Should animal fats be back on the table? A critical review of the human health effects of animal fat
William BarendseCSIRO Animal, Food and Health Sciences, 306 Carmody Road, St Lucia, Qld 4067, Australia. School of Veterinary Science, University of Queensland, Gatton, Qld 4343, Australia. Email: Bill.Barendse@csiro.au
Animal Production Science 54(7) 831-855 https://doi.org/10.1071/AN13536
Submitted: 13 December 2013 Accepted: 27 March 2014 Published: 5 May 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Humans hunt or raise a wide variety of animals for meat, which vary from free-range to intensively reared. These animals form a valuable part of human nutrition. Their tissues, including the fat, contain vitamin and other essential nutrients necessary for health. However, animal fat from ruminants and other land mammals is usually regarded as saturated. The purpose of this review is partly to examine the basis for the saturated fat hypothesis of cardiovascular disease given more recent research, to examine the human health effects of animal fats, and partly to draw into one place the diverse knowledge about animal fat and the effects of fat on metabolism. Mechanistic understanding of the initiation of the fatty streak and atherosclerosis calls into question the avoidance of ruminant or porcine fat. Due to high levels of oleic acid, a low n-6 : n-3 fatty acid ratio in some groups, and the presence of specific micronutrients including vitamins and essential fatty acids, animal fats are of benefit in human nutrition. Animal fats can be obtained in minimally processed form making them a convenient source of energy and micronutrients.
Additional keywords: cardiovascular disease, docosahexaenoic acid (DHA), ketogenic, obesity, saturated fat.
Introduction
What butter and whiskey will not cure there’s no cure for – Irish Proverb
‘For example, in Framingham, Mass, the more saturated fat one ate, the more cholesterol one ate, the more calories one ate, the lower the person’s serum cholesterol.’ – William Castelli, 3rd Director of the Framingham Heart Study (1992).
Humans have used animals and animal products for food including fish, seafood, insects, birds, reptiles, and mammals since time immemorial. These are either caught wild, are harvested as free-range animals, or are raised at varying degrees of intensity for a wide range of markets. They are a source not only of nutrition but also of gustatory pleasure, and many are symbols or are eaten during special occasions. But, with high rates of cardiovascular disease (CVD) and, more recently, high rates of obesity in the West, first fat of all kinds and then saturated fat have been put under the microscope as aetiological agents in chronic disease. Many observers have noted that the trend of usage of animal fats has been in the opposite direction to the rise in CVD or obesity (Yudkin 1957; Antar et al. 1964; Kritchevsky 1976; Enig et al. 1978; Blaxter and Webster 1991; Eisenmann 2003; Carlson et al. 2011; Chapman et al. 2011). Furthermore, with the discovery of the effects on CVD of industrial trans fats, animal fats have made a resurgence in the popular literature, on the internet, and in low carbohydrate diet books (Taubes 2001, 2007). The diet wars of the 1960s and 1970s have reappeared (Yudkin 1964; Keys 1971), with a renewed focus on the effects of sucrose and fructose in obesity, CVD, cancer and type 2 diabetes as opposed to fat (Miller et al. 2011; Hoenselaar 2012; Lustig et al. 2012; Basu et al. 2013). There is a renewed popularity of low carbohydrate high fat diets, as well as the scientific study of them (Foster et al. 2003; Volek et al. 2003; Yancy et al. 2004). Indeed, it now appears that genes contribute to whether one will drop out of a low calorie diet depending on whether it is low fat or high fat (Grau et al. 2009). Despite these changes in society, high quality food has always featured animal fats because of their taste and during the past 40 years there has been no deviation from that practice (Escoffier 1921; Bocuse 1988; Carluccio and Contaldo 2012). The purpose of this review is to investigate whether there are any health benefits in putting animal fats back on the table.
One justification for the unrestricted use of animal fats is the archaeological, ethnographic, and historical evidence that for 1–2 million years before the invention of agriculture humans were omnivorous hunter-gatherers consuming animal tissues including fat (Richards and Trinkaus 2009; Sponheimer and Dufour 2009; Stiner and Munro 2011; Ungar and Sponheimer 2011). Some humans still occupy this niche, and their hunting and gathering habits have been documented (Murdock 1967; Cordain et al. 2002a; Rouja et al. 2003). Organs and fat were generally preferred to lean meat by these hunter-gatherers who were well aware of the value of eating animal fat: the side effects of eating only lean meat have been replicated in the laboratory and they include diarrhoea and unsatisfied hunger (Stefansson 1912; McClellan and Du Bois 1930; Phinney 2004). During human evolution, how much fat was eaten on a daily or yearly basis is a matter for speculation, and would depend on a host of factors. Given the nausea limit in human responses to large amounts of fat (Man and Gildea 1932), one suspects as much fat as could be stomached. These ancestral patterns of food use do not prescribe any particular modern diet or lifestyle but they do show the likely human nutritional adaptations and responses to food, and point to the nutrients that need to be obtained from food (Cordain et al. 2005; Lindeberg 2009).
Saturated fat and cardiovascular disease
The degree of saturation of animal fat
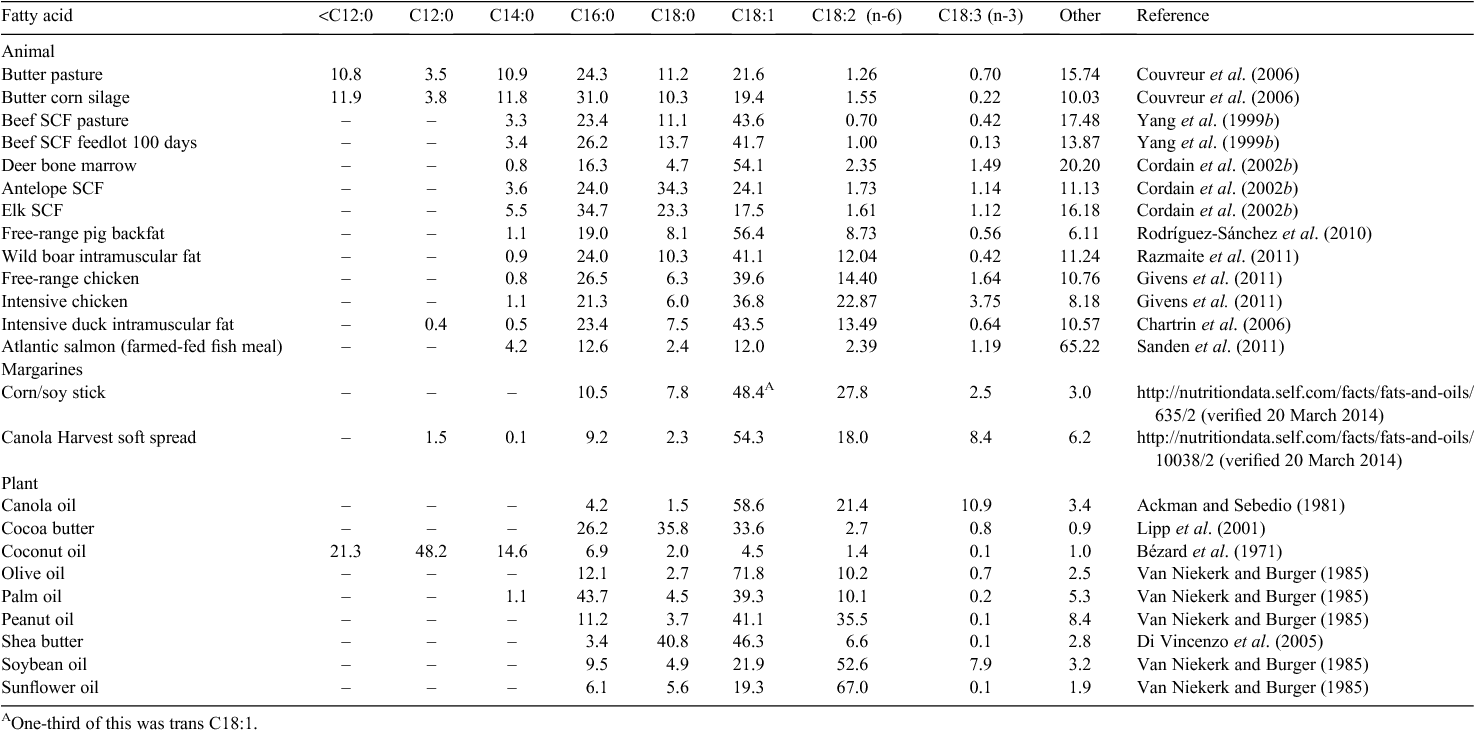
Although animal fats are described as saturated and containing cholesterol, apart from butter and some fatty fish, animal fat is best described as monounsaturated either by content or by function (Table 1) for its effect on serum cholesterol. First, dietary cholesterol makes a second order contribution to serum cholesterol, that is, its effect is proportional to the square root of dietary intake, leading to minor changes in serum cholesterol, its importance has been debunked, and it has long been ignored in prediction of CVD risk (Keys et al. 1965, 1974). Second, as can be seen from the table, the major component of the triacylglycerol (TAG, triglycerides) of animal fat is monounsaturated fatty acid (MUFA), mostly oleic acid, irrespective of how solid the fat is at room temperature, and in some cases oleic acid consists of more than 50% of all fatty acids (FA). Even when oleic acid is less than 50% of all the FA, there are other MUFA and FA that do not affect overall serum cholesterol. As an example from one of the hardest animal fats, approximately only 27% of tallow from pasture-fed beef is cholesterol-increasing saturated fatty acid (CISFA) (Yang et al. 1999b), i.e. chain length of 12–16 carbons, and which would raise serum cholesterol, 1% is polyunsaturated, ~4% is conjugated linoleic acid (CLA), and the rest is either MUFA or is the saturated fatty acid (SFA) stearic acid that causes the same effect on total serum cholesterol (TSC) as MUFA (Keys et al. 1965; Grande et al. 1970; Bonanome and Grundy 1988; Tholstrup et al. 1994a, 1994b; de Roos et al. 2001; Mensink et al. 2003). By comparison, in butter from pasture-fed cows, 42% of the fat is CISFA (Couvreur et al. 2006) and would raise serum cholesterol despite butter having a total of more than 60% SFA. Butter differs from other animal fats in having a large amount of short- and medium-chain SFA, which are rapidly oxidised by the liver instead of being stored in adipose tissue or transported by lipoprotein cholesterol particles (Bach and Babayan 1982; DeLany et al. 2000) – the amount of palmitate in butter is similar to other animal fats. The distinguishing features of most animal fats, compared with most plant oils, is the low variability in proportion of palmitate to the total amount of FA, compared with the large range in proportion of other FA, the relatively large proportion of FA with odd number of carbons in the acyl backbone or with variable numbers and locations of unsaturated bonds, and the low to very low levels of n-6 polyunsaturated fatty acids (PUFA), especially in ruminant fat.
The effects of fat on human blood lipids
Fat and cholesterol are carried by chylomicrons and lipoprotein cholesterol particles in circulation, and because serum cholesterol has been associated with risk of CVD, fat intake has been implicated in CVD, although detailed analyses show results contrary to this expectation. The effect of different FA on the average TSC of the sample, its sub-fractions, fasting triacylglycerides (FTG) and the number and size of low density lipoprotein cholesterol (LDL-C) particles have been determined on largely inactive people in studies in metabolic wards. The description of these average changes in TSC and FTG have been studied for more than 50 years, they are well known and are predictable using the Keys equation (Man and Gildea 1932; Havel et al. 1955; Ahrens et al. 1957; Havel 1957a, 1957b; Keys et al. 1957, 1965; Albrink and Man 1959; Kuo and Carson 1959; Grande et al. 1970; Acheson et al. 1988; Tholstrup et al. 1994a, 1994b; de Roos et al. 2001; McDevitt et al. 2001; Mensink et al. 2003; Matthan et al. 2004; Chapman et al. 2011; Miller et al. 2011). These changes can be summarised by saying, first, that substituting MUFA and stearic acid for starch causes no change in the average serum cholesterol of a population sample, although LDL-C concentration decreases while high density lipoprotein cholesterol (HDL-C) concentration increases. Second, average serum cholesterol is increased as the proportion of CISFA increases and is decreased as the proportion of PUFA increases. The increases due to CISFA are twice the size of decreases due to PUFA for each unit of CISFA or PUFA. Third, HDL-C and FTG of an individual are inversely related, so high FTG is usually correlated with both low HDL-C and a pattern of low fat plus high carbohydrate intake. High FTG is strongly correlated with small dense LDL-C, to expanded waistlines and to other features of the metabolic syndrome, which is strongly predictive of increased risk of CVD (Castelli 1986; Boerwinkle et al. 1994; Gardner et al. 1996; Stampfer et al. 1996; Lamarche et al. 1997; Barrows and Parks 2006; Roberts et al. 2008). While serum cholesterol and, particularly, LDL-C concentrations of the individual are used to calculate risk of CVD, of more importance to the mechanistic development of CVD is the size and number of LDL-C particles in circulation and the composition of cholesteryl-esters (cf. below).
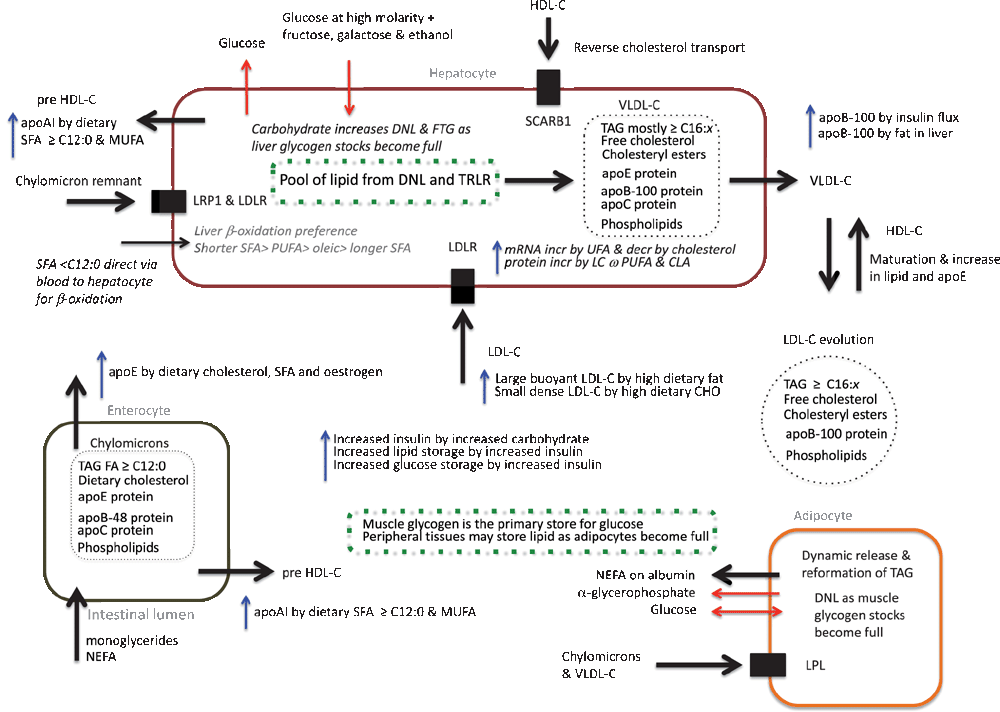
An overview of fat and cholesterol transport is shown in Fig. 1 and shows five major phases that are each affected by dietary and genetic factors. These phases are (1) the entry of FA into enterocytes and the formation of chylomicrons, (2) the transport of fat and cholesterol via chylomicrons in the circulation to tissues, (3) the processing of chylomicron remnants by the liver and the formation of very low density lipoprotein cholesterol (VLDL-C) in the liver, (4) the transport of fat and cholesterol via VLDL-C and its remnant, LDL-C, to tissues, and (5) the release of FA from adipocytes to be carried on albumin to tissues via the blood (Chapman et al. 2011). Starting with the enterocytes, for cholesterol and fat transport, the HDL-C fraction requires apolipoprotein (apo) A series molecules and the huge chylomicrons require apoB-48, apoC and apoE (Utermann 1988; Chapman et al. 2011). After being carried to all tissues via the circulation the remnants of chylomicrons are processed by the liver. Dietary FA shorter than 12 carbons are not carried by chylomicrons but are dissolved in blood and carried to the liver via the Portal vein (Bach and Babayan 1982). At the liver, the VLDL-C fraction, composed of fat and cholesterol from chylomicron remnants and from that synthesised in the liver, requires apoB-100, apoC, and apoE for transport (Utermann 1988). As these mature to LDL-C, the apoC and apoE proteins and cholesterol are lost to HDL-C, leaving only apoB-100 as well as progressively depleted LDL-C (Chapman et al. 2011). The longer the LDL-C particle stays in circulation the smaller and denser it becomes. HDL-C is part of the reverse transport of cholesterol, carrying it back to the liver where it is recycled (Fielding and Fielding 1995). Particles with apoE are taken up faster than particles with only apoB, because apoB can only be processed by the LDL receptor (LDLR) (Ishibashi et al. 1994). Thus chylomicrons and VLDL-C have a faster level of clearance from the circulation than LDL-C. Finally, when insulin levels decline, fat stored in adipocytes is released as non-esterified fatty acids (NEFA, also free fatty acids) transported on albumin (Cahill 2006; Hodson et al. 2008; Marinou et al. 2011).
The characteristic LDL-C particle number and LDL-C particle size distribution of an individual are affected by both genetics and diet. Each LDL-C particle has a single apoB molecule associated with it, and apoB expression is affected by insulin flux and the amount of lipid in the liver (Elam et al. 1999; Veniant et al. 1999), and so APOB gene expression and hence the number of LDL-C particles is partly driven by carbohydrate intake. It is also inversely related to the amount of apoE in circulation (Smit et al. 1988; Utermann 1988), and although there is a major genetic effect on apoE expression due to variation at the apolipoprotein E (APOE) coding sequence, apoE expression is positively correlated with dietary cholesterol, oestrogen and SFA (Utermann et al. 1979; Srivastava 1996; Srivastava et al. 1996).
There are three overall patterns of LDL-C particle size distribution in humans, stable pattern A is biased to larger less dense particles, stable pattern B is biased to smaller denser particles, and unstable pattern A, where individuals have pattern A at high fat levels and pattern B at low fat levels (Campos et al. 1995; Krauss and Dreon 1995; Dreon et al. 1999). Stable pattern B is governed by mutations at the LDLR (Austin et al. 1988; Nishina et al. 1992; Zhu et al. 2007), which causes delays in LDL-C clearance resulting in smaller, denser particles. Unstable pattern A is due to variation at the APOE locus, where the ε4 allele requires high fat levels to show pattern A (Dreon et al. 1995; Krauss and Dreon 1995), and reductions in LDL-C due to low fat diets are not accompanied by reductions in number of LDL-C particles. This is partly because the apoE*E4 protein has a higher affinity for low-density lipoprotein receptor related protein 1 as well as for VLDL-C than the E2 and E3 proteins (Egert et al. 2012), so chylomicrons are cleared at a faster rate and VLDL-C matures to LDL-C at a faster rate and so over its lifespan a lipoprotein cholesterol particle will be in the LDL-C form for longer for carriers of this allele.
High levels of CISFA cause the strongest increase in LDL-C particle size but unsaturated lipids also appear to maintain increased LDL-C particle size but to a lesser extent (Dreon et al. 1998; Kratz et al. 2002). Stable pattern B individuals respond to an increase in CISFA by increasing the number of small dense LDL-C particles in circulation, as seen by an increase in apoB levels (Krauss and Dreon 1995), because the genetic lesion is a reduction in recycling of LDL-C particles. Discordance between LDL-C and apoB concentrations in the blood is an indicator of these differences in LDL-C pattern, and discordance in apoB and LDL-C affects strongly the risk of CVD, with apoB level being the more accurate indicator (Dreon et al. 1995, 1998; Sniderman et al. 2012).
Unsaturated fatty acids (UFA) are known to increase the expression of the LDLR gene and, in addition, long-chain n-3 and n-6 PUFA and CLA increase the availability of the LDLR protein compared with CISFA (Fernandez and McNamara 1989; Yu-Poth et al. 2005; Dorfman and Lichtenstein 2006). This is consistent with the well known reduction of LDL-C concentration with increased UFA. A similar effect is expected for dietary stearic acid because its effects on serum cholesterol fractions are not statistically significantly different to that of oleic acid, it is associated with a reduction in concentration of apoB-100, and it is rapidly converted to oleic acid in the liver where the LDLR gene is expressed (Bonanome and Grundy 1988; Tholstrup et al. 1994a; Mensink et al. 2003).
Although LDL-C concentration of the blood is the most common risk factor used in prediction of CVD, the concentration of LDL-C per mL of blood is a combination of both the number of LDL particles per mL and the amount of cholesterol contained in each particle. That is, a particular LDL-C concentration of the blood could be made up of many particles each containing a small amount of cholesterol or few particles each containing a large amount of cholesterol. However, what counts for heightened risk of CVD is the number of LDL particles per mL in circulation rather than LDL-C concentration per se (Sniderman et al. 2012). Two individuals with the same LDL-C can have vastly different risks for CVD if one has many small dense particles with small amounts of cholesterol while the other has far fewer particles each carrying much larger amounts of cholesterol. LDL-C particles come in a range of densities. The larger the LDL-C particle the higher the concentration of free cholesterol and lipid, the lower the concentration of protein and cholesteryl-esters, the younger it is, and the longer it takes either for the apoB on the surface of the particle to be glycosylated, or for the particle to cross the vascular endothelium or to oxidise (Tribble et al. 1992; Reaven et al. 1994; Younis et al. 2013).
The development of cardiovascular disease
Plaque formation and atherosclerosis, which is at the heart of CVD, is mechanistically initiated by the peroxidation of PUFA in the cholesteryl-esters of LDL-C, and the first evidence of this was discovered 60 years ago (Glavind et al. 1952). The favoured PUFA for incorporation into cholesteryl-esters is linoleic acid (C18:2 n-6), the most common n-6 PUFA (Esterbauer et al. 1992). Restricting PUFA in the diet and providing high levels of oleic acid results in its replacement in cholesteryl-esters by oleic acid, the latter of which does not peroxidise (Nestel et al. 1992; Abbey et al. 1993; Sandker et al. 1993; Reaven et al. 1994). The PUFA-based cholesteryl-esters of LDL-C are peroxidised when they cross the endothelium, resulting in stepwise damage to apoB-100 on the surface of the LDL-C particle, which results in the LDL-C particle being taken up by macrophages, where the cholesterol accumulates (Brown and Goldstein 1983; Steinberg et al. 1989; Esterbauer et al. 1992; Stocker and Keaney 2004). The resulting foam cells form the basis of the atherosclerotic plaque. HDL-C particles act as antioxidants in this cascade, and in addition, do cross the vascular endothelium and accept cholesterol from foam cells, reversing the process, returning cholesterol to the liver where it is converted to bile salts (Brown and Goldstein 1983; Fielding and Fielding 1995). The smaller HDL3 particles are most efficient at this process, and their numbers are increased through consumption of CISFA (Nestel et al. 1992; Kontush et al. 2003). This mechanism explains to some extent why HDL-C is an independent risk factor to LDL-C for CVD (Castelli et al. 1986; Stampfer et al. 1996; Chapman et al. 2011; Miller et al. 2011). Antioxidants such as Vitamins E and C can slow the damage, but they only delay the peroxidation on the scale of minutes not hours (Esterbauer et al. 1992), consistent with the demonstrated weak effect of antioxidant use in cohort trials and the failure of antioxidant therapy to control atherosclerosis in random control trials (Jha et al. 1995; Knekt et al. 2004). Once the plaque forms, inflammatory immunological processes are engaged (Stocker and Keaney 2004), and anti-inflammatory and anti-platelet treatment can reduce the risk of vascular death (Bousser et al. 2011). The tendency for LDL-C to cross the vascular endothelium is increased if it is small dense LDL-C. High levels of CISFA are important when they increase the number of small dense LDL-C molecules, otherwise the damage is done by peroxidation of PUFA, and both factors can be rescued by having MUFA as the dominant FA.
The epidemiology of cardiovascular disease and saturated fat
These factors help to explain the success of the traditional Mediterranean diet and lifestyle, which has been described as largely lacto-vegetarian (Keys 1995). The Mediterranean diet and lifestyle as originally described, is a high lipid (40% of total calories) low protein (10%) diet based on olive oil, vegetables, fruit, whole-grain cereals and legumes, wine, nuts, full fat cheese, fish, and meat, especially pork fat and offal (Keys et al. 1970; Keys 1995). The fat in this specification is dominated by MUFA, with low levels of linoleic acid (2% of total calories), a ratio of n-6 to n-3 PUFA of ~2, and with modest levels of SFA (8% of total calories) (Keys and Kimura 1970; Keys et al. 1970, 1980). Given the composition of olive oil and other plant oils, this suggests a substantial part of the lipid (perhaps a third) was from animal sources. What counts is degree of adherence to that traditional Mediterranean diet and lifestyle (de Lorgeril et al. 1994, 1999; Trichopoulou et al. 2003; Scarmeas et al. 2006; Féart et al. 2009; Sofi et al. 2012), no single food group is the magic bullet, although there was evaluation of which particular factors are critical that might alleviate the effects of a Western diet and lifestyle (Keys 1980; Hertog et al. 1993; Sandker et al. 1993; Evans et al. 1995; de Lorgeril et al. 2002).
Altering the effects of large amounts of SFA on serum cholesterol by replacing it with large amounts of PUFA to control TSC and reduce CVD, which was the subject of a large random controlled trial (Multiple Risk Factor Intervention Trial Research Group 1982), was not envisaged as an option to control CVD and was explicitly criticised before it was performed (Keys et al. 1974). The trial was unsuccessful because it showed no significant change in rates of CVD in the trial versus the control sample. Worryingly, this trial showed increased rates of cancer in the test group compared to the control (Blaxter and Webster 1991). Indeed, although treatment of CVD has greatly improved over the last 50 years, the incidence has stayed the same over that time and reductions in serum cholesterol have played a small (11%) role in postponing deaths from CVD (Hunink et al. 1997; Ford et al. 2007; Gouda et al. 2012). Smaller trials showed some progress in treating CVD where the lipid composition approximated that of the Mediterranean diet by providing to participants rapeseed (canola) oil hydrogenated to a margarine that was dominated by MUFA, with more SFA and a lower ratio of n-6 to n-3 PUFA than olive oil (de Lorgeril et al. 1994, 1999).
Although they can be flawed, nutritional surveys have found little evidence to link reported fat consumption of any kind and CVD within countries (Siri-Tarino et al. 2010), and meta-analyses of random controlled trials have shown small or no benefit to replacing saturated fat with carbohydrate or unsaturated fat (Multiple Risk Factor Intervention Trial Research Group 1982; Micha and Mozaffarian 2010; Hooper et al. 2011). Average longevity, a key statistic in comparisons of different diets and lifestyles, does not increase when SFA is replaced by either carbohydrate or UFA (Hooper et al. 2011). Nevertheless, these surveys have extra-ordinary statistical power, covering many hundreds of thousands of people. Any association to SFA that is seen is variable and only at the very highest levels of consumption, which is consistent with the known mechanisms of the effect of CISFA on LDL-C structure in some individuals. On the other hand, very high body mass index (BMI: kg/m2) is reliably and consistently linked within populations to all forms of CVD (Keys et al. 1980; McGee and Diverse Populations Collaboration 2005), a marker of increased food consumption or decreased movement. Indeed, waist circumference and waist to hip ratio are stronger physical correlates to CVD than BMI by itself (de Hollander et al. 2012) and the relative risk of a large waist circumference easily exceeds the relative risk found for high consumption of SFA and is similar to being in the top quintile for non-HDL-C or the bottom quintile for HDL-C (Chapman et al. 2011; Hooper et al. 2011). In food overconsumption studies, excess fructose rather than excess glucose leads to an increase in intra-abdominal fat and hence expanded waist circumferences (Stanhope et al. 2009).
The within-cohort argument against animal or saturated fat became much weaker once the effects of trans fats in margarines derived from partial hydrogenation of C18 PUFA in vegetable oils were separated from animal fats. Animal fats and margarines had initially been grouped together in analyses, as representatives of fats that were solid at room temperature. These margarines contained substantial amounts of elaidic acid (C18:1 trans 9) (Hunter 2001; Hayes and Pronczuk 2010). The effect of industrial trans fat on risk of heart disease is powerful, the dose response predictable (Hu et al. 1997), the results marked (Willett et al. 1993; Dorfman et al. 2009; Mozaffarian et al. 2009), and industrial trans fats are the only fat to double the risk of CVD, all other fats change the risk by a small percentage. Consequently, cities in the United States have banned trans fats from publically prepared food (http://www.nbcnews.com/id/16051436/ns/health-diet_and_nutrition/t/new-york-city-passes-trans-fat-ban/#.UVpxVBm8yTw, accessed 1 April 2014). In effect, C18 trans MUFA, derived from the partial hydrogenation of C18:2 and C18:3 PUFA, show decreased HDL-C but similar LDL-C levels to CISFA (de Roos et al. 2001; Matthan et al. 2004), which explained to some extent the previously observed emergence of a pattern of low HDL-C, high LDL-C and high FTG seen in some populations (Castelli 1986). Many margarines have a large amount of PUFA, which would contribute to peroxidisable cholesteryl-esters, providing a double hit. Indeed, once trans fats were controlled, risk of CVD declined with increased number of beef, lamb, and pork stews consumed per week, implying a beneficial effect of these animal fats on CVD (Willett et al. 1993). Many manufacturers now create margarines with lower trans fat levels through interesterification of completely hydrogenated fats and unhydrogenated oils (Hunter 2001; Hayes and Pronczuk 2010). But there is always some level of trans fat due to the refinement of vegetable oils, while there would still be a high level of linoleic acid (Table 1).
Most of the epidemiological evidence for the role of fat in CVD comes from comparisons between countries and from the changes in food usage over time within countries. As noted above, if one were to use the evidence of changes in food usage, then animal fat consumption declined and plant oil consumption increased as CVD and obesity rates increased, which argues against the role of animal fat in CVD or obesity (Yudkin 1957; Antar et al. 1964; Oddy and Yudkin 1969; Kritchevsky 1976; Enig et al. 1978; Eisenmann 2003; Carlson et al. 2011; Chapman et al. 2011). Comparisons between countries are controversial, called ecological comparisons, because many factors change from one country to the next, and such comparisons result in incorrect inferences due to correlations based on mean values (Robinson 1950; Evans 2011). Moreover, the early ecological comparison of six countries was criticised for biased selection of countries, which had led to the reporting of very strong relationships between fat and CVD (Keys 1953; Yerushalmy and Hilleboe 1957; Yudkin 1957). Nevertheless, much of the prestige of the argument against SFA is based on a later version of that ecological comparison, the Seven Countries study, in which individuals within 16 cohorts were evaluated, which showed that within each cohort, smoking, blood pressure, and serum cholesterol were each important risk factors for CVD (Keys et al. 1980). Importantly, however, food intake of each individual was not measured, but gross food composition was determined for a representative sample of 30–50 households, and then cross-cohort correlations were made between average nutrient composition and average rates of CVD (Keys et al. 1980). This was justified on the basis of the known relationship between average fat composition of a ration and average serum cholesterol of individuals consuming that ration – that individual cholesterol values are too variable for accurate inference (Keys et al. 1980). Indeed, the first study of serum cholesterol and atherosclerosis at autopsy had shown no correlation between individual values for these two variables (Lande and Sperry 1936). The Seven Countries study is the basis for the Mediterranean diet and lifestyle.
What are the alternative explanations for the extremely high rates of CVD that occurred in the East Finland cohort in the Seven Countries study (Keys et al. 1980), if one were to assume that they were not due to SFA. There, the East Finland cohort had the highest proportion of saturated fat in the diet and nearly twice the incidence of coronary heart disease (CHD) compared with any other cohort, the SFA derived mainly from milk fat. The overall ration also had low levels of linoleic acid as a proportion of calories and a low ratio of n-6 to n-3 FA (Keys and Kimura 1970; Keys et al. 1970). First, the West Finland cohort had the same median TSC value as the East Finland cohort (Keys et al. 1970; Stengard et al. 1995) but had only a third the rate of age adjusted CHD compared with the East Finland cohort. Furthermore, West Finland had a higher average TSC than the Zutphen (Netherlands) or US Railroad cohorts, and the same or more SFA as a proportion of calories than either of those, but less CHD than either Zutphen or US Railroad. Second, the East Finland cohort had the most calorie intake per kilogram bodyweight, but this was not substantially greater than West Finland. East Finland did consume 32% more calories per kilo bodyweight than the Crete cohort (lowest CHD rate), and although both consumed ~40% of calories as fat, this implies substantially more fat in total (Keys et al. 1970). Third, the East Finland cohort was located in Karelia (Keys et al. 1958), then an isolated and icebound region for most of the year while the West Finland cohort was centred near Turku and included a substantial proportion of Swedes (Karvonen et al. 1970). During winter the rations were reduced to milk and other dairy products, bread, and potatoes with small amounts of other foodstuffs (Roine et al. 1958), hardly well balanced but hardly a death sentence, given the benefits of dairy product consumption for CVD and the metabolic syndrome (Evans et al. 1995; Elwood et al. 2010; Livingstone et al. 2013). Indeed, there was little difference between East and West Finland in their diets, apart from a small increase in diversity in the West, and reduced vitamin C, vitamin E, and iodine in the East. Finnish researchers in the 1950s suspected differences in iodine and subsequent goitre as a likely cause of the difference in CHD between the two Finnish samples (Roine et al. 1958; Uotila et al. 1958). Hypothyroidism is a known cause of elevated FTG with decreased particle size of the VLDL fraction (Nikkilä and Kekki 1972; Abrams et al. 1981; Castelli 1986), although these days vitamins C and E would also be thought important due to their antioxidant effects (Esterbauer et al. 1992). Fourth, the East Finland cohort was centred on the town of Ilomantsi, 3 km from the Finno-Russian border in an area in which Russians had seized territory and displaced Karelians had been repatriated by the Finnish government to other parts of Finland (Karvonen et al. 1970). While the report stated that the cohort itself consisted of a minimum of displaced persons, the stress associated with such events, which may have involved family members, should not be underestimated. Stress is a well known factor in CVD, especially stress where one is powerless to alter events (Marmot et al. 1997). None of the other cohorts experienced a similar event associated with the Second World War. Last, Karelians are a minority ethnic group and have one of the highest frequencies of the APOE ε4 allele in Europe (Fullerton et al. 2000). The APOE gene shows a North/South cline in the frequency of the ε4 allele in Europe, evidence of natural selection, while the lowest frequencies of ε4 of any population are found on the islands and shores of the Mediterranean (Corbo and Scacchi 1999; Singh et al. 2006; Lappalainen et al. 2010). Carriers of the ε4 allele have a 40% increased risk and homozygotes for this allele have approximately double the risk of CHD compared with the APOE ε3 homozygotes in Western countries (Menzel et al. 1983; Song et al. 2004; Mooijaart et al. 2006). While it may be coincidental that the age-adjusted death rates for CHD in East Finland and Crete were very similar to the frequencies of homozygotes for APOE ε4 in these two cohorts, APOE genotype has been shown to affect CHD in the East and West Finland populations of the Seven Countries study (Stengard et al. 1995). The response of APOE ε4 to SFA versus MUFA is significantly different to the response of APOE ε3, with SFA-dominated fats resulting in a substantially elevated LDL-C profile for this allele (Moreno et al. 2009; Egert et al. 2012). Given that APOE ε4 is ancestral (Hanlon and Rubinsztein 1995; Fullerton et al. 2000), may be a thrifty allele (Corbo and Scacchi 1999) and that the East Finland population was actually consuming the most calories per kilogram bodyweight, there is evidence of a genetic component to the observations and the differences between cohorts. Obviously, there would be other genes involved in blood lipids and response to fat consumption, not just APOE but would also include mutations such as the Karelian form of the LDLR gene (Vuorio et al. 1997), which would have a role in differences between populations (Teslovich et al. 2010). Taken together, any or all of these factors could be working to explain this ecological comparison.
Perhaps the final word on fat and cholesterol should be given to William Castelli (see quote at the beginning) on individuals living in the real world. In the real world there is only a tenuous link at best between consumption of SFA and serum cholesterol, with other factors overriding the relationship. For example, ‘The two factors that jump to mind are exercise and weight. In Framingham, for example, we found that the people who ate the most cholesterol, ate the most saturated fat, ate the most calories, weighed the least, and were the most physically active’ (Castelli 1992). As noted at the start, the more SFA consumed the lower the serum cholesterol, and in the Framingham Heart study (Castelli et al. 1986), not only did lower serum cholesterol and lower BMI equate to lower risk of CVD, but higher HDL-C, consistent with higher consumption of SFA, was shown to have protective effects.
Components of animal fat with known health benefits
The most important health benefit of fat is that it is a carrier for the vitamins A, D, E, and K and the precursors β-carotene and cholesterol. The transport of these into the body partly through active transport and passive diffusion in fat has been recently reviewed (Reboul and Borel 2011). Deficiency disorders for these vitamins are well known, blindness, rickets, and bleeding disorders are the well attested results of frank deficiency, but low levels of these vitamins are associated with bone weakness, osteoporosis, calcification of the arteries, CHD, type 2 diabetes, depression and other mental disorders (Rimm et al. 1993; Schaafsma et al. 2000; Holick 2007; Beulens et al. 2010; Flore et al. 2013). Animal tissues, especially organs such as the liver and adipose tissue, and dairy products are a valuable source for several of these fat soluble vitamins. Although the importance of fat soluble vitamins is extremely well known, with recommendations to consume less fat and less animal tissues on the one hand or absence of these foods in some developing countries on the other hand, vitamin supplementation is often needed.
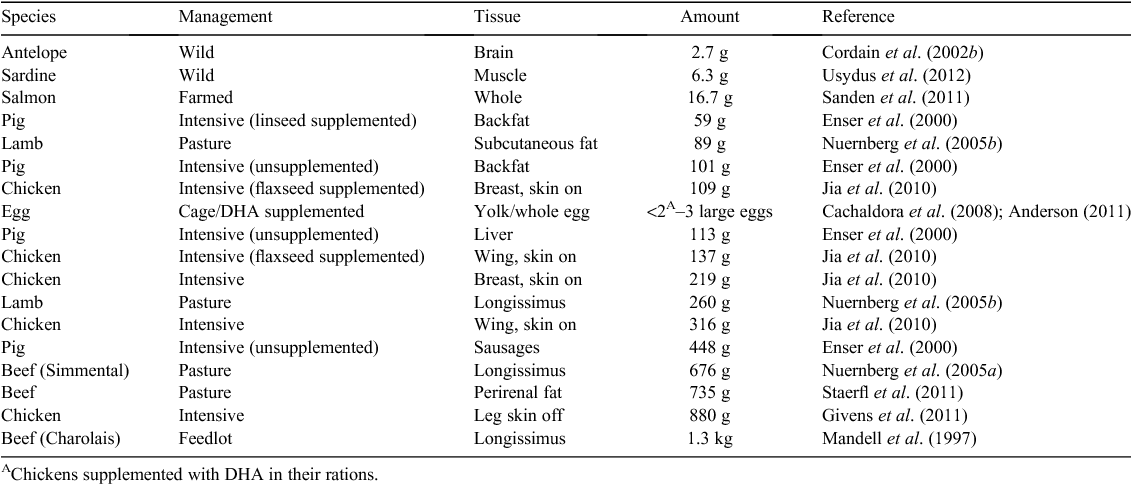
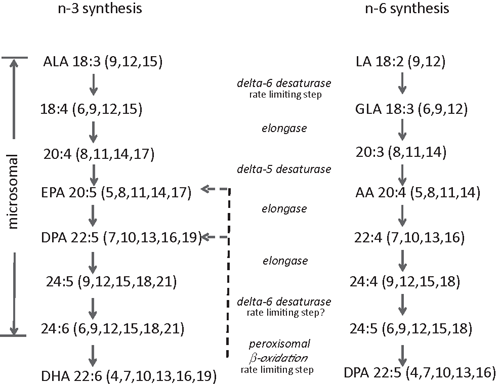
The fat and other tissues of a wide variety of land animals, not just oily fish, contain long-chain n-3 PUFA (Table 2). Given the pollution of the ocean by methyl mercury, ocean fish consumption needs to be modulated to achieve the benefits of eating oily fish (Mozaffarian and Rimm 2006). These PUFA are part of the phospholipid fraction, hence their presence in substantial amounts in tissues such as brain, liver, and egg yolks. Long-chain n-3 PUFA have a wide range of well attested health benefits, primarily in brain development in young children and as an important modulator of immune system function, especially the inflammatory response (Simopoulos 1991). As inflammation is central to many chronic disorders of aging and obesity, this subject is important and is regularly reviewed (MacLean et al. 2006; Schmitz and Ecker 2008; Simopoulos 2008; Nicholson et al. 2013). Although α-linolenic acid (C18:3 n-3) is found in leafy green vegetables, some nuts and some oil seed, it appears that only animals or algae can synthesise the long-chain n-3 PUFA docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) (Sprecher 2002). The sequence of reactions and rate-limiting steps are shown in Fig. 2. The human metabolism has a trivial ability to increase DHA given extra α-linolenic acid in the diet (Bézard et al. 1994; Brenna 2002; Burdge and Calder 2005; Harper et al. 2006), although levels of DHA in the serum of self-reported vegans is higher than expected, which if accurate suggests regulation of DHA levels and enhanced endogenous synthesis of DHA in the absence of dietary intake (Welch et al. 2010). The starting ratio of shorter-chain n-6 to n-3 PUFA in the diet largely determines the ratio of longer-chain PUFA that are synthesised, and the ratio stored in tissues or cholesteryl-esters is therefore a reflection of the intake ratio of these fats (Zock et al. 1997). Nevertheless, there is human genetic variation in the genes of these pathways, which bias processing towards n-3 PUFA over n-6 PUFA (Tanaka et al. 2009). High levels of circulating long-chain n-3 PUFA, especially DHA, therefore, generally requires a dietary source in humans, essentially from fat of other animals, with evidence of little additional benefit beyond a threshold intake of ~250 mg per day (Mozaffarian and Rimm 2006). EPA is interconverted to DHA via the intermediate n-3 docosapentaenoic acid (DPA) but not from n-6 DPA, and n-3 DPA is a long-chain PUFA found in ruminant tissues. However, the ratio of the n-6 to n-3 PUFA in the tissue depends largely on the food source of the animal, with grass-fed ruminants having approximately a 2 : 1 ratio of n-6 to n-3 FA, much lower than for grain-fed ruminants (Yang et al. 1999b; Couvreur et al. 2006; Sinclair 2007), compositional effects seen in other species as well (Sinclair et al. 2010). Given the decline and pollution of fisheries (Costello et al. 2012; Halpern et al. 2012) there are other ways of ensuring a sufficient amount of long-chain n-3 PUFA, especially since many populations, perhaps under the advice to cut back on saturated fat have reduced their consumption of animal fat and are deficient in DHA and EPA (Givens 2010). Better data on DHA, n-3 DPA, and EPA concentrations in a variety of animal tissues under different management systems would be welcome.
|
The ratio of n-6 to n-3 PUFA (OMR) in animal fats may make an important contribution to human health although it is not clear whether these are all direct effects or whether they are partial effects due to food overconsumption and obesity. Several studies have shown that a high OMR in tissues or cholesteryl-esters is associated with several diseases including cancers such as prostate, bowel and breast cancer, CVD, autoimmune diseases and inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease (Godley et al. 1996; Harvei et al. 1997; Gogos et al. 1998; Simonsen et al. 1998; Yang et al. 1999c; Simopoulos 2008; Murff et al. 2011). Intake of many types of fat have been examined, with a suggestion of a threshold of n-6 PUFA, and reduced rates of disease for fats or oils low in n-6 PUFA, including animal fat (Zock and Katan 1998; Freedman et al. 2008; Alexander et al. 2010; Brennan et al. 2010; Dong et al. 2011; Gilsing et al. 2011; Liu et al. 2011; Psaltopoulou et al. 2011; Chajes et al. 2012). Moreover, supplementation with long-chain n-3 FA fails where there are high rates of consumption of n-6 PUFA. Many plant oils have OMR >10, some with OMR >100 (Table 1). It has been estimated that the general consumption in Western countries is OMR ~15, while more traditional diets have much lower OMR, and the traditional Greek diet, on which the Mediterranean diet was modelled, had an OMR of 2–4 (Keys and Kimura 1970; Keys et al. 1970; Simopoulos 2008). In general, for the shorter 18-carbon n-3 and n-6 FA, animal fat can have much lower OMR than plant oils, especially if their rations are low in n-6 PUFA. For longer-chain n-3 and n-6 FA the ratio also depends on the tissue and the species. Ruminant fat has low PUFA due to bio-hydrogenation in the rumen, and from animals on pasture has OMR ≤2 (Table 1) and increased levels of α-linolenic acid, which suggests that ruminant fat has a role in reducing not just the amount of n-6 PUFA but also the OMR. However, the West and other countries with high rates of obesity have high rates of these diseases, and being obese greatly increases the risk of these disorders including increased rates for higher levels of intra-abdominal fat (Connolly et al. 2002; Key et al. 2004; Pischon et al. 2008; Renehan et al. 2008). In overfeeding, it is primarily dietary fat that is stored, due to the high metabolic cost of converting dietary glucose to lipid in humans, ~0.33 Mj per Mj of lipid deposited, although some samples of obese people showed a greater ability to convert carbohydrate to fat than non-obese people (Acheson et al. 1988; Pasquet et al. 1992; McDevitt et al. 2001; Stanhope et al. 2009). So analyses of the fat of obese individuals will usually show a high ratio of n-6 to n-3 fat in countries where seed oils are the dominant source of lipids. This acts to confound the analysis. Is it just that increased food consumption has resulted in higher BMI, which of itself predisposes the individual to these disorders, or is it truly the type of fat? Mechanistic studies of n-3 and n-6 fats show their role in respectively inhibiting and promoting inflammation and tumour growth (Xia et al. 2005; Kobayashi et al. 2006), while high levels of n-6 fats may inhibit the action of delta-9 desaturase (Yang et al. 1999b), critical for desaturating SFA. So while controlling bodyweight may be the more important strategy, reducing the level of n-6 PUFA to bring down the OMR of the diet appears worthwhile.
In addition to long-chain n-3 PUFA, ruminant fat contains various types of CLA, a series of bioactive compounds with well known positive effects on human health. CLA are derived from PUFA in animal feed that had been bio-hydrogenated in the rumen. Most of the CLA in the animal’s tissues are from vaccenic acid (C18:1 trans-11) absorbed from the rumen that is converted to the CLA rumenic acid (C18:2 cis-9 trans-11) by the host through the action of delta-9 desaturase, and a minor component of the CLA is produced by the rumen flora and absorbed by the host as one of a range of CLA (Griinari and Bauman 1999; Pariza et al. 2001). The richest source is ruminant milk fat (Parodi 1997), but CLA are found in all ruminant fat depots including substantial amounts in bone marrow (Cordain et al. 2002b), a traditional source of food for pre-agricultural humans (Morin 2007). CLA is produced from both n-6 and n-3 PUFA, so animals on grain versus pasture do not produce less CLA, just the kind of CLA is altered. On grain compared with pasture, most of the CLA is still cis-9, trans-11 C18:2 but there is a reduction of trans-11, cis-13 C18:2 and an increase in trans-7, cis-9 C18:2, trans-10, cis-12 C18:2, and cis-9, cis-11 C18:2 (Daley et al. 2010; Aldai et al. 2011). This is apparently due to changes in the bacterial composition of the rumen in response to changes in pH as a result of the amount of fibre and energy density of the feed (Daley et al. 2010). These CLA isomers have different 3-D structures and there is some evidence for different biological activities, with the cis-9, trans-11 C18:2 isomer seen as the most beneficial (Pariza et al. 2001). This CLA isomer is synthesised directly by humans from vaccenic acid (trans-11 C18:1), through the action of delta-9 desaturase in the liver and other tissues. CLA came to prominence in screens to find carcinogenic molecules in grilled steak (Ha et al. 1987), and they were targeted because they are trans fats. However, it proved to be one of the most anti-carcinogenic molecules in cell culture assay. In addition, due to the concern with trans fats of industrial hydrogenation, especially elaidic acid, any trans fat receives a great deal of interest, and the health benefits of CLA have been thoroughly studied and intensively reviewed (Parodi 1997; Pariza et al. 2001; Dilzer and Park 2012). A wide range of favourable health outcomes have been found, including anti-cancer and anti-obesity effects, improvements in glucose tolerance, cardiovascular health, bone density, immune system function and inflammation, and gut health. Much of this work is now focussed on CLA as a pharmaceutical preparation or food additive, consisting of the main isomers cis-9, trans-11 C18:2 and trans-10, cis-12 C18:2, with dosages from 0.5 to 7 g per day (Dilzer and Park 2012). Given the concentration of CLA in dairy foods or ruminant fat, therapeutic doses of CLA could be obtained from moderate consumption (e.g. 100 g of full fat matured cheese) of dairy products or ruminant fat (Fogerty et al. 1988; Mushtaq et al. 2010).
There are minor components of animal fat that have unverified human health benefits (Parodi 1997, 2004). In animals, fat is a metabolically active energy store, as adipose tissue, or a nutritive substance, as in milk. Apart from the major TAG and NEFA that constitute the fat, there are minor components of the fat such as phospholipids and sphingolipids that constitute part of the lipid structural element of cell membranes, the non-fat components of the adipocyte, or the fat micelles that constitute milk fat. Few health benefits have been identified for these except that phospholipids and sphingolipids do include long-chain n-3 FA (Lehninger 1982). Pathways involving ceramide and sphingomyelin, components and downstream metabolites of sphingolipids, are known to be involved in several pathogenic metabolic processes involved in cancer cell proliferation as well as insulin resistance (Holland et al. 2007). Butyrate (C4:0), a short-chain SFA, forms a substantial part of dairy fat. Butyrate is generated by fermentation from plant fibre and resistant starch in the colon of humans and is the preferred source of fuel for colon cells (Scheppach 1994), and increased levels of butyrate as a result of improved diet quality may reduce risk of colon cancer (Steinmetz and Potter 1991; Van Munster et al. 1994; Mathers et al. 2012). Although most butyrate from dairy fat is absorbed from the small intestine and metabolised in the liver, there is clear speculation about the potential role of dairy butyrate in not just colon but other cancers, not by surviving into the large colon but by increased levels delivered via the circulation (Parodi 1997, 2004). The human health effects of dairy derived butyrate, ceramide, and sphingomyelin, as well as other minor components of animal fat, have not been extensively studied.
Health effects of fat as a macronutrient and energy source
Calorie restriction, not lipid restriction, has been shown to affect longevity in a wide range of species (Fontana et al. 2010) but longevity studies of humans by humans are obviously impractical (Hursting et al. 2010), the benefits are questioned relative to sanitation and modern medicine (Everitt and LeCouteur 2007), and a recent trial in primates failed to find an effect (Mattison et al. 2012). It is well known that a low fat, low protein whole-plant diet, when supplemented with the essential nutrients vitamin B12 and long-chain n-3 PUFA, has been associated with reduction of the thickness of cardiac arteries (Ornish et al. 1998). While this gives enormous prestige to low fat whole-plant diets, reduction of the thickness of cardiac arteries can also be achieved using calorie restriction of a high quality diet, including meat, eggs, and dairy products, thereby consuming animal fat (Fontana et al. 2004, 2007). Furthermore, starvation itself also causes a reduction in thickness of cardiac arteries in addition to generalised tissue wasting, as was found in the Minnesota Semistarvation Experiment of humans. There, a more than 50% reduction in calories to an extremely low fat ration of root vegetables, cabbage, cereals, and a few grams of animal protein per week also showed substantial negative physical and physiological effects (Keys 1950; Taylor and Keys 1950). In calorie-restricted individuals, when protein was reduced to maintenance levels and replaced isocalorically with other macronutrients, the level of insulin-like growth factor-1 (IGF-1) dropped substantially (Fontana et al. 2008). This longevity marker, which is associated with cancer, diabetes, and dementia, including Alzheimer’s disease (Paolisso et al. 1997; Arai et al. 2001; Carro et al. 2002; Trejo et al. 2002; Longo and Finch 2003; Pollak et al. 2004; Carro and Torres-Aleman 2006; Fontana et al. 2010; Guevara-Aguirre et al. 2011; Duron et al. 2012; Rajpathak et al. 2012), is raised the least by lipids and most by protein consumption (Thissen et al. 1994). A study of raw food enthusiasts consuming 40% of calories as lipid and a maintenance level of 0.7 g/kg of protein (Rand et al. 2003), ~10% of calories, showed similar low levels of IGF-I without apparent calorie restriction (Fontana et al. 2008). This macronutrient composition is similar to the original description of the Mediterranean diet on Crete (Keys et al. 1970).
Although calorie restriction is a niche activity in the West, there is some evidence of increased longevity for communities that eat less food not less fat. Studies of the ‘Blue Zones’, areas of the world with proven enhanced aging, increased proportions of centenarians, and reduced morbidity from aging (Fraser and Shavlik 2001; Poulain et al. 2004), show first a huge range in average fat consumption, from <10 to >40% of calories (Willcox et al. 2007, 2009; Appel 2008; Pes et al. 2013), relatively low levels of protein, a diet that is plant-based but not vegan, with differences in staple foods and wide variations in the amount of saturated fat consumed. The individuals generally consume substantially fewer calories than in the West, and have traditions of eating until one was not quite full. There is little evidence of deficiency in essential nutrients (Polidori et al. 2007; Willcox et al. 2007; Buffa et al. 2010). It is by no means certain that the individuals are calorie restricted, and claims to that extent are a source of controversy, because the calorie and protein intake in some of these groups is consistent with their smaller size (Keys and Kimura 1970; Willcox et al. 2007; Pes et al. 2013). Nevertheless, these individuals consume on average substantially fewer calories than in the West, and less than individuals in surrounding regions (e.g. Sardinia vs Italy, Okinawa vs Japan). Furthermore, despite the lower calorie intake, the individuals were far more physically active than the average in the West, although their activity is not usually associated with particularly hard work but instead represents activity all day long (Fraser and Shavlik 2001; Willcox et al. 2009; Pes et al. 2013). Fat appears not to be important in explaining increases in longevity in these Blue Zones. Moreover, a study of Ashkenazi centenarians in the US showed that a non-significant smaller proportion of the centenarians reported eating a low fat diet (Rajpathak et al. 2011) compared with an age-matched cohort from NHANES I – the only statistically significant difference was that fewer of the men smoked. Eating less in the English-speaking West is an unpalatable recommendation that goes against centuries old traditions of ‘eat, drink and be merry, for tomorrow you die’, entrenched overconsumption of food, and the social prestige of large amounts of lean meat (Dickson Wright 2011).
While fat per se is not negatively associated with longevity, it is positively associated with digestion and gut performance. Low fat high carbohydrate diets of a high glycemic index have been associated with symptomatic gall bladder disease (Tsai et al. 2005) likely due to reduced cycling of cholesterol through bile salt release. Although fat or oil are often stated as affecting gastrooesophagial reflux disease, a systematic review of the literature failed to find an effect of fat consumption (Dent et al. 2005). In addition, fat has a unique ability to stimulate colonic contraction, not found for carbohydrate or protein, and these contractions are essential to the voiding of faeces (Wright et al. 1980). Indeed, remedies for constipation before the Second World War recommended, in addition to ‘roughage’, water and the avoidance of diuretics, the consumption of fat such as bacon fat, butter, or olive oil, especially if the faeces was small and dry (Hutchison 1936), and for spastic colon no fibre and large amounts of the above listed fat was recommended. This is not a recommendation that is in any current medical guideline. Yet several pieces of evidence show that it is valid. First, infants take in no fibre, so amount of fibre is not a variable. Animal fats in the formula result in stools that are softer, larger, and that are easier to pass than plant fats (Forsyth et al. 1999), and babies also grow better when the source of the fat is from animal rather than plant sources (López-López et al. 2001). The exact reasons for these effects are not known, but these effects are thought to be due partly to the characteristic stereoisomeric structure of animal fats compared with plant fats (Hunter 2001), and the presence of long-chain PUFA. Second, in a study of a large number of Swiss citizens, the small percentage of individuals with great difficulty in passing faeces were statistically more likely to have a high fibre diet than a low fibre diet (Curtin et al. 1998). Although individuals on a high fat diet were less likely to report difficulty in passing faeces this was not statistically significant. Third, animal fat also contains a substantial proportion of stearic acid. Up to 20% of stearic acid, depending on the stereoisomeric location of the FA, is not absorbed by the enterocytes compared with 0–2% for other FA (Bracco 1994). The unabsorbed stearic acid will crystallise in the lumen of the gut as the pH increases and be excreted as a calcium soap (Owen et al. 1995). This reduces the energy of the diet, and changes the consistency and increases the mass of the faeces (Dougherty et al. 1995). This is not to argue that dietary fibre is unimportant, rather that dietary fibre is often not sufficient to cure constipation and other ailments of the colon and rectum. Constipation underlies many of the diseases of the bowel, and it is long known to be a precursor to diverticulitis, varicose veins, and haemorrhoids, among other disorders (Burkitt 1973; Cleave 1974). Colorectal cancer is related to diet quality, risk is reduced through the consumption of vegetables, fruit and resistant starch (Macquart-Moulin et al. 1987; Steinmetz and Potter 1991; Van Munster et al. 1994; Archer et al. 1998; Topping and Clifton 2001; Mathers et al. 2012), and fat consumption has not been shown to be linked to its occurrence in large meta-analyses (Nelson et al. 1999; Liu et al. 2011). As an aside, colorectal cancer has been linked to red meat, preserved meat and beer consumption, which may implicate methods of preparation and preservation of food involving known factors such as nitrosamines and heterocyclic amines (McMichael et al. 1979; Potter 1999; Aune et al. 2013; Egeberg et al. 2013).
The macroscopic structure of a fat and its stereoisomeric composition of TAG and their abundances makes a substantial difference to the organoleptic and processing characteristics of that fat (Bracco 1994), there are differences between species and between plants and animals (Hunter 2001), but whether these make a substantial difference to health is not clear. A TAG is composed of a glycerol molecule covalently bound to three FA, numbered sn-1, sn-2 and sn-3 from the bottom to the top – which can be imagined as a glycerol molecule displayed vertically on the left-hand side of a visual image with the three FA displayed horizontally, bound to the hydroxyl groups of the glycerol molecule. The specific sn-x structures and varieties of TAG affect the properties of a fat. For example, beef tallow and cocoa butter have similar but not identical mouthfeel, and the property of melting on the tongue is due mainly to the reduced types of TAG and their specific combinations in cocoa butter (Bracco 1994). During digestion, lipases hydrolyse the bonds at the sn-1 and sn-3 position, resulting in a monoglyceride and two free FA, and the monoglycerides are taken up more efficiently. However, this preference is only likely to affect monoglycerides of stearic acid (Bracco 1994), since >99% of all the other FA are taken up irrespective of the sn-x position that they occur in. These monoglycerides preferentially form the backbones for new TAG in the host (Zock et al. 1996). Plant oils tend to have more PUFA in the sn-2 position than animal fat (Hunter 2001), which would then result in PUFA being preferentially incorporated into reformed TAG in the individual. Given the discussion on a high OMR and reactive oxygen species (see above and below) this may have unfortunate effects on stored and structural lipid in the individual. Stereoisomeric modifications of palmitic acid in a normal diet in adults appeared to make no significant difference to the HDL-C or LDL-C concentrations in the blood (Zock et al. 1995). However, in a review of the literature on interesterification, (Hayes and Pronczuk 2010) report that a large amount of stearic acid in the sn-2 position had negative effects on the relative HDL-C and LDL-C profile. As stearic acid is generally not found at high frequency in the sn-2 position in natural fats, and is usually considered as hypocholesterolemic (see above), they considered this a concern for manufactured fats. Furthermore, they report that interesterified fats alter the HDL-C and LDL-C ratios and concentrations in the serum of both infants and piglets away from the pattern seen when they are fed their mother’s milk (Hayes and Pronczuk 2010), which generally has SFA at the sn-2 position (Hunter 2001). This is a concern given the known improvement in growth of infants on animal versus plant fats (López-López et al. 2001). Finally, the overall structure of a fat may affect its digestion. For example, butter is a water-in-oil emulsion, compared with the lipid contents of the gut, which after secretion of bile, becomes an oil-in-water emulsion. This has been hypothesised to explain the result of a shorter period of elevated lipemia after a meal using butter compared with olive or sunflower oils, with an increased number of chylomicrons and faster processing of chylomicron remnants, a positive physiological result (Mekki et al. 2002). The alternative explanation is that the cholesterol and SFA in butter increased the expression of apoE (see above), resulting in more chylomicrons that were processed more efficiently by the body. Animal fats as a constituent of adipose or muscle tissue or dairy products and plant fats or oils in the form of nuts, seeds or oily fruits are absorbed slowly compared with refined cooking oils or fats added to food, due to the structures and digestibility of the items (Berry et al. 2008; Damasceno et al. 2011; Michalski et al. 2013; Garcia et al. 2014).
Where lipids are the overwhelming energy source then there is substantial generation of ketone bodies, and there are some benefits to be had from the metabolism of ketone bodies (Veech et al. 2001; Volek et al. 2008). The ketone bodies β-hydroxybutyrate and acetoacetate are deliberately generated from FA and are not a consequence of incomplete glucose metabolism via the tricarboxylic acid cycle (Krebs 1966; Krebs et al. 1971). These are a form of energy from fat that is directly soluble in aqueous solution rather than either being carried as NEFA attached to albumin or TAG carried via serum cholesterol or chylomicrons, and are the only metabolite from FA that can be directly metabolised by the brain (Cahill 2006). In a further parallel to serum glucose, levels of ketone bodies are regulated by insulin, so that a pulse of higher levels of ketone bodies increases insulin thereby reducing release of lipid from adipocytes (Hawkins et al. 1971). It is thought that ancestral human diets would have generated ketone bodies for parts of the day or for days (Krebs et al. 1971; Veech et al. 2001; Volek et al. 2008), because they are metabolites associated with starvation (Cahill 2006). Although levels of serum ketone bodies have not been measured in traditional hunter-gatherers, there is evidence from contemporary study of some hunter-gatherers of absence of food for days (Stefansson 1912; Marlowe 2005). In addition, measurements on Western volunteers of both sexes show the generation of ketone bodies a few hours after moderate to high fat meals if no snacks are taken (Marinou et al. 2011). As blood glucose and insulin levels gradually decline after a meal, levels of NEFA increase in the circulation, and the ketone bodies acetoacetate and β-hydroxybutyrate start to rise (Cahill 2006; Hodson et al. 2008; Marinou et al. 2011). The metabolism of ketone bodies requires less oxygen to generate the same physiological output and generates fewer free radicals (Sato et al. 1995), so is less damaging to tissues (Halliwell 2006), than the metabolism of serum glucose. In the presence of both serum glucose and ketone bodies, less glucose is used (Kashiwaya et al. 1994) suggesting that the body will protect its glucose sources in the presence of ketone bodies. In these mild ketotic states, the amount of acetocarnitine and ubiquinone-10 (Coenzyme Q10), the latter through its obligate relationship to the uncoupling proteins, are increased in tissues including the liver, heart, brain, and skeletal muscle (Pearson and Tubbs 1967; McGarry et al. 1975; Echtay et al. 2000; Sullivan et al. 2004). These are two important anti-oxidants in the body whose levels appear to decline with age and are associated with the decline of function of mitochondria due to age-related oxidative damage (Shigenaga et al. 1994; Hiatt 2001).
High fat low carbohydrate low to moderate protein diets, often called ketogenic diets, have been shown to reverse the metabolic syndrome and non-alcoholic fatty liver disease without medication and address a range of other biological indicators (Hite et al. 2011; Peréz-Guisado and Munoz-Serrano 2011a, 2011b). These diets have not only led to weight loss without direct calorie restriction they have resulted in improvement in the risk factors associated with CVD when animal and other fats are used (Foster et al. 2003; Volek et al. 2003; Herron et al. 2004; Sharman and Volek 2004; Volek and Sharman 2004; Yancy et al. 2004, 2010; Brinkworth et al. 2009; Sacks et al. 2009). Moreover, trials of ketogenic diets in the treatment of final stage cancer patients and in experimental systems (Tisdale et al. 1987; Breitkreutz et al. 2005; Zuccoli et al. 2010; Ho et al. 2011; Klement and Kaemmerer 2011; Schmidt et al. 2011; Chang et al. 2013) have occurred, with some interesting results of reduction in tumour size, reduction of cachexia, or delay of initiation of cancer. Ketogenic diets continue to be used to treat some classes of epilepsy (Freeman et al. 1998; Kossoff et al. 2006; Neal et al. 2008), and have been suggested for other mental disorders or dementias including Alzheimer’s disease and Parkinson’s disease (VanItallie and Nufert 2003; Kim et al. 2007; Kossoff and Hartman 2012). A random controlled trial of an additive to the normal diet, AC-1202, consisting of short- and medium-chain TAG derived from coconut oil and that are metabolised to ketone bodies, has shown some improvement in Alzheimer’s disease sufferers from ketone body production (Henderson et al. 2009). Therapeutic doses were 20 g per day, which generated similar levels of ketone bodies to a low carbohydrate diet. Butter fat consists of >10% of short-chain FA and a further 15% of the medium-chain SFA of C12:0 and C14:0, which are rapidly oxidised in the liver (Bach and Babayan 1982; MacDougall et al. 1996; DeLany et al. 2000) and would therefore generate increased levels of ketone bodies in a high fat but not necessarily low carbohydrate diet.
Finally, at the opposite extreme, consumption of large amounts of sugar, refined carbohydrate and food overconsumption in general can lead to obesity, high serum glucose and hyperinsulinaemia. The effects of high serum glucose and hyperinsulinaemia, hall marks of type 2 diabetes, are well known and are associated with a wide range of chronic diseases of Western civilisation (Kuusisto et al. 1997; Xu et al. 2004; Friberg et al. 2007; Xue and Michels 2007; Johnson et al. 2009; Orgel and Mittelman 2013). High serum glucose is functionally related to the glycosylation of protein, which increases the rate of aging of tissues. High serum glucose is also functionally related to the increased glycosylation of small dense LDL-C and subsequent damage to apoB-100 molecules within the blood stream (Younis et al. 2013), contributing to atherosclerosis. Serum glucose is needed for cancer cell growth, as cancerous cells are switched to obtaining energy from aerobic glycolysis, the Warburg effect. Cancerous cells use the intermediates of the glycolytic pathway to generate FA and other biosynthetic intermediates within the cell so as to generate cell membranes and organelles and thereby allow cell growth and division, and this is what most of the glucose is used for (Warburg 1956; Wang et al. 2012). Paradoxically, these cells do not derive much energy from either ketone bodies or β-oxidation of FA, irrespective of the circulating level of FA. Carbohydrate sources vary in their ability to maintain high serum glucose and promote insulin resistance, with recent research showing that fructose, and by association sucrose, have a much higher ability to promote insulin insensitivity and, consequently, a prolonged elevation in serum glucose than glucose itself (Stanhope et al. 2009). Individuals respond differently to these two factors, leading to correlations between disorders. For example, diabetic individuals are more likely to have CVD, or a cancer, or either vascular dementia or Alzheimer’s disease (Stamler et al. 1993; Luchsinger et al. 2001). Non-diabetic individuals with Alzheimer’s disease are more likely to have high serum glucose and hyperinsulinaemia, even though BMI is not associated to Alzheimer’s disease. Some of these disorders have historically been blamed on high fat consumption using evidence from ecological comparisons, but the evidence from high fat ketogenic diets (cf. above) suggests instead that these disorders are due to food overconsumption rather than fat overconsumption. Obviously, animal fat is not a panacea for all ills, but avoidance of animal fat is no panacea either.
Recommendations for animal industries
If humans are to consume animal fat then the best quality fat should be produced (Kelly et al. 2001; Tume 2004; Givens 2005; Shingfield et al. 2013). This review suggests that this results in some clear selection and production/nutrition goals for animals. These goals are that where possible animals should be produced with a low OMR, that long-chain n-3 PUFA should be increased, that higher levels of oleate or stearate and lower levels of palmitate should be encouraged, but that increased n-6 PUFA content need not be encouraged.
The composition of the fat of monogastric animals is changed by the feed they eat. Farmed fish are known to have lower levels of long-chain n-3 PUFA and many of the most valuable fish are fed combinations of fish meal. Some research suggests that farmed fish such as salmon have the ability to generate their own long-chain n-3 PUFA when the diet is limiting (Sanden et al. 2011). Approximately 35–45% of chicken and other poultry fat is MUFA, between 22 and 27% of the fat is CISFA, 13–26% of fat is PUFA depending on species and method of rearing, but there can be large changes in long-chain n-3 PUFA if the ration contains higher levels of α-linolenic acid (Chartrin et al. 2006; Jia et al. 2010; Givens et al. 2011). In pigs there are smaller changes in stearic acid, MUFA and PUFA with different rations and genotypes than in chickens but CISFA are ~27% of the fat and can as low as 21% for free-range pigs (Rodríguez-Sánchez et al. 2010; Razmaite et al. 2011; Barea et al. 2013). The value of the OMR is dependent on the food source. Interestingly, genetic modification of pigs with the nematode delta-3 desaturase results in animals with a lower OMR even if fed diets high in n-6 PUFA (Ren et al. 2011). This has not yet been reported in other food animal species.
Ruminants have low levels of PUFA in their fat due to bio-hydrogenation of dietary PUFA (Doreau and Ferlay 1994) although there are differences in ruminants in the amount of oleate depending on genetics and feed source (Yang et al. 1999a). One of the major effects on ruminant fat is the ratio of stearate to oleate, which depends on the location of the fat within the body: the closer to the surface the higher the oleate and the lower the stearate (Meng et al. 1969; West and Shaw 1975; Morin 2007; Staerfl et al. 2011). Despite the difference in total SFA between free-range chicken, pig and ruminant fat, they contain very similar levels of CISFA, in a range ~25%. Although the level of PUFA in ruminant fat is much lower than in monogastric fat, the OMR is still dependent on the ration or type of feed, with lower OMR achievable on feed if higher levels of n-3 fats are used (Bobe et al. 2007). Changing the OMR in ruminant feed will affect the OMR of PUFA available for human consumption, and this would be a benefit. Whether the feeding of cattle in feedlots will grow or decline in the future will be affected by a range of factors including amount of methane produced per kilogram meat produced, the cost of feedlot rations compared with the cost and requirement for biodiesel and other fuels, and the availability of rangeland for pasture for ruminants.
The palatability of animal foods is affected by the amount and composition of its fat, but at present, in Western countries like Australia, a lot of emphasis is placed on reducing the amount of fat in livestock. Palatability and eating quality of meat is related to increased fat content, as reported in tests of consumers, but consumers in many Western countries actively select leaner cuts when meat is presented raw (Wood et al. 1999; Thompson 2004; Dransfield et al. 2005; Polkinghorne and Thompson 2010; Utrilla et al. 2010). Although it is suggested that this reduction in meat fat will improve human health, as this review has shown, this claim is dubious. This demand for leaner meat has led to some distortions in selection and production goals. From poultry to cattle, animals are selected for more meat and less fat (Abasht et al. 2006; Lagarrigue et al. 2006; Givens et al. 2011), and meat is presented with more fat trimmed from the cuts. This has led to fat being discarded and, in some cases, excess animal fat burned for fuel (Fairlie 2010), which is wasted production. Selecting animals for more meat, greater efficiency, greater productivity, and less fat has occasionally resulted in negative consequences for the animals themselves, such as reductions in fertility and fecundity, dystocia, porcine stress syndrome, Rendement Napole, or skeletal problems (Carlson et al. 1980; Hanset et al. 1982; Enfält et al. 1997; Grobet et al. 1997; Pitchford 2004). Changing the selection goal of more meat and less fat may well be intractable, however, as it is partly a response to consumer preference, and consumer preference can take decades to change.
Nevertheless, it is not impossible for consumer sentiment to change, a newspaper article titled ‘Butter crisis spreads in Norway’ (Anonymous 2011) reported that due to a high fat low carbohydrate diet craze Norway ran out of butter in the lead in to the Christmas period. New formulations of products, such as Christmas Puddings and Chicken Liver Pate, are appearing on Australian supermarket shelves with ingredients including animal fats and margarines made from edible animal tallow (change observed between December 2012 and April 2013) that previously had potato starch as stabiliser or vegetable shortening only. Prominent medical professionals are starting to say that it is better to control sugar, especially fructose and thereby sucrose (Lustig et al. 2012), and that controlling dietary saturated fat is irrelevant, so it may be that the wheel has started to turn. Animal industries should be aware of these trends and need to take advantage of them when they arise, because changing selection goals or production practises does not occur overnight. More emphasis should be placed on healthy fat from animals, not just from fish, but also from livestock of all kinds.
Acknowledgements
I thank J. Henshall, D. Topping, and A. Yang for critically reading the manuscript. Although the author has been supported by funds from Meat & Livestock Australia, Dairy Australia, and the Beef Cooperative Research Centre in the past, no meat or dairy industry body commissioned, funded or had any part in this review. Any discussion of the role of animal food in human nutrition these days needs a declaration of biases: the author was raised a strict whole-food ovo-lacto vegetarian and occasionally relapses to that state; this review is a response to the author’s question of his own research, ‘Why are we trying to increase intramuscular fat if animal fat is so bad for us?’
References
Abasht B, Dekkers JCM, Lamont SJ (2006) Review of quantitative trait loci identified in the chicken. Poultry Science 85, 2079–2096.| Review of quantitative trait loci identified in the chicken.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlansL7P&md5=df9bec7ec57a2e02bd57ac5293570897CAS | 17135661PubMed |
Abbey M, Belling GB, Noakes M, Hirata F, Nestel PJ (1993) Oxidation of low-density liproteins – intraindividual variability and the effect of dietary linoleate supplementation. The American Journal of Clinical Nutrition 57, 391–398.
Abrams JJ, Grundy SM, Ginsberg H (1981) Metabolism of plasma triglycerides in hypothyroidism and hyperthyroidism in man. Journal of Lipid Research 22, 307–322.
Acheson KJ, Schutz Y, Bessard T, Anantharaman K, Flatt JP, Jequier E (1988) Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. The American Journal of Clinical Nutrition 48, 240–247.
Ackman RG, Sebedio J-L (1981) Fatty acids and sterols in oils from canola screenings. Journal of the American Oil Chemists’ Society 58, 594–598.
| Fatty acids and sterols in oils from canola screenings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXkt1eisLg%3D&md5=283f220ce743fc1ca9f3b5f1152cccb5CAS |
Ahrens EH, Hirsch J, Insull W, Tsaltas TT, Blomstrand R, Peterson ML (1957) The influence of dietary fats on serum-lipid levels in man. Lancet 269, 943–953.
| The influence of dietary fats on serum-lipid levels in man.Crossref | GoogleScholarGoogle Scholar |
Albrink MJ, Man EB (1959) Serum triglycerides in coronary artery disease. Archives of Internal Medicine 103, 4–8.
| Serum triglycerides in coronary artery disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1MXktVygtg%3D%3D&md5=f1fc0f2bd0c93f035c0e8b6c08ee53c8CAS | 13605296PubMed |
Aldai N, Dugan MER, Kramer JKG, Martinez A, Lopez-Campos O, Mantecon AR, Osoro K (2011) Length of concentrate finishing affects the fatty acid composition of grass-fed and genetically lean beef: an emphasis on trans-18:1 and conjugated linoleic acid profiles. Animal 5, 1643–1652.
| Length of concentrate finishing affects the fatty acid composition of grass-fed and genetically lean beef: an emphasis on trans-18:1 and conjugated linoleic acid profiles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1yms7rF&md5=0ee81a2ddf4fc326accc06d866bb44f4CAS | 22440357PubMed |
Alexander DD, Morimoto LM, Mink PJ, Lowe KA (2010) Summary and meta-analysis of prospective studies of animal fat intake and breast cancer. Nutrition Research Reviews 23, 169–179.
| Summary and meta-analysis of prospective studies of animal fat intake and breast cancer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotFeju7k%3D&md5=6ec005b54bafe82bd4123098dd5ed3f4CAS | 20181297PubMed |
Anderson KE (2011) Comparison of fatty acid, cholesterol, and vitamin A and E composition in eggs from hens housed in conventional cage and range production facilities. Poultry Science 90, 1600–1608.
| Comparison of fatty acid, cholesterol, and vitamin A and E composition in eggs from hens housed in conventional cage and range production facilities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsVGrt7Y%3D&md5=9d2b7b6cfd83913faa89cfb228d73d58CAS | 21673178PubMed |
Anonymous (2011) Butter crisis spreads in Norway. The Australian 15 November 2011, 10
Antar MA, Hodges RE, Ohlson MA (1964) Changes in retail market food supplies in the United States in the last seventy years in relation to the incidence of Coronary Heart Disease, with special reference to dietary carbohydrates and essential fatty acids. The American Journal of Clinical Nutrition 14, 169–178.
Appel LJ (2008) Dietary patterns and longevity: expanding the Blue Zones. Circulation 118, 214–215.
| Dietary patterns and longevity: expanding the Blue Zones.Crossref | GoogleScholarGoogle Scholar | 18625902PubMed |
Arai Y, Hirose N, Yamamura K, Shimizu K, Takayama M, Ebihara Y, Osono Y (2001) Serum insulin-like growth factor-1 in centenarians: implications of IGF-1 as a rapid turnover protein. Gerontology Series A 56, M79–M82.
Archer SY, Meng SF, Shei A, Hodin RA (1998) p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proceedings of the National Academy of Sciences, USA 95, 6791–6796.
| p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjslynur8%3D&md5=11d36c1e9d4a1f8d12bbbb3e29e23ce7CAS |
Aune D, Chan DSM, Vieira AR, Rosenblatt DAN, Vieira R, Greenwood DC, Kampman E, Norat T (2013) Red and processed meat intake and risk of colorectal adenomas: a systematic review and meta-analysis of epidemiological studies. Cancer Causes & Control 24, 611–627.
| Red and processed meat intake and risk of colorectal adenomas: a systematic review and meta-analysis of epidemiological studies.Crossref | GoogleScholarGoogle Scholar |
Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM (1988) Low density lipoprotein subclass patterns and risk of myocardial infarction. Journal of the American Medical Association 260, 1917–1921.
| Low density lipoprotein subclass patterns and risk of myocardial infarction.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1czjtlaktQ%3D%3D&md5=69f67c48e204f8ebe396979261b96939CAS | 3418853PubMed |
Bach AC, Babayan VK (1982) Medium chain triglycerides – an update. The American Journal of Clinical Nutrition 36, 950–962.
Barea R, Isabel B, Nieto R, Lopez-Bote C, Aguilera JF (2013) Evolution of the fatty acid profile of subcutaneous back-fat adipose tissue in growing Iberian and Landrace × Large White pigs. Animal 7, 688–698.
| Evolution of the fatty acid profile of subcutaneous back-fat adipose tissue in growing Iberian and Landrace × Large White pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjt1Wks70%3D&md5=a9f9742f4137381fe921a1ea1bca8e8cCAS | 23031353PubMed |
Barrows BR, Parks EJ (2006) Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. The Journal of Clinical Endocrinology and Metabolism 91, 1446–1452.
| Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjvFOktrY%3D&md5=ab5cf14469543a7cacb4dff5af89d121CAS | 16449340PubMed |
Basu S, Yoffe P, Hills N, Lustig RH (2013) The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. PLoS ONE 8, e57873
| The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjvFGqtLg%3D&md5=f6512e574217263298007e9b2825de12CAS | 23460912PubMed |
Berry SEE, Tydeman EA, Lewis HB, Phalora R, Roxborough J, Picout DR, Ellis PR (2008) Manipulation of lipid bioavailability of almond seeds influences postprandial lipemia in health human subjects. The American Journal of Clinical Nutrition 88, 922–929.
Beulens JWJ, Van der A DL, Grobbee DE, Sluijs I, Spijkerman AMW, van der Schouw YT (2010) Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care 33, 1699–1705.
| Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFahs7vF&md5=fd123cdca2844c69b509ff5609caba89CAS |
Bézard J, Bugaut M, Clement G (1971) Triglyceride composition of coconut oil. Journal of the American Oil Chemists’ Society 48, 134–139.
| Triglyceride composition of coconut oil.Crossref | GoogleScholarGoogle Scholar |
Bézard J, Blond JP, Bernard A, Clouet P (1994) The metabolism and availability of essential fatty acids in animal and human tissues. Reproduction, Nutrition, Development 34, 539–568.
| The metabolism and availability of essential fatty acids in animal and human tissues.Crossref | GoogleScholarGoogle Scholar | 7840871PubMed |
Blaxter KL, Webster AJF (1991) Animal production and food: real problems and paranoia. Animal Production 53, 261–269.
| Animal production and food: real problems and paranoia.Crossref | GoogleScholarGoogle Scholar |
Bobe G, Zimmerman S, Hammond EG, Freeman AE, Porter PA, Luhman CM, Beitz DC (2007) Butter composition and texture from cows with different milk fatty acid compositions fed fish oil or roasted soybeans. Journal of Dairy Science 90, 2596–2603.
| Butter composition and texture from cows with different milk fatty acid compositions fed fish oil or roasted soybeans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlvFOisLk%3D&md5=99bf18e5af0f4dbdd7f879da33ec4c2aCAS | 17517699PubMed |
Bocuse P (1988) ‘The cuisine of Paul Bocuse.’ (Grafton Books: London)
Boerwinkle E, Brown S, Sharrett AR, Heiss G, Patsch W (1994) Apolipoprotein E polymorphism influences postprandial retinyl palmitate but not triglyceride concentrations. American Journal of Human Genetics 54, 341–360.
Bonanome A, Grundy SM (1988) Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. The New England Journal of Medicine 318, 1244–1248.
| Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c3gt1SmsA%3D%3D&md5=0b4334bfac3f4998fce04e7af9b7d871CAS | 3362176PubMed |
Bousser MG, Amarenco P, Chamorro A, Fisher M, Ford I, Fox KM, Hennerici MG, Mattle HP, Rothwell PM, de Cordoue A, Fratacci MD, Investigators PS (2011) Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet 377, 2013–2022.
| Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntlGjtLs%3D&md5=27af1dbdcd6d75fe151cebbf58f7fc8fCAS | 21616527PubMed |
Bracco U (1994) Effect of triglyceride structure on fat absorption. The American Journal of Clinical Nutrition 60, 1002S–1009S.
Breitkreutz R, Tesdal K, Jentschura D, Haas O, Leweling H, Holm E (2005) Effects of a high-fat diet on body composition in cancer patients receiving chemotherapy: a randomized controlled study. Wiener Klinische Wochenschrift 117, 685–692.
| Effects of a high-fat diet on body composition in cancer patients receiving chemotherapy: a randomized controlled study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xms1Wrug%3D%3D&md5=57d31b7ed46c854032327b5ff14fcc1fCAS | 16416368PubMed |
Brenna JT (2002) Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Current Opinion in Clinical Nutrition and Metabolic Care 5, 127–132.
| Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitVKnu7c%3D&md5=d015d48db9fd4129040cb522895a1685CAS | 11844977PubMed |
Brennan SF, Cantwell MM, Cardwell CR, Velentzis LS, Woodside JV (2010) Dietary patterns and breast cancer risk: a systematic review and meta-analysis. The American Journal of Clinical Nutrition 91, 1294–1302.
| Dietary patterns and breast cancer risk: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlsVWrtbw%3D&md5=ab1be955218666c26f7290616d5ecaafCAS | 20219961PubMed |
Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM (2009) Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. The American Journal of Clinical Nutrition 90, 23–32.
| Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvFGmsL8%3D&md5=d7cbfc20bbf1eea8624527e20e0819e0CAS | 19439458PubMed |
Brown MS, Goldstein JL (1983) Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annual Review of Biochemistry 52, 223–261.
| Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXltVaqur8%3D&md5=721f9aa7936d2621341921df519bd3e1CAS | 6311077PubMed |
Buffa R, Floris G, Lodde M, Cotza M, Marini E (2010) Nutritional status in the healthy longeval population from Sardinia (Italy). The Journal of Nutrition, Health & Aging 14, 97–102.
| Nutritional status in the healthy longeval population from Sardinia (Italy).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c%2FotVKhsw%3D%3D&md5=4bed0be062aed22936990b4d260aebf0CAS |
Burdge GC, Calder PC (2005) Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reproduction, Nutrition, Development 45, 581–597.
| Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1CmurbJ&md5=440fb6de98f8f98c194d028234847326CAS | 16188209PubMed |
Burkitt DP (1973) Some diseases characteristic of modern western civilization. British Medical Journal 1, 274–278.
| Some diseases characteristic of modern western civilization.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE3s%2FptVKgtA%3D%3D&md5=68bd2ea1115ae64a9db5cf01377190e3CAS | 4568142PubMed |
Cachaldora P, Garcia-Rebollar P, Alvarez C, Mendez J, De Blas JC (2008) Double enrichment of chicken eggs with conjugated linoleic acid and n-3 fatty acids through dietary fat supplementation. Animal Feed Science and Technology 144, 315–326.
| Double enrichment of chicken eggs with conjugated linoleic acid and n-3 fatty acids through dietary fat supplementation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvVKjs7k%3D&md5=bca8232dc4197311b4a34497830368c7CAS |
Cahill GF (2006) Fuel metabolism in starvation. Annual Review of Nutrition 26, 1–22.
Campos H, Dreon DM, Krauss RM (1995) Associations of hepatic and lipoprotein lipase activities with changes in dietary composition and low density lipoprotein subclasses. Journal of Lipid Research 36, 462–472.
Carlson JP, Christian LL, Kuhlers DL, Rasmusen BA (1980) Influence of the porcine stress syndrome on production and carcass traits. Journal of Animal Science 50, 21–28.
Carlson JJ, Eisenmann JC, Norman GJ, Ortiz KA, Young PC (2011) Dietary fiber and nutrient density are inversely associated with the metabolic syndrome in US adolescents. Journal of the American Dietetic Association 111, 1688–1695.
| Dietary fiber and nutrient density are inversely associated with the metabolic syndrome in US adolescents.Crossref | GoogleScholarGoogle Scholar | 22027051PubMed |
Carluccio A, Contaldo G (2012) ‘Two greedy Italians eat Italy.’ (Quadrille Publishing: London)
Carro E, Torres-Aleman I (2006) Serum insulin-like growth factor I in brain function. The Keio Journal of Medicine 55, 59–63.
| Serum insulin-like growth factor I in brain function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xot1Cnsb4%3D&md5=b448c05b28e9bed8ffda5ff62151421cCAS | 16830417PubMed |
Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I (2002) Serum insulin-like growth factor I regulates brain amyloid-levels. Nature Medicine 8, 1390–1397.
| Serum insulin-like growth factor I regulates brain amyloid-levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XptVSgsb0%3D&md5=bb90ef1db831ef8355af33d24bfc2c55CAS | 12415260PubMed |
Castelli WP (1986) The triglyceride issue – a view from Framingham. American Heart Journal 112, 432–437.
| The triglyceride issue – a view from Framingham.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL283pt1Ojsw%3D%3D&md5=d8119190ae6d3d30d9aa037b9bfa874aCAS | 3739899PubMed |
Castelli WP (1992) Concerning the possibility of a nut. Archives of Internal Medicine 152, 1371–1372.
| Concerning the possibility of a nut.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38zivVKjsQ%3D%3D&md5=d8447893562dc2dac7cca1277238c14aCAS | 1303626PubMed |
Castelli WP, Garrison RJ, Wilson PWF, Abbott RD, Kalousdian S, Kannel WB (1986) Incidence of coronary heart disease and lipoprotein cholesterol levels – the Framingham study. Journal of the American Medical Association 256, 2835–2838.
| Incidence of coronary heart disease and lipoprotein cholesterol levels – the Framingham study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s%2FjslWktA%3D%3D&md5=3e5b708a689305d681e8caae8a9acf8aCAS | 3773200PubMed |
Chajes V, Torres-Mejia G, Biessy C, Ortega-Olvera C, Angeles-Llerenas A, Ferrari P, Lazcano-Ponce E, Romieu I (2012) Omega-3 and omega-6 polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: impact of obesity status. Cancer Epidemiology, Biomarkers & Prevention 21, 319–326.
| Omega-3 and omega-6 polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: impact of obesity status.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslOrt74%3D&md5=765989e29e49ab79a8568d4a19fc0ed5CAS |
Chang HT, Olson LK, Schwartz KA (2013) Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutrition & Metabolism 10, 47
| Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFymtL7O&md5=3e2b5963ad28d16995b21ff8738200a4CAS |
Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Nordestgaard BG, Ray KK, Reiner Z, Taskinen MR, Tokgozoglu L, Tybjaerg-Hansen A, Watts GF, Soc European Atherosclerosis (2011) Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. European Heart Journal 32, 1345–1361.
| Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntF2ht7k%3D&md5=b03ffbd5e6e9215ab0eaa41373dd0723CAS | 21531743PubMed |
Chartrin P, Bernadet MD, Guy G, Mourot J, Duclos MJ, Baeza E (2006) The effects of genotype and overfeeding on fat level and composition of adipose and muscle tissues in ducks. Animal Research 55, 231–244.
| The effects of genotype and overfeeding on fat level and composition of adipose and muscle tissues in ducks.Crossref | GoogleScholarGoogle Scholar |
Cleave TL (1974) ‘The saccharine disease.’ (John Wright & Sons: Bristol)
Connolly BS, Barnett C, Vogt KN, Li T, Stone J, Boyd NF (2002) A meta-analysis of published literature on waist-to-hip ratio and risk of breast cancer. Nutrition and Cancer 44, 127–138.
| A meta-analysis of published literature on waist-to-hip ratio and risk of breast cancer.Crossref | GoogleScholarGoogle Scholar | 12734058PubMed |
Corbo RM, Scacchi R (1999) Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Annals of Human Genetics 63, 301–310.
| Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c3gtlShuw%3D%3D&md5=86c9c31500b5ffaf5d0a36a5a12e031fCAS | 10738542PubMed |
Cordain L, Eaton SB, Miller JB, Mann N, Hill K (2002a) The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. European Journal of Clinical Nutrition 56, S42–S52.
| The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic.Crossref | GoogleScholarGoogle Scholar | 11965522PubMed |
Cordain L, Watkins BA, Florant GL, Kelher M, Rogers L, Li Y (2002b) Fatty acid analysis of wild ruminant tissues: evolutionary implications for reducing diet-related chronic disease. European Journal of Clinical Nutrition 56, 181–191.
| Fatty acid analysis of wild ruminant tissues: evolutionary implications for reducing diet-related chronic disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XislChsrY%3D&md5=1404c5d2a42092fc55a218ed7b56ef55CAS | 11960292PubMed |
Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J (2005) Origins and evolution of the Western diet: health implications for the 21st century. The American Journal of Clinical Nutrition 81, 341–354.
Costello C, Ovando D, Hilborn R, Gaines SD, Deschenes O, Lester SE (2012) Status and solutions for the world’s unassessed fisheries. Science 338, 517–520.
| Status and solutions for the world’s unassessed fisheries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFGmtbvI&md5=4cff9b278834833fd978c15ae2b2c8e9CAS | 23019613PubMed |
Couvreur S, Hurtaud C, Lopez C, Delaby L, Peyraud JL (2006) The linear relationship between the proportion of fresh grass in the cow diet, milk fatty acid composition, and butter properties. Journal of Dairy Science 89, 1956–1969.
| The linear relationship between the proportion of fresh grass in the cow diet, milk fatty acid composition, and butter properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xlt1eru7g%3D&md5=c5e807343044e4157b1d83343b7c22f4CAS | 16702259PubMed |
Curtin F, Morabia A, Bernstein M, Dederding J-P (1998) A population survey of bowel habits in urban Swiss men. European Journal of Public Health 8, 170–175.
| A population survey of bowel habits in urban Swiss men.Crossref | GoogleScholarGoogle Scholar |
Daley CA, Abbott A, Doyle PS, Nader GA, Larson S (2010) A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutrition Journal 9, 10
| A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef.Crossref | GoogleScholarGoogle Scholar | 20219103PubMed |
Damasceno NRT, Perez-Heras A, Serra M, Cofan M, Sala-Vila A, Salas-Salvado J, Ros E (2011) Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutrition, Metabolism, and Cardiovascular Diseases 21, S14–S20.
| Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntVequr4%3D&md5=e9d8d7f727220a916b7255b363929f47CAS |
de Hollander EL, Bemelmans WJE, Boshuizen HC, Friedrich N, Wallaschofski H, Guallar-Castillon P, Walter S, Zillikens MC, Rosengren A, Lissner L, Bassett JK, Giles GG, Orsini N, Heim N, Visser M, de Groot L, Collaborators WCE (2012) The association between waist circumference and risk of mortality considering body mass index in 65- to 74-year-olds: a meta-analysis of 29 cohorts involving more than 58 000 elderly persons. International Journal of Epidemiology 41, 805–817.
| The association between waist circumference and risk of mortality considering body mass index in 65- to 74-year-olds: a meta-analysis of 29 cohorts involving more than 58 000 elderly persons.Crossref | GoogleScholarGoogle Scholar | 22467292PubMed |
de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin J-L, Monjaud I, Guidollet J, Touboul P, Delaye J (1994) Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 343, 1454–1459.
| Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2c3mtVOrtg%3D%3D&md5=accc5b4db3bcf2b8a322d9cf8ba7e90eCAS | 7911176PubMed |
de Lorgeril M, Salen P, Martin J-L, Monjaud I, Delaye J, Mamelle N (1999) Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 99, 779–785.
| Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M7ksFOjsg%3D%3D&md5=fb49729fbdf4313ea1b921bf65dbc4a8CAS | 9989963PubMed |
de Lorgeril M, Salen P, Martin JL, Boucher F, Paillard F, de Leiris J (2002) Wine drinking and risks of cardiovascular complications after recent acute myocardial infarction. Circulation 106, 1465–1469.
| Wine drinking and risks of cardiovascular complications after recent acute myocardial infarction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XntVKksb8%3D&md5=a956a82a56fed281691662f049e33efdCAS | 12234949PubMed |
de Roos NM, Schouten EG, Katan MB (2001) Consumption of a solid fat rich in lauric acid results in a more favorable serum lipid profile in healthy men and women than consumption of a solid fat rich in trans-fatty acids. The Journal of Nutrition 131, 242–245.
DeLany JP, Windhauser MM, Champagne CM, Bray GA (2000) Differential oxidation of individual dietary fatty acids in humans. The American Journal of Clinical Nutrition 72, 905–911.
Dent J, El-Serag HB, Wallander MA, Johansson S (2005) Epidemiology of gastrooesophageal reflux disease: a systematic review. Gut 54, 710–717.
| Epidemiology of gastrooesophageal reflux disease: a systematic review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2M7pslylsA%3D%3D&md5=371972b3fdc15e08d9b5174491125235CAS | 15831922PubMed |
Di Vincenzo D, Maranz S, Serraiocco A, Vito R, Wiesman Z, Bianchi G (2005) Regional variation in shea butter lipid and triterpene composition in four African countries. Journal of Agricultural and Food Chemistry 53, 7473–7479.
| Regional variation in shea butter lipid and triterpene composition in four African countries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXot1Wjt7g%3D&md5=c14843c543a66ee4c9baf99f443a7f36CAS | 16159175PubMed |
Dilzer A, Park Y (2012) Implication of conjugated linoleic acid (CLA) in human health. Critical Reviews in Food Science and Nutrition 52, 488–513.
| Implication of conjugated linoleic acid (CLA) in human health.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xms1Whsb8%3D&md5=39b800de83b73e0a67c07f4b4addf440CAS | 22452730PubMed |
Dickson Wright C (2011) ‘A history of English food.’ (Random House Books: UK)
Dong JY, Zhang LJ, He K, Qin LQ (2011) Dairy consumption and risk of breast cancer: a meta-analysis of prospective cohort studies. Breast Cancer Research and Treatment 127, 23–31.
| Dairy consumption and risk of breast cancer: a meta-analysis of prospective cohort studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFegt7Y%3D&md5=35a824de36b8c792f8c464f14568a4a5CAS | 21442197PubMed |
Doreau M, Ferlay A (1994) Digestion and utilization of fatty-acids by ruminants. Animal Feed Science and Technology 45, 379–396.
| Digestion and utilization of fatty-acids by ruminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXktVOru7s%3D&md5=9dfa80e014aac13144c2af992b152700CAS |
Dorfman SE, Lichtenstein AH (2006) Dietary fatty acids differentially modulate messenger RNA abundance of low-density lipoprotein receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and microsomal triglyceride transfer protein in Golden-Syrian hamsters. Metabolism: Clinical and Experimental 55, 635–641.
| Dietary fatty acids differentially modulate messenger RNA abundance of low-density lipoprotein receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and microsomal triglyceride transfer protein in Golden-Syrian hamsters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjvVGmurk%3D&md5=f6bc2faa45f26893b7c6b3e60c044b78CAS |
Dorfman SE, Laurent D, Gounarides JS, Li X, Mullarkey TL, Rocheford EC, Sari-Sarraf F, Hirsch EA, Hughes TE, Commerford SR (2009) Metabolic implications of dietary trans-fatty acids. Obesity 17, 1200–1207.
| Metabolic implications of dietary trans-fatty acids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXot12murs%3D&md5=092ad8ff7ad8c1197a7f4c5957ba27a2CAS | 19584878PubMed |
Dougherty RM, Allman MA, Iacono JM (1995) Effects of diets containing high and low amounts of stearic acid on plasma lipoprotein fractions and fecal fatty acid excretion of men. The American Journal of Clinical Nutrition 61, 1120–1128.
Dransfield E, Ngapo TM, Nielsen NA, Bredahl L, Sjoden PO, Magnusson M, Campo MM, Nute GR (2005) Consumer choice and suggested price for pork as influenced by its appearance, taste and information concerning country of origin and organic pig production. Meat Science 69, 61–70.
| Consumer choice and suggested price for pork as influenced by its appearance, taste and information concerning country of origin and organic pig production.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsFWjtQ%3D%3D&md5=2c847164aca7189b153703f5d8ad42fdCAS | 22062640PubMed |
Dreon DM, Fernstrom HA, Miller B, Krauss RM (1995) Apolipoprotein E isoform phenotype and LDL subclass response to a reduced fat diet. Arteriosclerosis, Thrombosis, and Vascular Biology 15, 105–111.
| Apolipoprotein E isoform phenotype and LDL subclass response to a reduced fat diet.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlt1eks7k%3D&md5=c773cdaf7b3a9f45a2895b7c0e485f4dCAS | 7749804PubMed |
Dreon DM, Fernstrom HA, Campos H, Blanche P, Williams PT, Krauss RM (1998) Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. The American Journal of Clinical Nutrition 67, 828–836.
Dreon DM, Fernstrom HA, Williams PT, Krauss RM (1999) A very-low-fat diet is not associated with improved lipoprotein profiles in men with a predominance of large, low-density lipoproteins. The American Journal of Clinical Nutrition 69, 411–418.
Duron E, Funalot B, Brunel N, Coste J, Quinquis L, Viollet C, Belmin J, Jouanny P, Pasquier F, Treluyer JM, Epelbaum J, le Bouc Y, Hanon O (2012) Insulin-like growth factor-I and insulin-like growth factor binding protein-3 in Alzheimer’s disease. The Journal of Clinical Endocrinology and Metabolism 97, 4673–4681.
| Insulin-like growth factor-I and insulin-like growth factor binding protein-3 in Alzheimer’s disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVCgtL7N&md5=d114d1c2e5abaa74cea8c6dfaf1fbb55CAS | 23015654PubMed |
Echtay KS, Winkler E, Klingenberg M (2000) Coenzyme Q is an obligatory cofactor for uncoupling protein function. Nature 408, 609–613.
| Coenzyme Q is an obligatory cofactor for uncoupling protein function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXovFajtr0%3D&md5=ef35f0bfd9a06705954b074f72538dffCAS | 11117751PubMed |
Egeberg R, Olsen A, Christensen J, Halkjaer J, Jakobsen MU, Overvad K, Tjonneland A (2013) Associations between red meat and risks for colon and rectal cancer depend on the type of red meat consumed. The Journal of Nutrition 143, 464–472.
| Associations between red meat and risks for colon and rectal cancer depend on the type of red meat consumed.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlt1SnsLk%3D&md5=334f6b58a4484ec3c40ad183441ebbd6CAS | 23427329PubMed |
Egert S, Rimbach G, Huebbe P (2012) ApoE genotype: from geographic distribution to function and responsiveness to dietary factors. The Proceedings of the Nutrition Society 71, 410–424.
| ApoE genotype: from geographic distribution to function and responsiveness to dietary factors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVOjur7M&md5=3a21768a3e8a758118e1a1a14c7aae23CAS | 22564824PubMed |
Eisenmann JC (2003) Secular trends in variables associated with the metabolic syndrome of North American children and adolescents: a review and synthesis. American Journal of Human Biology 15, 786–794.
| Secular trends in variables associated with the metabolic syndrome of North American children and adolescents: a review and synthesis.Crossref | GoogleScholarGoogle Scholar | 14595870PubMed |
Elam MB, von Wronski MA, Cagen L, Thorngate F, Kumar P, Heimberg M, Wilcox HG (1999) Apolipoprotein B mRNA editing and apolipoprotein gene expression in the liver of hyperinsulinemic fatty Zucker rats: relationship to very low density lipoprotein composition. Lipids 34, 809–816.
| Apolipoprotein B mRNA editing and apolipoprotein gene expression in the liver of hyperinsulinemic fatty Zucker rats: relationship to very low density lipoprotein composition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmtFKisL0%3D&md5=5ed0488b855216f50a8a5d0eec4c9a13CAS | 10529091PubMed |
Elwood PC, Pickering JE, Givens DI, Gallacher JE (2010) The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids 45, 925–939.
| The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1GjtLrM&md5=c552017d28615a1feb9d503a13c2ddd6CAS | 20397059PubMed |
Enfält AC, Lundstrom K, Hansson I, Johansen S, Nystrom PE (1997) Comparison of non-carriers and heterozygous carriers of the RN(–) allele for carcass composition, muscle distribution and technological meat quality in Hampshire-sired pigs. Livestock Production Science 47, 221–229.
| Comparison of non-carriers and heterozygous carriers of the RN(–) allele for carcass composition, muscle distribution and technological meat quality in Hampshire-sired pigs.Crossref | GoogleScholarGoogle Scholar |
Enig MG, Munn RJ, Keeney M (1978) Dietary fat and cancer trends – critique. Federation Proceedings 37, 2215–2220.
Enser M, Richardson RI, Wood JD, Gill BP, Sheard PR (2000) Feeding linseed to increase the n-3 PUFA of pork: fatty acid composition of muscle, adipose tissue, liver and sausages. Meat Science 55, 201–212.
| Feeding linseed to increase the n-3 PUFA of pork: fatty acid composition of muscle, adipose tissue, liver and sausages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXit1yrs7k%3D&md5=a21cd462bd975ebe5aa5e2b444104285CAS | 22061086PubMed |
Escoffier A (1921) ‘The complete guide to the art of modern cookery.’ (Heinemann: London)
Esterbauer H, Gebicki J, Puhl H, Jurgens G (1992) The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radical Biology & Medicine 13, 341–390.
| The role of lipid peroxidation and antioxidants in oxidative modification of LDL.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXjs1OnsQ%3D%3D&md5=bbf8671718bb88081ff0abfdcbf4d1eeCAS |
Evans A (2011) The French paradox and other ecological fallacies. International Journal of Epidemiology 40, 1486–1489.
| The French paradox and other ecological fallacies.Crossref | GoogleScholarGoogle Scholar | 22268235PubMed |
Evans AE, Ruidavets JB, McCrum EE, Cambou JP, McClean R, Dousteblazy P, McMaster D, Bingham A, Patterson CC, Richard JL, Mathewson ZM, Cambien F (1995) Autre pays, autre coeurs? Dietary patterns, risk factors and ischaemic heart disease in Belfast and Toulouse. QJM: An International Journal of Medicine 88, 469–477.
Everitt AV, LeCouteur DG (2007) Life extension by calorie restriction in humans. Annals of the New York Academy of Sciences 1114, 428–433.
| Life extension by calorie restriction in humans.Crossref | GoogleScholarGoogle Scholar | 17717102PubMed |
Fairlie S (2010) ‘Meat: a benign extravagance.’ (Permanent Publications: East Meon, UK)
Féart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues J-F, Scarmeas N, Barberger-Gateau P (2009) Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. Journal of the American Medical Association 302, 638–648.
| Adherence to a Mediterranean diet, cognitive decline, and risk of dementia.Crossref | GoogleScholarGoogle Scholar | 19671905PubMed |
Fernandez ML, McNamara DJ (1989) Dietary fat mediated changes in hepatic apoprotein B/E receptor in the guinea pig – effect of polyunsaturated, monounsaturated, and saturated fat. Metabolism: Clinical and Experimental 38, 1094–1102.
| Dietary fat mediated changes in hepatic apoprotein B/E receptor in the guinea pig – effect of polyunsaturated, monounsaturated, and saturated fat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXlvFemsbw%3D&md5=b7dff7937c02a1721a8531f74608622aCAS |
Fielding CJ, Fielding PE (1995) Molecular physiology of reverse cholesterol transport. Journal of Lipid Research 36, 211–228.
Flore R, Ponziano FR, Di Rienzo TA, Zocco MA, Flex A, Gerardino L, Lupascu A, Santoro L, Santoliquido A, Di Stasio E, Chierici E, Lanti A, Tondi P, Gasbarrini A (2013) Something more to say about calcium homeostasis: the role of vitamin K2 in vascular calcification and osteoporosis. European Review for Medical and Pharmacological Sciences 17, 2433–2440.
Fogerty AC, Ford GL, Svoronos D (1988) Octadeca-9,11-dienoic acid in foodstuffs and in the lipids of human blood and breast milk. Nutrition Reports International 38, 937–943.
Fontana L, Meyer TE, Klein S, Holloszy JO (2004) Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proceedings of the National Academy of Sciences, USA 101, 6659–6663.
| Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVyiu7c%3D&md5=74c91253f75b064567ce9be7fddfa00cCAS |
Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO, Washington U (2007) Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. American Journal of Physiology. Endocrinology and Metabolism 293, E197–E202.
| Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXosV2msbo%3D&md5=3617a29458b1aa30b5bec0ee25b6a72aCAS | 17389710PubMed |
Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO (2008) Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 7, 681–687.
| Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1ClsLfE&md5=a6802d2a6e4abb7ea68ec381904b6809CAS | 18843793PubMed |
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span – from yeast to humans. Science 328, 321–326.
| Extending healthy life span – from yeast to humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXks1Snsro%3D&md5=4bf0622d76e96645c4134a2c3fe0f783CAS | 20395504PubMed |
Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S (2007) Explaining the decrease in US deaths from coronary disease, 1980–2000. The New England Journal of Medicine 356, 2388–2398.
| Explaining the decrease in US deaths from coronary disease, 1980–2000.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmt1Wnsbs%3D&md5=34d3e1c4c8f1fe9571e02f03db73d3ebCAS | 17554120PubMed |
Forsyth JS, Varma S, Colvin M (1999) A randomised controlled study of the effect of long chain polyunsaturated fatty acid supplementation on stool hardness during formula feeding. Archives of Disease in Childhood 81, 253–256.
| A randomised controlled study of the effect of long chain polyunsaturated fatty acid supplementation on stool hardness during formula feeding.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MvitFGmsA%3D%3D&md5=d8b4f27af07ece9ae638ee0c06861d4eCAS | 10451400PubMed |
Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S (2003) A randomized trial of a low-carbohydrate diet for obesity. The New England Journal of Medicine 348, 2082–2090.
| A randomized trial of a low-carbohydrate diet for obesity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjvVGlurY%3D&md5=463efaec8c00205703b965e1d8363e75CAS | 12761365PubMed |
Fraser GE, Shavlik DJ (2001) Ten years of life is it a matter of choice? Archives of Internal Medicine 161, 1645–1652.
| Ten years of life is it a matter of choice?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MvhsFalsA%3D%3D&md5=8901061f69ae4ee374a94c0dd85682e7CAS | 11434797PubMed |
Freedman LS, Kipnis V, Schatzkin A, Potischman N (2008) Methods of epidemiology: evaluating the fat-breast cancer hypothesis – comparing dietary instruments and other developments. Cancer Journal (Sudbury, Mass.) 14, 69–74.
| Methods of epidemiology: evaluating the fat-breast cancer hypothesis – comparing dietary instruments and other developments.Crossref | GoogleScholarGoogle Scholar |
Freeman JM, Vining EPG, Pillas DJ, Pyzik PL, Casey JC, Kelly MT (1998) The efficacy of the ketogenic diet – 1998: a prospective evaluation of intervention in 150 children. Pediatrics 102, 1358–1363.
| The efficacy of the ketogenic diet – 1998: a prospective evaluation of intervention in 150 children.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M%2FlsFCqsQ%3D%3D&md5=0cdda6dfc4d97a5c0cf873440bdd3f8dCAS | 9832569PubMed |
Friberg E, Orsini N, Mantzoros CS, Wolk A (2007) Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 50, 1365–1374.
| Diabetes mellitus and risk of endometrial cancer: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2szjslCjsQ%3D%3D&md5=939601cdf689e18c5a3f242e28325cbaCAS | 17476474PubMed |
Fullerton SM, Clark AG, Weiss KM, Nickerson DA, Taylor SL, Stengard JH, Salomaa V, Vartiainen E, Perola M, Boerwinkle E, Sing CF (2000) Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. American Journal of Human Genetics 67, 881–900.
| Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnsVCgs7c%3D&md5=adb8e7620f811e13c418217da2fbf8dfCAS | 10986041PubMed |
Garcia C, Antona C, Robert B, Lopez C, Armand M (2014) The size and interfacial composition of milk fat globules are key factors controlling triglycerides bioavailability in simulated human gastro-duodenal digestion. Food Hydrocolloids 35, 494–504.
| The size and interfacial composition of milk fat globules are key factors controlling triglycerides bioavailability in simulated human gastro-duodenal digestion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1CrtrrF&md5=953dde2f965eaf2dfac813349bc70f1aCAS |
Gardner CD, Fortmann SP, Krauss RM (1996) Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. Journal of the American Medical Association 276, 875–881.
| Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28zns1Oltg%3D%3D&md5=f8d0b094faf4bcefbd2a33f5b94fe8f3CAS | 8782636PubMed |
Gilsing AMJ, Weijenberg MP, Goldbohm RA, van den Brandt PA, Schouten LJ (2011) Consumption of dietary fat and meat and risk of ovarian cancer in the Netherlands cohort study. The American Journal of Clinical Nutrition 93, 118–126.
| Consumption of dietary fat and meat and risk of ovarian cancer in the Netherlands cohort study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXls1Ckug%3D%3D&md5=31ffdce4d9cde9c83ef883900e788d35CAS |
Givens DI (2005) The role of animal nutrition in improving the nutritive value of animal-derived foods in relation to chronic disease. The Proceedings of the Nutrition Society 64, 395–402.
| The role of animal nutrition in improving the nutritive value of animal-derived foods in relation to chronic disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVKgt7jM&md5=3e5612c8e8410eda2ca076277bd4fc75CAS | 16048674PubMed |
Givens DI (2010) Milk and meat in our diet: good or bad for health? Animal 4, 1941–1952.
| Milk and meat in our diet: good or bad for health?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlGjsr%2FL&md5=ea609693260baec7f51f48de05aad9a6CAS | 22445367PubMed |
Givens DI, Gibbs RA, Rymer C, Brown RH (2011) Effect of intensive vs free range production on the fat and fatty acid composition of whole birds and edible portions of retail chickens in the UK. Food Chemistry 127, 1549–1554.
| Effect of intensive vs free range production on the fat and fatty acid composition of whole birds and edible portions of retail chickens in the UK.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvVSqtrk%3D&md5=2c8239945410cf4f55f26d4f67f54cccCAS |
Glavind J, Hartmann S, Clemmesen J, Jessen KE, Dam H (1952) Studies on the role of lipoperoxides in human pathology II. The presence of peroxidized lipids in the atherosclerotic aorta. Acta Pathologica et Microbiologica Scandinavica 30, 1–6.
| Studies on the role of lipoperoxides in human pathology II. The presence of peroxidized lipids in the atherosclerotic aorta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG38XjtVersA%3D%3D&md5=e77b7be153b3f84a99a876b3e668d47cCAS | 14933036PubMed |
Godley PA, Campbell MK, Gallagher P, Martinson FEA, Mohler JL, Sandler RS (1996) Biomarkers of essential fatty acid consumption and risk of prostatic carcinoma. Cancer Epidemiology, Biomarkers & Prevention 5, 889–895.
Gogos CA, Ginopoulos P, Salsa B, Apostolidou E, Zoumbos NC, Kalfarentzos F (1998) Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy – a randomized control trial. Cancer 82, 395–402.
| Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy – a randomized control trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlslymsQ%3D%3D&md5=23c3712b30099350e0ac6564106bd432CAS | 9445198PubMed |
Gouda HN, Critchley J, Powles J, Capewell S (2012) Why choice of metric matters in public health analyses: a case study of the attribution of credit for the decline in coronary heart disease mortality in the US and other populations. BMC Public Health 12, 88
| Why choice of metric matters in public health analyses: a case study of the attribution of credit for the decline in coronary heart disease mortality in the US and other populations.Crossref | GoogleScholarGoogle Scholar | 22284813PubMed |
Grande F, Anderson JT, Keys A (1970) Comparison of effects of palmitic and stearic acids in diet on serum cholesterol in man. The American Journal of Clinical Nutrition 23, 1184–1193.
Grau K, Hansen T, Holst C, Astrup A, Saris WHM, Arner P, Rossner S, Macdonald I, Polak J, Oppert JM, Langin D, Martinez JA, Pedersen O, Sorensen TIA (2009) Macronutrient-specific effect of FTO rs9939609 in response to a 10-week randomized hypo-energetic diet among obese Europeans. International Journal of Obesity 33, 1227–1234.
| Macronutrient-specific effect of FTO rs9939609 in response to a 10-week randomized hypo-energetic diet among obese Europeans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVWls7nI&md5=ca3e130222899bfa41daf6077797dbdeCAS | 19687793PubMed |
Griinari JM, Bauman DE (1999) Biosynthesis of conjugated linoleic acid and its incorporation into meat and milk in ruminants. In ‘Advances in conjugated linoleic acid research. Vol. 1’. (Eds MP Yurawecz, MM Mossoba, JKG Kramer, MW Pariza, GJ Nelson) pp. 180–200. (American Oil Chemists Society: Champaign, IL)
Grobet L, Martin LJR, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, Fries R, Hanset R, Georges M (1997) A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nature Genetics 17, 71–74.
| A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlvVGhtr8%3D&md5=55f9ba5334848284a7dfbd02da4b48dbCAS | 9288100PubMed |
Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD (2011) Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Science Translational Medicine 3, 70ra13
| Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans.Crossref | GoogleScholarGoogle Scholar | 21325617PubMed |
Ha YL, Grimm NK, Pariza MW (1987) Anticarcinogens from fried ground-beef: heat-altered derivatives of linoleic acid. Carcinogenesis 8, 1881–1887.
| Anticarcinogens from fried ground-beef: heat-altered derivatives of linoleic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXitVentbg%3D&md5=5c6e43f81310a964d4088a9014811c5fCAS | 3119246PubMed |
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? Journal of Neurochemistry 97, 1634–1658.
| Oxidative stress and neurodegeneration: where are we now?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xnt1Ons7g%3D&md5=51a07450bd0bcedd7dd8d75d88885e5cCAS | 16805774PubMed |
Halpern BS, Longo C, Hardy D, McLeod KL, Samhouri JF, Katona SK, Kleisner K, Lester SE, O’Leary J, Ranelletti M, Rosenberg AA, Scarborough C, Selig ER, Best BD, Brumbaugh DR, Chapin FS, Crowder LB, Daly KL, Doney SC, Elfes C, Fogarty MJ, Gaines SD, Jacobsen KI, Karrer LB, Leslie HM, Neeley E, Pauly D, Polasky S, Ris B, St Martin K, Stone GS, Sumaila UR, Zeller D (2012) An index to assess the health and benefits of the global ocean. Nature 488, 615–620.
| An index to assess the health and benefits of the global ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtF2rtL7N&md5=4cf580ff6b402ec075ebfca02e0cdf5bCAS | 22895186PubMed |
Hanlon CS, Rubinsztein DC (1995) Arginine residues at codon 112 and codon 158 in the apolipoprotein E gene correspond to the ancestral state in humans. Atherosclerosis 112, 85–90.
| Arginine residues at codon 112 and codon 158 in the apolipoprotein E gene correspond to the ancestral state in humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXisVShtLs%3D&md5=45d80e955c69b15c22d3a46dce6883eeCAS | 7772071PubMed |
Hanset R, Michaux C, Dessy-Doize C, Burtonboy G (1982) Studies on the 7th rib cut in double muscled and conventional cattle. Anatomical, histological and biochemical aspects. In ‘Muscle hypertrophy of genetic origin and its use to improve beef production’. (Eds JWB King, F Menissier) pp. 341–349. (Martinus Nijhoff Publishers: The Hague, The Netherlands)
Harper CR, Edwards MJ, DeFilipis AP, Jacobson TA (2006) Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. The Journal of Nutrition 136, 83–87.
Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE, Vatten L (1997) Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. International Journal of Cancer 71, 545–551.
| Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2szit1WmsA%3D%3D&md5=83358cb9335b152ad6b29194e519f319CAS |
Havel RJ (1957a) Early effects of fasting and of carbohydrate ingestion on lipids and lipoproteins of serum in man. The Journal of Clinical Investigation 36, 855–859.
| Early effects of fasting and of carbohydrate ingestion on lipids and lipoproteins of serum in man.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG2sXnslOnuw%3D%3D&md5=f3b30fb33339dc31131708b88c8f21edCAS | 13439026PubMed |
Havel RJ (1957b) Early effects of fat ingestion on lipids and lipoproteins of serum in man. The Journal of Clinical Investigation 36, 848–854.
| Early effects of fat ingestion on lipids and lipoproteins of serum in man.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG2sXnslylsA%3D%3D&md5=b06dd4c1c12365432ddf506fb6f07488CAS | 13439025PubMed |
Havel RJ, Eder HA, Bragdon JH (1955) Distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. The Journal of Clinical Investigation 34, 1345–1353.
| Distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG28XitFyn&md5=31ca428448d23126a3b6c09edbe34b31CAS | 13252080PubMed |
Hawkins RA, Alberti KGM, Houghton CR, Williams DH, Krebs HA (1971) Effect of acetoacetate on plasma insulin concentration. Biochemical Journal 125, 541
Hayes KC, Pronczuk A (2010) Replacing trans fat: the argument for palm oil with a cautionary note on interesterification. Journal of the American College of Nutrition 29, 253S–284S.
| Replacing trans fat: the argument for palm oil with a cautionary note on interesterification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1KrurzL&md5=128dfbba05bec4e603f5f4067c23f790CAS | 20823487PubMed |
Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC (2009) Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutrition & Metabolism 6, 31
| Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial.Crossref | GoogleScholarGoogle Scholar |
Herron KL, Lofgren IE, Sharman M, Volek JS, Fernandez ML (2004) High intake of cholesterol results in less atherogenic low-density lipoprotein particles in men and women independent of response classification. Metabolism: Clinical and Experimental 53, 823–830.
| High intake of cholesterol results in less atherogenic low-density lipoprotein particles in men and women independent of response classification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXkt1CmsL4%3D&md5=766dc88c2eb378259a067cf2cc50c30cCAS |
Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D (1993) Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen elderly study. Lancet 342, 1007–1011.
| Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen elderly study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2c%2FhvVajsQ%3D%3D&md5=c11ee2cb2b6f323aa77973b4de857c70CAS |
Hiatt WR (2001) Medical treatment of peripheral arterial disease and claudication. The New England Journal of Medicine 344, 1608–1621.
| Medical treatment of peripheral arterial disease and claudication.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkt1OktrY%3D&md5=e7b2d7712431c81b7c99c7370d70e721CAS | 11372014PubMed |
Hite AH, Berkowitz VG, Berkowitz K (2011) Low-carbohydrate diet review: shifting the paradigm. Nutrition in Clinical Practice 26, 300–308.
| Low-carbohydrate diet review: shifting the paradigm.Crossref | GoogleScholarGoogle Scholar | 21586415PubMed |
Ho VW, Leung K, Hsu A, Luk B, Lai J, Shen SY, Minchinton AI, Waterhouse D, Bally MB, Lin W, Nelson BH, Sly LM, Krystal G (2011) A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Research 71, 4484–4493.
| A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotlersbY%3D&md5=a068b1008533f04296675d977a95ebe7CAS | 21673053PubMed |
Hodson L, Skeaff CM, Fielding BA (2008) Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Progress in Lipid Research 47, 348–380.
| Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnt1yjtr4%3D&md5=6ee7e4071ba1d1317c1efa238c49991eCAS | 18435934PubMed |
Hoenselaar R (2012) Saturated fat and cardiovascular disease: the discrepancy between the scientific literature and dietary advice. Nutrition 28, 118–123.
| Saturated fat and cardiovascular disease: the discrepancy between the scientific literature and dietary advice.Crossref | GoogleScholarGoogle Scholar | 22208554PubMed |
Holick MF (2007) Vitamin D deficiency. The New England Journal of Medicine 357, 266–281.
| Vitamin D deficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXotVejsbc%3D&md5=f1fe34995387769be093ce61eaf4409fCAS | 17634462PubMed |
Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu YQ, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA (2007) Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metabolism 5, 167–179.
| Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjsF2qtL8%3D&md5=a180f6d85b90f78089f414993a6158eaCAS | 17339025PubMed |
Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore H, Smith GD (2011) Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database of Systematic Reviews CD002137
Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC (1997) Dietary fat intake and the risk of coronary heart disease in women. The New England Journal of Medicine 337, 1491–1499.
| Dietary fat intake and the risk of coronary heart disease in women.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c%2Fis1Oltw%3D%3D&md5=521d1ff93971425edb93a5fd36cd4fb1CAS | 9366580PubMed |
Hunink MGM, Goldman L, Tosteson ANA, Mittleman MA, Goldman PA, Williams LW, Tsevat J, Weinstein MC (1997) The recent decline in mortality from coronary heart disease, 1980–1990. The effect of secular trends in risk factors and treatment. Journal of the American Medical Association 277, 535–542.
| The recent decline in mortality from coronary heart disease, 1980–1990. The effect of secular trends in risk factors and treatment.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s7osVemtQ%3D%3D&md5=810796f8fe630260dce1c2a60ac96c19CAS |
Hunter JE (2001) Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids 36, 655–668.
| Studies on effects of dietary fatty acids as related to their position on triglycerides.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmtFKlt7o%3D&md5=d0edb3b808fce2dbd6ea7fc272d869e1CAS | 11521963PubMed |
Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN (2010) Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis 31, 83–89.
| Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktlCgtQ%3D%3D&md5=82b9b4df7f03b2bdf300f24251672c0bCAS | 19969554PubMed |
Hutchison R (1936) Treatment of chronic constipation. British Medical Journal I, 374–375.
| Treatment of chronic constipation.Crossref | GoogleScholarGoogle Scholar |
Ishibashi S, Herz J, Maeda N, Goldstein JL, Brown MS (1994) The two-receptor model of lipoprotein clearance: tests of the hypothesis in ‘knockout’ mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proceedings of the National Academy of Sciences, USA 91, 4431–4435.
| The two-receptor model of lipoprotein clearance: tests of the hypothesis in ‘knockout’ mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXktlWksLo%3D&md5=1d13e66a5e9431f97fd9aed68ecea001CAS |
Jha P, Flather M, Lonn E, Farkouh M, Yusuf S (1995) The antioxidant vitamins and cardiovascular disease: a critical review of epidemiological and clinical trial data. Annals of Internal Medicine 123, 860–872.
| The antioxidant vitamins and cardiovascular disease: a critical review of epidemiological and clinical trial data.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28%2FmtFyltQ%3D%3D&md5=3f24f3fd89855d04049cddc012777ad8CAS | 7486470PubMed |
Jia W, Rogiewicz A, Bruce HL, Slominski BA (2010) Feeding flaxseed enhances deposition of omega-3 fatty acids in broiler meat portions in different manner. Canadian Journal of Animal Science 90, 203–206.
| Feeding flaxseed enhances deposition of omega-3 fatty acids in broiler meat portions in different manner.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVKisbjM&md5=36d2ffd03fb23e002bf827893745a39eCAS |
Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J, Council Nutr Phys Activity Metab, Council Epidemiology Prevention (2009) Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 120, 1011–1020.
| Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1WntbbP&md5=80409da122cde7f0d9d588464a71f410CAS | 19704096PubMed |
Karvonen MJ, Orma E, Punsar S, Kallio V, Arstila M, Luomanmaki K, Takkunen J (1970) VI. Five-year experience in Finland. Circulation 41, I-52–I-62.
| VI. Five-year experience in Finland.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE3c7nvFejtw%3D%3D&md5=e34a7b831aae470b5f533e4eb80909eaCAS |
Kashiwaya Y, Sato K, Tsuchiya N, Thomas S, Fell DA, Veech RL, Passonneau JV (1994) Control of glucose utilization in working perfused rat heart. The Journal of Biological Chemistry 269, 25 502–25 514.
Kelly MJ, Tume RK, Newman SA, Thompson JM (2001) Environmental effects on the fatty acid composition of subcutaneous beef fat. Australian Journal of Experimental Agriculture 41, 1023–1031.
| Environmental effects on the fatty acid composition of subcutaneous beef fat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotlGhur8%3D&md5=01538073334ebb24c388c280033d4019CAS |
Key T, Schatzkin A, Willett WC, Allen NE, Spencer EA, Travis RC (2004) Diet, nutrition and the prevention of cancer. Public Health Nutrition 7, 187–200.
| Diet, nutrition and the prevention of cancer.Crossref | GoogleScholarGoogle Scholar | 14972060PubMed |
Keys A (1950) The residues of malnutrition and starvation. Science 112, 371–373.
Keys A (1953) Atherosclerosis: a problem in newer public health. Journal of the Mount Sinai Hospital, New York 20, 118–139.
Keys A (1971) Sucrose in the diet and coronary heart disease. Atherosclerosis 14, 193–202.
| Sucrose in the diet and coronary heart disease.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE38%2FktVWrsg%3D%3D&md5=a379054262aaab39a8e18261de1aea0fCAS | 4940760PubMed |
Keys A (1980) Wine, garlic, and CHD in seven countries. Lancet 315, 145–146.
| Wine, garlic, and CHD in seven countries.Crossref | GoogleScholarGoogle Scholar |
Keys A (1995) Mediterranean diet and public health: personal reflections. The American Journal of Clinical Nutrition 61, S1321–S1323.
Keys A, Kimura N (1970) Diets of middled-aged farmers in Japan. The American Journal of Clinical Nutrition 23, 212–223.
Keys A, Anderson JT, Grande F (1957) Prediction of serum-cholesterol response of man to changes in fats in the diet. Lancet 270, 959–966.
| Prediction of serum-cholesterol response of man to changes in fats in the diet.Crossref | GoogleScholarGoogle Scholar |
Keys A, Karvonen MJ, Fidanza F (1958) Serum-cholesterol studies in Finland. Lancet 272, 175–178.
| Serum-cholesterol studies in Finland.Crossref | GoogleScholarGoogle Scholar |
Keys A, Anderson JT, Grande F (1965) Serum cholesterol response to changes in the diet. IV. Particular saturated fatty acids in the diet. Metabolism: Clinical and Experimental 14, 776–787.
| Serum cholesterol response to changes in the diet. IV. Particular saturated fatty acids in the diet.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2MXksVygsrg%3D&md5=99b59e243b870ac7816eb80ad1755b98CAS |
Keys A, Blackburn H, Menotti A, Buzina R, Mohaček I, Karvonen MJ, Punsar S, Aravanis C, Corcondilas A, Dontas AS, Lekos D, Fidanza F, Puddu V, Taylor HL, Monti M, Kimura N, van Buchem FSP, Djordjević BS, Strasser T, Anderson JT, den Hartog C, Pekkarinen M, Roine P, Sdrin H (1970) Coronary heart disease in 7 countries. Circulation 41, I1–I209.
Keys A, Grande F, Anderson JT (1974) Bias and misrepresentation revisited – perspective on saturated fat. The American Journal of Clinical Nutrition 27, 188–212.
Keys A, Aravanis C, Blackburn H, Buzina R, Djordjević BS, Dontas AS, Fidanza F, Karvonen MJ, Kimura N, Menotti A, Mohaček I, Nedeljković S, Puddu V, Punsar S, Taylor HL, van Buchem FSP (1980) ‘Seven Countries: a multivariate analysis of death and coronary heart disease.’ (Harvard University Press: Cambridge, MA)
Kim DY, Davis LM, Sullivan PG, Maalouf M, Simeone TA, van Brederode J, Rho JM (2007) Ketone bodies are protective against oxidative stress in neocortical neurons. Journal of Neurochemistry 101, 1316–1326.
| Ketone bodies are protective against oxidative stress in neocortical neurons.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmsFensLw%3D&md5=d3fa2e9955772836acfdf1dd8ff5b942CAS |
Klement RJ, Kaemmerer U (2011) Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutrition & Metabolism 8, 75
| Is there a role for carbohydrate restriction in the treatment and prevention of cancer?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XisFertbk%3D&md5=c4de3c8aaf659d1e557130cd9bf9072fCAS |
Knekt P, Ritz J, Pereira MA, O’Reilly EJ, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Liu SM, Spiegelman D, Stevens J, Virtamo J, Willett WC, Rimm EB, Ascherio A (2004) Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. The American Journal of Clinical Nutrition 80, 1508–1520.
Kobayashi N, Barnard RJ, Henning SM, Elashoff D, Reddy ST, Cohen P, Leung P, Hong-Gonzalez J, Freedland SJ, Said J, Gui D, Seeram NP, Popoviciu LM, Bagga D, Heber D, Glaspy JA, Aronson WJ (2006) Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E-2. Clinical Cancer Research 12, 4662–4670.
| Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E-2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnvVGksL4%3D&md5=a3507036a8b41f0802e94c86d6ed272dCAS | 16899616PubMed |
Kontush A, Chantepie S, Chapman MJ (2003) Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arteriosclerosis, Thrombosis, and Vascular Biology 23, 1881–1888.
| Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnslWitr4%3D&md5=0962e2c7eae0b7d178d9fda81a48dd5aCAS | 12920049PubMed |
Kossoff EH, Hartman AL (2012) Ketogenic diets: new advances for metabolism-based therapies. Current Opinion in Neurology 25, 173–178.
| Ketogenic diets: new advances for metabolism-based therapies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsVCrsbw%3D&md5=f6c864e6de1a02417284bb7571466d80CAS | 22322415PubMed |
Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP (2006) A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia 47, 421–424.
| A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy.Crossref | GoogleScholarGoogle Scholar | 16499770PubMed |
Kratz M, Cullen P, Kannenberg F, Kassner A, Fobker M, Abuja PM, Assmann G, Wahrburg U (2002) Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. European Journal of Clinical Nutrition 56, 72–81.
| Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xht1yrsbk%3D&md5=5fce6e8baae192b6b88f041ca2a2d739CAS | 11840183PubMed |
Krauss RM, Dreon DM (1995) Low density lipoprotein subclasses and response to a low fat diet in healthy men. The American Journal of Clinical Nutrition 62, S478–S487.
Krebs HA (1966) The regulation of the release of ketone bodies by the liver. Advances in Enzyme Regulation 4, 339–353.
| The regulation of the release of ketone bodies by the liver.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2sXpt12n&md5=bc97dbbb65e77feb0e58de08564fc2b8CAS | 4865971PubMed |
Krebs HA, Williamson DH, Bates MW, Page MA, Hawkins RA (1971) The role of ketone bodies in caloric homeostasis. Advances in Enzyme Regulation 9, 387–409.
| The role of ketone bodies in caloric homeostasis.Crossref | GoogleScholarGoogle Scholar |
Kritchevsky D (1976) Diet and atherosclerosis. American Journal of Pathology 84, 615–632.
Kuo PT, Carson JC (1959) Dietary fats and the diurnal serum triglyceride levels in man. The Journal of Clinical Investigation 38, 1384–1393.
| Dietary fats and the diurnal serum triglyceride levels in man.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1MXhtFCjtro%3D&md5=1fd07ecbc7fabdc793ad336dbdf70096CAS | 13673095PubMed |
Kuusisto J, Koivisto K, Mykkanen L, Helkala EL, Vanhanen M, Hanninen T, Kervinen K, Kesaniemi YA, Riekkinen PJ, Laakso M (1997) Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. British Medical Journal 315, 1045–1049.
| Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXnt1Klsbg%3D&md5=e2448c3fa672793252f0a6a83c59960bCAS | 9366728PubMed |
Lagarrigue S, Pitel F, Carre W, Abasht B, Le Roy P, Neau A, Amigues Y, Sourdioux M, Simon J, Cogburn L, Aggrey S, Leclercq B, Vignal A, Douaire M (2006) Mapping quantitative trait loci affecting fatness and breast muscle weight in meat-type chicken lines divergently selected on abdominal fatness. Genetics, Selection, Evolution 38, 85–97.
| Mapping quantitative trait loci affecting fatness and breast muscle weight in meat-type chicken lines divergently selected on abdominal fatness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xps1ejug%3D%3D&md5=ccc4d8cd1446331180da1bf84019ce02CAS | 16451793PubMed |
Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP (1997) Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men – prospective results from the Quebec Cardiovascular Study. Circulation 95, 69–75.
| Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men – prospective results from the Quebec Cardiovascular Study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s7kslKntQ%3D%3D&md5=ba2dcb9d5b7696ac92aa9f29d5dc1b64CAS | 8994419PubMed |
Lande KE, Sperry WM (1936) Human atherosclerosis in relation to the cholesterol content of the blood serum. Archives of Pathology 22, 301–312.
Lappalainen T, Salmela E, Andersen PM, Dahlman-Wright K, Sistonen P, Savontaus ML, Schreiber S, Lahermo P, Kere J (2010) Genomic landscape of positive natural selection in northern European populations. European Journal of Human Genetics 18, 471–478.
| Genomic landscape of positive natural selection in northern European populations.Crossref | GoogleScholarGoogle Scholar | 19844263PubMed |
Lehninger AL (1982) ‘Principles of biochemistry.’ (Worth Publishers: New York)
Lindeberg S (2009) Modern human physiology with respect to evolutionary adaptations that relate to diet in the past. In ‘Evolution of hominin diets’. (Eds JJ Hublin, MP Richard) pp. 43–57. (Springer: Dordrecht, The Netherlands)
Lipp M, Simoneau C, Ulberth F, Anklam E, Crews C, Brereton P, de Greyt W, Schwack W, Wiedmaier C (2001) Composition of genuine cocoa butter and cocoa butter equivalents. Journal of Food Composition and Analysis 14, 399–408.
| Composition of genuine cocoa butter and cocoa butter equivalents.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmt1Kntr0%3D&md5=e02b0c1b8b0029fda84dab58473bfe5bCAS |
Liu L, Zhuang W, Wang R-Q, Mukherjee R, Xiao S-M, Chen X, We X-T, Zhou Y, Zhang H-Y (2011) Is dietary fat associated with the risk of colorectal cancer? A meta-analysis of 13 prospective cohort studies. European Journal of Nutrition 50, 173–184.
| Is dietary fat associated with the risk of colorectal cancer? A meta-analysis of 13 prospective cohort studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXovFChtLw%3D&md5=d39d6bb758c68b09065078404e15741bCAS | 20697723PubMed |
Livingstone KM, Lovegrove JA, Cockcroft JR, Elwood PC, Pickering JE, Givens DI (2013) Does dairy food intake predict arterial stiffness and blood pressure in men? Evidence from the Caerphilly prospective study. Hypertension 61, 42–47.
| Does dairy food intake predict arterial stiffness and blood pressure in men? Evidence from the Caerphilly prospective study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVahsrnI&md5=797a80452119baaa4cc9925b5e25a272CAS | 23150503PubMed |
Longo VD, Finch CE (2003) Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 299, 1342–1346.
| Evolutionary medicine: from dwarf model systems to healthy centenarians?Crossref | GoogleScholarGoogle Scholar | 12610293PubMed |
López-López A, Castellote-Bargallo AI, Campoy-Folgoso C, Rivero-Urgell M, Tormo-Carnice R, Infante-Pina D, López-Sabater MC (2001) The influence of dietary palmitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newborn faeces. Early Human Development 65, S83–S94.
| The influence of dietary palmitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newborn faeces.Crossref | GoogleScholarGoogle Scholar | 11755039PubMed |
Luchsinger JA, Tang M-X, Stern Y, Shea S, Mayeux R (2001) Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. American Journal of Epidemiology 154, 635–641.
| Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MrjvVKnsA%3D%3D&md5=d06acb6f4404e5fc70c7ebcbb3ea660cCAS | 11581097PubMed |
Lustig RH, Schmidt LA, Brindis CD (2012) The toxic truth about sugar. Nature 482, 27–29.
| The toxic truth about sugar.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1Cqu7w%3D&md5=f505e8d6356dcdb9e7a3aaccf2e57d13CAS | 22297952PubMed |
MacDougall DE, Jones PJH, Kitts DD, Phang PT (1996) Effect of butter compared with tallow consumption on postprandial oxidation of myristic and palmitic acids. The American Journal of Clinical Nutrition 63, 918–924.
MacLean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM, Suttorp MJ, Lim YW, Traina SB, Hilton L, Garland R, Morton SC (2006) Effects of omega-3 fatty acids on cancer risk – a systematic review. Journal of the American Medical Association 295, 403–415.
| Effects of omega-3 fatty acids on cancer risk – a systematic review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnsFOlsw%3D%3D&md5=1a4292c5d931c6686a7f25ee092b3d31CAS | 16434631PubMed |
Macquart-Moulin G, Riboli E, Cornee J, Kaaks R, Berthezene P (1987) Colorectal polyps and diet – a case control study in Marseilles. International Journal of Cancer 40, 179–188.
| Colorectal polyps and diet – a case control study in Marseilles.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s3nvVKrtA%3D%3D&md5=1074a13b1cdf7300011b7e989db84094CAS |
Man EB, Gildea EF (1932) The effect of the ingestion of a large amount of fat and of a balanced meal on the blood lipids of normal man. The Journal of Biological Chemistry 99, 61–69.
Mandell IB, Buchanan Smith JG, Holub BJ, Campbell CP (1997) Effects of fish meal in beef diets on growth performance, carcass characteristics, and fatty acid composition of longissimus muscle. Journal of Animal Science 75, 910–919.
Marinou K, Adiels M, Hodsen L, Frayn KN, Karpe F, Fielding BA (2011) Young women partition fatty acids towards ketone body production rather than VLDL–TAG synthesis, compared with young men. The British Journal of Nutrition 105, 857–865.
| Young women partition fatty acids towards ketone body production rather than VLDL–TAG synthesis, compared with young men.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXis1yitb8%3D&md5=45a466d8883a9292091f27ce3d9b37d1CAS | 21251339PubMed |
Marlowe FW (2005) Hunter-gatherers and human evolution. Evolutionary Anthropology 14, 54–67.
| Hunter-gatherers and human evolution.Crossref | GoogleScholarGoogle Scholar |
Marmot MG, Bosma H, Hemingway H, Brunner E, Stansfield S (1997) Contribution of job control and other risk factors to social variations in coronary heart disease incidence. Lancet 350, 235–239.
| Contribution of job control and other risk factors to social variations in coronary heart disease incidence.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2szos1Ojuw%3D%3D&md5=c462985dcae7ae88571e3f0c4187d97dCAS | 9242799PubMed |
Mathers JC, Movahedi M, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans G, Maher ER, Bertario L, Bisgaard ML, Dunlop M, Ho JWC, Hodgson S, Lindblom A, Lubinski J, Morrison PJ, Murday V, Ramesar R, Side L, Scott RJ, Thomas HJW, Vasen H, Gerdes AM, Barker G, Crawford G, Elliott F, Pylvanainen K, Wijnen J, Fodde R, Lynch H, Bishop DT, Burn J, Investigators C (2012) Long-term effect of resistant starch on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. The Lancet Oncology 13, 1242–1249.
| Long-term effect of resistant starch on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslKktL%2FL&md5=66e83a2b4a0902ba2c7a242e31d088a9CAS | 23140761PubMed |
Matthan NR, Welty FK, Barrett PHR, Harausz C, Dolnikowski GG, Parks JS, Eckel RH, Schaefer EJ, Lichtenstein AH (2004) Dietary hydrogenated fat increases high-density lipoprotein apoA-I catabolism and decreases low-density lipoprotein apoB-100 catabolism in hypercholesterolemic women. Arteriosclerosis, Thrombosis, and Vascular Biology 24, 1092–1097.
| Dietary hydrogenated fat increases high-density lipoprotein apoA-I catabolism and decreases low-density lipoprotein apoB-100 catabolism in hypercholesterolemic women.Crossref | GoogleScholarGoogle Scholar | 15087307PubMed |
Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, De Cabo R (2012) Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321.
| Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1KkurnK&md5=8eef6663129d431e49bb299c2fb5f492CAS | 22932268PubMed |
McClellan WS, Du Bois EF (1930) Clinical calorimetry. XLV. Prolonged meat diets with a study of kidney function and ketosis. The Journal of Biological Chemistry 87, 651–668.
McDevitt RM, Bott SJ, Harding M, Coward WA, Bluck LJ, Prentice AM (2001) De novo lipogenesis during controlled overfeeding with sucrose or glucose in lean and obese women. The American Journal of Clinical Nutrition 74, 737–746.
McGarry JD, Robles-Valdes C, Foster DW (1975) Role of carnitine in hepatic ketogenesis. Proceedings of the National Academy of Sciences, USA 72, 4385–4388.
| Role of carnitine in hepatic ketogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XivVGgug%3D%3D&md5=04e3ab2e4bc706985587bb9999daedeaCAS |
McGee DL, Diverse Populations Collaboration (2005) Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Annals of Epidemiology 15, 87–97.
| Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies.Crossref | GoogleScholarGoogle Scholar | 15652713PubMed |
McMichael AJ, Potter JD, Hetzel BS (1979) Time trends in colo-rectal cancer mortality in relation to food and alcohol consumption: United States, United Kingdom, Australia and New Zealand. International Journal of Epidemiology 8, 295–303.
| Time trends in colo-rectal cancer mortality in relation to food and alcohol consumption: United States, United Kingdom, Australia and New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3c7lvFShtg%3D%3D&md5=fb9f8fcf64ef0d354225f26158cfaa0cCAS | 541153PubMed |
Mekki N, Charbonnier M, Borel P, Leonardi J, Juhel C, Portugal H, Lairon D (2002) Butter differs from olive oil and sunflower oil in its effects on postprandial lipemia and triacylglycerol-rich lipoproteins after single mixed meals in healthy young men. The Journal of Nutrition 132, 3642–3649.
Meng MS, West GC, Irving L (1969) Fatty acid composition of caribou bone marrow. Comparative Biochemistry and Physiology 30, 187–191.
| Fatty acid composition of caribou bone marrow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1MXksFynt70%3D&md5=265bf11e70226213d827dcc3d34dbad7CAS | 5804481PubMed |
Mensink RP, Zock PL, Kester ADM, Katan MB (2003) Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. The American Journal of Clinical Nutrition 77, 1146–1155.
Menzel HJ, Kladetzky RG, Assmann G (1983) Apolipoprotein E polymorphism and coronary artery disease. Arteriosclerosis 3, 310–315.
| Apolipoprotein E polymorphism and coronary artery disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXlt1Shsrg%3D&md5=b3e386a2f37b8b5c5a24bcc19cbc883bCAS | 6882285PubMed |
Micha R, Mozaffarian D (2010) Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids 45, 893–905.
| Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1GjtL3E&md5=83a9b0bb2c89c5700be6d98a76f0cdffCAS | 20354806PubMed |
Michalski MC, Genot C, Gayet C, Lopez C, Fine F, Joffre F, Vendeuvre JL, Bouvier J, Chardigny JM, Raynal-Ljutovac K, , for the Steering Committee of RMT LISTRAL (2013) Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Progress in Lipid Research 52, 354–373.
| Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslGmur%2FM&md5=6665443ab49ffd334828fdda7be4129bCAS | 23624223PubMed |
Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S (2011) Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123, 2292–2333.
| Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association.Crossref | GoogleScholarGoogle Scholar | 21502576PubMed |
Mooijaart SP, Berbee JFP, van Heemst D, Havekes LM, de Craen AJM, Slagboom PE, Rensen PCN, Westendorp RGJ (2006) ApoE plasma levels and risk of cardiovascular mortality in old age. PLoS Medicine 3, e76
| ApoE plasma levels and risk of cardiovascular mortality in old age.Crossref | GoogleScholarGoogle Scholar |
Moreno JA, Perez-Jimenez F, Moreno-Luna R, Perez-Martinez P, Fuentes-Jimenez F, Marin C, Portugal H, Lairon D, Lopez-Miranda J (2009) The effect of apoE genotype and sex on ApoE plasma concentration is determined by dietary fat in healthy subjects. The British Journal of Nutrition 101, 1745–1752.
| The effect of apoE genotype and sex on ApoE plasma concentration is determined by dietary fat in healthy subjects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXos1Cqs7s%3D&md5=afaf7bf4ad37903dba72bf769abe7ffaCAS | 19025720PubMed |
Morin E (2007) Fat composition and Nunamiut decision-making: a new look at the marrow and bone grease indices. Journal of Archaeological Science 34, 69–82.
| Fat composition and Nunamiut decision-making: a new look at the marrow and bone grease indices.Crossref | GoogleScholarGoogle Scholar |
Mozaffarian D, Rimm EB (2006) Fish intake, contaminants, and human health – evaluating the risks and the benefits. Journal of the American Medical Association 296, 1885–1899.
| Fish intake, contaminants, and human health – evaluating the risks and the benefits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFWmt7%2FF&md5=c43dabd7b5b87b03b7ab36924bd361c6CAS | 17047219PubMed |
Mozaffarian D, Aro A, Willett WC (2009) Health effects of trans-fatty acids: experimental and observational evidence. European Journal of Clinical Nutrition 63, S5–S21.
| Health effects of trans-fatty acids: experimental and observational evidence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsF2ht7o%3D&md5=886111fcb9a34b7a0f6837eb89718703CAS | 19424218PubMed |
Multiple Risk Factor Intervention Trial Research Group (1982) Multiple risk factor intervention trial risk factor changes and mortality results. Journal of the American Medical Association 248, 1465–1477.
| Multiple risk factor intervention trial risk factor changes and mortality results.Crossref | GoogleScholarGoogle Scholar | 7050440PubMed |
Murdock GP (1967) Ethnographic atlas – a summary. Ethnology 6, 109–236.
| Ethnographic atlas – a summary.Crossref | GoogleScholarGoogle Scholar |
Murff HJ, Shu XO, Li HL, Yang G, Wu XY, Cai H, Wen WQ, Gao YT, Zheng W (2011) Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women: a prospective cohort study. International Journal of Cancer 128, 1434–1441.
| Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women: a prospective cohort study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFKrtrc%3D&md5=3bb526c2393a89890674fd6fc860f0feCAS |
Mushtaq S, Mangiapane EH, Hunter KA (2010) Estimation of cis-9, trans-11 conjugated linoleic acid content in UK foods and assessment of dietary intake in a cohort of healthy adults. The British Journal of Nutrition 103, 1366–1374.
| Estimation of cis-9, trans-11 conjugated linoleic acid content in UK foods and assessment of dietary intake in a cohort of healthy adults.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlvVensLc%3D&md5=6121314684094c882ee461914bd76e53CAS | 19968893PubMed |
Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH (2008) The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurology 7, 500–506.
| The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial.Crossref | GoogleScholarGoogle Scholar | 18456557PubMed |
Nelson RL, Persky V, Turyk M (1999) Determination of factors responsible for the declining incidence of colorectal cancer. Diseases of the Colon and Rectum 42, 741–752.
| Determination of factors responsible for the declining incidence of colorectal cancer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MzgsV2nsQ%3D%3D&md5=6ed2fddc7ee175525dec45bae7c6b93fCAS | 10378598PubMed |
Nestel P, Noakes M, Belling B, McArthur R, Clifton P, Janus E, Abbey M (1992) Plasma lipoprotein lipid and Lp[a] changes with substitution of elaidic for oleic acid in the diet. Journal of Lipid Research 33, 1029–1036.
Nicholson T, Khademi H, Moghadasian MH (2013) The role of marine n-3 fatty acids in improving cardiovascular health: a review. Food & Function 4, 357–365.
| The role of marine n-3 fatty acids in improving cardiovascular health: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjtV2ktLs%3D&md5=a40698258e7f0f7c8f1b26bef58964bcCAS |
Nikkilä EA, Kekki M (1972) Plasma triglyceride metabolism in thyroid disease. The Journal of Clinical Investigation 51, 2103–2114.
| Plasma triglyceride metabolism in thyroid disease.Crossref | GoogleScholarGoogle Scholar | 4341014PubMed |
Nishina PM, Johnson JP, Naggert JK, Krauss RM (1992) Linkage of atherogenic lipoprotein phenotype to the low density lipoprotein receptor locus on the short arm of chromosome 19. Proceedings of the National Academy of Sciences, USA 89, 708–712.
| Linkage of atherogenic lipoprotein phenotype to the low density lipoprotein receptor locus on the short arm of chromosome 19.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhtVSnur4%3D&md5=20982462086f84ec0816202137053643CAS |
Nuernberg K, Dannenberger D, Nuernberg G, Ender K, Voigt J, Scollan ND, Wood JD, Nute GR, Richardson RI (2005a) Effect of a grass-based and a concentrate feeding system on meat quality characteristics and fatty acid composition of longissimus muscle in different cattle breeds. Livestock Production Science 94, 137–147.
| Effect of a grass-based and a concentrate feeding system on meat quality characteristics and fatty acid composition of longissimus muscle in different cattle breeds.Crossref | GoogleScholarGoogle Scholar |
Nuernberg K, Nuernberg G, Ender K, Dannenberger D, Schabbel W, Grumbach S, Zupp W, Steinhart H (2005b) Effect of grass vs. concentrate feeding on the fatty acid profile of different fat depots in lambs. European Journal of Lipid Science and Technology 107, 737–745.
| Effect of grass vs. concentrate feeding on the fatty acid profile of different fat depots in lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtF2itLjL&md5=cf230ba15381dfd445fdf31fb407f553CAS |
Oddy DJ, Yudkin J (1969) An evaluation of English diets of the 1860s. The Proceedings of the Nutrition Society 28, A13–A14.
Orgel E, Mittelman SD (2013) The links between insulin resistance, diabetes, and cancer. Current Diabetes Reports 13, 213–222.
| The links between insulin resistance, diabetes, and cancer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjvFeru7s%3D&md5=05c96e9fb196f900005d3df8c9986611CAS | 23271574PubMed |
Ornish D, Scherwitz LW, Billings JH, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, Hogeboom C, Brand RJ (1998) Intensive lifestyle changes for reversal of coronary heart disease. Journal of the American Medical Association 280, 2001–2007.
| Intensive lifestyle changes for reversal of coronary heart disease.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M%2FnvFGmtw%3D%3D&md5=fb88f0494161f26a19b8a41863b550bdCAS | 9863851PubMed |
Owen RW, Weisgerber UM, Carr J, Harrison MH (1995) Analysis of calcium–lipid complexes in faeces. European Journal of Cancer Prevention 4, 247–255.
| Analysis of calcium–lipid complexes in faeces.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2MznsFKksQ%3D%3D&md5=3a4314ada7bc5fa598a598afcb7d7298CAS | 7647693PubMed |
Paolisso G, Ammendola S, DelBuono A, Gambardella A, Riondino M, Tagliamonte MR, Rizzo MR, Carella C, Varricchio M (1997) Serum levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 in healthy centenarians: relationship with plasma leptin and lipid concentrations, insulin action, and cognitive function. The Journal of Clinical Endocrinology and Metabolism 82, 2204–2209.
| Serum levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 in healthy centenarians: relationship with plasma leptin and lipid concentrations, insulin action, and cognitive function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXksVCntbs%3D&md5=c7699dbdfb815665f9cd3323f4cdbaabCAS | 9215295PubMed |
Pariza MW, Park Y, Cook ME (2001) The biologically active isomers of conjugated linoleic acid. Progress in Lipid Research 40, 283–298.
| The biologically active isomers of conjugated linoleic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltFWhurs%3D&md5=ed18b59e0670185a0f9c8d27132a8d10CAS | 11412893PubMed |
Parodi PW (1997) Cows’ milk fat components as potential anticarcinogenic agents. The Journal of Nutrition 127, 1055–1060.
Parodi PW (2004) Milk fat in human nutrition. Australian Journal of Dairy Technology 59, 3–59.
Pasquet P, Brigant L, Froment A, Koppert GA, Bard D, Degarine I, Apfelbaum M (1992) Massive overfeeding and energy balance in men – the Guru-Walla model. The American Journal of Clinical Nutrition 56, 483–490.
Pearson DJ, Tubbs PK (1967) Carnitine and derivatives in rat tissues. Biochemical Journal 105, 953–963.
Peréz-Guisado J, Munoz-Serrano A (2011a) The effect of the Spanish ketogenic Mediterranean diet on nonalcoholic fatty liver disease: a pilot study. Journal of Medicinal Food 14, 677–680.
| The effect of the Spanish ketogenic Mediterranean diet on nonalcoholic fatty liver disease: a pilot study.Crossref | GoogleScholarGoogle Scholar | 21688989PubMed |
Peréz-Guisado J, Munoz-Serrano A (2011b) A pilot study of the Spanish ketogenic Mediterranean diet: an effective therapy for the metabolic syndrome. Journal of Medicinal Food 14, 681–687.
| A pilot study of the Spanish ketogenic Mediterranean diet: an effective therapy for the metabolic syndrome.Crossref | GoogleScholarGoogle Scholar | 21612461PubMed |
Pes GM, Tolu F, Poulain M, Errigo A, Masala S, Pietrobelli A, Battistini NC, Maioli M (2013) Lifestyle and nutrition related to male longevity in Sardinia: an ecological study. Nutrition, Metabolism, and Cardiovascular Diseases 23, 212–219.
| Lifestyle and nutrition related to male longevity in Sardinia: an ecological study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38zmtFCiuw%3D%3D&md5=011dd91973876a3148c2a18d8a3461cdCAS | 21958760PubMed |
Phinney SD (2004) Ketogenic diets and physical performance. Nutrition & Metabolism 1, 2
| Ketogenic diets and physical performance.Crossref | GoogleScholarGoogle Scholar |
Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KGM, Tjonneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PHM, May AM, Bueno-De-Mesquita HB, van Duijnhoven FJB, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quiros JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E (2008) General and abdominal adiposity and risk of death in Europe. The New England Journal of Medicine 359, 2105–2120.
| General and abdominal adiposity and risk of death in Europe.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlOrsrnJ&md5=457d04dbd368312ca7f2fc8fe41e9d4aCAS | 19005195PubMed |
Pitchford WS (2004) Genetic improvement of feed efficiency of beef cattle: what lessons can be learnt from other species? Australian Journal of Experimental Agriculture 44, 371–382.
| Genetic improvement of feed efficiency of beef cattle: what lessons can be learnt from other species?Crossref | GoogleScholarGoogle Scholar |
Polidori MC, Mariani E, Baggio G, Deiana L, Carru C, Pes GM, Cecchetti R, Franceschi C, Senin U, Mecocci P (2007) Different antioxidant profiles in Italian centenarians: the Sardinian peculiarity. European Journal of Clinical Nutrition 61, 922–924.
| Different antioxidant profiles in Italian centenarians: the Sardinian peculiarity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnt1WlsLY%3D&md5=7ca74275ceee3048539b72d18429f418CAS | 17228351PubMed |
Polkinghorne RJ, Thompson JM (2010) Meat standards and grading: a world view. Meat Science 86, 227–235.
| Meat standards and grading: a world view.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cnntFensg%3D%3D&md5=584f593747e2f1d6f710a104760557dfCAS | 20541325PubMed |
Pollak MN, Schernhammer ES, Hankinson SE (2004) Insulin-like growth factors and neoplasia. Nature Reviews. Cancer 4, 505–518.
| Insulin-like growth factors and neoplasia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlt1Oktro%3D&md5=86a3cfaa2d7659e0b7f3e6326f209002CAS | 15229476PubMed |
Potter JD (1999) Colorectal cancer: molecules and populations. Journal of the National Cancer Institute 91, 916–932.
| Colorectal cancer: molecules and populations.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M3os1WjsQ%3D%3D&md5=adf845ff069a1700ed0d31bd9425bd0cCAS | 10359544PubMed |
Poulain M, Pes GM, Grasland C, Carru C, Ferrucci L, Baggio G, Franceschi C, Deiana L (2004) Identification of a geographic area characterized by extreme longevity in the Sardinia island: the AKEA study. Experimental Gerontology 39, 1423–1429.
| Identification of a geographic area characterized by extreme longevity in the Sardinia island: the AKEA study.Crossref | GoogleScholarGoogle Scholar | 15489066PubMed |
Psaltopoulou T, Kosti RI, Haidopoulos D, Dimopoulos M, Panagiotakos DB (2011) Olive oil intake is inversely related to cancer prevalence: a systematic review and a meta-analysis of 13 800 patients and 23340 controls in 19 observational studies. Lipids in Health and Disease 10, 127
| Olive oil intake is inversely related to cancer prevalence: a systematic review and a meta-analysis of 13 800 patients and 23340 controls in 19 observational studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFCjs73J&md5=d922111c54ccb11c572085b7c800fdf0CAS | 21801436PubMed |
Rajpathak SN, Liu YH, Ben-David O, Reddy S, Atzmon G, Crandall J, Barzilai N (2011) Lifestyle factors of people with exceptional longevity. Journal of the American Geriatrics Society 59, 1509–1512.
| Lifestyle factors of people with exceptional longevity.Crossref | GoogleScholarGoogle Scholar | 21812767PubMed |
Rajpathak SN, He MA, Sun Q, Kaplan RC, Muzumdar R, Rohan TE, Gunter MJ, Pollak M, Kim M, Pessin JE, Beasley J, Wylie-Rosett J, Hu FB, Strickler HD (2012) Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes 61, 2248–2254.
| Insulin-like growth factor axis and risk of type 2 diabetes in women.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlantbrE&md5=3bf9127ab73aff805586e625ef878ccaCAS | 22554827PubMed |
Rand WM, Pellett PL, Young VR (2003) Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. The American Journal of Clinical Nutrition 77, 109–127.
Razmaite V, Svirmickas GJ, Siukscius A, Sveistiene R (2011) Comparative characterization of fatty acid profiles in intramuscular lipids from different domestic and wild monogastric animal species. Veterinarija Ir Zootechnika 53, 45–50.
Reaven PD, Grasse BJ, Tribble DL (1994) Effects of linoleate enriched and oleate enriched diets in combination with alpha-tocopherol on the susceptibility of LDL and LDL subfractions to oxidative modification in humans. Arteriosclerosis and Thrombosis 14, 557–566.
| Effects of linoleate enriched and oleate enriched diets in combination with alpha-tocopherol on the susceptibility of LDL and LDL subfractions to oxidative modification in humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXkvVWkt7Y%3D&md5=19186ad818632deae6ea4000318f3184CAS | 8148354PubMed |
Reboul E, Borel P (2011) Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Progress in Lipid Research 50, 388–402.
| Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1OisL%2FO&md5=34c88878a9acbd626e26f6791a674e17CAS | 21763723PubMed |
Ren HY, Zheng XM, Chen HX, Li K (2011) Transgenic pigs carrying a synthesized fatty acid desaturase gene yield high level of omega-3 PUFAs. Agricultural Sciences in China 10, 1603–1608.
| Transgenic pigs carrying a synthesized fatty acid desaturase gene yield high level of omega-3 PUFAs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlKrs7fI&md5=3224db943288d51cce09eaabe09bebcdCAS |
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371, 569–578.
| Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies.Crossref | GoogleScholarGoogle Scholar | 18280327PubMed |
Richards MP, Trinkaus E (2009) Isotopic evidence for the diets of European Neanderthals and early modern humans. Proceedings of the National Academy of Sciences, USA 106, 16 034–16 039.
| Isotopic evidence for the diets of European Neanderthals and early modern humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1OjsbvK&md5=680e4da4fe71b7db67e844f38562a664CAS |
Rimm EB, Stampfer MJ, Ascherso A, Giovannucci E, Colditz GA, Willett WC (1993) Vitamin E consumption and the risk of coronary heart disease in men. The New England Journal of Medicine 328, 1450–1456.
| Vitamin E consumption and the risk of coronary heart disease in men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s3ktFagsw%3D%3D&md5=d17ebb97e2308f29835fc56de380d2caCAS | 8479464PubMed |
Roberts R, Bickerton AS, Fielding BA, Blaak EE, Wagenmakers AJ, Chong MFF, Gilbert M, Karpe F, Frayn KN (2008) Reduced oxidation of dietary fat after a short term high-carbohydrate diet. The American Journal of Clinical Nutrition 87, 824–831.
Robinson WS (1950) Ecological correlations and the behaviour of individuals. American Sociological Review 15:351–357, reprinted in International. Journal of Epidemiology 38, 337–341.
| Ecological correlations and the behaviour of individuals. American Sociological Review 15:351–357, reprinted in International.Crossref | GoogleScholarGoogle Scholar |
Rodríguez-Sánchez JA, Ripoll G, Latorre MA (2010) The influence of age at the beginning of Montanera period on meat characteristics and fat quality of outdoor Iberian pigs. Animal 4, 289–294.
| The influence of age at the beginning of Montanera period on meat characteristics and fat quality of outdoor Iberian pigs.Crossref | GoogleScholarGoogle Scholar | 22443883PubMed |
Roine P, Pekkarinen M, Karvonen MJ, Kihlberg J (1958) Diet and cardiovascular disease in Finland. Lancet 2, 173–175.
| Diet and cardiovascular disease in Finland.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaG1c7gsl2jtw%3D%3D&md5=0f017c1890ce5a0154ef9882b46b8fffCAS | 13564790PubMed |
Rouja PM, Dewailly É, Blanchet C, Bardi Community (2003) Fat, fishing patterns, and health among the Bardi people of north western Australia. Lipids 38, 399–405.
| Fat, fishing patterns, and health among the Bardi people of north western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvFShs78%3D&md5=28d2c1684b12ca93e0b4d73597c13c66CAS | 12848285PubMed |
Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA (2009) Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. The New England Journal of Medicine 360, 859–873.
| Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXis1Cnsbs%3D&md5=30e6c48d6c6550d19a3c1c5f547e2770CAS | 19246357PubMed |
Sanden M, Stubhaug I, Berntssen MHG, Lie Ø, Torstensen BE (2011) Atlantic salmon (Salmo salar L.) as a net producer of long-chain marine omega-3 fatty acids. Journal of Agricultural and Food Chemistry 59, 12697–12706.
| Atlantic salmon (Salmo salar L.) as a net producer of long-chain marine omega-3 fatty acids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlGgur7N&md5=f7f9137a7bea1389772ba35414fe998bCAS | 22017199PubMed |
Sandker GW, Kromhout D, Aravanis C, Bloemberg BPM, Mensink RP, Karalias N, Katan MB (1993) Serum cholesteryl ester fatty acids and their relation with serum lipids in elderly men in Crete and The Netherlands. European Journal of Clinical Nutrition 47, 201–208.
Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL (1995) Insulin, ketone bodies, and mitochondrial energy transduction. The FASEB Journal 9, 651–658.
Scarmeas N, Stern Y, Tang M-X, Mayeux R, Luchsinger JA (2006) Mediterranean diet and risk for Alzheimer’s disease. Annals of Neurology 59, 912–921.
| Mediterranean diet and risk for Alzheimer’s disease.Crossref | GoogleScholarGoogle Scholar | 16622828PubMed |
Schaafsma A, Muskiet FAJ, Storm H, Hofstede GJH, Pakan I, Van der Veer E (2000) Vitamin D3 and vitamin K1 supplementation of Dutch postmenopausal women with normal and low bone mineral densities: effect on serum 25-hydroxyvitamin D and carboxylated osteocalcin. European Journal of Clinical Nutrition 54, 626–631.
| Vitamin D3 and vitamin K1 supplementation of Dutch postmenopausal women with normal and low bone mineral densities: effect on serum 25-hydroxyvitamin D and carboxylated osteocalcin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmtFOmtLc%3D&md5=bc8b44c4ae4020a7564cd1b3524a6b37CAS | 10951511PubMed |
Scheppach W (1994) Effects of short chain fatty acids on gut morphology and function. Gut 35, S35–S38.
| Effects of short chain fatty acids on gut morphology and function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXjt1Wru7Y%3D&md5=6f714ac29d668b31878cf78b50c353b5CAS | 8125387PubMed |
Schmidt M, Pfetzer N, Schwab M, Strauss I, Kammerer U (2011) Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutrition & Metabolism 8, 54
| Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFCkurrE&md5=2de7aa7c8d529b5d5392915d5da4372cCAS |
Schmitz G, Ecker J (2008) The opposing effects of n-3 and n-6 fatty acids. Progress in Lipid Research 47, 147–155.
| The opposing effects of n-3 and n-6 fatty acids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXislKnsLk%3D&md5=9b4b193471f52b2580de770f8f6c8b14CAS | 18198131PubMed |
Sharman MJ, Volek JS (2004) Weight loss leads to reductions in inflammatory biomarkers after a very-low-carbohydrate diet and a low-fat diet in overweight men. Clinical Science 107, 365–369.
| Weight loss leads to reductions in inflammatory biomarkers after a very-low-carbohydrate diet and a low-fat diet in overweight men.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnslOqu7o%3D&md5=def6e2c9936d46277c92484894c55b6bCAS | 15265001PubMed |
Shigenaga MK, Hagen TM, Ames BN (1994) Oxidative damage and mitochondrial decay in aging. Proceedings of the National Academy of Sciences, USA 91, 10771–10778.
| Oxidative damage and mitochondrial decay in aging.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmvFyjtL4%3D&md5=745db82ddc55dd21a3930351251872b4CAS |
Shingfield KJ, Bonnet M, Scollan ND (2013) Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 7, 132–162.
| Recent developments in altering the fatty acid composition of ruminant-derived foods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjsFeisbg%3D&md5=9d0bb65fd1e098821c8e000c0d77549cCAS | 23031638PubMed |
Simonsen N, van’t Veer P, Strain JJ, Martin-Moreno JM, Huttunen JK, Navajas JFC, Martin BC, Thamm M, Kardinaal AFM, Kok FJ, Kohlmeier L (1998) Adipose tissue omega-3 and omega-6 fatty acid content and breast cancer in the EURAMIC study. American Journal of Epidemiology 147, 342–352.
| Adipose tissue omega-3 and omega-6 fatty acid content and breast cancer in the EURAMIC study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7mslGhtA%3D%3D&md5=5e6e534abcdcc68f088b53092b8f9d83CAS | 9508101PubMed |
Simopoulos AP (1991) Omega-3 fatty acids in health and disease and in growth and development. The American Journal of Clinical Nutrition 54, 438–463.
Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Experimental Biology and Medicine 233, 674–688.
| The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmslWjtbk%3D&md5=5d9444518d8a3bab0c758ba44f5625b5CAS | 18408140PubMed |
Sinclair LA (2007) Nutritional manipulation of the fatty acid composition of sheep meat: a review. The Journal of Agricultural Science 145, 419–434.
| Nutritional manipulation of the fatty acid composition of sheep meat: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpslGisrk%3D&md5=751377d9f1a154411f86c555e431cb05CAS |
Sinclair AJ, Barone S, Stobaus T, Tume R, Beilken S, Mueller W, Cunningham J, Barnes JA, Greenfield H (2010) Lipid composition of Australian pork cuts 2005/2006. Food Chemistry 121, 672–681.
| Lipid composition of Australian pork cuts 2005/2006.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXivVOrtr0%3D&md5=33713e8302358949354bd652c693a3c2CAS |
Singh PP, Singh M, Mastana SS (2006) APOE distribution in world populations with new data from India and the UK. Annals of Human Biology 33, 279–308.
| APOE distribution in world populations with new data from India and the UK.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28nlvVerug%3D%3D&md5=3342f318d5469c90b70509a69f630364CAS | 17092867PubMed |
Siri-Tarino PW, Sun Q, Hu FB, Krauss RM (2010) Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. The American Journal of Clinical Nutrition 91, 535–546.
| Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFyntLk%3D&md5=ea8f3c4a75e962b6309526bb2076aa7dCAS | 20071648PubMed |
Smit M, Deknijff P, Rosseneu M, Bury J, Klasen E, Frants R, Havekes L (1988) Apolipoprotein E polymorphism in the Netherlands and its effect on plasma lipid and apolipoprotein levels. Human Genetics 80, 287–292.
| Apolipoprotein E polymorphism in the Netherlands and its effect on plasma lipid and apolipoprotein levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXlsV2rsg%3D%3D&md5=f9d3fd79887762418aa115a2726f7ff6CAS | 3192216PubMed |
Sniderman AD, Islam S, Yusuf S, McQueen MJ (2012) Discordance analysis of apolipoprotein B and non-high density lipoprotein cholesterol as markers of cardiovascular risk in the INTERHEART study. Atherosclerosis 225, 444–449.
| Discordance analysis of apolipoprotein B and non-high density lipoprotein cholesterol as markers of cardiovascular risk in the INTERHEART study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFWit7zI&md5=c01a639fcc504b1245d1c661d5dab3ceCAS | 23068583PubMed |
Sofi F, Abbate R, Gensini GF, Casini A, Trichopoulou A, Bamia C (2012) Identification of change-points in the relationship between food groups in the Mediterranean diet and overall mortality: an ‘a posteriori’ approach. European Journal of Nutrition 51, 167–172.
| Identification of change-points in the relationship between food groups in the Mediterranean diet and overall mortality: an ‘a posteriori’ approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xislaqu7Y%3D&md5=b0bc02d63b1a0b066b281f4be7cddec2CAS | 21541730PubMed |
Song YQ, Stampfer MJ, Liu SM (2004) Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Annals of Internal Medicine 141, 137–147.
| Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease.Crossref | GoogleScholarGoogle Scholar |
Sponheimer M, Dufour DL (2009) Increased dietary breadth in early hominin evolution: revisiting arguments and evidence with a focus on biogeochemical contributions. In ‘Evolution of hominin diets’. (Eds JJ Hublin, MP Richard) pp. 229–240. (Springer: Dordrecht, The Netherlands)
Sprecher H (2002) The roles of anabolic and catabolic reactions in the synthesis and recycling of polyunsaturated fatty acids. Prostaglandins, Leukotrienes, and Essential Fatty Acids 67, 79–83.
| The roles of anabolic and catabolic reactions in the synthesis and recycling of polyunsaturated fatty acids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnvFGrsrg%3D&md5=f8c34788d02d1db6605917723414715eCAS | 12324224PubMed |
Srivastava RAK (1996) Regulation of the apolipoprotein E by dietary lipids occurs by transcriptional and post-transcriptional mechanisms. Molecular and Cellular Biochemistry 155, 153–162.
| Regulation of the apolipoprotein E by dietary lipids occurs by transcriptional and post-transcriptional mechanisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhvFKiu7Y%3D&md5=62083a3f2cea07632536b79011daa079CAS |
Srivastava RAK, Bhasin N, Srivastava N (1996) Apolipoprotein E gene expression in various tissues of mouse and regulation by estrogen. Biochemistry and Molecular Biology International 38, 91–101.
Staerfl SM, Soliva CR, Leiber F, Kreuzer M (2011) Fatty acid profile and oxidative stability of the perirenal fat of bulls fattened on grass silage and maize silage supplemented with tannins, garlic, maca and lupines. Meat Science 89, 98–104.
| Fatty acid profile and oxidative stability of the perirenal fat of bulls fattened on grass silage and maize silage supplemented with tannins, garlic, maca and lupines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtl2gsrs%3D&md5=10ecbeb633db05c6c5c41dd50e4761a9CAS | 21550730PubMed |
Stamler J, Vaccaro O, Neaton JD, Wentworth D (1993) Diabetes, other risk factors, and 12-yr cardiovascular mortaility for mean screened in the multiple risk factor intervention trial. Diabetes Care 16, 434–444.
| Diabetes, other risk factors, and 12-yr cardiovascular mortaility for mean screened in the multiple risk factor intervention trial.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s7mtFyrtQ%3D%3D&md5=9ee876aa1633c579a5e270d114d08742CAS | 8432214PubMed |
Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH (1996) A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. Journal of the American Medical Association 276, 882–888.
| A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28zns1Oltw%3D%3D&md5=fd938ebd958aebe624611394c892e8c4CAS | 8782637PubMed |
Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ (2009) Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. The Journal of Clinical Investigation 119, 1322–1334.
| Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsFyrtrg%3D&md5=163555d8f7b84603fd8cc536fdb276b0CAS | 19381015PubMed |
Stefansson V (1912) ‘My life with the Eskimo.’ (Harper and Brothers, Norwood Press: Norwood, MA)
Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL (1989) Beyond cholesterol modifications of low-density lipoprotein that increase its atherogenicity. The New England Journal of Medicine 320, 915–924.
Steinmetz KA, Potter JD (1991) Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes & Control 2, 427–442.
| Vegetables, fruit, and cancer. II. Mechanisms.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK387htVCqug%3D%3D&md5=bf21932e3f7c3eb8c8f42652453f4fe6CAS |
Stengard JH, Zerba KE, Pekkanen J, Ehnholm C, Nissinen A, Sing CF (1995) Apolipoprotein-E polymorphism predicts death from coronary heart disease in a longitudinal study of elderly Finnish men. Circulation 91, 265–269.
| Apolipoprotein-E polymorphism predicts death from coronary heart disease in a longitudinal study of elderly Finnish men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M7gtlOnsw%3D%3D&md5=f192329b7bdd0ff87759523f5f2cd4d9CAS | 7805227PubMed |
Stiner MC, Munro ND (2011) On the evolution of diet and landscape during the Upper Paleolithic through Mesolithic at Franchthi Cave (Peloponnese, Greece). Journal of Human Evolution 60, 618–636.
| On the evolution of diet and landscape during the Upper Paleolithic through Mesolithic at Franchthi Cave (Peloponnese, Greece).Crossref | GoogleScholarGoogle Scholar | 21371735PubMed |
Stocker R, Keaney JF (2004) Role of oxidative modifications in atherosclerosis. Physiological Reviews 84, 1381–1478.
| Role of oxidative modifications in atherosclerosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovVyltbo%3D&md5=b6d7db6bccd097723e38ed5e2c9053c2CAS | 15383655PubMed |
Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM (2004) The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Annals of Neurology 55, 576–580.
| The ketogenic diet increases mitochondrial uncoupling protein levels and activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjs1ChtLk%3D&md5=b3a26668c78b4ca37e14e47d47038d2fCAS | 15048898PubMed |
Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, Singleton A, Bandinelli S, Cherubini A, Arnett D, Tsai MY, Ferrucci L (2009) Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI study. PLOS Genetics 5, e1000338
| Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI study.Crossref | GoogleScholarGoogle Scholar | 19148276PubMed |
Taubes G (2001) The soft science of dietary fat. Science 291, 2536–2545.
| The soft science of dietary fat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXisFeltLc%3D&md5=01c51e1f54362c563958278a0da543dfCAS | 11286266PubMed |
Taubes G (2007) ‘Good calories, bad calories: challenging the conventional wisdom on diet, weight control, and disease.’ (A.A. Knopf: New York)
Taylor HL, Keys A (1950) Adaptation to caloric restriction. Science 112, 215–218.
| Adaptation to caloric restriction.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaG3c%2FltF2gug%3D%3D&md5=5888546c30f3f616ca7c604b036686f0CAS | 15442306PubMed |
Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li XH, Guo XQ, Li MY, Cho YS, Go MJ, Kim YJ, Lee JY, Park T, Kim K, Sim X, Ong RTH, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Zhao JH, Yuan X, Luan JA, Lamina C, Ziegler A, Zhang W, Zee RYL, Wright AF, Witteman JCM, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJG, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx B, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PKE, Lucas G, Luben R, Loos RJF, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, Konig IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens A, Ingelsson E, Igi W, Hovingh GK, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Doering A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJC, de Faire U, Crawford G, Collins FS, Chen YDI, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJP, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713.
| Biological, clinical and population relevance of 95 loci for blood lipids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVSmurfI&md5=2c5733d8a4c7bc70af334a941c3bad1fCAS | 20686565PubMed |
Thissen JP, Ketelslegers JM, Underwood LE (1994) Nutrional regulation of the insulin-like growth factors. Endocrine Reviews 15, 80–101.
Tholstrup T, Marckmann P, Jespersen J, Sandstrom B (1994a) Fat high in stearic acid favorably affects blood lipids and factor VII coagulation activity in comparison with fats high in palmitic acid or high in myristic and lauric acids. The American Journal of Clinical Nutrition 59, 371–377.
Tholstrup T, Marckmann P, Jespersen J, Vessby B, Jart A, Sandstrom B (1994b) Effect on blood lipids, coagulation, and fibrinolysis of a fat high in myristic acid and a fat high in palmitic acid. The American Journal of Clinical Nutrition 60, 919–925.
Thompson JM (2004) The effects of marbling on flavour and juiciness scores of cooked beef, after adjusting to a constant tenderness. Australian Journal of Experimental Agriculture 44, 645–652.
| The effects of marbling on flavour and juiciness scores of cooked beef, after adjusting to a constant tenderness.Crossref | GoogleScholarGoogle Scholar |
Tisdale MJ, Brennan RA, Fearon KC (1987) Reduction of weight loss and tumor size in a cachexia model by a high fat diet. British Journal of Cancer 56, 39–43.
| Reduction of weight loss and tumor size in a cachexia model by a high fat diet.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s3pvFCitA%3D%3D&md5=928e83f16b0ba3a6c816b4508e99db82CAS | 3620317PubMed |
Topping DL, Clifton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiological Reviews 81, 1031–1064.
Trejo JL, Carro E, Nunez A, Torres-Aleman I (2002) Sedentary life impairs self-reparative processes in the brain: the role of serum insulin-like growth factor-I. Reviews in the Neurosciences 13, 365–374.
| Sedentary life impairs self-reparative processes in the brain: the role of serum insulin-like growth factor-I.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotVeqsw%3D%3D&md5=cc68f38636df89424ebe35bc76f115a6CAS | 12542262PubMed |
Tribble DL, Holl LG, Wood PD, Krauss RM (1992) Variations in oxidative susceptibility among 6 low density lipoprotein subfractions of differing density and particle size. Atherosclerosis 93, 189–199.
| Variations in oxidative susceptibility among 6 low density lipoprotein subfractions of differing density and particle size.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XksVGkur4%3D&md5=d76708070567489f8031369d9215e742CAS | 1590824PubMed |
Trichopoulou A, Costacou T, Bamia C, Trichopoulou D (2003) Adherence to a Mediterranean diet and survival in a Greek population. The New England Journal of Medicine 348, 2599–2608.
| Adherence to a Mediterranean diet and survival in a Greek population.Crossref | GoogleScholarGoogle Scholar | 12826634PubMed |
Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL (2005) Dietary carbohydrates and glycaemic load and the incidence of symptomatic gall stone disease in men. Gut 54, 823–828.
| Dietary carbohydrates and glycaemic load and the incidence of symptomatic gall stone disease in men.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltlOmsbk%3D&md5=2ac744a164bb2427655a61d52eec2c4dCAS | 15888792PubMed |
Tume RK (2004) The effects of environmental factors on fatty acid composition and the assessment of marbling in beef cattle: a review. Australian Journal of Experimental Agriculture 44, 663–668.
| The effects of environmental factors on fatty acid composition and the assessment of marbling in beef cattle: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmsVCgtLc%3D&md5=5c506b4e6533ddf19b7bb758c75cacebCAS |
Ungar PS, Sponheimer M (2011) The diets of early hominins. Science 334, 190–193.
| The diets of early hominins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1yktrzI&md5=f85fbaeeeb4e442a42ee9644e9deba69CAS | 21998380PubMed |
Uotila U, Raekallio J, Ehrnrooth W (1958) Goitre and atherosclerosis. Lancet 2, 171–173.
| Goitre and atherosclerosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaG1c7gsl2jtg%3D%3D&md5=04e5c5ddc52e85c3a77884f54b4ef49aCAS | 13564789PubMed |
Usydus Z, Szlifder-Richert J, Adamczyk M (2012) Variation in proximate composition and fatty acid profiles of Baltic sprat (Sprattus sprattus balticus). Food Chemistry 130, 97–103.
| Variation in proximate composition and fatty acid profiles of Baltic sprat (Sprattus sprattus balticus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVWjsbvO&md5=9b1cf9cdbdb067991d74de8d47c4e3bbCAS |
Utermann G (1988) Apolipoprotein polymorphism and multifactorial hyperlipemia. Journal of Inherited Metabolic Disease 11, 74–86.
| Apolipoprotein polymorphism and multifactorial hyperlipemia.Crossref | GoogleScholarGoogle Scholar | 3141688PubMed |
Utermann G, Pruin N, Steinmetz A (1979) Polymorphism of apolipoprotein E. III. Effect of a single polymorphic gene locus on plasma lipid levels in man. Clinical Genetics 15, 63–72.
| Polymorphism of apolipoprotein E. III. Effect of a single polymorphic gene locus on plasma lipid levels in man.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXhtlGlsr0%3D&md5=131882a3d2f40a1c2afc7e0438cca6a3CAS | 759055PubMed |
Utrilla MC, Soriano A, Ruiz AG (2010) Quality attributes of pork loin with different levels of marbling from Duroc and Iberian cross. Journal of Food Quality 33, 802–820.
| Quality attributes of pork loin with different levels of marbling from Duroc and Iberian cross.Crossref | GoogleScholarGoogle Scholar |
Van Niekerk PJ, Burger AEC (1985) The estimation of the composition of edible oil mixtures. Journal of the American Oil Chemists’ Society 62, 531–538.
| The estimation of the composition of edible oil mixtures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhs1GgsL8%3D&md5=a20df2b0d7a403474bd8a096cbeae88bCAS |
VanItallie TB, Nufert TH (2003) Ketones: metabolism’s ugly duckling. Nutrition Reviews 61, 327–341.
| Ketones: metabolism’s ugly duckling.Crossref | GoogleScholarGoogle Scholar | 14604265PubMed |
Van Munster IP, Tangerman A, Nagengast FM (1994) Effect of resistant starch on colonic fermentation, bile acid metabolism, and mucosal proliferation. Digestive Diseases and Sciences 39, 834–842.
| Effect of resistant starch on colonic fermentation, bile acid metabolism, and mucosal proliferation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXksFCmurw%3D&md5=24b03cc8d70b1a9491718360a8621ed0CAS | 8149850PubMed |
Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GR Jr (2001) Ketone bodies, potential therapeutic uses. IUBMB Life 51, 241–247.
| Ketone bodies, potential therapeutic uses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmvF2mtrg%3D&md5=83907b0eac0b714c33e91c40c4335a53CAS | 11569918PubMed |
Veniant MM, Kim E, McCormick S, Boren J, Nielsen LB, Raabe M, Young SG (1999) Insights into apolipoprotein B biology from transgenic and gene-targeted mice. The Journal of Nutrition 129, 451S–455S.
Volek JS, Sharman MJ (2004) Cardiovascular and hormonal aspects of very-low-carbohydrate ketogenic diets. Obesity Research 12, 115S–123S.
| Cardiovascular and hormonal aspects of very-low-carbohydrate ketogenic diets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmt12ktA%3D%3D&md5=4570f6e3ebc2b9107b8601985d4fde39CAS | 15601959PubMed |
Volek JS, Sharman MJ, Gomez AL, Scheett TP, Kraemer WJ (2003) An isoenergetic very low carbohydrate diet improves serum HDL cholesterol and triacylglycerol concentrations, the total cholesterol to HDL cholesterol ratio and postprandial lipemic responses compared with a low fat diet in normal weight, normolipidemic women. The Journal of Nutrition 133, 2756–2761.
Volek JS, Fernandez ML, Feinman RD, Phinney SD (2008) Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Progress in Lipid Research 47, 307–318.
| Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnt1yjsbg%3D&md5=7fa2c897dc010dcdb0375e7ae454de8fCAS | 18396172PubMed |
Vuorio AF, Turtola H, Piilahti K-M, Repo P, Kanninen T, Kontula K (1997) Familial hypercholesterolemia in the Finnish North Karelia. Arteriosclerosis, Thrombosis, and Vascular Biology 17, 3127–3138.
| Familial hypercholesterolemia in the Finnish North Karelia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c%2FntlOltQ%3D%3D&md5=fdb047e6f2feb4ade78cf942f8dd5219CAS | 9409302PubMed |
Wang ZX, Jeon HY, Rigo F, Bennett CF, Krainer AR (2012) Manipulation of PK-M mutually exclusive alternative splicing by antisense oligonucleotides. Open Biology 2, 120133
| Manipulation of PK-M mutually exclusive alternative splicing by antisense oligonucleotides.Crossref | GoogleScholarGoogle Scholar |
Warburg O (1956) On the origin of cancer cells. Science 123, 309–314.
| On the origin of cancer cells.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaG28%2FltV2ktQ%3D%3D&md5=b6d650188bfaab87c4b62e7428bf939cCAS | 13298683PubMed |
Welch AA, Shrestha SS, Lentjes MAH, Wareham NJ, Khaw KT (2010) Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the precursor-product ratio of alpha-linolenic acid to long-chain n-3 polyunsaturated fatty acids results from the EPIC-Norfolk cohort. The American Journal of Clinical Nutrition 92, 1040–1051.
| Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the precursor-product ratio of alpha-linolenic acid to long-chain n-3 polyunsaturated fatty acids results from the EPIC-Norfolk cohort.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVWgt7nE&md5=b00167ad164a0b858039c64aa6087b7cCAS | 20861171PubMed |
West GC, Shaw DL (1975) Fatty acid composition of Dall sheep bone marrow. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology 50, 599–601.
| Fatty acid composition of Dall sheep bone marrow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2MXkslKjsLw%3D&md5=cd80400e522eb0e193f0df2f10d98f8cCAS |
Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, He Q, Curb JD, Suzuki M (2007) Caloric restriction, the traditional Okinawan diet, and healthy aging. Annals of the New York Academy of Sciences 1114, 434–455.
| Caloric restriction, the traditional Okinawan diet, and healthy aging.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVKjsr7N&md5=ceedb71009a5f9c7a5193d9b8fb2066dCAS | 17986602PubMed |
Willcox DC, Willcox BJ, Todoriki H, Suzuki M (2009) The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. Journal of the American College of Nutrition 28, 500S–516S.
| The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlvFOhu70%3D&md5=f67f22a7e33de8b0035901a335b648f0CAS | 20234038PubMed |
Willett WC, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Rosner BA, Sampson LA, Hennekens CH (1993) Intake of trans fatty acids and risk of coronary heart disease among women. Lancet 341, 581–585.
| Intake of trans fatty acids and risk of coronary heart disease among women.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s7nt1yltQ%3D%3D&md5=3679925ac35260e67d19377a475ee025CAS | 8094827PubMed |
Wood JD, Enser M, Fisher AV, Nute GR, Richardson RI, Sheard PR (1999) Manipulating meat quality and composition. The Proceedings of the Nutrition Society 58, 363–370.
| Manipulating meat quality and composition.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MzpsFKksA%3D%3D&md5=916ff91ca92766346fdfcef70589b73dCAS | 10466178PubMed |
Wright SH, Snape JW Jr, Battle W, Cohen S, London RL (1980) Effect of dietary components on gastrocolonic response. The American Journal of Physiology 238, G228–G232.
Xia SH, Wang JD, Kang JX (2005) Decreased n-6/n-3 fatty acid ratio reduces the invasive potential of human lung cancer cells by downregulation of cell adhesion/invasion-related genes. Carcinogenesis 26, 779–784.
| Decreased n-6/n-3 fatty acid ratio reduces the invasive potential of human lung cancer cells by downregulation of cell adhesion/invasion-related genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXis1yjsbs%3D&md5=b9919136600a4d79a6aaf6308fc71049CAS | 15661810PubMed |
Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L (2004) Diabetes mellitus and risk of dementia in the Kungsholmen project – a 6-year follow-up study. Neurology 63, 1181–1186.
| Diabetes mellitus and risk of dementia in the Kungsholmen project – a 6-year follow-up study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2crgvVWhuw%3D%3D&md5=d6117afd181c5a1703eb62fe7e85f4c3CAS | 15477535PubMed |
Xue F, Michels KB (2007) Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. The American Journal of Clinical Nutrition 86, 823S–835S.
Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC (2004) A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia – a randomized, controlled trial. Annals of Internal Medicine 140, 769–777.
| A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia – a randomized, controlled trial.Crossref | GoogleScholarGoogle Scholar | 15148063PubMed |
Yancy WS, Westman EC, McDuffie JR, Grambow SC, Jeffreys AS, Bolton J, Chalecki A, Oddone EZ (2010) A randomized trial of a low-carbohydrate diet vs Orlistat Plus a low-fat diet for weight loss. Archives of Internal Medicine 170, 136–145.
| A randomized trial of a low-carbohydrate diet vs Orlistat Plus a low-fat diet for weight loss.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitVOiurg%3D&md5=74c4b8309743b9a0ce4260c1a9e970e7CAS | 20101008PubMed |
Yang A, Larsen TW, Powell VH, Tume RK (1999a) A comparison of fat composition of Japanese and long-term grain-fed Australian steers. Meat Science 51, 1–9.
| A comparison of fat composition of Japanese and long-term grain-fed Australian steers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVWruw%3D%3D&md5=f663d9a9529337c50825a8b8bbba4592CAS | 22061530PubMed |
Yang A, Larsen TW, Smith SB, Tume RK (1999b) Delta(9) desaturase activity in bovine subcutaneous adipose tissue of different fatty acid composition. Lipids 34, 971–978.
| Delta(9) desaturase activity in bovine subcutaneous adipose tissue of different fatty acid composition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmvVaht7g%3D&md5=6b9c49c20d587ec4c87f177c83ae876aCAS | 10574662PubMed |
Yang YJ, Lee SH, Hong SJ, Chung BC (1999c) Comparison of fatty acid profiles in the serum of patients with prostate cancer and benign prostatic hyperplasia. Clinical Biochemistry 32, 405–409.
| Comparison of fatty acid profiles in the serum of patients with prostate cancer and benign prostatic hyperplasia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnvFylsrs%3D&md5=1fe6a585715a3c00d25139006dc186dfCAS | 10667474PubMed |
Yerushalmy J, Hilleboe HE (1957) Fat in the diet and mortality from heart disease a methodological note. New York State Journal of Medicine 57, 2343–2354.
Younis NN, Soran H, Pemberton P, Charlton-Menys V, Elseweidy MM, Durrington PN (2013) Small dense LDL is more susceptible to glycation than more buoyant LDL in Type 2 diabetes. Clinical Science 124, 343–349.
| Small dense LDL is more susceptible to glycation than more buoyant LDL in Type 2 diabetes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs12rsbjI&md5=5a5ece0ec9c6f153cab16e49817360e4CAS | 22985435PubMed |
Yu-Poth S, Yin DZ, Kris-Etherton PM, Zhao GX, Etherton TD (2005) Long-chain polyunsaturated fatty acids upregulate LDL receptor protein expression in fibroblasts and HepG2 cells. The Journal of Nutrition 135, 2541–2545.
Yudkin J (1957) Diet and coronary thrombosis hypothesis and fact. Lancet 2, 155–162.
| Diet and coronary thrombosis hypothesis and fact.Crossref | GoogleScholarGoogle Scholar |
Yudkin J (1964) Dietary fat and dietary sugar in relation to ischaemic heart-disease and diabetes. Lancet 2, 4–5.
| Dietary fat and dietary sugar in relation to ischaemic heart-disease and diabetes.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF2c7gslWnuw%3D%3D&md5=b48281d1854281c7a178689f1fbd7d35CAS | 14149218PubMed |
Zhu H, Tucker HM, Grear KE, Simpson JF, Manning AK, Cupples LA, Estus S (2007) A common polymorphism decreases low-density lipoprotein receptor exon 12 splicing efficiency and associates with increased cholesterol. Human Molecular Genetics 16, 1765–1772.
| A common polymorphism decreases low-density lipoprotein receptor exon 12 splicing efficiency and associates with increased cholesterol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXosFektbg%3D&md5=df5d93d5df476dfe263c0372f3913d84CAS | 17517690PubMed |
Zock PL, Katan MB (1998) Linoleic acid intake and cancer risk: a review and meta-analysis. The American Journal of Clinical Nutrition 68, 142–153.
Zock PL, de Vries JHM, de Fouw NJ, Katan MB (1995) Positional distribution of fatty acids in dietary triglycerides: effects on fasting blood lipoprotein concentrations in humans. The American Journal of Clinical Nutrition 61, 48–55.
Zock PL, Gerritsen J, Katan MB (1996) Partial conservation of the sn-2 position of dietary triglycerides in fasting plasma lipids in humans. European Journal of Clinical Investigation 26, 141–150.
| Partial conservation of the sn-2 position of dietary triglycerides in fasting plasma lipids in humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xhs1Kjsbg%3D&md5=b0b4135bab27c35bfa13f5e9a688866bCAS | 8904524PubMed |
Zock PL, Mensink RP, Harryvan J, de Vries JHM, Katan MB (1997) Fatty acids in serum cholesteryl esters as quantitative biomarkers of dietary intake in humans. American Journal of Epidemiology 145, 1114–1122.
| Fatty acids in serum cholesteryl esters as quantitative biomarkers of dietary intake in humans.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2szkt1yqtg%3D%3D&md5=3808f75a3773d27f5ebdae3b0a86e278CAS | 9199541PubMed |
Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, Seyfried TN (2010) Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutrition & Metabolism 7, 33
| Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report.Crossref | GoogleScholarGoogle Scholar |

William Barendse is a senior principal research scientist at CSIRO Animal, Food and Health Sciences and an adjunct Professor in the School of Veterinary Science, the University of Queensland. Bill was born in Cape Town, South Africa, came to Perth as a teenager, and finished a Bachelor’s Degree in Science in the Zoology Department of the University of Western Australia. That led to a PhD in the same department, in Genetics and Systematics of the species Mygalopsis , with Mike Johnson. A Macquarie University Fellowship followed, to study the human disorder pre-eclampsia with Des Cooper, which introduced him to Molecular Genetics and Linkage Analysis. That led to a position in Rockhampton with CSIRO Tropical Animal Production to make a linkage map of the cow, in Jay Hetzel’s group. The cow maps acted as the seed for the genome sequence and genetic history of the cow, to which he contributed. He and his group have been involved in the mapping of genes affecting production traits of cattle. These include the Calpastatin gene for meat tenderness. Since 1994, he has been studying marbling of beef and fatness of cattle, which has led almost inexorably to this review. He has authored or co-authored more than 140 refereed articles in the primary scientific literature.