The great divide: population comparison using 3D and 2D geometric morphometrics of Petaurus breviceps and Petaurus notatus along Australia’s eastern Great Dividing Range
Meagan Powley A * and Katarina M Mikac A
A * and Katarina M Mikac A
A
Abstract
The geographic distribution of many Australian terrestrial species is poorly understood and after taxonomic revision, new distributional limits are often unclear. This study examined skull size and shape of Petaurus breviceps (senso stricto) and Petaurus notatus, to clarify species distribution in regions where it remains unresolved. We used two regions of known distributions; east (P. breviceps) and west of the Great Dividing Range (GDR) (P. notatus) in New South Wales and compared these specimens to the untested region within the mid-GDR (previously assumed to be P. breviceps). The skull shape of the mid-region was found to be consistent with the west population P. notatus, rather than the anticipated east populations. The results suggest P. breviceps is restricted to the eastern coastal region. This revised distribution of P. breviceps emphasises the importance of identifying biogeographical barriers to refine species distribution and contribute to future conservation efforts.
Keywords: biogeography, conservation, marsupial, morphology, Petauridae, spatial ecology, 3D, 2D.
Introduction
Accurate identification of species and their distribution provides ecologists the information required to understand species biology and to plan species specific conservation strategies. The Petaurus breviceps species complex, once considered a single widespread arboreal petaurid species, recently underwent taxonomic revision. From Petaurus breviceps (Waterhouse 1838) (sensu lato) in Australia, three genetically and morphologically divergent species emerged (Cremona et al. 2021), though the geographic distribution of these species has yet to be fully determined.
The previous distribution of Petaurus breviceps (senso lato) was extensive, occurring through eastern and northern Australia, throughout New Guinea, and on some islands of eastern Indonesia (Smith 1973). Petaurus breviceps (senso lato) currently remains listed as a species of Least Concern with no additional sustainability assessment attempted since 2016 (IUCN 2023). Following taxonomic review of the Australian populations, three distinct species were recognised; Petaurus breviceps (Waterhouse 1838) (sensu stricto), Petaurus notatus (Peters 1859) and Petaurus ariel (Gould 1842) (Cremona et al. 2021) (Fig. 1). While P. ariel remains likely isolated in north-western Australia, the boundaries of the geographic distribution between P. breviceps (sensu stricto) and P. notatus feasibly overlap in some regions. The distribution of P. notatus in Australia represents the largest of the three species, extending throughout eastern Queensland, inland New South Wales, Victoria and south-eastern South Australia (Cremona et al. 2021). There is also an introduced population in Tasmania (Campbell et al. 2018). After the taxonomic revision the distribution of P. breviceps (sensu stricto) was limited to was the eastern side of the peak of the Great Dividing Range (GDR) in New South Wales and southeast Queensland as far north as the Sunshine Coast (Cremona et al. 2021). This revised distribution proposed a continuous narrow coastal lowland range, no greater than 130 km wide from north of the NSW/Victorian border (Eden −37.08°, 149.86°) to southeast Queensland (Sunshine Coast −26.98°, 152.93°) with the GDR forming the western boundary. North of the Sunshine Coast P. notatus is distributed from the coast westward throughout most of Queensland (Cremona et al. 2021; Powley and Mikac 2024).
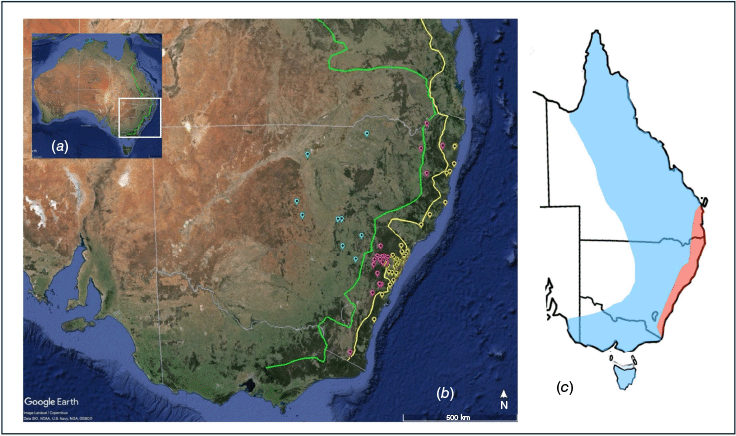
The Great Dividing Range (GDR) in eastern Australia with locations of examined specimens. (a) Australia with state boundaries indicated in light grey, green line indicates Great Divide (GD). (b) Enlarged view of southeastern – Green line indicates GDR peak, Great Escarpment yellow line and GDR between green and yellow lines. Eastern specimens (yellow markers), mid specimens (pink) and western specimens (blue). (c) Current distribution for P. notatus (blue) and P. breviceps (pink) for east coast of Australia (Cremona et al. 2021; Powley and Mikac 2024). (Image developed using Google Earth Pro (2022) 7.3.6.9345 (64-bit)).
Climatic and geographic changes over time have prevented or enabled dispersal even at a regional level (Glor and Warren 2011). A physical change in the environment that interrupts species dispersal is known as a biogeographical barrier, and is described as an area or region that is difficult to cross, and may occur at different times throughout history (Briggs 1974; Procheş 2006). This barrier disrupts gene flow between regions that are isolated from each other and conceivably lead to their vicariance due to allopatry as the species evolves independently. More notably, the prevention of gene flow between populations can eventually lead to extinction of those populations. Some examples a biogeographical barrier are mountain ranges, oceans and rivers, however, soil and vegetation types, climate and altitude are also potential barriers (Schneider et al. 1999; Jouventin et al. 2006; Glor and Warren 2011; Milner et al. 2012; Bui and Henderson 2013; Bryant and Krosch 2016).
The GDR runs along the eastern margin of Australia for approximately 3500 km and has two geological features: the Great Divide (GD) and to the east the Great Escarpment (GE). The GE is a single escarpment that runs alongside the GD, in some regions up to hundreds of kilometres to the east and other areas as little as 15 km (Oilier 1982; Johnson 2009). The GE is the initial elevation zone separating the coast from the Tablelands prior to reaching the maximum Australian Height Datum (AHD) of the GDR (Pulsford et al. 2003). The abrupt edge of the GE forms a barrier between the tablelands and the coast in many places up to 1000 m AHD (Oilier 1982).
Typically, the GDR elevation ranges from 300 to 1600 m with a maximum height of 2300 m AHD. Its highest peaks are concentrated in southern New South Wales and northern Victoria (Johnson 2009). The lowest GDR heights are in central Queensland where some low-lying hills are 10 m AHD. The elevation of the GDR is conservative when compared to other international mountain ranges, however, this elevation is responsible for considerable climatic and environmental variation seen along the range and differentiating it from the mainly low topographical features observed throughout the rest of Australia (Keast 1981; Taylor 1994). Between high and low elevation, vegetation varies greatly under the influence of a range of climatic, topographic and geological conditions.
Species distribution along the GDR is strongly influenced by altitude, with species richness generally peaking at mid-elevations and declining at higher elevations (Keith and Bedward 1999; Mallen-Cooper and Pickering 2008a, 2008b; Beck et al. 2017). This pattern is observed not only in vegetation but also in fauna, including insects and vertebrates, in southern New South Wales and is consistent with global trends. Vertebrates are particularly difficult to categorise within narrow altitude bands due to their varied ecological requirements. These include vegetation type, habitat structure, diet and thermoregulatory needs, all of which shape species’ altitudinal preferences and restrict the availability of suitable habitat. The GDR functions as a significant biogeographic barrier, limiting east-west dispersal. This is especially evident in reptiles, amphibians and some mammals, for which the GDR defines the western extent of their distribution (James and Moritz 2000; Symula et al. 2008; Frankham et al. 2012, 2016). Morphological differences across the range further support the presence of population divergence (Furlan et al. 2013). Species west of the GDR tend to be highly endemic with limited dispersal capacity (Cracraft 1982; Hazlitt et al. 2014). Comparable patterns in the Andes suggest that mountain ranges can promote niche-specific speciation rather than simply acting as barriers (López-Aguirre et al. 2015).
The distribution for P. breviceps and P. notatus east and west of the GDR was partially resolved using morphological and genetic analysis (Cremona et al. 2021). The authors identified specimens from the eastern and western margins of the distribution, however, specimens west of the GE and east of the GD were not analysed. Another potentially confounding factor in accurately identifying species distribution is hybridisation, which has been identified between some co-located species within the Petaurus (Zuckerman 1952; Knipler et al. 2021) and may be more widespread. Hybridisation may contribute to variation in shape and size of skulls.
A contemporary working hypothesis is that the population located along the GDR from the GE to the GD, currently identified as P. breviceps, may have niche specific characteristics that could differentiate them from either the western P. notatus or the eastern P. breviceps.
The aim of this research is to assess the morphological variation of skulls from P. breviceps on the east and P. notatus on the west of the GDR in NSW, and compare these to the population located along the GDR in order to identify them to species.
Materials and methods
Specimens
A review of all Petaurus skulls from accessible institutions in Australia identified 175 skulls suitable for analysis from the Australian Museum, Museums Victoria, South Australia Museum and Queensland Museum. Adult skulls were determined by the complete eruption of the permanent molars (Cremona et al. 2021). Specimen location and sex were obtained from museum records. To compare skull size and shape, each specimen was allocated to east, mid or west GDR regions based on unprojected latitude and longitude coordinates (Fig. 1). The following categories were used for analysis; east (E), n = 90, mid (M), n = 34 and west (W), n = 9.
3D models and landmarks
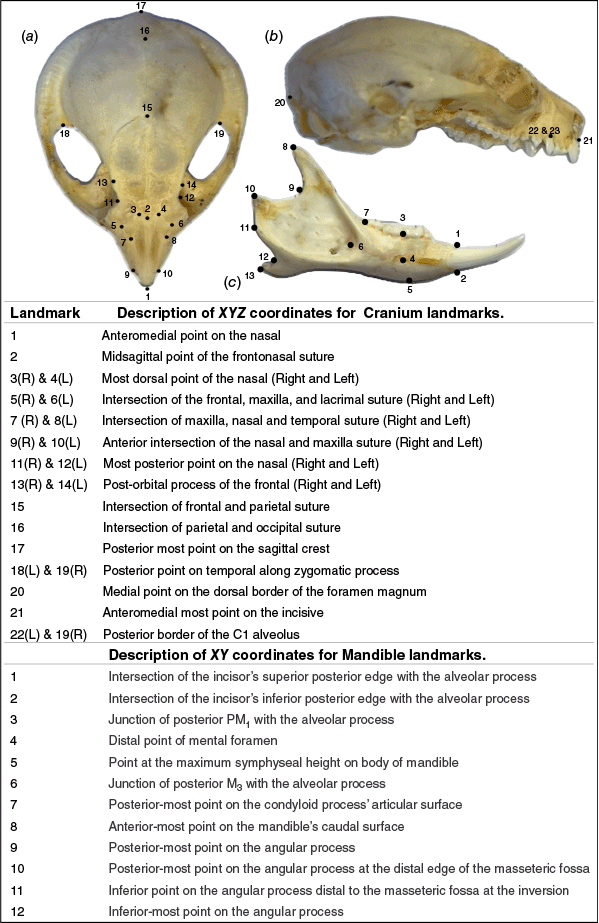
Using complete adult P. breviceps and P. notatus skulls, 95 three-dimensional (3D) craniums models and 131 two-dimensional (2D) images of mandibles were collected. Landmarks used in this analysis were consistent with previous research on similar species (Cardini et al. 2005; Dawson and Milne 2012; Viacava et al. 2020; Powley and Mikac 2024). Incomplete or damaged cranium and mandible were used to collect linear measurements where available. Cranium specimens were scanned in a standard anatomical position using a SOL PRO 3D Scanner (Scan Dimension, Global Scanning Denmark) following manufacturers recommendations. Individual 3D scans were taken in a 360° pass in the ‘High Accuracy’ setting in the near lens position. Scans were processed and aligned in SOL PRO Viewer 2.0.0 and the final meshed object was used for landmarking. Landmarking the 3D model was guided by textured layers in Slicer V5.4.0 (Fedorov et al. 2012). Photographs obtained of the specimens also aided in the identification of landmark anatomical locations. Images for 2D analysis of the mandibles were obtained using standardised image acquisition techniques (Cardini et al. 2022). Landmarks were applied to images using ImageJ V1.54g (Schneider et al. 2012) (Fig. 2).
Landmark anatomical positions used for geometric morphometrics for P. breviceps and P. notatus. Upper shows placement of linear measurements and lower are abbreviations and definitions used here. (a) Dorsal view of cranium showing position of landmarks 1–19, (b) Lateral view of cranium showing position of landmarks 20–23, (c) Laterial (buccal) view of mandible showing position of mandible landmarks 1–12.
Linear measurements
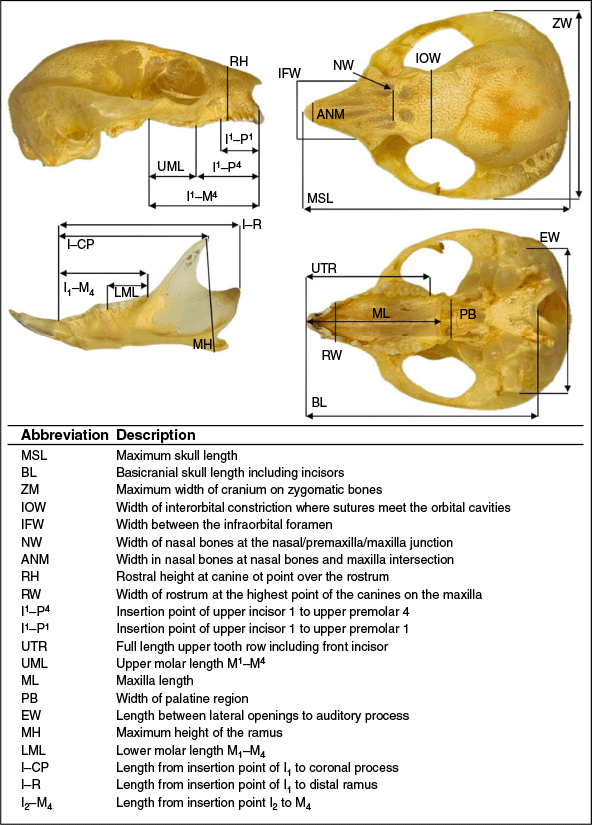
Linear measurements of the cranium and mandible were collected by the primary author using Protech Digital Vernier Callipers. The measurements chosen were consistent with studies conducted on similar species (Van Dyck 1990; Feijó and Cordeiro-Estrela 2016; Cremona et al. 2021; Powley and Mikac 2024). Characteristics of size were analysed using discriminant function analysis (DFA) and T-test using JMP® Version 16.2.0. (SAS Institute Inc., Cary, NC, 1989–2023) (Fig. 3).
Geometric morphometrics
The geometric morphometric analysis for the 3D models and the 2D images was performed using MorphoJ v1.07a (Klingenberg 2011). All landmarks were applied to models and images twice to test for digitising error (von Cramon-Taubadel et al. 2007). Digitising error was rendered to non-significant using revision where required. Geometric morphometric analyses were conducted separately for the cranium and mandible to investigate shape variation among specimens from different geographic regions. Groups were defined by location as east, mid and west of the GDR. Because preliminary tests revealed no significant sexual dimorphism, males and females were pooled for all analyses.
For the cranium, 22 3D anatomical landmarks were collected, while 12 2D anatomical landmarks were used for the mandible. A Generalised Procrustes Analysis (GPA) was carried out separately for the cranial and mandibular datasets to remove variation related to position, orientation and scale, standardising all specimens to unit centroid size. Principal Component Analysis (PCA) was performed to summarise the major axes of shape variation within each structure. Group differences were tested using DFA for the GDR (east, mid and west) groupings. Mahalanobis and Procrustes distances between group centroids were calculated, and the significance of group separation was assessed using permutation tests (10,000 iterations). Canonical Variates Analysis (CVA) was also used to visualise patterns of shape divergence among regional groups for each anatomical structure.
Sexual dimorphism
The size and shape of specimens were analysed for characteristics of sexual dimorphism using DFA with 1000 permutations. DFA was used to assess sexual dimorphism in size. The analysis showed significant sexual dimorphic size characteristics for mid (P < 0.001) and west (P < 0.001); no significant result was found for the east (P = 0.1750). Further analysis of the linear measurements used separate sexes. DFA was used for the coordinate data for each region (east, mid and west). There was no significant shape characteristics of sexual dimorphism identified for either the cranium or mandible for labelled populations of P. breviceps or P. notatus. All subsequent analysis of shape used combined male and female populations for each region (east, mid and west).
Allometry
Any effects of allometry (size on shape) were assessed by regression in MorphoJ. The Procrustes coordinates and centroid size were compared to quantify the amount a shape variation was influenced as the size of the specimen increased. Less than 5% of the shape change was explained by the size increase, and when the residuals from the regression analysis were compared among the three regions, no significant differences were found. The shape coordinates were hence suitable to use without size correction in all further analysis.
Results
Linear measurements
DFA was performed to assess regional variation in cranium and mandible size across male and female specimens using linear measurements (Table 1).
| Regions | Eigenvalues | Approx. F | Num d.f. | Prob > F | |
|---|---|---|---|---|---|
| Male cranium size | |||||
| E–W | 6.89 | 1.72 | 16 | 0.3191 | |
| M–W | 340.51 | -127.69 | 16 | <0.0001 | |
| E–M | 2.45 | 1.84 | 16 | 0.1854 | |
| Regions | Eigenvalues | Approx. F | d.f. | Prob > F | |
|---|---|---|---|---|---|
| Female cranium size | |||||
| E–W | 3.53 | 4.41 | 16 | 0.0011 | |
| M–W | 1,018,719.26 | -63,669.95 | 16 | <0.0001 | |
| E–M | 1.64 | 2.87 | 16 | 0.0071 | |
| Male mandible size | |||||
| E–W | 0.27 | 1.36 | 5 | 0.2717 | |
| M–W | 0.25 | 0.59 | 5 | 0.7073 | |
| E–M | 0.39 | 2.81 | 5 | 0.0115 | |
| Female mandible size | |||||
| E–W | 0.18 | 1.70 | 5 | 0.1545 | |
| M–W | 0.86 | 2.06 | 5 | 0.1409 | |
| E–M | 0.23 | 2.49 | 5 | 0.0422 | |
Regional comparisons include east (E; east of the Great Dividing Range (GDR)), mid (M; middle of the GDR), and west (W; west of the GDR) populations. Shown are the eigenvalues, approximate F-statistics, degrees of freedom (d.f.) and associated P values for each pairwise comparison. Statistically significant differences (P < 0.05) are indicated in bold.
For males, significant regional variation in overall cranium size was detected between the mid and west regions (M–W; Approx. F = −127.69, P < 0.0001), while no significant differences between the E–W and E–M was found. In females, cranium size varied significantly across all regional comparisons. For mid and west regions (M–W; Approx. F = −63,669.95, P < 0.0001), east and west (E–W; Approx. F = 4.41, P = 0.0011) and east and mid comparisons (E–M; Approx. F = 2.87, P = 0.0071).
For mandible size, males size showed a significant difference only between the east and mid regions (E–M; Approx. F = 2.81, P = 0.0115). No significant variation was found between the E–W or M–W regions. In females, mandible size differed significantly between the east and mid regions (E–M; Approx. F = 2.49, P = 0.0422), but comparisons between E–W and M–W regions were not significantly different.
Regional variation in linear measurements was observed in both males and females (Table 2). In males, individuals from the mid region generally exhibited the largest mean values for cranial and mandibular traits (e.g. MSL, EW, I1–P4), while east males were intermediate, and west males were typically smallest (based on limited sample size). Similarly, females from the mid region showed consistently larger means across most traits compared to east and west populations. Independent t-tests were conducted to compare linear measurements between mid-east regions for both males and females (Supplementary Information). Among males, significant regional differences were observed for EW (t = 3.58, d.f. = 10.81, P = 0.0044), RH (t = 2.85, d.f. = 14.47, P = 0.0125) and I2–M4 (t = −2.41, d.f. = 11.15, P = 0.0346), indicating population-level variation in cranial morphology. A near-significant difference was also noted for MSL (t = 2.11, d.f. = 11.88, P = 0.0563). In females, regional comparisons revealed a broader pattern of increased linear dimensions in the mid population. Statistically significant differences were detected in a range of cranial and mandibular traits, including MSL (P = 0.0029), BL, ZW, IOW, IFW, RW, UTR, I1–P1, EW, PB, ML, I–CP, and I–R (all P < 0.05), reflecting an overall increase in skull size. Only EW showed a statistically significant difference in both sexes, suggesting consistent regional differentiation in this trait (Supplementary Information).
| East | Mid | West | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trait | N | Mean | s.d. | N | Mean | s.d. | N | Mean | s.d. | |
| Males | ||||||||||
| MSL | 17 | 38.6 | 1.12 | 4 | 39.74 | 0.18 | 1 | 39.51 | ||
| BL | 17 | 34.17 | 1.12 | 4 | 35.92 | 1.0 | 1 | 34.06 | ||
| ZW | 18 | 26.59 | 0.81 | 4 | 27.37 | 0.46 | 1 | 28.29 | ||
| IOW | 18 | 8.15 | 0.41 | 4 | 8.29 | 0.43 | 1 | 8.21 | ||
| LW | 16 | 8.56 | 0.34 | 4 | 8.91 | 0.79 | 1 | 8.19 | ||
| NW | 15 | 6.98 | 1.04 | 4 | 6.8 | 0.77 | 1 | 6.25 | ||
| ANM | 18 | 2.84 | 0.29 | 4 | 2.98 | 0.16 | 1 | 2.69 | ||
| RH | 17 | 9.48 | 0.5 | 4 | 9.93 | 0.2 | 1 | 9.61 | ||
| ROW | 17 | 6.63 | 0.47 | 4 | 7.1 | 0.28 | 1 | 6.69 | ||
| UTR | 17 | 17.97 | 0.49 | 4 | 18.35 | 0.15 | 1 | 16.97 | ||
| UML | 17 | 6.72 | 0.48 | 4 | 6.66 | 0.19 | 1 | 6.58 | ||
| I1-P4 | 17 | 11.81 | 0.54 | 4 | 12.56 | 0.17 | 1 | 10.04 | ||
| I1-P1 | 17 | 8.58 | 0.69 | 4 | 8.84 | 0.43 | 1 | 8.2 | ||
| EW | 18 | 22.76 | 0.7 | 4 | 23.72 | 0.36 | 1 | 24.71 | ||
| PW | 16 | 7.01 | 0.33 | 4 | 7.48 | 0.38 | 1 | 7.39 | ||
| ML | 16 | 19.54 | 0.64 | 4 | 20.38 | 0.24 | 1 | 19.06 | ||
| LML | 18 | 7.61 | 0.68 | 6 | 7.49 | 0.26 | 2 | 7.23 | 0.8 | |
| MH | 18 | 13.44 | 0.67 | 6 | 13.54 | 0.75 | 2 | 11.5 | 3.08 | |
| I-CP | 18 | 20.04 | 0.9 | 6 | 20.69 | 1.28 | 2 | 18.28 | 1.9 | |
| I-R | 18 | 142.0 | 506.87 | 6 | 23.28 | 0.81 | 2 | 21.53 | 1.9 | |
| I2-M4 | 18 | 11.5 | 0.42 | 6 | 11.07 | 0.44 | 2 | 10.86 | 0.83 | |
| Females | ||||||||||
| MSL | 30 | 37.457 | 1.064 | 12 | 38.297 | 0.953 | 2 | 35.825 | 0.898 | |
| BL | 30 | 33.055 | 1.194 | 12 | 33.988 | 1.198 | 2 | 31.405 | 1.662 | |
| ZW | 30 | 25.844 | 0.758 | 12 | 26.518 | 0.887 | 2 | 25.41 | 0.297 | |
| IOW | 30 | 7.808 | 0.435 | 12 | 8.139 | 0.28 | 2 | 7.145 | 0.601 | |
| LW | 29 | 8.228 | 0.518 | 11 | 8.709 | 0.325 | 2 | 8.455 | 1.69 | |
| NW | 28 | 6.662 | 0.751 | 12 | 6.926 | 0.737 | 2 | 6.03 | 0.071 | |
| ANM | 29 | 2.773 | 0.252 | 12 | 2.744 | 0.282 | 2 | 2.565 | 0.332 | |
| RH | 30 | 9.375 | 0.497 | 11 | 9.306 | 0.415 | 2 | 8.58 | 0.58 | |
| ROW | 28 | 6.364 | 0.4 | 11 | 6.733 | 0.308 | 2 | 6.55 | 0.467 | |
| UTR | 30 | 17.411 | 0.658 | 12 | 18.022 | 0.552 | 2 | 16.62 | 0.608 | |
| UML | 30 | 6.511 | 0.39 | 12 | 6.652 | 0.358 | 2 | 6.435 | 0.431 | |
| I1-P4 | 29 | 11.669 | 0.4 | 12 | 11.762 | 0.648 | 2 | 9.655 | 1.846 | |
| I1-P1 | 30 | 8.25 | 0.617 | 12 | 8.879 | 0.837 | 2 | 7.23 | 1.711 | |
| EW | 30 | 22.291 | 0.896 | 12 | 22.803 | 0.874 | 2 | 22.46 | 0.028 | |
| PW | 30 | 6.922 | 0.305 | 11 | 7.275 | 0.4 | 2 | 6.865 | 0.318 | |
| ML | 30 | 19.242 | 0.61 | 11 | 19.792 | 0.546 | 2 | 18.31 | 0.636 | |
| LML | 34 | 7.389 | 0.441 | 13 | 7.595 | 0.385 | 3 | 6.65 | 0.55 | |
| MH | 34 | 12.844 | 0.881 | 13 | 13.104 | 0.42 | 3 | 12.42 | 0.213 | |
| I-CP | 34 | 19.622 | 0.963 | 12 | 20.402 | 0.653 | 3 | 18.637 | 1.075 | |
| I-R | 34 | 22.089 | 1.012 | 12 | 22.777 | 0.654 | 3 | 21.463 | 0.25 | |
| I2-M4 | 34 | 11.269 | 0.567 | 12 | 11.303 | 0.602 | 3 | 10.897 | 0.567 | |
All measurements are in millimetres.
Geometric morphometrics
Analyses were used to assess shape differences in crania and mandibles among east, mid and west populations of P. breviceps and P. notatus. Procrustes distances, Mahalanobis distances, and Hotelling’s T2 statistics were calculated for each pairwise regional comparison (Table 3). For cranium shape, significant differences were observed between the east and west regions (Procrustes distance = 0.03, Mahalanobis distance = 2.52, T2 = 92.28, P < 0.0001) and between the east and mid regions (Procrustes distance = 0.03, Mahalanobis distance = 3.64, T2 = 163.54, P = 0.0170). No significant shape differences were detected between mid and west populations. For mandible shape, a comparable pattern was found. East and west populations were significantly differentiated (Procrustes distance = 0.03, Mahalanobis distance = 2.57, T2 = 97.69, P < 0.0001), as were east and mid populations (Procrustes distance = 0.02, Mahalanobis distance = 2.44, T2 = 78.12, P = 0.0020). Differences between mid and west regions were not statistically significant (Table 3 and Fig. 4).
| Region | Procrustes distance | Mahalanobis distance | T2 | Procrustes distance | P value (T2) | |
|---|---|---|---|---|---|---|
| Cranium shape | ||||||
| EW | 0.03 | 2.52 | 92.28 | 0.002 | <0.0001 | |
| MW | 0.03 | 3.11 | 85.94 | 0.11 | 0.2020 | |
| EM | 0.03 | 3.64 | 163.54 | 0.33 | 0.0170 | |
| Mandible shape | ||||||
| EW | 0.03 | 2.57 | 97.69 | 0.002 | <0.0001 | |
| MW | 0.03 | 2.79 | 71.68 | 0.09 | 0.3140 | |
| EM | 0.02 | 2.44 | 78.12 | 0.08 | 0.0020 | |
For each comparison, Procrustes distance, Mahalanobis distance, and Hotelling’s T2 statistic are reported, along with associated P-values for Procrustes and T2 tests. Significant differences (P < 0.05) are shown in bold.
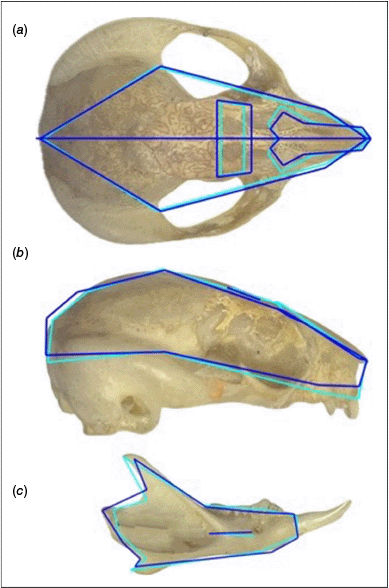
Significant morphological variation in cranium and mandible for east and mid labelled P. breviceps specimens. Shape represented by wireframes for cranial (a), lateral (b) and mandible (c). Light blue is the east specimens, and dark blue is mid specimens. East and mid populations divided by elevation of the Great Escapement and throughout the Great Dividing Ranges and south of Brisbane Valley Barrier.
Discussion
Multiple lines of evidence from the analysis of P. breviceps and P. notatus skulls have resulted in the identification of regionally specific variation in shape and size of skulls both within and between two species. Previous research based on specimens from the east and west margins of the distribution recognised specimens located along the GDR as P. breviceps (Cremona et al. 2021). Our research has found that the population currently recognised as P. breviceps, distributed along the GDR has a significantly different skull shape and size when compared with known P. breviceps on the coastal east of the GDR. Consistently, the mid GDR population has no significant skull shape difference when compared with the western P. notatus specimens.
The identification of skull shape characteristics in specimens within this previously unstudied region is inconsistent with the current identified distribution for P. breviceps. Our findings indicate that the distribution for P. notatus begins at the peak of the elevation of the GE, continues west and includes the GDR. The skull shape analysis reveals that the GDR population are more closely associated with the inland P. notatus rather than the coastal P. breviceps. Therefore, and from herein, we recognise those occurring from the GE and west as P. notatus.
The mid GDR female population were significantly larger than the west P. notatus females; there was also a significant size difference found for males. One identified climatic response in Petaurus, Bergmann’s Rule, could be an explanation for the increase in skull size for the GDR female population (Stobo-Wilson et al. 2020). This response is observed in faunal species typically responding to colder climates with an increase in size when compared to conspecifics in warmer climates. An increase in size (volume to surface area) facilitates improvement in body temperature regulation and therefore improves heat conservation (Mayr 1956; Thomas 2009; Olalla-Tárraga 2011). A more likely response explaining the difference in skull size found between the elevated GDR and western specimens is the climatic response in the western specimens. Central western regions experience higher seasonal temperatures compared with the GDR region (NSWDPI 2023). A likely climatic response for western specimens is a reduction in size, more suited to managing their body temperature in hotter conditions and previously identified in Petaurus species in other hotter regions in Australia (Quin et al. 1996; Stobo-Wilson et al. 2020; Powley and Mikac 2024). However, the adaptation has not been confirmed in males, indicating that it is unlikely that P. notatus located in the mid-GDR or west region are responding to this climatic effect alone.
Hybridisation offers a potential explanation for regional differences in skull form. Here hybridisation between P. notatus and Petaurus norfolcensis could occur in their shared distribution along the GDR (Crane et al. 2017; Baker and Gynther 2023), it has been reported in captivity (Zuckerman 1952) and in the wild (Knipler et al. 2021). P. norfolcensis have an approximately 30% longer skull length than P. notatus (Stobo-Wilson et al. 2020) and if hybridisation was occurring along the GDR, an increase in skull size may be expected. However, we found no regional evidence of hybridisation based on male skull size, or, for shape of the skull in either sex along the GDR. Consequently, it is unlikely that hybridisation is the only contributing factor for the size differences we found in females between western and mid-GDR regions.
A key finding from our research is the mid-GDR population species are morphologically consistent with P. notatus (Fig. 5). There is no significant shape difference found between the mid and west P. notatus populations. In NSW, the incline of the GE may disrupt distribution between mid P. notatus and eastern P. breviceps. It rises abruptly separating the coastline from the tablelands by several hundred, to over a thousand metres, in some regions (Pulsford et al. 2003). The significant shape differences found between the mid and east previously labelled P. breviceps specimens make the likelihood of these being the same species improbable. Geometric morphometric methods are effective for discriminating between cryptic species by providing evidence for a net difference (average shape difference) between groups (Zelditch et al. 2012), and has been effective for differentiating between species including mammals (Sztencel-Jabłonka et al. 2009; Calahorra-Oliart et al. 2021; Viacava et al. 2023; Alhajeri 2025). Further support for this finding would be gained with genetical sampling from within the identified regions.
East coast of Australia indicating proposed revised distribution for P. notatus and P. breviceps. Blue area indicates distribution for P. notatus based on consistency of shape of the cranium between east, mid or west regions of Great Dividing Range (GDR) (green line representing peak of GDR) and Brisbane Valley Barrier indicates end of distribution (red area) (Cremona et al. 2021; Flores-Rentería et al. 2021; Powley and Mikac 2024). Pink area indicates likely reduced geographic distribution for P. breviceps.
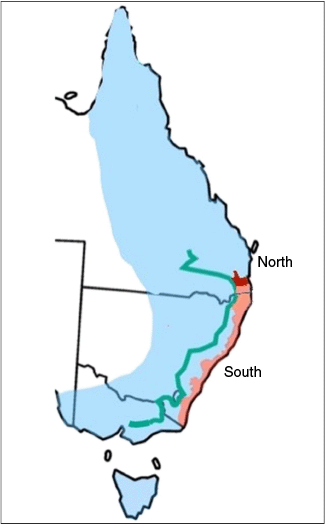
Conclusions
The geometric morphometric methods and analysis of linear measurements were used in the examination of skulls of P. breviceps and P. notatus throughout New South Wales. We found that P. notatus (previously labelled P. breviceps) specimens along the mid-GDR were significantly different to the east P. breviceps specimens. No significant shape difference between the mid-GDR populations with inland (west) P. notatus was found. The specimens provided a robust characterisation of size and shape for mid and eastern populations; however, a limited number of western specimen availability may be a limitation of this research. A future direction to further validate these findings would be the analysis of genetic material from live captured specimens from throughout the described distribution. Overall, these findings suggest that the geographic distribution of the east population of P. breviceps is likely again reduced from the defined region described in the recent taxonomic review.
Data availability
Most data that support this study are available in the article and accompanying online supplementary material. Other data that support this study will be shared upon reasonable request to the corresponding author.
Acknowledgements
The authors would like to thank the curators from the following Australian museums for access to the specimens; Australian Museum, Queensland Museum, Museums Victoria and South Australia Museum.
Author contributions
Conceptualisation Katarina Mikac, Data curation Meagan Powley, Formal analysis Meagan Powley, Funding acquisition Meagan Powley, Investigation Meagan Powley, Methodology Meagan Powley, Project administration Meagan Powley, Resources Katarina Mikac, Software Meagan Powley, Supervision Katarina Mikac, Validation Meagan Powley & Katarina Mikac, Visualisation Meagan Powley, Writing – original draft Meagan Powley, Writing – review & editing Meagan Powley & Katarina Mikac.
References
Alhajeri, B. H. (2025[In English]). Cranial variation in species and subspecies of kangaroo rats (Dipodomys, Dipodomyinae, Rodentia) according to geometric morphometrics. Integrative Zoology 20(1), 108-134.
| Crossref | Google Scholar | PubMed |
Beck, J., McCain, C. M., Axmacher, J. C., Ashton, L. A., Bärtschi, F., Brehm, G., Choi, S.-W., Cizek, O., Colwell, R. K., Fiedler, K., Francois, C. L., Highland, S., Holloway, J. D., Intachat, J., Kadlec, T., Kitching, R. L., Maunsell, S. C., Merckx, T., Nakamura, A., Odell, E., Sang, W., Toko, P. S., Zamecnik, J., Zou, Y., and Novotny, V. (2017). Elevational species richness gradients in a hyperdiverse insect taxon: a global meta-study on geometrid moths. Global Ecology and Biogeography 26(4), 412-424.
| Crossref | Google Scholar |
Briggs, J. C. (1974). Operation of Zoogeographic Barriers. Systematic Biology 23(2), 248-256.
| Crossref | Google Scholar |
Bryant, L. M., and Krosch, M. N. (2016). Lines in the land: a review of evidence for eastern Australia’s major biogeographical barriers to closed forest taxa. Biological Journal of the Linnean Society 119(2), 238-264.
| Crossref | Google Scholar |
Bui, E., and Henderson, B. (2013). C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant and Soil 373, 553-568.
| Crossref | Google Scholar |
Calahorra-Oliart, A., Ospina-Garcés, S. M., and León-Paniagua, L. (2021). Cryptic species in Glossophaga soricina (Chiroptera: Phyllostomidae): do morphological data support molecular evidence? Journal of Mammalogy 102(1), 54-68.
| Crossref | Google Scholar |
Campbell, C., Sarre, S., Stojanovic, D., Gruber, B., Medlock, K., Harris, S., Macdonald, A., and Holleley, C. (2018). When is a native species invasive? Incursion of a novel predatory marsupial detected using molecular and historical data. Diversity and Distributions 24, 831-840.
| Crossref | Google Scholar |
Cardini, A., Hoffmann, R. S., and Thorington, R. W., Jr (2005). Morphological evolution in marmots (Rodentia, Sciuridae): size and shape of the dorsal and lateral surfaces of the cranium. Journal of Zoological Systematics and Evolutionary Research 43(3), 258-268.
| Crossref | Google Scholar |
Cardini, A., de Jong, Y. A., and Butynski, T. M. (2022[In English]PMCID: PMC9298422). Can morphotaxa be assessed with photographs? Estimating the accuracy of two-dimensional cranial geometric morphometrics for the study of threatened populations of African monkeys. The Anatomical Record 305(6), 1402-1434.
| Crossref | Google Scholar | PubMed |
Cracraft, J. (1982). Geographic Differentiation, Cladistics, and Vicariance Biogeography: Reconstructing the Tempo and Mode of Evolution. American Zoologist 22(2), 411-424.
| Crossref | Google Scholar |
Crane, M., Lindenmayer, D. B., and Banks, S. C. (2017). Conserving and restoring endangered southern populations of the Squirrel Glider (Petaurus norfolcensis) in agricultural landscapes. Ecological Management & Restoration 18(1), 15-25.
| Crossref | Google Scholar |
Cremona, T., Baker, A. M., Cooper, S. J. B., Montague-Drake, R., Stobo-Wilson, A. M., and Carthew, S. M. (2021). Integrative taxonomic investigation of Petaurus breviceps (Marsupialia: Petauridae) reveals three distinct species. Zoological Journal of the Linnean Society 191, 503-527.
| Crossref | Google Scholar |
Dawson, R., and Milne, N. (2012). Cranial size and shape variation in mainland and island populations of the quokka. Journal of Zoology 288(4), 267-274.
| Crossref | Google Scholar |
Fedorov, A., Beichel, R., Kalpathy-Cramer, J., Finet, J., Fillion-Robin, J.-C., Pujol, S., Bauer, C., Jennings, D., Fennessy, F., Sonka, M., Buatti, J., Aylward, S., Miller, J.V., Pieper, S., and Kikinis, R. (2012). 3d Slicer as an image computing platform for the quantitative imaging network. Magnetic Resonance Imaging 30, 1323-1341.
| Crossref | Google Scholar | PubMed |
Feijó, A., and Cordeiro-Estrela, P. (2016). Taxonomic revision of the Dasypus kappleri complex, with revalidations of Dasypus pastasae (Thomas, 1901) and Dasypus beniensis Lönnberg, 1942 (Cingulata, Dasypodidae). Zootaxa 4170, 271-297.
| Crossref | Google Scholar | PubMed |
Flores-Rentería, L., Rymer, P.D., Ramadoss, N., and Riegler, M. (2021). Major biogeographic barriers in eastern Australia have shaped the population structure of widely distributed Eucalyptus moluccana and its putative subspecies. Ecology and Evolution 11, 14828-14842.
| Crossref | Google Scholar | PubMed |
Frankham, G. J., Handasyde, K. A., and Eldridge, M. D. B. (2012). Novel insights into the phylogenetic relationships of the endangered marsupial genus Potorous. Molecular Phylogenetics and Evolution 64(3), 592-602.
| Crossref | Google Scholar |
Frankham, G. J., Handasyde, K. A., and Eldridge, M. D. B. (2016). Evolutionary and contemporary responses to habitat fragmentation detected in a mesic zone marsupial, the long-nosed potoroo (Potorous tridactylus) in south-eastern Australia. Journal of Biogeography 43(4), 653-665.
| Crossref | Google Scholar |
Furlan, E., Griffiths, J., Gust, N., Handasyde, K., Grant, T., Gruber, B., and Weeks, A. (2013). Dispersal patterns and population structuring among platypuses, Ornithorhynchus anatinus, throughout south-eastern Australia. Conservation Genetics 14, 837-853.
| Crossref | Google Scholar |
Glor, R. E., and Warren, D. (2011). Testing ecological explanations for biogeographic boundaries. Evolution 65(3), 673-683.
| Crossref | Google Scholar | PubMed |
Hazlitt, S. L., Goldizen, A. W., Nicholls, J. A., and Eldridge, M. D. (2014[In English] PMCID: PMC3997325). Three divergent lineages within an Australian marsupial (Petrogale penicillata) suggest multiple major refugia for mesic taxa in southeast Australia. Ecology and Evolution 4(7), 1102-1116.
| Crossref | Google Scholar | PubMed |
IUCN (2023). The IUCN Red list of Threatened Species. Version 2022-2. Available at https://www.iucnredlist.org
James, C. H., and Moritz, C. (2000). Intraspecific phylogeography in the sedge frog Litoria fallax (Hylidae) indicates pre-Pleistocene vicariance of an open forest species from eastern Australia. Molecular Ecology 9(3), 349-358.
| Crossref | Google Scholar |
Jouventin, P., Cuthbert, R. J., and Ottvall, R. (2006). Genetic isolation and divergence in sexual traits: evidence for the northern rockhopper penguin Eudyptes moseleyi being a sibling species. Molecular Ecology 15(11), 3413-3423.
| Crossref | Google Scholar |
Keith, D. A., and Bedward, M. (1999). Native vegetation of the South East Forests region, Eden New South Wales. Cunninghamia 6, 1-218.
| Google Scholar |
Klingenberg, C.P. (2011). MorphoJ: An Integrated Software Package for Geometric Morphometrics. Molecular Ecology Resources 11, 353-357.
| Crossref | Google Scholar | PubMed |
Knipler, M. L., Dowton, M., and Mikac, K. M. (2021). Genome-Wide SNPs Detect Hybridisation of Marsupial Gliders (Petaurus breviceps breviceps × Petaurus norfolcensis) in the Wild. Genes 12(9), 1327.
| Crossref | Google Scholar | PubMed |
López-Aguirre, C., Pérez-Torres, J., and Wilson, L.A. (2015). Cranial and mandibular shape variation in the genus Carollia (Mammalia: Chiroptera) from Colombia: biogeographic patterns and morphological modularity. PeerJ 3, e1197.
| Crossref | Google Scholar | PubMed |
Mallen-Cooper, J., and Pickering, C. M. (2008a[In English]). Decline in Species Richness and Cover of Exotic Plants with Increasing Altitude. The Victorian Naturalist 125(3), 64-75.
| Google Scholar |
Mallen-Cooper, J., and Pickering, C. M. (2008b). Linear declines in exotic and native plant species richness along an increasing altitudinal gradient in the Snowy Mountains, Australia. Austral Ecology 33(5), 684-690.
| Crossref | Google Scholar |
Mayr, E. (1956). Geographical Character Gradients and Climatic Adaptation. Evolution 10(1), 105-108.
| Crossref | Google Scholar |
Milner, M. L., Rossetto, M., Crisp, M. D., and Weston, P. H. (2012). The impact of multiple biogeographic barriers and hybridization on species-level differentiation. American Journal of Botany 99(12), 2045-2057.
| Crossref | Google Scholar | PubMed |
NSWDPI (2023). ‘NSW State Seasonal Update - December 2022. Vol. 10.’ (NSW Department of Primary Industries: Orange, NSW.) Available at https://www.dpi.nsw.gov.au/climate-landing/ssu/nsw-state-seasonal-update-december-2022 [verified 22 January 2025].
Oilier, C. D. (1982). The Great Escarpment of eastern Australia: Tectonic and geomorphic significance. Journal of the Geological Society of Australia 29(1–2), 13-23.
| Crossref | Google Scholar |
Olalla-Tárraga, M. Á. (2011). “Nullius in Bergmann” or the pluralistic approach to ecogeographical rules: a reply to Watt et al. (2010). Oikos 120(10), 1441-1444.
| Crossref | Google Scholar |
Powley, M., and Mikac, K. (2024). Comparative Analysis of Petaurus Cryptic Species of ‘Sugar Glider’ from Australia and New Guinea Using 3D Geometric Morphometrics. Animals 14(24), 3680.
| Crossref | Google Scholar | PubMed |
Procheş, S. (2006[In English]). Latitudinal and longitudinal barriers in global biogeography. Biology Letters 2(1), 69-72.
| Crossref | Google Scholar | PubMed |
Pulsford, I., Worboys, G., Hudsn, J., and Shepherd, T. (2003). A Potential New Continental-scale Conservation Corridor for Australia: Combining the Australian Alps and the Great Escarpment of Eastern Australia Conservation Corridors. Mountain Research and Development 23, 292-294.
| Crossref | Google Scholar |
Quin, D. G., Smith, A. P., and Norton, T. W. (1996). Eco-geographic variation in size and sexual dimorphism in sugar gliders and squirrel gliders (marsupialia: Petauridae). Australian Journal of Zoology 44(1), 19-45.
| Crossref | Google Scholar |
Schneider, C. J., Smith, T. B., Larison, B., and Moritz, C. (1999). A test of alternative models of diversification in tropical rainforests: ecological gradients vs. rainforest refugia. Proceedings of the National Academy of Sciences 96(24), 13869-13873.
| Crossref | Google Scholar | PubMed |
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9(7), 671-675.
| Crossref | Google Scholar | PubMed |
Smith, M. J. (1973). Petaurus breviceps. Mammalian Species [30] 1-5.
| Crossref | Google Scholar |
Stobo-Wilson, A. M., Cremona, T., Murphy, B. P., and Carthew, S. M. (2020). Geographic variation in body size of five Australian marsupials supports Bergmann’s thermoregulation hypothesis. Journal of Mammalogy 101(4), 1010-1020.
| Crossref | Google Scholar |
Symula, R., Keogh, J. S., and Cannatella, D. C. (2008). Ancient phylogeographic divergence in southeastern Australia among populations of the widespread common froglet, Crinia signifera. Molecular Phylogenetics and Evolution 47(2), 569-580.
| Crossref | Google Scholar | PubMed |
Sztencel-Jabłonka, A., Jones, G., and Bogdanowicz, W. (2009). Skull Morphology of Two Cryptic Bat Species: Pipistrellus pipistrellus and P. pygmaeus — A 3D Geometric Morphometrics Approach with Landmark Reconstruction. Acta Chiropterologica 11(1), 113-126.
| Crossref | Google Scholar |
Thomas, G. H. (2009). Bergmann’s idiosyncratic rule: a role for fecundity selection?. Molecular Ecology 18(6), 1027-1029.
| Crossref | Google Scholar |
Van Dyck, S. (1990). Belideus gracilis - Soaring Problems for an Old De Vis glider. Memoirs of the Queensland Museum 28, 329-336.
| Google Scholar |
Viacava, P., Blomberg, S. P., Sansalone, G., Phillips, M. J., Guillerme, T., Cameron, S. F., Wilson, R. S., and Weisbecker, V. (2020). Skull shape of a widely distributed, endangered marsupial reveals little evidence of local adaptation between fragmented populations. Ecology and Evolution 10(18), 9707-9720.
| Crossref | Google Scholar |
Viacava, P., Blomberg, S. P., and Weisbecker, V. (2023). The relative performance of geometric morphometrics and linear-based methods in the taxonomic resolution of a mammalian species complex. Ecology and Evolution 13(3), e9698.
| Crossref | Google Scholar |
von Cramon-Taubadel, N., Frazier, B. C., and Lahr, M. M. (2007[In English]). The problem of assessing landmark error in geometric morphometrics: theory, methods, and modifications. American Journal of Physical Anthropology 134(1), 24-35.
| Crossref | Google Scholar | PubMed |
Zuckerman, S. (1952). The breeding seasons of mammals in captivity. Proceedings of the Zoological Society of London 122(3), 827-950.
| Crossref | Google Scholar |