Assessing the detectability of a cryptic arboreal marsupial by using a novel survey approach
Cassie Thompson A * , Leroy Gonsalves B , Brad Law B and Peter B. Banks A
A * , Leroy Gonsalves B , Brad Law B and Peter B. Banks A
A
B
Abstract
Non-detection of a species arising from inadequate sampling effort or ineffective techniques, may have serious consequences for its conservation, particularly of those that are declining. The threatened and cryptic eastern pygmy possum (Cercartetus nanus), despite its widespread distribution, is infrequently detected using standard trapping techniques (e.g. Elliott traps and spotlighting). There are no survey guidelines for the species, and published literature suggest detection often requires significant survey effort and therefore cost. In this study, we investigated the detectability of the eastern pygmy possum by using wildlife cameras focussed on nest boxes and nectar food resources. We collected detection data in bushland remnants in northern Sydney over 5 years by using these methods and modelled detection probability. Detection probability was highest during winter in each year, which coincided with banksia flowering and breeding events, but detectability varied across survey years. We found that cameras targeting flowering banksia achieved a 95% detection probability from an average trapping effort of 117 camera nights, compared with 237 camera nights at nest boxes. We conclude that targeted use of wildlife cameras may be a cost-effective alternative to labour-intensive standard survey methods or to supplement existing survey approaches (e.g. nest box checks) and improve detection probability.
Keywords: banksia, camera, Cercartetus nanus, cryptic, detectability, eastern pygmy possum, novel method, survey method.
Introduction
Accurate detection of presence or absence is important for the conservation and management of fauna species (Burns et al. 2019). Environmental impact assessments rely on accurate species detection to ensure that species are properly assessed, appropriate mitigation measures are applied, and any biodiversity losses resulting from development are effectively offset (Brownlie et al. 2013). In such instances, methods need to be species-specific, with a high level of accuracy, and appropriate to employ in a cost-efficient manner. The level of survey effort required to determine the presence or true absences at a location can be significant for some species, particularly for those that are considered to be cryptic (Burns et al. 2019; Harley and Eyre 2024). Small arboreal mammals are often difficult to survey by using conventional techniques such as trapping and spotlighting (Moore et al. 2021). These methods have known sampling biases (Tasker and Dickman 2001), even for terrestrial fauna (Johnstone et al. 2021), and high rates of false absences for arboreal mammals (Wintle et al. 2005).
The arboreal eastern pygmy possum (Cercartetus nanus) is a threatened species native to eastern Australia and is commonly considered to be cryptic and hard to survey (Bladon et al. 2002; Harris and Goldingay 2005b; Harris et al. 2007b). It generally occurs in low-density populations (Bowen and Goldingay 2000), which may be missed and assumed absent because of inappropriate survey techniques or survey effort. Despite its seemingly widespread occurrence, the eastern pygmy possum is rarely recorded during fauna surveys, particularly using standard mammal survey techniques (e.g. Elliott trapping and spotlighting) (Bowen and Goldingay 2000). For example, Suckling (1978) used spotlighting, ground-based Elliott trapping and trialled the use of baited hair tubes (30 mm wide PVC pipe open at both ends) at a survey site where the species was known to occur; yet, no individuals were recorded. Bowen and Goldingay (2000) reviewed previous surveys in New South Wales (NSW) and found at that time, that only five extensive surveys recorded more than 10 individuals. Similarly, other burramyids such as the little pygmy possum (Cercartetus lepidus) and long-tailed pygmy possum (Cercartetus caudatus) appear difficult to detect, owing to the lack of studies recording them; however, pitfall traps have shown success for surveying the western pygmy possum (Cercartetus concinnus) (Pestell and Petit 2007) and the little pygmy possum (Ward 1992; Duncan and and Taylor 2001).
Traditional methods for detecting eastern pygmy possums, such as Elliott and pitfall trapping, have shown limited and inconsistent success. Elliott trapping appears more effective when used repeatedly in the same location (Ward 1990; Laidlaw and Wilson 1996), particularly near flowering banksias (Bowen and Goldingay 2000; Harris and Goldingay 2005a), although this increases effort and the standard bait mix may be inadequate because it competes with natural foods. Pitfall traps have reported higher capture rates than spotlighting or Elliott trapping in some studies (Bennett et al. 1989; Bowen and Goldingay 2000; Tasker and Dickman 2001; Tulloch and Dickman 2006); yet, large-scale surveys in NSW State Forests recorded only one capture from over 10,000 pitfall trap nights (Bowen and Goldingay 2000). Spotlighting, although common for arboreal mammals, is considered less reliable for this species (Davey 1990; Kavanagh and Webb 1998). The literature offers little clear guidance on the most effective survey methods, and the species is regarded as ‘very difficult to detect, especially via spotlighting’ (eastern pygmy possum; Ecological Data, BioNet Atlas, NSW). Traditional trapping techniques are also labour-intensive and potentially harmful to animal welfare (Garden et al. 2007). Given this and the low detectability of the eastern pygmy possum with these methods, alternative reliable approaches are needed.
There has been an increase in the use of, and it has been suggested that the species is most reliably detected in nest boxes (Bowen and Goldingay 2000; Goldingay 2023). Nest boxes have been successfully applied to study the effects of fragmentation on demography of the eastern pygmy possum (Bladon et al. 2002), life history (Ward 1990) and the effects of habitat, movements and social organisation (Harris and Goldingay 2005b; Law et al. 2013; Harris et al. 2014; Goldingay and Keohan 2017; Goldingay and Rueegger 2018; Law et al. 2018; Goldingay 2019, 2023). Previous studies have also investigated optimal box design to record eastern pygmy possums (Beyer and Goldingay 2006; Rueegger et al. 2012). A recent study found a detection probability of 0.35 per survey visit for a cluster of five nest boxes (Chew et al. 2024). However, like conventional techniques, nest boxes can represent a labour-intensive survey method because of the ongoing need for physical checks at a site, ongoing maintenance, and these have not always proven effective for detection, particularly in areas where hollows are abundant (Harris and Goldingay 2005a).
Camera traps are rapidly becoming a preferred tool for recording mammal species (Bowler et al. 2017). Camera traps have been found to be more effective than live trapping (Bondi et al. 2010) and hair tunnels (Paull et al. 2011) for detecting small mammals, and despite high initial costs, camera traps have been found to be less costly than labour-intensive conventional methods in the long term (Welbourne et al. 2015). Even though camera trapping can be as effective and efficient in determining occupancy for some arboreal mammals as it is for terrestrial species (Harley and Eyre 2024), few studies have specifically used camera traps to detect the eastern pygmy possum. Cameras have been used to identify behaviour and assess nest box preferences of eastern pygmy possums (Rueegger et al. 2012). Another study in Jervis Bay (NSW) used a range of standard methods (Elliott and cage traps) but only detected the eastern pygmy possum on camera traps on the ground by using drift netting (Harris et al. 2007). Cameras with infrared triggers have also been used to record pollinators on banksia inflorescence and successfully recorded eastern pygmy possums (Carthew 1993). Infrared cameras also found that eastern pygmy possums were the most frequent small mammal visitor spending significant periods at inflorescence of heath-leaved banksia (Banksia ericifolia) in bushland reserves in northern Sydney (Saul 2013; O’Rourke et al. 2020), suggesting cameras focussed at resources may optimise detections.
In this study, we assess the efficacy of camera traps for detecting the eastern pygmy possum at nest boxes and flowering food resources, in all seasons and over a 5-year period that spanned a range of climatic conditions, including a significant drought that resulted in the 2019/2020 megafires across eastern Australia, although the study area was not burnt. The use of cameras has the potential to reduce field effort to detect cryptic species, given that these can be set up to collect several months of data from one installation. We modelled detection probability by using camera traps focused on banksia inflorescences and nest boxes (artificial hollows), to assess which method is best to maximise the likelihood of detection for eastern pygmy possums when using cameras. We also assessed how detection probability varied among seasons and years. Cumulative detection probability curves were generated to guide survey effort required to achieve a high degree of confidence in absences for the species at surveyed sites.
Materials and methods
Our study area comprised seven bushland remnant localities on the urban edge in northern Sydney, Australia, located within the Northern Beaches local government area on the Ingleside escarpment (Fig. 1). This area is dominated by a mixture of urban and semi-rural land uses interspersed with remnant vegetation. Vegetation at the seven localities comprised heathland or heathy woodland on sandstone dominated by flowering Myrtaceae and Proteaceae species, with an abundance of Banksia spp. Cameras were installed at or near nest boxes that had successfully captured eastern pygmy possums as part of an ongoing population study (C. Thompson, unpubl. data) (Table 1). Nest boxes were made from salvaged hollows of varying sizes (but with a minimum internal diameter of 10 cm), end caps made of steel, and a drilled opening of 3 cm near the top of the box. These were installed between 0.5 and 1.8 m from the ground on banksia or eucalypt trees, to allow for checking without a ladder. Groups of 5 or 10 nest boxes were installed at each bushland remnant, along a transect at a spacing of 50–100 m apart. Given that a typical home range for an eastern pygmy possum is approximately 3–4 ha (Harris et al. 2007a; Law et al. 2013), each transect was separated by at least 500 m, where there was no impermeable barrier (e.g. large roads, development and fences). Not all nest boxes were used for this study, because these were installed and are being monitored for an ongoing population study (C. Thompson et al., unpubl. data) associated with road crossing structures (‘underpass’ and ‘overpass’). The crossing structure study had not recorded any tagged eastern pygmy possums crossing the road over the 8 years of survey, thus the ‘north’ and ‘south’ localities associated with the crossing structure locations were considered independent for this study. Similarly, Goldingay (2023) recorded only two road crossings (road width of approximately 10 m) between nest boxes from >100 tagged individuals.
| Site name | Approximate patch size (ha) | Number of nest boxes installed at site | Number of camera locations included in analysis per method (across all years) | ||
|---|---|---|---|---|---|
| Nestbox | Banksia | ||||
| Laurel Road Reserve | 98 | 5 | 3 | 0 | |
| Overpass north | 25 | 10 | 4 | 2 | |
| Overpass south | 98 | 10 | 4 | 3 | |
| Powderworks Road Quarry | 9 | 5 | 0 | 1 | |
| Underpass north | 25 | 10 | 4 | 3 | |
| Underpass south | 12 | 10 | 2 | 4 | |
| Wesley Road Reserve | 98 | 5 | 2 | 0 | |
Reconyx hyperfire (HC600 and PC800) covert infrared cameras were set to detect eastern pygmy possums by directing them at either flowering banksias or nest boxes. In total, 16 camera locations at nest boxes, and 12 camera locations at banksia inflorescence in proximity to nest box locations were established. Cameras sampling flowering banksia were installed in proximity to installed nest boxes (within 20 m); however, both camera types were not used concurrently. The cameras have inbuilt passive infrared LED motion sensors and were installed ~0.5 m in front of the boxes or banksia inflorescence on trees and secured with straps, with a high passive infrared (PIR) sensitivity. Cameras were not changed for close-proximity photos. On the basis of pilot testing of the methods, this appeared to be a sufficient distance to avoid overexposed images and to allow for identification of all mammals to species level. Cameras were set to record a sequence of 10 photos; however, this was reduced to five photos after the first year of survey given the number of false triggers and number of images to process. A quiet period of 1 min was set before they could be triggered again. For each site (nest box/banksia inflorescence), a detection of at least one eastern pygmy possum per night was scored as ‘presence’. This was done to reduce counting the same individual multiple times and to provide a ‘detection’ or ‘non-detection’ score per survey night per camera for detection modelling. Capture rates at each camera location were calculated on the basis of the number of detections (i.e. one image of an eastern pygmy possum per camera per night) divided by the total camera nights and expressed as a percentage.
Heath-leaved banksia was the dominant banksia species in the study area. This species is a known important local food resource for eastern pygmy possums (Tulloch and Dickman 2007; Goldingay and Keohan 2017; Goldingay 2023) and its inflorescences produce copious amounts of sugary nectar at night (Carpenter 1978). Heath-leaved banksia flowers in autumn through winter and sometimes into spring (Copland and Whelan 1989; Goldingay 2023); thus, cameras were installed only within these seasons for the banksia cameras. Flowering duration of individual inflorescences varies considerably, but may be less than 4 weeks (Copland and Whelan 1989). Banksia inflorescences were targeted for survey on the basis of flower maturity and nectar secretion, and to allow a minimum recording period of 30 days, so as to allow for the highest nectar availability to be sampled over the camera deployment across autumn, winter and spring (Fig. 2). Nest box cameras were not limited to the flowering season of heath-leaved banksia and recorded across several seasons. Images were individually processed using an image viewer and tagged. Detected fauna were identified to species level where possible, using reference images and texts (Van Dyck and Strahan 2008). Data were then collated as a detection history (presence/absence) for input into the modelling.
Covert infrared camera trained on a flowering heath-leaved banksia (Banksia ericifolia) inflorescence in northern Sydney (left). Covert infrared camera trained on a salvaged log nest box in northern Sydney (right).

An occupancy model was used to assess detection probabilities by using data generated by cameras at the seven bushland localities, with data collected over 5 years. Because camera numbers for the study were limited, cameras were not installed consistently within localities over the survey period, but shifted among nest boxes and banksia flowers across the survey period, resulting in detection/non-detection data and periods of no survey for each camera location (Table 2). To ensure independence, detection histories were prepared for each camera location for input into the model. A ‘site’ was included for each camera type (banksia or nest box) within a locality (e.g. Laurel Road Reserve), resulting in one record (detection/non-detection) or NA if the camera was moved to another site, per night. In total, 32 sites were sampled. Data analyses were run using the RPresence package (ver. 2.15.17; http://www.mbr-pwrc.usgs.gov/software/presence.html; MacKenzie and Hines 2023), by using a single season occupancy modelling framework, because we were modelling only detection probabilities. Site occupancy modelling accounts for the potential for imperfect detectability (i.e. false absences that result when a species is present but not detected), by using information from repeated observations at sample sites occupied to estimate detection probabilities (Mackenzie 2005).
| Year | Number of eastern pygmy possum detection nights (total camera nights) by camera survey method | ||
|---|---|---|---|
| Nestbox | Banksia | ||
| 2018 | 12 (98) | 0 (0) | |
| 2019 | 55 (590) | 0 (0) | |
| 2020 | 61 (1239) | 12 (60) | |
| 2021 | 5 (1130) | 9 (113) | |
| 2022 | 0 (107) | 9 (396) | |
Detection probability (p) was modelled with occupancy (psi) held constant. Detection was modelled to compare covariate models with a null model. We assessed single covariates and built on the most supported single covariate model by adding an additional covariate until there was no improvement (i.e. adding an additional variable did not improve the top model by >2 AIC points). We included year as a covariate in all detection models to account for any changes in detection probability associated with year of sampling. Variables assessed were season (austral spring, summer, autumn and winter) and survey method (camera on nest box or camera on banksia inflorescence). Banksia inflorescence could be sampled only during particular seasons, i.e. when flowering; however, nest boxes were sampled across a range of seasons. Detection models were ranked from the lowest to highest on the basis of Akaike’s information criterion (AIC) (Burnham and Anderson 2004), with the difference in AIC (ΔAIC) calculated between each model and the top-ranked model. Models with ΔAIC of <2 were considered equally plausible to explain the data (Burnham and Anderson 2004).
To evaluate the minimum survey effort required to confidently detect the eastern pygmy possum at a site when present, we calculated the cumulative detection probability (P) by using the standard formula from MacKenzie and Royle (2005), as follows:
where
P is the cumulative probability of detecting the species at least once over k independent surveys,
p is the estimated single-survey detection probability, and
k is the number of surveys (or camera nights in this context).
This approach allowed us to estimate the number of repeated surveys required to achieve a desired cumulative detection probability (typically ≥0.95) under different conditions and detection methods, in this case for different detection methods (e.g. cameras trained on banksia inflorescences vs nest boxes), stratified by season and year to reflect observed variability. Given the strong effect of year of survey on detection probability, estimates from 2020 were chosen to guide minimum survey duration to achieve high detection probability (95%) for eastern pygmy possum. This year was chosen because it provided an intermediate detection probability value (i.e. not the extreme ends) and represented a year where both banksia and nest box cameras were used.
Results
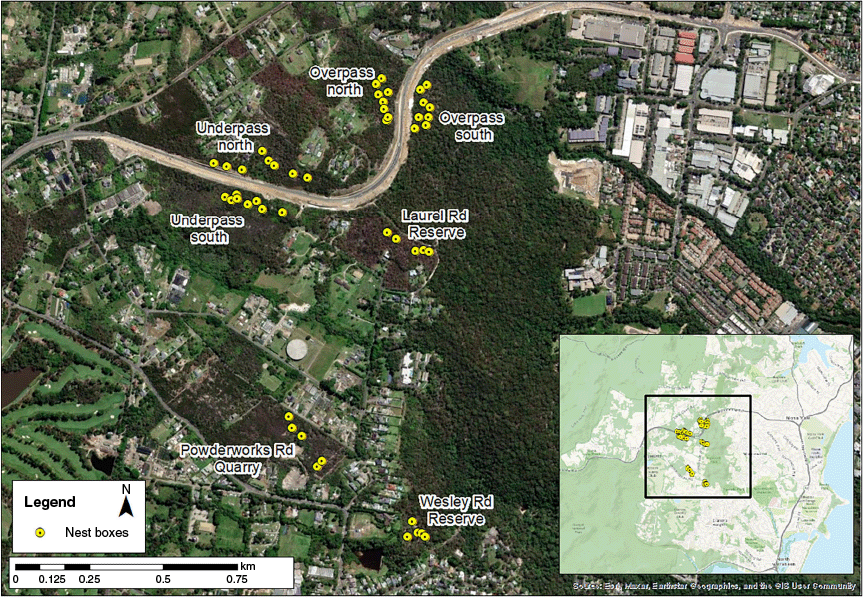
During 2018–2022, 133 eastern pygmy possum detection nights were recorded by using cameras at nest boxes (during 2147 camera nights), whereas cameras at banksia inflorescences recorded 30 eastern pygmy possum detection nights (703 camera nights) (Fig. 3). Detection nights decreased across the survey period for nest box cameras and banksia cameras (Fig. 4).
Example images of the eastern pygmy possum (Cercartetus nanus) by using covert infrared cameras trained on nest boxes (left) and flowering banksia inflorescence (right).

Nightly eastern pygmy possum (Cercartetus nanus) activity (total detections/total camera type × 100 per year) across years for both banksia and nest box cameras in northern Sydney. NA, no banksia cameras were used in 2018 and 2019 and only one nest box camera was used in 2022, but did not get any detections in the period deployed.
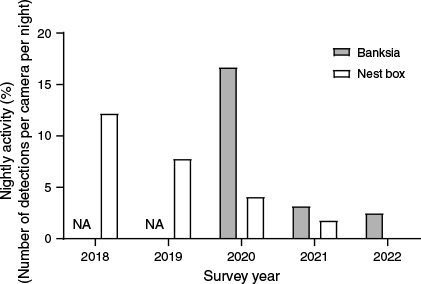
Modelling showed that a single model explained the data better than any other (Table 3). This additive model allowed detection probability to vary among years of survey, among seasons and between surveys by using cameras trained on banksia inflorescences or nest boxes. The detection probability was highest in winter and lowest in spring (Fig. 5a). Modelled detection probability was more than 2.7 times higher for cameras on banksia inflorescences than for cameras on nest boxes (Fig. 5b). This trend was found for all years in which both survey methods were employed (Fig. 6). Detection probability for banksia cameras in winter ranged from 0.23 in 2000, to 0.03 in 2022, and for nest box cameras in winter from 0.29 in 2018 to 0.01 in 2022 (Fig. 6).
(a) Detection probability versus season for eastern pygmy possum (Cercartetus nanus) in northern Sydney by using cameras (assuming sampling was undertaken in 2020 and with camera on banksia). (b) Detection probability versus camera type for eastern pygmy possum in northern Sydney (assuming sampling was undertaken in 2020 and in winter).
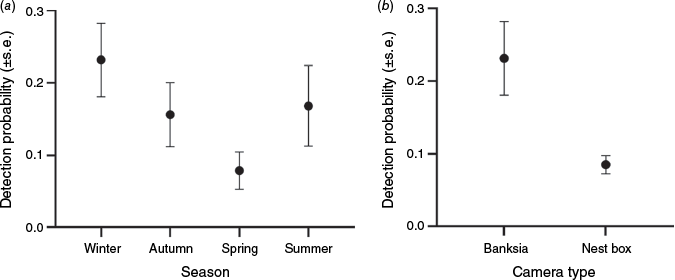
Influence of survey year on detection probabilities for eastern pygmy possum (Cercartetus nanus) in northern Sydney by using banksia and nest box cameras.
| Model | DAIC | AIC | wgt | npar | neg2ll | |
|---|---|---|---|---|---|---|
| psi(.), p(Year + Season + Banksia) | 0.00 | 1183.1 | 0.99 | 10 | 1163.10 | |
| psi(.), p(Year + Season) | 10.76 | 1193.86 | 0.01 | 9 | 1175.86 | |
| psi(.), p(Year + Spring) | 13.91 | 1197.01 | 0.00 | 7 | 1183.01 | |
| psi(.), p(Year + Winter) | 17.44 | 1200.54 | 0.00 | 7 | 1186.54 | |
| psi(.), p(Year + Banksia) | 28.93 | 1212.03 | 0 | 7 | 1198.03 | |
| psi(.), p(Year) | 48.97 | 1232.07 | 0 | 6 | 1220.07 | |
| psi(.), p(Year + Autumn) | 50.94 | 1234.04 | 0 | 7 | 1220.04 |
Supported models shaded in grey. DAIC is the difference in Akaike information criterion (AIC) values between a given model and the model with the lowest AIC. Season is autumn, winter, spring, or summer, where only one season (e.g. winter) is included in a model, that season is being contrasted to all others, which are equal. wgt, model weight; npar, number of parameters; neg2ll, negative log-likelihood; psi, occupancy; and p, detection probability.
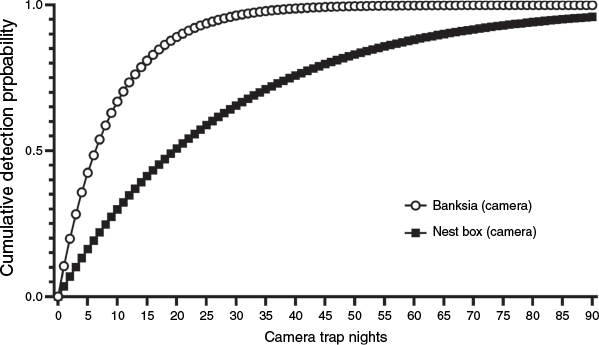
Minimum camera night requirements at an occupied site to be 95% confident of detecting eastern pygmy possum with a camera trained on a banksia inflorescence in winter was 28 nights (assuming detection probabilities recorded in 2020) or, on average, 117 nights (when detection probability was averaged across the 3 years of surveys) for one camera. The minimum level of sampling needed to achieve a similar detection probability for a camera on a nest box was 85 nights in winter (assuming detection probabilities recorded in 2020) or 237 nights, when averaging across the 5 years of survey for one camera (Fig. 7).
Survey duration versus cumulative detection probability (2020 + season + banksia, with occupancy held constant) for the eastern pygmy possum (Cercartetus nanus) in northern Sydney bushland remnants on the basis of single-visit detection by using nest box cameras or banksia inflorescence cameras in winter.
Discussion
Influence of survey method on detection probability
Wildlife cameras are an invaluable tool for the detection of rare and elusive arboreal species if used correctly, particularly for species that are small and nocturnal, making observations from the ground at night extremely difficult (Bowler et al. 2017). We found that camera traps trained on banksia inflorescence yielded a 95% detection probability, on average, from 117 camera nights (with a range of 28–222 camera nights across the three survey years) and outperformed cameras trained on nest boxes. Camera traps may provide a more cost-effective alternative than do traditional survey methods that require daily trap checks, particularly for cryptic species with reported low capture rates (Bowen and Goldingay 2000). Although image processing adds to effort, advances in artificial intelligence (AI) may reduce this burden (Vélez et al. 2023).
Few studies have estimated detection probabilities for eastern pygmy possums while accounting for imperfect detection. However, studies on similar cryptic marsupials have demonstrated the value of modelling detectability, including sugar gliders (Petaurus notatus) where bait type significantly influences detection rates (Owens et al. 2024), and broader vertebrate communities in northern Australia where occupancy models account for low detection probabilities in small mammals (Einoder et al. 2018). For the eastern pygmy possum, a recent study found a detection probability of 0.35 per visit for clusters of nest boxes when spread across all seasons on the Central Coast, NSW (Chew et al. 2024). This translates to an estimated survey effort of seven nest box checks to a cluster of five nest boxes at a site to have confidence of 95% of detecting the species. Another study reported detection probability of 0.21 per visit in autumn/winter (translating to 13 nest box checks) and 0.48 or five nest box checks for any age–sex-class males (Goldingay 2023). Both studies were completed ina similar habitat to that of the study area within myrtaceous-rich woodland with a dominance of banksia. We found comparable detection probabilities by using single cameras trained on banksia flowers, although this was year-dependent (0.01–0.10 per night per camera) and for cameras trained on nest box cameras (0.01–0.14 per night per camera).
A potential limitation of remote methods is the species’ use of torpor (Nowack et al. 2016; Geiser et al. 2018), which may lead to non-detection on camera, but not during physical checks. However, repeat sampling by using cameras may offset this limitation. Eastern pygmy possums can enter deep, multi-day torpor throughout the year (Geiser 1993; Turner et al. 2012); yet, in some areas torpor was rare (e.g. 1 in 499 observations near Sydney; Goldingay and Rueegger 2018). Increased survey duration can help mitigate false absences owing to torpor. Additionally, cameras may be less intrusive for torpid individuals, avoiding energetic costs associated with disturbance (Sørås et al. 2022).
Capture rates vary widely across survey methods, from 0.93% (pitfall traps), to 1.7% (Elliot traps), and 9.1% (nest boxes). The nest box capture rate drops to 4.3% by excluding an outlier Bladon et al. (2002), which had pre-clearing trapping rates of 33.5% that have not been replicated in any other study (Bowen and Goldingay 2000) (Table 4). In our study, single cameras on nest boxes had a 5.2% capture rate, whereas banksia cameras reached 7.5%. Rueegger et al. (2012) found positive detections at 72.5% of nest boxes deployed by using cameras, including 10 with no records from manual checks. This supports the value of cameras in improving detection and cost-effectiveness.
| Method | Average capture rate for method (%) | Average capture rate (detections per method night, %) when detected (study), location | |
|---|---|---|---|
| Nestboxes | 9.13 | 33.5% (Bladon et al. 2002 pre-clearing), Dorrigo NSW | |
| 7.8% (Bladon et al. 2002 post-clearing), Dorrigo NSW | |||
| 4.46% (Chew et al. 2024), Central Coast, NSW | |||
| 3.82% (Rueegger et al. 2012), Royal National Park, NSW | |||
| 3% (Harris and Goldingay 2005b), Barren Grounds Nature Reserve, NSW | |||
| 2.2% (Harris et al. 2014), Barren Grounds Nature Reserve, NSW | |||
| Pitfall traps | 0.93 | 2.22% (Shelly 1998), central-west, NSW | |
| 0.33% (Goldingay and Daly 1998), Queenbeyan, NSW | |||
| 0.5% (Braithwaite 1983), Eden, NSW | |||
| 0.65% (Rueegger et al. 2012), Royal National Park | |||
| Elliott traps | 1.7 | 0.07% (Whelan et al. 1996), Royal National Park, NSW ground Elliots | |
| 2.24% (Goldingay et al. 1987), Barren Grounds Nature Reserve, NSW | |||
| 1.94% (Goldingay et al. 1991), Budderoo National Park, NSW | |||
| 0.44%.(Rueegger et al. 2012), Royal National Park, NSW | |||
| 3.8% (Harris et al. 2014), Barren Grounds Nature Reserve, NSW adjacent to flowering banksia and with honey-water mixture near trap (none in ground traps) | |||
| 4.03% (Harris and Goldingay 2005b), Barren Grounds Nature Reserve, NSW | |||
| 0.3% (Laidlaw and Wilson 1996), Otways, Victoria (VIC), with ground Elliots | |||
| 2.5% (Evans and Bunce 2000), Wilsons Promontory National Park, VIC, in banksia trees | |||
| 0.02% (Tasker and Dickman 2001), north-eastern NSW | |||
| Pitfalls and Elliots | 0.84 | 0.68% (Tulloch and Dickman 2006), Royal and Heathcote National Park, NSW | |
| 0.14% (Harris et al. 2007a), ground and tree Elliots and pitfalls in Jervis Bay, NSW | |||
| 1.7% (Tulloch and Dickman 2007), Royal and Heathcote National Park, NSW | |||
| Spotlighting | 1.29 | 1.29% (Davey 1990), southern coast, NSW | |
| Baited hairtubes | 0.17 | 0.04% (Dickman and Happold 1987), Australian Capital Territory (ACT) | |
| 0.3% (Tulloch and Dickman 2006), Royal National Park, NSW |
Influence of season on detection probability
Detection probability peaked in winter and was lowest in spring for nest box cameras. Although Chew et al. (2024) found no seasonal effect in nest box detectability, in that study sample imbalances may have masked trends. Goldingay (2023) reported a four-fold increase in detection of breeding females in nest boxes in autumn/winter compared with spring/summer. This trend is likely to align with flowering of key banksia and breeding periods, as has been observed elsewhere (Ward 1990; Bladon et al. 2002; Tulloch and Dickman 2006; Law et al. 2013; Goldingay 2023). We recommend that camera surveys coincide with peak flowering of dominant local nectar resources.
Influence of year on detection probability
Detectability can reflect population abundance (Kéry and Schmidt 2008; McCarthy et al. 2012). As populations decline, previously adequate survey effort may no longer suffice (Burns et al. 2019), highlighting the need to account for detection probability when monitoring trends (Gonsalves et al. 2024). We observed declining detectability over time, which resulted in increased survey effort required for 95% detection, from 21 to 715 camera nights (nest box cameras) from 2018 to 2022, and 28 to 222 camera nights (banksia cameras) from 2020 to 2022. This may be reflecting possum abundance over the survey period. The study period coincided with a prolonged drought and the 2019–2020 bushfires. Although the study area was unburnt, drought is likely to have affected local possum populations. Heathlands dominated by banksia are vulnerable to declining precipitation and climate change (Yates et al. 2010), and banksias show reduced reproduction in drought (Poot et al. 2012). Thus, declines in detectability may reflect drought-driven reductions in plant productivity and, subsequently, possum abundance. Eastern pygmy possums may have also shifted to alternative habitats outside camera detection zones, which is known to occur in western pygmy possums, which can travel long distances in times of drought to forage (Morrant and Petit 2012). It is also possible that individuals may move out of the typical banksia-rich microhabitat areas where cameras were located on both nest boxes and on banksias, in search of other food resources.
Implications for survey effort
Targeting nest boxes or flowering banksias with cameras offers a low-cost alternative to live trapping, especially with advances in AI and cheaper equipment. Welbourne et al. (2015) found that camera trapping used ~62% fewer consumables and only ~27% of the labour compared with live trapping. Achieving 95% detection by using nest box checks of clusters of five boxes requires seven monthly visits (Chew et al. 2024), whereas our study indicated similar confidence with 3 months of banksia camera data or 8 months from nest box cameras, requiring only two site visits if using suitable cameras with sufficient storage. Use of more than one camera per site would reduce this sampling effort.
Traditional methods for sampling small arboreal mammals often require canopy access (Moore et al. 2021). Our cameras were deployed at 0.5–1.8 m and were easily accessible. This aligns with known height preferences of eastern pygmy possums (Evans and Bunce 2000; Law et al. 2018). Remote cameras are increasingly used to detect arboreal mammals and can be more cost-effective than are traditional approaches (Gonsalves et al. 2024). Accurate placement within the camera’s PIR detection band is essential, because most are designed for large terrestrial species (Debruille et al. 2020). Although baits and lures may improve detection, their success varies because they compete with background natural foods (Garvey et al. 2020; Johnstone et al. 2021). Banksia flowers, being both abundant and a known food resource, acted as an effective natural lure. Unlike standard baits (e.g. peanut butter/oats or sugar spray), native floral resources may be more attractive to nectarivores such as the eastern pygmy possum, but this remains untested. This method could benefit other hard-to-detect nectarivores, including other burramyids and cryptic small mammals such as feathertail gliders, although none was detected in our study.
The efficacy of cameras in habitats lacking heath-leaved banksias (e.g. inland regions or other banksia species such as B. serrata or B. integrifolia) is not clear. The species also uses other food plants (e.g. Xylomelum pyriforme, Doryanthes excelsa, Corymbia gummifera, Lambertia formosa) (Carthew 1993; Tulloch and Dickman 2007; Law et al. 2018). Future studies could test floral resources with seasonal blooms, although camera placement height may pose challenges. Outside banksia-dominated habitats, alternative natural food sources and bait strategies should be investigated. We recommend pilot studies in different regions and habitats to determine appropriate survey effort and reduce false absences. Long-term monitoring should account for changes in detectability related to survey method, camera technology, and population changes (Gonsalves et al. 2024).
In conclusion, wildlife cameras offer a non-invasive, cost-effective method to detect cryptic arboreal species when used to tap into natural behaviours. We tested a novel camera-based approach for eastern pygmy possums targeting natural food sources and found comparable or higher detection probabilities and capture rates than those reported using standard methods, particularly in banksia-rich areas. However, cameras provide limited data on demography or population dynamics essential for management. Modelling techniques may compensate for this to some extent (Gracanin and Mikac 2022). Nonetheless, we recommend continued use of nest box checks to complement cameras, especially outside banksia-dominated habitats.
Data availability
The data that support this study are available in the BioNet Atlas at https://www2.environment.nsw.gov.au/topics/animals-and-plants/biodiversity/nsw-bionet
References
Bennett, A. F., Schulz, M., Lumsden, L. F., Robertson, P., and Johnson, P. G. (1989). Pitfall trapping of small mammals in temperate forests of southeastern Australia. Australian Mammalogy 12(1), 37-39.
| Crossref | Google Scholar |
Beyer, G. L., and Goldingay, R. L. (2006 [in English]). The value of nest boxes in the research and management of Australian hollow-using arboreal marsupials. Wildlife Research 33(3), 161-174.
| Crossref | Google Scholar |
Bladon, R. V., Dickman, C. R., and Hume, I. D. (2002). Effects of habitat fragmentation on the demography, movements and social organisation of the eastern pygmy-possum Cercartetus nanus) in northern New South Wales. Wildlife Research 29(1), 105-116.
| Crossref | Google Scholar |
Bondi, N., White, J., Stevens, M., and Cooke, R. (2010). A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities. Wildlife Research 37, 456-465.
| Crossref | Google Scholar |
Bowen, M., and Goldingay, R. (2000). Distribution and Status of The Eastern Pygmy Possum (Cercartetus nanus) in New South Wales. Australian Mammalogy 21(2), 153-164.
| Crossref | Google Scholar |
Bowler, M. T., Tobler, M. W., Endress, B. A., Gilmore, M. P., and Anderson, M. J. (2017). Estimating mammalian species richness and occupancy in tropical forest canopies with arboreal camera traps. Remote Sensing in Ecology and Conservation 3(3), 146-157.
| Crossref | Google Scholar |
Braithwaite, L. W. (1983). Studies on the arboreal marsupial fauna of eucalypt forests being harvested for woodpulp at Eden, NSW. I. The species and distribution of animals. Wildlife Research 10(2), 219-229.
| Crossref | Google Scholar |
Brownlie, S., Nicholas, K., and Treweek, J. (2013). Biodiversity tradeoffs and offsets in impact assessment and decision making: can we stop the loss? Impact Assessment and Project Appraisal 31(1), 24-33.
| Crossref | Google Scholar |
Burnham, K. P., and Anderson, D. R. (2004). Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociological Methods & Research 33(2), 261-304.
| Crossref | Google Scholar |
Burns, P. A., McCall, C., Rowe, K. C., Parrott, M. L., and Phillips, B. L. (2019). Accounting for detectability and abundance in survey design for a declining species. Diversity and Distributions 25(10), 1655-1665.
| Crossref | Google Scholar |
Carthew, S. M. (1993). An assessment of pollinator visitation to Banksia spinulosa. Australian Journal of Ecology 18(3), 257-268.
| Crossref | Google Scholar |
Chew, D. J. I., Law, B., Leo, V., Southwell, D. M., Anson, J. R., and Hayward, M. W. (2024). Eastern pygmy possum (Cercartetus nanus) populations persist in Central Coast forests after the Black Summer bushfires. Australian Mammalogy 46(3), AM24011.
| Crossref | Google Scholar |
Copland, B. J., and Whelan, R. J. (1989). Seasonal Variation in Flowering Intensity and Pollination Limitation of Fruit Set in Four Co-Occurring Banksia Species. Journal of Ecology 77(2), 509-523.
| Crossref | Google Scholar |
Davey, S. (1990). Methods for Surveying the Abundance and Distribution of Arboreal Marsupials in a South Coast Forest of New-South-Wales. Wildlife Research 17(4), 427-445.
| Crossref | Google Scholar |
Debruille, A., Kayser, P., Veron, G., Vergniol, M., and Perrigon, M. (2020). Improving the detection rate of binturongs (Arctictis binturong) in Palawan Island, Philippines, through the use of arboreal camera-trapping. Mammalia 84(6), 563-567.
| Crossref | Google Scholar |
Dickman, C. R., and Doncaster, C. P. (1987). The Ecology of Small Mammals in Urban Habitats. I. Populations in a Patchy Environment. Journal of Animal Ecology 56(2), 629-640.
| Crossref | Google Scholar |
Duncan, A. M. R., and Taylor, R. J. (2001). Occurrence of pygmy possums, Cercartetus lepidus and C. nanus, and their nest sites in logged and unlogged dry and wet eucalypt forest in Tasmania. Australian Forestry 64(3), 159-164.
| Crossref | Google Scholar |
Einoder, L. D., Southwell, D. M., Lahoz-Monfort, J. J., Gillespie, G. R., Fisher, A., and Wintle, B. A. (2018). Occupancy and detectability modelling of vertebrates in northern Australia using multiple sampling methods. PLoS One 13(9), e0203304.
| Crossref | Google Scholar | PubMed |
Evans, K., and Bunce, A. (2000). A comparison of the foraging behaviour of the eastern pygmy-possum (Cercartetus nanus) and nectarivorous birds in a Banksia integrifolia woodland. Australian Mammalogy 22(1), 81-86.
| Crossref | Google Scholar |
Garden, J. G., McAlpine, C. A., Possingham, H. P., and Jones, D. N. (2007). Using multiple survey methods to detect terrestrial reptiles and mammals: what are the most successful and cost-efficient combinations? Wildlife Research 34(3), 218-227.
| Google Scholar |
Garvey, P. M., Banks, P. B., Suraci, J. P., Bodey, T. W., Glen, A. S., Jones, C. J., McArthur, C., Norbury, G. L., Price, C. J., Russell, J. C., and Sih, A. (2020). Leveraging Motivations, Personality, and Sensory Cues for Vertebrate Pest Management. Trends in Ecology & Evolution 35(11), 990-1000.
| Crossref | Google Scholar | PubMed |
Geiser, F. (1993). Hibernation in the Eastern Pygmy Possum, Cercartetus-Nanus (Marsupialia, Burramyidae). Australian Journal of Zoology 41(1), 67-75.
| Crossref | Google Scholar |
Geiser, F., Stawski, C., Doty, A. C., Cooper, C. E., and Nowack, J. (2018 [in English]). A burning question: what are the risks and benefits of mammalian torpor during and after fires? Conservation Physiology 6, coy057.
| Crossref | Google Scholar | PubMed |
Goldingay, R. L. (2019). Does temperature variation influence nest box use by the eastern pygmy-possum? Australian Mammalogy 42, 77-84.
| Crossref | Google Scholar |
Goldingay, R. L. (2023). Habitat use by the eastern pygmy-possum in a coastal woodland–heathland mosaic. Australian Mammalogy 45, 275-284.
| Crossref | Google Scholar |
Goldingay, R., and Daly, G. (1998). Surveys of Arboreal and Terrestrial Mammals in The Montane Forests of Queanbeyan, New South Wales. Australian Mammalogy 20(1), 9-19.
| Crossref | Google Scholar |
Goldingay, R. L., and Keohan, J. (2017). Population density of the eastern pygmy-possum in a heath–woodland habitat. Australian Journal of Zoology 65(6), 391-397.
| Crossref | Google Scholar |
Goldingay, R. L., and Rueegger, N. (2018). Elevation induced variation in the breeding traits of a nectar-feeding non-flying mammal. Ecological Research 33(5), 979-988.
| Crossref | Google Scholar |
Goldingay, R., Carthew, S. M., and Whelan, R. J. (1987). Transfer of Banksia-Spinulosa Pollen by Mammals: Implications for Pollination. Australian Journal of Zoology 35, 319-325.
| Google Scholar |
Goldingay, R. L., Carthew, S. M., and Whelan, R. J. (1991). The Importance of Non-Flying Mammals in Pollination. Oikos 61(1), 79-87.
| Crossref | Google Scholar |
Gonsalves, L., Law, B., and Slade, C. (2024). Landscape-scale monitoring for forest fauna is achievable: a case study using remote sensors, artificial intelligence and robust analytics. Australian Zoologist 43(4), 526-544.
| Crossref | Google Scholar |
Gracanin, A., and Mikac, K. M. (2022). The Use of Selfie Camera Traps to Estimate Home Range and Movement Patterns of Small Mammals in a Fragmented Landscape. Animals 12(7), 912.
| Google Scholar |
Harley, D., and Eyre, A. (2024). Ten years of camera trapping for a cryptic and threatened arboreal mammal: a review of applications and limitations. Wildlife Research 51(2), WR23054.
| Crossref | Google Scholar |
Harris, J., and Goldingay, R. (2005a). Detection of the eastern pygmy-possum Cercartetus nanus (Marsupialia: Burramyidae) at Barren Grounds Nature Reserve, New South Wales. Australian Mammalogy 27(1), 85-88.
| Crossref | Google Scholar |
Harris, J., and Goldingay, R. (2005b). Distribution, habitat and conservation status of the eastern pygmy-possum Cercartetus nanus in Victoria. Australian Mammalogy 27(2), 185-210.
| Crossref | Google Scholar |
Harris, J. M., Goldingay, R. L., Broome, L., Craven, P., and Maloney, K. S. (2007a). Aspects of the ecology of the eastern pygmy-possum Cercartetus nanus at Jervis Bay, New South Wales. Australian Mammalogy 29(1), 39-46.
| Crossref | Google Scholar |
Harris, J. M., Munks, S. A., Goldingay, R. L., Wapstra, M., and Hird, D. (2007b). Distribution, habitat and conservation status of the eastern pygmy-possum Cercartetus nanus in Tasmania. Australian Mammalogy 29(2), 213-232.
| Crossref | Google Scholar |
Harris, J. M., Goldingay, R. L., and Brooks, L. O. (2014 [in English]). Population ecology of the eastern pygmy-possum (Cercartetus nanus) in a montane woodland in southern New South Wales. Australian Mammalogy 36(2), 212-218.
| Crossref | Google Scholar |
Johnstone, K. C., McArthur, C., and Banks, P. B. (2021). Behavioural drivers of survey bias: interactive effects of personality, the perceived risk and device properties. Oecologia 197(1), 117-127.
| Crossref | Google Scholar | PubMed |
Kavanagh, R., and Webb, G. (1998). Effects of variable-intensity logging on mammals, reptiles and amphibians at Waratah Creek, southeastern New South Wales. Pacific Conservation Biology 4, 326-347.
| Crossref | Google Scholar |
Kéry, M., and Schmidt, B. R. (2008). Imperfect detection and its consequences for monitoring for conservation. Community Ecology 9(2), 207-216.
| Crossref | Google Scholar |
Laidlaw, W. S., and Wilson, D. B. A. (1996). The home range and habitat utilisation of Cercartetus nanus (Marsupialia: Burramyidae) in coastal heathland, Anglesea, Victoria. Australian Mammalogy 19, 63-68.
| Google Scholar |
Law, B., Chidel, M., Britton, A., and Brassil, T. (2013). Response of eastern pygmy possums, Cercartetus nanus, to selective logging in New South Wales: home range, habitat selection and den use. Wildlife Research 40(6), 470.
| Crossref | Google Scholar |
Law, B., Chidel, M., Britton, A., and Threlfall, C. (2018). Comparison of microhabitat use in young regrowth and unlogged forest by the eastern pygmy-possum (Cercartetus nanus). Australian Mammalogy 40(1), 1-9.
| Crossref | Google Scholar |
Carpenter, F. L. (1978). Hooks for mammal pollination? Oecologia 35(2), 123-132.
| Crossref | Google Scholar | PubMed |
Mackenzie, D. I. (2005). Was it there? Dealing with imperfect detection for species presence/absence data. Australian & New Zealand Journal of Statistics 47(1), 65-74.
| Crossref | Google Scholar |
Mackenzie, D. I., and Royle, J. A. (2005). Designing occupancy studies: general advice and allocating survey effort. Journal of Applied Ecology 42(6), 1105-1114.
| Crossref | Google Scholar |
Moore, J. F., Soanes, K., Balbuena, D., Beirne, C., Bowler, M., Carrasco-Rueda, F., Cheyne, S. M., Coutant, O., Forget, P.-M., Haysom, J. K., Houlihan, P. R., Olson, E. R., Lindshield, S., Martin, J., Tobler, M., Whitworth, A., and Gregory, T. (2021). The potential and practice of arboreal camera trapping. Methods in Ecology and Evolution 12(10), 1768-1779.
| Crossref | Google Scholar |
Morrant, D. S., and Petit, S. (2012). Strategies of a small nectarivorous marsupial, the western pygmy-possum, in response to seasonal variation in food availability. Journal of Mammalogy 93(6), 1525-1535.
| Crossref | Google Scholar |
Nowack, J., Delesalle, M., Stawski, C., and Geiser, F. (2016). Can hibernators sense and evade fires? Olfactory acuity and locomotor performance during deep torpor. The Science of Nature 103(9), 73.
| Crossref | Google Scholar | PubMed |
O’Rourke, R. L., Anson, J. R., Saul, A. M., and Banks, P. B. (2020). Limits to alien black rats (Rattus rattus) acting as equivalent pollinators to extinct native small mammals: the influence of stem width on mammal activity at native Banksia ericifolia inflorescences. Biological Invasions 22(2), 329-338.
| Crossref | Google Scholar |
Owens, G., Gracanin, A., Potts, J., Young, C. M., Heinsohn, R., Gibbons, P., and Stojanovic, D. (2024). Detection and density estimation for a cryptic species. Austral Ecology 49(2), e13467.
| Crossref | Google Scholar |
Paull, D. J., Claridge, A. W., and Barry, S. C. (2011). There’s no accounting for taste: bait attractants and infrared digital cameras for detecting small to medium ground-dwelling mammals. Wildlife Research 38(3), 188-195.
| Crossref | Google Scholar |
Pestell, A. J. L., and Petit, S. (2007). Methods and ethical considerations of pitfall trapping for the western pygmy possum (Cercartetus concinnus Gould) (Marsupialia:Burramyidae), with observations on capture patterns and nest sites. Wildlife Research 34(4), 296-305.
| Crossref | Google Scholar |
Rueegger, N. N., Goldingay, R. L., and Brookes, L. O. (2012). Does nest box design influence use by the eastern pygmy-possum? Australian Journal of Zoology 60(6), 372.
| Crossref | Google Scholar |
Shelly, D. (1998 [in English]). Survey of vertebrate fauna and habitats in a cypress pine-ironbark forest in Central-West New South Wales. Australian Zoologist 30(4), 426-436.
| Crossref | Google Scholar |
Sørås, R., Fjelldal, M. A., Bech, C., van der Kooij, J., Skåra, K. H., Eldegard, K., and Stawski, C. (2022). State dependence of arousal from torpor in brown long-eared bats (Plecotus auritus). Journal of Comparative Physiology B 192(6), 815-827.
| Crossref | Google Scholar | PubMed |
Suckling, G. (1978). A Hair Sampling Tube for the Detection of Small Mammals in Trees. Wildlife Research 5(2), 249-252.
| Crossref | Google Scholar |
Tasker, E., and Dickman, C. (2001). A review of Elliott trapping methods for small mammals in Australia. Australian Mammalogy 23(2), 77-87.
| Crossref | Google Scholar |
Tulloch, A. I., and Dickman, C. R. (2006 [in English]). Floristic and structural components of habitat use by the eastern pygmy-possum (Cercartetus nanus) in burnt and unburnt habitats. Wildlife Research 33(8), 627-637.
| Crossref | Google Scholar |
Tulloch, A. I., and Dickman, C. R. (2007 [in English]). Effects of food and fire on the demography of a nectar-feeding marsupial: a field experiment. Journal of Zoology 273(4), 382-388.
| Crossref | Google Scholar |
Turner, J. M., Körtner, G., Warnecke, L., and Geiser, F. (2012 [in English]). Summer and winter torpor use by a free-ranging marsupial. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology 162(3), 274-280.
| Crossref | Google Scholar | PubMed |
Vélez, J., McShea, W., Shamon, H., Castiblanco-Camacho, P. J., Tabak, M. A., Chalmers, C., Fergus, P., and Fieberg, J. (2023). An evaluation of platforms for processing camera-trap data using artificial intelligence. Methods in Ecology and Evolution 14(2), 459-477.
| Crossref | Google Scholar |
Ward, S. (1990). Life-History of the Eastern Pygmy-Possum, Cercartetus-Nanus (Burramyidae, Marsupialia), in South-Eastern Australia. Australian Journal of Zoology 38(3), 287-304.
| Crossref | Google Scholar |
Ward, S. (1992). Life-History of the Little Pygmy-Possum, Cercartetus-Lepidus (Marsupialia, Burramyidae), in the Big Desert, Victoria. Australian Journal of Zoology 40(1), 43-55.
| Crossref | Google Scholar |
Welbourne, D. J., MacGregor, C., Paull, D., and Lindenmayer, D. B. (2015). The effectiveness and cost of camera traps for surveying small reptiles and critical weight range mammals: a comparison with labour-intensive complementary methods. Wildlife Research 42(5), 414-425.
| Crossref | Google Scholar |
Wintle, B. A., Kavanagh, R. P., McCarthy, M. A., and Burgman, M. A. (2005). Estimating and dealing with detectability in occupancy surveys for forest owls and arboreal marsupials. Journal of Wildlife Management 69(3), 905-917.
| Google Scholar |
Yates, C. J., Mcneill, A., Elith, J., and Midgley, G. F. (2010). Assessing the impacts of climate change and land transformation on Banksia in the South West Australian Floristic Region. Diversity and Distributions 16(1), 187-201.
| Crossref | Google Scholar |