. E., Ross, A., and Kingsford, R. T. (2022). Using DNA metabarcoding as a novel approach for analysis of platypus diet. Scientific Reports 12(1), 2247.
| Crossref | Google Scholar | PubMed |
Jayson, S., Ferguson, A., Goetz, M., Routh, A., Tapley, B., Harding, L., Michaels, C. J., and Dawson, J. (2018). Comparison of the nutritional content of the captive and wild diets of the critically endangered mountain chicken frog (Leptodactylus fallax) to improve its captive husbandry. Zoo Biology 37(5), 332-346.
| Crossref | Google Scholar | PubMed |
Leray, M., Yang, J. Y., Meyer, C. P., Mills, S. C., Agudelo, N., Vincent, R., Boehm, J. T., and Machida, R. J. (2013). A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: application for characterizing coral reef fish gut contents. Frontiers in Zoology 10, 34.
| Crossref | Google Scholar | PubMed |
Lyon, J. P., and O’connor, J. P. (2008). Smoke on the water: can riverine fish populations recover following a catastrophic fire‐related sediment slug? Austral Ecology 33(6), 794-806.
| Crossref | Google Scholar |
Marsh, J. R., and Glatz, R. V. (2022). Assessing the impact of the black summer fires on Kangaroo Island threatened invertebrates: Towards rapid habitat assessments for informing targeted post-fire surveys. Australian Zoologist 42(2), 479-501.
| Crossref | Google Scholar |
Maunder, M. N., and Punt, A. E. (2004). Standardizing catch and effort data: a review of recent approaches. Fisheries Research 70(2-3), 141-159.
| Crossref | Google Scholar |
McLachlan‐Troup, T. A., Dickman, C. R., and Grant, T. R. (2010). Diet and dietary selectivity of the platypus in relation to season, sex and macroinvertebrate assemblages. Journal of Zoology 280(3), 237-246.
| Crossref | Google Scholar |
Minshall, G. W. (2003). Responses of stream benthic macroinvertebrates to fire. Forest Ecology and Management 178(1–2), 155-161.
| Crossref | Google Scholar |
Mitchell, L. J., Horsburgh, G. J., Dawson, D. A., Maher, K. H., and Arnold, K. E. (2022). Metabarcoding reveals selective dietary responses to environmental availability in the diet of a nocturnal, aerial insectivore, the European Nightjar (Caprimulgus europaeus). Ibis (London, England) 164(1), 60-73.
| Crossref | Google Scholar |
Royer, T. V., and Minshall, G. W. (1997). Temperature patterns in small streams following wildfire. Archiv Für Hydrobiologie 140, 237-242.
| Crossref | Google Scholar |
Rust, A. J., Hogue, T. S., Saxe, S., and McCray, J. (2018). Post-fire water-quality response in the western United States. International Journal of Wildland Fire 27(3), 203-216.
| Crossref | Google Scholar |
Serena, M., and Grant, T. R. (2017). Effect of flow on platypus (Ornithorhynchus anatinus) reproduction and related population processes in the upper Shoalhaven River. Australian Journal of Zoology 65(2), 130-139.
| Crossref | Google Scholar |
Serena, M., and Williams, G. A. (1997). Population attributes of platypus (Ornithorhynchus anatinus) in Flinders Chase National Park, Kangaroo Island. South Australian Naturalist 72, 28-34.
| Google Scholar |
Serena, M., and Williams, G. A. (2012). Effect of sex and age on temporal variation in the frequency and direction of platypus (Ornithorhynchus anatinus) captures in fyke nets. Australian Mammalogy 34(1), 75-82.
| Crossref | Google Scholar |
Serena, M., Lyon, J. P., Tonkin, Z. D., Lieschke, J., and Williams, G. A. (2022). Differential impacts of a wildfire and post-fire sedimentation event on platypus and fish populations in a Victorian upland river. Marine and Freshwater Research 74(1), 86-94.
| Crossref | Google Scholar |
Shackleton, M. E., Dafforn, K. A., Murphy, N. P., Greenfield, P., Cassidy, M., and Besley, C. H. (2021). How does molecular taxonomy for deriving river health indices correlate with traditional morphological taxonomy? Ecological Indicators 125, 107537.
| Crossref | Google Scholar |
Sheldon, F., Boulton, A. J., and Puckridge, J. T. (2002). Conservation value of variable connectivity: aquatic invertebrate assemblages of channel and floodplain habitats of a central Australian arid-zone river, Cooper Creek. Biological Conservation 103(1), 13-31.
| Crossref | Google Scholar |
Silva, L. G., Doyle, K. E., Duffy, D., Humphries, P., Horta, A., and Baumgartner, L. J. (2020). Mortality events resulting from Australia’s catastrophic fires threaten aquatic biota. Global Change Biology 26(10), 5345-5350.
| Crossref | Google Scholar | PubMed |
Stewart, J., Bino, G., Hawke, T., and Kingsford, R. T. (2021). Seasonal and geographic variation in packed cell volume and selected serum chemistry of platypuses. Scientific Reports 11(1), 15932.
| Crossref | Google Scholar |
Thomas, J. L., Handasyde, K. A., Temple-Smith, P. D., and Parrott, L. (2017). Seasonal changes in food selection and nutrition of captive platypuses (Ornithorhynchus anatinus). Australian Journal of Zoology 65(5), 319-327.
| Crossref | Google Scholar |
Whiting, J., and Green, D. (2015) Hydrological investigations to inform surface water use limits and generalised environmental water requirements for key ecological assets on Kangaroo Island. Available at https://www.waterconnect.sa.gov.au/Content/Publications/DEW/DEWNR-TR-2015-07.pdf
Whittington, R. J., and Grant, T. R. (1983). Haematology and blood chemistry of the free-living platypus, Ornithorhynchus anatinus (Shaw) (Monotremata: Ornithorhynchidae). Australian Journal of Zoology 31(4), 475-482.
| Crossref | Google Scholar |
Whittington, R. J., and Grant, T. R. (1984). Haematology and blood chemistry of the conscious platypus, Ornithorhynchus anatinus (Shaw) (Monotremata: Ornithorhynchidae). Australian Journal of Zoology 32(5), 631-635.
| Crossref | Google Scholar |
Williams, G. A., Serena, M., and Grant, T. R. (2013). Age-related change in spurs and spur sheaths of the platypus (Ornithorhynchus anatinus). Australian Mammalogy 35(1), 107-114.
| Crossref | Google Scholar |
Appendix 1.River gauge volumes at Rocky River
Monthly maximum (red), mean (black), and minimum (blue) discharge at Rocky River upstream Gorge Falls River Gauge (https://water.data.sa.gov.au/Data/Location/Summary/Location/A5130501).
Appendix 2.Platypus capture history
| Site | Unique | Total | Unique male | Count male | Unique female | Count female | Count adult | Count juvenile | |
|---|---|---|---|---|---|---|---|---|---|
| Surveys 1998–2000 | |||||||||
| T024 | 1 | 2 | 0 | 0 | 1 | 2 | 2 | 0 | |
| T026 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 0 | |
| T028 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T037 | 6 | 6 | 4 | 4 | 2 | 2 | 6 | 0 | |
| T039 | 3 | 4 | 2 | 2 | 1 | 2 | 4 | 0 | |
| T046 | 4 | 4 | 2 | 2 | 2 | 2 | 4 | 0 | |
| T050 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | |
| T055 | 2 | 3 | 1 | 1 | 1 | 2 | 3 | 0 | |
| T060 | 4 | 4 | 3 | 3 | 1 | 1 | 4 | 0 | |
| T065 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | |
| T070 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T071 | 4 | 4 | 2 | 2 | 2 | 2 | 4 | 0 | |
| T072 | 5 | 7 | 2 | 2 | 3 | 5 | 7 | 0 | |
| T074 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | |
| T075 | 4 | 6 | 1 | 1 | 3 | 5 | 6 | 0 | |
| T076 | 4 | 4 | 3 | 3 | 1 | 1 | 4 | 0 | |
| T080 | 4 | 5 | 1 | 1 | 3 | 4 | 5 | 0 | |
| T088 | 6 | 13 | 4 | 10 | 2 | 3 | 13 | 0 | |
| T090 | 6 | 12 | 3 | 6 | 3 | 6 | 12 | 0 | |
| T092 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T093 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T094 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T096 | 2 | 3 | 0 | 0 | 2 | 3 | 3 | 0 | |
| T100 | 3 | 3 | 3 | 3 | 0 | 0 | 3 | 0 | |
| T104 | 5 | 5 | 2 | 2 | 3 | 3 | 4 | 1 | |
| T105 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T106 | 5 | 12 | 0 | 0 | 5 | 12 | 12 | 0 | |
| T107 | 1 | 2 | 1 | 2 | 0 | 0 | 2 | 0 | |
| T108 | 10 | 14 | 5 | 7 | 5 | 7 | 11 | 3 | |
| T109 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T110 | 19 | 33 | 12 | 22 | 7 | 11 | 33 | 0 | |
| T150 | 4 | 5 | 3 | 4 | 1 | 1 | 4 | 1 | |
| T152 | 1 | 3 | 0 | 0 | 1 | 3 | 3 | 0 | |
| Surveys 2021–2022 | |||||||||
| R1.1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | |
| R1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R1.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R1.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R2.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R2.2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | |
| R2.3 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | |
| R2.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R3.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R3.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R3.3 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | |
| R3.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R4.1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | |
| R4.2 | 1 | 2 | 0 | 0 | 1 | 2 | 2 | 0 | |
| R4.3 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | |
| R4.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R5 | 4 | 4 | 2 | 2 | 2 | 2 | 3 | 1 | |
| R6.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R6.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R6.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R6.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R7 | 2 | 2 | 1 | 1 | 1 | 1 | 0 | 2 | |
The table includes the number of unique individuals, total captures, and breakdowns by sex and age class: number of unique males and females, total male and female captures, and counts of adults and juveniles.
| Capture year | Platypus | Sex | Age | Tail volume index | |
|---|---|---|---|---|---|
| 2021 | KI1 | Male | Juvenile | 2 | |
| KI2 | Female | Juvenile | 2 | ||
| KI3 | Female | Adult | 3 | ||
| KI4 | Male | Adult | 3 | ||
| KI5 | Male | Sub-adult | 2 | ||
| KI6 | Female | Adult | 3 | ||
| KI7 | Male | Juvenile | 2 | ||
| KI8 | Female | Juvenile | |||
| 2022 | KI9 | Male | Adult | 3 | |
| KI10 | Male | Adult | 3 | ||
| KI11 | Female | Adult | 2 | ||
| KI12 | Female | Adult | 2 | ||
| KI2 | Female | Adult | 2 | ||
| KI14 | Male | Juvenile | 4 |