Towards a new phenotype for tick resistance in beef and dairy cattle: a review
Heather M. Burrow A M , Ben J. Mans B C H , Fernando F. Cardoso D , Michael A. Birkett E , Andrew C. Kotze F , Ben J. Hayes G , Ntanganedzeni Mapholi H , Kennedy Dzama I , Munyaradzi C. Marufu J , Naftaly W. Githaka K and Appolinaire Djikeng L M
A M , Ben J. Mans B C H , Fernando F. Cardoso D , Michael A. Birkett E , Andrew C. Kotze F , Ben J. Hayes G , Ntanganedzeni Mapholi H , Kennedy Dzama I , Munyaradzi C. Marufu J , Naftaly W. Githaka K and Appolinaire Djikeng L M
A Faculty of Science, Agriculture, Business and Law, W40, University of New England 2351, NSW, Australia.
B Onderstepoort Veterinary Institute, Agricultural Research Council, 100 Old Soutpan Road, Onderstepoort 0084, South Africa.
C Department of Veterinary Tropical Diseases, University of Pretoria, M35, Onderstepoort 0110, South Africa.
D Embrapa Pecuária Sul (South Livestock), Bagé 96401-970, Brazil.
E Biointeractions and Crop Protection Department, Rothamsted Research, Harpenden, Herts, AL5 2JQ, UK.
F CSIRO Agriculture and Food, Queensland Bioscience Precinct, 306 Carmody Road, St Lucia 4067, Qld, Australia.
G Queensland Alliance for Agriculture and Food Innovation, University of Queensland, 306 Carmody Road, St Lucia 4067, Qld, Australia.
H Department of Life and Consumer Sciences, University of South Africa, Private Bag X6, Florida 1710, South Africa.
I Department of Animal Sciences, Stellenbosch University, Merriman Street, Private Bag X1, Matieland 7602, Stellenbosch, South Africa.
J Department of Production Animal Studies, University of Pretoria, Private Bag X4, Onderstepoort 0110, South Africa.
K International Livestock Research Institute, Old Naivasha Road, Kabete PO Box 30709, Nairobi, Kenya.
L Centre for Tropical Livestock Genetics and Health, University of Edinburgh, Roslin, Midlothian EH25 9RG, UK.
M Corresponding authors. Email: Heather.Burrow@une.edu.au; appolinaire.djikeng@ctlgh.org
Animal Production Science 59(8) 1401-1427 https://doi.org/10.1071/AN18487
Submitted: 7 August 2018 Accepted: 3 March 2019 Published: 4 July 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
About 80% of the world’s cattle are affected by ticks and tick-borne diseases, both of which cause significant production losses. Cattle host resistance to ticks is the most important factor affecting the economics of tick control, but it is largely neglected in tick-control programs due to technical difficulties and costs associated with identifying individual-animal variation in resistance. The present paper reviews the scientific literature to identify factors affecting resistance of cattle to ticks and the biological mechanisms of host tick resistance, to develop alternative phenotype(s) for tick resistance. If new cost-effective phenotype(s) can be developed and validated, then tick resistance of cattle could be genetically improved using genomic selection, and incorporated into breeding objectives to simultaneously improve cattle productive attributes and tick resistance. The phenotype(s) could also be used to improve tick control by using cattle management. On the basis of the present review, it is recommended that three possible phenotypes (haemolytic analysis; measures of skin hypersensitivity reactions; simplified artificial tick infestations) be further developed to determine their practical feasibility for consistently, cost-effectively and reliably measuring cattle tick resistance in thousands of individual animals in commercial and smallholder farmer herds in tropical and subtropical areas globally. During evaluation of these potential new phenotypes, additional measurements should be included to determine the possibility of developing a volatile-based resistance phenotype, to simultaneously improve cattle resistance to both ticks and biting flies. Because the current measurements of volatile chemistry do not satisfy the requirements of a simple, cost-effective phenotype for use in commercial cattle herds, consideration should also be given to inclusion of potentially simpler measures to enable indirect genetic selection for volatile-based resistance to ticks.
Additional keywords: blood parameters, host resistance, immune response, skin hypersensitivity, tick count, volatiles.
Introduction
About 80% of the world’s cattle are at risk of ticks and tick-borne diseases, both of which cause significant production losses. Economic losses from ticks and tick-borne diseases were estimated in 1996 to range from US$13.9 to US$18.7 billion per annum (de Castro 1997), with current estimates ranging from US$20 to US$30 billion per annum (Lew-Tabor and Rodriguez Valle 2016).
In a review of tick-control methods, Frisch (1999) suggested that cattle host resistance was the single most important factor affecting the economics of tick control. On the basis of recent reviews of the literature, host resistance is moderately to highly heritable (Burrow 2014; Burrow and Henshall 2014) and represents a low-cost, permanent solution requiring no extra labour or resources and incurring no additional costs to deliver beef and milk products (Frisch 1999). Acaricides or an anti-tick vaccine (developed in the 1990s, but subsequently withdrawn from the market in 2010) do not offer a permanent solution to tick control. Attempts to develop new anti-tick vaccines are ongoing (Guerrero et al. 2014; Lew-Tabor and Rodriguez Valle 2016), but new vaccines are highly unlikely to confer total protection against ticks. High host resistance is the key to effective long-term tick control, with total resistance being the ultimate aim (Frisch 1999). However, while improvements to acaricides and vaccines are continuously being pursued, improvements to the most important single factor controlling ticks, i.e. host resistance, is largely neglected (Frisch 1999).
The primary reason for such neglect is the difficulty and expense of identifying individual-animal variation in resistance to ticks. This constraint applies in research herds as well as in beef and dairy commercial and smallholder farms in both developed and developing countries. The purpose of the present paper is, therefore, to review information from the scientific literature to gain a better understanding of the factors affecting resistance of cattle to ticks as well as the biological mechanisms of host tick resistance, with the aim of identifying potential new phenotypes that can be evaluated in very large numbers of animals for tick resistance. The review will also briefly examine possibilities for the genetic improvement of tick resistance and incorporating economic weightings for tick resistance in breeding objectives aimed at simultaneously improving economically important productive and adaptive traits.
The need for a new phenotype for tick resistance
Cattle researchers have used single or repeated counts of the number of engorging ticks (i.e. ticks between 4.5 and 8 mm in diameter) on one or both sides of each animal following artificial or field infestation (Wharton and Utech 1970), so as to identify individual-animal variation in tick resistance. Tick counts are very time-consuming and require skilled animal technicians as well as expensive infrastructure to constrain animals simultaneously if reasonable numbers of animals are to be counted in a single day. The presence of ticks is also highly seasonal and, hence, animals need to be mustered at times when variation in tick numbers occurs across animals in a cohort group if valid tick counts are to be achieved using natural tick infestations. Alternately, artificial tick infestations can be used, but they require tick-breeding facilities and skilled laboratory technicians to deliver specific quantities of tick larvae for on-farm infestations. In regions where multiple tick species infest cattle, use of artificial infestations are also unlikely to be representative of tick loads under natural infestations.
In an attempt to simplify the tick-counting process, a system of single or repeated visual scores of the number of engorging ticks on one side of the animal following field infestations was implemented (Prayaga et al. 2009). Scores ranging from 0 (low) to 5 (high) were used. Tick scores do not require the same level of infrastructure as tick counts and, consequently, the rate of throughput of animals per day can be increased. However, the heritability of tick score is considerably lower than the heritability of tick count (Burrow 2014) and tick scores are subject to most of the other constraints that apply to tick counts. Hence, they are also very difficult to implement under research, commercial and smallholder production systems. A simpler, more cost-effective method of identifying individual animal variation in resistance to ticks (phenotyping) under research, commercial and smallholder production systems is urgently required to enable genetic and management improvements in host resistance to ticks.
Desirable features of a new tick-resistance phenotype
Desirable aspects of any new tick-resistance phenotype were foremost among considerations of this review. Those desirable features include the need for the new phenotype(s) to be
-
moderately to highly heritable and strongly correlated with the current ‘gold standard’ tick-count phenotype, to enable ongoing application of previous research results based on tick counts, rather than having to repeat much of that previous research; to enable assessment of heritabilities and genetic correlations, large numbers of animals will need to be simultaneously recorded in well designed contemporary groups for both tick count and the new phenotype(s), and ideally also for other economically important traits where feasible;
-
cost-effective for use in extensive pastoral production systems, and ideally without the need to muster animals on repeated occasions to derive the phenotype;
-
capable of being reliably scheduled at a nominated point in time that suits management requirements, rather than at times when animals are known to have at least moderate levels of tick infestation, as is the current requirement;
-
identifiable on the day of measurement, to enable management decisions while animals are still in hand (e.g. to draft off susceptible animals for ongoing closer management and culling from the breeding herd); and
-
able to accommodate non-linear relationships (e.g. threshold responses that might trigger differential management decisions).
Applying potential new phenotype(s) through genomic selection
Recent reviews of the literature indicate that resistance of beef and dairy cattle to ticks is moderately to highly heritable, providing good opportunities to directly improve resistance of cattle to ticks through crossbreeding and within-breed selection (Burrow 2014; Burrow and Henshall 2014). On the basis of several independent studies in tropically adapted cattle in northern Australia, it was also concluded that selection to improve resistance to any one stressor of tropical environments will improve resistance to other stressors. That conclusion was particularly true for resistance to ticks, worms and heat stress, where genetic correlations were consistently moderately positive, suggesting that the same or closely linked genes affect all three traits (Burrow 2012).
The existence of favourable genetic correlations among adaptive traits does not extend to genetic correlations between adaptive and productive traits. Except for heat stress measured by rectal temperatures under conditions of high ambient temperatures, resistance to most environmental stressors appears to be largely genetically independent of productive traits such as growth, reproduction and product quality, albeit the conclusions are based on only a small number of Australian studies (Burrow 2014). It can, therefore, be concluded that, in cattle well adapted to tropical and subtropical environments, there are no major strongly antagonistic correlations among the traits that would preclude simultaneous genetic improvement in all the traits in tropical cattle-breeding objectives.
Gibson and Bishop (2005) suggested that adaptive traits are ideal candidate traits for use of genomic information due to the difficulty and expense of collecting the essential phenotypes needed for conventional phenotype-based selection. Those authors predicted that the use of genomic information based on single-nucleotide polymorphisms (SNPs) or quantitative trait loci would be most beneficial for traits of low heritability or traits that are difficult, expensive or impossible to record routinely. They also suggested that the use of genetic markers could be particularly beneficial in the low- to medium-input systems of the developing world, where disease resistance and adaptation of livestock are critically important for the sustainable livelihoods of poor farmers. Although their predictions about usage remain valid, it has since become clear that it is very unlikely that any individual SNP will account for a significant proportion of the genetic variation for economically important traits, as was hypothesised at that time. Rather, genetic variation for most traits of economic importance is underpinned by a large number of mutations, each of small effect (MacLeod et al. 2016).
Resistance of cattle to ticks appears to follow this genetic architecture, i.e. genome-wide association studies (see e.g. Gasparin et al. 2007; Machado et al. 2010; Porto-Neto et al. 2010, 2011a, 2011b, 2012, 2014; Turner et al. 2010; Cardoso et al. 2015; Mota et al. 2016; Mapholi et al. 2016) have identified few, if any, associations of large effect.
Given this trait architecture, genomic selection (Meuwissen et al. 2001, 2016) is likely to be a more effective way of improving tick resistance. Genomic selection uses a large reference population of animals genotyped with genome-wide SNP markers, and phenotyped for the trait of interest, to derive the effect of each SNP (reflecting the effect of the causal mutations affecting the trait captured through linkage disequilibrium with the SNP). However, it has not been possible to develop genomic-prediction equations for resistance of cattle to ticks, largely due to the ongoing inability to cost-effectively identify individual-animal variation in resistance.
To achieve the levels of accuracy required for traits such as cattle resistance to ticks, given the moderate heritabilities of the trait, very large cattle resource populations that have been accurately recorded for the trait are needed (Goddard and Hayes 2009). Such populations are already being established in several countries where ticks are endemic. If a cost-effective measure of tick resistance can be developed and validated, those resource populations would readily include the new tick-resistance phenotype in their recording programs.
Incorporating tick resistance as a trait in breeding objectives
Once a practical and cost-effective tick phenotype is available for on-farm use and sufficiently large reference populations are developed, tick resistance could be incorporated into the breeding objectives of cattle genetic-improvement programs. In most cases, it could be expected that tick resistance will be an additional economic trait for consideration in already complex breeding objectives (Reis et al. 2017). Since tick counts are, in general, weakly correlated with other economic traits in cattle (Burrow 2001; Biegelmeyer et al. 2017), the emphasis to be given in a selection index to the tick-related criterion will be critical to determine the genetic progress that can be achieved for tick resistance.
Deriving economic values for tick resistance simultaneously with other adaptive and productive traits is the most appropriate way to incorporate this new trait in cattle-selection programs (e.g. Amer et al. 2001). Nonetheless, bio-economic modelling of tick parasitism and epidemiology for specific cattle-production systems and environments is challenging and scarce in the recent livestock literature (Grisi et al. 2014; Mapholi et al. 2014). Recent work from Brazil, using a stochastic model to account for death risk and reduced productivity, has demonstrated a low importance for tick counts in a global breeding objective, with values of 12.9% for Brangus (Simoes 2017) and 3.8% for Hereford and Braford cattle (Costa et al. 2018). However, these values were derived under production systems in subtropical environments where ticks (and, therefore, tick-borne diseases) were controlled without major difficulties by chemical treatments and appropriate management. Therefore, additional research is required to evaluate the economic values of tick resistance under more challenging environments, with larger tick loads and widespread resistance of ticks to chemicals, particularly in taurine herds raised under tropical conditions or where cattle are managed in production systems where ticks cannot be controlled by management options due to expense or logistical difficulties. In systems where ticks cannot be controlled, then economic values and the relative importance of tick resistance in breeding objectives will certainly be much higher.
Reis et al. (2017) proposed different scenarios to include tick resistance as an extra breeding goal for Brazilian Hereford and Braford cattle. They concluded that availability of highly accurate genomic information from multi-trait calibration sets would be desirable to achieve faster genetic progress for complex breeding goals. Moreover, due to the low correlation with other economically important productive traits, they suggested that if the goal is to breed specific lines of tick-resistant cattle, assigning a relative importance of 50% for counts in the selection index would be a suitable alternative. This would assure the necessary genetic progress for resistance to ticks (more than 0.5 genetic s.d. per generation) in the target population, while retaining the necessary balance to ensure genetic progress in other economically relevant traits. This could become a strategy to create market differentiation and advantage for taurine cattle breeds or composites selected under tropical and subtropical conditions. Genomic selection could accelerate development of such specialised lines. However, such a high weight on tick resistance would greatly compromise genetic gain for other traits such as meat quality. An alternative use of the genomic information would be to develop new composite breeds that stack tick-resistance chromosome segments together with meat quality- and reproduction-improving segments, so as to maximise performance of the resulting animals.
Tick species that infest cattle
Ticks are small to medium-sized blood-sucking arachnids, with ~900 tick species occurring worldwide, although most have a regional distribution (Guglielmone et al. 2010). Tick species affecting cattle are ‘hard ticks’ (Ixodids, see for example Horak et al. 2009) and are found in tropical and subtropical regions of the world. They vary across regions, but include subspecies of Rhipicephalus (previously known as Boophilus), Amblyomma and Hyalomma. Cattle in Asia, Australia and Central and South America are affected by members of the Asian tick Rhipicephalus microplus complex, whereas cattle across Africa are affected by species from all three genera. Infestation of cattle by these species has a direct impact on production, such as, for example, growth and milk production, efficiency of feed utilisation, reproductive performance (Seebeck et al. 1971; O’Kelly and Kennedy 1981; O’Kelly et al. 1988) as well as affecting indirectly through transmission of tick-borne diseases (Lehmann 1993). Additional hide and udder damage resulting from tick bites reduces or negates the sale of cattle hides and reduces milking ability in the affected cattle.
Of major concern across Africa is the recent partial or complete displacement of some indigenous tick species with the more aggressive Asian tick (R. microplus), which was introduced with the importation of cattle to Africa, and which has proven to be a very efficient vector of tick-borne diseases, particularly of Babesia bovis. Displacement of species has been reported from Ivory Coast and Cameroon (Madder et al. 2011; Mamoudou et al. 2016; Boka et al. 2017), South and southern Africa (Tønnesen et al. 2004; Nyangiwe et al. 2011, 2013) and Tanzania and eastern Africa more generally (Lynen et al. 2008; Horak et al. 2009). In addition to the displacement of some tick species, the Tanzanian data, in particular, indicate that the advance of R. microplus and the retreat of R. decoloratus are associated with the 58-mm isohyet and the 22–23°C isotherm and suggest a higher-temperature tolerance for R. microplus (Lynen et al. 2008). Those authors anticipated that climate change is likely to increase the spread of R. microplus, and, consequently, Babesia bovis, into new areas of Africa.
In Australia, the Australian cattle tick, Rhipicephalus australis, formerly known as R. microplus, has been determined to be a distinct species and can be distinguished from R. microplus on the basis of morphological, genetic and mating criteria (Labruna et al. 2009; Estrada-Peña et al. 2012). The R. microplus complex now consists of at least three genotypes or species (Clades A–C) based on molecular systematics, named R. microplus, including Rhipicephalus annulatus and R. australis (Burger et al. 2014; Low et al. 2015; Roy et al. 2018). It is not yet known how their biological differences may affect the selection of tick resistance in cattle, but any genetic-improvement program will need to consider this.
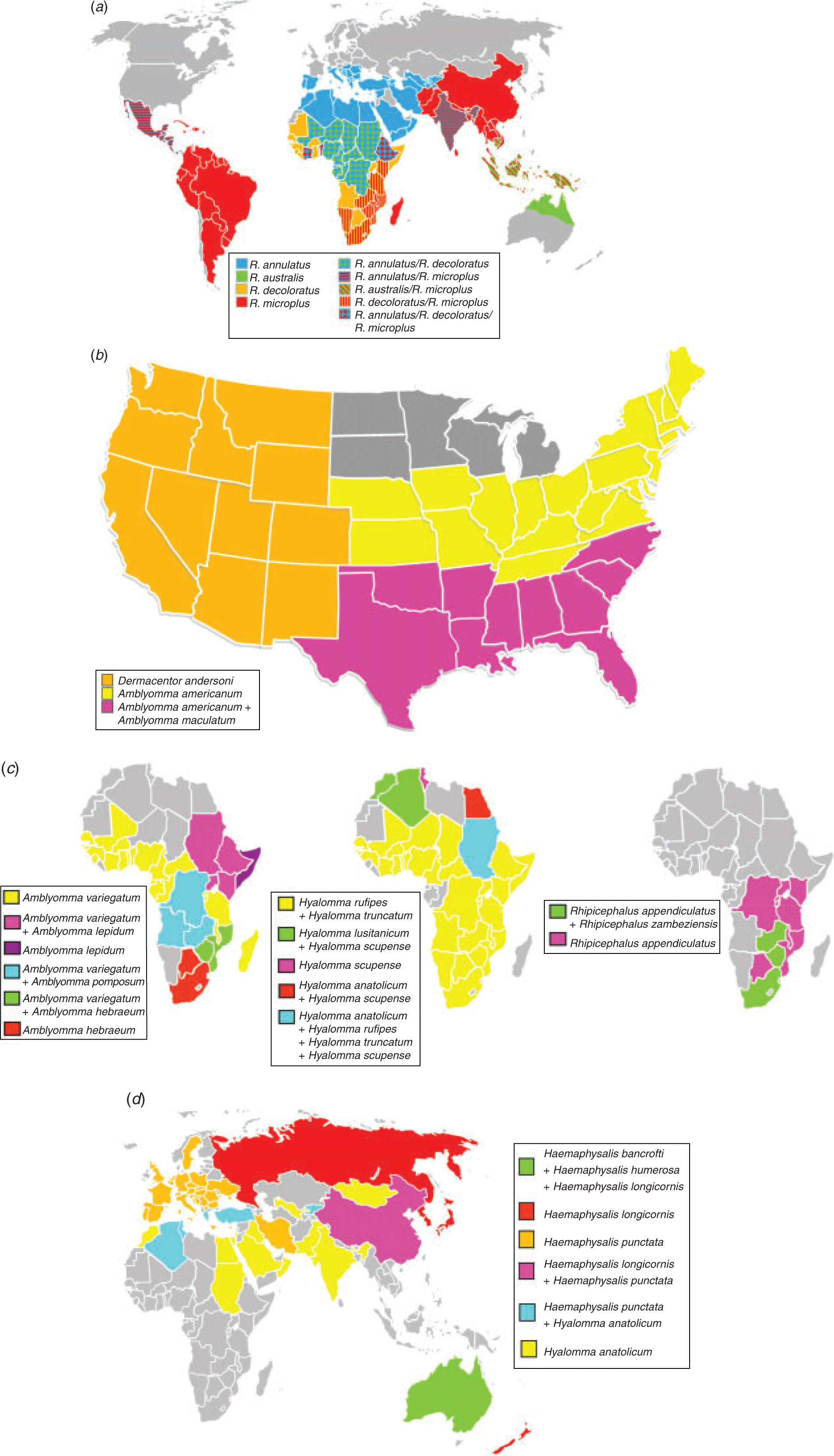
As indicated above, the tick of major concern for cattle in regions excluding Africa is R. microplus. However, several other ticks in these regions also affect cattle. In the United States, the main tick species of concern to cattle include Amblyomma americanum, which causes hide damage ‘tick worry’ (Barnard et al. 1992), while Amblyomma maculatum causes a condition known as ‘gotch ear’ (Edwards 2011) and has a potential role in the spread of heartwater. Both species occur in the south-eastern states (Sonenshine 2018; Raghavan et al. 2019). Dermacentor andersoni (western Canada and United States) can cause tick paralysis in cattle (James et al. 2006; Lysyk et al. 2009). Recently, Haemaphysalis longicornis, the vector for the virulent Ikeda strain of Theileria buffeli/orientalis, has been introduced into the United States, but has not yet been established endemically (Beard et al. 2018). Both Rhipicephalus annulatus and R. microplus remain serious problems in Mexico as well as southern Texas (Lohmeyer et al. 2011). Rhipicephalus annulatus occurs endemically in the Mediterranean, and middle-eastern, central, northern and West Africa (Walker et al. 2003). Both tick species can transmit Anaplasma marginale (the causative agent for bovine anaplasmosis), B. bovis (the causative agent for Asiatic red water) and Babesia bigemina (the causative agent for African red water; Walker et al. 2003). In Central and South America, in addition to R. microplus, Amblyomma sculptum, A. tonelliae and A. triste are the main ticks that affect cattle (Nava 2017). However, given recent changes in the taxonomic status of various Amblyomma species in the Neotropics, their geographic distributions need to be reassessed (Nava et al. 2014; Lado et al. 2018). For example, it has been proposed that A. maculatum and A. triste should be synonymised (Lado et al. 2018), while A. sculptum and A. tonelliae have only recently been reinstated or described as new species (Nava et al. 2014). Their economic importance lies in the damage to hides and general impact on host productivity.
In Australia and New Zealand, the introduction of H. longicornis from Asia has introduced pathogenic genotypes of Theileria buffeli/orientalis, notably Ikeda (Izzo et al. 2010; Kamau et al. 2011; McFadden et al. 2011). This introduction has been responsible for oriental theileriosis outbreaks along the eastern coast of Australia and the North Island of New Zealand and has recently spread to the South Island as well (Kamau et al. 2011; McFadden et al. 2011, 2016). Ticks that are endemic to Australia and are vectors of the more benign genotypes of T. buffeli/orientalis (e.g. buffeli, chitose and Type C) include Haemaphysalis bancrofti and Haemaphysalis humerosa (Barker and Walker 2014). In Asia, H. longicornis is the main vector of the Ikeda strain of T. buffeli/orientalis and occurs in Japan, Korea, and parts of China and Russia (Barker and Walker 2014). The Ikeda strain has been imported into Pakistan and Vietnam from Australia, but it remains to be seen whether an appropriate tick vector is present (Gebrekidan et al. 2017a, 2017b).
In Africa, in addition to R. microplus, a large number of tick species are of economic and veterinary importance for cattle. Members of the genus Amblyomma mainly transmit Ehrlichia ruminantium, the causative agent of heartwater and include A. hebraeum (Botswana, southern Mozambique, South Africa, southern Zimbabwe, Swaziland), A. lepidum (central and eastern Sudan, Ethiopia, southern Somalia, eastern Uganda, Kenya and the northern region of central Tanzania), A. pomposum (Angola, western Zambia and southern Democratic Republic of Congo) and A. variegatum (central, eastern, southern Africa extralimital to South Africa and West Africa; Walker and Olwage 1987; Walker et al. 2003). These species also transmit Theileria mutans and T. velifera, causative agents of benign bovine theileriosis, while A. variegatum is a vector of Ehrlichia bovis that causes bovine ehrlichiosis. Haemaphysalis punctata transmits Theileria buffeli, the cause of bovine theileriosis and occurs mainly in Europe, the northern Mediterranean, eastward into central Asia, but also extends into northern Africa (Walker et al. 2003). Hyalomma anatolicum transmits Theileria annulata as well as Trypanosoma theileri, the causative agents for tropical theileriosis and benign bovine trypanosomiasis in cattle respectively. It is widespread from northern Africa, the Mediterranean, the Middle East, India, Iran, China and southern Russia. Hyalomma scupense (=H. detritum) vectors Theileria annulata and occurs in the Mediterranean parts of northern Africa as well as northern-central Sudan. Hyalomma rufipes is the vector for Anaplasma marginale and occurs in most parts of Africa and extends into southern Europe and eastward to central Asia. Hyalomma lusitanicum also vectors T. annulata and is found in Mediterranean regions of Algeria and Morocco. Hyalomma truncatum occurs in Africa south of the Sahara and causes toxicoses known as sweating sickness in cattle (Walker et al. 2003). Rhipicephalus annulatus occurs in central, northern and West Africa and south-eastern Sudan. Rhipicephalus (Boophilus) decoloratus transmits Babesia bigemina, Anaplasma marginale and Borrelia theileri, the cause of spirochaetosis in cattle. It occurs in most regions south of the Sahara (Walker et al. 2003). Rhipicephalus appendiculatus and R. zambeziensis are the vectors for Theileria parva, the causative agent for Corridor disease, East Coast fever and Zimbabwean theileriosis (January disease), occurring from southern to central Africa (Walker et al. 2000). East Coast fever is considered to have the largest economic impact on cattle in Africa, with an estimated 1 000 000 deaths per annum (Nene et al. 2016). Corridor disease is found wherever African buffalo (Syncerus caffer), R. appendiculatus, and cattle co-occur. The only exception to this is in South Africa where large Corridor disease-free African buffalo herds are kept within the endemic region for R. appendiculatus, due to strict disease and movement management by the Department of Agriculture, Forestry and Fisheries of South Africa (Pienaar et al. 2011; Laubscher and Hoffman 2012).
Figure 1a shows the worldwide distribution of the economically important members of the Boophilus subgenus, indicating the endemic nature of the tick problem globally. Additional distributions for the major economic ticks are also provided in Fig. 1b–d. Given the large number of tick species that can transmit the same pathogens, selection for tick resistance across species boundaries should be an important consideration to prevent replacement of one tick species with another.
|
Factors affecting cattle tick resistance
Breed effects
Cattle evolved into two distinct geographic groupings ~610 000–850 000 years ago (MacHugh et al. 1997). Bos taurus breeds are adapted mostly to temperate environments in Europe and the Near East, and include British and European breeds most suited for milk (e.g. Holstein–Friesian, Jersey) or beef (e.g. Angus, Hereford, Charolais) production. Zebu or Bos indicus breeds evolved in more tropical environments in southern Asia (MacHugh et al. 1997) and include breeds that have evolved for specialist milk (e.g. Sahiwal, Red Sindhi) and beef (e.g. Brahman, Nellore) production. A third distinct grouping evolved more recently in tropical environments, and those breeds are now commonly referred to as tropically adapted taurine breeds. These are true Bos taurus (Frisch et al. 1997; Hanotte et al. 2003; Gibbs et al. 2009) that retain some of the productive attributes of Bos taurus, but they are better adapted to tropical environments than is European Bos taurus. They include the southern African Sanga breeds (e.g. Afrikaner, N’guni, Tuli), West African humpless breeds (e.g. N’dama), and Criollo breeds of Latin America and the Caribbean (e.g. Romosinuano). Historical reports describing these breeds suggested that they were admixtures of Bos indicus and Bos taurus. However, on the basis of recently available molecular-genetic tools, it is now widely accepted these breeds are true Bos taurus, although some degree of admixture of the breed types may have occurred over recent decades.
Numerous historical and more recent studies have indicated that large differences exist between beef- and dairy-cattle breeds in resistance to a wide range of tropical environmental stressors and factors that affect animal performance, including resistance to ticks. As indicated by Hewetson (1972), it has long been recognised that some breeds of cattle are more resistant to infestations with ticks than are others (e.g. Lush 1927; Zawadowsky 1931; Kelley 1932; Bonsma 1940; as cited by Hewetson 1972). These and more recent reports (e.g. Seifert 1971; Hewetson 1972; Utech et al. 1978; Frisch 1981, 1987; Utech and Wharton 1982; Madalena et al. 1985, 1990; Sahibi et al. 1997; Frisch and O’Neill 1998; Mwangi et al. 1998; Wambura et al. 1998; Frisch et al. 2000; Berman 2011; Ibelli et al. 2012) from Australia, Brazil and Africa have indicated that Bos indicus breeds have a greater resistance to ticks than do European Bos taurus breeds, with the tropically adapted taurine breeds being more resistant than European breeds, but not as resistant as Bos indicus (e.g. Spickett et al. 1989; Scholtz et al. 1991; Latif 2006; Muchenje et al. 2008).
These and many other studies have shown that, in temperate environments, there are substantial differences in growth, milking ability, reproduction and product quality among these different cattle breeds. However, in cattle grazed at pasture in tropical environments, the differences in performance are generally masked by the effects of environmental stressors on those productive attributes. This led to recommendations that, for most purposes in the tropics, comparisons of performance should be made across general breed types or groupings (Bos taurus, British and European; Bos indicus; and tropically adapted taurine) rather than across specific breeds (Burrow et al. 2001). These results also allowed development of a table of comparative rankings of the different breed types for different productive and adaptive attributes in both temperate and tropical environments (see Burrow 2012). It was concluded that any breeding program designed for cattle grazed at pasture in tropical environments must consider the impacts of both productive and adaptive attributes, even though adaptive traits (and some productive traits) are generally very difficult and expensive to measure (Burrow 2012).
Other effects
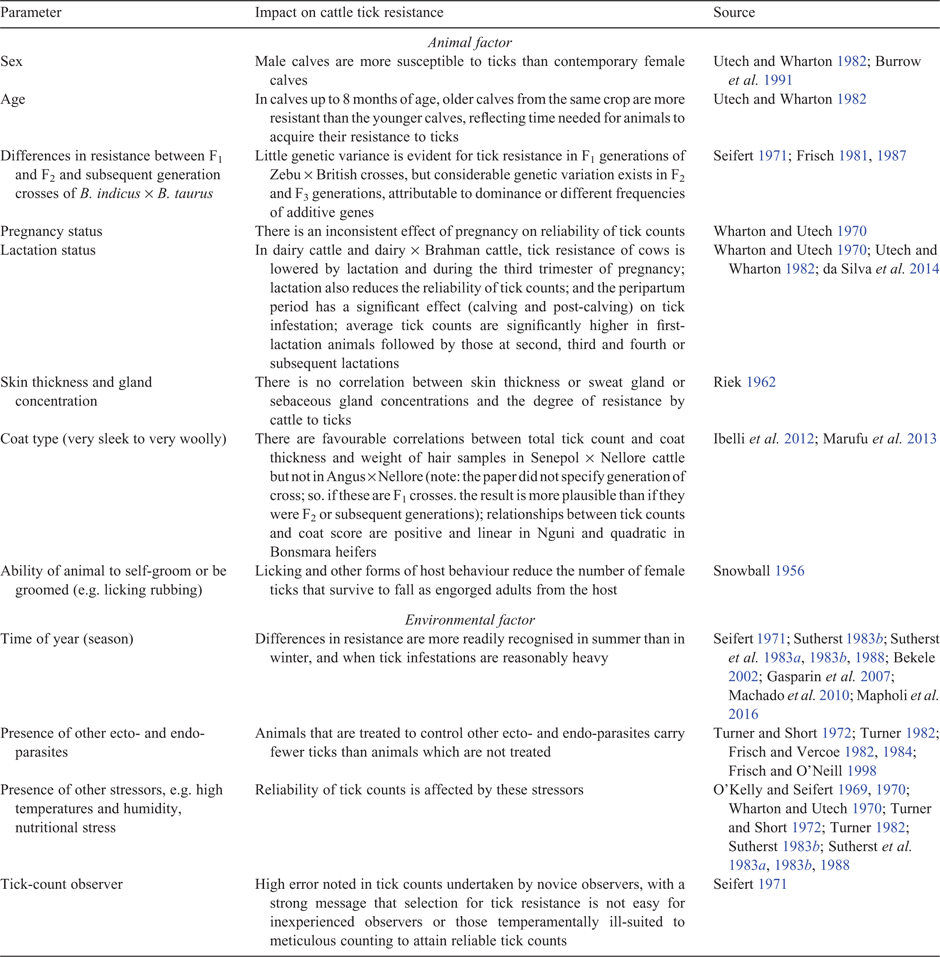
There are several additional animal and environmental factors that affect resistance of cattle to ticks, and, hence, must be considered in the design of cattle-resource population(s) established to measure cattle tick resistance. Table 1 briefly summarises those factors.
|
Cattle resistance to ticks
Innate or acquired resistance and is it the same in different breeds?
It is important to understand whether resistance of cattle to ticks is innate or acquired resistance. If it is an acquired resistance, then the timing of any assessment of an animal’s resistance will be critically important (i.e. it should occur only after all animals have experienced repeated exposures to ticks, so that the assessment is undertaken after the animals have acquired their resistance).
Legg (1930) was the first person known to report that aspects of tick resistance indicated that all cattle show considerable resistance to ticks in the sense that only a relatively small percentage of larval ticks ever reach maturity. However, most studies have indicated that all animals are susceptible to tick infestations at their first challenge and, subsequently, develop different degrees of resistance. Later studies (e.g. Hewetson 1972; Sutherst 1983a) have indicated that resistance of cattle to ticks is acquired rather than innate, with all cattle being susceptible at their first (and early) exposures to ticks. Hewetson (1972) showed that Bos indicus Sahiwal cattle were as susceptible to ticks as were crossbred Sahiwal × Jersey cattle at the time of their first infestation, but, over the following three infestations, the Sahiwals became more resistant. This was reflected in heritability estimates, with the heritability of tick resistance being zero at the first three infestations, and the estimates increasing to 0.28 and 0.42 at the fourth and fifth infestations respectively.
The only study known to suggest that cattle tick resistance could be an innate resistance is that of Riek (1962). In that study, the resistance of Bos taurus and Bos indicus and of their crosses to tick infestation was assessed by repeated experimental infestation. Two types of resistance were observed, namely, an acquired resistance that became evident after repeated exposure and an innate resistance that was present in some animals, never previously exposed, on their first infestation (Riek 1962). Acquired resistance was least apparent in purebred Bos taurus, but considerable variation in the degree of resistance was observed among individuals within the respective breed groups. Innate resistance was observed in some Bos indicus. The mechanism appeared to persist in subsequent exposures, but its significance was difficult to assess (Riek 1962).
On the basis of these findings, it should be assumed that cattle tick resistance is acquired for all cattle-breed types (beef and dairy cattle and across Bos taurus and Bos indicus breeds), with allowance needing to be made for animals to acquire resistance before assessments of tick resistance are undertaken. On the basis of practical research experience, cattle that are reared on pasture in tick-endemic areas can be assumed to have acquired their resistance to ticks by the time they are weaned at 6–9 months of age. In production systems where weaning is routinely practiced, weaning tends to occur around the start of the tropical ‘dry season’, to ensure that cows are not lactating during periods of nutritional stress. The start of the ‘dry season’ also tends to coincide with the start of cooler months when tick infestations are lower and less reliable, on the basis of studies summarised in Table 1. Hence, it is recommended that assessments of cattle tick resistance be undertaken from the commencement of warmer periods in post-weaning cattle (e.g. in the southern hemisphere that would equate to calf weaning from May to July and tick-resistance assessments undertaken from August or September, when the calves are 9–12 months of age).
Is cattle tick resistance the same for different tick species?
An additional question that needs consideration is whether cattle tick resistance is similar for the different tick species. Only the studies of Wagland et al. (1985), de Castro et al. (1989) and Miranpuri (1989) are known to have examined this question.
Wagland et al. (1985) exposed cattle simultaneously to both Haemaphysalis longicornis (bush tick) and Boophilus microplus (cattle tick). Cattle that had never been exposed to either tick species were equally susceptible to both species. Cattle with acquired resistance to both species ranked consistently for levels of resistance to each species when infested separately. Concurrent infestation with H. longicornis had no effect on ranking for resistance to B. microplus. The coefficient of concordance between the rankings of individuals on their levels of resistance to both species of tick was positive but not statistically significant. The authors concluded the tick antigens stimulating host resistance were species-specific and, therefore, did not provide cross-protection, an important consideration in the development of anti-tick vaccines. However, the favourable correlation in rankings of individual animals for resistance to the two species suggested that co-selection for resistance to different tick species was achievable (Wagland et al. 1985).
de Castro et al. (1989) co-infested R. appendiculatus-resistant cattle with R. appendiculatus and R. pulchellus, limiting each tick species to an ear. Intense pruritis, grooming and acute inflammation were observed in the ears infested with R. appendiculatus, but no reaction was observed in the opposite ears infested with R. pulchellus. More R. pulchellus than R. appendiculatus nymphs were obtained from resistant animals, although more nymphs of both species were obtained from susceptible cattle than from resistant cattle. However, the mean engorged weights of R. pulchellus nymphs obtained from resistant and susceptible cattle were not significantly different. The authors concluded that different antigens may be involved in development of resistance to these tick species.
Miranpuri (1989) investigated the effect of repeated pure infestations with Boophilus microplus on susceptibility to subsequent pure infestations with Hyalomma anatolicum, and the effects of infestations with both tick species on susceptibility to a series of mixed infestations. Results showed that cattle acquired resistance to both tick species after repeated pure infestations, but animals with acquired resistance were as susceptible to the other tick species as were animals that had never been exposed to ticks of either species. After repeated pure infestations with both tick species, cattle responded to five mixed infestations, showing a high degree of resistance to B. microplus and low resistance to H. a. anatolicum (mean yield for B. microplus was 10 ± 8.1 ticks per host after the first mixed exposure and declined to 1.3 ± 1.7 after the fifth, whereas the mean yield for H. a. anatolicum was 71.4 ± 11.3 ticks per host following the first exposure and declined to 37.3 ± 7.8 after the fifth). The author concluded that host responses elicited to one species did not provide cross-resistance to the second species used in that study.
On the basis of these studies, it is clear that further studies are needed to determine whether co-selection for resistance to different tick species is feasible. Hence, any studies aimed at development of a more cost-effective phenotype for cattle tick resistance should be undertaken in a region where cattle are infested by multiple tick species. In addition, assessments of host tick resistance should take account of the number of ticks of each species infesting individual animals.
Biological mechanisms of cattle tick resistance
Clinical signs of cattle tick resistance
Resistance of cattle to ticks depends on the animal’s capacity to acquire immunity in response to tick exposure, but how the immunological mechanism affects tick development and mortality is not well defined. Johnston and Bancroft (1918, cited by Hewetson 1972) suggested the following criteria to assess resistance of cattle to ticks:
-
ticks failing to complete their life-cycles;
-
cattle that have light tick infestations relative to other animals that are heavily infested;
-
female ticks failing to engorge in numbers similar to those on susceptible animals under the same conditions; and
-
engorged ticks failing to lay a normal number of eggs or to lay eggs with a normal fertility.
-
Hewetson (1972) added the following two additional criteria:
-
an increase in the time taken for female ticks to complete their parasitic life cycles; and
-
a decrease in the mean weight of replete female ticks.
Other clinical signs of tick resistance that could be useful in defining a new phenotype include:
-
anaemia affecting productive and reproductive traits (Lehmann 1993), with the removal of blood by the ticks (0.3 mL/tick; Seifert et al. 1968) accounting for depletion of some blood constituents (e.g. albumin, haemoglobin and cholesterol);
-
depressed appetite and reduced dietary-nitrogen utilisation, reducing growth rates, milk production and reproductive performance (Seebeck et al. 1971; O’Kelly and Kennedy 1981; O’Kelly et al. 1988); and
-
transmission of diseases through tick saliva, which in turn suppresses immune function and results in cattle mortality and morbidity (Lehmann 1993).
Of these, Criterion 2 is the basis of the current ‘gold standard’ for assessing cattle tick resistance. The remaining criteria have until now been regarded as being either too difficult to measure or too poorly correlated with tick counts to warrant consideration as a phenotype for tick resistance. However, as discussed further later in this review, new protocols for assessing anaemia could contribute to a new and cost-effective tick-resistance phenotype.
Immunological mechanisms
Early studies from Belmont Research Station in north-eastern Australia on the mechanisms of acquired tick resistance in cattle have provided a large number of indicators of immunological causes. Tick-susceptible animals have decreased protein and dry-matter digestibility and increased nitrogen loss in urine. Infestation also depresses blood concentrations of albumin, haemoglobin, cholesterol and the enzymes alkaline phosphatase, lactate dehydrogenase and amylase (O’Kelly and Seifert 1969, 1970; O’Kelly et al. 1971). Other changes suggesting the immune system is implicated include increases in serum globulin, lymphocytes and eosinophils and a decrease in neutrophils (O’Kelly et al. 1971). Tick infestation also causes loss of proteins, which is not entirely explained by blood loss (Springell et al. 1971). The failure of the host to replenish depleted albumin and haemoglobin suggests a probable effect of tick toxin on protein metabolism. In addition, amylase type was moderately associated with tick resistance (Ashton et al. 1968). A further finding is that tick resistance is also correlated with lipid constituents of blood (O’Kelly 1968).
Subsequently, Wikel (1996, p. 1) undertook a detailed review of host immunity to ticks and developed the following conclusive summary:
‘… the tick–host–pathogen interface is characterised by complex immunological interactions. Tick feeding induces host immune regulatory and effector pathways involving antibodies, complement, antigen-presenting cells, T-lymphocytes and other bioactive molecules. Acquired resistance impairs tick engorgement, ova production and viability. Tick countermeasures to host defences reduce T-lymphocyte proliferation, elaboration of the TH1 cytokines interleukein-1 and interferon-y, production of macrophage cytokines interleukin-1 and tumour necrosis factor and antibody responses. The dynamic balance between acquired resistance and tick modulation of host immunity affects engorgement and pathogen transmission.’
With this wide array of effects, it is understandable why development of a simple, cost-effective phenotype for tick resistance still remains to be achieved.
However, many researchers have since investigated different aspects of the immunological function associated with cattle tick resistance, using different approaches as summarised briefly below. Their results have provided greater insight into specific aspects of immune competence, which might be targeted when developing a new phenotype for cattle tick resistance.
Stear et al. (1989) assessed two consecutive crops of 75% Brahman × 25% Shorthorn calves for resistance to Boophilus microplus in Australia in May, July and October 1983. Although the level of resistance to artificial tick infestation varied considerably among seasons, the animals maintained very similar rankings for resistance in all three seasons. The cattle were typed for 30 bovine Class-1 lymphocyte antigens, with antigens W6 and CA31 found to be associated with susceptibility to tick infestation. However, none of the other lymphocyte antigens showed strong associations with either resistance or susceptibility. The authors believed that W6 was unlikely to be useful as an indicator of susceptibility on its own because there could be other unidentified factors that play a role in determining whether or not W6 exerts a significant effect on tick resistance. The other significant antigen, CA31, is a subgroup of W6, i.e. all cattle with CA31 also possess W6. Hence, the association of W6 with tick susceptibility may be due to the presence of CA31 in some W6-positive cattle.
In a follow-up study, Stear et al. (1990) used natural infestations of the cattle tick and levels of buffalo fly and faecal-nematode egg concentrations to assess resistance in tropically adapted male and female taurine animals in the post-weaning period, when the animals were between 9 and 19 months of age. In addition, the male animals were artificially challenged with B. microplus tick larvae on two separate occasions. Cattle with bovine major histocompatibility (BoLA) antigens W6.1 and W7 had significantly fewer ticks than did cattle lacking those antigens. Cattle with BoLA antigens W7 and CA36 had lower concentrations of nematode eggs in their faeces than did cattle lacking these BoLA antigens. The authors concluded that BoLA is one of the genetic systems that influences resistance to B. microplus, although it was premature to recommend a role for BoLA in selective breeding to increase tick resistance.
Studies based on gene expression and other methods provide additional supporting evidence of an immunological basis to tick resistance in cattle, although it is similarly premature to recommend evidence from those studies as the basis of a phenotype for the trait. Results from these studies are summarised in Table 2.

|
Skin hypersensitivity reactions
Acquired tick resistance by cattle is associated with development of a hypersensitivity response to the salivary secretion of the tick. It is manifested by serous exudation and is usually accompanied by a popular reaction at the site of attachment of the tick (Riek 1962). Histological changes in the skin following attachment are also evident, although lesions indicative of an allergic reaction are found only in animals with some degree of resistance. Those changes comprise cellular invasion, predominantly eosinophilic, which extends deep into the dermis (Riek 1962). In highly resistant cattle, blood histamine concentration reached a peak 48 h after infestation with larvae, and subsided to normal levels 7–8 days later, providing additional evidence of a hypersensitivity reaction (Riek 1962). In susceptible cattle, there was little or no variation in histamine concentration during the parasitic life cycle of the tick. In animals with varying degrees of resistance, temporary increases occurred following the larval and nymphal moults (Riek 1962).
The total local concentration of histamine available in the skin is related to resistance, with higher concentrations linked to higher resistance, with detachment of R. microplus larvae precipitated by histamine release (Willadsen et al. 1979; Kemp and Bourne 1980). The possibility of using local histamine concentrations in the skin as possible criteria for selection of resistance should be considered, given that biosensors for biogenic amines are becoming feasible (Rong et al. 2017).
A recent updated review considering bovine immune factors that underlie tick resistance concluded that the single most important mediator of resistance remains histamine and its associated cell types and molecules (Robbertse et al. 2017). It is, therefore, of interest that at least some tick species secrete abundant histamine-binding proteins in their saliva during feeding, and these act as scavengers to inhibit inflammation (Paesen et al. 1999; Mans 2005). Ticks with experimentally confirmed histamine-binding activity, histamine-binding proteins or histamine countering activity include the hard ticks (Amblyomma americanum, Dermacentor reticulatus, Haemaphysalis spinigera, Hyalomma asiaticum, R. appendiculatus, R. evertsi evertsi and R. sanguineus; Chinery and Ayitey-Smith 1977; Chinery 1981; Neitz et al. 1993; Paesen et al. 1999; Sangamnatdej et al. 2002; Aljamali et al. 2003; Wang et al. 2016) and the soft ticks (Argas monolakensis, Argas reflexus, Ornithodoros savignyi and O. turicata; Mans et al. 2008; Neelakanta et al. 2018). The histamine-binding proteins belong to the lipocalin family and while some family members may not bind histamine per se, they might bind other bioactive molecules involved in the regulation of host defences. Most species probably possess related biogenic amine-binding proteins that scavenge serotonin, an important platelet agonist (Mans et al. 2017).
In some cases, such as for the hard tick Ixodes scapularis, serotonin-binding but not histamine-binding activity is present in adult tick saliva (Mans et al. 2008). In other ticks, histamine-binding proteins have been inferred on the basis of sequence identity, homology or molecular docking experiments (Díaz-Martín et al. 2011; Rodriguez-Valle et al. 2013; Valdés 2014). However, the results of these studies need to be confirmed experimentally, since it has been indicated that sequence similarity or even the presence of a biogenic amine-binding motif does not guarantee histamine binding (Mans et al. 2017). This is especially relevant for ticks such as R. australis (previously R. microplus), where tick rejection has been associated with an increased histamine concentration at the feeding site (Tatchell and Bennett 1969; Willadsen et al. 1979; Kemp and Bourne 1980), suggesting that these ticks do not have significant concentrations of histamine-binding proteins in their saliva. Alternately, histamine-binding proteins may be present in other members of the R. microplus complex, and this may be determined only empirically. For the majority of studies, the presence of histamine-binding lipocalins has not been confirmed in larval, nymphal or adult stages. The possibility, therefore, exists that even for ticks that possess histamine-binding proteins, a single life stage may be susceptible to resistant cattle. Also, field responses to resistant cattle may be complex, given that multiple genes with biogenic amine-binding motifs are present in tick transcriptomes and regulation of salivary genes may vary at individual tick level (Mans et al. 2017). The choice of tick species, the origin of the tick population and the initial diversity of the tick population may all influence the outcome of genetic selection for resistance.
Riek (1962) also provided some evidence that it might be possible to induce the hypersensitive state, with a consequent reduction in tick burden, by repeated daily subcutaneous injections of 0.5 mL of a 1 in 10 dilution of larval extract. This evidence was subsequently used by Bechara et al. (2000) to test the use of unfed larval extract as a measure of tick resistance in a small number of animals. Marufu et al. (2013) subsequently used the method described by Bechara et al. (2000) in larger numbers of animals, as described in more detail in a later section of this review.
Induction of this hypersensitive state is probably related to acquired immunity and may suggest that other antigens could elicit the hypersensitive response. In this regard, transcriptome studies have indicated a high degree of tick salivary-gland complexity with potentially hundreds to thousands of proteins secreted over the course of a feeding event (Mans 2016; Mans et al. 2016). Many of these proteins target central molecules involved in inflammation, blood coagulation and platelet aggregation. Several of them are scavengers of important inflammatory and platelet-aggregation mediators such as thromboxane A2, an important platelet-aggregation agonist that mediates collagen-induced platelet aggregation (Mans and Ribeiro 2008a); leukotriene B4, an important stimulant of neutrophil migration, aggregation and degranulation (Beaufays et al. 2008; Mans and Ribeiro 2008a); or the cysteinyl leukotrienes LTC4, LTD4 and LTE4, that induce oedema (Mans and Ribeiro 2008b). The complement pathway, specifically C3 or C5, is also targeted by ticks (Ribeiro 1987; Valenzuela et al. 2000; Nunn et al. 2005; Daix et al. 2007; Schroeder et al. 2007; Mans and Ribeiro 2008a; Schuijt et al. 2011; Franco et al. 2016; Silva et al. 2016). Several chemokine and cytokine inhibitors have also been characterised in ticks (Hajnická et al. 2001; Vančová et al. 2007, 2010; Frauenschuh et al. 2007; Déruaz et al. 2008; Bonvin et al. 2016; Hayward et al. 2017). Most ticks also possess apyrase that hydrolyses ADP, a platelet-aggregation agonist, and ATP, a pro-inflammatory molecule (Mans 2016). In addition, most tick species possess at least a thrombin inhibitor or possibly even more than one inhibitor of the clotting cascade (Mans et al. 2016). The number of inhibitors are not surprising since inflammation, the complement cascade, blood clotting and platelet aggregation are not independent processes, but comprise an interdependent and highly integrated defence mechanism requiring a multi-targeted attack by the tick (Mans 2016). The ability of ticks to modulate the host’s immune system is, therefore, much more advanced and complex, suggesting that resistance in cattle may be multifactorial.
In addition to this hypersensitivity reaction, Carvalho et al. (2010b) showed that, relative to normal skin, cattle that are genetically susceptible to tick infestations have an increased clotting time of blood collected from the immediate vicinity of haemorrhagic feeding pools in skin infested with different developmental stages of the tick. Conversely, the clotting time of tick-infested skin from genetically resistant cattle was shorter than that of normal skin. Their data indicated that ticks are able to modulate their host’s local haemostatic reactions. In the resistant phenotype, larger numbers of inflammatory cells (eosinophils and basophils) were recruited and the authors speculated that increased tissue factor levels due to elevated eosinophils might result in greater coagulation (Carvalho et al. 2010b). The authors also suggested that resistant hosts may downregulate the expression of anti-haemostatics in tick salivary glands. More recently, the same approach was repeated and similar trends were observed (Franzin et al. 2017; Maruyama et al. 2017). The possibility that resistant hosts may manipulate tick expression patterns is an exciting observation with regard to selection of resistant breeds. This phenotype (downregulation of tick expression) may also be considered as a potential criterion for genetic improvement of tick resistance.
Volatile semiochemicals
Locating of vertebrate hosts by ectoparasitic insects (flies) and acarines (ticks, mites) is mediated by several different stimuli emanating from the host, including visual cues (size, skin colour and pattern), heat, carbon dioxide and volatile organic compounds. The latter, which are known to play a major role in vertebrate–ectoparasite interactions, are detected by olfactory organs located in the peripheral nervous system of the ectoparasites, e.g. insect antennae. Manipulation of these behaviour-modifying chemical signals, otherwise known as semiochemicals, has long been viewed as a target for practical development of new interventions against ectoparasite pests that exhibit nuisance behaviour and can transmit causative agents of infectious diseases (Logan and Birkett 2007).
Gothe (1987) and Sonenshine (2004, 2006) each reviewed the role of semiochemicals in mediating important aspects of tick behaviour. These information-containing compounds include pheromones (used for conspecific communication), allomones (defence secretions) and kairomones (used for host identification and location). Pheromones, the best known and most intensively studied of these semiochemicals, include arrestment (assembly) pheromones, attraction–aggregation–attachment pheromones and sex pheromones. Ticks also produce an allomone that protects against some insect predators. Ticks use kairomones for host identification, including, for example, volatiles such as CO2 and NH3 and various oils such as glandular secretions from the host. Over the past 10–20 years, knowledge of different tick pheromones, allomones and kairomones has been used to develop novel tick-control products by incorporating tick pheromones and small amounts of pesticide to attract and kill ticks on their hosts or in vegetation (see Latha (2012), for a detailed summary of these various uses of semiochemicals). However, rather than using the semiochemicals for the production of anti-tick treatments, our interest is in exploring the possibility of quantifying semiochemical production by hosts, to examine whether those concentrations are related to different levels of host resistance to ticks and, hence, could be used as an alternative phenotype for assessing host tick resistance.
The interest in quantifying host-derived semiochemical production stems from earlier research in disease-transmitting cattle flies and dairy cattle in the Netherlands and Denmark. For example, Holstein–Friesian heifers can be ranked according to fly load, and retain their rankings across years, with some heifers consistently attracting flies, whereas others in the same herd carry only a few flies (Jensen et al. 2004). In addition, that study demonstrated that it was possible to manipulate overall fly loads in herds to increase or decrease fly numbers by removing or adding susceptible or resistant heifers to the different herds, clearly suggesting a genetic basis to resistance or susceptibility to flies. This finding is in line with the low to moderate estimates of heritability for resistance of cattle to buffalo flies (Haematobia irritans exigua) in northern Australia (Burrow 2014), with buffalo flies being similar to the fly species tested in these European studies. Subsequently, Birkett et al. (2004) used data from the Jensen et al. (2004) study to test the hypothesis that natural differential attractiveness for cattle flies in Holstein–Friesian cattle is partly due to differences in volatile semiochemicals emitted by the host. Coupled gas chromatography–electrophysiology (GC–EAG), coupled gas chromatography–mass spectrometry (GC–MS), electrophysiology (EAG), laboratory behaviour and field studies were used. In volatile cattle extracts collected by air entrainment, several active peaks were located by coupled GC–EAG for two different fly species, namely, Musca autumnalis and Haematobia irritans. Further collection and testing of different volatile compounds showed that only some of the compounds were physiologically active across the range of flies tested. In field studies using small herds of heifers ranked on their fly load, individual slow-release formulations of 6-methyl-5-hepten-2-one (MHO), when applied to low and high fly-ranked heifers, reduced fly loads on those individuals. The authors concluded that natural differential attractiveness for cattle flies in Holstein–Friesian cattle was due to enhanced production of MHO as a non-host semiochemical in fly-resistant cattle, and suggested that the phenomenon would be likely to also apply to other vertebrate host species and their associated insect pests. The hypothesis of differential volatile signalling for ectoparasites has also been tested for the interaction between humans and mosquitos and biting midges, with results showing that MHO, together with a second compound (geranylacetone) accounts for reduced attractiveness of less-bitten people (Logan et al. 2008, 2009). The researchers have, subsequently, hypothesised that the differential volatile signalling in vertebrates derives from oxidative biochemical cleavage of steroidal compounds at the skin surface (M. A. Birkett, pers. comm.). Pertinent to the present review, the hypothesis of differential host signalling has, subsequently, been tested in tick populations in resistant Girolando and Nelore (both Bos indicus) and susceptible Holstein (Bos taurus) cattle in Brazil, with the study demonstrating that more resistant animals produce greater amounts of MHO plus other ‘repellent’ molecules (M. A. Birkett, pers. comm.). Similar results have been reported for differential attractiveness of different dog breeds to ticks (Borges et al. 2015; de Oliveira Filho et al. 2016, 2017), with the same hypothesis of differential volatile signalling testing true, albeit the repellents that were identified were different from those in cattle.
While the identification of compounds such as MHO represents the discovery of new repellents for livestock protection against ectoparasites, their use as repellent sprays, such as, for example, pour-ons and eartags, is not sustainable because of technical difficulties with formulations (high volatility causing reduced efficacy). Hence, the long-term aim of this research is to breed animals to produce enhanced concentrations of the volatile compound that confers resistance to flies (M. A. Birkett, pers. comm.). Further development of the approach might enable the genetics for volatile-repellent production to be defined. This may provide an entirely new opportunity to breed animals for volatile-based resistance, either as a new trait or in conjunction with a more cost-effective method of phenotyping ticks that would potentially improve resistance of cattle for ticks and also biting flies.
Possible new phenotypes for cattle tick resistance
As summarised in earlier sections of the present paper, genetic variation in host resistance is reflected in several different aspects of tick development. However, the early literature was unequivocal that the most important measure was the total count of replete female ticks (i.e. engorging Rhipicephalus microplus ticks between 4.5 mm and 8 mm in size). Since total tick counts are affected by a large number of factors (summarised in Table 1), the only reliable comparisons will be those made under contemporary conditions, as suggested in the following practical guide based on infestation with R. microplus (Burrow 1997), noting that different guidelines may be required for other tick species:
-
Cattle should be managed together as a single management group without dipping to control ticks for at least 6 months before tick assessment.
-
Cattle should have had sufficient exposure to ticks to have acquired immunity. On the basis of data from Belmont Research Station in north-eastern Australia, animals need continuous low-level infestations of ticks for a period of ~3 months before tick counts stabilise and animals are considered to have acquired resistance. If tick counts on all animals are very high, it is likely that the animals are still acquiring resistance and if tick counts on all animals are very low, it is likely that there is insufficient tick challenge to measure resistance. As a guide, there should be an average tick count of at least 20 ticks per side of each animal, averaged over at least 15 animals. If the average count is <20 ticks, then the level of infestation is too low to accurately determine tick resistance and an artificial infestation may be necessary. Once resistance to ticks has been acquired, tick resistance is stable across time.
-
Animals to be compared must be of a similar age and sex class, i.e. steers should not be compared with bulls, young cattle should not be compared with older cattle, and lactating cows should not be compared with non-lactating cows. Similarly, tick counts on animals from one paddock should not be compared with tick counts on animals from another paddock, even if the paddocks are adjacent, because environmental factors (e.g. previous stocking rates) are likely to affect the tick populations in the different paddocks.
-
Nutritional stress should be minimised at the time of assessment because animals undergoing nutritional stress often demonstrate an impaired immune system.
As suggested above, development and validation of a new phenotype(s) for tick resistance should initially be undertaken using the cattle-management guidelines above, with half-body tick counts as the standard against which any new phenotype(s) are compared. Options that could be considered as the basis of a new tick-resistance phenotype(s) could include technological approaches to counting ticks (e.g. high-resolution imagery in combination with electronic animal identification and sensor networks), use of clinical signs of cattle susceptibility to ticks (e.g. anaemia and decreased immune function and obvious hide and udder damage), measures of hypersensitive skin reactions, or animal behavioural assessments (e.g. amount of movement with more lethargic animals possibly also being those with greater susceptibility to ticks).
Measures of haematological parameters
The first possible new phenotype considered is development or modification of a method used by Andronicos et al. (2014) to examine relationships between haematological parameters and the ability of sheep to resist infection with the parasitic nematode Haemonchus contortus. In their study, blood samples from individual sheep that had been challenged by worms earlier in their lives were analysed for a standard haematological panel. The blood parameters measured were haematological (including red blood cell count, haematocrit, haemoglobin concentration), as well as immunological (including numbers of eosinophils, basophils, neutrophils and monocytes). Thereafter, a multivariate analytical approach was used to define algorithms on the basis of the combined blood parameters to rank the ability of sheep to resist nematode infection, in a single blood sample. The algorithms were shown to classify susceptible sheep with 100% accuracy and resistant sheep with 80% accuracy. However, no attempt was made to evaluate the resistance or susceptibility of individual animals, as only representative animals from extreme lines were tested. The novelty of this approach was the use of multivariate models of analysis and a machine-learning approach to combine all of the blood parameters relative to the traditional approach of examining each parameter on a case-by-case basis.
Subsequent experiments by this same research group (Bell et al. 2019) applied this multivariate approach to large flocks of outbred sheep (i.e. animals not previously selected for parasite resistance or susceptibility). Initially, blood parameters were measured in animals under an artificial worm challenge, and the data were used to derive a regression model defining the relationship between the level of worm infection and the various blood parameters. This model was then applied to data collected from a second experiment where animals acquired natural worm infections in the field over a period of several months. The ability of the model to predict the worm-infection status of individual animals was assessed by comparing model predictions with actual infection levels at the end of the experimental period. Approximately 65% of the cohort of animals that were most susceptible to worm infection (highest worm-egg counts) were predicted to be susceptible on the basis of the haematology-based model.
The biological basis of the worm resistance test described by Andronicos et al. (2014) is presumed to lie in the following two aspects of the interaction of H. contortus with their sheep host: first, the blood-feeding habit of this worm species that results in anaemia in infected animals; and second, the role of leukocytes in the host response to nematode infection. Eosinophils, mast cells, basophils and neutrophils have been implicated in the immune response to helminths (Anthony et al. 2007; Voehringer 2011; Allen et al. 2015). In general terms, the interaction of ticks with their host animals involves both these aspects seen with blood-feeding helminths, i.e. anaemia and the involvement of leukocyte cell types, including eosinophils, basophils and mast cells in the immune response (Wikel 1996; Brossard and Wikel 1997; Jonsson et al. 2014). This general level of concordance between the two host–parasite interactions, combined with the novelty of multivariate analysis and machine-learning approaches to derive algorithms to relate infection levels to blood parameters, provides the rationale behind our suggestion that the approach of Andronicos et al. (2014) may be applicable to tick-resistance phenotyping.
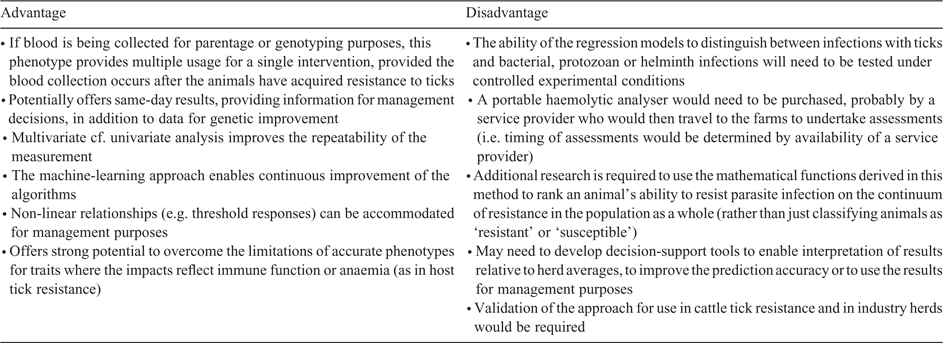
A limitation of this approach is that the use of haematological parameters to predict parasite-resistance status, either specifically for worms or ticks, will need to operate under conditions of co-infection with other parasites or diseases. In some cases, where a phenotypic test for tick resistance may be required, the animals may also be affected by tick-borne disease (bacterial or protozoan) or parasitic worms. Ticks at different life stages will also likely be present. However, it is possible that the different feeding habits of the various parasites (e.g. blood-feeding of ticks versus mucosal grazing of many worm species), as well as the different generalised immune response (Type 1 helper T-cell for protozoa and bacteria vs Type 2 for worms and ticks), will result in different ‘signatures’ of response as defined by a regression model derived from all the red and white blood cell parameters. Testing of this will require comparisons of controlled experiments (tick infections only) with co-infection studies. The usefulness of a haematological tick-phenotype test will depend on the regression model being able to show a degree of specificity in accounting for tick-mediated effects as distinct from the effects of the other potential infections. Advantages and disadvantages of this possible phenotype are summarised in Table 3.
|
Measures of skin hypersensitivity reactions
A second possible new phenotype is based on the skin hypersensitivity reaction described previously. Bechara et al. (2000) used measurements of skin hypersensitivity to correlate host resistance to ticks in 20 Bos indicus and Bos taurus (10 of each breed type) animals. The method was subsequently used by Marufu et al. (2013, 2014) to distinguish levels of tick resistance in larger numbers of Nguni and Bonsmara heifers. The test used by these studies is based on the reaction elicited by intradermal inoculation of 0.1 mL unfed larval extract from the tick in the animal’s ear. Ear thickness is measured using callipers before and after inoculation.
The process to derive the unfed larval extract requires skilled laboratory technicians, with unfed tick larvae (2 months old) from a laboratory colony of ticks killed by immersion in liquid nitrogen, homogenised with a ground glass homogeniser in phosphate buffered saline (PBS, pH 7.4) and sonicated three times for 10 s each and once for 60 s (20 MHz). The extract is then centrifuged at 12 000g (4°C) for 1 h and the supernatant filtered through a 0.22-µm Millex-GV (Millipore) filter and stored at −40°C until use.
Thereafter, animals are given a 0.1-mL intradermal injection (50 µg of protein) of the larval extract in a shaved area on the outer surface of the left ear. An equal volume of PBS, pH 7.4, is inoculated in the contralateral ear to provide a control. Ear thickness is measured in triplicate with the aid of a Mitutoyo® precision instrument just before the injection and at various times post-inoculation. Results are expressed as the percentage of increase in ear thickness compared with the initial measurement. Values obtained from PBS-injected ears are subtracted from those for unfed larval extract-injected ears (Marufu et al. 2013).
In the Bechara et al. (2000) study, Bos taurus calves with acquired tick resistance showed an immediate reaction with maximum response (75% increase in ear thickness) at 10-min post-inoculation. Resistant Bos indicus calves presented an immediate response with maximum reaction (70% increase in ear thickness) between 10 min and 1 h post-inoculation.
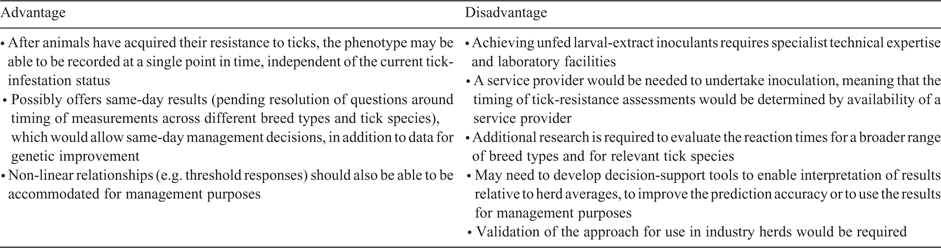
However, Marufu et al. (2013) indicated that Bonsmara cattle showed a more intense immediate reaction and no delayed hypersensitivity reaction to larval extracts of Rhipicephalus ticks, whereas Nguni heifers presented a less intense immediate reaction and a delayed hypersensitivity reaction at 72 h post-inoculation with larval extracts of the same tick species. Reactions to R. decoloratus larval extract produced a more intense skin response at all time intervals in both breeds than did that to R. microplus. These results indicate that additional research is needed to identify the best time of measurement of the hypersensitivity reaction across different breed types and different tick species. In addition, for further development of the hypersensitivity phenotype per sé, consideration could also be given to the possible use of infrared thermography with a portable device (e.g. phone or tablet) and development of an ‘app’ to measure skin responses to inoculation with larval extracts as an alternative to measurements of skin thickness. Advantages and disadvantages of this possible phenotype are summarised in Table 4.
|
Simplified artificial tick infestation (‘tick-bag test’)
As outlined above, artificial tick infestations have been used for several decades to assess cattle resistance to ticks when insufficient variation in tick numbers occurs during natural infestations. Artificial infestations overcome the problem of seasonal variation in tick numbers, but generate their own disadvantages, such as, for example, the need for tick-breeding facilities and skilled laboratory technicians to deliver the tick larvae for use on-farm, the strong likelihood of infesting pastures with ticks, thereby exacerbating the tick problem, and a need to muster animals on at least two occasions to assess animal resistance to ticks.
A simplified test (referred to here as the ‘tick-bag test’) was developed by Heyne et al. (1987) to overcome at least one of those disadvantages, i.e. contamination of pastures with ticks. The test involves the following: (1) restraining the animals in a crush to enable a circular part of their upper back to be shaved for easier attachment of ticks (although for assessments of host resistance to ticks, this may not be ideal as it does not replicate the conditions ticks would encounter when animals are grazing); (2) a calico bag (Fig. 2) is attached to the clean-shaven area using a contact adhesive applied to the outer ring, followed by a 24-h drying period; (3) a specified number of tick larvae (estimated by weight of larvae) are placed into opened plastic vials (to allow the ticks to exit), which are then inserted into the calico bags before the bag is twisted shut and secured with a rubber castration ring; and (4) the bags are monitored twice daily until larvae are ready for counting (e.g. Days 18–21 for the Rhipicephalus spp.) following removal of the bags.

|
The test was developed specifically to facilitate evaluation of the transmission of diseases by ticks and, subsequently, to test efficacy of chemicals to control tick infestations in many different animal species, but with no attempt made as yet to determine its role in evaluating individual host resistance to ticks. If the test is to be useful for phenotyping animals for their resistance to ticks, genetic correlations will need to be estimated between the test- and individual-animal tick counts to establish the test as a reliable indicator of tick resistance. Consideration may also need to be given to the species of tick larvae used in the test and whether the larval composition is representative of natural tick infestations in the region. There are no known published reports indicating the magnitude and direction of either phenotypic or genetic correlations between half- or whole-body tick counts and results from the tick-bag test. Advantages and disadvantages of this possible phenotype are summarised in Table 5.
|
Measures of semiochemicals
As outlined above, determining whether a relationship exists between tick counts and levels of semiochemicals is regarded as a strongly synergistic opportunity to investigate the possibility of developing an entirely novel trait (volatile-based resistance), with potential to improve cattle resistance to several ectoparasites such as ticks and biting flies. While measurement of semiochemicals using current methods does not satisfy the desirable features of a new phenotype for tick resistance suggested earlier, it is recommended that any research program established to evaluate alternative measures of tick resistance in cattle should include measures of the volatile chemistry of those animals. Should the volatile-based resistance phenotype prove to be useful for genetic-improvement programs, then it would also be necessary to identify more practical ways of assessing volatile-based resistance in commercial and smallholder herds. Thus, consideration should also be given, during the design of any research program, to inclusion of potentially simpler measures that might be correlated with volatile-based resistance to evaluate the phenotype, so as to enable those correlations to also be tested.
Other possible phenotypes for tick resistance
There are several additional potential phenotypes for tick resistance that could be considered for development and testing using high-resolution imagery (digital and infrared) based on different data-capture methods (such as e.g. in conjunction with walk-over weighing or similar ‘self-measurement’ technologies, use of drones), in conjunction with mobile data-acquisition systems such as sensor networks. It may also be possible to remotely monitor animal-behaviour patterns to determine whether, for example, the amount of movement or rubbing or grooming behaviours are associated with the degree of tick infestation on individual animals. Recent studies in Brazil investigated the use of low-resolution infrared images to detect ticks in cattle, as well as introducing a new automatic method to analyse the images and count the ticks captured in the images (McManus et al. 2016; Barbedo et al. 2017). However, the authors suggested that the infrared thermographic images have limited potential for detecting ticks in cattle because they enable just a rough estimate for the degree of infestations. However, their algorithm was able to emulate the visual estimates using the infrared thermal images ‘reasonably well’, suggesting that improvements in the image capture should increase the accuracies for automatic counting (Barbedo et al. 2017). In addition to these phenotypes, measurement of skin histamine concentration, or downregulation of tick salivary-gland expression may also be considered in future.
Once phenotype(s) correlated with the ‘gold-standard’ tick counts have been validated, it is expected that those new phenotype(s) will then be incorporated into new ‘-omics’ studies (e.g. functional genomics, gene expression, transcriptomics, proteomics and metabolomics, along the lines of the results summarised in Table 2) to identify more precisely cattle that are either resistant or susceptible to ticks, potentially enabling simpler and more cost-effective diagnostic tests.
Recent advances in genomic sciences are now also providing unique opportunities to survey microbial communities, especially in various living organisms. These relationships (e.g. symbiotic, commensal or parasitic) have evolved to drive important and measurable phenotypes such as disease susceptibility, for example Ruiz-Rodríguez et al. (2009) and Pearce et al. (2017). Microbial communities characterised from the skin, gut, vagina and other parts of humans, animals and birds have shown interesting genetic variations linked to distinct phenotypic outcomes. As the field of livestock microbiome-community study emerges, it will be opportunistic to consider studies of microbiome communities (on the skin and parts of the bodies) to further detect and dissect heritable variations of cattle to resistance to tick infestations. Embarking on metagenomics to support quantitative and qualitative analyses of tick communities on cattle that display differences in tick resistance will be most likely to provide additional options for novel phenotyping methods.
Regardless of the potential of any of these technologies, currently all of these approaches fail with regard to some of the desirable features of a new phenotype outlined previously. While all are potentially capable of being applied under extensive pastoral production systems if the systems can be developed sufficiently well to reliably assess the number of ticks on a particular site(s) of the animal (and potentially distinguish among different tick species if that is identified as being important), none satisfies the requirement of being able to determine the level of acquired host tick resistance when tick infestations are low. Hence, these technologies should continue to be considered, but they are not included in the recommendations for developing and validating new phenotypes in the following section.
Assuming that it is possible to develop and validate new phenotype(s) for host tick resistance, application in beef and dairy cattle-resource populations in tropical and subtropical areas of the world would follow a pathway similar to that shown diagrammatically in Fig. 3.
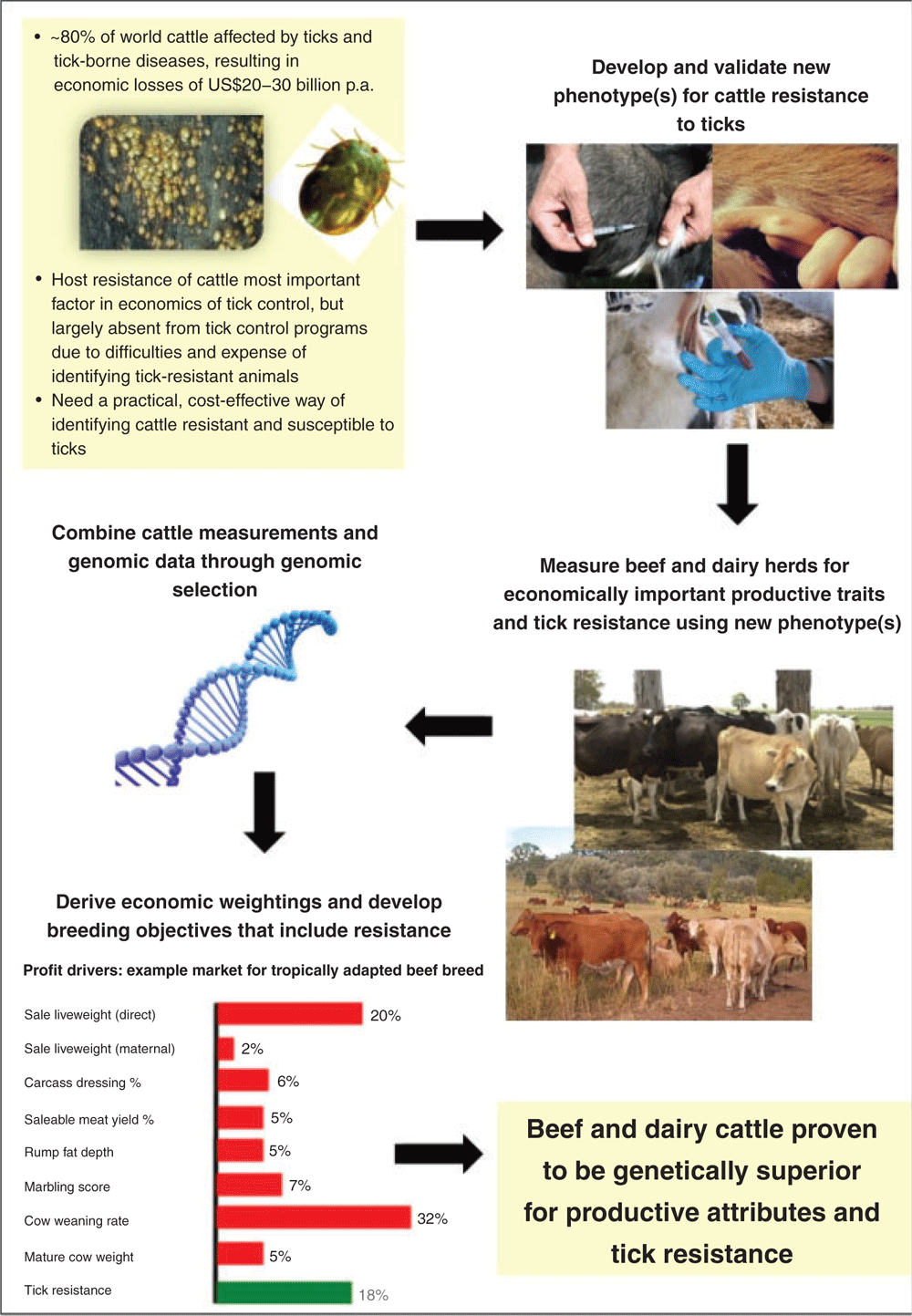
|
Recommendations
On the basis of the present review, it is recommended that three potential new phenotypes (same-day haemolytic analysis; same-day measures of skin hypersensitivity reactions; and artificial infestations using the ‘tick bag’) be investigated and further developed to determine their practical feasibility for consistently, cost-effectively and reliably measuring cattle tick resistance in thousands of individual animals in commercial and smallholder farmer herds in tropical and subtropical areas globally. During evaluation of these potential new phenotypes for tick resistance, additional measurements should be included in any experimental protocol to determine the possibility of developing a volatile-based resistance phenotype to simultaneously improve cattle resistance to both ticks and biting flies. Because the current measurements of volatile chemistry do not satisfy the requirements of a simple, cost-effective phenotype for use in commercial cattle herds, consideration should also be given to inclusion of potentially simpler measures that might enable indirect selection for volatile-based resistance.
We propose that the best method to incorporate all populations, novel phenotypes and gold-standard phenotypes to produce the most accurate genomic predictions for tick resistance is a multi-trait genomic restricted maximum-likelihood approach (G-REML; Maier et al. 2015; Hayes et al. 2017). In this approach, each phenotype and phenotype × population (where populations can even be in different countries, as demonstrated by Hayes et al. 2017) is treated as a separate trait. Genetic (or genomic) correlations are estimated among the traits, and among the populations, provided there is sufficient genomic relationship between them (in human studies, elements of the genomic relationship between pairs of individuals can be as low as 0.025 and still contribute information; Maier et al. 2015). The multiple-trait G-REML approach has the advantage that genomic predictions for all individuals are made on the ‘gold-standard’ trait scale, with the novel phenotypes contributing accuracy to these phenotypes according to the genetic correlations.
The sample size required for accurate genomic predictions (of non-phenotyped individuals) is:
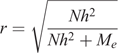
where r is the accuracy of genomic prediction, N is the number of individuals genotyped and phenotyped, h2 is the heritability of the trait, and Me is the number of independent chromosome segments, which is ~2NeL, where Ne is the effective population size and L is the length of the genome in Morgans (Hayes et al. 2009). This equation implies that thousands of animals must be phenotyped and genotyped to enable accurate genomic predictions of tick resistance, given moderate to low h2, and large Ne for tropical cattle populations. Using this formula and an estimated h2 of 0.19, Reis et al. (2017) indicated that sample sizes of 20 870 and 28 939 animals would be required to achieve a 0.90 accuracy for tick-resistance genomic prediction for Hereford and Braford cattle respectively, in Brazil. Using this multiple-trait approach, all available data can be combined across traits and across countries to achieve the required accuracy for reliable predictions.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This review was undertaken to inform an international workshop organised as part of a Global Challenges Research Fund Impact Accelerator Award through the Roslin Institute and the Centre for Tropical Livestock Genetics and Health (CTLGH) at the University of Edinburgh, UK. Sincere thanks are due to the Roslin Institute for partial funding of the research and to staff of the CTLGH and the Agricultural Research Council in South Africa for their assistance with organisation of the international workshop held in South Africa in December 2017. The research was also partially funded by the Bill and Melinda Gates Foundation, with UK aid from the UK Government’s Department for International Development (Grant Agreement OPP1127286) under the auspices of the Centre for Tropical Livestock Genetics and Health (CTLGH), established jointly by the University of Edinburgh, SRUC (Scotland’s Rural College), and the International Livestock Research Institute. The findings and conclusions contained herein are those of the authors and do not necessarily reflect positions or policies of the Bill and Melinda Gates Foundation nor the UK Government.
References
Aljamali MN, Bior AD, Sauer JR, Essenberg RC (2003) RNA interference in ticks: a study using histamine binding protein dsRNA in the female tick Amblyomma americanum. Insect Molecular Biology 12, 299–305.| RNA interference in ticks: a study using histamine binding protein dsRNA in the female tick Amblyomma americanum.Crossref | GoogleScholarGoogle Scholar | 12752664PubMed |
Allen JE, Sutherland TE, Rückerl D (2015) IL-17 and neutrophils: unexpected players in the type 2 immune response. Current Opinion in Immunology 34, 99–106.
| IL-17 and neutrophils: unexpected players in the type 2 immune response.Crossref | GoogleScholarGoogle Scholar | 25794823PubMed |
Amer PR, Emmans GC, Simm G (2001) Breeding objectives for beef cattle in Ireland. Livestock Production Science 67, 223–239.
| Breeding objectives for beef cattle in Ireland.Crossref | GoogleScholarGoogle Scholar |
Andronicos NM, Henshall JM, Le Jambre LF, Hunt PW, Ingham AB (2014) A one shot blood phenotype can identify sheep that resist Haemonchus contortus challenge. Veterinary Parasitology 205, 595–605.
| A one shot blood phenotype can identify sheep that resist Haemonchus contortus challenge.Crossref | GoogleScholarGoogle Scholar | 25200384PubMed |
Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Gause WC (2007) Protective immune mechanisms in helminth infection. Nature Reviews. Immunology 7, 975–987.
| Protective immune mechanisms in helminth infection.Crossref | GoogleScholarGoogle Scholar | 18007680PubMed |
Ashton GC, Seifert GW, Francis J (1968) An association between serum amylase phenotype and tick infestation in cattle. Australian Journal of Biological Sciences 21, 303–308.
| An association between serum amylase phenotype and tick infestation in cattle.Crossref | GoogleScholarGoogle Scholar | 5691119PubMed |
Bagnall N, Gough J, Cadogan L, Burns B, Kongsuwan K (2009) Expression of intracellular calcium signalling genes in cattle skin during tick infestation. Parasite Immunology 31, 177–187.
| Expression of intracellular calcium signalling genes in cattle skin during tick infestation.Crossref | GoogleScholarGoogle Scholar | 19292769PubMed |
Barbedo JGA, Gulias Gomes CC, Cardoso FF, Domingues R, Ramos JV, McManus CM (2017) The use of infrared images to detect ticks in cattle and proposal of an algorithm for quantifying the infestation. Veterinary Parasitology 235, 106–112.
| The use of infrared images to detect ticks in cattle and proposal of an algorithm for quantifying the infestation.Crossref | GoogleScholarGoogle Scholar |
Barker SC, Walker AR (2014) Ticks of Australia. The species that infest domestic animals and humans. Zootaxa 3816, 1–144.
| Ticks of Australia. The species that infest domestic animals and humans.Crossref | GoogleScholarGoogle Scholar |
Barnard DR, Ervin RT, Epplin FM (1992) Bio-economic impact of Amblyomma americanum in beef cattle production systems. In ‘Tick vector biology’. (Eds B Fivaz, T Petney, I Horak) pp. 55–69. (Springer: Berlin)
Beard CB, Occi J, Bonilla DL, Egizi AM, Fonseca DM, Mertins JW, Backenson BP, Bajwa WI, Barbarin AM, Bertone MA, Brown J, Connally NP, Connell ND, Eisen RJ, Falco RC, James AM, Krell RK, Lahmers K, Lewis N, Little SE, Neault M, Pérez de León AA, Randall AR, Ruder MG, Saleh MN, Schappach BL, Schroeder BA, Seraphin LL, Wehtje M, Wormser GP, Yabsley MJ, Halperin W (2018) Multistate infestation with the exotic disease-vector tick Haemaphysalis longicornis. United States, August 2017–September 2018. Morbidity and Mortality Weekly Report 67, 1310–1313.
| Multistate infestation with the exotic disease-vector tick Haemaphysalis longicornis. United States, August 2017–September 2018.Crossref | GoogleScholarGoogle Scholar | 30496158PubMed |
Beaufays J, Adam B, Menten-Dedoyart C, Fievez L, Grosjean A, Decrem Y, Prévôt PP, Santini S, Brasseur R, Brossard M, Vanhaeverbeek M, Bureau F, Heinen E, Lins L, Vanhamme L, Godfroid E (2008) Ir-LBP, an Ixodes ricinus tick salivary LTB4-binding lipocalin, interferes with host neutrophil function. PLoS One 3, e3987
| Ir-LBP, an Ixodes ricinus tick salivary LTB4-binding lipocalin, interferes with host neutrophil function.Crossref | GoogleScholarGoogle Scholar | 19096708PubMed |
Bechara GH, Morelli Júnior J, Szabó MP (2000) Skin test and tick immune status in susceptible and resistant cattle in Brazil. Annals of the New York Academy of Sciences 916, 570–575.
| Skin test and tick immune status in susceptible and resistant cattle in Brazil.Crossref | GoogleScholarGoogle Scholar | 11193675PubMed |
Bekele T (2002) Studies on seasonal dynamics of ticks of ogaden cattle and individual variation in resistance to ticks in eastern Ethiopia. Journal of Veterinary Medicine: Infectious Diseases and Veterinary Public Health 49, 285–288.
| Studies on seasonal dynamics of ticks of ogaden cattle and individual variation in resistance to ticks in eastern Ethiopia.Crossref | GoogleScholarGoogle Scholar |
Bell A, McNally J, Smith DV, Rahman A, Hunt P, Kotze AC, Dominik S, Ingham A (2019) Quantification of differences in resistance to gastrointestinal nematode infections in sheep using a multivariate blood parameter. Veterinary Parasitology 270, 31–39.
Berman A (2011) Are adaptations present to support dairy cattle productivity in warm climates? Journal of Dairy Science 94, 2147–2158.
| Are adaptations present to support dairy cattle productivity in warm climates?Crossref | GoogleScholarGoogle Scholar | 21524505PubMed |
Biegelmeyer P, Gulias-Gomes CC, Roso VM, Dionello NJL, Cardoso FF (2017) Tick resistance genetic parameters and its correlations with production traits in Hereford and Braford cattle. Livestock Science 202, 96–100.
| Tick resistance genetic parameters and its correlations with production traits in Hereford and Braford cattle.Crossref | GoogleScholarGoogle Scholar |
Birkett MA, Agelopoulos N, Jensen K-MV, Jespersen JB, Pickett JA, Prijs HJ, Thomas G, Trapman JJ, Wadhams LJ, Woodcock CM (2004) The role of volatile semiochemicals in mediating host location and selection by nuisance and disease-transmitting cattle flies. Medical and Veterinary Entomology 18, 313–322.
| The role of volatile semiochemicals in mediating host location and selection by nuisance and disease-transmitting cattle flies.Crossref | GoogleScholarGoogle Scholar | 15641996PubMed |
Boka OM, Achi L, Adakal H, Azokou A, Yao P, Yapi YG, Kone M, Dagnogo K, Kaboret YY (2017) Review of cattle ticks (Acari, Ixodida) in Ivory Coast and geographic distribution of Rhipicephalus (Boophilus) microplus, an emerging tick in West Africa. Experimental & Applied Acarology 71, 355–369.
| Review of cattle ticks (Acari, Ixodida) in Ivory Coast and geographic distribution of Rhipicephalus (Boophilus) microplus, an emerging tick in West Africa.Crossref | GoogleScholarGoogle Scholar |
Bonsma JC (1940) The influence of climatological factors on cattle. Observations on cattle in tropical regions. Farming in South Africa 15, 373–385.
Bonvin P, Power CA, Proudfoot AE (2016) Evasins: therapeutic potential of a new family of chemokine-binding proteins from ticks. Frontiers in Immunology 7, Article 208
| Evasins: therapeutic potential of a new family of chemokine-binding proteins from ticks.Crossref | GoogleScholarGoogle Scholar | 27375615PubMed |
Borges LMF, de Oliveira Filho JG, Ferreira LL, Braz Louly CC, Pickett JA, Birkett MA (2015) Identification of non-host semiochemicals for the brown dog tick, Rhipicephalus sanguineus sensu lato (Acari: Ixodidae), from tick-resistant beagles, Canis lupus familiaris. Ticks and Tick-Borne Diseases 6, 676–682.
| Identification of non-host semiochemicals for the brown dog tick, Rhipicephalus sanguineus sensu lato (Acari: Ixodidae), from tick-resistant beagles, Canis lupus familiaris.Crossref | GoogleScholarGoogle Scholar |
Brossard M, Wikel SK (1997) Immunology of interactions between ticks and hosts. Medical and Veterinary Entomology 11, 270–276.
| Immunology of interactions between ticks and hosts.Crossref | GoogleScholarGoogle Scholar | 9330259PubMed |
Burger TD, Shao R, Barker SC (2014) Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Molecular Phylogenetics and Evolution 76, 241–253.
| Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species.Crossref | GoogleScholarGoogle Scholar | 24685498PubMed |
Burrow HM (1997) ‘Tropical beef breeds and performance assessment: a producer’s guide.’ (Meat Research Corporation and Belmont Red Association of Australia: Rockhampton, Queensland)
Burrow HM (2001) Variances and covariances between productive and adaptive traits and temperament in a composite breed of tropical beef cattle. Livestock Production Science 70, 213–233.
| Variances and covariances between productive and adaptive traits and temperament in a composite breed of tropical beef cattle.Crossref | GoogleScholarGoogle Scholar |
Burrow HM (2012) Importance of adaptation and genotype × environment interactions in tropical beef breeding systems. Animal – Special Issue on Sustainable Animal Production in the Tropics 6, 729–740.
Burrow HM (2014) Genetic aspects of cattle adaptation in the tropics. In ‘The genetics of cattle’. 2nd edn. (Eds DJ Garrick, A Ruvinsky) pp. 571–597. (CAB International: Oxfordshire, UK)
Burrow HM, Henshall JM (2014) Relationships between adaptive and productive traits in cattle, goats and sheep in tropical environments. In ‘Proceedings of the 10th world congress on genetics applied to livestock production’, 17–22 August 2014, Vancouver, Canada. Available at http://www.wcgalp.org/proceedings/2014invited paper 044-9213 [Verified 17 June 2019]
Burrow HM, Seifert GW, Hetzel DJS (1991) Consequences of selection for weaning weight in Zebu, Bos taurus and Zebu × Bos taurus cattle in the tropics. Australian Journal of Agricultural Research 42, 295–307.
| Consequences of selection for weaning weight in Zebu, Bos taurus and Zebu × Bos taurus cattle in the tropics.Crossref | GoogleScholarGoogle Scholar |
Burrow HM, Moore SS, Johnston DJ, Barendse W, Bindon BM (2001) Quantitative and molecular genetic influences on properties of beef. Australian Journal of Experimental Agriculture 41, 893–919.
| Quantitative and molecular genetic influences on properties of beef.Crossref | GoogleScholarGoogle Scholar |
Cardoso FF, Gomes CCG, Sollero BP, Oliveira MM, Roso VM, Piccoli ML, Higa RH, Yokoo MJ, Caetano AR, Aguilar I (2015) Genomic prediction for tick resistance in Braford and Hereford cattle. Journal of Animal Science 93, 2693–2705.
| Genomic prediction for tick resistance in Braford and Hereford cattle.Crossref | GoogleScholarGoogle Scholar | 26115257PubMed |
Carvalho WA, Franzin AM, Abatepaulo ARR, de Oliveira CJ, Moré DD, da Silva JS, Ferreira BR, de Miranda Santos IK (2010a) Modulation of cutaneous inflammation induced by ticks in contrasting phenotypes of infestation in bovines. Veterinary Parasitology 167, 260–273.
| Modulation of cutaneous inflammation induced by ticks in contrasting phenotypes of infestation in bovines.Crossref | GoogleScholarGoogle Scholar | 19836891PubMed |
Carvalho WA, Maruyama SR, Franzin AM, Rodrigues Abatepaulo AR, Anderson JM, Rossetti Ferreira B, Chaves Ribeiro JM, Dantas Moré D, Mendes Maia AA, Valenzuela JG, Rocha Garcia G, Ferreira de Miranda Santos IK (2010b) Rhipicephalus (Boophilus) microplus: clotting time in tick-infested skin varies according to local inflammation and gene expression patterns in tick salivary glands. Experimental Parasitology 124, 428–435.
| Rhipicephalus (Boophilus) microplus: clotting time in tick-infested skin varies according to local inflammation and gene expression patterns in tick salivary glands.Crossref | GoogleScholarGoogle Scholar | 20045690PubMed |
Carvalho WA, Ianella P, Arnold FGC, Rodrigues Caetano A, Maruyama SR, Rossetti Ferreira B, Andreucci Conti LH, Monteiro da Silva MR, Paula JOF, Mendes Maia AA, Ferreira de Miranda Santos IK (2011) Haplotypes of the bovine IgG2 heavy gamma chain in tick-resistant and tick-susceptible breeds of cattle. Immunogenetics 63, 319–324.
| Haplotypes of the bovine IgG2 heavy gamma chain in tick-resistant and tick-susceptible breeds of cattle.Crossref | GoogleScholarGoogle Scholar | 21301827PubMed |
Carvalho WA, Domingues R, de Azevedo Prata MC, Vinícius M, da Silva GB, de Oliveira GC, Facioni Guimarães SE, Machao MA (2014) Microarray analysis of tick-infested skin in resistant and susceptible cattle confirms the role of inflammatory pathways in immune activation and larval rejection. Veterinary Parasitology 205, 307–317.
| Microarray analysis of tick-infested skin in resistant and susceptible cattle confirms the role of inflammatory pathways in immune activation and larval rejection.Crossref | GoogleScholarGoogle Scholar | 25108850PubMed |
Chinery WA (1981) Observation on the saliva and salivary gland extract of Haemaphysalis spinigera and Rhipicephalus sanguineus sanguineus. The Journal of Parasitology 67, 15–19.
| Observation on the saliva and salivary gland extract of Haemaphysalis spinigera and Rhipicephalus sanguineus sanguineus.Crossref | GoogleScholarGoogle Scholar | 7229815PubMed |
Chinery WA, Ayitey-Smith E (1977) Histamine blocking agent in the salivary gland homogenate of the tick Rhipicephalus sanguineus sanguineus. Nature 265, 366–367.
| Histamine blocking agent in the salivary gland homogenate of the tick Rhipicephalus sanguineus sanguineus.Crossref | GoogleScholarGoogle Scholar | 834285PubMed |
Costa RF, Reis AP, Yokoo MJ, van der Werf JHJ, MacNeil MD, Cardoso FF (2018). Economic indexes including tick resistance for Hereford and Braford breeds raised in southern Brazil. In ‘Proceedings of the world congress on genetics applied to livestock production 11.667’, 11–16 February 2018, Auckland, New Zealand.
da Silva JB, Rangel CP, de Azevedo Baêta B, Da Fonseca AH (2014) Analysis of the risk factors relating to cows’ resistance to Rhipicephalus microplus ticks during the peripartum. Experimental & Applied Acarology 63, 551–557.
| Analysis of the risk factors relating to cows’ resistance to Rhipicephalus microplus ticks during the peripartum.Crossref | GoogleScholarGoogle Scholar |
Daix V, Schroeder H, Praet N, Georgin JP, Chiappino I, Gillet L, de Fays K, Decrem Y, Leboulle G, Godfroid E, Bollen A, Pastoret PP, Gern L, Sharp PM, Vanderplasschen A (2007) Ixodes ticks belonging to the Ixodes ricinus complex encode a family of anticomplement proteins. Insect Molecular Biology 16, 155–166.
| Ixodes ticks belonging to the Ixodes ricinus complex encode a family of anticomplement proteins.Crossref | GoogleScholarGoogle Scholar | 17298559PubMed |
de Castro JJ (1997) Sustainable tick and tick-borne disease control in livestock development in developing countries. Veterinary Parasitology 71, 77–97.
| Sustainable tick and tick-borne disease control in livestock development in developing countries.Crossref | GoogleScholarGoogle Scholar | 9261972PubMed |
de Castro JJ, Newson RM, Herbert IV (1989) Resistance in cattle against Rhipicephalus appendiculatus with an assessment of cross-resistance to R. pulchellus (Acari: Ixodidae). Experimental & Applied Acarology 6, 237–244.
| Resistance in cattle against Rhipicephalus appendiculatus with an assessment of cross-resistance to R. pulchellus (Acari: Ixodidae).Crossref | GoogleScholarGoogle Scholar |
de Oliveira Filho JG, Franceschini Sarria AL, Ferreira LL, Caulfield JC, Powers SJ, Pickett JA, de Leon AAP, Birkett MA, Borges LMF (2016) Quantification of brown dog tick repellents, 2-hexanone and benzaldehyde, and release from tick-resistant beagles Canis lupus familiaris. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 1022, 64–69.
| Quantification of brown dog tick repellents, 2-hexanone and benzaldehyde, and release from tick-resistant beagles Canis lupus familiaris.Crossref | GoogleScholarGoogle Scholar | 27085013PubMed |
de Oliveira Filho JG, Ferreira LL, Franceschini Sarria AL, Pickett JA, Birkett MA, Mascarin GM, Pérez de León AA, Borges LMF (2017) Brown dog tick, Rhipicephalus sanguineus sensu lato, infestation of susceptible dog hosts is reduced by slow release of semiochemicals from a less susceptible host. Ticks and Tick-Borne Diseases 8, 139–145.
| Brown dog tick, Rhipicephalus sanguineus sensu lato, infestation of susceptible dog hosts is reduced by slow release of semiochemicals from a less susceptible host.Crossref | GoogleScholarGoogle Scholar | 28340941PubMed |
Déruaz M, Frauenschuh A, Alessandri AL, Dias JM, Coelho FM, Russo RC, Ferreira BR, Graham GJ, Shaw JP, Wells TN, Teixeira MM, Power CA, Proudfoot AE (2008) Ticks produce highly selective chemokine binding proteins with nti-inflammatory activity. The Journal of Experimental Medicine 205, 2019–2031.
| Ticks produce highly selective chemokine binding proteins with nti-inflammatory activity.Crossref | GoogleScholarGoogle Scholar | 18678732PubMed |
Díaz-Martín V, Manzano-Román R, Siles-Lucas M, Oleaga A, Pérez-Sánchez R (2011) Cloning, characterization and diagnostic performance of the salivary lipocalin protein TSGP1 from Ornithodoros moubata. Veterinary Parasitology 178, 163–172.
| Cloning, characterization and diagnostic performance of the salivary lipocalin protein TSGP1 from Ornithodoros moubata.Crossref | GoogleScholarGoogle Scholar | 21216104PubMed |
Domingues R, Wohlres-Viana S, Reis DR, Teixeira HC, Ferreira AP, Guimarães SEF, Prata MC, Furlong J, Verneque RS, Machado MA (2014) Expression of immune response genes in peripheral blood of cattle infested with Rhipicephalus microplus. Genetics and Molecular Research 13, 4013–4021.
| Expression of immune response genes in peripheral blood of cattle infested with Rhipicephalus microplus.Crossref | GoogleScholarGoogle Scholar | 24938612PubMed |
Edwards KT (2011) Gotch ear: a poorly described, local, pathologic condition of livestock associated primarily with the Gulf Coast tick, Amblyomma maculatum. Veterinary Parasitology 183, 1–7.
| Gotch ear: a poorly described, local, pathologic condition of livestock associated primarily with the Gulf Coast tick, Amblyomma maculatum.Crossref | GoogleScholarGoogle Scholar | 22047764PubMed |
Estrada-Peña A, Venzal JM, Nava S, Mangold A, Guglielmone AA, Labruna MB, de la Fuente J (2012) Reinstatement of Rhipicephalus (Boophilus) australis (Acari: Ixodidae) with redescription of the adult and larval stages. Journal of Medical Entomology 49, 794–802.
| Reinstatement of Rhipicephalus (Boophilus) australis (Acari: Ixodidae) with redescription of the adult and larval stages.Crossref | GoogleScholarGoogle Scholar | 22897039PubMed |
Estrada-Peña A, Farkas R, Jaenson TG, Koenen F, Madder M, Pascucci I, Salman M, Tarrés-Call J, Jongejan F (2013) Association of environmental traits with the geographic ranges of ticks (Acari: Ixodidae) of medical and veterinary importance in the western Palearctic. A digital data set. Experimental & Applied Acarology 59, 351–366.
| Association of environmental traits with the geographic ranges of ticks (Acari: Ixodidae) of medical and veterinary importance in the western Palearctic. A digital data set.Crossref | GoogleScholarGoogle Scholar |
Franco PF, Silva NC, Fazito do Vale V, Abreu JF, Santos VC, Gontijo NF, Valenzuela JG, Pereira MH, Sant’Anna MR, Gomes AP, Araujo RN (2016) Inhibition of the classical pathway of the complement system by saliva of Amblyomma cajennense (Acari: Ixodidae). Experimental Parasitology 164, 91–96.
| Inhibition of the classical pathway of the complement system by saliva of Amblyomma cajennense (Acari: Ixodidae).Crossref | GoogleScholarGoogle Scholar | 26948715PubMed |
Franzin AM, Maruyama SR, Garcia GR, Oliveira RP, Ribeiro JMC, Bishop R, Maia AAM, Moré DD, Ferreira BR, Miranda Santos IKF (2017) Immune and biochemical responses in skin differ between bovine hosts genetically susceptible and resistant to the cattle tick Rhipicephalus microplus. Parasites & Vectors 10, 51
| Immune and biochemical responses in skin differ between bovine hosts genetically susceptible and resistant to the cattle tick Rhipicephalus microplus.Crossref | GoogleScholarGoogle Scholar |
Frauenschuh A, Power CA, Déruaz M, Ferreira BR, Silva JS, Teixeira MM, Dias JM, Martin T, Wells TN, Proudfoot AE (2007) Molecular cloning and characterization of a highly selective chemokine-binding protein from the tick Rhipicephalus sanguineus. The Journal of Biological Chemistry 282, 27250–27258.
| Molecular cloning and characterization of a highly selective chemokine-binding protein from the tick Rhipicephalus sanguineus.Crossref | GoogleScholarGoogle Scholar | 17640866PubMed |
Frisch JE (1981) Factors affecting resistance to ecto- and endoparasites of cattle in tropical areas and the implications for selection. In ‘Isotopes and radiation in parasitology IV’. pp. 17–32. (International Atomic Energy Agency: Vienna)
Frisch JE (1987) Physiological reasons for heterosis in growth of Bos indicus x Bos taurus. Journal of Agricultural Science 109, 213–230.
| Physiological reasons for heterosis in growth of Bos indicus x Bos taurus.Crossref | GoogleScholarGoogle Scholar |
Frisch JE (1999) Towards a permanent solution for controlling cattle ticks. International Journal for Parasitology 29, 57–71.
| Towards a permanent solution for controlling cattle ticks.Crossref | GoogleScholarGoogle Scholar | 10048820PubMed |
Frisch JE, O’Neill CJ (1998) Comparative evaluation of beef cattle breeds of African, European and Indian origins. 2. Resistance to cattle ticks and gastrointestinal nematodes. Animal Science 67, 39–48.
| Comparative evaluation of beef cattle breeds of African, European and Indian origins. 2. Resistance to cattle ticks and gastrointestinal nematodes.Crossref | GoogleScholarGoogle Scholar |
Frisch JE, Vercoe JE (1982) Consideration of adaptive and productive components of productivity in breeding beef cattle for tropical Australia. In ‘Proceedings second world congress on genetics applied to livestock production’, vol. 6, Madrid, Spain. pp. 307–321. Available at http://www.wcgalp.org/proceedings/1982 [Verified 17 June 2019]
Frisch JE, Vercoe JE (1984) An analysis of growth of different cattle genotypes reared in different environments. Journal of Agricultural Science 103, 137–153.
| An analysis of growth of different cattle genotypes reared in different environments.Crossref | GoogleScholarGoogle Scholar |
Frisch JE, Drinkwater R, Harrison B, Johnson S (1997) Classification of the southern African Sanga and East African shorthorned zebu. Animal Genetics 28, 77–83.
| Classification of the southern African Sanga and East African shorthorned zebu.Crossref | GoogleScholarGoogle Scholar | 9172304PubMed |
Frisch JE, O’Neill CJ, Kelly MJ (2000) Using genetics to control cattle parasites: the Rockhampton experience. International Journal for Parasitology 30, 253–264.
| Using genetics to control cattle parasites: the Rockhampton experience.Crossref | GoogleScholarGoogle Scholar | 10719118PubMed |
Gasparin G, Miyata M, Coutinho LL, Martinez ML, Teodoro RL, Furlong J, Machado MA, Silva MVGB, Sonstegard TS, Regitano LCA (2007) Mapping of quantitative trait loci controlling tick [Riphicephalus (Boophilus) microplus] resistance on chromosomes 5, 7 and 14. Animal Genetics 38, 453–459.
| Mapping of quantitative trait loci controlling tick [Riphicephalus (Boophilus) microplus] resistance on chromosomes 5, 7 and 14.Crossref | GoogleScholarGoogle Scholar | 17894560PubMed |
Gebrekidan H, Nelson L, Smith G, Gasser RB, Jabbar A (2017a) An outbreak of oriental theileriosis in dairy cattle imported to Vietnam from Australia. Parasitology 144, 738–746.
| An outbreak of oriental theileriosis in dairy cattle imported to Vietnam from Australia.Crossref | GoogleScholarGoogle Scholar | 27938442PubMed |
Gebrekidan H, Abbas T, Wajid M, Ali A, Gasser RB, Jabbar A (2017b) Molecular characterisation of Theileria orientalis in imported and native bovines from Pakistan. Infection, Genetics and Evolution 47, 19–25.
| Molecular characterisation of Theileria orientalis in imported and native bovines from Pakistan.Crossref | GoogleScholarGoogle Scholar | 27838527PubMed |
Gibbs RA, Taylor JF, Van Tassell CP, Barendse W, Eversole KA, Gill CA, Green RS, Hamernik DL, Kappes SM, Lien S, the Bovine HapMap Consortium (2009) Genome-wide survey of SNP variation uncovers the genetic structure of cattle breeds. Science 324, 528–532.
| Genome-wide survey of SNP variation uncovers the genetic structure of cattle breeds.Crossref | GoogleScholarGoogle Scholar | 19390050PubMed |
Gibson JP, Bishop SC (2005) Use of molecular markers to enhance resistance of livestock to disease: a global approach. Revue Scientifique et Technique (International Office of Epizootics) 24, 343–353.
| Use of molecular markers to enhance resistance of livestock to disease: a global approach.Crossref | GoogleScholarGoogle Scholar |
Goddard ME, Hayes BJ (2009) Mapping genes for complex traits in domestic animals and their use in breeding programmes. Nature Reviews. Genetics 10, 381–391.
| Mapping genes for complex traits in domestic animals and their use in breeding programmes.Crossref | GoogleScholarGoogle Scholar | 19448663PubMed |
Gothe R (1987) Tick pheromones. The Onderstepoort Journal of Veterinary Research 54, 439–441.
Grisi L, Leite RC, Souza Martins JR, Medeiros de Barros AT, Andreotti R, Duarte Cancado PH, Perez de Leon AA, Pereira JB, Villela HS (2014) Reassessment of the potential economic impact of cattle parasites in Brazil. Revista Brasileira de Parasitologia Veterinária 23, 150–156.
| Reassessment of the potential economic impact of cattle parasites in Brazil.Crossref | GoogleScholarGoogle Scholar | 25054492PubMed |
Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, Pérez de León AA (2014) Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasites & Vectors 7, 475
| Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations.Crossref | GoogleScholarGoogle Scholar |
Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Pena A, Horak IG, Shao RF, Barker SC (2010) The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa 2528, 1–28.
| The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names.Crossref | GoogleScholarGoogle Scholar |
Hajnická V, Kocáková P, Sláviková M, Slovák M, Gasperík J, Fuchsberger N, Nuttall PA (2001) Anti-interleukin-8 activity of tick salivary gland extracts. Parasite Immunology 23, 483–489.
| Anti-interleukin-8 activity of tick salivary gland extracts.Crossref | GoogleScholarGoogle Scholar | 11589777PubMed |
Hanotte O, Ronin Y, Agaba M, Nilsson P, Gelhaus A, Horstmann R, Sugimoto Y, Kemp S, Gibson J, Korol A, Soller M, Teale A (2003) Mapping of quantitative trait loci controlling trypanotolerance in a cross of tolerant West African N’dama and susceptible East African Boran cattle. Proceedings of the National Academy of Sciences, USA 100, 7443–7448.
| Mapping of quantitative trait loci controlling trypanotolerance in a cross of tolerant West African N’dama and susceptible East African Boran cattle.Crossref | GoogleScholarGoogle Scholar |
Hayes BJ, Visscher PM, Goddard ME (2009) Increased accuracy of artificial selection by using the realized relationship matrix. Genetical Research 91, 47–60.
| Increased accuracy of artificial selection by using the realized relationship matrix.Crossref | GoogleScholarGoogle Scholar |
Hayes BJ, Nieuwhof G, Haile Mariam M (2017) A multi-trait approach to incorporating foreign phenotypes and genotypes in genomic predictions to increase accuracy and reduce bias. Proceedings Australian Association of Animal Breeding and Genetics 22, 265-268
Hayward J, Sanchez J, Perry A, Huang C, Rodriguez Valle M, Canals M, Payne RJ, Stone MJ (2017) Ticks from diverse genera encode chemokine-inhibitory evasin proteins. The Journal of Biological Chemistry 292, 15670–15680.
| Ticks from diverse genera encode chemokine-inhibitory evasin proteins.Crossref | GoogleScholarGoogle Scholar | 28778927PubMed |
Hewetson RW (1972) The inheritance of resistance by cattle to cattle tick. Australian Veterinary Journal 48, 299–303.
| The inheritance of resistance by cattle to cattle tick.Crossref | GoogleScholarGoogle Scholar | 5068812PubMed |
Heyne H, Elliott EGR, Bezuidenhout JD (1987) Rearing and infection techniques for Amblyomma species to be used in heartwater transmission experiments. The Onderstepoort Journal of Veterinary Research 54, 461–471.
Horak IG, Nyangiwe N, De Matos C, Neves L (2009) Species composition and geographic distribution of ticks infesting cattle, goats and dogs in a temperate and in a subtropical region of south-east Africa. The Onderstepoort Journal of Veterinary Research 76, 263–276.
| Species composition and geographic distribution of ticks infesting cattle, goats and dogs in a temperate and in a subtropical region of south-east Africa.Crossref | GoogleScholarGoogle Scholar | 21105593PubMed |
Ibelli AMG, Ribeiro ARB, Giglioti R, Regitano LCA, Alencar MM, Chagas ACS, Paço AL, Oliveira HN, Duarte JMS, Oliveira MCS (2012) Resistance of cattle of various genetic groups to the tick Rhipicephalus microplus and the relationship with coat traits. Veterinary Parasitology 186, 425–430.
| Resistance of cattle of various genetic groups to the tick Rhipicephalus microplus and the relationship with coat traits.Crossref | GoogleScholarGoogle Scholar |
Izzo MM, Poe I, Horadagoda N, De Vos AJ, House JK (2010) Haemolytic anaemia in cattle in NSW associated with Theileria infections. Australian Veterinary Journal 88, 45–51.
| Haemolytic anaemia in cattle in NSW associated with Theileria infections.Crossref | GoogleScholarGoogle Scholar | 20148827PubMed |
James AM, Freier JE, Keirans JE, Durden LA, Mertins JW, Schlater JL (2006) Distribution, seasonality, and hosts of the Rocky Mountain wood tick in the United States. Journal of Medical Entomology 43, 17–24.
| Distribution, seasonality, and hosts of the Rocky Mountain wood tick in the United States.Crossref | GoogleScholarGoogle Scholar | 16506443PubMed |
Jensen K-MV, Jespersen JB, Birkett MA, Pickett JA, Thomas G, Wadhams LJ, Woodcock CM (2004) Variation in the load of the horn fly, Haematobia irritans, in cattle herds is determined by the presence or absence of individual heifers. Medical and Veterinary Entomology 18, 275–280.
| Variation in the load of the horn fly, Haematobia irritans, in cattle herds is determined by the presence or absence of individual heifers.Crossref | GoogleScholarGoogle Scholar |
Johnston TH, Bancroft MJ (1918) A tick resistant condition in cattle. Proceedings of the Royal Society of Queensland 30, 219–317.
Jonsson NN, Piper EK, Constantinoiu CC (2014) Host resistance in cattle to infestation with the cattle tick Rhipicephalus microplus. Parasite Immunology 36, 553–559.
| Host resistance in cattle to infestation with the cattle tick Rhipicephalus microplus.Crossref | GoogleScholarGoogle Scholar | 25313455PubMed |
Kamau J, de Vos AJ, Playford M, Salim B, Kinyanjui P, Sugimoto C (2011) Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasites & Vectors 4, 22
| Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks.Crossref | GoogleScholarGoogle Scholar |
Kelley RB (1932) ‘Zebu (Brahman) cross cattle and their possibilities in north Australia.’ Pamphlet no. 27. (Council for Scientific and Industrial Research: Rockhampton, Qld)
Kemp DH, Bourne A (1980) Boophilus microplus: the effect of histamine on the attachment of cattle-tick larvae - studies in vivo and in vitro. Parasitology 80, 487–496.
| Boophilus microplus: the effect of histamine on the attachment of cattle-tick larvae - studies in vivo and in vitro.Crossref | GoogleScholarGoogle Scholar | 7393620PubMed |
Kongsuwan K, Josh P, Colgrave ML, Bagnall NH, Gough J, Burns B, Pearson R (2010) Activation of several key components of the epidermal differentiation pathway in cattle following infestation with the cattle tick, Rhipicephalus (Boophilus) microplus. International Journal for Parasitology 40, 499–507.
| Activation of several key components of the epidermal differentiation pathway in cattle following infestation with the cattle tick, Rhipicephalus (Boophilus) microplus.Crossref | GoogleScholarGoogle Scholar | 19909754PubMed |
Labruna MB, Naranjo V, Mangold AJ, Thompson C, Estrada-Peña A, Guglielmone AA, Jongejan F, de la Fuente J (2009) Allopatric speciation in ticks: genetic and reproductive divergence between geographic strains of Rhipicephalus (Boophilus) microplus. BMC Evolutionary Biology 9, 46
| Allopatric speciation in ticks: genetic and reproductive divergence between geographic strains of Rhipicephalus (Boophilus) microplus.Crossref | GoogleScholarGoogle Scholar | 19243585PubMed |
Lado P, Nava S, Mendoza-Uribe L, Caceres AG, Delgado-de la Mora J, Licona-Enriquez JD, Delgado-de la Mora D, Labruna MB, Durden LA, Allerdice MEJ, Paddock CD, Szabó MPJ, Venzal JM, Guglielmone AA, Beati L (2018) The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae) group of ticks: phenotypic plasticity or incipient speciation? Parasites & Vectors 11, 610
| The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae) group of ticks: phenotypic plasticity or incipient speciation?Crossref | GoogleScholarGoogle Scholar |
Latha BR (2012) Semiochemical: a novel thought for tick control. Journal of Veterinary & Animal Sciences (Lahore) 43, 1–10.
Latif AA (2006) ‘Sustainable control methods for ticks and tick-borne diseases in Africa.’ (Veterinary Research Laboratory: Harare, Zimbabwe)
Laubscher L, Hoffman L (2012) An overview of disease-free buffalo breeding projects with reference to the different systems used in South Africa. Sustainability 4, 3124–3140.
| An overview of disease-free buffalo breeding projects with reference to the different systems used in South Africa.Crossref | GoogleScholarGoogle Scholar |
Legg J (1930) Some observations on the life history of the cattle tick (Boophilus australis). Proceedings of the Royal Society of Queensland 41, 121–132.
Lehmann T (1993) Ectoparasites: direct impact on host fitness. Parasitology Today 9, 8–13.
| Ectoparasites: direct impact on host fitness.Crossref | GoogleScholarGoogle Scholar | 15463655PubMed |
Lew-Tabor AE, Rodriguez Valle M (2016) A review of reverse vaccinology approaches for the development of vaccines against ticks and tick-borne diseases. Ticks and Tick-Borne Diseases 7, 573–585.
| A review of reverse vaccinology approaches for the development of vaccines against ticks and tick-borne diseases.Crossref | GoogleScholarGoogle Scholar | 26723274PubMed |
Logan JG, Birkett MA (2007) Semiochemicals for biting fly control: their identification and exploitation. Pest Management Science 63, 647–657.
| Semiochemicals for biting fly control: their identification and exploitation.Crossref | GoogleScholarGoogle Scholar | 17549674PubMed |
Logan JG, Birkett MA, Clark SJ, Mordue (Luntz) AJ, Pickett JA, Powers S, Seal NJ, Wadhams LJ (2008) Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. Journal of Chemical Ecology 34, 308–322.
| Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes.Crossref | GoogleScholarGoogle Scholar | 18306972PubMed |
Logan JG, Seal NJ, Cook JI, Stanczyk NM, Birkett MA, Clark SJ, Gezan SA, Wadhams LJ, Pickett JA, Mordue (Luntz) AJ (2009) Identification of human-derived volatile chemicals that interfere with attraction of the Scottish biting midge and their potential use as repellents. Journal of Medical Entomology 46, 208–219.
| Identification of human-derived volatile chemicals that interfere with attraction of the Scottish biting midge and their potential use as repellents.Crossref | GoogleScholarGoogle Scholar | 19351071PubMed |
Lohmeyer KH, Pound JM, May MA, Kammlah DM, Davey RB (2011) Distribution of Rhipicephalus (Boophilus) microplus and Rhipicephalus (Boophilus) annulatus (Acari: Ixodidae) infestations detected in the United States along the Texas/Mexico border. Journal of Medical Entomology 48, 770–774.
| Distribution of Rhipicephalus (Boophilus) microplus and Rhipicephalus (Boophilus) annulatus (Acari: Ixodidae) infestations detected in the United States along the Texas/Mexico border.Crossref | GoogleScholarGoogle Scholar | 21845935PubMed |
Low VL, Tay ST, Kho KL, Koh FX, Tan TK, Lim YA, Ong BL, Panchadcharam C, Norma-Rashid Y, Sofian-Azirun M (2015) Molecular characterisation of the tick Rhipicephalus microplus in Malaysia: new insights into the cryptic diversity and distinct genetic assemblages throughout the world. Parasites & Vectors 8, 341
| Molecular characterisation of the tick Rhipicephalus microplus in Malaysia: new insights into the cryptic diversity and distinct genetic assemblages throughout the world.Crossref | GoogleScholarGoogle Scholar |
Lush JL (1927) ‘Percentage of blood’ and Mendelish: value of ‘fractions of blood’ as an index of the genetic constitution of an animal. The Journal of Heredity 18, 351–367.
| ‘Percentage of blood’ and Mendelish: value of ‘fractions of blood’ as an index of the genetic constitution of an animal.Crossref | GoogleScholarGoogle Scholar |
Lynen G, Zeman P, Bakuname C, Di Giulio G, Mtui P, Sanka P, Jongejan F (2008) Shifts in the distributional ranges of Boophilus ticks in Tanzania: evidence that a parapatric boundary between Boophilus microplus and B. decoloratus follows climate gradients. Experimental & Applied Acarology 44, 147–164.
| Shifts in the distributional ranges of Boophilus ticks in Tanzania: evidence that a parapatric boundary between Boophilus microplus and B. decoloratus follows climate gradients.Crossref | GoogleScholarGoogle Scholar |
Lysyk TJ, Veira DM, Majak W (2009) Cattle can develop immunity to paralysis caused by Dermacentor andersoni. Journal of Medical Entomology 46, 358–366.
| Cattle can develop immunity to paralysis caused by Dermacentor andersoni.Crossref | GoogleScholarGoogle Scholar | 19351088PubMed |
Machado MA, Azevedo AL, Teodoro RL, Pires MA, Cd Peixoto MG, de Freitas C, Prata MC, Furlong J, da Silva MV, Guimarães SE, Regitano LC, Coutinho LL, Gasparin G, Verneque RS (2010) Genome wide scan for quantitative trait loci affecting tick resistance in cattle (Bos taurus × Bos indicus). BMC Genomics 11, 280
| Genome wide scan for quantitative trait loci affecting tick resistance in cattle (Bos taurus × Bos indicus).Crossref | GoogleScholarGoogle Scholar | 20433753PubMed |
MacHugh DE, Shriver MD, Loftus RT, Cunningham P, Bradley DG (1997) Microsatellite DNA variation and the evolution, domestication and phylogeography of taurine and zebu cattle (Bos taurus and Bos indicus). Genetics 146, 1071–1086.
MacLeod IM, Bowman PJ, Vander Jagt CJ, Haile-Mariam M, Kemper KE, Chamberlain AJ, Schrooten C, Hayes BJ, Goddard ME (2016) Exploiting biological priors and sequence variants enhances QTL discovery and genomic prediction of complex traits. BMC Genomics 17, 144
| Exploiting biological priors and sequence variants enhances QTL discovery and genomic prediction of complex traits.Crossref | GoogleScholarGoogle Scholar | 26920147PubMed |
Madalena FE, Teodoro RL, Lemos AM, Oliveira GP (1985) Causes of variation of field burdens of cattle tick (Boophilus microplus). Revista Brasileira de Genetica 8, 361–375.
Madalena FE, Teodoro RL, Lemos AM, Monteiro JBN, Barbosa RT (1990) Evaluation of strategies for crossbreeding of dairy cattle in Brazil. Journal of Dairy Science 73, 1887–1901.
| Evaluation of strategies for crossbreeding of dairy cattle in Brazil.Crossref | GoogleScholarGoogle Scholar |
Madder M, Thys E, Achi L, Touré A, De Deken R (2011) Rhipicephalus (Boophilus) microplus: a most successful invasive tick species in West-Africa. Experimental & Applied Acarology 53, 139–145.
| Rhipicephalus (Boophilus) microplus: a most successful invasive tick species in West-Africa.Crossref | GoogleScholarGoogle Scholar |
Maier R, Moser G, Chen GB, Ripke S, Cross-Disorder Working Group of the Psychiatric Genomics Consortium Coryell W, Potash JB, Scheftner WA, Shi J, Weissman MM, Hultman CM, Landén M, Levinson DF, Kendler KS, Smoller JW, Wray NR, Lee SH (2015) Joint analysis of psychiatric disorders increases accuracy of risk prediction for schizophrenia, bipolar disorder, and major depressive disorder. American Journal of Human Genetics 96, 283–294.
| Joint analysis of psychiatric disorders increases accuracy of risk prediction for schizophrenia, bipolar disorder, and major depressive disorder.Crossref | GoogleScholarGoogle Scholar | 25640677PubMed |
Mamoudou A, Nguetoum NC, Zoli PA, Sevidzem SL (2016) Identification and infestation of ticks on cattle in the peri-urban area of Ngaoundere, Cameroon. Journal of Veterinary Science and Medical Diagnostics 5,
| Identification and infestation of ticks on cattle in the peri-urban area of Ngaoundere, Cameroon.Crossref | GoogleScholarGoogle Scholar |
Mans BJ (2005) Tick histamine-binding proteins and related lipocalins: potential as therapeutic agents. Current Opinion in Investigational Drugs 6, 1131–1135.
Mans BJ (2016) Glandular matrices and secretions: blood-feeding arthropods. In ‘Extracellular composite matrices in arthropods’. (Eds E Cohen, B Moussian) pp. 625–688. (Springer: Cham)
Mans BJ, Ribeiro JM (2008a) Function, mechanism and evolution of the moubatin-clade of soft tick lipocalins. Insect Biochemistry and Molecular Biology 38, 841–852.
| Function, mechanism and evolution of the moubatin-clade of soft tick lipocalins.Crossref | GoogleScholarGoogle Scholar | 18694828PubMed |
Mans BJ, Ribeiro JM (2008b) A novel clade of cysteinyl leukotriene scavengers in soft ticks. Insect Biochemistry and Molecular Biology 38, 862–870.
| A novel clade of cysteinyl leukotriene scavengers in soft ticks.Crossref | GoogleScholarGoogle Scholar | 18675910PubMed |
Mans BJ, Ribeiro JM, Andersen JF (2008) Structure, function, and evolution of biogenic amine-binding proteins in soft ticks. The Journal of Biological Chemistry 283, 18721–18733.
| Structure, function, and evolution of biogenic amine-binding proteins in soft ticks.Crossref | GoogleScholarGoogle Scholar | 18445596PubMed |
Mans BJ, de Castro MH, Pienaar R, de Klerk D, Gaven P, Genu S, Latif AA (2016) Ancestral reconstruction of tick lineages. Ticks and Tick-Borne Diseases 7, 509–535.
| Ancestral reconstruction of tick lineages.Crossref | GoogleScholarGoogle Scholar | 26868413PubMed |
Mans BJ, Featherston J, de Castro MH, Pienaar R (2017) Gene duplication and protein evolution in tick–host interactions. Frontiers in Cellular and Infection Microbiology 7, 413
| Gene duplication and protein evolution in tick–host interactions.Crossref | GoogleScholarGoogle Scholar | 28993800PubMed |
Mapholi NO, Marufu MC, Maiwashe A, Banga CB, Muchenje V, MacNeil MD, Chimonyo M, Dzama K (2014) Towards a genomics approach to tick (Acari: Ixodidae) control in cattle: a review. Ticks and Tick-Borne Diseases 5, 475–483.
| Towards a genomics approach to tick (Acari: Ixodidae) control in cattle: a review.Crossref | GoogleScholarGoogle Scholar | 24954600PubMed |
Mapholi NO, Maiwashe A, Matika O, Riggio V, Bishop SC, MacNeil MD, Banga C, Taylor JF, Dzama K (2016) Genome-wide association study of tick resistance in South African Nguni cattle. Ticks and Tick-Borne Diseases 7, 487–497.
| Genome-wide association study of tick resistance in South African Nguni cattle.Crossref | GoogleScholarGoogle Scholar | 26897394PubMed |
Marufu MC, Chimonyo M, Mans BJ, Dzama K (2013) Cutaneous hypersensitivity responses to Rhipicephalus tick larval antigens in pre-sensitized cattle. Ticks and Tick-Borne Diseases4 311316
Marufu MC, Dzama K, Chimonyo M (2014) Cellular responses to Rhipicephalus microplus infestations in pre-sensitised cattle with differing phenotypes of infestation. Experimental & Applied Acarology 62, 241–252.
| Cellular responses to Rhipicephalus microplus infestations in pre-sensitised cattle with differing phenotypes of infestation.Crossref | GoogleScholarGoogle Scholar |
Maruyama SR, Garcia GR, Teixeira FR, Brandão LG, Anderson JM, Ribeiro JMC, Valenzuela JG, Horackova J, Veríssimo CJ, Katiki LM, Banin TM, Zangirolamo AF, Gardinassi LG, Ferreira BR, de Miranda-Santos IKF (2017) Mining a differential sialotranscriptome of Rhipicephalus microplus guides antigen discovery to formulate a vaccine that reduces tick infestations. Parasites & Vectors 10, 206
| Mining a differential sialotranscriptome of Rhipicephalus microplus guides antigen discovery to formulate a vaccine that reduces tick infestations.Crossref | GoogleScholarGoogle Scholar |
McFadden AM, Rawdon TG, Meyer J, Makin J, Morley CM, Clough RR, Tham K, Mullner P, Geysen D (2011) An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naive cattle. New Zealand Veterinary Journal 59, 79–85.
| An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naive cattle.Crossref | GoogleScholarGoogle Scholar | 21409734PubMed |
McFadden A, Gias E, Heuer C, Stevens McFadden FJ, Pulford DJ (2016) Prevalence and spatial distribution of cattle herds infected with Theileria orientalis in New Zealand between 2012 and 2013. New Zealand Veterinary Journal 64, 55–59.
| Prevalence and spatial distribution of cattle herds infected with Theileria orientalis in New Zealand between 2012 and 2013.Crossref | GoogleScholarGoogle Scholar | 26436469PubMed |
McManus C, Tanure CB, Peripolli V, Seixas L, Fischer V, Gabbi AM, Menegassi SRO, Stumpf MT, Kolling GJ, Dias E, Costa JBG (2016) Infrared thermography in animal production: an overview. Computers and Electronics in Agriculture 123, 10–16.
| Infrared thermography in animal production: an overview.Crossref | GoogleScholarGoogle Scholar |
Meuwissen THE, Hayes BJ, Goddard ME (2001) Prediction of total genetic value using genome-wide dense marker maps. Genetics 157, 1819–1829.
Meuwissen T, Hayes B, Goddard M (2016) Genomic selection: a paradigm shift in animal breeding. Animal Frontiers 6, 6–14.
Miranpuri GS (1989) Relationship between the resistance of crossbred cattle to ticks, Boophilus microplus (Canestrini, 1887) and Hyalomma anatolicum anatolicum (Koch, 1844). Veterinary Parasitology 31, 289–301.
| Relationship between the resistance of crossbred cattle to ticks, Boophilus microplus (Canestrini, 1887) and Hyalomma anatolicum anatolicum (Koch, 1844).Crossref | GoogleScholarGoogle Scholar | 2763448PubMed |
Mota RR, Lopes PS, Tempelman RJ, Silva FF, Aguilar I, Gomes CCG, Cardoso FF (2016) Genome-enabled prediction for tick resistance in Hereford and Braford beef cattle via reaction norm models. Journal of Animal Science 94, 1834–1843.
| Genome-enabled prediction for tick resistance in Hereford and Braford beef cattle via reaction norm models.Crossref | GoogleScholarGoogle Scholar | 27285681PubMed |
Muchenje V, Dzama K, Chimonyo M, Raats JG, Strydom PE (2008) Tick susceptibility and its effects on growth performance and carcass characteristics of Nguni, Bonsmara and Angus steers raised on natural pasture. Animal 2, 298–304.
| Tick susceptibility and its effects on growth performance and carcass characteristics of Nguni, Bonsmara and Angus steers raised on natural pasture.Crossref | GoogleScholarGoogle Scholar | 22445024PubMed |
Mwangi DM, McKeever DJ, Nyanjui JK, Barbet AF, Mahan SM (1998) Major antigenic proteins 1 and 2 of Cowdria ruminantium are targets for T-lymphocyte responses of immune cattle. Annals of the New York Academy of Sciences 849, 372–374.
| Major antigenic proteins 1 and 2 of Cowdria ruminantium are targets for T-lymphocyte responses of immune cattle.Crossref | GoogleScholarGoogle Scholar | 9668489PubMed |
Nava S (2017) Epidemiology and control of ticks with medical and veterinary importance in South America. Revista Colombiana de Ciencias Pecuarias 30, 294
Nava S, Beati L, Labruna MB, Cáceres AG, Mangold AJ, Guglielmone AA (2014) Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum, and Amblyomma sculptum (Ixodida: Ixodidae). Ticks and Tick-Borne Diseases 5, 252–276.
| Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum, and Amblyomma sculptum (Ixodida: Ixodidae).Crossref | GoogleScholarGoogle Scholar | 24556273PubMed |
Neelakanta G, Sultana H, Sonenshine DE, Andersen JF (2018) Identification and characterization of a histamine-binding lipocalin-like molecule from the relapsing fever tick Ornithodoros turicata. Insect Molecular Biology 27, 177–187.
| Identification and characterization of a histamine-binding lipocalin-like molecule from the relapsing fever tick Ornithodoros turicata.Crossref | GoogleScholarGoogle Scholar | 29164729PubMed |
Neitz AW, Gothe R, Pawlas S, Groeneveld HT (1993) Investigations into lymphocyte transformation and histamine release by basophils in sheep repeatedly infested with Rhipicephalus evertsi evertsi ticks. Experimental & Applied Acarology 17, 551–559.
| Investigations into lymphocyte transformation and histamine release by basophils in sheep repeatedly infested with Rhipicephalus evertsi evertsi ticks.Crossref | GoogleScholarGoogle Scholar |
Nene V, Kiara H, Lacasta A, Pelle R, Svitek N, Steinaa L (2016) The biology of Theileria parva and control of East Coast fever: current status and future trends. Ticks and Tick-Borne Diseases 7, 549–564.
| The biology of Theileria parva and control of East Coast fever: current status and future trends.Crossref | GoogleScholarGoogle Scholar | 26972687PubMed |
Nunn MA, Sharma A, Paesen GC, Adamson S, Lissina O, Willis AC, Nuttall PA (2005) Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. Journal of Immunology (Baltimore, Md.: 1950) 174, 2084–2091.
| Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata.Crossref | GoogleScholarGoogle Scholar |
Nyangiwe N, Goni S, Hervé-Claude LP, Ruddat I, Horak IG (2011) Ticks on pastures and on two breeds of cattle in the Eastern Cape Province, South Africa. The Onderstepoort Journal of Veterinary Research 78, 1–9.
| Ticks on pastures and on two breeds of cattle in the Eastern Cape Province, South Africa.Crossref | GoogleScholarGoogle Scholar |
Nyangiwe N, Harrison A, Horak IG (2013) Displacement of Rhipicephalus decoloratus by Rhipicephalus microplus (Acari: Ixodidae) in the Eastern Cape Province, South Africa. Experimental & Applied Acarology 61, 371–382.
| Displacement of Rhipicephalus decoloratus by Rhipicephalus microplus (Acari: Ixodidae) in the Eastern Cape Province, South Africa.Crossref | GoogleScholarGoogle Scholar |
O’Kelly JC (1968) Comparative studies of lipid metabolism in zebu and British cattle in a tropical environment. Australian Journal of Biological Sciences 21, 1013–1024.
| Comparative studies of lipid metabolism in zebu and British cattle in a tropical environment.Crossref | GoogleScholarGoogle Scholar | 5701182PubMed |
O’Kelly JC, Kennedy PM (1981) Metabolic changes in cattle due to the specific effect on the tick, Boophilus microplus. British Journal of Nutrition 45, 557–566.
| Metabolic changes in cattle due to the specific effect on the tick, Boophilus microplus.Crossref | GoogleScholarGoogle Scholar | 7236581PubMed |
O’Kelly JC, Seifert GW (1969) Relationship between resistance to Boophilus microplus, nutritional status and blood composition in Shorthorn × Hereford cattle. Australian Journal of Biological Sciences 22, 1497–1506.
| Relationship between resistance to Boophilus microplus, nutritional status and blood composition in Shorthorn × Hereford cattle.Crossref | GoogleScholarGoogle Scholar |
O’Kelly JC, Seifert GW (1970) The effects of tick (Boophilus microplus) infestations on the blood composition of Shorthorn × Hereford cattle on high and low planes of nutrition. Australian Journal of Biological Sciences 23, 681–690.
| The effects of tick (Boophilus microplus) infestations on the blood composition of Shorthorn × Hereford cattle on high and low planes of nutrition.Crossref | GoogleScholarGoogle Scholar |
O’Kelly JC, Seebeck RM, Springell PH (1971) Alterations in host metabolism by the specific and anorectic effects of the cattle tick (Boophilus microplus). II. Changes in blood composition. Australian Journal of Biological Sciences 24, 381–389.
| Alterations in host metabolism by the specific and anorectic effects of the cattle tick (Boophilus microplus). II. Changes in blood composition.Crossref | GoogleScholarGoogle Scholar | 5280978PubMed |
O’Kelly JC, Post TB, Bryan RP (1988) The influence of parasitic infestations on metabolism, puberty and first mating performance of heifers grazing in a tropical area. Animal Reproduction Science 16, 177–189.
| The influence of parasitic infestations on metabolism, puberty and first mating performance of heifers grazing in a tropical area.Crossref | GoogleScholarGoogle Scholar |
Paesen GC, Adams PL, Harlos K, Nuttall PA, Stuart DI (1999) Tick histamine-binding proteins: isolation, cloning and three-dimensional structure. Molecular Cell 3, 661–671.
| Tick histamine-binding proteins: isolation, cloning and three-dimensional structure.Crossref | GoogleScholarGoogle Scholar | 10360182PubMed |
Pearce DS, Hoover BA, Jennings S, Nevitt GA, Docherty KM (2017) Morphological and genetic factors shape the microbiome of a seabird species (Oceanodroma leucorhoa) more than environmental and social factors. Microbiome 5, 146
| Morphological and genetic factors shape the microbiome of a seabird species (Oceanodroma leucorhoa) more than environmental and social factors.Crossref | GoogleScholarGoogle Scholar | 29084611PubMed |
Pienaar R, Potgieter FT, Latif AA, Thekisoe OM, Mans BJ (2011) Mixed Theileria infections in free-ranging buffalo herds: implications for diagnosing Theileria parva infections in Cape buffalo (Syncerus caffer). Parasitology 138, 884–895.
| Mixed Theileria infections in free-ranging buffalo herds: implications for diagnosing Theileria parva infections in Cape buffalo (Syncerus caffer).Crossref | GoogleScholarGoogle Scholar | 21524322PubMed |
Piper EK, Jonsson NN, Gondro C, Lew-Tabor EE, Moolhuijzen P, Vance ME, Jackson LA (2009) Immunological profiles of Bos taurus and Bos indicus cattle infested with the cattle tick, Rhipicephalus (Boophilus) microplus. Clinical and Vaccine Immunology; CVI 16, 1074–1086.
| Immunological profiles of Bos taurus and Bos indicus cattle infested with the cattle tick, Rhipicephalus (Boophilus) microplus.Crossref | GoogleScholarGoogle Scholar | 19474263PubMed |
Piper EK, Jackson LA, Bielefeldt-Ohmann H, Gondro C, Lew-Tabor AE, Jonsson NN (2010) Tick-susceptible Bos taurus cattle display an increased cellular response at the site of larval Rhipicephalus (Boophilus) microplus attachment, compared with tick-resistant Bos indicus cattle. International Journal for Parasitology 40, 431–441.
| Tick-susceptible Bos taurus cattle display an increased cellular response at the site of larval Rhipicephalus (Boophilus) microplus attachment, compared with tick-resistant Bos indicus cattle.Crossref | GoogleScholarGoogle Scholar | 19852965PubMed |
Piper EK, Jonsson NN, Gondro C, Vance ME, Lew-Tabor A, Jackson LA (2017) Peripheral cellular and humoral responses to infestation with the cattle tick Rhipicephalus microplus in Santa Gertrudis cattle. Parasite Immunology 39,
| Peripheral cellular and humoral responses to infestation with the cattle tick Rhipicephalus microplus in Santa Gertrudis cattle.Crossref | GoogleScholarGoogle Scholar | 27862028PubMed |
Porto Neto L, Bunch R, Harrison BE, Prayaga K, Barendse W (2010) Haplotypes that include the integrin alpha 11 gene associated with tick burden in cattle. BMC Genetics 11, 55
| Haplotypes that include the integrin alpha 11 gene associated with tick burden in cattle.Crossref | GoogleScholarGoogle Scholar | 20565915PubMed |
Porto Neto LR, Bunch RJ, Harrison BE, Barendse W (2011a) DNA variation in the gene ELTD1 is associated with tick burden in cattle. Animal Genetics 42, 50–55.
| DNA variation in the gene ELTD1 is associated with tick burden in cattle.Crossref | GoogleScholarGoogle Scholar | 20880337PubMed |
Porto Neto LR, Jonsson NN, D’Occhio MJ, Barendse W (2011b) Molecular genetic approaches for identifying the basis of variation in resistance to tick infestation in cattle. Veterinary Parasitology 180, 165–172.
| Molecular genetic approaches for identifying the basis of variation in resistance to tick infestation in cattle.Crossref | GoogleScholarGoogle Scholar | 21700395PubMed |
Porto Neto LR, Jonsson NN, Ingham A, Bunch RJ, Harrison BE, Barendse W (2012) The RIPK2 gene: a positional candidate for tick burden supported by genetic associations in cattle and immunological response of knockout mouse. Immunogenetics 64, 379–388.
| The RIPK2 gene: a positional candidate for tick burden supported by genetic associations in cattle and immunological response of knockout mouse.Crossref | GoogleScholarGoogle Scholar | 22314416PubMed |
Porto-Neto LR, Reverter A, Prayaga KC, Chan EKF, Johnston DJ, Bolormaa S, Goddard ME, Hawken RJ, Fordyce G, Garcia JF, Sonstegard TS, Burrow HM, Henshall JM, Lehnert SA, Barendse W (2014) The genetic architecture of climatic adaptation in tropical cattle. PLoS One
| The genetic architecture of climatic adaptation in tropical cattle.Crossref | GoogleScholarGoogle Scholar | 25419663PubMed |
Prayaga KC, Corbet NJ, Johnston DJ, Wolcott ML, Fordyce G, Burrow HM (2009) Genetics of adaptive traits in heifers and their relationship to growth, pubertal and carcass traits in two tropical beef cattle genotypes. Animal Production Science 49, 413–425.
| Genetics of adaptive traits in heifers and their relationship to growth, pubertal and carcass traits in two tropical beef cattle genotypes.Crossref | GoogleScholarGoogle Scholar |
Raghavan RK, Peterson AT, Cobos ME, Ganta R, Foley D (2019) Current and future distribution of the Lone Star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS One 14, e0209082
| Current and future distribution of the Lone Star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America.Crossref | GoogleScholarGoogle Scholar | 30601855PubMed |
Reis APA, Boligon AA, Yokoo MJ, Cardoso FF (2017) Design of selection schemes to include tick resistance in the breeding goal for Hereford and Braford cattle. Journal of Animal Science 95, 572–583.
Ribeiro JM (1987) Ixodes dammini: salivary anti-complement activity. Experimental Parasitology 64, 347–353.
| Ixodes dammini: salivary anti-complement activity.Crossref | GoogleScholarGoogle Scholar | 3119364PubMed |
Riek RF (1962) Studies on the reactions of animals to infestation with ticks. VI. Resistance of cattle to infestation with the tick Boophilus microplus (Canestrini). Australian Journal of Agricultural Research 13, 532–550.
| Studies on the reactions of animals to infestation with ticks. VI. Resistance of cattle to infestation with the tick Boophilus microplus (Canestrini).Crossref | GoogleScholarGoogle Scholar |
Robbertse L, Richards SA, Maritz-Olivier C (2017) Bovine immune factors underlying tick resistance: integration and future directions. Frontiers in Cellular and Infection Microbiology 7, 522
| Bovine immune factors underlying tick resistance: integration and future directions.Crossref | GoogleScholarGoogle Scholar | 29312898PubMed |
Robbertse L, Richards SA, Clift SJ, Barnard AC, Leisewitz A, Crafford JE, Maritz-Oliver C (2018) Comparison of the differential regulation of T and B-lymphocyte subsets in the skin and lymph nodes amongst three cattle breeds as potential mediators of immune resistance to Rhipicephalus microplus. Ticks and Tick Borne Diseases 9, 976–987.
Rodriguez-Valle M, Moolhuijzen P, Piper EK, Weiss O, Vance M, Bellgard M, Lew-Tabor A (2013) Rhipicephalus microplus lipocalins (LRMs): genomic identification and analysis of the bovine immune response using in silico predicted B and T cell epitopes. International Journal for Parasitology 43, 739–752.
| Rhipicephalus microplus lipocalins (LRMs): genomic identification and analysis of the bovine immune response using in silico predicted B and T cell epitopes.Crossref | GoogleScholarGoogle Scholar | 23747800PubMed |
Rong G, Corrie SR, Clark HA (2017) In vivo biosensing: progress and perspectives. ACS Sensors 2, 327–338.
| In vivo biosensing: progress and perspectives.Crossref | GoogleScholarGoogle Scholar | 28723197PubMed |
Roy BC, Estrada-Peña A, Krücken J, Rehman A, Nijhof AM (2018) Morphological and phylogenetic analyses of Rhipicephalus microplus ticks from Bangladesh, Pakistan and Myanmar. Ticks and Tick-Borne Diseases
| Morphological and phylogenetic analyses of Rhipicephalus microplus ticks from Bangladesh, Pakistan and Myanmar.Crossref | GoogleScholarGoogle Scholar | 29661691PubMed |
Ruiz-Rodríguez M, Valdivia E, Soler JJ, Martin-Vivaldi M, Martin-Platero AM, Martinex-Bueno M (2009) Symbiotic bacteria living in the hoopoe’s uropygial gland prevent feather degradation. The Journal of Experimental Biology 212, 3621–3626.
| Symbiotic bacteria living in the hoopoe’s uropygial gland prevent feather degradation.Crossref | GoogleScholarGoogle Scholar | 19880722PubMed |
Sahibi H, Rhalem A, Tikki N, Ben Kouka F, Barriga O (1997) Hyalomma ticks: bovine resistance under field conditions as related to host age and breed. Parasite 4, 159–165.
| Hyalomma ticks: bovine resistance under field conditions as related to host age and breed.Crossref | GoogleScholarGoogle Scholar | 9296059PubMed |
Sangamnatdej S, Paesen GC, Slovak M, Nuttall PA (2002) A high affinity serotonin- and histamine-binding lipocalin from tick saliva. Insect Molecular Biology 11, 79–86.
| A high affinity serotonin- and histamine-binding lipocalin from tick saliva.Crossref | GoogleScholarGoogle Scholar | 11841505PubMed |
Scholtz MM, Spickett AM, Lombard PE, Enslin CB (1991) The effect of tick infestation on the productivity of cows of three breeds of cattle. The Onderstepoort Journal of Veterinary Research 58, 71–74.
Schroeder H, Daix V, Gillet L, Renauld JC, Vanderplasschen A (2007) The paralogous salivary anti-complement proteins IRAC I and IRAC II encoded by Ixodes ricinus ticks have broad and complementary inhibitory activities against the complement of different host species. Microbes and Infection 9, 247–250.
| The paralogous salivary anti-complement proteins IRAC I and IRAC II encoded by Ixodes ricinus ticks have broad and complementary inhibitory activities against the complement of different host species.Crossref | GoogleScholarGoogle Scholar | 17223370PubMed |
Schuijt TJ, Coumou J, Narasimhan S, Dai J, Deponte K, Wouters D, Brouwer M, Oei A, Roelofs JJ, van Dam AP, van der Poll T, Van’t Veer C, Hovius JW, Fikrig E (2011) A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell Host & Microbe 10, 136–146.
| A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent.Crossref | GoogleScholarGoogle Scholar |
Seebeck RM, Springell PH, O’Kelly JC (1971) Alterations in host metabolism by the specific and anorectic effects of the cattle tick (Boophilus microplus) I. Food intake and body weight growth. Australian Journal of Biological Sciences 24, 373–380.
| Alterations in host metabolism by the specific and anorectic effects of the cattle tick (Boophilus microplus) I. Food intake and body weight growth.Crossref | GoogleScholarGoogle Scholar | 5280977PubMed |
Seifert GW (1971) Variations between and within breeds of cattle in resistance to field infestations on the cattle tick (Boophilus microplus). Australian Journal of Agricultural Research 22, 159–168.
| Variations between and within breeds of cattle in resistance to field infestations on the cattle tick (Boophilus microplus).Crossref | GoogleScholarGoogle Scholar |
Seifert GW, Springell PH, Tatchell RJ (1968) Radioactive studies on the feeding of larvae, nymphs and adults of the cattle tick Boophilus microplus (Canestrini). Parasitology 58, 415–430.
| Radioactive studies on the feeding of larvae, nymphs and adults of the cattle tick Boophilus microplus (Canestrini).Crossref | GoogleScholarGoogle Scholar |
Silva NC, Vale VF, Franco PF, Gontijo NF, Valenzuela JG, Pereira MH, Sant’Anna MR, Rodrigues DS, Lima WS, Fux B, Araujo RN (2016) Saliva of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) inhibits classical and alternative complement pathways. Parasites & Vectors 9, 445
| Saliva of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) inhibits classical and alternative complement pathways.Crossref | GoogleScholarGoogle Scholar |
Simoes MRS (2017) Bioeconomic models for genetic improvement of the Brangus breed. MSc Thesis, Federal University of Pelotas, Pelotas, Rio Grande do Sul, Brazil.
Snowball GJ (1956) The effect of self-licking by cattle on infestations of cattle tick, Boophilus microplus (Canestrini). Australian Journal of Agricultural Research 7, 227–232.
| The effect of self-licking by cattle on infestations of cattle tick, Boophilus microplus (Canestrini).Crossref | GoogleScholarGoogle Scholar |
Sonenshine DE (2004) Pheromones and other semiochemicals of ticks and their use in tick control. Parasitology 129, S405–S425.
| Pheromones and other semiochemicals of ticks and their use in tick control.Crossref | GoogleScholarGoogle Scholar | 15938521PubMed |
Sonenshine DE (2006) Tick pheromones and their use in tick control. Annual Review of Entomology 51, 557–580.
| Tick pheromones and their use in tick control.Crossref | GoogleScholarGoogle Scholar | 16332223PubMed |
Sonenshine DE (2018) Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. International Journal of Environmental Research and Public Health 15,
| Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease.Crossref | GoogleScholarGoogle Scholar | 29522469PubMed |
Spickett AM, De Klerk D, Enslin CB, Scholtz MM (1989) Resistance of Nguni, Bonsmara and Hereford cattle to ticks in a bushveld region of South Africa. The Onderstepoort Journal of Veterinary Research 56, 245–250.
Springell PH, O’Kelly JC, Seebeck RM (1971) Alterations in host metabolism by the specific and anorectic effects of the cattle tick (Boophilus microplus). III. Metabolic implications of blood volume, body water and carcass composition changes. Australian Journal of Biological Sciences 24, 1033–1045.
| Alterations in host metabolism by the specific and anorectic effects of the cattle tick (Boophilus microplus). III. Metabolic implications of blood volume, body water and carcass composition changes.Crossref | GoogleScholarGoogle Scholar | 5159198PubMed |
Stear MJ, Nicholas FW, Brown SC, Holroyd RG (1989) Class I antigens of the bovine major histocompatibility system and resistance to the cattle tick (Boophilus microplus) assessed in three different seasons. Veterinary Parasitology 31, 303–315.
| Class I antigens of the bovine major histocompatibility system and resistance to the cattle tick (Boophilus microplus) assessed in three different seasons.Crossref | GoogleScholarGoogle Scholar | 2763449PubMed |
Stear MJ, Hetzel DJSH, Brown SC, Gershwin LJ, Mackinnon MJ, Nicholas FW (1990) The relationships among ecto- and endoparasite levels, class I antigens of the bovine major histocompatibility system, immunoglobulin E levels and weight gain. Veterinary Parasitology 34, 303–321.
| The relationships among ecto- and endoparasite levels, class I antigens of the bovine major histocompatibility system, immunoglobulin E levels and weight gain.Crossref | GoogleScholarGoogle Scholar | 2316176PubMed |
Sutherst RW (1983a) The numbers of bush ticks, Haemaphysalis longicornis, parasitic on grazing cattle before and after the acquisition of host resistance. Australian Veterinary Journal 60, 20–21.
| The numbers of bush ticks, Haemaphysalis longicornis, parasitic on grazing cattle before and after the acquisition of host resistance.Crossref | GoogleScholarGoogle Scholar | 6681952PubMed |
Sutherst RW (1983b) Variation in the numbers of the cattle tick, Boophilus microplus (Canestrini), in a moist habitat made marginal by low temperatures. Austral Entomology 22, 1–5.
| Variation in the numbers of the cattle tick, Boophilus microplus (Canestrini), in a moist habitat made marginal by low temperatures.Crossref | GoogleScholarGoogle Scholar |
Sutherst RW, Maywald GF, Kerr JD, Stegeman DA (1983a) The effect of cattle tick (Boophilus microplus) on the growth of Bos indicus × B. taurus steers. Australian Journal of Agricultural Research 34, 317–327.
| The effect of cattle tick (Boophilus microplus) on the growth of Bos indicus × B. taurus steers.Crossref | GoogleScholarGoogle Scholar |
Sutherst RW, Kerr JD, Maywald GF, Stegeman DA (1983b) The effect of season and nutrition on the resistance of cattle to the tick Boophilus microplus. Australian Journal of Agricultural Research 34, 329–339.
| The effect of season and nutrition on the resistance of cattle to the tick Boophilus microplus.Crossref | GoogleScholarGoogle Scholar |
Sutherst RW, Maywald GF, Bourne AS, Sutherland ID, Stegeman DA (1988) Ecology of the cattle tick (Boophilus microplus) in subtropical Australia. II. Resistance of different breeds of cattle. Australian Journal of Agricultural Research 39, 299–308.
| Ecology of the cattle tick (Boophilus microplus) in subtropical Australia. II. Resistance of different breeds of cattle.Crossref | GoogleScholarGoogle Scholar |
Tatchell RJ, Bennett GF (1969) Boophilus microplus: antihistaminic and tranquilizing drugs and cattle resistance. Experimental Parasitology 26, 369–377.
| Boophilus microplus: antihistaminic and tranquilizing drugs and cattle resistance.Crossref | GoogleScholarGoogle Scholar | 4396213PubMed |
Tønnesen MH, Penzhorn B, Bryson NR, Masibigiri T (2004) Displacement of Boophilus decoloratus by Boophilus microplus in the Soutpansberg region, Limpopo Province, South Africa. Experimental & Applied Acarology 32, 199–208.
| Displacement of Boophilus decoloratus by Boophilus microplus in the Soutpansberg region, Limpopo Province, South Africa.Crossref | GoogleScholarGoogle Scholar |
Turner HG (1982) Genetic variation of rectal temperature in cows and its relationship to fertility. Animal Production 35, 401–412.
| Genetic variation of rectal temperature in cows and its relationship to fertility.Crossref | GoogleScholarGoogle Scholar |
Turner HG, Short AJ (1972) Effects of field infestations of gastrointestinal helminths and of the cattle tick (Boophilus microplus) on growth of three breeds of cattle. Australian Journal of Agricultural Research 23, 177–193.
| Effects of field infestations of gastrointestinal helminths and of the cattle tick (Boophilus microplus) on growth of three breeds of cattle.Crossref | GoogleScholarGoogle Scholar |
Turner LB, Harrison BE, Bunch RJ, Porto Neto LR, Li Y, Barendse W (2010) A genome-wide association study of tick burden and milk composition in cattle. Animal Production Science 50, 235–245.
| A genome-wide association study of tick burden and milk composition in cattle.Crossref | GoogleScholarGoogle Scholar |
Utech KBW, Wharton RH (1982) Breeding for resistance to Boophilus microplus in Australian Illawarra Shorthorn and Brahman × Australia Illawarra Shorthorn cattle. Australian Veterinary Journal 58, 41–46.
| Breeding for resistance to Boophilus microplus in Australian Illawarra Shorthorn and Brahman × Australia Illawarra Shorthorn cattle.Crossref | GoogleScholarGoogle Scholar |
Utech KBW, Wharton RH, Kerr JD (1978) Resistance to Boophilus microplus (Canestrini) in different breeds of cattle. Australian Journal of Agricultural Research 29, 885–895.
| Resistance to Boophilus microplus (Canestrini) in different breeds of cattle.Crossref | GoogleScholarGoogle Scholar |
Valdés JJ (2014) Antihistamine response: a dynamically refined function at the host-tick interface. Parasites & Vectors 7, 491
| Antihistamine response: a dynamically refined function at the host-tick interface.Crossref | GoogleScholarGoogle Scholar |
Valenzuela JG, Charlab R, Mather TN, Ribeiro JM (2000) Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. The Journal of Biological Chemistry 275, 18717–18723.
| Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis.Crossref | GoogleScholarGoogle Scholar | 10749868PubMed |
Vančová I, Slovák M, Hajnická V, Labuda M, Simo L, Peterková K, Hails RS, Nuttall PA (2007) Differential anti-chemokine activity of Amblyomma variegatum adult ticks during blood-feeding. Parasite Immunology 29, 169–177.
| Differential anti-chemokine activity of Amblyomma variegatum adult ticks during blood-feeding.Crossref | GoogleScholarGoogle Scholar | 17371454PubMed |
Vančová I, Hajnická V, Slovák M, Nuttall PA (2010) Anti-chemokine activities of ixodid ticks depend on tick species, developmental stage, and duration of feeding. Veterinary Parasitology 167, 274–278.
| Anti-chemokine activities of ixodid ticks depend on tick species, developmental stage, and duration of feeding.Crossref | GoogleScholarGoogle Scholar | 19836889PubMed |
Voehringer D (2011) Basophils in immune responses against helminths. Microbes and Infection 13, 881–887.
| Basophils in immune responses against helminths.Crossref | GoogleScholarGoogle Scholar | 21658463PubMed |
Wagland BM, Sutherst RW, Roberts JA (1985) Relationship between the resistance of cattle to Haemaphysalis longicornis and to Boophilus microplus. Australian Veterinary Journal 62, 308–310.
| Relationship between the resistance of cattle to Haemaphysalis longicornis and to Boophilus microplus.Crossref | GoogleScholarGoogle Scholar | 4074218PubMed |
Walker JB, Olwage A (1987) The tick vectors of Cowdria ruminantium (Ixodoidea, Ixodidae, genus Amblyomma) and their distribution. The Onderstepoort Journal of Veterinary Research 54, 353–379.
Walker JB, Keirans JE, Horak IG (2000) ‘The genus Rhipicephalus (Acari: Ixodidae): a guide to the brown ticks of the world.’ (Cambridge University Press: Cambridge, UK)
Walker AR, Bouattour A, Camicas J-L, Estrada-Peña A, Horak IG, Latif AA, Pegram RG, Preston P (2003) Ticks of domestic animals in Africa: a guide to identification of species. Bioscience reports, Edinburgh, UK.
Wambura PN, Gwakisa PS, Silayo RS, Rugaimumamu EA (1998) Breed-associated resistance to tick infestation in Bos indicus and their crosses with Bos taurus. Veterinary Parasitology 77, 63–70.
| Breed-associated resistance to tick infestation in Bos indicus and their crosses with Bos taurus.Crossref | GoogleScholarGoogle Scholar | 9652384PubMed |
Wang YH, Reverter A, Kemp D, McWilliam SM, Ingham A, Davis CA, Moore RJ, Lehnert SA (2007) Gene expression profiling of Hereford Shorthorn cattle following challenge with Boophilus microplus tick larvae. Australian Journal of Experimental Agriculture 47, 1397–1407.
| Gene expression profiling of Hereford Shorthorn cattle following challenge with Boophilus microplus tick larvae.Crossref | GoogleScholarGoogle Scholar |
Wang Y, Li Z, Zhou Y, Cao J, Zhang H, Gong H, Zhou J (2016) Specific histamine binding activity of a new lipocalin from Hyalomma asiaticum (Ixodidae) and therapeutic effects on allergic asthma in mice. Parasites & Vectors 9, 506
| Specific histamine binding activity of a new lipocalin from Hyalomma asiaticum (Ixodidae) and therapeutic effects on allergic asthma in mice.Crossref | GoogleScholarGoogle Scholar |
Wharton RH, Utech KBW (1970) The relation between engorgement and dropping of Boophilus microplus (Canestrini) (Ixodidae) to the assessment of tick numbers on cattle. Journal of the Australian Entomological Society 9, 171–182.
| The relation between engorgement and dropping of Boophilus microplus (Canestrini) (Ixodidae) to the assessment of tick numbers on cattle.Crossref | GoogleScholarGoogle Scholar |
Wikel SK (1996) Host immunity to ticks. Annual Review of Entomology 41, 1–22.
| Host immunity to ticks.Crossref | GoogleScholarGoogle Scholar | 8546443PubMed |
Willadsen P, Wood GM, Riding GA (1979) The relation between skin histamine concentration, histamine sensitivity, and the resistance of cattle to the tick, Boophilus microplus. Parasitology Research 59, 87–93.
Zawadowsky MM (1931) Zebu-Yak hybrids: sterility of bulls, fertility of cows and material on the genetics of Zebu-Yak hybrids. The Journal of Heredity 22, 297–313.
| Zebu-Yak hybrids: sterility of bulls, fertility of cows and material on the genetics of Zebu-Yak hybrids.Crossref | GoogleScholarGoogle Scholar |


